Abstract
Objectives:
Chronic pain is one of the most common and challenging symptoms in fibromyalgia (FM). Currently, self-reported pain is the main criterion used by clinicians assessing patients with pain. However, it is subjective, and multiple factors can affect pain levels. In this study, we investigated the neural correlates of FM pain using conditioned pain modulation (CPM), electroencephalography (EEG), and transcranial magnetic stimulation (TMS).
Methods:
In this cross-sectional neurophysiological analysis of a randomized, double-blind controlled trial, 36 patients with fibromyalgia were included. We analyzed CPM, EEG variables and TMS measures and their correlation with pain levels as measured by a visual analog scale. Univariate and multivariate linear regression analyses were performed to identify the predictors of pain severity.
Results:
We found: (1) no association between pain levels and CPM; (2) an association between reduced alpha and beta power over the central region in resting-EEG and higher pain levels; (3) an association between smaller event-related desynchronization (ERD) responses in theta and delta bands over the central region and higher pain levels; (4) an association between smaller ERD responses in theta and delta bands and smaller intracortical inhibition and higher intracortical facilitation ratios; (5) an association between smaller ERD responses in delta band and reduced CPM.
Conclusions:
Our results do not support CPM as a biomarker for pain in FM. Although a disrupted endogenous pain system plays a major role in chronic pain, it seems that CPM is dissociated from clinical manifestations of pain. Specific EEG findings related to pain, CPM and TMS measures suggest that FM leads to a disruption of inhibitory neural modulators. These neural targets could be explored in potential future treatment or as biomarkers of pain in FM.
Keywords: biomarker, chronic pain, conditioned pain modulation, electroencephalography, event-related desynchronization, fibromyalgia, neural inhibitory state, transcranial magnetic stimulation
Introduction
Fibromyalgia syndrome (FM) is a condition characterized by widespread chronic pain and hyperalgesia, along with psychological distress [4, 69]. Even though the diagnostic criteria for FM were revised in 2016 [68], diagnosis and follow-up still present challenges for objective assessment due to clinical heterogeneity and lack of specific confirmatory tests. At present, self-reported pain is the main criterion used by clinicians assessing patients with pain [12]. However, it is subjective, and multiple intrinsic and extrinsic factors can affect pain perception [53]. Therefore, it is important to identify the underlying mechanisms and investigate physiological markers to develop better treatments.
Even though the etiology of FM is still not fully understood, a few recent studies shed some light on the mechanisms of FM. Functional neuroimaging studies and biochemical abnormalities in cerebrospinal fluid, such as decreased serotonin (5HT) and noradrenaline (NA), suggest pathogenesis of central origin [56, 25]. Also, a decrease in 5HT and NA supports the idea that dysfunction of the descending inhibitory systems is responsible for the widespread chronic pain of fibromyalgia [28]. This chronic pain is considered as a consequence not only of peripheral sensitization, but also neuroplastic changes in the central nervous system (CNS) [58]. Conditioned pain modulation (CPM), which is part of quantitative sensory testing (QST), is believed to reflect the endogenous inhibitory pain modulation mechanism of the CNS [42] and has been widely used in chronic pain syndromes as evidence of a defective endogenous inhibitory pain system [34]. Normand et al. showed that FM patients have less CPM efficacy compared to healthy controls and patients with major depressive disorder, suggesting that a deficit of pain inhibition could be more specific to fibromyalgia, and could be distinguished from other hyperalgesia syndromes [45]. Even though an increasing number of studies show that FM patients have less CPM efficacy than the healthy population, other studies seem to contradict these findings [11, 52]. Also, the severity of clinical pain and CPM are often not correlated [70]. Even though the difference of CPM between chronic pain patients and the healthy population has been well studied, more studies are needed before considering CPM as a valid biomarker of chronic pain among these patients. In this respect, the critical points that need to be elucidated are whether this variability in results may be related to clinical characteristics of patients and whether these characteristics can be determined, so that CPM can be used as a biomarker for characterizing patients with chronic pain.
Quantitative electroencephalography (qEEG) is a potential biomarker to help in understanding this pain-CPM association. Although the general hypothesis of EEG patterns related to chronic pain has been widely studied, the EEG signatures related to CPM response remain unclear. qEEG is a marker that can provide information on central mechanisms involved in chronic pain [50]. It provides reliable and relevant information about brain functioning during rest, sensory stimulation, and cognitive tasks [13]. Previous research has established the presence of thalamocortical dysrhythmia (TCD) in resting state-EEG, characterized by increases in theta and beta power along with slowing of the dominant alpha peak in chronic pain patients [18, 36]. Considering that patients with chronic pain have central sensitization and disruptions of inhibitory brain networks [5, 33, 38], available biomarkers to evaluate the cortical inhibitory tonus are limited and EEG task-related evoked potentials could be an option. Some studies have tried to validate EEG correlates with inhibitory networks in chronic pain patients using pain-related evoked potentials [59, 24]. However, the influences of stimulus type, stimulation location and complex experimental set-up, reduce the reproducibility and applicability in future clinical practice [65, 2]. One alternative is the use of the EEG oscillations related to motor tasks, indexed by event-related desynchronization (ERD) [64]. It has been reported that just before and during a motor-related task (motor execution, observation or imagery), cortical activation can be seen, as measured by an absolute and relative power decrease [49]. Given that long-term adaptive changes in cortical activation associated with sensorimotor behavior exist in chronic pain even in the absence of peripheral stimulation, it would be essential and more helpful to understand and explore how chronic pain in FM influences brain activation patterns during performance of tasks that require sensorimotor processing without acute sensorial stimulation. Therefore, besides resting EEG, the use of motor tasks can show altered sensorimotor activation without peripheral nociceptive stimulation, commonly referred to as event-related desynchronization (ERD) [40].
Transcranial magnetic stimulation (TMS) is another biomarker that can help elucidate central nervous system (CNS) changes associated with a deficit in inhibitory control in chronic pain. Therefore, TMS becomes a potential biomarker for chronic pain and can also be used to explore the association between pain and CPM. Studies of TMS in chronic pain have shown abnormal cortical excitability as expressed by decreased short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) [39]. It has been suggested that in chronic pain states, there is an imbalance between excitation and inhibition mechanisms induced by reduction in GABA activity, increase in glutamate activity and activation of NMDA-dependent activity [44]. This association of changes has been described as central sensitivity syndrome (CSS) [8]. However, previously published studies have been limited to showing a relationship between neurophysiological changes and the CPM. It is therefore still unknown how CNS changes (evaluated by EEG and TMS) are affected when this system is disturbed.
To establish the neurophysiological signatures (EEG and TMS) related to pain perception and CPM function in FM subjects is important, since this could create an objective parameter to help discriminate different pain phenotypes and treatment responder subgroups, allowing clinicians to better personalize pain management.
In this study, therefore, we aimed to assess the relationship between clinical pain perception and CPM response and to establish the neurophysiological signatures related to these processes in FM subjects. It is hoped that this research will contribute to a deeper understanding of robust and specific neurophysiological markers (by EEG and TMS) to pain and CPM response, considering pain and clinical characteristics. Our main hypothesis is that FM is associated with commonly seen markers of less inhibitory activity (at cortical and spinal levels); thus, these markers would display certain patterns expected to be associated with pain perception and CPM.
Methods
Study design
This study is a cross-sectional analysis of a randomized, double-blind clinical trial investigating the effects of tDCS in combination with aerobic exercise, on pain in fibromyalgia patients (NCT03371225) [7]. This study was approved by the IRB at the Partners Human Research Committee (Protocol approval number: 2017P002524). All participants have given their written informed consent.
Participants
Inclusion criteria: adults (18–65 years); diagnosis of fibromyalgia pain according to the American College of Rheumatology (ACR) 2010 criteria: existing pain for more than 6 months with an average of at least 4 on a 0–10 Visual Analogue Scale (VAS) scale) without other comorbid chronic pain diagnosis; pain resistant to common analgesics and medications for chronic pain; patients must have the ability to feel sensation by stimulation. Exclusion criteria: clinically significant or unstable medical or psychiatric disorder; history of substance abuse within the past 6 months as self-reported; previous significant neurological history (e.g., traumatic brain injury), resulting in neurological deficits; previous neurosurgical procedure with craniotomy; severe depression (with a score of >30 on the Beck Depression Inventory); pregnancy, as the safety of tDCS in pregnant population (and children) has not been assessed (though the risk is believed to be non-significant); current opiate use in large doses; and an increased risk for exercise defined as not fulfilling the American College of Sports Medicine criteria (i.e., risk of cardiovascular complication) and in this case not cleared by a licensed physician. Written informed consent was obtained from each participant.
Demographic and clinical variables
Demographical and clinical variables were obtained from all the subjects, such as: information on age; gender; revised Fibromyalgia Impact Questionnaire (FIQ) -instrument that is useful to assess current health status in fibromyalgia patients with clinical and research relevance; quality of life assessed by Quality of Life Scale (QoL) - composed by fifteen areas that have impact in chronic conditions; Beck Depression Inventory (BDI) - formed by twenty-one questions to estimate depression features, sleepiness and anxiety measurements (visual analog scale from 0 to 10).
We evaluated the pain outcome through a pain visual analog scale (VAS) (from 0 to 10), which is a validated, subjective measure for acute and chronic pain [14].
Conditioned pain modulation (CPM)
During the CPM protocol, heat pulses were generated by a TSA-II Stimulator (Medoc Advanced Medical Systems, Ramat Yishai, Israel) delivered to the right proximal volar forearm using a 30 mm × 30 mm embedded heat pain (HP) thermode.
We followed the adapted protocol suggested by Granot et al (2008) [22] and Nir et al (2011) [43]. We first determined the pain-60 test temperature (which is the temperature that induces pain sensation at a magnitude of 60 on a 0–100 numerical pain scale (NPS) by applying a Peltier thermode (Medoc Advanced Medical Systems, Ramat Yishai, Israel) on the right forearm and delivering three short heat stimuli (43°C, 44°C, and 45°C), each lasting 7 s. Subjects were asked to rate the level of pain intensity using an NPS ranging from 0=‘no pain’ to 100=‘the worst pain imaginable’. If the first temperature of 43°C was considered too painful (>60/100), we stopped the series and provided additional stimuli at lower temperatures of 41°C and 42°C. If the three temperatures (43°C, 44°C and 45°C) are unable to achieve pain-60, we delivered additional stimuli at 46°C, 47°C and 48°C until reaching the desired pain level of 60/100; in the unlikely event that none of those temperatures elicited pain-60, we considered it to be 48°C.
On determining the pain-60 temperature, we administered the test stimulus at that temperature for 30 s, and subjects were asked to rate their pain intensity at 10, 20 and 30s after the thermode reached the pain-60 temperature (mean scores of the three pain ratings were calculated). Five minutes after delivering the test stimulus, the conditioning stimulus was applied: the subject’s left hand was immersed for 30 s in a water bath set at 10°C–12°C. Then, the same pain-60 temperature was applied to the right forearm (left hand was immersed) for 30 s and the subject was again asked to rate their pain intensity three times after the thermode reached the pain-60 temperature: at 10, 20 and 30 s (mean scores of the three pain ratings were calculated). CPM response was calculated as the difference between the average of pain ratings from the test stimulus minus the average of pain ratings during the conditioned stimulus.
Electroencephalography (EEG) assessment
EEG was performed over approximately 45 min: 25 min of participant and software preparation, 10 min of EEG recording divided into a resting EEG condition and a task-related condition (8 min). Participants were asked to relax, and the investigator ensured they did not fall asleep.
Resting-state EEG protocol
We recorded the EEG in a standardized way [46]. Resting-state EEG was recorded for 10 minutes (5 min with eyes open, 5 min with eyes closed) using a 64-channel EGI system (Electrical Geodesics, Inc) (EGI, Eugene, USA). The EEG was recorded with a band-pass filter of 0.3–200 Hz and digitized at the sampling rate of 250 Hz [37]. We averaged the spectrum values for each frequency bin (1/Hz) and then averaged the signals from the channels for each region (frontal, central, and parietal). The EEG data were analyzed visually by an expert EEG clinical neurophysiologist to exclude the existence of epileptiform discharges and artifacts.
Resting-state power analysis
We used a high-pass filter of 1 Hz and a low-pass filter of 50 Hz, followed by manual artifact detection and rejection by a blinded assessor. The data were then exported and analyzed offline with EEGLab [15] and MATLAB (MATLAB R2012a, The MathWorks Inc. Natick, MA, 2000).
We performed independent component analysis (ICA) decomposition as a spatial filter to exclude muscular and/or ocular artifacts together with an inspection and manual rejection by an experienced clinical neurophysiologist. The artifact-free data was next processed using Fast Fourier transformation (averaged windows of 5 s with 50% overlap) to calculate absolute power (μV2) and relative power (specific band power/total power) for the following EEG bands: delta (1 – 3.9 Hz), theta (4 – 7.9 Hz), alpha (8–12.9 Hz), beta (13–30 Hz), and the sub-bands: low alpha (8–9.9 Hz), high alpha (10–12.9 Hz), low beta (13–19.9 Hz), and high beta (20–30 Hz). Also, we used the ICA decomposition to assess the spatial distribution of the brain oscillations in EEG’s bands. Therefore, we chose the components with maximal percent relative variance for each channel at each frequency band to construct the Figure 1. All the EEG-related measurements were calculated from the central, parietal, and frontal areas since they are important cortical regions involved in pain perception [10]. Electrodes representing these regions were selected and averaged (supplementary figure; S1).
Figure1:
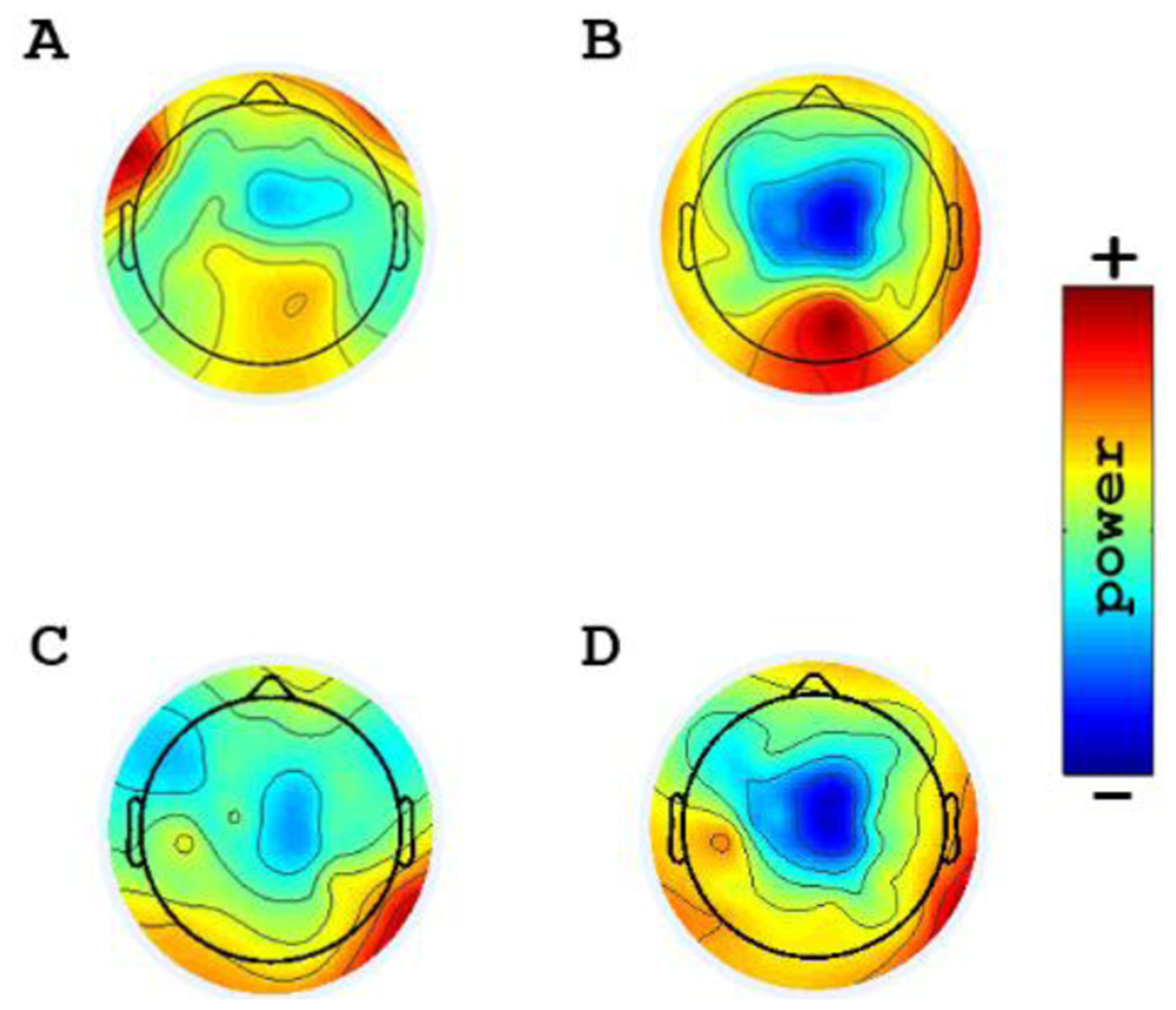
Topoplots, showing the topographic distribution of the alpha and beta power in resting-EEG for representative patients with less and higher pain. Blue areas represent lower activity. A: Relatively increased alpha power in the central area in a patient with less pain B: Relatively decreased alpha power in the central area in a patient with higher pain C: Relatively increased beta power in the central area in a patient with less pain D: Relatively decreased beta power in the central area in a patient with higher pain
Event-Related Spectral Perturbations Protocol
We performed an event-related spectral perturbations (ERSP) protocol separately from resting-state EEG. The protocol included movement observation (MO), movement imagery (MI), and movement execution (ME). These were recorded by connecting the Net Station software (for EGI) with E-Prime to present the visual stimuli. The entire task-related condition part consisted of 60 trials, with 20 trials for each of MO, MI and ME in a randomized order [35, 41]. Each trial lasted 8 seconds (one second of fixation, three seconds of motor task, and four seconds of rest). It involved initial fixation (on a cross on a screen), followed by a visual cue stating the task to be performed (‘imagine’ and ‘clench’), and a video was automatically played for the observation task. During each ME task, the subject was asked to clench her/his right hand once; during the MO trial, the participant viewed a video of right-hand clenching; during the MI task, the participant was asked to imagine clenching her/his right hand once. Subjects were instructed on how to perform tasks during the previous practice time and were instructed to avoid producing artifacts such as blinks or head movements.
Event-related Desynchronization (ERD) analysis
EEG data were segmented into successive 250-point (1,000ms) windows with 230-point overlapping. After that, the data were epoched. We calculated ERD from the central area and it was calculated at each segment with a frequency resolution of 1 Hz. We analyzed the 3 seconds of each motor task (used as the intra-experimental event condition) and the 4 seconds of each resting period (used as the intra-experimental reference). The time-frequency decomposition was obtained using a Short Time Fourier Transformation in the frequency range of 1–30 Hz and a Morlet Wavelets was done to assess the reference power spectrum. ERD values were calculated for each of the subjects and each of the trial periods (fixation and task) as relative power decrease with respect to a reference period using a bootstrap resampling method [41]. For the ERD calculation the classic method was used adapted from Pfurtscheller and colleagues [48, 49]: ERD% = (R−A) / R × 100, where R = power in the reference period and A = power in the task phase. ERD was defined as the percentage decrease of the power during the task with respect to the baseline value (rest). Accordingly, event-related decrements that are representative of a decrease in power and indicate cortical activation are expressed as negative values [60].
Transcranial magnetic stimulation (TMS)
We measured motor cortex excitability using TMS. Single-pulse TMS was performed to acquire resting motor threshold (rMT) and motor evoked potentials (MEPs), and paired pulse techniques were used to measure short interval cortical inhibition (SICI) and intracortical facilitation (ICF). We used a Magstim Rapid2 device with a figure-of-eight magnetic stimulator coil placed at 45 degrees of the scalp, to send a perpendicular pulse over the right and left motor cortex (for all assessments), the coil stability and direction was managed by the assessor without neuronavigation; we simultaneously recorded surface electromyogram from the contralateral first dorsal interosseous muscle. TMS data was recorded and stored in a computer for off-line analysis.
rMT:Initially, we investigated rMT following the technique described by Rossini and colleagues, where rMT is defined as the lowest stimulus intensity to evoke a MEP of 100 ìV in 3/5 trials in the relaxed muscle [54].
MEPs:We adjusted the TMS machine output intensity at 120% of MT to achieve a baseline MEP of 1 mV peak-to-peak amplitude before the intervention. The time between the MEP trials was 7 seconds. The assessor assures as much as possible a constant participant level of arousal. We recorded 10 MEPs and averaged their peak-to-peak amplitudes.
SICI and ICF:We used paired pulse protocols with a subthreshold conditioning stimulus (80% rMT) followed by a suprathreshold test stimulus of 120% of rMT. Interstimulus intervals were 2 ms for SICI and 10 ms for ICF. Ten randomized stimuli were applied at each interval and the percentage of inhibition or facilitation for each interstimulus interval was calculated (MEP ratio).
Statistical analysis
We used descriptive statistics to report baseline characteristics. Data were expressed as mean and standard deviation for the analysis. Histogram and Shapiro-Wilk test assessed data distribution for normality. After determining that data had a sufficiently normal distribution, we conducted univariate analyses to explore relationships between pain outcomes, demographic/clinical characteristics, TMS, CPM, and EEG-related variables. Then, we conducted independent linear regression models to test the association between pain and the biomarkers of interest (CPM, TMS and EEG variables) as dependent variables.
Confounders assessment
We determined the effects of confounders in these models by adding independent variables (demographic and clinical) in subsequent multivariate regression models. Considering our main predictors CPM, EEG and TMS, we assessed for confounding variables if they changed the β coefficient more than 10 %; the variable that was not a confounder was kept in the model if the p value was <0.05 and if it did not substantially inflate the standard error of the main predictors. We also tested the interaction of demographic and clinical variables with the main predictors’ variables, and this was included in the final models if significant. We used Stata Statistical Software 15 (Stata Corp LLC) for the statistical analyses. Because this was an exploratory study and to minimize the risk of type II errors, no correction for multiple comparisons was done.
Results
Demographics and clinical characteristics
Twenty-six subjects were included. Further clinical data are provided in Table 1.
Table 1.
Demographics and clinical characteristics (n=26)
| Characteristic | Mean ± SD or Median (IQR) or % |
|---|---|
| Age | 53 (47 to 58) |
| Gender (women, %) | 23 (88.5%) |
| Pain level (VAS) | 5.98 ± 2.01 |
| BDI | 17.46 ± 8.81 |
| Vas (anxiety) | 4.79 ± 2.79 |
| QoL | 67.81 ± 15.21 |
| FIQR | 55.74 ± 16.88 |
VAS=visual analog scale, BDI=beck depression scale, QoL=quality of life scale, FIQR=Fibromyalgia Impact Questionnaire-revised.
Neurophysiological findings
One subject for the TMS and five subjects for the EEG analysis had to exclude because of the unavailability of the data. CPM and TMS findings were provided in Table 2 and EEG findings were provided in Table 3.
Table 2.
CPM and TMS findings (n=26)
| Measurements | Mean ± SD |
|---|---|
| CPM response (vas diff) | 0.70 ± 1.20 |
| MEP | 1.03 ± 0.40 |
| SICI ratio | 0.61 ± 0.40 (39% of inhibition) |
| LICF ratio | 1.70 ± 0.87 (70% of facilitation) |
CPM=conditioned pain modulation, MEP=motor-evoked potential, SICI= short-interval intracortical inhibition, ICF= intracortical facilitation.
Table 3.
EEG relative power (in percentage)
| Frequency band | Central (Mean ± SD) | Parietal (Mean ± SD) | Frontal (Mean ± SD) |
|---|---|---|---|
| Delta | 20 ± 18 | 20 ± 15 | 26 ± 20 |
| Theta | 11 ± 8 | 11 ± 7 | 10 ± 6 |
| Alpha | 45 ± 25 | 49 ± 22 | 43 ± 24 |
| Beta | 13 ± 9 | 12 ± 6 | 11 ± 8 |
Multivariate analyses models to identify the association between neurophysiological markers and pain scores on fibromyalgia
CPM response and pain: we did not find an association between CPM response and pain levels (p=0.83). Additionally, FIQ, QoL, BDI, sleepiness and anxiety were not confounders in this association.
Resting-EEG and pain: we found a negative association of pain intensity with the alpha power in frontal, central and parietal areas (β=0.042, p=0.004; β=−0.045, p=0.017; β=−0.037, p=0.018, respectively) and beta power in central area (β=−0.028, p=0.031). We adjusted the models by the main confounder, FIQ. No other demographical/clinical variable fulfilled the confounder criteria. The multivariate regression lines are defined in table 4. (Figure S1 represents the different topographical maps of representative patients with higher and lower pain levels).
Table 4.
Resting-EEG and Pain models
| Models | Multivariate regression lines definition | R-squared | p-value |
|---|---|---|---|
| Central Area | |||
| Alpha power | Pain (vas) = 1.91 – 0.045alpha power + 0.07FIQ | 44% | p=0.017 |
| Beta power | Pain (vas) = 2.32 – 0.28beta power + 0.06FIQ | 40% | p=0.031 |
| Frontal Area | |||
| Alpha power | Pain (vas) = 2.23 – 0.042alpha power + 0.07FIQ | 53% | p=0.004 |
| Parietal Area | |||
| Alpha power | Pain (vas) = 2.20 – 0.037alpha power+ 0.07FIQ | 43% | p=0.018 |
ERD and pain: we found a negative association of theta and delta ERD during the fixation period of MI and MO tasks (for theta ERD: β=0.028, p=0.001; β=0.027. p= 0.023; for delta ERD: β=0.08, p=0.016; β=0.022, p= 0.01, respectively) with pain intensity in the central area adjusted by FIQ. Because ERD is defined as power decrease, negative numbers indicate bigger ERDs; in other words, a smaller theta ERD predicts higher pain. The multivariate regression lines are defined in table 5.
Table 5.
ERD and Pain models
| Models | Multivariate regression lines definition | R-squared | p-value |
|---|---|---|---|
| Fixation period of MI in the central area | |||
| Theta ERD | Pain (vas) = 1.43 – 0.028theta ERD + 0.06FIQ. | 69% | p=0.001 |
| Delta ERD | Pain (vas) = 2.02 – 0.08delta ERD + 0.05FIQ | 57% | p=0.016 |
| Fixation period of MO in the central area | |||
| Theta ERD | Pain (vas) = 2.3 – 0.027theta ERD + 0.06FIQ | 55% | p=0.023 |
| Delta ERD | Pain (vas) = 1.37 – 0.022deltaERD + 0.07FIQ | 58% | p=0.01 |
ERD and TMS: we found a positive correlation of theta ERD during MO with the SICI (Pearson coefficient=0.53, p= 0.013) and negative correlation of delta ERD during ME with the ICF ratios (Pearson coefficient=−0.46, p= 0.034) in central area.
CPM response and ERD: we found a positive association delta ERD during actual MO task (β=−0.015, p=0.025) with CPM responses in central area adjusted by FIQ. The multivariate regression lines are defined in table 6.
Table 6.
CPM and ERD Models
| Models | Multivariate regression lines definition | R-squared | p-value |
|---|---|---|---|
| MO period in the central area | |||
| Delta ERD | CPM = 1.35 – 0.015delta ERD - 0.015FIQ. | 25% | p=0.025 |
Discussion
This study explored neural markers for chronic pain in FM patients using different techniques, namely CPM, EEG, and TMS. Our results provide several valuable insights into chronic pain markers in FM: (1) no association was shown between pain levels and CPM; (2) lower alpha and beta power over the central region in resting EEG were associated with higher pain levels; (3) lower ERD in theta and delta bands over the central region was associated with higher pain levels; (4) lower ERD in theta and delta bands was correlated with smaller SICI and higher ICF ratios; and (5) lower ERD in the delta band was associated with lower CPM efficacy.
The first important result of the current study is that there was no correlation between pain severity and CPM efficacy. Although in the literature, CPM efficacy has been shown as a promising marker for pain status [8, 26, 45, 53], this data must be interpreted with caution because of the samples included in studies. Most of them have investigated CPM comparing chronic pain patients and healthy population; as such, the high variability of clinical expression of pain among patients has not usually been considered. Given that FM is a complex pain disease accompanied by fatigue, sleep disturbance, memory and mood problems, it can thus be suggested that FM effects on CPM may be a combination of pain and the behavioral characteristics of the disease. This also accords with a recent review, which showed that the majority of the results reported non-significant correlations between CPM efficiency and pain intensity [19]. In this respect, our findings do not support the validity of CPM as a biomarker of clinical pain among patients with FM and highlight the need for more objective markers for pain.
Another important finding is that alpha (frontal, central and parietal) and beta (central) power was relatively decreased in patients who had higher pain levels. Even though it has been shown that EEG power tends to shift toward slower frequencies as an indicator of maladaptive plasticity in chronic pain patients [50], a certain ratio between slower and faster oscillations has yet to be elucidated. The current study found resting-state alpha activity slowing down to theta frequencies, corresponding to earlier descriptions of TCD, even though here the association between theta frequency and pain levels was a non-significant trend. Besides alpha and theta oscillations, another interesting finding was the negative correlation between pain levels and beta band power. At present, beta oscillations, one of the main sensorimotor rhythms, are still not well understood in terms of their functional significance [17] but are thought to reflect changes in the balance of excitatory and inhibitory systems due to disrupted GABAergic inhibition [55]. Therefore, given that CSS is one of the most specific clinical pictures of disrupted neuronal circuits in which the defective inhibitory function stands out, it is reasonable to consider that decreased beta power may indicate less cortical organization and thus decreased modulatory effect. Here, beta oscillations need to be considered as a dynamic state. They usually increase during periods of intense physical or motor activity, and also once there is increased cortical demand [9, 1, 30]. Thus, decreased beta oscillations likely indicate a state of chronic cortical disengagement that leads to less cortical modulatory effects in the chronic pain state. Also, other studies have shown a decrease in beta power during ictal states in migraine, while it is increased during painless states [6]. This also goes along with our findings, which showed that higher beta power was correlated with decreased pain levels.
Moreover, we found that higher levels of pain and lower CPM efficacy were associated with smaller ERD during motor tasks (imagery, observation and execution) in theta and delta bands, over the central region (smaller theta and delta for higher pain, smaller delta ERD for lower CPM). It is known that during motor control (including the execution and planification) the relationship between inhibitory and excitatory networks is critical [62]. It seems that increase of inhibitory tonus in the motor and premotor cortex during motor-related tasks is needed in order to facilitate subcortical activation and release motor activity [3, 16, 23]. In this respect, the ERD could be a surrogate of this inhibitory tonus, which could potentially be used to evaluate inhibitory networks in the sensorimotor cortex of chronic pain patients. Our results support this hypothesis, whereby higher level of pain was associated with small ERD during motor tasks (imagery, observation and execution) in the theta and delta bands, over the central region. These findings could represent an EEG signature of the inhibitory tonus disruption in fibromyalgia patients. Since a higher ERD needs optimal cortical inhibitory activity, and given that these populations with widespread pain are associated with dysfunction of cortical inhibitory networks [18, 21, 66], we would expect a low ERD, even less if the pain levels are higher. Besides, these findings are predominant in the lower frequency ranges (theta and delta bands), which is consistent with previous literature on the association of theta and delta brain oscillations with chronic pain patients [50] and with the intensity of pain [63]. However, to our knowledge, this is the first report using a motor task paradigm.
To date, little is known about whether theta and delta ERD have any role in the motor task paradigm. Igarashi et al. showed that theta oscillations play a role in neuronal coordination during motor preparation and action in rats [27]. Also, Popovych et al. suggested that phase-locked delta and theta neural oscillations in the motor cortex could be an indicator for the preparation and execution of motor actions [51]. However, another opinion, suggested by Sarnthein et al., is that slower oscillations are likely to reflect the underlying central pain, and can be an indicator of this disruption in resting and active states [57]. The presence of theta ERD in sensorimotor regions has been found only in spinal cord injury patients with pain during an MI task in comparison to patients without pain and healthy controls [67].
Moreover, delta ERD activity is generated by cortico-cortical interactions and is a product of the distributed network system of the brain involved in cognitive processes, mainly in decision-making and attentional processes. Besides, theta ERD activity is related to cortico-hippocampal or fronto-limbic interactions [29] and is associated with a complex set of cognitive processes including alertness, arousal or readiness, selective attention and error processing, and reward processing. Pandey et al. investigated the activation-inhibition dimension in alcoholics using the Go/No-Go task [47] and found that alcoholics had smaller evoked delta, theta, slow alpha, and fast alpha frequency band power compared to controls, suggesting deficits in activation/inhibition activity of neural circuits underlying the desired/required behavior. In this respect, it is reasonable to think that these activities could indicate a disruption in inhibitory activities in FM patients.
Surprisingly, ERD differences related to pain levels were found only in theta and delta oscillations. A possible explanation for this might be that theta and delta ERD may be more specific to pain pathophysiology compared to alpha and beta ERD.
Another interesting point about the role of ERD as a biomarker of inhibitory network function is the correlation with SICI and ICF. We found that higher ERD to observation and motor execution over the central area was correlated with high SICI and small ICF ratios. TMS paired-pulse protocols have been used in several studies to assess inhibitory and excitatory responses in the motor cortex [31, 32]. Our results suggest that an EEG task-related experiment using motor tasks could be an alternative and feasible biomarker to evaluate the inhibitory brain tonus in chronic pain patients. Also, to the best our knowledge, this is the first report showing the relationship between delta/theta ERD and SICI/ICF. Therefore, our findings could shed new light on the underlying neural processes in FM patients. However, one limitation of our analysis is that ERD calculation is based on a comparison of power spectrum assessed by two different types of spectrum analysis, although both transformations have been reported to display adequate frequency resolution at low frequencies [61]. More studies with homogenous calculation, with larger sample sizes and healthy participants as a control group are needed to confirm these preliminary observations.
It is important to highlight that disease activity indexed by FIQ was the main confounder in our models, suggesting that clinical characteristics play a critical role in pain perception in FM.
This study has identified the neurophysiological biomarkers, particularly EEG, which are related to both pain perception and CPM efficacy. These findings have important implications for developing valid biomarkers for pain in FM. Therefore, the potential use of these markers could be helpful to individualize the treatment response, in particular for future research into a novel approach for the treatment of chronic pain, such as EEG-based neurofeedback applications.
Subjects in this study were not asked to discontinue their usual medications due to ethical considerations, but they were asked to inform us in case of any changes in their usual treatment. Although Gervasoni et al. showed that there was no difference between EEGs of rats that were being given selective serotonin reuptake inhibitors, tricyclic antidepressants, norepinephrine inhibitors, and a control group [20], investigation of a much larger population in future studies might allow evaluation of whether there is any influence of different medications.
Supplementary Material
S1: The EGI 64-channel HydroCel Geodesic Sensor Net is displayed below.
Acknowledgments
The authors are thankful to Judah Leao Barouh for his help on processing TMS data.
Funding Sources
This work is supported by a NIH grant (1R01AT009491-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors report no conflict of interest.
References
- [1].Baker SN. Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol 2007;17:649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baumgartner U, Greffrath W, Treede RD. Contact heat and cold, mechanical, electrical and chemical stimuli to elicit small fiber-evoked potentials: merits and limitations for basic science and clinical use. Neurophysiol Clin 2012;42:267–80. [DOI] [PubMed] [Google Scholar]
- [3].Begum T, Mima T, Oga T, Hara H, Satow T, Ikeda A, et al. Cortical mechanisms of unilateral voluntary motor inhibition in humans. Neurosci Res 2005;53:428–35. [DOI] [PubMed] [Google Scholar]
- [4].Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord 2007;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum 2014;44:68–75. [DOI] [PubMed] [Google Scholar]
- [6].Cao Z, Lin CT, Chuang CH, Lai KL, Yang AC, Fuh JL, et al. Resting-state EEG power and coherence vary between migraine phases. J Headache Pain 2016;17:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Castelo-Branco L, Kucukseymen EU, Duarte D, El-Hagrassy MM, Pinto CB, Gunduz ME, et al. Optimised transcranial direct current stimulation (tDCS) for fibromyalgia—targeting the endogenous pain control system: a randomised, double-blind, factorial clinical trial protocol. BMJ Open 2019;9:e032710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Caumo W, Deitos A, Carvalho S, Leite J, Carvalho F, Dussan-Sarria JA, et al. Motor Cortex Excitability and BDNF Levels in Chronic Musculoskeletal Pain According to Structural Pathology. Front Hum Neurosci 2016;10:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chakarov V, Naranjo JR, Schulte-Monting J, Omlor W, Huethe F, Kristeva R. Beta-range EEG-EMG coherence with isometric compensation for increasing modulated low-level forces. J Neurophysiol 2009;102:1115–20. [DOI] [PubMed] [Google Scholar]
- [10].Christmann C, Koeppe C, Braus DF, Ruf M, Flor H. A simultaneous EEG-fMRI study of painful electric stimulation. Neuroimage 2007;34:1428–37. [DOI] [PubMed] [Google Scholar]
- [11].Coppieters I, Ickmans K, Cagnie B, Nijs J, De Pauw R, Noten S, et al. Cognitive Performance Is Related to Central Sensitization and Health-related Quality of Life in Patients with Chronic Whiplash-Associated Disorders and Fibromyalgia. Pain Physician 2015;18:E389–401. [PubMed] [Google Scholar]
- [12].Davis KD, Racine E, Collett B. Neuroethical issues related to the use of brain imaging: can we and should we use brain imaging as a biomarker to diagnose chronic pain? Pain 2012;153:1555–9. [DOI] [PubMed] [Google Scholar]
- [13].de Vries M, Wilder-Smith OH, Jongsma ML, van den Broeke EN, Arns M, van Goor H, et al. Altered resting state EEG in chronic pancreatitis patients: toward a marker for chronic pain. J Pain Res 2013;6:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Delgado DA, Lambert BS, Boutris N, McCulloch PC, Robbins AB, Moreno MR, et al. Validation of Digital Visual Analog Scale Pain Scoring With a Traditional Paper-based Visual Analog Scale in Adults. J Am Acad Orthop Surg Glob Res Rev 2018;2:e088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Meth 2004;134:9–21. [DOI] [PubMed] [Google Scholar]
- [16].Ebbesen CL, Doron G, Lenschow C, Brecht M. Vibrissa motor cortex activity suppresses contralateral whisking behavior. Nat Neurosci 2017;20:82–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol 2010;20:156–65. [DOI] [PubMed] [Google Scholar]
- [18].Fallon N, Chiu Y, Nurmikko T, Stancak A. Altered theta oscillations in resting EEG of fibromyalgia syndrome patients. Eur J Pain 2018;22:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fernandes C, Pidal-Miranda M, Samartin-Veiga N, Carrillo-de-la-Pena MT. Conditioned pain modulation as a biomarker of chronic pain: a systematic review of its concurrent validity. Pain 2019;160:2679–90. [DOI] [PubMed] [Google Scholar]
- [20].Gervasoni D, Panconi E, Henninot V, Boissard R, Barbagli B, Fort P, et al. Effect of chronic treatment with milnacipran on sleep architecture in rats compared with paroxetine and imipramine. Pharmacol Biochem Behav 2002;73:557–63. [DOI] [PubMed] [Google Scholar]
- [21].Gonzalez-Roldan AM, Cifre I, Sitges C, Montoya P. Altered Dynamic of EEG Oscillations in Fibromyalgia Patients at Rest. Pain Med 2016;17:1058–68. [DOI] [PubMed] [Google Scholar]
- [22].Granot M, Weissman-Fogel I, Crispel Y, Pud D, Granovsky Y, Sprecher E, et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? Pain 2008;136:142–9. [DOI] [PubMed] [Google Scholar]
- [23].Guo JZ, Graves AR, Guo WW, Zheng J, Lee A, Rodriguez-Gonzalez J, et al. Cortex commands the performance of skilled movement. Elife 2015;4:e10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hansen N, Obermann M, Uceyler N, Zeller D, Mueller D, Yoon MS, et al. [Clinical application of pain-related evoked potentials]. Schmerz 2012;26:8–15. [DOI] [PubMed] [Google Scholar]
- [25].Harte SE Harris RE, Clauw DJ. The neurobiology of central sensitization. J Appl Behav Res 2018;23:e12137. [Google Scholar]
- [26].Hilgenberg-Sydney PB, Kowacs PA, Conti PC. Somatosensory evaluation in Dysfunctional Syndrome patients. J Oral Rehabil 2016;43:89–95. [DOI] [PubMed] [Google Scholar]
- [27].Igarashi J, Isomura Y, Arai K, Harukuni R, Fukai T. A theta-gamma oscillation code for neuronal coordination during motor behavior. J Neurosci 2013;33:18515–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 2005;114:295–302. [DOI] [PubMed] [Google Scholar]
- [29].Karakas S, Erzengin OU, Basar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neurophysiol 2000;111:1719–32. [DOI] [PubMed] [Google Scholar]
- [30].Kilner JM, Baker SN, Salenius S, Jousmaki V, Hari R, Lemon RN. Task-dependent modulation of 15–30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol 1999;516:559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Klomjai W, Katz R, Lackmy-Vallee A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med 2015;58:208–13. [DOI] [PubMed] [Google Scholar]
- [32].Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol 1993;471:501–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci 2017;18:113. [DOI] [PubMed] [Google Scholar]
- [34].Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain 2012;13:936–44. [DOI] [PubMed] [Google Scholar]
- [35].Li H, Huang G, Lin Q, Zhao J-L, Lo W-LA, Mao Y-R, et al. Combining movement-related cortical potentials and event-related desynchronization to study movement preparation and execution. Front Neurol 2018;9:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lim M, Kim JS, Kim DJ, Chung CK. Increased Low- and High-Frequency Oscillatory Activity in the Prefrontal Cortex of Fibromyalgia Patients. Front Hum Neurosci 2016;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu C, Wang H, Pu H, Zhang Y, Zou L. EEG feature extraction and pattern recognition during right and left hands motor imagery in brain-computer interface. In Proceedings of the International Conference on BioMedical Engineering and Informatics (BMEI) IEEE; 2012. p. 506–510. [Google Scholar]
- [38].Lopez-Sola M, Woo CW, Pujol J, Deus J, Harrison BJ, Monfort J, et al. Towards a neurophysiological signature for fibromyalgia. Pain 2017;158:34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mhalla A, de Andrade DC, Baudic S, Perrot S, Bouhassira D. Alteration of cortical excitability in patients with fibromyalgia. Pain 2010;149:495–500. [DOI] [PubMed] [Google Scholar]
- [40].Neuper C, Wortz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res 2006;159:211–22. [DOI] [PubMed] [Google Scholar]
- [41].Neuper C, Wörtz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res 2006;159:211–22. [DOI] [PubMed] [Google Scholar]
- [42].Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care 2015;9:131–7. [DOI] [PubMed] [Google Scholar]
- [43].Nirl RR, Granovskyl Y, Yarnitskyl D, Sprecherl E, Granotl M. A psychophysical study of endogenous analgesia: the role of the conditioning pain in the induction and magnitude of conditioned pain modulation. Eur J Pain 2011;15:491–7. [DOI] [PubMed] [Google Scholar]
- [44].Nitsche MA, Monte-Silva K, Kuo MF, Paulus W. Dopaminergic impact on cortical excitability in humans. Rev Neurosci 2010;21:289–98. [DOI] [PubMed] [Google Scholar]
- [45].Normand E, Potvin S, Gaumond I, Cloutier G, Corbin JF, Marchand S. Pain inhibition is deficient in chronic widespread pain but normal in major depressive disorder. J Clin Psychiatry 2011;72:219–24. [DOI] [PubMed] [Google Scholar]
- [46].Nuwer MR, Lehmann D, Silva FLd, Matsuoka S, Sutherling W, Vibert J-F. IFCN guidelines for topographic and frequency analysis of EEGs and EPs. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 1994;91:1–5. [DOI] [PubMed] [Google Scholar]
- [47].Pandey AK, Kamarajan C, Manz N, Chorlian DB, Stimus A, Porjesz B. Delta, theta, and alpha event-related oscillations in alcoholics during Go/NoGo task: Neurocognitive deficits in execution, inhibition, and attention processing. Prog Neuropsychopharmacol Biol Psychiatry 2016;65:158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pfurtscheller G, Aranibar A. Event-related cortical desynchronization detected by power measurements of scalp EEG. Electroencephalogr Clin Neurophysiol 1977;42:817–26. [DOI] [PubMed] [Google Scholar]
- [49].Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 1999;110:1842–57. [DOI] [PubMed] [Google Scholar]
- [50].Pinheiro ES, de Queiros FC, Montoya P, Santos CL, do Nascimento MA, Ito CH, et al. Electroencephalographic Patterns in Chronic Pain: A Systematic Review of the Literature. PLoS One 2016;11:e0149085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Popovych S, Rosjat N, Toth TI, Wang BA, Liu L, Abdollahi RO, et al. Movement-related phase locking in the delta-theta frequency band. Neuroimage 2016;139:439–49. [DOI] [PubMed] [Google Scholar]
- [52].Potvin S, Marchand S. Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia and in healthy controls. Pain 2016;157:1704–10. [DOI] [PubMed] [Google Scholar]
- [53].Rajapakse D, Liossi C, Howard RF. Presentation and management of chronic pain. Arch Dis Child 2014;99:474–80. [DOI] [PubMed] [Google Scholar]
- [54].Rossini PM, Micera S, Benvenuto A, Carpaneto J, Cavallo G, Citi L, et al. Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin Neurophysiol 2010;121:777–83. [DOI] [PubMed] [Google Scholar]
- [55].Rossiter HE, Davis EM, Clark EV, Boudrias MH, Ward NS. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. Neuroimage 2014;91:360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum 1992;35:550–6. [DOI] [PubMed] [Google Scholar]
- [57].Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain 2006;129:55–64. [DOI] [PubMed] [Google Scholar]
- [58].Sharif-Naeini R, Basbaum AI. Targeting pain where it resides … In the brain . Sci Transl Med 2011;3:65ps1. [DOI] [PubMed] [Google Scholar]
- [59].Siedler G, Sommer C, Uceyler N. Pain-related evoked potentials in patients with large, mixed, and small fiber neuropathy. Clin Neurophysiol 2020;131:635–41. [DOI] [PubMed] [Google Scholar]
- [60].Storti SF, Formaggio E, Manganotti P, Menegaz G. Brain Network Connectivity and Topological Analysis During Voluntary Arm Movements. Clin EEG Neurosci 2016;47:276–90. [DOI] [PubMed] [Google Scholar]
- [61].Sun P Comparison of STFT and Wavelet Transform inTime-frequency Analysis. University of Gävle, Faculty of Engineering; 2015. [Google Scholar]
- [62].Svoboda K, Li N. Neural mechanisms of movement planning: motor cortex and beyond. Curr Opin Neurobiol 2018;49:33–41. [DOI] [PubMed] [Google Scholar]
- [63].Ta Dinh S, Nickel MM, Tiemann L, May ES, Heitmann H, Hohn VD, et al. Brain dysfunction in chronic pain patients assessed by resting-state electroencephalography. Pain 2019;160:2751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Toro C, Deuschl G, Thatcher R, Sato S, Kufta C, Hallett M. Event-related desynchronization and movement-related cortical potentials on the ECoG and EEG. Electroencephalogr Clin Neurophysiol 1994;93:380–9. [DOI] [PubMed] [Google Scholar]
- [65].Valeriani M, Pazzaglia C, Cruccu G, Truini A. Clinical usefulness of laser evoked potentials. Neurophysiol Clin 2012;42:345–53. [DOI] [PubMed] [Google Scholar]
- [66].Vanneste S, Ost J, Van Havenbergh T, De Ridder D. Resting state electrical brain activity and connectivity in fibromyalgia. PLoS One 2017;12:e0178516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Vuckovic A, Hasan MA, Fraser M, Conway BA, Nasseroleslami B, Allan DB. Dynamic oscillatory signatures of central neuropathic pain in spinal cord injury. J Pain 2014;15:645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RL, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016;46:319–29. [DOI] [PubMed] [Google Scholar]
- [69].Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]
- [70].Yarnitsky D Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 2015;156 Suppl 1:S24–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1: The EGI 64-channel HydroCel Geodesic Sensor Net is displayed below.


