Abstract
Hypophosphatasia (HPP) is a rare disorder resulting from biallelic loss-of-function variants or monoallelic dominant negative variants in the ALPL gene. We herein describe the clinical outcome of a 32-year-old woman with childhood-onset HPP caused by compound heterozygous variants in ALPL. Her chief complaints were severe musculoskeletal pain, muscle weakness, and impaired daily activities necessitating assistance in housework and child-rearing in addition to a history of early tooth loss and mildly short stature. Asfotase alfa therapy produced a remarkable increase in muscle strength and daily activities and markedly reduced musculoskeletal pain. Drug efficacy was clearly demonstrated through multiple test batteries (muscle strength test using microFET®2, six-minute walking test, Stair Climb Test, rising-from-floor-time test, and number-of-steps test using Actigraph®) currently adopted as standardized evaluations in Duchenne muscular dystrophy clinical trials since no test batteries for HPP have been established to date. These tests may also be promising for the assessment of HPP.
Keywords: Hypophosphatasia, Alkaline phosphatase, Genetic disease, Asfotase alfa, Recombinant gene therapy, Motor function
1. Introduction
Hypophosphatasia (HPP) is a rare disorder caused by biallelic loss-of-function variants or monoallelic dominant negative variants in the ALPL gene encoding tissue non-specific alkaline phosphatase (TNSALP) [1]. HPP is classified into perinatal lethal, prenatal benign, infantile, childhood, adult, and odontohypophosphatasia forms [2]. Asfotase alfa is a human recombinant TNSALP enzyme replacement agent approved for patients with pediatric-onset HPP [3].
An open-label study of infants and young children with severe HPP found that treatment with asfotase alfa for up to 7 years improved HPP-related skeletal abnormalities [4]. Asfotase alfa for infantile and childhood HPP was also shown to ameliorate motor function and height gains [5]. To date, however, there are no data on the efficacy of asfotase alfa in adult patients with childhood-onset HPP exhibiting musculoskeletal complications and impaired daily activities.
We herein describe the clinical outcome of a woman with HPP who had experienced the loss of her deciduous teeth in early childhood and displayed severe musculoskeletal pain, muscle weakness, and decreased daily activities. Successful treatment with asfotase alfa was clearly demonstrated using multiple detailed test batteries for motor function, pain, and activities of daily living (ADL) currently adopted in clinical trials of Duchenne muscular dystrophy (DMD).
2. Material and method
2.1. Subject
The patient was a 32-year-old woman born to non-consanguineous Japanese parents. At one year of age, she began losing her deciduous teeth, which had erupted from the age of 7 months. A concomitant serum ALP level of under 10 IU/L (normal range: 395–1289 IU/L according to Japan Society of Clinical Chemistry [JSCC] consensus) resulted in the diagnosis of HPP. She showed mild growth restriction during childhood; her height was 75.5 cm (−1.7 standard deviation [SD]) at 19 months of age and 86.2 cm (−1.9 SD) at the age of 3 years and 1 month. She lost her adult teeth during college and experienced significant restrictions in ADL due to severe musculoskeletal pain and generalized fatigue. Genetic testing revealed compound heterozygous pathogenic variants in ALPL, namely c.[572A>G];[1276G>A], p.[Glu191Gly];[Gly426Ser].
Upon presentation at our department at the age of 31 years, her height was 147 cm (−2.0 SD) and weight was 39 kg (−2.5 SD). She complained of severe headaches and neck pain that caused nausea and had generalized musculoskeletal pain leading to difficulties in housework and child-rearing. Her serum ALP level was 6 IU/L (normal range: 106–322 IU/L according to JSCC consensus). She commenced asfotase alpha (Strensiq®) therapy at 6 mg/kg/week subcutaneously, which produced remarkable decreases in plasma pyridoxal 5′-phosphate (PLP) and PLP/pyridoxal ratio from 145.4 nmol/L and 27.8 to 8.4 nmol/L and 0.5, respectively [6]. Dual-energy X-ray absorptiometry showed a mild increase in bone mineral density from 1.001 g/cm2 at the lumbar 1–4 region (L1–4) (Z-score: −1.0) and 0.615 g/cm2 at the right total hip (Z-score: −2.5) before treatment to 1.042 g/cm2 at L1–4 (Z-score: −0.8) and 0.651 g/cm2 at the right total hip (Z-score: −2.3) after 7 months of therapy. A high-precision body component analyzer (InBody770®; InBody Japan, Tokyo, Japan) revealed slightly increased skeletal muscle mass from 14.8 kg before treatment to 15.2 kg after 12 months of therapy (normal range: 16.8–20.6 kg).
2.2. Motor function measurements
Motor function was measured using the following five test batteries, which have been adopted in DMD clinical trials [7]. MicroFET®2 (Hoggan Scientific, LLC, Salt Lake City, USA) was used to measure abduction strength of the hip joints, flexion and extension of the knee joints, flexion of the shoulder joints, and flexion and extension of the elbow joints. The six-minute walking test (6MWT), Stair Climb Test, and rising-from-floor-time test were employed to measure overall motor function. The number-of-steps test via Actigraph® (MTN-220, ESTERA, Saitama, Japan) was used to evaluate the total amount of physical activity. All measurements were performed by an experienced physiotherapist (HN) engaged in clinical trials of DMD. Additionally, a visual analog scale (VAS) was used to obtain total pain values and locations of the most severe pain in her daily life. This study was approved by the Ethics Committee of Shinshu University School of Medicine (#4654). Written consent was obtained from the patient.
3. Results
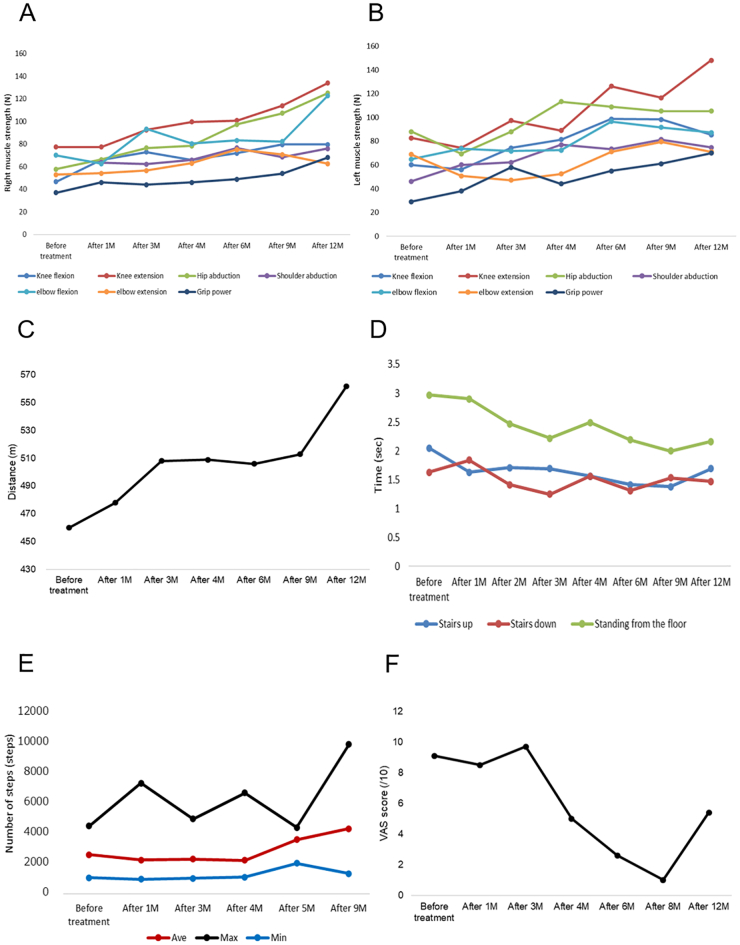
MicroFET®2 analysis revealed gradually increased strength in all muscles investigated (Fig. 1A, B). The 6MWT showed a marked increase in distance from 460 m before treatment to 562 m after 12 months of therapy (Fig. 1C). Both Stair Climb Test (up and down) and rising-from-floor-time test scores were improved by the treatment (Fig. 1D). The average number of steps she could climb increased from 2591 to 4307 (Fig. 1E). Her VAS score for pain was 9.5/10 before therapy, with multiple joints of the extremities affected and the most severe pain being in the neck. Although her pain score was drastically reduced to 1.0 after 8 months of treatment (Fig. 1F), it increased to 5.4 at 12 months of therapy due to greater daily activity from improved motor function and pain.
Fig. 1.
Efficacy of asfotase alfa evaluated through multiple test batteries.
Muscle strength measured by microFET®2 on (A) the right side and (B) the left side. Strength in various muscles showed consistent improvement. (C) Distances in the 6-min walking test. Walking distance increased during 1–3 months and 9–12 months of treatment, with a plateau at 3–9 months. (D) Results of the Stair Climb Test and rising-from-floor-time test.
The time for climbing up or down stairs and the time for standing from the floor decreased.
(E) Number of steps measured by Actigraph®. Average and minimum step numbers increased after 4 months of treatment, while the maximum number of steps fluctuated. (F) VAS scores for whole-body pain. Pain scores reduced after 4 months of the treatment. However, pain increased after 12 months of therapy, presumably due to an increase in activities associated with improved pain and motor function.
4. Discussion
This is the first report to present detailed data on improved motor function and relevant symptoms in an adult patient with childhood-onset HPP by asfotase alfa therapy. Drug efficacy was clearly demonstrated using multiple test batteries already adopted in DMD clinical trials due to no established testing for motor function in HPP [8,9,10,11].
Instantaneous muscle strength as measured by microFET®2 showed marked enhancements in the upper (shoulders and elbows) and lower (hips and knees) limbs, which was consistent with the increased muscle mass detected by the InBody770®. The general strength and endurance of anti-gravity muscles as evaluated by the 6MWT, Stair Climb Test, and rising-from-floor-time test also revealed gains from the first month of therapy. Regarding total activities of daily life, Actigraph® showed an increase in the average number of steps from approximately four months of treatment. Pain levels estimated by VAS scores indicated marked relief until 8 months, with subsequent worsening that was attributed to an increase in daily activity levels. Asfotase alfa therefore appeared to exert noticeable effects on instantaneous and endurable muscle strength. However, the precise mechanism remains unknown due to no available muscle histopathology or laboratory results. Moreover, her pain relief might have been associated with increased muscle strength in addition to other independent effects.
The efficacy of 5-year asfotase alfa therapy on motor function was earlier demonstrated in 19 adolescent or adult patients with infantile or pediatric HPP. The 6MWT was used as a validated measure in combination with multiple exploratory tests, including the Bruininks-Oseretsky Test of Motor Proficiency (Running Speed and Agility subset and Strength subtest) for evaluating gross motor function, hand-held dynamometry for testing muscle strength of the hip extensors and abductors, the Lower Extremity Functional Scale to calculate the performance of transitional movements, locomotion, climbing stairs, and squatting, and the Brief Pain Inventory-Short Form for assessing pain [12]. Phillips et al. also showed high test-retest reliability (r = 0.95; p < 0.0001) for the 6MWT in HPP as well as sufficient validity (i.e., walking distance increased as the severity of skeletal disease decreased) [13].
Indicating the smallest benefit of value to the patient [14], the minimally clinically important difference (MCID) of the 6MWT is reportedly 31 m in adults with pediatric-onset HPP and 43 m in adolescents with pediatric-onset HPP [13]. In the present case, the 6MWT distance surpassed both MCID values: 48 m at 3 months of treatment and 102 m at 12 months.
5. Conclusion
Asfotase alfa treatment was successful in an adult woman with childhood-onset HPP by markedly increasing muscle strength and daily activities while reducing musculoskeletal pain. Multiple test batteries, such as those adopted in DMD clinical trials, may also be useful to comprehensively evaluate motor function in HPP.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
None.
Acknowledgements
We thank the patient for her participation in this study. We are also grateful to Trevor Ralph, M.S., from Ez Communications (http://www.ezcomm.biz/teacher/index.html), for editing a draft of this manuscript.
Contributor Information
Hitomi Nishizawa, Email: hitnishi@shinshu-u.ac.jp.
Tomoki Kosho, Email: ktomoki@shinshu-u.ac.jp.
References
- 1.Whyte M.P. Hypophosphatasia. In: Glorieux J.M., Juppner H., editors. Pediatric Bone: Biology & Diseases. 3rd ed. Academic Press; San Diego: 2012. pp. 771–794. [Google Scholar]
- 2.Whyte M.P. Heritable metabolic and dysplastic bone diseases. Endocrinol. Metab. Clin. N. Am. 1990;19:133–173. [PubMed] [Google Scholar]
- 3.Whyte M.P., Valdes R., Jr., Ryan L.M. Infantile hypophosphatasia: enzyme replacement therapy by intravenous infusion of alkaline phosphatase-rich plasma from patients with Paget bone disease. J. Pediatr. 1982;101:379–386. doi: 10.1016/s0022-3476(82)80061-9. [DOI] [PubMed] [Google Scholar]
- 4.Whyte M.P., Simmons J.H., Moseley S. Asfotase alfa for infants and young children with hypophosphatasia: 7 year outcomes of a single-arm, open-label, phase 2 extension trial. Lancet Diabetes Endocrinol. 2019;7:93–105. doi: 10.1016/S2213-8587(18)30307-3. [DOI] [PubMed] [Google Scholar]
- 5.Whyte M.P., Madson K.L., Phillips D. Asfotase alfa therapy for children with hypophosphatasia. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama T., Kubota T., Ozono K. Pyridoxal 5′-phosphate and related metabolites in hypophosphatasia: effects of enzyme replacement therapy. Mol. Genet. Metab. 2018;125:174–180. doi: 10.1016/j.ymgme.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Shieh P.B., Mcintosh J., Jin F. Deflazacort versus prednisone/prednisolone for maintaining motor function and delaying loss of ambulation: a post HOC analysis from the ACT DMD trial. Muscle Nerve. 2018;58:639–645. doi: 10.1002/mus.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merlini L., Sabatelli P. Improving clinical trial design for Duchenne muscular dystrophy. BMC Neurol. 2015;153:1–6. doi: 10.1186/s12883-015-0408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald C.M., Henricson E.K., Abresch R.T. The 6-minute walk test and other clinical endpoints in Duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve. 2013;48:357–368. doi: 10.1002/mus.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy S., Schädelin S., Hafner P. Longitudinal reliability of outcome measures in patients with Duchenne muscular dystrophy. Muscle Nerve. 2020;61:63–68. doi: 10.1002/mus.26690. [DOI] [PubMed] [Google Scholar]
- 11.De Lattre C., Payan C., Vuillerot C. Motor function measure: validation of a short form for young children with neuromuscular diseases. Arch. Phys. Med. Rehabil. 2013;94:2218–2226. doi: 10.1016/j.apmr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Kishnani P.S., Rockman-Greenberg C., Rauch F. Five-year efficacy and safety of asfotase alfa therapy for adults and adolescents with hypophosphatasia. Bone. 2019;121:149–162. doi: 10.1016/j.bone.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Phillips D., Tomazos I.C., Moseley S. Reliability and validity of the 6-minute walk test in Hypophosphatasia. JBMR Plus. 2019;3 doi: 10.1002/jbm4.10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGlothlin A.E., Lewis R.J. Minimal clinically important difference. JAMA. 2014;312:1342–1343. doi: 10.1001/jama.2014.13128. [DOI] [PubMed] [Google Scholar]