Abstract
Oxidative stress (OS) in non-alcoholic fatty liver disease (NAFLD) promotes liver injury and inflammation. Treatment with vitamin E (α-tocopherol, αT), a lipid-soluble antioxidant, improves liver injury but also decreases steatosis, thought to be upstream of OS, through an unknown mechanism. To elucidate the mechanism, we combined a mechanistic human trial interrogating pathways of intrahepatic triglyceride (IHTG) accumulation and in vitro experiments. 50% of NAFLD patients (n = 20) treated with αT (200–800 IU/d) for 24 weeks had a ≥ 25% relative decrease in IHTG by magnetic resonance spectroscopy. Paired liver biopsies at baseline and week 4 of treatment revealed a decrease in markers of hepatic de novo lipogenesis (DNL) that strongly predicted week 24 response. In vitro, using HepG2 cells and primary human hepatocytes, αT inhibited glucose-induced DNL by decreasing SREBP-1 processing and lipogenic gene expression. This mechanism is dependent on the antioxidant capacity of αT, as redox-silenced methoxy-αT is unable to inhibit DNL in vitro. OS by itself was sufficient to increase S2P expression in vitro, and S2P is upregulated in NAFLD livers. In summary, we utilized αT to demonstrate a vicious cycle in which NAFLD generates OS, which feeds back to augment DNL and increases steatosis.
Clinicaltrials.gov: NCT01792115.
Keywords: Non-alcoholic fatty liver disease, NAFLD, Vitamin E, Oxidative stress, de novo lipogenesis, S1P, S2P
1. Introduction
Non-alcoholic fatty liver disease (NAFLD), characterized by accumulation of intrahepatic triglycerides (IHTG), is the most common liver disorder in Western Nations [1]. Its progressive form, non-alcoholic steatohepatitis (NASH), is associated with liver injury partially driven by oxidative stress [2], suggesting the use of antioxidants as potential therapy. Vitamin E (α-tocopherol, αT) is a lipid soluble antioxidant [3,4] which localizes to membranes [5,6] and effectively treats NAFLD-associated injury, inflammation [7] and disease progression [8] in clinical trials. However, αT also decreases IHTG both in clinical [7,9,10] and animal studies [11,12] through an unknown mechanism. It's further unclear if this mechanism is dependent on its antioxidant capacity. Vitamin E's biological function is not fully understood [4,13] and it is still debated if the antioxidant activity is the only function in vivo. Putative non-antioxidative functions of vitamin E include inhibition of protein kinase C [14], decrease of platelet aggregation [15] and downregulation of macrophage scavenger receptors [16].
Excess energy intake and peripheral lipolysis induce steatosis and promote oxidative stress in NAFLD [17].
However, this fat-to-oxidative stress unidirectional model does not explain how vitamin E, an antioxidant, decreases IHTG. If the decrease in IHTG is mediated though the antioxidative effect of vitamin E, it would suggest a two-directional model, where oxidative stress, generated by IHTG or its precursors, also drives lipid accumulation. It has been estimated that in NAFLD the majority of fatty acids found in the liver arise from non-esterified fatty acids (NFA, 59%) released from the adipose tissue, followed by hepatic de novo lipogenesis (DNL) (26%) and dietary sources (14%) [18]. While dietary fat uptake is unlikely to be affected by oxidative stress, changes in adipose tissue metabolism, especially mitochondrial respiration [19], or hepatic DNL could potentially be affected by oxidative stress. Finally inhibition of VLDL secretion by oxidative stress [20] could also lead to IHTG accumulation.
We aimed to identify the mechanism by which vitamin E reduces IHTG in patients with NAFLD and whether these changes are mediated by its antioxidant activity. Furthermore, we utilized vitamin E treatment to uncover a pathway by which oxidative stress modulates hepatic lipid content.
2. Materials and methods
2.1. Clinical trial design
A single-center prospective trial (clinicaltrials.gov NCT01792115) with two distinct and separate primary aims, one clinical and one scientific. The primary clinical aim was to determine the minimal effective dose of vitamin E for steatosis reduction and its long-term effect (up to 144 weeks) and will be reported separately. The primary scientific aim of the trial, the focus of this manuscript, was to understand and characterize the mechanism of steatosis reduction by vitamin E. The primary endpoint for the scientific aim was reduction of IHTG by 1H-magnetic resonance spectroscopy (MRS) from baseline (immediately prior to starting vitamin E) to week 24 of treatment. For binary analyses we defined patients as responders if they achieved a relative reduction of ≥ 25% in IHTG. Adult (age > 18) patients were eligible for inclusion if they met 2 of 3 criteria: (1) imaging consistent with steatosis; (2) elevated ALT; and (3) the presence of metabolic syndrome and/or diabetes. Patients were enrolled between May 2013 and November 2016 by hepatologists at the NIDDK Liver Clinic. Exclusion criteria included chronic infections with hepatitis B or C virus, excessive alcohol consumption, substance abuse within the past 12 months, decompensated liver disease, uncontrolled hypo- or hyperthyroidism, uncontrolled diabetes mellitus, current treatment with Vitamin E, coagulopathies, maldigestion, pregnancy or inability to practice contraception, breast feeding. Anemia or history of gastrointestinal bleeding were added as exclusion criteria on June 2015, after we noticed and reported to the Institutional Review Board higher-than expected rates of iron deficiency developing after starting treatment.
All patients underwent a 12 weeks run-in period of lifestyle intervention (Supplementary Fig. S1) which included an individual counseling session with a Registered Dietitian (RD), focused on a balanced diet and individualized recommendations for weight loss, followed by group nutrition education sessions every 2 weeks. Following the run-in phase, patients underwent a baseline evaluation including blood tests, glucose tolerance testing, measurement of liver fat content by 1H-magnetic resonance spectroscopy (MRS) and a percutaneous liver biopsy. Patients with no evidence of NAFLD on biopsy ended their participation at that point. Patients with histological confirmation of NAFLD (either NAFL or NASH) on the baseline biopsy were randomized at a 1:1:1 ratio to receive one of 3 doses of αT (200, 400 or 800 IU/d), for 24 weeks, and were followed at a 1–8 week intervals in a parallel assigned design. Randomization was performed by the NIH Clinical Center Pharmaceutical Development Section using a random numbers’ table and block randomization with block size 6:3:3:3, stratified by NASH vs. NAFL and by diabetes. While on vitamin E treatment, patients were encouraged to continue and adhere to the lifestyle and dietary guidelines of the run-in phase and had repeat individual follow-up sessions with the study RD every 2 months. Compliance to the use of vitamin E was assessed with drug diaries and pill counts. A similar evaluation to baseline was repeated on week 4 of vitamin E treatment, including MRS and a liver biopsy. On week 24, a final MRS was performed to assess treatment response.
Our goal, defined prior to study initiation, was to identify early (week 4) molecular hepatic changes in response to aT treatment that occur prior to major histological improvement, and to determine if these changes predict the clinical response at week 24.
The trial was approved by the NIDDK Institutional Review Board and all subjects provided written informed consent prior to inclusion.
2.2. Human sample processing
Plasma and serum samples were aliquoted and stored at −-80 °C until analysis. Liver biopsy samples were placed in RNALater (Qiagen) or liquid N2 at bedside and stored at −80 °C. Histological samples were scored by a single pathologist (DEK) according to NASH-CRN scoring system [21]. Serum αT was determined by HPLC at the NIH Clinical Center Department of Laboratory Medicine.
2.3. Liver fat measurement
Hepatic fat content was measured using 1H-magnetic resonance spectroscopy (MRS) at 3T (MAGNETOM Verio; Siemens, Tarrytown, NY) [22].
2.4. Lipidomic analysis of liver biopsies
16 paired liver tissue samples (~1 mg) were available for lipidomic analysis using LC-MS (Metabolon Inc. USA). DNL was estimated using the ratio of triglyceride C16:0/C18:2 [[23], [24], [25]], C16:1 and the C16:1/C16:0 ratio [24,[26], [27], [28]].
2.5. De novo lipogenesis in isolated VLDL by LC-MS analysis
VLDL were isolated from fasting plasma and total fatty acids were detected by UPLC-MS/MS. The ratio of C16:0/C18:2, C16:1 and C16:1/C16:0 were used as estimates for DNL.
2.6. Cell culture
HepG2 cells were grown in DMEM normal glucose medium (5.5 mmol/L, NGM), or high glucose medium (25 mmol/L, HGM). Primary human hepatocytes (Lonza, USA, PHH) were cultivated in Lonza HBM TM Basal Media with HCM SingleQuots (Lonza, USA). Cells were allowed to attach overnight, incubated in serum free media for 24 h (except primary hepatocytes) followed by 48 h incubation with test compounds. To induce oxidative stress, cells were incubated overnight with 0.5 μmol/L cumene hydroperoxide (Sigma Aldrich).
2.7. In vitro de novo lipogenesis assays
Cellular triglycerides were extracted using a modified Bligh and Dyer method and triglyceride content was quantified using Infinity triglyceride reagent (Thermo Fisher Scientific). Alternatively, cells were incubated with D-[14C(U)]-glucose (PerkinElmer, USA) and activity was counted in the lipid fraction.
2.8. Statistics
Data was analyzed using Graph Pad Prism 8 for Mac (GraphPad Software, San Diego). Normality was assessed using D'Agostino-Pearson omnibus normality test and significance was tested by paired Student's t-test or Wilcoxon, as appropriate. Fold-change differences between non-responders and responders were analyzed by t-test or Wilcoxon rank sum test, as appropriate. Frequency of reduction of hepatic fat in response to different doses of vitamin E at week 4 was analyzed using Chi-square test for trend while fold-change differences were analyzed by ANOVA testing for trend. For cell culture data with more than two groups, data were analyzed by ANOVA with Tukey's multiple comparison correction. Significance testing of immunofluorescent data was performed by either a nested t-test or a nested ANOVA with a Tukey's post-test. All statistical tests are two-sided.
See supplementary materials for power and sample size calculations, additional methods and reagent information.
3. Results
3.1. Clinical response to αT
28 patients with NAFLD completed the run-in phase and had a baseline liver biopsy (Fig. S1). At the end of the lifestyle intervention run-in phase, normal liver histology was found in 5 patients; one additional patient had mild disease and elected not to receive treatment. Of 22 biopsy-confirmed patients who were randomized and received αT, one withdrew from the study after one week and another completed the study but was withdrawn from analysis due to a missing baseline MRS, resulting in 20 patients analyzed (Fig. S2). Baseline patient characteristics are presented in Table 1.
Table 1.
Clinical characteristics.a.
| Baseline (n = 20) | Week 4 (n = 20) | Week 24 (n = 20) | p-value (Baseline vs Week 4) | p-value (Baseline vs Week 24) | |
|---|---|---|---|---|---|
| Age [years] | 49 ± 11a | – | – | – | – |
| Female | 8 (40%) | – | – | – | – |
| Hispanic Ethnicity | 12 (60%) | – | – | – | – |
| Diabetes | 4 (20%) | ||||
| Liver Histology | |||||
| NASH vs Steatosis | 11/9 (55%/45%) | – | – | - | - |
| Cirrhosis | 2 (10%) | – | – | - | - |
| NAFLD Activity Score (NAS)b | 3.5 ± 1.3 | 2.8 ± 1.3 | – | 0.04 | – |
| Portal Inflammation | 0.9 ± 0.6 | 0.8 ± 0.5 | – | 0.73 | – |
| Lobular Inflammation | 1.5 ± 0.7 | 1.2 ± 0.4 | – | 0.19 | – |
| Ballooning | 0.7 ± 0.6 | 0.6 ± 0.5 | – | >0.99 | – |
| Mallory Bodiesc | 1.1 ± 1.3 | 0.8 ± 1.2 | – | 0.42 | – |
| Steatosis | 1.5 ± 0.8 | 1.0 ± 0.9 | – | 0.02 | – |
| Fibrosis | 1.3 ± 1.4 | 1.4 ± 1.1 | – | >0.99 | – |
| BMI [kg/m2] | 33.3 ± 6.9 | 33.4 ± 7.0 | 33.7 ± 6.8 | >0.99 | 0.36 |
| IHTG (%) | 15.6 ± 10.1 | 14.9 ± 10.7 | 12.6 ± 8.6 | >0.99 | 0.41 |
| ALT [U/L] | 43 ± 24 | 34 ± 19 | 29 ± 14 | 0.08 | 0.003 |
| Glucose [mg/dL] | 107.2 ± 15.9 | 106.0 ± 19.6 | 107.2 ± 20.8 | >0.99 | >0.99 |
| Serum αT [μmol/L] | 27.8 ± 7.2 | 56.6 ± 18.3 | 60.8 ± 25.7d | <0.0001 | <0.0001 |
Data expressed as mean ± SD or n (%). Semi-quantitative histology scores presented as mean ± SD to facilitate assessment of effect size. Significance testing was performed using non-parametric Wilcoxon signed-rank test.
Histological scoring using NASH-CRN scores. One week 4 sample was too small for scoring.
Mallory bodies scored semi-quantitatively (0–4).
Vitamin E not quantified in one hemolyzed sample.
During the 24 weeks of αT treatment patients were highly compliant, taking 98.5 ± 1.5% of their prescribed dose as assessed by pill count. After 24 weeks of αT treatment, IHTG showed a relative decrease of 27% (median, IQR -46%–27%) with 4 patients (20%) normalizing IHTG (≤5% fat). Using a pre-defined response criterion of ≥ 25% relative decrease in IHTG, ten (50%) patients were classified as responders while 10 (50%) did not respond, consistent with previously reported response rates [29]. Although neither IHTG nor ALT changed significantly by week 4, a mild improvement of histological steatosis scores was already seen (Table 1), likely related to underscoring of biopsies with borderline steatosis. By week 24 ALT showed a significant decrease (Table 1). Serum αT levels showed a dose-dependent increase starting at week 4 and remained increased at week 24, although without a difference between doses (Table 1, Figs. S3A–B). The effect on IHTG showed no dose-dependency (Figs. S3C–D). We therefore pooled all doses for the mechanistic analysis. Serum vitamin E levels did not differ between responders and non-responders at any timepoint (Figure S3 F-H).
As expected, αT treatment was not associated with a reduction in bodyweight (Table 1) nor with improvement in insulin sensitivity (Table S1) [7]. Subjects treated with αT did not change their energy expenditure, reported caloric or macronutrient intake (Tables S1 and S2). αT did not decrease levels of fasting circulating non-esterified fatty acids (NEFA), a major source of hepatic fat, nor did it affect suppression of NEFA release from adipose tissue during IVGTT (Tables S1 and S2) or the levels of fasting triglycerides which are a proxy for VLDL. Thus, the decrease in IHTG was not driven by decreased supply from extra-hepatic sources or increased hepatic lipid export, suggesting an intra-hepatic process.
3.2. αT has minor effects on hepatic gene expression in the fasting state
To examine if αT affects hepatic gene expression, we analyzed 19 pairs of liver samples (baseline and week 4) by RNA sequencing from 9 responders and 10 non-responders. No genes were significantly changed by αT treatment (Fig. S4A). We performed a nested analysis to investigate changes of gene expression over time by treatment response (Fig. S4B) and identified suppressor of cytokine signaling 2 (SOCS2) and prolyl 4-hydroxylase subunit alpha 1 (P4HA1A) which were significantly decreased by αT treatment in responders compared to non-responders, as confirmed by qPCR (Figs. S4B–H). SOCS2 expression is induced by proinflammatory cytokines as a regulator to moderate inflammatory response [30] while P4HA1 is expressed by stellate cells and facilitates collagen synthesis [31]. A decrease of both is likely related to an overall improvement of hepatic injury but not likely a direct treatment response to αT. Of note, biopsies were obtained in the fasting state and are thus not likely to identify an effect of αT treatment on expression of post-prandially regulated genes.
3.3. Decrease in de novo lipogenesis during αT treatment predicts future decrease in steatosis
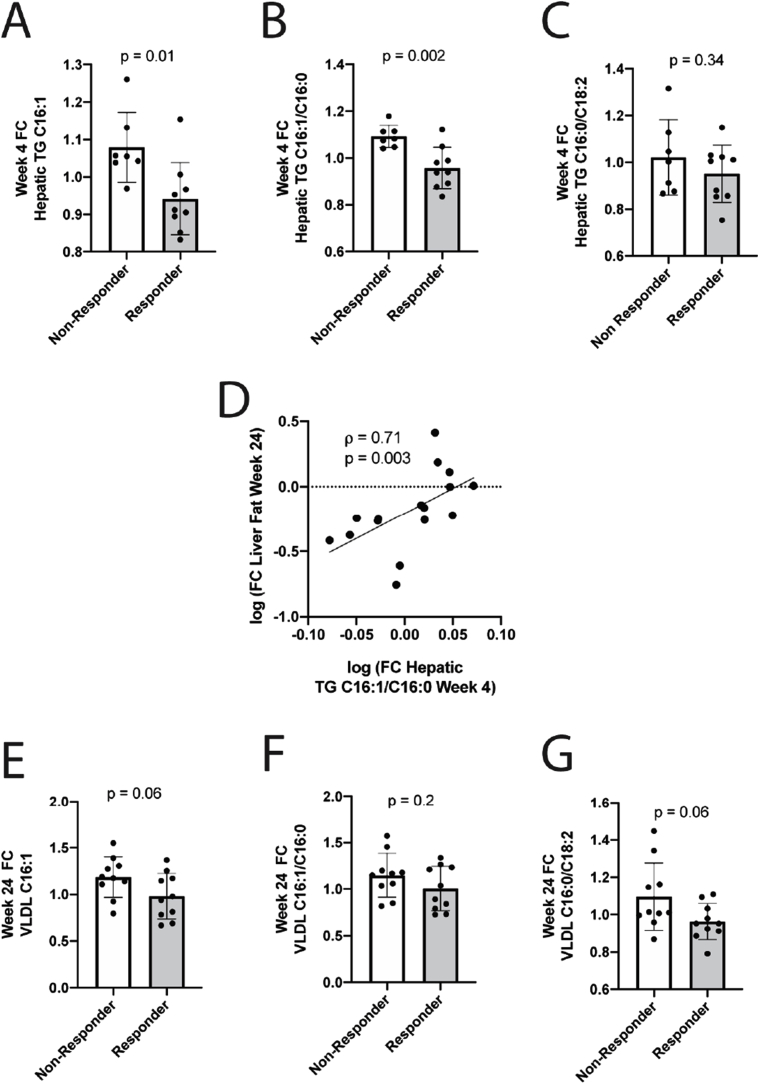
As αT did not affect NEFA delivery to the liver, we investigated whether it affects hepatic DNL, the second major source of IHTG [18]. Lipidomic analysis of paired liver biopsies showed that by week 4, responders significantly decreased hepatic TG-C16:1.
(p = 0.01), TG-C16:1/C16:0 ratio (p = 0.002; Fig. 1A–B) and numerically TG-C16:0/C18:2 ratio (Fig. 1C), suggesting a decrease in hepatic DNL compared to non-responders. Importantly, the decrease in hepatic TG-C16:1/C16:0 ratio at week 4 predicted a later (week 24) decrease in IHTG, the primary endpoint (Fig. 1D, rho = 0.71, p = 0.003). As with clinical response parameters, αT dose did not affect hepatic DNL markers (Fig. S3E).
Fig. 1.
αT treatment decreases hepatic DNL in patients with NAFLD. Markers of DNL (C16:1, C16:1/C16:0 and C16:0/C18:2) were quantified in triglycerides from human liver samples before and after 4 weeks of αT treatment (n = 16). Responders (≥25% reduction of IHTG at week 24) had significantly decreased hepatic C16:1 (A) and C16:1/C16:0 ratio (B) and numerically lower C16:0/C18:2 (C). Week 4 changes in hepatic TG C16:1/C16:0 ratio predict change in IHTG content at week 24 (D). DNL markers were measured in VLDL at baseline and week 24 (n = 20). At week 24, responders had a non-significant decrease in VLDL C16:1 (E) and C16:0/C18:2 (G) with little effect on VLDL C16:1/C16:0 (F). A-C, E-G. mean ± SD. Change from baseline to week 4 presented as fold-change (FC). IHTG, intrahepatic triglyceride.
Average weight change during treatment differed between groups (−0.7 kg in responders vs. +2.5 kg in non-responders, p = 0.02, Table S2). However, by week 24, only 2 subjects had meaningful (>5%) weight loss, both in the responder group, and most subjects in both groups actually gained weight (Fig. S5A). Weight change trended for association with IHTG change in non-responders, as expected, but was not associated in responders (Fig. S5B). Furthermore, a decrease in hepatic TG-C16:1/C16:0 at week 4 was 100% sufficient to predict IHTG response at week 24, even in subjects who gained weight. Thus, we conclude that the decrease in steatosis with vitamin E cannot be attributed to weight loss.
To investigate if the impact of αT on DNL persisted to week 24 we quantified the same DNL markers in very low-density lipoproteins (VLDL). Responders continued to show a non-significant trend for a decrease in VLDL C16:1 (Fig. 1F, p = 0.06), and C16:0/C18:2 (Fig. 1H, p = 0.06) but not in C16:1/C16:0 (Fig. 1G, p = 0.2). These changes were not driven by a change in total energy or carbohydrate intake (Table S2). In summary, αT treatment is associated with an early decrease in hepatic DNL which predicts subsequent decrease in IHTG.
3.4. αT suppresses glucose-induced triglyceride accumulation and de novo lipogenesis through down-regulation of DNL genes
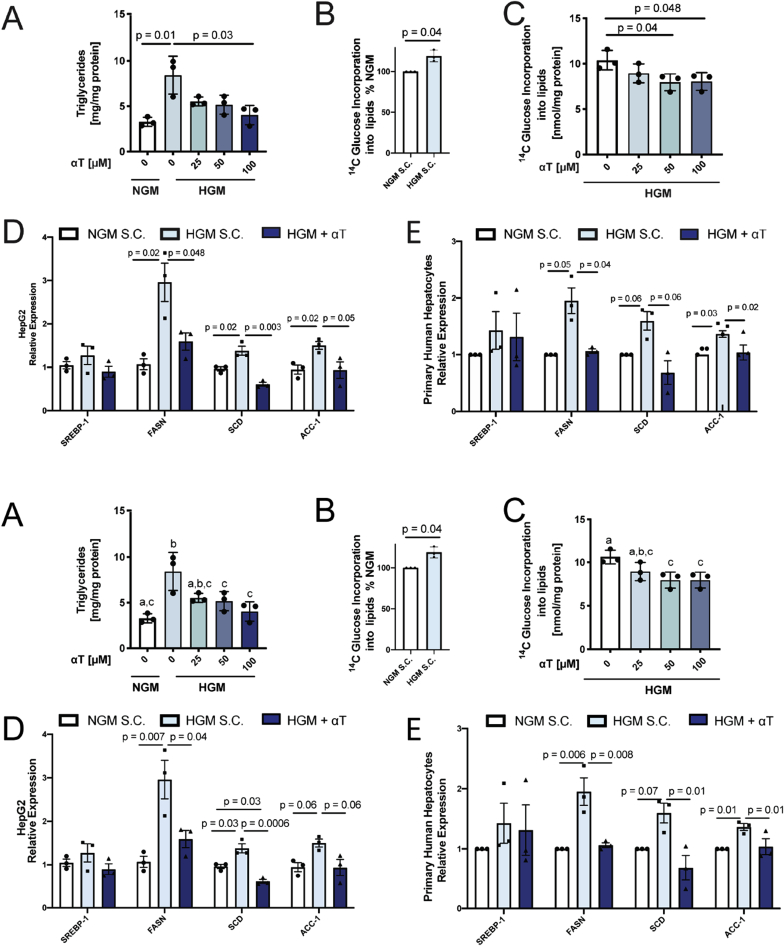
Since we found αT to affect DNL in vivo, we established an in vitro DNL model to study its mechanism. HepG2 hepatoma cells cultured to induce DNL with high glucose medium (HGM, 25 mmol/L glucose) significantly increased TG accumulation compared to normal medium (NGM, 5.5 mmol/L glucose, Fig. 2A) and this could be counteracted by αT supplementation (Fig. 2A). To directly assess DNL, we cultured cells with 14C-labeled glucose. HGM increased incorporation of glucose-derived 14C into lipids by 19% (Fig. 2B) and again this was decreased by αT (Fig. 2C). αT supplementation did not affect cell viability or proliferation (Figs. S6A–B).
Fig. 2.
αT inhibits de novo lipogenesis (DNL) in vitro. HepG2 cells were incubated with normal growth medium (NGM, white) or high glucose medium (HGM, 25 mmol/L glucose, blue shades) for 48 h. Incorporation of 14C-Glucose into lipids was used as a measure of DNL. Concurrent incubation of cells with 25–100 μmol/L αT decreased cellular triglycerides (A) and DNL (C). αT (100 μmol/L) inhibits HGM-induced upregulation of fatty acid synthase (FASN) and stearoyl-CoA desaturase (SCD) mRNA expression in HepG2 (D) and primary human hepatocytes (PHH, n = 3 donors) (E). PHH experiments were normalized within each donor. All n = 3 experiments; A-C mean ± SD, D-E mean ± SEM. All samples were compared to HGM solvent control (S·C.). Bars not sharing the same letter are significantly different p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
DNL is a tightly controlled process, regulated partially by transcription. HepG2 cells cultured in HGM induced FASN and SCD mRNA expression, and this was blocked by αT treatment (Fig. 2D) while ACC-1 showed a similar, but albeit non-significant, trend (p = 0.06 for both increase by HGM and decrease by αT). We confirmed this effect in primary human hepatocytes (PHH), where HGM (25 mmol/L) incubation significantly increased FASN and ACC-1 expression with a trend for SCD (p = 0.07) compared to standard PHH medium (containing 11.5 mmol/L glucose) and all were significantly decreased by the addition of αT (Fig. 2E). αT did not inhibit glucose uptake nor did it increase glucose or fatty acid metabolism (Fig. S7). Taken together, we show that αT is able to decrease the expression of DNL genes in liver cells and decrease glucose-induced lipid accumulation.
3.5. αT suppresses SREBP-1 maturation
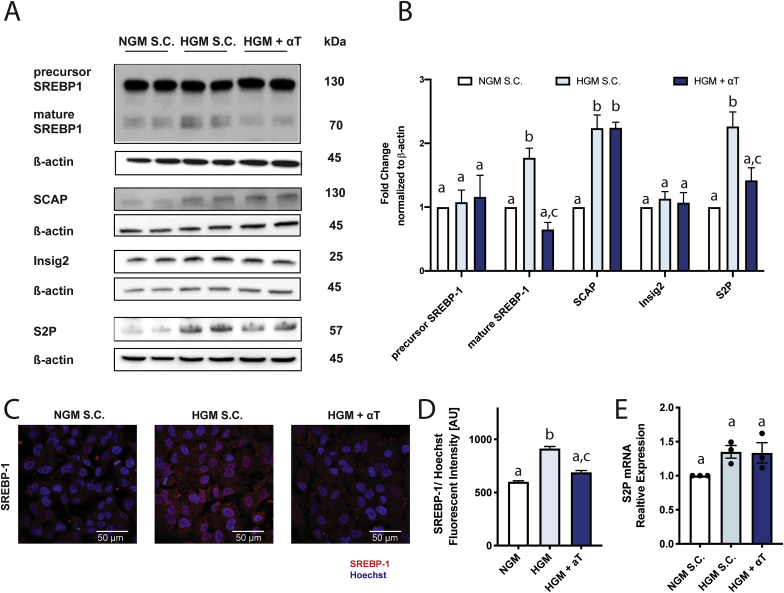
A major regulator of DNL genes (including FASN and SCD) is the transcription factor SREBP-1, which is regulated transcriptionally and post-transcriptionally. SREBP-1 mRNA was not affected by αT in HepG2 or PHH (Fig. 2D–E). SREBP-1 protein is retained as an inactive precursor bound to the ER membrane and requires proteolytic cleavage to translocate from ER to nucleus. HGM treatment of HepG2 cells induced SREBP-1 maturation (Fig. 3A–B) and nuclear translocation (Fig. 3C–D) which was prevented by αT treatment without affecting levels of SREBP-1 precursor protein.
Fig. 3.
αT decreases SREBP-1 maturation. HepG2 cells incubated with normal growth medium (NGM, white) or high glucose medium (HGM, 25 mmol/L, blue shades) with or without αT (100 μmol/L) for 48 h αT decreased HGM-induced maturation of SREBP-1 without affecting precursor protein levels (A,B) and decreased HGM-induced nuclear translocation (C,D). αT did not affect SCAP or Insig2 (A–B). αT inhibits HGM-driven upregulation S2P at protein (A–B) but not mRNA (E) level. qPCR results were normalized to 18s (E). A - representative Western blot images for respective proteins; B – corresponding quantification. C- representative imaging of nuclear SREBP-1 in cells; D – Corresponding quantification. All data mean ± SEM. B, E n = 3; D n = 2. Solvent Control (S·C.). Bars not sharing the same letter are significantly different p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
SREBP-1 resides in the ER in a complex with SCAP and Insig. Insig retains the SREBP-1/SCAP/Insig complex in the ER while SCAP facilitates transport of SREBP-1 from the ER to Golgi apparatus. HGM increased SCAP protein expression as previously reported [32] but both SCAP and Insig2 were unaffected by αT (Fig. 3A–B). Maturation and translocation of SREBP-1 from Golgi to nucleus requires cleavage by site-1 and site-2 protease (S1P and S2P). HGM significantly induced S2P protein levels, and this was abolished by αT treatment (Fig. 3A–B; Fig. S8-AB). A similar effect was noted for S1P protein by immunofluorescence only (Fig. S8-AB) as the S1P transmembrane form was not detectable by western blot. Changes in S1P and S2P protein levels were not paralleled at the gene expression level (Fig. 3E, Fig. S8C), indicating that the induction by HGM and decrease by αT are likely mediated by a post-transcriptional mechanism. S1P activation and increased DNL can also results from ER stress [33]; however αT treatment did not affect ER stress markers (Fig. S9), suggesting this is not the pathway by which it modulates DNL. Nuclear translocation of another key DNL transcription factor, ChREBP, was increased by HGM but was not affected by αT (Fig. S10). In summary, αT decreases SREBP-1 maturation, possibly through reduction of the terminal cleavage enzymes S1P and S2P.
3.6. Hydroxyl group and lipophilic side chain of αT are necessary to inhibit glucose-induced lipid accumulation
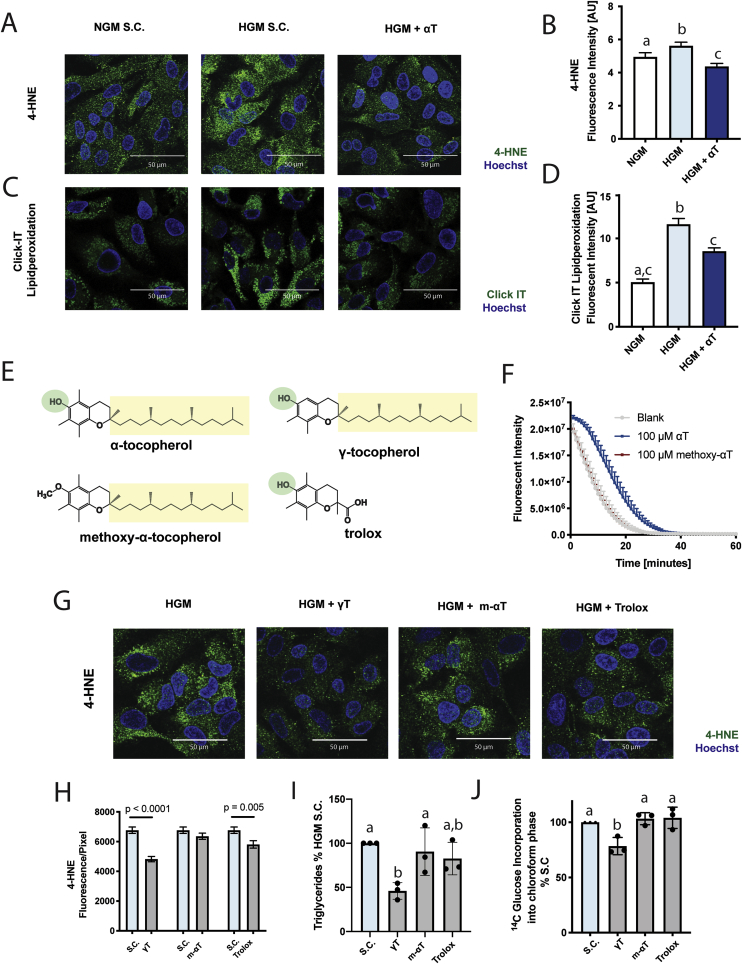
HGM treatment of HepG2 cells induces lipid peroxidation which is mitigated by αT (Fig. 4A–D). To investigate whether the observed reduction in DNL requires the antioxidant capacity of αT, we synthesized an αT derivate, methoxy-α-tocopherol (m-αT), where the.
Fig. 4.
αT inhibits de novo lipogenesis through an antioxidant effect. HepG2 cells incubated with normal growth medium (NGM, white) or high glucose medium (HGM, 25 mmol/L, blue shades) with or without αT (100 μmol/L), γ-tocopherol (γT, 75 μmol/L), methoxy-α-tocopherol (m-αT, 100 μmol/L) or trolox (100 μmol/L) for 48 h. HGM significantly induced 4-HNE adduct formation (A, B) and oxidation of ClickIT linoleic acid probe (C, D). Structural formulas of investigated compounds (E). αT, but not m-αT is able to quench oxygen radicals in the ORAC assay (F). γT and trolox inhibit HGM-induced 4-HNE adduct formation, while m-αT does not (G, H). αT and γT are able to decrease glucose-induced triglyceride formation (I) and DNL (J), while m-αT and trolox are ineffective. B, D, H mean ± SEM; I,J mean ± SD. B,D,H n = 2; I-J n = 3. A, C, G show representative images; B, D, I show corresponding quantification. Solvent Control (S·C.). Bars not sharing the same letter are significantly different p < 0.05. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Electron-donating hydroxyl group is blocked by methylation (Fig. 4E). We verified that m-αT lacks antioxidant activity in vitro (Fig. 4F) and found it unable to decrease HGM-driven 4-HNE formation (Fig. 4G–H). We further verified uptake of the compound by HPLC (Fig. S14). In contrast to αT, m-αT did not prevent HGM-induced lipid accumulation and 14C-glucose lipid incorporation (Fig. 4I–J). Thus, our findings are consistent with the assumption that the antioxidant capacity of αT is required for its effect on DNL. To assess the importance of αT localization to the lipid compartment, we investigated the water-soluble Trolox, which has the same chromanol ring structure and antioxidant capacity as αT [34], but lacks the lipophilic side chain (Fig. 4E). Trolox decreased 4-HNE adducts (Fig. 4G–H) but did not affect DNL (Fig. 4I–J). Gamma-tocopherol (γT), another lipid-soluble antioxidant (Fig. 4E) [35] with similar subcellular distribution [6] as αT, was able to decrease 4-HNE, TG accumulation, and DNL (Fig. 4H–J). Taken together the data highlight the requirement of antioxidant capacity and lipophilicity of αT for the inhibition of DNL.
3.7. S2P protein expression is increased by oxidative stress and in NAFLD
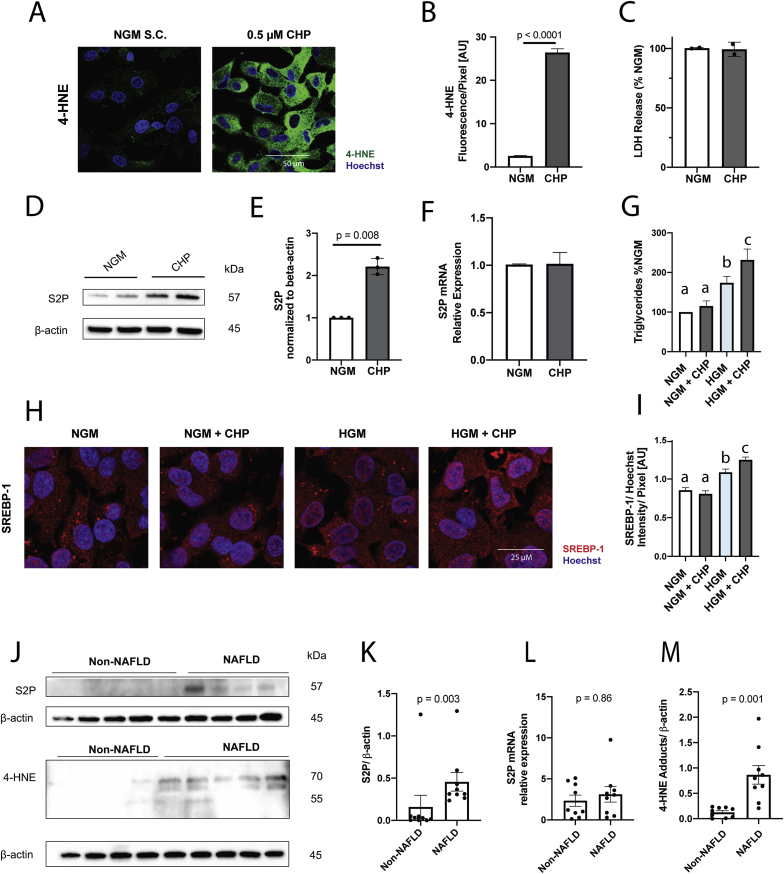
Since the antioxidant capacity of αT is required for its effect on DNL, and since HGM induces lipid peroxidation, we hypothesized that oxidative stress by itself can exacerbate SREBP-1 translocation through increased expression of S2P. Cumene hydroperoxide (CHP) was used to induce oxidative stress in HepG2 cells under NGM (Fig. 5A–B) at a non-cytotoxic dose (Fig. 5C). CHP treatment increased S2P protein (Fig. 5D–E, Fig. S9) without affecting its mRNA expression (Fig. 5F). Similar results were obtained for S1P using immunofluorescence (Fig. S11). S2P and S1P facilitate final SREBP-1 processing, after SREBP-1 has translocated to the Golgi (Fig. 6A). As such, we predicted that isolated S2P/S1P upregulation will not be sufficient to increase SREBP-1 processing, in the absence of SREBP-1 release from the ER. Indeed, CHP by itself did not increase SREBP-1 translocation and lipid accumulation (Fig. 5G–I) under normal glucose concentrations, despite its effects on S2P and S1P, but was able to increase SREBP-1 translocation and triglyceride accumulation under HGM, when SREBP-1 is accessible for processing (Fig. 5G–I). Our data suggest that high substrate availability is required to drive SREBP-1 maturation, with oxidative stress modulating its cleavage by increasing S2P (and likely S1P) protein levels. Thus, oxidative stress exacerbates substrate-driven DNL in an SREBP-1 mediated fashion possibly through upregulation of S2P (and S1P).
Fig. 5.
Oxidative stress increases S2P protein. HepG2 cells were incubated with cumene hydroperoxide (CHP, 0.5 μmol/L, grey) overnight. CHP increased 4-HNE adducts (A,B) without changes in viability (C) and increased S2P protein expression (D,E) without changes in mRNA (F). CHP requires high glucose media (25 mmol/L glucose) to induce triglyceride accumulation (I) and increase SREBP-1 nuclear translocation (G,H). Control (n = 9) and NAFLD (n = 9) liver tissue samples were obtained through the Liver Tissue and Cell Distribution System (LTCDS). NAFLD patients had significantly higher protein levels of S2P (J,K) as well as 4-HNE (M) compared to controls without changes in S2P mRNA (L). qPCR data was normalized to 18s. B,I mean ± SEM, n = 2; C, mean ± SD, n = 2; E,F mean ± SEM, n = 3; G mean ± SD, n = 3; K, L, M mean ± SEM, n = 17. Bars not sharing the same letter are significantly different p < 0.05.
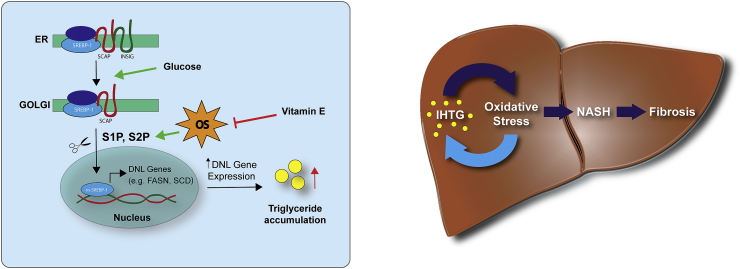
Fig. 6.
Proposed mechanism and NAFLD progression model. (Left) SREBP-1 resides complexed with SCAP and INSIG in the ER. Upon activation, INSIG dissociates and SCAP facilitates transport to Golgi where cleavage by S1P and S2P leads to mature SREBP-1 which translocates to the nucleus and activates lipogenic genes. Vitamin E blocks SREBP-1 translocation through reduced protein expression of S1P and S2P by lowering oxidative stress (OS). (Right) Intra-hepatic triglyceride accumulation (IHTG) induces OS which can lead to progression to non-alcoholic steatohepatitis (NASH) and fibrosis. Our data supports a bi-directional model in which oxidative stress contributes to disease progression and exacerbates IHTG.
Consistent with our in vitro findings, 4-HNE adducts and S2P protein were upregulated in livers from patients with NAFLD compared to controls, without an effect on S1P or S2P mRNA (Fig. 5J-M, Fig. S12). The increase in S2P protein expression without corresponding increase in mRNA is consistent with our hypothesis of a posttranscriptional regulation of S2P by oxidative stress.
4. Discussion
Currently no U.S.-FDA-approved treatment for NAFLD is available [36]. αT improves NASH-associated injury, inflammation [29] and increases transplant free survival [8]. Unexpectedly, it also decreases liver fat [7,10].
In this work, we used a clinical mechanistic trial and in vitro models to demonstrate that αT decreases IHTG by inhibiting DNL through its antioxidant activity. In vitro, αT decreased SREBP-1 maturation and lipogenic gene expression (Fig. 6A), possibly through a decrease in S2P, a terminal SREBP-1 processing enzyme that is upregulated by oxidative stress. In human NAFLD, DNL [18,25] and oxidative stress ([37] and Fig. 5M) are increased and we observed an increase of hepatic S2P protein expression, confirming our in vitro data.
Silencing redox activity of αT by methylation or removal of its lipophilic side chain eliminated the protective effect on glucose-induced lipid accumulation. This, as well as parallel effects of γT, another lipid soluble antioxidant, strongly suggest that the mechanism is related to αT's antioxidant capacity and lipid solubility. Effects of oxidative stress on lipid accumulation have been previously described. For example, an increase in DNL gene expression was demonstrated in animals experiencing increased oxidative stress due to knock out of the antioxidant enzyme superoxide dismutase (SOD) [38] as well as in HepG2 cells treated with H2O2 [39].
The SREBP-1 pathway is regulated transcriptionally and post-transcriptionally. Our data suggest that oxidative stress augments DNL through modulation of S2P and likely S1P. Modulation of SREBP-1 activity and DNL by terminal proteases has been previously shown. Disruption of S1P in mice led to decreased hepatocyte TG content and DNL [40], and mutations in S1P protected zebrafish embryos from alcohol-induced steatosis [41]. Furthermore, an S1P inhibitor, PF-429242, reduces FASN expression and DNL in HepG2 cells and in mice [42]. Finally, activation of S1P by ER stress was also shown to drive DNL [33]. The effect of S2P on DNL has not been investigated, but our data suggest that S2P may also play an important role. Thus, existing data support our hypothesis that changes in terminal processing can control SREBP-1 translocation and DNL.
The ability of αT to decrease DNL was seen in vivo as well as in vitro. Although our in vitro findings identified SREBP-1-driven changes in lipogenic gene expression as a likely mechanism, we did not observe similar gene expression changes in vivo. In vitro, oxidative stress modulated SREBP-1 processing and DNL only in the presence of high substrate availability, mimicking the physiological post-prandial state. In contrast, human liver biopsies were performed after an overnight (≥12 h) fast, when DNL is suppressed [43]. This may have limited our ability to detect changes in gene expression which fluctuate with nutrient availability, especially if the detrimental effects of oxidative stress and the beneficial effect of αT on gene expression predominantly occur post-prandially. In contrast to gene expression studies which capture a “snapshot” of a fluctuating metabolic state, our lipidomic analysis of human liver samples was able to show an impact on DNL, likely as it reflects an averaged effect over a longer time.
The strength of our study is the detailed in vitro analysis and the clinical trial. By performing a mechanistic study, we were able to obtain repeat liver samples early, to investigate effects of vitamin E prior to significant histological improvement and compare future responders and non-responders, suggesting causality. However, some of the interpretations are limited by small sample size, which was an inherent requirement for feasibility. Another potential limitation is the absence of a placebo arm. This was driven by ethical concerns, as the study was not designed to demonstrated the efficacy of vitamin E in reducing steatosis in NAFLD, which was previously shown [10,29]. Instead, we focused on how vitamin E reduces IHTG. In lieu of a placebo arm, we included a lifestyle run-in phase as lifestyle likely accounts for the majority of “placebo” responses seen in clinical trials for NAFLD [44]. Through inclusion of a run-in phase we aimed to eliminate competing behavioral changes during vitamin E treatment. Another limitation is the indirect measurement of DNL. The gold standard for DNL assessment is quantification of isotopically-labeled palmitate in VLDL from precursors or deuterated water but these experiments were not included in the study design. Instead, we quantified DNL using established fatty acid ratios [[23], [24], [25],27,28] in liver biopsies and VLDL which have been shown, especially for C16:1, to correlate well with isotopically-determined DNL [25].
Our subjects were free-living and weight loss is expected in some, which can impact IHTG and DNL. αT treatment did not lead to weight loss. Although on-treatment weight change significantly differed between responders and non-responders, the vast majority of subjects in both groups showed no meaningful weight loss. A > 5% decrease in bodyweight was previously shown to improve steatosis [45] but in our study only 3 subjects lost >1% of bodyweight (Fig. S5A), and even subjects who gained weight (Fig. S5B) had a week 24 decrease in IHTG if their DNL markers improved by week 4 of treatment. The reduction of bodyweight is thus unlikely to be a driving factor for the reduction of steatosis, although a larger study would be needed to formally prove independence.
The clinical response rate to vitamin E in our study was 50%. In clinical trials, only a fraction of patients with NAFLD or NASH (typically less than 60%) achieve histological or radiological response to interventions [36] and vitamin E is no exception [7]. What makes a patient a non-responder, whether this can be predicted from baseline characteristics, and whether non-response to one agent predicts non-response to others is unknown and our study was neither designed, nor powered, to answer that question. For vitamin E this is even applicable to experimental animal models where vitamin E supplementation has alleviated steatosis in some animal models of NAFLD and NASH [[46], [47], [48]] but not in others [49,50]. Different genetic backgrounds, disease models or species differences may be to blame [51].
We did not find an association of vitamin E dose (200, 400, 800 IU/d) with response, even though αT serum levels differed. The absence may be due to saturation of hepatic αT levels, as 200 IU/d is almost 9-times the recommended daily allowance for adults [4]. Furthermore, it is not clear that NAFLD is a vitamin E deficiency disorder or the result of local vitamin E deficiency. In fact, in NAFLD and possibly in hepatic steatosis in general, vitamin E appears to be sequestered in lipid droplets [[52], [53], [54]] which may prevent it from performing its physiological role in other subcellular compartments. Supplementation may be able to restore vitamin E concentration in other organelles although the appropriate dose needs to be elucidated in larger studies.
Overall, we show that vitamin E reduces IHTG by reducing DNL in vitro and in vivo. Importantly, our findings demonstrate a bi-directional pathway, where oxidative stress (traditionally thought to be caused by IHTG or processes leading to it) exacerbates DNL and IHTG formation, generating a vicious cycle of steatosis and injury (Fig. 6B). Our data point to a role of oxidative stress in metabolic diseases beyond being a consequence of excessive metabolism. If SREBP-1 is indeed influenced by oxidative stress in vivo, DNL may be exacerbated under high substrate availability, like obesity, generating a vicious cycle in which high substrate availability increases metabolic demand and radical production which then in turn leads to activation of DNL increasing IHTG.
Author contributions
MCP contributed to the study concept and design; the acquisition of data; the analysis and the interpretation of data; statistical analysis and the preparation of the manuscript. AR synthesized methoxy-αT. KV and RU performed statistical analysis for RNA-Seq data. MG, HC, PW developed and performed the fatty acid analysis for isolated VLDL. PCV and ML quantified methoxy-αT levels. DEK evaluated liver histology. RO and AMG quantified liver fat by MRI. SY, MS designed and managed the lifestyle intervention and SL, NS, DDL, SZF, LM, YM, RS, NM, WCAH, and ASA contributed to the acquisition of data. YR contributed to the study concept and design; supervision of the clinical trial; the analysis and the interpretation of data; the critical revision of the manuscript for important intellectual content; obtained funding; the administrative, technical, or material support; the study supervision and is the overall guarantor of the study.
Funding
This research was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Cancer Institute (NCI), USA. MCP received funding from the NIH Office of Dietary Supplements (ODS), USA. Vitamin E was provided by Pharmavite under a Clinical Trial Agreement. The Liver Tissue and Cell Distribution System (LTCDS), Department of Pediatric Gastroenterology, Hepatology, and Nutrition, University of Minnesota Medical School, was funded by NIH Contract #HHSN276201200017C.
Declaration of competing interest
The authors have declared no conflict of interest.
Acknowledgments
The authors would like to thank the patients in the clinical trial for their participation and the clinical support staff and Lita Freeman for technical assistance. MCP would also like to acknowledge Dr. Audrey Boyer, ReneDohl. and Lady DAG. for their encouragement during the project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101710.
Data and material availability
All data generated or analyzed during the current study are available from the corresponding author (Y.R.) upon request, human data sharing may be contingent upon Institutional Review Board approval. RNA Sequencing data has been uploaded to dbGaP (phs001930) and can be requested via authorized access. R Code for the analysis of the RNA Sequencing Data is available upon request.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Masarone M., Rosato V., Dallio M., Gravina A.G., Aglitti A., Loguercio C., Federico A., Persico M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxid. Med. Cell. Longev. 2018:9547613. doi: 10.1155/2018/9547613. (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton G.W., Joyce A., Ingold K.U. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet (North Am. Ed.) 1982;2:327. doi: 10.1016/s0140-6736(82)90293-8. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine . Natl. Acad. Press. Washingt. DC; 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids; pp. 186–283. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Quinn P.J. Vitamin E and its functions in membranes. Prog. Lipid Res. 1999;38:309–336. doi: 10.1016/s0163-7827(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 6.Irías-Mata A., Sus N., Flory S., Stock D., Woerner D., Podszun M., Frank J. α-Tocopherol transfer protein does not regulate the cellular uptake and intracellular distribution of α- and γ-tocopherols and -tocotrienols in cultured liver cells. Redox Biol. 2018;19:28–36. doi: 10.1016/j.redox.2018.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanyal A.J., Chalasani N., V Kowdley K., McCullough A., Diehl A.M., Bass N.M., Neuschwander-Tetri B. a, Lavine J.E., Tonascia J., Unalp A., Van Natta M., Clark J., Brunt E.M., Kleiner D.E., Hoofnagle J.H., Robuck P.R. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vilar‐Gomez E., Vuppalanchi R., Gawrieh S., Ghabril M., Saxena R., Cummings O.W., Chalasani N. Vitamin E improves transplant‐free survival and hepatic decompensation among patients with nonalcoholic steatohepatitis and advanced fibrosis. Hepatology. 2020;71:495–509. doi: 10.1002/hep.30368. [DOI] [PubMed] [Google Scholar]
- 9.Lavine J.E. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J. Pediatr. 2000;136:734–738. doi: 10.1067/mpd.2000. [DOI] [PubMed] [Google Scholar]
- 10.Bril F., Biernacki D.M., Kalavalapalli S., Lomonaco R., Subbarayan S.K., Lai J., Tio F., Suman A., Orsak B.K., Hecht J., Cusi K. Role of vitamin E for nonalcoholic steatohepatitis in patients with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2019;42:1481–1488. doi: 10.2337/dc19-0167. [DOI] [PubMed] [Google Scholar]
- 11.Raso G.M., Esposito E., Iacono A., Pacilio M., Cuzzocrea S., Canani R.B., Calignano A., Meli R. Comparative therapeutic effects of metformin and vitamin E in a model of non-alcoholic steatohepatitis in the young rat. Eur. J. Pharmacol. 2009;604:125–131. doi: 10.1016/j.ejphar.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Podszun M.C., Grebenstein N., Spruss A., Schlueter T., Kremoser C., Bergheim I., Frank J. Dietary α-tocopherol and atorvastatin reduce high-fat-induced lipid accumulation and down-regulate CD36 protein in the liver of Guinea pigs. J. Nutr. Biochem. 2014;25:573–579. doi: 10.1016/j.jnutbio.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Galli F., Azzi A., Birringer M., Cook-Mills J.M., Eggersdorfer M., Frank J., Lorkowski S., Özer N.K. Vitamin E: emerging aspects and new directions. Free Radic. Biol. Med. 2016;102:16–36. doi: 10.1016/j.freeradbiomed.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Boscoboinik D., Szewczyk A., Henseys C., Azzi A. Inhibition of cell proliferation by α-tocopherol. J. Biol. Chem. 1991;266:6188–6194. [PubMed] [Google Scholar]
- 15.Williams J.C., Forster L.A., Tull S.P., Wong M., Bevan R.J., Ferns G.A. Dietary vitamin E supplementation inhibits thrombin-induced platelet aggregation, but not monocyte adhesiveness, in patients with hypercholesterolaemia. Int. J. Exp. Pathol. 1997;78:259–266. doi: 10.1046/j.1365-2613.1997.260359.x. http://www.ncbi.nlm.nih.gov/pubmed/9505937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teupser D., Thiery J., Seidel D. Alpha-tocopherol down-regulates scavenger receptor activity in macrophages. Atherosclerosis. 1999;144:109–115. doi: 10.1016/s0021-9150(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 17.Muriel P. Role of free radicals in liver diseases. Hepatol. Int. 2009;3:526–536. doi: 10.1007/s12072-009-9158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI200523621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masschelin P.M., Cox A.R., Chernis N., Hartig S.M. The impact of oxidative stress on adipose tissue energy balance. Front. Physiol. 2020;10:1–8. doi: 10.3389/fphys.2019.01638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan M., Cederbaum A.I., Zhang Y.L., Ginsberg H.N., Williams K.J., Fisher E.A. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J. Clin. Invest. 2004;113:1277–1287. doi: 10.1172/JCI19197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.C., Torbenson M.S., Unalp-Arida A., Yeh M., McCullough A.J., Sanyal A.J. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Ouwerkerk R., Pettigrew R.I., Gharib A.M. Liver metabolite concentrations measured with 1H MR spectroscopy. Radiology. 2012;265:565–575. doi: 10.1148/radiol.12112344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudgins L.C., Hellerstein M., Seidman C., Neese R., Diakun J., Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J. Clin. Invest. 1996;97:2081–2091. doi: 10.1172/JCI118645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong M.F.-F., Hodson L., Bickerton A.S., Roberts R., Neville M., Karpe F., Frayn K.N., Fielding B.A. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am. J. Clin. Nutr. 2008;87:817–823. doi: 10.1093/ajcn/87.4.817. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.J., Lambert J.E., Hovhannisyan Y., Ramos-Roman M.A., Trombold J.R., Wagner D.A., Parks E.J. Palmitoleic acid is elevated in fatty liver disease and reflects hepatic lipogenesis. Am. J. Clin. Nutr. 2015;101:34–43. doi: 10.3945/ajcn.114.092262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peter A., Cegan A., Wagner S., Lehmann R., Stefan N., Königsrainer A., Königsrainer I., Häring H.U., Schleicher E. Hepatic lipid composition and stearoyl-coenzyme A desaturase 1 mRNA expression can be estimated from plasma VLDL fatty acid ratios. Clin. Chem. 2009;55:2113–2120. doi: 10.1373/clinchem.2009.127274. [DOI] [PubMed] [Google Scholar]
- 27.Peter A., Cegan A., Wagner S., Elcnerova M., Konigsrainer A., Konigsrainer I., Haring H.-U., Schleicher E.D., Stefan N. Relationships between hepatic stearoyl-CoA desaturase-1 activity and mRNA expression with liver fat content in humans. AJP Endocrinol. Metab. 2011;300:E321–E326. doi: 10.1152/ajpendo.00306.2010. [DOI] [PubMed] [Google Scholar]
- 28.Klawitter J., Bek S., Zakaria M., Zeng C., Hornberger A., Gilbert R., Shokati T., Klawitter J., Christians U., Boernsen K.O. Fatty acid desaturation index in human plasma: comparison of different analytical methodologies for the evaluation of diet effects. Anal. Bioanal. Chem. 2014;406:6399–6408. doi: 10.1007/s00216-014-8020-4. [DOI] [PubMed] [Google Scholar]
- 29.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., Neuschwander-Tetri B.A., Lavine J.E., Tonascia J., Unalp A., Van Natta M., Clark J., Brunt E.M., Kleiner D.E., Hoofnagle J.H., Robuck P.R. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott J., Johnston J.A. SOCS: role in inflammation, allergy and homeostasis. Trends Immunol. 2004;25:434–440. doi: 10.1016/J.IT.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Li J., Ghazwani M., Zhang Y., Lu J., Li J., Fan J., Gandhi C.R., Li S. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J. Hepatol. 2013;58:522–528. doi: 10.1016/j.jhep.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H., Zhao M., Sud N., Christian P., Shen J., Song Y., Pashaj A., Zhang K., Carr T., Su Q. Glucagon regulates hepatic lipid metabolism via cAMP and Insig-2 signaling: implication for the pathogenesis of hypertriglyceridemia and hepatic steatosis. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep32246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J.Y., Garcia-Carbonell R., Yamachika S., Zhao P., Dhar D., Loomba R., Kaufman R.J., Saltiel A.R., Karin M. ER stress drives lipogenesis and steatohepatitis via caspase-2 activation of S1P. Cell. 2018;175:133–145. doi: 10.1016/j.cell.2018.08.020. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cort W.M., Scott J.W., Araujo M., Mergens W.J., Cannalonga M.A., Osadca M., Harley H., Parrish D.R., Pool W.R. Antioxidant activity and stability of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid. J. Am. Oil Chem. Soc. 1975;52:174–178. doi: 10.1007/BF02672164. [DOI] [PubMed] [Google Scholar]
- 35.Kamal-Eldin A., Appelqvist L. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 36.Rotman Y., Sanyal A.J. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2016:312431. doi: 10.1136/gutjnl-2016-312431. gutjnl-2016- [DOI] [PubMed] [Google Scholar]
- 37.Feldstein A.E., Lopez R., Tamimi T.A.-R., Yerian L., Chung Y.-M., Berk M., Zhang R., McIntyre T.M., Hazen S.L. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J., Homma T., Kurahashi T., Kang E.S., Fujii J. Oxidative stress triggers lipid droplet accumulation in primary cultured hepatocytes by activating fatty acid synthesis. Biochem. Biophys. Res. Commun. 2015;464:229–235. doi: 10.1016/j.bbrc.2015.06.121. [DOI] [PubMed] [Google Scholar]
- 39.Sekiya M., Hiraishi A., Touyama M., Sakamoto K. Oxidative stress induced lipid accumulation via SREBP1c activation in HepG2 cells. Biochem. Biophys. Res. Commun. 2008;375:602–607. doi: 10.1016/j.bbrc.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 40.Yang J., Goldstein J.L., Hammer R.E., Moon Y.-A., Brown M.S., Horton J.D. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc. Natl. Acad. Sci. Unit. States Am. 2002;98:13607–13612. doi: 10.1073/pnas.201524598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passeri M.J., Cinaroglu A., Gao C., Sadler K.C. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology. 2009;49:443–452. doi: 10.1002/hep.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hawkins J.L., Robbins M.D., Warren L.C., Xia D., Petras S.F., Valentine J.J., Varghese A.H., Wang I.-K., Subashi T.A., Shelly L.D., Hay B.A., Landschulz K.T., Geoghegan K.F., Harwood H.J. Pharmacologic inhibition of site 1 protease activity inhibits sterol regulatory element-binding protein processing and reduces lipogenic enzyme gene expression and lipid synthesis in cultured cells and experimental animals. J. Pharmacol. Exp. Therapeut. 2008;326:801–808. doi: 10.1124/jpet.108.139626. [DOI] [PubMed] [Google Scholar]
- 43.Horton J.D., Bashmakov Y., Shimomura I., Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl. Acad. Sci. Unit. States Am. 1998;95:5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han M.A.T., Altayar O., Hamdeh S., Takyar V., Rotman Y., Etzion O., Lefebvre E., Safadi R., Ratziu V., Prokop L.J., Murad M.H., Noureddin M. Rates of and factors associated with placebo response in trials of pharmacotherapies for nonalcoholic steatohepatitis: systematic Review and meta-analysis. Clin. Gastroenterol. Hepatol. 2019;17:616–629. doi: 10.1016/j.cgh.2018.06.011. e26. [DOI] [PubMed] [Google Scholar]
- 45.Vilar-Gomez E., Martinez-Perez Y., Calzadilla-Bertot L., Torres-Gonzalez A., Gra-Oramas B., Gonzalez-Fabian L., Friedman S.L., Diago M., Romero-Gomez M. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378. doi: 10.1053/j.gastro.2015.04.005. e5. [DOI] [PubMed] [Google Scholar]
- 46.Phung N., Pera N., Farrell G., Leclercq I., Hou J.Y., George J. Pro-oxidant-mediated hepatic fibrosis and effects of antioxidant intervention in murine dietary steatohepatitis. Int. J. Mol. Med. 2009;24:171–180. doi: 10.3892/ijmm. [DOI] [PubMed] [Google Scholar]
- 47.Podszun M.C., Grebenstein N., Spruss A., Schlueter T., Kremoser C., Bergheim I., Frank J. Dietary α-tocopherol and atorvastatin reduce high-fat-induced lipid accumulation and down-regulate CD36 protein in the liver of Guinea pigs. J. Nutr. Biochem. 2014;25:573–579. doi: 10.1016/j.jnutbio.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Nan Y.M., Wu W.J., Fu N., Liang B.L., Wang R.Q., Li L.X., Zhao S.X., Zhao J.M., Yu J. Antioxidants vitamin e and 1-aminobenzotriazole prevent experimental non-alcoholic steatohepatitis in mice. Scand. J. Gastroenterol. 2009;44:1121–1131. doi: 10.1080/00365520903114912. [DOI] [PubMed] [Google Scholar]
- 49.Chung M.Y., Yeung S.F., Park H.J., Volek J.S., Bruno R.S. Dietary α- and γ-tocopherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis. J. Nutr. Biochem. 2010;21:1200–1206. doi: 10.1016/j.jnutbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Presa N., Clugston R.D., Lingrell S., Kelly S.E., Merrill A.H., Jana S., Kassiri Z., Gómez-Muñoz A., Vance D.E., Jacobs R.L., van der Veen J.N. Vitamin E alleviates non-alcoholic fatty liver disease in phosphatidylethanolamine N-methyltransferase deficient mice. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2019;1865:14–25. doi: 10.1016/j.bbadis.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Ma Y., Brown P.M., Lin D.D., Ma J., Feng D., Belyaeva O.V., Podszun M.C., Roszik J., Allen J., Umarova R., Kleiner D.E., Kedishvili N.Y., Gavrilova O., Gao B., Rotman Y. Hsd17b13 deficiency does not protect mice from obesogenic diet injury. Hepatology. 2020 doi: 10.1002/hep.31517. hep.31517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Violet P., Ebenuwa I.C., Wang Y., Niyyati M., Padayatty S.J., Head B., Wilkins K., Chung S., Thakur V., Ulatowski L., Atkinson J., Ghelfi M., Smith S., Tu H., Bobe G., Liu C., Herion D.W., Shamburek R.D., Manor D., Traber M.G., Levine M. Vitamin E sequestration by liver fat in humans. JCI Insight. 2020;5:17. doi: 10.1172/jci.insight.133309. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagita A., Ando M. Assessment of hepatic vitamin E status in adult patients with liver disease. Hepatology. 1997;26:392–397. doi: 10.1002/hep.510260220. [DOI] [PubMed] [Google Scholar]
- 54.Bartolini D., Torquato P., Barola C., Russo A., Rychlicki C., Giusepponi D., Bellezza G., Sidoni A., Galarini R., Svegliati-Baroni G., Galli F. Nonalcoholic fatty liver disease impairs the cytochrome P-450-dependent metabolism of α-tocopherol (vitamin E) J. Nutr. Biochem. 2017;47:120–131. doi: 10.1016/j.jnutbio.2017.06.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.