Abstract
Adults with mobility related disabilities (MRDs) represent an underserved group with a high prevalence of overweight/obesity and limited options for weight management. We previously demonstrated clinically meaningful 12-month weight loss in adults with MRDs ( −6.2%, 36% ≥ 5% of baseline weight) using an enhanced Stop Light Diet (eSLD) delivered using at home face-to-face behavioral sessions and optional physical activity. However, the costs/logistics associated with intervention delivery by individual home visits limits the potential for scaling and implementation of this approach. Thus, we will conduct a two-arm randomized trial in 128 overweight/obese adults with MRDs to compare weight loss (6 mos.) and maintenance (12 mos.) between interventions utilizing the eSLD, behavioral counseling, and increased physical activity delivered to individual participants in their homes or delivered to groups of participants in their homes remotely via video conferencing. The primary aim will compare weight loss between interventions arms across 6 months. Secondarily, we will compare weight loss (0-18 mos.), the proportion of participants who achieve clinically meaningful weight loss (≥ 5%) from 0 to 6 and 0 to18 months, and changes in quality of life from 0 to 6 and 0 to 18 months between interventions arms. We will also conduct cost, cost-effectiveness and contingent valuation comparisons and explore the influence of behavioral session attendance, compliance with the recommendations for diet and physical activity, self-monitoring of diet and physical activity, barriers to physical activity, sleep quality, and medications on weight change across 6 and 18 months.
Keywords: mobility related disabilities, physical disabilities, weight loss, diet, weight maintenance, physical activity
1. Introduction
Adults with mobility related disabilities (MRDs) represent a sizeable and growing segment of the population, with a high prevalence of overweight/obesity (~51%), and limited empirically based options for effective weight management (1, 2). The combination of MRDs and obesity may limit the ability to ambulate and perform transfers (wheelchair users) (3), increases the risk for pressure-sores (4, 5), respiratory problems (6), carpal tunnel syndrome (7), and interferes with self-care, reduces quality of life, and increases health care expenditures (8–10).
Several barriers, including lack of accessible and affordable transportation to attend on-site meetings (3), difficulty with food shopping and meal preparation (11–13), and lack of accessible facilities for participation in physical activity (14–17) preclude extrapolation of weight management guidelines developed for non-disabled adults (18) to adults with MRDs. A limited number of trials have addressed different weight management strategies for adults with MRDs. The majority of these trials have employed 1-group, pre-post designs, in small samples (n ≤ 16), over relatively short time frames (≤ 20 wks.) (19–22), and have demonstrated unimpressive results in terms of both mean weight loss, and the percentage of participants losing ≥ 5% of baseline weight. Previously, we compared weight loss (6 mos.) and maintenance (6 mos.) in a sample of 126 overweight/obese, adults with MRDs randomized to either a reduced energy (1,200-1,500 kcal/d) meal plan, or an enhanced Stop Light Diet (eSLD) (23). We enhanced the original Stop Light Diet, which categorizes foods by energy content corresponding to a traffic light, i.e., red (eat sparingly), yellow (eat moderately), and green (eat freely) (24), by encouraging daily consumption of 2 portion-controlled entrees (≤ 300 kcal each), 2 high volume low energy shakes (100 kcal each), and ≥ five 1-cup servings of fruits/vegetables. Behavioral counseling was delivered during monthly home visits conducted by a trained health educator, and included self-monitoring (logs) and optional physical activity. Significantly greater mean weight loss was observed in the eSLD compared with the meal plan group at both 6 months (eSLD = −4.1%, meal plan = −2.3%, p = 0.024) and 12 months (eSLD = −6.2%, meal plan = −0.6%, p = 0.005). Additionally, the percentage of participants losing ≥ 5% of baseline weight at 12 mos. was significantly higher in the eSLD (36%) compared with the meal plan groups (13%, p = 0.003).
These results are encouraging; however, the cost and logistics associated with intervention delivery to individuals in their home limits the potential for scaling and implementation of this approach. The evaluation of alternative strategies for the delivery of weight management to greater numbers of adults with MRDs, potentially improve weight loss outcomes, and reduce costs are warranted. Thus, we will conduct a two-arm randomized trial in overweight/obese adults with MRDs to compare weight loss (6 mos.) and maintenance (12 mos.) between interventions utilizing the eSLD, behavioral counseling, and increased physical activity delivered to individual participants in their homes or delivered remotely to groups of participants in their homes via video conferencing.
2. Methods and materials
2.1. Overview of study design (Table 1)
Table 1:
Design Overview for an 18-month Weight Loss and Maintenance Intervention in Adults with Mobility Related Disabilities
| INTERVENTION GROUPS | ||
|---|---|---|
| Group Remote | Individual home visit | |
| Diet | SLD enhanced to include portion-controlled entrées and low kcal shakes | SLD enhanced to include portion-controlled entrées and low kcal shakes |
| Behavioral change sessions | ||
| Delivery method | Group video conferencing (iPad®) | Individual home visit |
| Meeting frequency | ||
| Weight loss (0-6 mos.) | 2/mo. | 2/mo. |
| Maintenance (7-12 mos.) | 2/mo. | 2/mo. |
| Maintenance (13-18 mos.) | 1/mo. | 1/mo. |
| Physical Activity | ||
| Recommendation | Aerobic:150 min/wk.; RT: 2/wk.; Reduced sedentary time | Aerobic:150 min/wk.; RT: 2 /wk.; Reduced sedentary time |
| Delivery method | Group video conferencing (iPad®) | Self-directed with support phone calls/text messages |
| Session frequency/duration | ||
| Weight loss (0-6 mos.) | 2-30 min sessions/wk. | 1-call (5-10 min) and 1 text message/wk. |
| Maintenance (7-18 mos.) | 1-30 min session/wk. | 1-text message/wk. |
| Self-Monitoring | ||
| Diet | LoseIt! app® | Self-report/pencil/paper |
| Physical activity | Fitbit® | Self-report/pencil/paper |
| Body weight | Electronic scale | Electronic scale |
Note: SLD=Stop Light Diet, RT=resistance training
We will conduct a 2-arm randomized trial (group remote (GR) vs. individual home visit (IH)) using intent-to-treat principles, to compare body weight following weight loss (6 mos.) and maintenance (18 mos.) in overweight/obese adults with MRDs. Both interventions will be delivered by trained health educators and will include behavioral sessions, a reduced energy diet (eSLD), increased physical activity, and self-monitoring of body weight, diet and physical activity. The primary outcome of weight will be assessed during home visits at baseline (mo. 0), following weight loss (6 mos.), during (12 mos.) and at the end of maintenance (18 mos.). The primary aim will compare mean weight loss between the GR and IH arms across 6 months. Secondarily, we will compare mean weight loss (0-18 mos.), the proportion of participants who achieve clinically meaningful weight loss (≥ 5%) from 0 to 6 and 0 to18 months, and changes in quality of life from 0 to 6 and 0 to 18 months between the GR and IH arms. We will also conduct cost, cost-effectiveness and contingent valuation comparisons between intervention arms. Finally, we will explore the influence of behavioral session attendance, compliance with the recommendations for diet (energy intake, number of entrees /shakes, servings of fruits/vegetables), physical activity (min of moderate-vigorous physical activity and sedentary time), self-monitoring of diet and physical activity, barriers to physical activity, sleep quality, and medications on weight change across 6 and 18 months.
2.2. Intervention-theoretical model
The intervention will focus on the aspects of Social Cognitive Theory (25) which have been shown to be most effective for changing diet and physical activity including intention formation, goal setting (10% weight loss at 6 mos., minimal regain at 12, and 18 mos.), self-monitoring (diet, physical activity, weight), and feedback (26). These techniques will be enhanced by employing behavior shaping (gradually modifying diet and physical activity), social support, stimulus control (strategies to decrease cues for less desirable and increase cues for more desirable diet and physical activity behaviors), cognitive strategies to increase self-efficacy and to deal with social relationships affected by weight, such as identifying/confronting self-sabotage attempts, and responding to stress with non-food techniques. Relapse prevention strategies to teach participants to recognize precursors and consequences of lapses, and to develop plans for addressing high risk situations will be included. The intervention is also informed by the social ecologic theory (27) as content will be tailored to the intrapersonal (individual’s knowledge, demographics, attitudes, values, skills, behavior), interpersonal (group support, health educator support, strategies for peer/family communication related to diet and physical activity, strategies for handling social situations), and community (community resources for physical activity, farmers markets, community advisory panel for intervention development/dissemination) levels of influence on behavior change. The weight management intervention content used in the study is based on previous research by our group and has been manualized.
2.3. Participant eligibility
Primary care physician clearance will be required for all participants. To enhance generalizability, individuals receiving treatment for prevalent chronic diseases, or with risk factors for chronic diseases such as hypertension, tobacco use, lipid abnormalities, or who take other medications that impact body weight, will not be excluded. Inclusion/exclusion criteria are presented in Table 2.
Table 2:
Participant Eligibility Criteria for an 18-month Weight Loss and Maintenance Intervention in Adults with Mobility Related Disabilities
| INCLUSION CRITERIA | |
|---|---|
| Disability Status | A permanent MRD (≥1 yr. duration) requiring the use of a wheelchair or resulting in the inability to walk 0.25 miles without stopping, with or without assistive devices, as documented by 7 items from the National Health and Nutrition Examination Survey Physical Function Survey (28), and confirmed by the primary care physician (PCP). |
| Age | ≥ 18 years |
| Overweight/obese | Body mass index (BMI) > 25 kg/m2. We are aware of the difficulty in the assessment of BMI and issues related to the use of BMI for classifying overweight/obesity in individuals with spinal cord injury or amputations (29, 30). In questionable cases regarding eligibility based on weight status, we will defer to the PCP. |
| Internet | Internet access in the home |
| EXCLUSION CRITERIA | |
| Health status | Serious medical risk, e.g., cancer, recent heart attack, or stroke; use of antipsychotics, untreated depression, or other psychiatric illness that would preclude participation in weight management; cognitive visual or hearing impairments that may interfere with compliance to the study protocol as determined by the PCP. |
| Recent weight loss | Participation in a weight loss program within the past 6 months. |
| Weight stability | Not weight stable defined as weight variation over the 3 months prior to baseline as less than ± 4.6 kg. |
| Weight | Baseline weight >400lbs as these individuals require more aggressive interventions (e.g., surgery, medication, etc.). |
| Current physical activity | Complete more than 3, 30-minute sessions of planned exercise per week. |
| Specialized Diet | Serious food allergies, consuming special diets (vegetarian, Atkins etc.), aversion to common foods (e.g., unwilling to consume dairy products, vegetables), |
| Functional status | Unable to participate in light-to moderate intensity physical activity, e.g., seated aerobics, resistance exercise, or physically unable to use the iPad®. |
| Eating disorders | Binge eating (Binge eating scale) or other eating disorders (EATs-26. |
| Pregnancy | Pregnancy during the previous 6 months, currently lactating or planned pregnancy in the following 18 months. Participants who become pregnant will we removed from the trial and referred to appropriate agencies for consultation. |
2.4. Participant recruitment/randomization/retention
We will recruit through health care providers (hospitals/physician’s offices, medical equipment vendors) email list serves, and community agencies including independent living and vocational rehabilitation centers. Potential participants will be asked to contact project staff via email, our website, or a dedicated study phone number that will be included in all recruitment materials. Interested participants will be asked to complete an on-line initial eligibility screening questionnaire to determine type of MRD, chronic diseases, medications, previous weight loss attempts, and current level of physical activity. Home visits will be scheduled with those deemed to be initially eligible to describe the project, answer questions, obtain consent, and measure height and weight to determine eligibility based on BMI. Project staff will send a form (fax/email) to the potential participant’s primary care physician describing the study and requesting clearance for participation. Overweight/obese individuals found to be ineligible will be referred to other available weight management options. We will recruit cohorts of 20-25 individuals. Participants are recruited in cohorts due to the design and logistics of the intervention delivery. Cohorts are necessary when you have a group design to ensure all participants in the cohort are on the same week and to minimize staff burden. Cohorts will have different health educators. Recruitment continues between each cohort but enrollment into the study only occurs once a full cohort has been recruited. Following baseline testing, participants will be stratified by their primary mode of locomotion outside the home, i.e., ambulatory or assistive device (wheelchair, scooter etc.) and computer randomized with equal allocation to the GR and IH arms. Treatment allocation sequences for each cohort will be concealed in envelopes and delivered to the study coordinator. Our intervention includes several components that may assist with participant retention such eliminating the need to travel to attend group meetings, providing frequent contact between participants and health educators (phone calls/video conference/in-person), and education designed to increase self-efficacy and motivation for diet and physical activity as part of the curriculum. We will also ask participants to complete a form that will include contact information (name, address, phone number, email) for themselves and a least two family members or close friends. Participants missing more than two consecutive behavioral sessions will be contacted by phone/text/email (maximum 3 attempts) to encourage participation and to identify barriers to participation. We will also mail birthday/holiday cards and provide intervention news and updates via monthly emails. Undelivered mailings retuned with a forwarding address will be used to track participants potentially lost to follow-up.
2.5. Intervention components
2.5.1. Diet
The Stop Light Diet (SLD), developed for use in children, categorizes foods by energy content: green (eat freely), yellow (eat in moderation) and red (avoid) (24). The SLD is easy to understand and implement when compared with a traditional meal plan diet which requires choosing appropriate foods from an unlimited array of options, making it difficult to prepare a nutritionally adequate reduced energy diet on a consistent basis. We will “enhance” the Stop Light Diet (eSLD) using a combination of commercially available portion-controlled meals (entrees/shakes) and fruits and vegetables. Portion-controlled meals include nutritional information on the label (calories, fat, protein etc.) making it easier to adhere to specific energy and nutrient prescriptions. During weight loss (0-6 mos.) participants will be asked to consume a minimum daily total of 2 entrees (~200 to 300 kcal each, saturated fat ≤ 3g), 2 shakes (~100 kcal each), five 1-cup servings of fruits/vegetables, and ad libitum non-caloric beverages. Participants will be asked to purchase entrees from a list meeting these caloric/fat requirements, as well as fruits/vegetables, and non-caloric beverages. The recommended entrees/shakes are affordable and available at most grocery stores at a cost of $2-$4 each, which may be an important consideration for individuals with MRDs where household income is often low (31). Two low-calorie shakes per day will be provided as part of the eSLD only during weight loss (0-6 months) (Profile by Sanford Health, Sioux Falls, SD). Dr. Ptomey, RDN will assure all diets are energy and nutritionally adequate, and will follow the Academy of Dietetics and Nutrition (AND) Evidence Library Nutritional Guidelines for individuals with spinal cord injury (SCI) (32). The use of portion-controlled meals for weight loss has been criticized for inadequately preparing participants to deal with meal planning and portion control required for successful weight loss maintenance. However, the eSLD does not eliminate decision making regarding shopping and preparation of fruit/vegetables, beverages and other foods using the SLD as a guide. Portion controlled entrees also provide participants with an understanding of appropriate portion size. During weight maintenance (7-18 mos.) participants will be encouraged to continue using the eSLD, or transition to a meal plan, or combination eSLD/meal plan diet. At the end of the weight loss phase (mo. 6) counseling will include strategies for complying with a conventional diet, i.e., meal planning, food shopping, meal preparation, portion control, etc.
2.5.3. Energy intake
Energy intake during weight loss (0-6 mos.) will be prescribed at 1,200-1,500 kcal/d for women and 1,500-1,800 kcal/d for men as recommended by current weight management guidelines from the American Heart Association/American College of Cardiology/The Obesity Society (18). We are aware that these recommendations were not designed specifically for adults with MRDs, and may be inappropriate for individuals with SCI due to variable reductions in energy expenditure associated with level and duration of injury, adrenergic function, and fat-free-mass (33–36). We will estimate energy needs for individuals with paraplegia (27.9 kcal/kg/d) and tetraplegia (22.7 kcal/kg/d) as recommended by Cox et al (37) with upward adjustment for individuals with pressure ulcers as suggested by the AND Evidence Library Nutritional Guidelines for individuals with SCI (38). These levels of energy intake provide a reasonable starting point and will be adjusted based on the observed weight change and participant feedback regarding hunger/satiation. Energy intake for weight maintenance (7-18 mos.) will be based on the resting metabolic rate (RMR) equation of Mifflin-St Jeor (39) adjusted for activities of daily living (RMR x 1.4-1.6) as participants will have a routine physical activity program. As described for weight loss, we will use the energy need estimates of Cox et al (37) and the AND Evidence Library for individuals with SCI (38). In our experience, some participants not meeting their weight loss goals (0-6 mos.) will volitionally attempt additional weight loss during maintenance (7-18 mos.) which will not be encouraged or discouraged. Participants experiencing weight gain will be counseled to improve compliance with the diet and physical activity protocols.
2.5.5. Behavioral sessions
Behavioral sessions (45-60 min) with groups of 10-15 participants will be conducted 2/mo. during weight loss and the first 6 mos. of maintenance (mos. 0-12), and 1/mo. during the last 6 mos. of maintenance (mos. 13-18) in the GR arm using Zoom® video conferencing software (Zoom® Inc. San Francisco, CA) and to individual participants in the IH arm during home visits. Content of the behavioral sessions will be identical in both intervention arms. Each session will include a review of participants self-monitored data for diet, physical activity, and weight recorded since the previous session (described below), a question to generate discussion regarding diet and physical activity, a lesson on a weight management topic, and an experiential learning assignment requiring problem solving or the practice of behavioral strategies, to be completed prior to the next session. Lessons will include topics such as goal setting, benefits of fruits and vegetables, cost and time efficient strategies for food shopping and preparation, specific strategies for increasing daily physical activity and decreasing sedentary time, maintaining adequate hydration, eating away from home etc., with examples and discussion questions specific to individuals with MRDs. Behavioral sessions during maintenance (7-18 mos.) will focus on transitioning to a conventional meal plan diet using SLD principles, with or without incorporation of portion-controlled meals, and cardinal behaviors for successful weight maintenance including relapse prevention, self-monitoring, regular physical activity, eating on weekends/vacations, and controlling energy intake on special occasions and holidays, etc. During each behavioral session, health educators will also answer questions, problem solve and provide support.
2.5.6. Physical activity.
Participants will be asked to complete 150 min./wk. of moderate-intensity aerobic activity (3-6 METs), and 2 d/wk. of resistance exercise as recommended for adults with/without disabilities by the American College of Sports Medicine/American Heart Association (40) and the U.S. Department of Health and Human Services (41). This level of exercise is well within the capability of most adults with MRDs, including those with SCI(42, 43); however, participants unable to complete 150 min./wk. will be encouraged to exercise to their individual capacities. We recognize that 150 min./wk. is less than the 300 min./wk. recommended for long-term weight management (44). However, given the limits to the levels of energy expenditure, from what will be predominantly upper extremity exercise, and the increased potential for overuse injuries with higher volumes of exercise (45), we felt that 300 min./wk. would be unrealistic in this sample. Aerobic exercise will progress from 60 min/wk. (3 d/wk., 20 min./d) to 150 min. /wk. (5 d/wk., 30 min./d) at the beginning of mo. 4 and remain at 150 min./wk. through mo. 18. RT (3 sets/8-10 reps, 4-5 exercises) using resistance bands (8 levels) provided by the study will be prescribed (Theraband®, Akron OH). Resistance exercise, as well as a stretching regime, will focus on scapular stabilizers and posterior shoulder muscles, and major lower extremity muscles for participants with this capability. During the first 2 weeks, participants will use a band that provides a resistance allowing completion of 3 sets with minimal fatigue. Resistance will be increased to the next level at wk. 3, and increased subsequently, when participants are able to complete 3 sets of 8-10 reps with minimal fatigue. The negative health consequences of sedentary behavior (46, 47) may be especially relevant for individuals with MRDs (15, 48). Therefore, strategies for decreasing sedentary time, such as increasing sit-to-stand transitions and standing time, in those who are able, and increasing nonexercise upper extremity activity, e.g., daily light activities for wheelchair users, will be recommended.
2.5.7. Self-monitoring
All participants will be asked to self-monitor diet and physical activity daily, and body weight weekly across the 18-month trial. This data will be used for participant motivation and accountability, and to inform counseling by health educators. Participants will be reminded to complete self-monitoring during behavioral sessions across 18 mos., and during the weight loss phase (0-6 mos.) via reminders sent an iPad® tablet computer (Apple Inc., Cupertino, CA) provided by the trial.
2.5.7.1. Body weight
Self-monitoring body weight is difficult for individuals with MRDs, specifically those who use wheelchairs, due to the lack of affordable wheelchair scales for home use (currently ~$600 each), and limited availability of wheelchair scales in the community (49–51). Given the importance of self-monitoring of body weight in weight management (52), and the potential safety concerns associated with prescribing a reduced energy diet without monitoring weight, we will provide all participants with electronic scales. Participants will be asked to weigh weekly: wheelchair users (Prime Scales®, Ultra-Portable Light Weight Wheelchair Scale, Ontario, CA); non-wheelchair users (Fitbit® Aria 2, Fitbit Inc., San Francisco, CA). The Fitbit® Aria 2 transmits the participant’s weight through Bluetooth using the Fitbit app.
2.5.7.2. Diet
Participants in the GR arm will be asked to log all food and beverages consumed (meals/snacks) using the Lose It!® app (Fitnow Inc., Boston MA) by entering the food name and selecting the portion size, or by scanning the bar code of the food item using the iPad®. A bar graph comparing the recommended and actual dietary intake for each participant is displayed, providing immediate feedback of how much food participants have consumed during the day and how much more or less they should consume. Participants in the IH arm will be asked record all food and beverages consumed (meals/snacks) on standard paper forms provided by the trial.
2.5.7.3. Physical activity
Participants in the GR arm will wear a Fitbit® Versa Lite activity tracker (Fitbit® Inc., San Francisco, CA) on their non-dominant wrist. This device tracks physical activity with minimal participant burden and provides real-time data, via the Fitbit app. This data is available to both participants and health educators to provide feedback during behavioral sessions. We realize that the validity of the Fitbit as a measure of physical activity may be questioned (53, 54)particularly for wheelchair users. However, the Fitbit will be used only to provide participant feedback, i.e., is physical activity increasing or decreasing over time, and not to assess compliance with physical activity recommendations. Participants in the IH arm will be asked to record the type/duration of physical activity on paper forms provided by the trial.
2.6. Intervention delivery
2.6.1. Consumer advisory panel
Prior to initiating the intervention, a member of the research team will convene a group of 4-5 individuals with MRDs, who will not participate in this trial, to review the intervention protocols, materials etc., and offer suggestions for modification. This group will reconvene annually to offer input regarding any disability specific issues that may arise, discuss results, and provide insight as to logical next steps, including strategies for dissemination.
2.6.2. Health educator training/intervention fidelity
New health educators will train by shadowing experience health educators for a minimum of 3 months prior to delivering the interventions. All health educators will participate in weekly 2-hour staff meetings to discuss issues relevant to intervention delivery. The same health educator will deliver both the GR and IH arms in each cohort to reduce the potential for health educator effects. Recordings of all behavioral and group physical activity sessions (GR-video, IH-audio) will be reviewed by the project coordinator. Intervention content that was delivered will be compared with a checklist of scheduled content (Table 3). Health educators covering less than 80% of scheduled content or failing to deliver physical activity as prescribed, will receive additional training and will be dismissed if the problem recurs.
Table 3.
Health Educator Observation Form
| Monthly Meeting Activities | Task Completed by Health Educator? | ||
|---|---|---|---|
| I. | Weigh In & Tracking of Progress | ||
| A. Obtain weight | YES | NO | |
| B. Plot weight and/or discuss progress | YES | NO | |
| COMMENTS | |||
| II. | Review diet and physical activity / Progress from previous meeting | ||
| A. Talks about progress from last session/beginning of program | YES | NO | |
| B. If barriers identified, helps the participant problem solve | YES | NO | |
| C. IH – review logs; GR – access LoseIt!® data, provides feedback | YES | NO | |
| COMMENTS | |||
| III | Education / Lesson | ||
| A. Adequately covered the main points of the lesson | YES | NO | |
| B. Generated good discussion with participant (or group if GR) | YES | NO | |
| C. Reads verbatim from the lesson plan | YES | NO | |
| COMMENTS | |||
| IV | Goal Setting | ||
| A. Followed up on goals set at previous meeting | YES | NO | |
| B. Set new goals for the next month | YES | NO | |
| C. Did the health educator involve the participant when setting the new goals? | YES | NO | |
| COMMENTS | |||
| V | Incentives | ||
| A. Were the shakes distributed and encouragement provided? | YES | NO | |
| B. Did the health educator provide support and encouragement for the next two weeks? | YES | NO | |
| COMMENTS | |||
| VII | Next Appointment | ||
| A. Was the next meeting scheduled? | YES | NO | |
| COMMENTS | |||
2.6.3. Orientation
Health educators will conduct home visits (~90 min) with all participants prior to initiating the intervention to provide a detailed description of both the dietary (eSLD) and physical activity components of the intervention and delivery formats (remote or home visit). All participants will be provided with an iPad® tablet computer and electronic scales; wheelchair users (Prime Scales®); non-wheelchair users (Fitbit® Aria 2). Access to non-study related materials, e.g. app store etc., will be blocked on all iPads® until completion of the study. The iPads® for the GR arm will be pre-loaded with the video conferencing software (Zoom®), the Lose It!® and Fitbit® applications, and behavioral session materials (described later). Participants in the IH arm will be provided with hard copies of the intervention materials and will be shown how to self-monitor diet and physical activity using hard copy records. During orientation, both intervention arms will receive a tutorial on the use of the iPad® and electronic scales. Participants in the GR arm will be oriented to the video conferencing software and both the Lose It!® and Fitbit® apps/devices used for self-monitoring diet and physical activity with time allotted for practice and questions. Per inclusion/exclusion criteria, those unable to perform these tasks will be ineligible. Tutorials describing trouble shooting for common technical problems, e.g., internet connectivity, data entry using Fitbit®, Lose It!®, will be loaded on the iPad®. Technical issues can also be resolved during behavioral sessions, or by contacting research staff by phone or email. The GR arm will be provided with an HDMI adaptor allowing video conference sessions to be displayed on a larger TV screen, if desired. Reminders regarding upcoming behavioral/physical activity sessions etc., or to prompt participants in the GR arm who are non-compliant with the study protocol, will be sent via the iPad®.
2.6.4. Physical activity delivery – group remote arm
Participants will be asked to attend 30-minute group exercise sessions led by a trained exercise leader that will be delivered to participants in their homes using video conferencing software (Zoom®) 2 x/wk. during weight loss (0-6 mos.) and 1 x/wk. during maintenance (7-18 mos.) (Table 1). Group sessions will include a warm-up, 20 min. of moderate intensity aerobic activity, 10 min of resistance exercise, and stretching and cool-down. Exercise sessions will include individuals with a range of functional abilities, e.g., wheelchair users/ambulatory. Thus, activities will be modified, as necessary, to insure they are applicable for all participants. Exercise sessions will be conducted in conjunction with behavioral sessions when schedules coincide. In such cases, the total session time (behavioral+exercise) will not exceed 60 minutes. Group sessions will provide 40 minutes of the 150 min./wk. recommendation for aerobic activity. To assist participants in both study arms in meeting the 150 min./wk. goal, all group sessions will be video recorded and uploaded remotely to participant’s iPads® where they can be accessed for use at any time. In addition, information regarding exercise resources available from the National Center on Health, Physical Activity and Disability, Craig Hospital, and the Christopher and Dana Reeve Foundation will be pre-loaded on the iPads® of participants in both study arms.
2.6.5. Physical activity delivery – individual home visit arm
Participants will be asked to complete the exercise recommendations on their own, as is customary in standard care weight management. Participants will receive one brief support/problem solving phone call (5-10 mins.) and one support text message per week from the health educator during weight loss (0-6 mos.) and one supportive text message per week during maintenance (7-18 mos.). Phone support has been shown to increase exercise in adults with MRDs (55).
2.6.6. Medical management
Physician clearance via a consent form will be required to participate in this trial. Study staff will alert the participant’s personal physician who will be responsible for any medication adjustments necessitated by weight change (~10%) associated with participation in this trial.
2.6.7. Participant incentives
Participants will be allowed to keep the iPad®, Fitbit® and electronic scale on study completion, and will receive $50 for completion of each of the four outcome assessments.
2.7. Outcome assessments
Most outcomes will be assessed during a 30-minute home visit; however; some assessments will occur across the 18-month intervention (Table 4). Physical measures (height, weight, waist circumference, blood pressure) will be obtained by trained staff blinded to condition, between 7-10 a.m., following a minimum 12-hour fast. Weight will be measured to the nearest 0.1 kg with participants wearing shorts and a t-shirt. BMI will be calculated as weight (kg)/height (m2). Staff will receive refresher training, and complete reliability assessments for physical measures 2-3 times/yr.
Table 4:
Outcomes Assessments Across the 18 Month Intervention
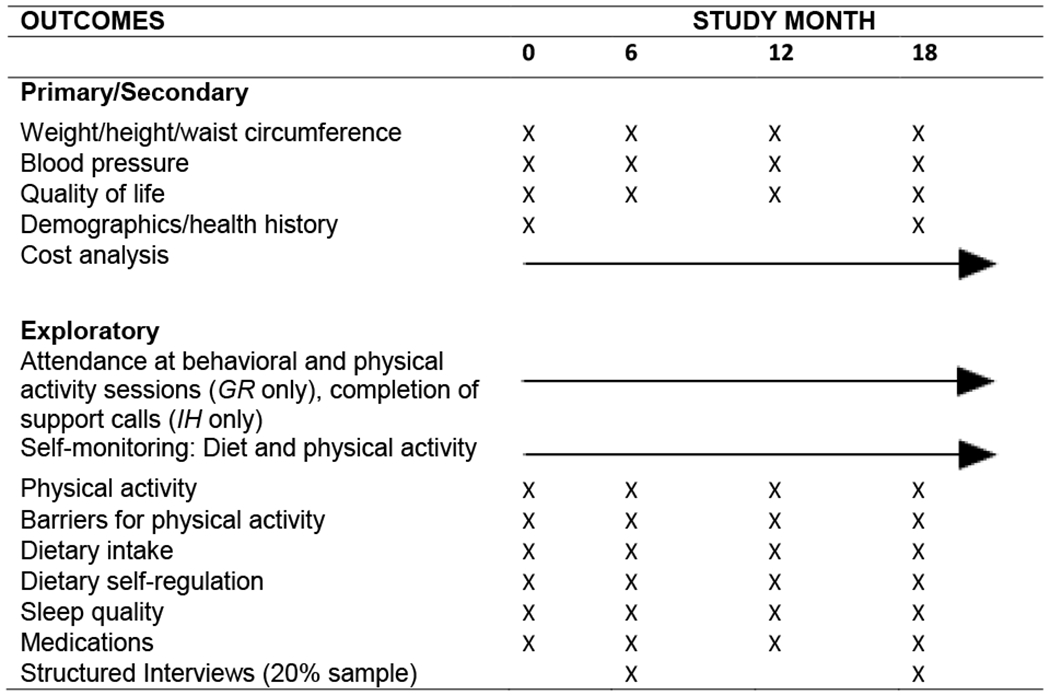 |
2.7.1. Primary/secondary outcomes
2.7.1.1. Weight/height: ambulatory participants.
Weight will be measured in duplicate with a portable, calibrated digital scale. (Model #PS6600, Befour, Saukville, WI.). Standing height will be measured in duplicate with a portable stadiometer (Model #IP0955, Invicta Plastics Limited, Leicester, UK).
2.7.1.2. Weight/height: participants who use wheelchairs.
Weight will be measured in duplicate using a portable calibrated, digital wheelchair scale (Model MX420, Befour, Saukville, WI.). Participant weight will be calculated as participant + chair weight minus chair weight. Digital photos of the chair will be obtained at baseline to ensure that the same chair (and accessories) is used for subsequent assessments, thus negating the need for repeated assessments of chair weight. Supine height will be assessed with a Shorr Knee Height Caliper (Weight and Measure, LLC) with legs outstretched and feet in dorsiflexion. If contractures prevent assessment of supine height, we will estimate total height based on knee height as described by Froehlich-Grobe et al (56).
2.7.1.3. Waist circumference
Waist circumference, as a surrogate for abdominal adiposity, will be assessed using the procedures described by Lohman et al. (57). Three measurements will be obtained with the outcome recorded as the average of the closest two measures.
2.7.1.4. Quality of life
Quality of life will be assessed with the Short Form Health Survey (SF-36E), a version of the SF-36 (58, 59) in which the physical activity section has been modified for individuals with MRDs. Internal consistency for SF-36E scale items range from 0.76 to 0.94 while reliability estimates for the summary scale range from 0.90 to 0.95 (60).
2.7.1.5. Cost analysis
We will conduct a cost effectiveness analysis using between group differences in mean weight loss at 6 mos. as our measure of effectiveness (61). Based on the results of a previous weight loss trial conducted by our research group (62), we expect greater weight loss and lower costs in the GR compared with the IH arms. The cost perspective for all analyses will be societal. We will collect data on both program costs, e.g. health educator time, supplies, iPads®, and participant costs, i.e. time, prospectively. Participant time, is a component of intervention costs (63) ,as time devoted to an intervention cannot be used for work or leisure. These costs may represent half of the total costs; (64) and will be gathered via survey (65). We will conduct sensitivity analyses that vary the value of participant time from $0.00 to the local median hourly wage. The gold standard for measuring cost is a time study based on a validated flowchart (66, 67). We will validate flowcharts for both study arms and use time studies to estimate health educator time, and program records to measure cost of supplies. Weight management programs have effects in addition to weight loss, e.g. increased vitality or improved appearance, thus the value of a program to participants may be imperfectly assessed by the objective outcome of body weight. Contingent valuation, in which participant’s express preferences for programs, will be used to examine the perceived worth of the GR and IH formats at 6 and 18 months (68). Conditional logistic regressions, with adjustments for clustering by health educator, will be used to analyze how differences in client attributes, anticipated costs, and anticipated gains affect preferences for the two intervention arms.
2.7.2. Process measures/exploratory outcomes
2.7.2.1. Adherence with diet recommendations
Adherence with dietary recommendations will be assessed using 3-day food records collected and analyzed using the Automated Self-Administered 24-hour (ASA24®) Dietary Assessment Tool, version 2018, developed by the National Cancer Institute (69). This self-administered, web-based tool, which has demonstrated acceptable psychometric properties (69–71) enables the recording of detailed information about all foods and beverages consumed over the collection period using the iPad® that will be provided to all participants. Participants will be asked to record all food, beverages, and supplements consumed for 3 consecutive days (2 wk. days and 1 wk. end day) starting the weekend prior to scheduled outcome assessments. The ASA24® provides prompts for the reporting of eating occasion and time, the type of food/beverages consumed, food preparation methods, and image-assisted assessment of portion size for both foods and beverages. Average energy (kcal/day) and macronutrient intake (% carbs, fat, protein) and the number of portion-controlled entrees/shakes, and fruits/vegetables consumed will be calculated using the ASA24®data.
2.7.2.2. Adherence with physical activity recommendations
Physical Activity and Disability Survey will be used to assess adherence with the physical activity recommendations (72). This semi-structured interview includes subscales for exercise, leisure time physical activity, household activity, and inactivity (sleeping, TV, computer use). Acceptable validity (significant correlation with cardiovascular fitness), internal consistency (Chronbach’s alpha .67 to .77), test-retest reliability (.78 to .95), inter-rater reliability (.92 to .99) and sensitivity to change in response to an intervention to increase physical activity have been demonstrated (72, 73).
2.7.2.3. Behavioral session attendance
Attendance at behavioral sessions will be obtained from records maintained by the health educator, and will be expressed as the percentage of possible sessions attended.
2.7.2.4. Dietary self-regulation
Dietary self-regulation will be assessed using the 18-item version of the Three-Factor Eating Inventory (TFEQ-R18) (74, 75). This instrument is a widely-used measure of eating behavior (restraint, disinhibition, hunger) with acceptable and well-established psychometric properties (74, 76)
2.7.2.5. Sleep quality
The Pittsburgh Sleep Quality Index (77) (78) has been used previously in adults with MRDs (79, 80) and has shown acceptable measures of internal homogeneity, consistency, and validity (78).
2.7.2.6. Barriers to Exercise
Barriers to exercise will be assessed using the Barriers to Exercise for Disabled Persons scale which has acceptable internal consistency and discriminant validity (81).
2.7.2.7. Medications
The name, amount, and frequency of medications will be obtained by research staff at each outcome assessment. Medications will be classified (yes/no) as to their association with weight gain/loss (82).
2.7.2.8. Structured interviews
Study staff will conduct structured interviews by phone with a 20% random sample of participants from both intervention arms following the completion of weight loss (6 months) and maintenance (18 months) to gather information which may be useful in improving the intervention and/or implementing the intervention in settings serving adults with MDRs. Topics will include preference for intervention arms (GH or IH), intervention length, difficulties in complying with intervention components, suggestions for improving the intervention, and overall satisfaction with health educators, behavior sessions, and the diet and physical activity recommendations. All interviews will be recorded, transcribed verbatim and entered into a qualitative data analysis program (Atlas.ti. v 8.4, Berlin, Germany). Coding disagreements will be reviewed and discussed by the research team until 100% agreement is achieved. A content analysis of transcribed interviews to explore trends and identify common themes across interviews will be conducted using methods suggested by Miles and Huberman (83) and further described by Ryan and Bernard (84).
2.8. Statistical power/analysis plan
2.8.1. Power
Randomization of 128 participants, with equal allocation to the GR and IH arms, will provide 80% power to demonstrate a clinically meaningful between arm difference in 6 month weight loss (3 kg) (85, 86), our primary aim, using an intent-to-treat analysis, assuming a common standard deviation of 6 kg and a type I error rate of 5%. This sample size will also provide 70% power to detect a between arm difference of 3 kg in a completer only analysis with an attrition rate of 20%. However, based on our experience with similar interventions (62, 87, 88) and our retention/incentive strategies to minimize loss to follow-up, we expect that the attrition rate will be < 20%.
2.8.2. Missing data
Analysis for our primary aim will be based on intent-to-treat principles using imputation for missing data. If the proportion of participants lost to follow-up differs by treatment arm we will determine if there are differences (p<0.05) between completers and those lost to follow-up in demographic characteristics, i.e., age, sex, baseline weight. If missing data are related to treatment arm and or demographic characteristics, we will use model based multiple imputation; otherwise, traditional imputation (k-nearest neighbors) will be used. All statistical analysis will be performed following completion of data collection using SAS version 9.4 or higher.
2.8.3. Analysis-primary aim
A two-sample independent t-test will be used to compare 6-month weight loss between the GR and IH arms in both intent-to-treat and completer only analyses. The impact of baseline characteristics including age, sex, race/ethnicity, weight, type of MRD, wheelchair use, employment status, and living situation on 6-month weight loss will be examined using linear regression controlling for intervention arm. The main effect of each of these variables, and any potential interaction with intervention arm, will be examined.
2.8.4. Analysis-secondary aims.
We will compare 0 to 18-month weight loss between intervention arms using a two-sample independent t-test. We will also use mixed linear models to compare 0 to 18-month weight loss controlling for baseline weight. Greater loss to follow-up over time is anticipated; however, we expect data to be missing at random. Under this assumption, mixed linear models will provide unbiased comparisons between intervention arms. An autoregressive correlation structure of the dependent variable (weight) over time will be assumed. The potential for treatment-by-time interactions will also be evaluated.
We will evaluate between arm differences in the proportion of participants achieving ≥ 5% weight loss (0-18 months) using a chi-square test. Participants without a weight at 18 months will be classified as not meeting the ≥ 5% goal. Logistic regression will be used to examine the impact of baseline characteristics, as previously defined, on the proportion of participants achieving ≥ 5% weight loss at 18 months.
Between intervention arm comparisons of blood pressure and quality of life (0-6 and 0-18 months) will be evaluated using a two-sample t-test.
2.8.5. Analysis-exploratory aims
We will examine the influence of the following variables on 6 and 18 month weight loss: behavioral session attendance, adherence with dietary (energy intake, number of entrees/shakes, servings of fruits/vegetables) and physical activity recommendations (minutes of moderate-vigorous activity and sedentary time), self-monitoring of diet and physical activity, and medications assessed over the time period of interest, i.e., (0-6 and 0-18 months), and the change in sleep quality, dietary self-regulation and barriers for physical activity (0-6 and 0-18 months). The influence of these factors as covariates, in addition to treatment will be examined. Our sample size (n=128) will only allow for the identification of approximately 4 variables that are related to weight loss in a single model, thus judicious model selection procedures will be implemented. Only variables that differ by treatment arm (p ≤0.10) will be considered for inclusion in the model. Any potential variable by treatment interactions (p ≤0.10) in a two-factor model will be considered for future modeling to examine main effect and interaction effects along with treatment in a joint model. We will utilize best subsets criteria based on Mallows’s Cp statistic (89) to produce the most parsimonious and least biased model of variables associated with weight loss at both 6 and 18 months.
3.0. Discussion
Adults with MRDs represent a sizeable and growing segment of the population, with a high prevalence of overweight/obesity, and limited empirically based options for effective weight management. The majority of weight loss trials that have included both ambulatory and non-ambulatory adults with MRDs have used 1-group, pre-post designs, in small samples (n ≤ 16), over relatively short time frames (≤ 20 wks.) (19–22). These trials have shown unimpressive results in terms of both mean weight loss, and the percentage of participants losing ≥ 5% of baseline weight. In contrast to previous work, results from our 12-month trial in adults with MRDs suggested the potential effectiveness of a simplified dietary approach (eSLD) combined with monthly, home visit behavioral sessions (23). Although these results are encouraging, intervention delivery to individuals in their home limits the potential for scaling and implementation of this approach by community and other agencies serving adults with MRDs. Thus, alternative strategies to provide weight management to greater numbers of adults with MRDs, potentially improve outcomes, and reduce costs are warranted.
Adults with MRDs face several barriers to participation in weight management. For example, the lack of affordable, accessible transportation represents a significant barrier for both attending on-site meetings required in traditional weight management programs, and for engaging in physical activity (3, 90, 91). Adults with MRDs may have a limited understanding regarding what constitutes a healthy diet, and experience difficulty with food shopping and meal preparation (11–13). A survey of 1,096 women with disabilities indicated that ~33% experienced barriers to achieving optimal nutrition; being too tired to cook was the most frequently reported barrier (~55%)(92). In addition to barriers to physical activity experienced by the general population, e.g., lack of time and motivation etc., adults with MRDs are confronted with inaccessible recreational facilities, lack of affordable and accessible fitness equipment, and poor infrastructure for physical activity (lack of sidewalks and curb cuts, narrow/damaged sidewalks etc.) (14–17). The interventions in this trial will use remote or home visit delivery and will include a diet that simplifies food shopping and preparation which will eliminate/reduce important barriers to successful weight management.
The IH arm in this trial is a modified version of the intervention that demonstrated clinically meaningful 12 month weight loss in our previous trial in adults with MRDs (23), and may be considered standard care. Participants in the IH arm will self-monitor body weight weekly and attend in-person behavioral sessions twice a month instead of monthly as in our previous trial. The GR, or experimental arm, takes advantage of currently available technologies to deliver behavioral counseling and physical activity to groups of adults with MRDs in their homes, and to self-monitor diet, physical activity and body weight. In addition to eliminating the need to travel for both behavioral sessions and physical activity, remote delivery provides weight management simultaneously to groups of individuals, thus offering reduced delivery costs/person. The group format allows interaction between participants, which has the potential to increase accountability, social support, and rapport, and has been associated with reduced drop-out (93) and improved weight loss in non-disabled adults (94, 95). The group format may be especially desirable for individuals with MRDs who are often socially isolated (96, 97). If shown to be effective, remote delivery offers a potentially cost-effective approach to provide weight management for adults with MRDs irrespective of geographic location.
The delivery formats in both intervention arms (home visits, video conferencing) eliminate the need to travel to attend behavioral sessions, and both groups will utilize a dietary approach (eSLD) which simplifies food shopping and preparation. Remotely delivered weight management interventions, utilizing currently available technology for delivery of group based behavioral counseling and physical activity, and self-monitoring of diet, physical activity, and weight, have not been evaluated in adults with MRDs. Based on results from weight management trials in non-disabled adults, (18, 98, 99) we hypothesize significantly greater weight loss in the GR compared with the IH arms. Results from our previous trial in adults with MRDs demonstrated clinically meaningful 12 mo. weight loss using a monthly home visit intervention similar to the IH arm in this trial (23). Thus, if our expectations are confirmed, adults with MRDs, and weight management practitioners, will be able to choose from two effective weight management strategies depending on participant/provider preferences for delivery format and cost considerations. Our primary aim is to compare weight loss and maintenance between GR and IH arms; however, we are interested in the major components that drive any observed differences. Therefore, we will identify variables that most highly influence weight along with the treatment and/or the mechanism(s) of action that are impacting weight loss
We chose the eSLD for 3 reasons: 1) Compared with a meal plan diet, the eSLD, which uses the Stop light diet + commercially available portion controlled meals (entrées /shakes) and fruits and vegetables, simplifies meal planning, food shopping and meal preparation, which represent significant barriers to weight management in adults with MRDs (11–13). 2) The AND Evidence Analysis Library indicates strong, consistent, supportive evidence (Grade I) that portion controlled meals result in significantly greater weight loss and maintenance compared with meal plan diets, and are especially useful in individuals who have difficulty with food selection and portion control (100). 3) Our previous trial in adults with MRDs demonstrated ~6% weight loss at 12 mos. using the eSLD, which was significantly greater than achieved with a meal plan diet (−0.6%) (23). Additionally, the Veterans Administrations (VA) “Aspire” weight loss intervention (101, 102) has also demonstrated significantly greater weight loss at 12 mos. using a SLD compared with the standard VA MOVE! meal plan diet (102).
The following design attributes ensure the scientific rigor of this trial: 1) Randomized design with equal allocation to groups, with allocation assignment concealed. 2) Adequate statistical power to address the primary aim. 3) Strategies to ensure the recruitment of sufficient participants to achieve our desired sample size. 4) Retention/incentive strategies to reduce loss to follow-up. 5) Controlling for attention by maintaining equal contact in both groups. 6) Secondary/exploratory aims to maximize the use of data, e.g., analysis to examine the impact of process variables/participant characteristics on weight change. 7) The use of a theory-based intervention delivered by trained health educators. 8) Delivery of both intervention arms by the same health educator to cohorts of participants to minimize potential health educator effects. 9) Procedures to ensure intervention fidelity. 10) Assessments completed by trained staff blind to intervention arm. 11) Evaluation of staff inter-rater reliability for all physical assessments (2-3 times/yr.). 12) Structured interviews at both 6 and 18 mos. to gather information that might be useful in improving the intervention and/or implementing the intervention in settings serving individuals with MRDs.
In summary, this two-arm randomized trial will compare weight loss (6 mos.) and maintenance (12 mos.) between an individual home visit intervention (IH), using a protocol similar to our previous trial, and an intervention delivered remotely via video conferencing to groups of overweight/obese adults with MRDs in their homes (GR), and utilizing available technology for self-monitoring and increasing physical activity. We expect clinically meaningful weight loss in both intervention arms, i.e. ≥ 5%, with significantly greater weight loss in the GR arm. If successful, the GR intervention has the potential to be implemented in a variety of settings to provide effective weight management for adults with MRDs at a reasonable cost.
Acknowledgments
Funding: National Institute of Diabetes and Digestive and Kidney Diseases (DK116669)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
NCT registration: NCT04046471
REFERENCES
- 1.Taylor DM. Americans with Disabilities: 2014. In: United States Census Bureau, editor. Washington, DC: 2018. p. 70–152. [Google Scholar]
- 2.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity amoung adults and youth: United States, 2015–2016. In: Statistics NCfH, editor. Hyattsville, MD: National Center for Health Statistics; 2017. [Google Scholar]
- 3.Locatelli SM, Gerber BS, Goldstein B, Weaver FM, LaVela SL. Health care provider practices, barriers, and facilitators for weight management for individuals with spinal cord injuries and disorders. Top Spinal Cord Inj Rehabil. 2014;20(4):329–37. Epub 2014/12/06. doi: 10.1310/sci2004-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liou TH, Pi-Sunyer FX, Laferrere B. Physical disability and obesity. Nutrition reviews. 2005;63(10):321–31 [DOI] [PubMed] [Google Scholar]
- 5.Liusuwan RA, Widman LM, Abresch RT, Johnson AJ, McDonald CM. Behavioral intervention, exercise, and nutrition education to improve health and fitness (BENEfit) in adolescents with mobility impairment due to spinal cord dysfunction. The journal of spinal cord medicine. 2007;30 Suppl 1:S119–26. Epub 2007/09/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald SJ, Kelleher AR. Mobility challenges in individuals with a spinal cord injury with increased body weight. Topics in Spinal Cord Injury Relabilitation. 2007;12(4):54–63. [Google Scholar]
- 7.Gater DR Jr. Obesity after spinal cord injury. Physical medicine and rehabilitation clinics of North America. 2007;18(2):333–51, vii Epub 2007/06/05. doi: 10.1016/j.pmr.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Holmgren M, Lindgren A, de Munter J, Rasmussen F, Ahlstrom G. Impacts of mobility disability and high and increasing body mass index on health-related quality of life and participation in society: a population-based cohort study from Sweden. BMC public health. 2014;14:381 Epub 2014/04/20. doi: 10.1186/1471-2458-14-381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karpur A, Bruyere SM. Health care expendiure among people with disabiities: Potential role of workplace health promoion and implications for rehabilitation counseling. Rehabilitation Counseling Bulletin. 2012;56(1):7–22. [Google Scholar]
- 10.Anderson WL, Wiener JM, Khatutsky G, Armour BS. Obesity and people with disabilities: the implications for health care expenditures. Obesity (Silver Spring, Md). 2013;21(12):E798–804. Epub 2013/06/28. doi: 10.1002/oby.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertoli S, Battezzati A, Merati G, Margonato V, Maggioni M, Testolin G, Veicsteinas A. Nutritional status and dietary patterns in disabled people. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2006;16(2):100–12 [DOI] [PubMed] [Google Scholar]
- 12.Tomey KM, Chen DM, Wang X, Braunschweig CL. Dietary intake and nutritional status of urban community-dwelling men with paraplegia. Archives of physical medicine and rehabilitation. 2005;86(4):664–71. Epub 2005/04/14. doi: 10.1016/j.apmr.2004.10.023 [DOI] [PubMed] [Google Scholar]
- 13.Mojtahedi MC, Boblick P, Rimmer JH, Rowland JL, Jones RA, Braunschweig CL. Environmental barriers to and availability of healthy foods for people with mobility disabilities living in urban and suburban neighborhoods. Archives of physical medicine and rehabilitation. 2008;89(11):2174–9. Epub 2008/11/11. doi: 10.1016/j.apmr.2008.05.011 [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg Y, Vanderbom KA, Vasudevan V. Does the built environment moderate the relationship between having a disability and lower levels of physical activity? A systematic review. Preventive medicine. 2017;95s:S75–s84. Epub 2016/07/30. doi: 10.1016/j.ypmed.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 15.Rimmer JH, Schiller W, Chen MD. Effects of disability-associated low energy expenditure deconditioning syndrome. Exercise and sport sciences reviews. 2012;40(1):22–9. Epub 2011/10/22. doi: 10.1097/JES.0b013e31823b8b82 [DOI] [PubMed] [Google Scholar]
- 16.Rimmer JH, Padalabalanarayanan S, Malone LA, Mehta T. Fitness facilities still lack accessibility for people with disabilities. Disability and health journal. 2016. Epub 2017/02/02. doi: 10.1016/j.dhjo.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 17.Martin Ginis KA, Ma JK, Latimer-Cheung AE, Rimmer JH. A systematic review of review articles addressing factors related to physical activity participation among children and adults with physical disabilities. Health psychology review. 2016;10(4):478–94. Epub 2016/10/30. doi: 10.1080/17437199.2016.1198240 [DOI] [PubMed] [Google Scholar]
- 18.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Journal of the American College of Cardiology. 2014;63(25 Pt B):2985–3023. Epub 2013/11/19. doi: 10.1016/j.jacc.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Henson S, Jackson AB, Richards JS. Obesity intervention in persons with spinal cord injury. Spinal cord. 2006;44(2):82–91. Epub 2005/08/17. doi: 10.1038/sj.sc.3101818 [DOI] [PubMed] [Google Scholar]
- 20.Betts AC, Froehlich-Grobe K. Accessible weight loss: Adapting a lifestyle intervention for adults with impaired mobility. Disability and health journal. 2017;10(1):139–44. Epub 2016/07/20. doi: 10.1016/j.dhjo.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 21.Radomski MV, Finkelstein M, Hagel S, Masemer S, Theis J, Thompson M. A pilot wellness and weight managment program for individuals with spinal cord injury: Participants’ goals and outcomes. Topics in spinal cord injury rehabilitation. 2011;17(2):59–69. [Google Scholar]
- 22.Brochetti AM, Brose SW, Kuemmel AM, Dang DJ, Bourbeau DJ. Interdisciplinary bodyweight management program for persons with SCI. The journal of spinal cord medicine. 2018:1–7. Epub 2018/12/06. doi: 10.1080/10790268.2018.1547860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichard A, Saunders MD, Saunders RR, Donnelly JE, Lauer E, Sullivan DK, Ptomey L. A comparison of two weight management programs for adults with mobility impairments. Disability and health journal. 2015;8(1):61–9. Epub 2014/09/23. doi: 10.1016/j.dhjo.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 24.Epstein L, Squires S. The Stoplight Diet for Children: An Eight-Week Program for Parents and Children. Boston, MA: Little Brown & Company; 1988. [Google Scholar]
- 25.Bandura A Social Foundations of Thought and Action: A Social Cognitive Theory. Englewood Cliffs, New Jersey: Prentice-Hall; 1986. [Google Scholar]
- 26.Michie S, Abraham C, Whittington C, McAteer J, Gupta S. Effective techniques in healthy eating and physical activity interventions: a meta-regression. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2009;28(6):690–701. Epub 2009/11/18. doi: 10.1037/a0016136 [DOI] [PubMed] [Google Scholar]
- 27.Golden SD, Earp JA. Social ecological approaches to individuals and their contexts: twenty years of health education & behavior health promotion interventions. Health education & behavior : the official publication of the Society for Public Health Education. 2012;39(3):364–72. Epub 2012/01/24. doi: 10.1177/1090198111418634 [DOI] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics. Physical Function items of the National Health and Nutrition Examination Survey1999-2000. [Google Scholar]
- 29.Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal cord. 2009;47(10):757–62. Epub 2009/04/08. doi: 10.1038/sc.2009.33 [DOI] [PubMed] [Google Scholar]
- 30.Ravensbergen HR, Lear SA, Claydon VE. Waist circumference is the best index for obesity-related cardiovascular disease risk in individuals with spinal cord injury. Journal of neurotrauma. 2014;31(3):292–300. Epub 2013/09/28. doi: 10.1089/neu.2013.3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okoro CA, Hollis ND, Cyrus AC, Griffin-Blake S. Prevalence of Disabilities and Health Care Access by Disability Status and Type Among Adults - United States, 2016. MMWR Morbidity and mortality weekly report. 2018;67(32):882–7. Epub 2018/08/17. doi: 10.15585/mmwr.mm6732a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Academy of Dietetics and Nutrition Evidence Analysis Library. Practice Guidelines for Nutrition Care for Patients with Spinal Cord Injury. http://adaevidencealibrary.com: 2009.
- 33.Bauman WA, Spungen AM, Wang J, Pierson RN Jr. The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury. Journal of rehabilitation research and development. 2004;41(1):1–8. [DOI] [PubMed] [Google Scholar]
- 34.Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. The American journal of clinical nutrition. 2003;77(2):371–8 [DOI] [PubMed] [Google Scholar]
- 35.Feasel S, Groah SL. The impact of diet on cardiovascular disease risk in individuals wih spinal cord injury. Topics in Spinal Cord Injury Relabilitation. 2009;14(3):58–68. [Google Scholar]
- 36.Khalil RE, Gorgey AS, Janisko M, Dolbow DR, Moore JR, Gater DR. The role of nutrition in health status after spinal cord injury. Aging and disease. 2013;4(1):14–22 [PMC free article] [PubMed] [Google Scholar]
- 37.Cox SA, Weiss SM, Posuniak EA, Worthington P, Prioleau M, Heffley G. Energy expenditure after spinal cord injury: an evaluation of stable rehabilitating patients. The Journal of trauma. 1985;25(5):419–23 [PubMed] [Google Scholar]
- 38.Library AAoDaNEA. Practice Guidelines for Nutrition Care for Patients with Spinal Cord Injury. http://adaevidencealibrary.com: 2009.
- 39.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. American Journal of Clinical Nutrition. 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- 40.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine & Science in Sports & Exercise. 2007;39(8):1423–34. [DOI] [PubMed] [Google Scholar]
- 41.United States Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition Washington, DC: U.S. Department of Health and Human Services; 2018. [Google Scholar]
- 42.Ginis KA, Arbour-Nicitopoulos KP, Latimer AE, Buchholz AC, Bray SR, Craven BC, Hayes KC, Hicks AL, McColl MA, Potter PJ, Smith K, Wolfe DL. Leisure time physical activity in a population-based sample of people with spinal cord injury part II: activity types, intensities, and durations. Archives of physical medicine and rehabilitation. 2010;91(5):729–33. Epub 2010/05/04. doi: 10.1016/j.apmr.2009.12.028 [DOI] [PubMed] [Google Scholar]
- 43.Wilroy J, Knowlden A. Systematic review of theory-based interventions aimed at increasing physical ativity in individuals with spinal cord injury. American Journal of Health Education. 2016;47(3):163–74. [Google Scholar]
- 44.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine and science in sports and exercise. 2009;41(2):459–71. Epub 2009/01/08. doi: 10.1249/MSS.0b013e3181949333 [DOI] [PubMed] [Google Scholar]
- 45.Kressler J, Cowan RE, Bigford GE, Nash MS. Reducing cardiometabolic disease in spinal cord injury. Physical medicine and rehabilitation clinics of North America. 2014;25(3):573–604, viii. Epub 2014/07/30. doi: 10.1016/j.pmr.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 46.Shuval K, Finley CE, Barlow CE, Nguyen BT, Njike VY, Pettee Gabriel K. Independent and joint effects of sedentary time and cardiorespiratory fitness on all-cause mortality: the Cooper Center Longitudinal Study. BMJ open. 2015;5(10):e008956 Epub 2015/11/04. doi: 10.1136/bmjopen-2015-008956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pulsford RM, Stamatakis E, Britton AR, Brunner EJ, Hillsdon MM. Sitting behavior and obesity: evidence from the Whitehall II study. American journal of preventive medicine. 2013;44(2):132–8. doi: 10.1016/j.amepre.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manns PJ, Dunstan DW, Owen N, Healy GN. Addressing the nonexercise part of the activity continuum: a more realistic and achievable approach to activity programming for adults with mobility disability? Physical therapy. 2012;92(4):614–25. Epub 2011/12/14. doi: 10.2522/ptj.20110284 [DOI] [PubMed] [Google Scholar]
- 49.Graham CL, Mann JR. Accessibility of primary care physician practice sites in South Carolina for people with disabilities. Disability and health journal. 2008;1(4):209–14. Epub 2008/10/01. doi: 10.1016/j.dhjo.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 50.Mudrick NR, Breslin ML, Liang M, Yee S. Physical accessibility in primary health care settings: results from California on-site reviews. Disability and health journal. 2012;5(3):159–67. Epub 2012/06/26. doi: 10.1016/j.dhjo.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 51.Pharr J Accessible medical equipment for patients with disabilities in primary care clinics: why is it lacking? Disability and health journal. 2013;6(2):124–32. Epub 2013/03/20. doi: 10.1016/j.dhjo.2012.11.002 [DOI] [PubMed] [Google Scholar]
- 52.Zheng Y, Klem ML, Sereika SM, Danford CA, Ewing LJ, Burke LE. Self-weighing in weight management: a systematic literature review. Obesity (Silver Spring, Md). 2015;23(2):256–65. Epub 2014/12/19. doi: 10.1002/oby.20946 [DOI] [PubMed] [Google Scholar]
- 53.Bai Y, Welk GJ, Nam YH, Lee JA, Lee JM, Kim Y, Meier NF, Dixon PM. Comparison of Consumer and Research Monitors under Semistructured Settings. Medicine and science in sports and exercise. 2016;48(1):151–8. Epub 2015/07/15. doi: 10.1249/mss.0000000000000727 [DOI] [PubMed] [Google Scholar]
- 54.Nelson MB, Kaminsky LA, Dickin DC, Montoye AH. Validity of Consumer-Based Physical Activity Monitors for Specific Activity Types. Medicine and science in sports and exercise. 2016;48(8):1619–28. Epub 2016/03/26. doi: 10.1249/mss.0000000000000933 [DOI] [PubMed] [Google Scholar]
- 55.Froehlich-Grobe K, Lee J, Aaronson L, Nary DE, Washburn RA, Little TD. Exercise for everyone: a randomized controlled trial of project workout on wheels in promoting exercise among wheelchair users. Archives of physical medicine and rehabilitation. 2014;95(1):20–8. Epub 2013/07/23. doi: 10.1016/j.apmr.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Froehlich-Grobe K, Nary DE, Van Sciver A, Lee J, Little TD. Measuring height without a stadiometer: empirical investigation of four height estimates among wheelchair users. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2011;90(8):658–66. Epub 2011/06/18. doi: 10.1097/PHM.0b013e31821f6eb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign,Ill: Human Kinetics Books; 1988. [Google Scholar]
- 58.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 59.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey: manual and interpretation guide. In: Institute TH, editor. Boston, MA: 1993. [Google Scholar]
- 60.Froehlich-Grobe K, Andresen EM, Caburnay C, White GW. Measuring health-related quality of life for persons with mobility impairments: an enabled version of the short-form 36 (SF-36E). Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2008;17(5):751–70. Epub 2008/04/23. doi: 10.1007/s11136-008-9342-5 [DOI] [PubMed] [Google Scholar]
- 61.Drummond MF, Sculpher MJ, Torrance GW. Methods for Economic Evlauation of Health Care Programs. Oxford, UK: Oxford University Press; 2005. [Google Scholar]
- 62.Donnelly JE, Goetz J, Gibson C, Sullivan DK, Lee R, Smith BK, Lambourne K, Mayo MS, Hunt S, Lee JH, Honas JJ, Washburn RA. Equivalent weight loss for weight management programs delivered by phone and clinic. Obesity (Silver Spring, Md). 2013;21(10):1951–9. Epub 2013/02/15. doi: 10.1002/oby.20334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russell LB, Gold MR, Siegel JE, Daniels N, Weinstein MC. The role of cost-effectiveness analysis in health and medicine. Panel on Cost-Effectiveness in Health and Medicine. Jama. 1996;276(14):1172–7 [PubMed] [Google Scholar]
- 64.Frew E, Wolstenholme JL, Atkin W, Whynes DK. Estimating time and travel costs incurred in clinic based screening: flexible sigmoidoscopy screening for colorectal cancer. Journal of medical screening. 1999;6(3):119–23 [DOI] [PubMed] [Google Scholar]
- 65.Reuben DB, Wong RC, Walsh KE, Hays RD. Feasibility and accuracy of a postcard diary system for tracking healthcare utilization of community-dwelling older persons. Journal of the American Geriatrics Society. 1995;43(5):550–2 [DOI] [PubMed] [Google Scholar]
- 66.Sloan FA. Valuing Health Care: Costs, Benefits, and Effectiveness of Pharmaceuticals and other Medical Technologies. Cambridge, UK: Cambridge University Press; 1996. [Google Scholar]
- 67.Lee RH, Bott MJ, Forbes S, Redford L, Swagerty DL, Taunton RL. Process-based costing. Journal of nursing care quality. 2003;18(4):259–66 [DOI] [PubMed] [Google Scholar]
- 68.Narbro K, Sjostrom L. Willingness to pay for obesity treatment. International journal of technology assessment in health care. 2000;16(1):50–9 [DOI] [PubMed] [Google Scholar]
- 69.Automated Self-Administered 24-Hour (ASA24) Dietary Assessment Tool. In: Services USDoHaH, editor. Washington, DC: National Cancer Institute; 2017. [Google Scholar]
- 70.Kirpatrick SI, Subar AF, Douglass D, Zimmerman TP, Thompson FE, Kahle LL, George SM, Dodd KW, Potischman N. Performance of the automated self-administered 24-hour recall relative to a measure of true intakes and to an interviewer-administered 24-hour recall. The American journal of clinical nutrition. 2014;100(1):233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park Y, Dodd KW, Thompson FE, Potischman N, Schoeller DA, Baer DJ, Midthune D, Troiano RP, Bowles HR, Subar AF. Comparison of self-reported dietary intakes from the 24- r recall, 4-d food records, and food-frequency questionnaires against recovery biomarkers. The American Journal of Clinical Nutrition. 2018;107(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rimmer JH, Riley BB, Rubin SS. A new measure for assessing the physical activity behaviors of persons with disabilities and chronic health conditions: the Physical Activity and Disability Survey. American journal of health promotion : AJHP. 2001;16(1):34–42 [DOI] [PubMed] [Google Scholar]
- 73.Rimmer JH, Rauworth A, Wang E, Heckerling PS, Gerber BS. A randomized controlled trial to increase physical activity and reduce obesity in a predominantly African American group of women with mobility disabilities and severe obesity. Preventive medicine. 2009;48(5):473–9 [DOI] [PubMed] [Google Scholar]
- 74.Karlsson J, Persson LO, Sjostrom L, Sullivan M. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjests (SOS) study. International Journal of Obesity 2000;24:1715–25. [DOI] [PubMed] [Google Scholar]
- 75.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. Journal of psychosomatic research. 1985;29(1):71–83 [DOI] [PubMed] [Google Scholar]
- 76.de Lauzon B, Romon M, Descamps V, Lafay L, Borys JM, Karlsson K, Ducimetiere P, Charles MA. The three-factor eating questionnaire-R18 is able to distinguish among different eating patterns in a general population. Journal of Nutrition. 2004;134:2372–80. [DOI] [PubMed] [Google Scholar]
- 77.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 78.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatric Research. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 79.Lobentanz I, Asenbaum S, Vass K, Sauter C, Klosch G, Kollegger H, Kristoferitsch W, Ziethofer J. Factors influencing quality of life in multiple sclerosis patients: disability, depressive mood, fatigue and sleep quality. Acta Neuroloica Scandinavica. 2004;110(1):6–13. [DOI] [PubMed] [Google Scholar]
- 80.Chien M-Y, Chen H-C. Poor sleep quality is independentl associated with physical disability in older adults. JCSM: Official publication of the American Academy of Sleep Medicine. 2015;11(3):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Becker H, Stuifbergen AK, Sands D. Development of a scale to measure barriers to health promotion activities among persons with disabilities. American journal of health promotion : AJHP. 1991;5(6):449–54 [DOI] [PubMed] [Google Scholar]
- 82.Domecq JP, Prutsky G, Leppin A, Sonbol MB, Altayar O, Undavalli C, Wang Z, Elraiyah T, Brito JP, Mauck KF, Lababidi MH, Prokop LJ, Asi N, Wei J, Fidahussein S, Montori VM, Murad MH. Clinical review: Drugs commonly associated with weight change: a systematic review and meta-analysis. The Journal of clinical endocrinology and metabolism. 2015;100(2):363–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miles MB, Huberman AM. Qualitative data analysis: A sourcebook for new methods. Newbury Park: Sage Publishers; 1984. [Google Scholar]
- 84.Ryan GW, Bernard HR. Data Management and Analysis Methods In: Denzin NK, Lincoln S, editors. Handbook of Qualitative Research. 2nd ed Thousand Oaks, CA: Sage Publications; 2000. p. 769–802. [Google Scholar]
- 85.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine & Science in Sports & Exercise. 2009;41(2):459–71. [DOI] [PubMed] [Google Scholar]
- 86.Lalonde L, Gray-McDonald K, Lowensteyn I, Marchand S, Dorais M, Michaels G, Llewellyn-Thomas HA, O’Connor A, Grover SA, Group atCCCA. Comparing the benefits of diet and exercise in the treatment of dyslipidemia. Preventive medicine. 2002;36:16–24. [DOI] [PubMed] [Google Scholar]
- 87.Saunders RR, Saunders MD, Donnelly JE, Smith BK, Sullivan DK, Guilford B, Rondon MF. Evaluation of an approach to weight loss in adults with intellectual or developmental disabilities. Intellectual and developmental disabilities. 2011;49(2):103–12. Epub 2011/03/31. doi: 10.1352/1934-9556-49.2.103 [DOI] [PubMed] [Google Scholar]
- 88.Ptomey LT, Sullivan DK, Lee J, Goetz JR, Gibson C, Donnelly JE. The use of technology for delivering a weight loss program for adolescents with intellectual and developmental disabilities. Journal of the Academy of Nutrition and Dietetics. 2015;115(1):112–8. Epub 2014/12/03. doi: 10.1016/j.jand.2014.08.031 [DOI] [PubMed] [Google Scholar]
- 89.Mallows CL. Some comments on Cp. Technometrics. 1973;15(4):661–75. doi: doi: 10.2307/1267380 JSTOR 1267380. [DOI] [Google Scholar]
- 90.Cardenas DD, Yilmaz B. Recruitment of spinal cord injury patients to clinical trials: Challenges and solutions. Topics in spinal cord injury rehabilitation. 2006;11(2):12–23. [Google Scholar]
- 91.Nosek MA, Hughes RB, Robinson-Whelen S, Taylor HB, Howland CA. Physical activity and nutritional behaviors of women with physical disabilities: physical, psychological, social, and environmental influences. Women’s health issues : official publication of the Jacobs Institute of Women’s Health. 2006;16(6):323–33. Epub 2006/12/26. doi: 10.1016/j.whi.2006.08.002 [DOI] [PubMed] [Google Scholar]
- 92.Hall L, Colantonio A, Yoshida K. Barriers to nutrition as a health promotion practice for women with disabilities. International journal of rehabilitation research Internationale Zeitschrift fur Rehabilitationsforschung Revue internationale de recherches de readaptation. 2003;26(3):245–7. Epub 2003/09/23. doi: 10.1097/01.mrr.0000088450.78481.aa [DOI] [PubMed] [Google Scholar]
- 93.Minniti A, Bissoli L, Di Francesco V, Fantin F, Mandragona R, Olivieri M, Fontana G, Rinaldi C, Bosello O, Zamboni M. Individual versus group therapy for obesity: comparison of dropout rate and treatment outcome. Eating and weight disorders : EWD. 2007;12(4):161–7 [DOI] [PubMed] [Google Scholar]
- 94.Renjilian DA, Perri MG, Nezu AM, McKelvey WF, Shermer RL, Anton SD. Individual versus group therapy for obesity: effects of matching participants to their treatment preferences. Journal of consulting and clinical psychology. 2001;69(4):717–21 [PubMed] [Google Scholar]
- 95.Cresci B, Tesi F, La Ferlita T, Ricca V, Ravaldi C, Rotella CM, Mannucci E. Group versus individual cognitive-behavioral treatment for obesity: results after 36 months. Eating and weight disorders : EWD. 2007;12(4):147–53 [DOI] [PubMed] [Google Scholar]
- 96.Lysandropoulos AP, Havrdova E. ‘Hidden’ factors influencing quality of life in patients with multiple sclerosis. European journal of neurology. 2015,’22 Suppl 2:28–33. Epub 2015/09/17. doi: 10.1111/ene.12801 [DOI] [PubMed] [Google Scholar]
- 97.Russell D Living arrangements, social integration, and loneliness in later life: the case of physical disability. Journal of health and social behavior. 2009;50(4):460–75 [DOI] [PubMed] [Google Scholar]
- 98.Hartmann-Boyce J, Johns DJ, Jebb SA, Aveyard P. Effect of behavioural techniques and delivery mode on effectiveness of weight management: systematic review, meta-analysis and meta-regression. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2014;15(7):598–609. Epub 2014/03/19. doi: 10.1111/obr.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hartmann-Boyce J, Johns DJ, Jebb SA, Summerbell C, Aveyard P. Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2014;15(11):920–32. Epub 2014/08/13. doi: 10.1111/obr.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nutrition AAoDa. How effective (in terms of client adherence and weight loss and maintenance) are meal replacements (liquid meals, meal bars, frozen prepackaged means)? 2011. [Google Scholar]
- 101.Lutes LD, Dinatale E, Goodrich DE, Ronis DL, Gillon L, Kirsh S, Richardson CR, Damschroder LJ. A randomized trial of a small changes approach for weight loss in veterans: design, rationale, and baseline characteristics of the ASPIRE-VA trial. Contemporary clinical trials. 2013;34(1):161–72. Epub 2012/10/09. doi: 10.1016/j.cct.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 102.Damschroder LJ, Lutes LD, Kirsh S, Kim HM, Gillon L, Holleman RG, Goodrich DE, Lowery JC, Richardson CR. Small-changes obesity treatment among veterans: 12-month outcomes. American journal of preventive medicine. 2014;47(5):541–53. Epub 2014/09/14. doi: 10.1016/j.amepre.2014.06.016 [DOI] [PubMed] [Google Scholar]


