Abstract
Background:
Volatile anesthetics moderately depress respiratory function at clinically relevant concentrations. Phox2b-expressing chemosensitive neurons in the retrotrapezoid nucleus, a respiratory control center, are activated by isoflurane but the underlying mechanisms remain unclear. Here we hypothesize that the sodium (Na+) leak channel (NALCN) contributes to the volatile anesthetics-induced modulation of retrotrapezoid nucleus neurons and to respiratory output.
Methods:
The contribution of Na+ leak channels to isoflurane-, sevoflurane- and propofol-evoked activity of Phox2b-expressing retrotrapezoid nucleus neurons and respiratory output were evaluated in wild-type and genetically modified mice lacking Na+ leak channels (both sexes). Patch-clamp recordings were performed in acute brain slices. Whole-body plethysmography was used to measure the respiratory activity.
Results:
Isoflurane at 0.42–0.50 mM (~1.5 MAC) increased the NALCN-mediated holding currents and conductance from −75.0 ± 12.9 to −130.1 ± 34.9 pA (mean ± SD, P = 0.002, n = 6) and 1.8 ± 0.5 to 3.6 ± 1.0 nS (P = 0.001, n = 6), respectively. At these concentrations, isoflurane increased activity of Phox2b-expressing retrotrapezoid nucleus neurons from 1.1 ± 0.2 to 2.8 ± 0.2 Hz (P < 0.001, n = 5), which was eliminated by bath application of Gd3+ or genetic silencing of NALCN. Genetic silencing of NALCN in the retrotrapezoid nucleus resulted in a diminished ventilatory response to carbon dioxide in mice under control conditions and during isoflurane anesthesia. Sevoflurane produced an effect comparable to that of isoflurane, whereas propofol did not activate NALCN-mediated holding conductance.
Conclusions:
Isoflurane and sevoflurane increase neuronal excitability of chemosensitive retrotrapezoid nucleus neurons partly by enhancing NALCN conductance. NALCN expression in the retrotrapezoid nucleus is required for the ventilatory response to carbon dioxide during anesthesia by isoflurane and sevoflurane, thus identifying NALCN as a requisite determinant of respiratory output during anesthesia of volatile anesthetics.
Introduction
General anesthetics have been used for more than 170 years, yet the cellular and molecular basis for how these drugs work remains poorly understood.1–4 Understanding how general anesthetics affect respiratory function is an important concern for the safety of patients undergoing general anesthesia.5 Compared to intravenous general anesthetic like propofol, volatile anesthetics including isoflurane and sevoflurane produce only a modest depression on respiratory function.6–8 Therefore, potent inhaled volatile anesthetics are frequently chosen when spontaneous ventilation is required during general anesthesia, such as airway intubation and pediatric bronchial foreign body retrieval.9–11 However, the mechanisms contributing to maintenance of breathing during inhalation anesthesia are unclear. Understanding how volatile anesthetics affect respiratory activity has immediate clinical implications, and may provide insight into therapeutic strategies to prevent anesthetic-induced respiratory failure.
The retrotrapezoid nucleus (RTN) is an important respiratory control center located in the rostral medulla oblongata. Neurons in this region that express the transcription factor Phox2b function as respiratory chemoreceptors by sensing CO2/H+ changes and sending excitatory glutamatergic drive to multiple levels of the respiratory circuits.12–14 Genetic ablation of Phox2b in retrotrapezoid nucleus neurons compromised respiratory function and increased mortality during exposure to ketamine, propofol or fentanyl, which indicates that normal function of retrotrapezoid nucleus Phox2b neurons is essential for breathing under general anesthesia.15,16 Previous study also suggests that retrotrapezoid nucleus Phox2b neurons are activated by isoflurane and help maintain respiratory activity during general anesthesia.7,15 The volatile anesthetic isoflurane can increase the firing rate of retrotrapezoid nucleus phox2b neurons at clinically relevant concentrations by inhibiting a THIK-1-like conductance and activating a yet unknown Na+ cation current.7
The sodium leak channel (NALCN) is a voltage-independent inward leak channel that is widely expressed throughout the central nervous system. NALCN produces background cation leak currents at resting membrane potential and thus strongly influences neuronal excitability.17–20 Previous work indicated that NACLN is required for normal respiratory activity21 and regulates excitability and transmitter modulation of chemosensitive retrotrapezoid nucleus neurons.22,23 Our recently study indicates that isoflurane can enhance NALCN conductance in forebrain neurons, which may contribute to the hyperactivity during induction of isoflurane.24 However, whether NALCN in retrotrapezoid nucleus neurons contributes to the modulation of volatile anesthetics on breathing under general anesthesia is still unclear. Therefore, the present study was designed to investigate the hypothesis that volatile anesthetics can activate NALCN in retrotrapezoid nucleus neurons in vitro and thereby contribute to maintenance of breathing during anesthesia by volatile anesthetics in vivo.
Materials and Methods
Animals
The protocol was reviewed and approved by the Committee of Animal Welfare of West China Hospital of Sichuan University (Chengdu, China) and relevant aspects of the ARRIVE guidelines were followed throughout. C57BL/6 mice were housed in standard conditions of a 12-h light/dark cycle, with free access to food and water. Twelve-week-old mice (injected with virus at 8 weeks, see below) were used to measure the breathing parameters. Neonatal mice at 7–12 days old were used for patch-clamp recordings.
Immunofluorescence staining
Mice were anesthetized with ketamine/xylazine (60/10 mg/kg) and then transcardially perfused with 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The perfused brains were removed and put into 4% paraformaldehyde solution overnight, followed by 30% sucrose for one day. Transverse sections of brain stem (−5.5 to −7.5 mm to Bregma) were cut (12 μm) using a freezing microtome (CM1850; Leica, Buffalo Grove, IL, USA). Sections were double-labeled by incubating at 4°C overnight with primary antibodies, including: NALCN (1:800, mouse, SMC-417, StressMarq Biosciences, Canada), NeuN (1:400, rabbit, ab104225, Abcam, Cambridge, MA, USA), NeuN (1:400, mouse, MAB377, Merck Millipore, Darmstadt, Germany) and Phox2b (1:500, rabbit, A92903N, Thermo Fisher, MA, USA). Then, the sections were incubated with secondary antibodies at room temperature for 2 h: Alexa Fluor 647 goat anti-mouse 1:200 (115-605-003), Alexa Fluor 488 goat anti-rabbit 1:200 (111-545-003) (Jackson ImmunoResearch, West Grove, PA, USA 19390). All photographs were captured using a Zeiss AxioImager Z.2. and prepared using Image J (NIH, Bethesda, MD).
Preparation of brain stem slices
Brain stem slices containing the retrotrapezoid nucleus area were prepared as previously described.25 Briefly, neonatal mice were decapitated under ketamine/xylazine (60/10 mg/kg) anesthesia, and transverse brain stem slices (300 μm) were cut using a vibratome (VT1000 A; Leica, USA) in ice-cold substituted Ringer solution containing (in mM): 260 sucrose, 3 KCl, 5 MgCl2, 1 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, and 1 kynurenic acid. The slices were incubated for 30 min at 37°C and subsequently at room temperature in normal Ringer solution (in mM): 130 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 26 NaHCO3 and 10 glucose. Both the substituted and normal Ringer solutions were equilibrated with 95% O2: 5% CO2, with pH 7.35. Two to 3 neurons were recorded from each animal. In total, 69 wild-type neonatal mice (postnatal 7–12 days, 32 males and 37 females) and 36 NALCN-shRNA-injected mice (postnatal 10–12 days, 17 males and 19 females) were included in the study.
Electrophysiological recordings
Each individual brain slice was mounted in a recording chamber submerged in a continuously perfused external solution (~2 ml/min), and bubbled with 95% O2/5% CO2 (pH = 7.35). Recordings were established using pipettes which had a resistance of 5–6 MΩ. Electrophysiological recordings were conducted using an Axopatch 700B amplifier and Digidata1440 digitizer linked to a computer running pClamp 10.2 software (Molecular Devices, Sunnyvale, CA, USA). The currents were sampled at 20 kHz and filtered at 10 kHz. Recordings were performed in either cell-attached or whole-cell configurations at room temperature (23–25°C). Holding currents and conductance were monitored over time by delivering −60 mV voltage steps every 170 ms. The current-voltage (I-V) relationship was determined using voltage steps between −60 and +80 mV (Δ 10 mV). The pipette internal solution used for recording spontaneous firing rate (in cell-attached mode) contained the following (in mM): 120 KCH3SO3, 4 NaCl, 1 MgCl2, 0.5 CaCl2, 10 HEPES, 10 EGTA, 3 Mg-ATP, and 0.3 GTP-Tris, pH 7.2 (adjusted with KOH). NALCN-mediated currents were measured in whole-cell voltage clamp (Vholding −60 mV) using a Cs+-based internal solution which contained the following (in mM): 104 Cs CH3SO3, 1 MgCl2, 0.5 CaCl2, 30 TEA-Cl, 10 EGTA, 3 Mg-ATP, 0.3 GTP-Tris, 10 HEPES, pH 7.2 (adjusted with CsOH). The external solution was the same as the normal Ringer solution but with the addition of TEA-Cl (20 mM), 4-aminopyridine (4-AP; 5 mM), tetrodotoxin (TTX; 500 nM), bicuculline (10 μM), picrotoxin (100 μM) and CNQX (10 μM) to block K+ channels, Nav and isolate retrotrapezoid nucleus neurons from synaptic/extra synaptic GABAergic and AMPA inputs. All recordings were maintained for several minutes to establish stable baseline conditions. Series resistance was typically < 10 MΩ. The neuron was discarded and the data not collected if the series resistance exceeded 15 MΩ. For wild-type neurons, evoked firing rates of less than 1.0 Hz under 10% CO2 were excluded.
Preparation of general anesthetics
Propofol was purchased from AstraZeneca (London, UK). Isoflurane and sevoflurane were obtained from Abbott Pharmaceutical Co., Ltd. (Shanghai, China). Saturated stock solutions of isoflurane (10–12 mM) and sevoflurane (4–6 mM) were prepared by adding liquid isoflurane and sevoflurane into exocellular solution and shaken overnight. Concentrations of saturated stock solutions were confirmed by gas chromatography.26 Stock solutions of isoflurane, sevoflurane or propofol (10 mg/ml) were diluted in extracellular solution to the desired final concentrations in gas-tight glass syringes by exocellular solution. Isoflurane at 0.30 mM and sevoflurane at 0.35 mM27 were used as the predicted minimum alveolar concentration (MAC) for rodents. Propofol at 20 μM was used in the patch-clamp recordings as this concentration is relevant to the plasma EC50 concentration of propofol in vitro.28,29
Virus injection
Mice were randomly assigned to an experimental group using a random number table generator. For the neonatal mice used in the patch-clamp recordings, the pups were anesthetized with 2% isoflurane and fixed in a stereotaxic frame (RWD, Life Science, Shenzhen, China). A micropipette loaded with adenovirus pDC316-CMV-ZsGreen1-U6-shRNA (Nalcn) or pDC316-CMV-ZsGreen1-U6-shRNA (scrambled) (2 × 1010 TU/ml) (Xuanzun Bioscience, Chongqing, China) was guided to the retrotrapezoid nucleus area (2.5 mm caudal to lambdoid, 1 mm lateral to the midline, and 4.0 mm ventral to the surface). The rate of injection was 500 nL/min, with a total volume of 100 nL.
For the behavioral experiments, mice were anesthetized with 2% isoflurane and fixed in a stereotaxic frame. A small hole was drilled in the skull and a pipette filled with pAAV2-H1-shRNA-(NALCN)-CAG-eGFP or pAAV2-scrambled-CAG-eGFP virus (2 × 1013 TU/ml) (Taitool Bioscience, Shanghai, China) was injected bilaterally into the retrotrapezoid nucleus area (6.5 mm caudal to Bregma, 1.3–1.4 mm lateral to the midline, and 5.2–5.5 mm ventral to the surface). The rate of injection was 500 nL/min, with total volume of 200 nL. After injection, all the mice were allowed to recover from anesthesia under a heating pad and treated with ampicillin (100 mg/kg, s.c.), atipamezole (2 mg/kg, s.c.), and ketoprofen (4 mg/kg, s.c.). The NALCN and scrambled shRNA were selected as previously described:22 AAGATCGCACAGCCTCTTCAT (NALCN); GCTCAGTACGATCATACTCAC (scrambled). The adult mice were available for respiratory activity recording 4 weeks after injection, and the pups were used for patch-clamp recordings 3 days after injection.
Whole-body plethysmography
Respiratory activity was measured using a whole-body plethysmograph system (Data Scientific International; DSI, MN, USA), utilizing an animal chamber maintained at room temperature and ventilated with air (0.5 L/min) at 10:00 to 16:00. For the original behavioral experiments, male adult mice (20–25 g, ~8 weeks old, n = 17, 7 in the NALCN-shRNA group and 7 in the scrambled-shRNA group, 3 used for location of fluorescence) were used. For the additional experiments in response to peer review, both male and female adult mice (20–25 g, ~8 weeks old; 3 females and 4 males in the NALCN-shRNA group and 3 females and 4 males in the scrambled-shRNA group) were used. All the mice were individually placed into the chamber and allowed 2 h to acclimate prior to the starting the measurements. Respiratory activity was recorded using Buxco® FinePointe™ software 2.0 for a period of 10 min in room air followed by exposure (10 min per condition) to graded increases in carbon dioxide (0, 3, 5% CO2, balance O2) under control conditions and in the presence of general anesthetics. MAC values (for loss of righting reflex) of isoflurane and sevoflurane were used as 0.8% and 1.5% in mice, respectively.30 The intraperitoneal dose of propofol was 70 mg/kg (ED50 for loss of righting reflex in mice).31 Respiratory frequency (FR, breaths/minute), tidal volume (VT, mL/g, normalized to body weight), and minute ventilation (VE, mL/min/g, normalized to body weight) were measured during a 30 s period of relative quiescence during the last minute of each condition. One technician who was blinded to the animal groups measured the respiratory outputs in vivo and another researcher analyzed the data.
Quantitative real-time polymerase chain reaction (qRT-PCR)
NALCN mRNA was measured by qRT-PCR. The retrotrapezoid nucleus area was dissected under a microscope. Total RNAs was extracted using the Eastep® Super RNA extraction kit (Promega, Shanghai, China). cDNA was synthesized with a GoScript™ Reverse Transcription Kit (Promega, Shanghai, China). Then, the cDNA was used as a template to quantify the NALCN expression level using GoTaq® qPCR Master Mix (Promega, Shanghai, China) and specific primers (Sangon Biotech, Shanghai, China) according to the manufacturer’s protocol. The forward and reverse primers of NALCN were: (5’−3’) GTCCTGACGAATCTCTGTCAGA and CTGAGATGACGCTGATGATGG, respectively. The GAPDH gene was used as an internal control. The PCR conditions were as follows: 3 min at 95°C; 40 cycles: 15 s, 95°C; 30 s, 55°C; 30 s, 72°C.
Statistical analysis
The sample sizes of the electrophysiological recordings were based on similar experimental designs and no power calculation was conducted. Power analysis was used to determine the number of animals used in each experiment in vivo. Preliminary results (n = 4) showed that under room air conditions, the respiratory frequency of NALCN genetically silenced mice and control mice was 249.3 ± 11.2 and 183.2 ± 6.3 bpm, respectively. Based on this to detect a 20% change in our primary outcome measures with 80% power (α = 0.05; β = 0.10), we needed 7 animals per group for the plethysmography experiments. Note that the preliminary data used for power analysis were not included in the final data; therefore, the power analysis based on the preliminary test was not an interim analysis for final analysis and the P values were not adjusted. Electrophysiological data were analyzed using Clampfit 10.2 software (Molecular Devices) and Graph-Pad Prism 8 (Graph-Pad Software, San Diego, CA, USA). All data are presented as mean ± SD, and outliers, if any, were also included in the analyses. Statistical analysis was performed using SPSS version 23.0 (SPSS Inc., Chicago, Illinois, USA) and GraphPad Prism 8. Normality of the data was tested by the Shapiro-Wilk test. Repeated measures data were analyzed by two-way ANOVA with a Bonferroni post hoc test. Two-tailed independent-samples or paired Student’s t tests were used for comparison between control and anesthetized animals. No animal data were missing or excluded from the behavioral analysis. Approximately 10–15% cells from wild-type mice were discarded due to a change of firing rate of less than 1 Hz in response to 10% CO2 in the patch-clamp recordings. The specific test used for each comparison is reported in the figure legend. Statistical significance was set at P < 0.05.
Results
Isoflurane activation of NALCN increases the activity of chemosensitive neurons in the retrotrapezoid nucleus
To determine whether NALCN contributes to isoflurane modulation of retrotrapezoid nucleus neurons, we first confirmed the expression and function of NALCN in Phox2b-positive retrotrapezoid nucleus neurons. Consistent with previous work,22,23 we found that NALCN was expressed by Phox2b-immunoreactive neurons in the retrotrapezoid nucleus (Fig. 1A). We also confirmed that NALCN was expressed by neurons functionally identified as retrotrapezoid nucleus chemoreceptors based on their firing response to CO2/H+. In cell-attached voltage-clamp mode, chemosensitive retrotrapezoid nucleus neurons were spontaneously active (1.5 ± 1.0 Hz) under control conditions (5% CO2) and respond to 10% CO2 with ≥ 1.0 Hz increase in firing rate (3.2 ± 1.0 Hz, P < 0.001, n = 10; Fig. 1C, D).
Fig. 1. NALCN in retrotrapezoid nucleus Phox2b neurons contributes to the neuronal firing rate.
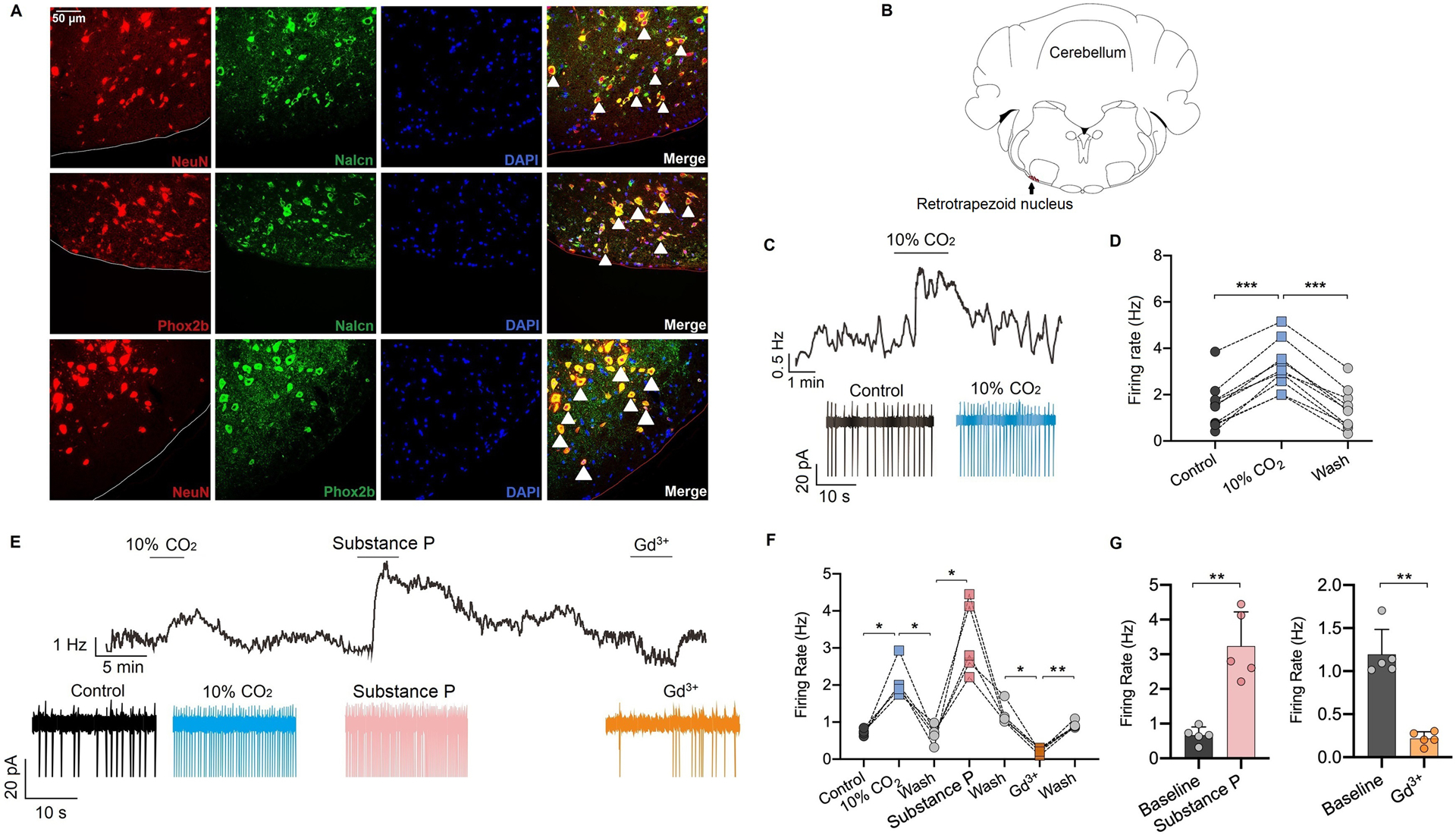
(A) The expression profile of NALCN in the retrotrapezoid nucleus area was detected by immunofluorescence staining. NALCN is widely expressed in retrotrapezoid nucleus neurons, which are also labeled with Phox2b (white arrows represent typical double-labeled neurons). (B) Mouse brain map (−6.5 mm to Bregma) indicates the relative location of the retrotrapezoid nucleus. (C) A recording from Phox2b neurons shows that the firing rate are increased when the brain was perfused with external solution aerated with 10% CO2, recorded by cell-attached mode. (D) Summary data of the firing rate of retrotrapezoid nucleus Phox2b neurons perfused with the external solution aerated with 10% CO2 (n = 10 neurons). (E-G) Representative traces (E) and quantification (F-G) for firing rate under control, 10% CO2, substance P (SP, 10 μM) and Gd3+ (50 μM) conditions. The firing rate was increased by perfusion of SP and diminished by Gd3+ (n = 5 neurons). Data are mean ± SD (G). * P < 0.05, ** P < 0.01, *** P < 0.001 by one-way repeated measures ANOVA (D, F) or two-tailed paired t-test (G).
Consistent with previous studies,22,23 we found that pharmacological blockade of NALCN by bath application of Gd3+ (50 μM) decreased baseline activity of chemosensitive retrotrapezoid nucleus neurons from 1.2 ± 0.2 to 0.2 ± 0.1 Hz (P =0.001, n = 5; Fig. 1E, F, G), whereas activation of NALCN by application of substance P (SP, 10 μM) increased firing rate from 0.7 ± 0.2 to 3.2 ± 0.9 Hz (P =0.005 by paired t test, n = 5; Fig. 1E, F, G).
Once a chemosensitive cell was identified, we went on to characterize the neuronal firing response to isoflurane. We found that exposure to isoflurane at both sub-anesthetic (0.20–0.25 mM, ~0.8 MAC) and anesthetic (0.42–0.50 mM, ~1.5 MAC) concentrations increased the firing rate of Phox2b-expressing retrotrapezoid nucleus neurons from 1.5 ± 0.8 to 2.9 ± 0.8 Hz (P = 0.020, n = 5, Fig. 2 B, C) and 1.0 ± 0.4 to 2.7 ± 0.4 Hz (P < 0.001, n = 5, Fig. 2 A, D, E), respectively. The effects of isoflurane on retrotrapezoid nucleus neuronal activity was dose-dependent (P = 0.041, Fig. 2F). Furthermore, bath application of Gd3+ (50 μM) decreased isoflurane-stimulated neuronal activity of Phox2b neurons in the retrotrapezoid nucleus from 2.7 ± 0.5 to 0.2 ± 0.1 Hz (P = 0.002, n = 5, Fig. 2G, J). In whole-cell current-clamp mode and in the presence of TTX (500 nM) to block neuronal action potentials, isoflurane at an anesthetic concentration of 0.42–0.50 mM (~1.5 MAC) depolarized resting membrane potential from −59.8 ± 2.5 to −55.0 ± 3.3 mV (P = 0.028, n = 5, Fig. 2K). In addition, NALCN was genetically silenced by NALCN-shRNA in brain slices, and neurons with ZsGreen fluorescence were selected for recording (supplementary Fig. 1A–B). Isoflurane at 0.42–0.50 mM (~1.5 MAC) did not increase firing rate of neurons in the retrotrapezoid nucleus in brain slices from NALCN genetically silenced mice (supplementary Fig. 1C). Of note, ~33% of the NALCN-silenced neurons were not spontaneously active and failed to respond to carbon dioxide. These results show that NALCN regulates basal activity and transmitter modulation of retrotrapezoid nucleus neurons and is a candidate substrate responsible for isoflurane activation of neurons of the retrotrapezoid nucleus.
Fig. 2. Isoflurane increases the firing rate of retrotrapezoid nucleus Phox2b neurons and may involve NALCN.
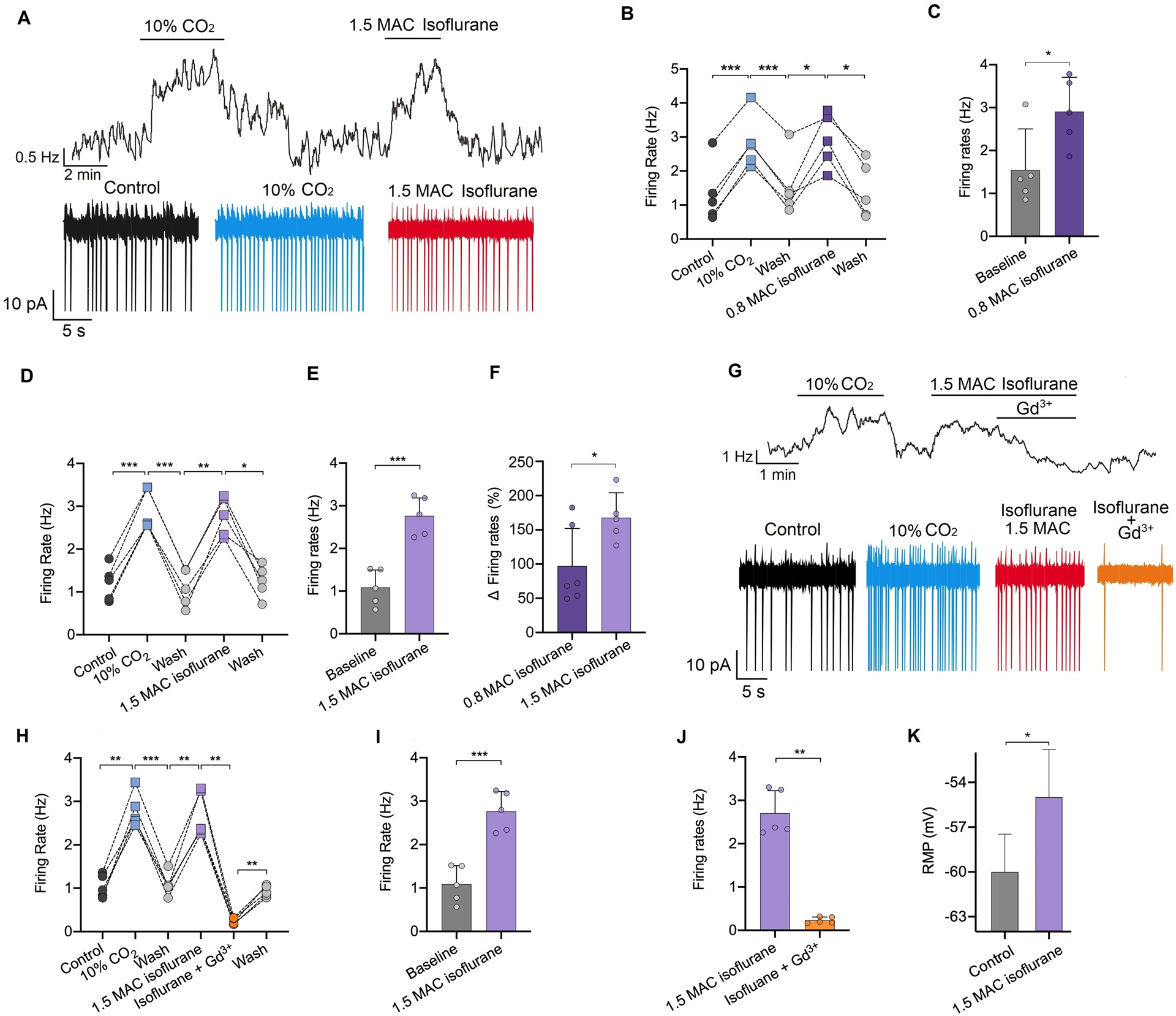
(A) Representative trace of firing rate from a chemosensitive retrotrapezoid nucleus neuron shows that exposure to 10% CO2 or isoflurane increases the neuronal firing rate. (B-E) Summary data indicate that isoflurane at ~0.8 MAC (minimum alveolar concentration, 0.20–0.25 mM) (B-C) or ~1.5 MAC (0.42–0.50 mM) (D-E) increases neuronal activity. (F) Comparison of the changed firing rate between ~0.8 MAC and ~1.5 MAC isoflurane (n = 5 or 6 neurons). (G-J) Representative trace of the firing rate (G) and summary data (H-J) indicates that Gd3+ (50 μM) can diminish the isoflurane (0.42–0.50 mM, ~1.5 MAC)-stimulated firing rate (n = 5 neurons). (K) Isoflurane (0.42–0.50 mM, ~1.5 MAC) can depolarize the resting membrane potential (RMP) of neurons (n = 5 neurons). Data are mean ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001 by one-way repeated measures ANOVA (B, D, H), two-tailed paired t test (C, E, I, J, K) or independent samples t test (F).
Isoflurane enhances NALCN conductance at clinically relevant concentrations in retrotrapezoid nucleus Phox2b neurons
The effects of isoflurane on NALCN-mediated currents in retrotrapezoid nucleus Phox2b neurons was characterized in whole-cell voltage-clamp (Vholding = −60 mV) using a Cs+-based internal solution. Consistent with our cell-attached voltage-clamp data (Fig. 2F), we found that isoflurane activation of NALCN conductance was concentration-dependent (P = 0.008, Fig. 3F). Isoflurane at 0.20–0.25 mM (~0.8 MAC) increased holding current from −68.9 ± 6.7 to −109.8 ± 11.6 pA (P = 0.005, n = 5, Fig. 3B) and increased conductance from 2.5 ± 0.8 to 3.7 ± 1.2 nS (P = 0.014, n = 5, Fig. 3B). Higher concentrations of isoflurane (0.42–0.50 mM, ~1.5 MAC) increased holding current from −75.0 ± 12.9 to −130.1 ± 34.9 pA (P = 0.002, n = 6, Fig. 3C) and increased conductance from 1.8 ± 0.5 to 3.6 ± 1.0nS (P = 0.001, n = 6, Fig. 3D). Bath application of SP (10 μM) increased holding current by 166.7% ± 56.4% and increased conductance by 189.1% ± 70.1% (Fig. 3E). Replacing extracellular Na+ with N-methyl D-glucamine (NMDG) apparently decreased holding current and conductance by 56.3% ± 22.7% (Fig. 3H) and 28.6% ± 18.7% (Fig. 3I), respectively. The effects of isoflurane on holding current and conductance were eliminated when extracellular Na+ was replaced with NMDG (Fig. 3H). Furthermore, isoflurane increased the slope of the I-V relationship, which appears voltage-independent and reverses potential near 0 mV (Fig. 3J, K). The effects of isoflurane on holding current and conductance was diminished in retrotrapezoid nucleus neurons in brain slices from NALCN genetically silenced mice (Fig. 3L–M).
Fig. 3. Isoflurane enhances NALCN conductance in retrotrapezoid nucleus Phox2b neurons.

(A) Whole-cell voltage-clamp recordings with Cs+-based internal solution in retrotrapezoid nucleus Phox2b neurons indicate that isoflurane (0.42–0.50 mM, ~1.5 MAC) enhances the holding currents and conductance. Perfusion of substance P (SP, 10 μM) also enhances the holding currents and conductance. (B) Summary data indicate that isoflurane (0.20–0.25 mM, ~0.8 MAC) increases both the holding current (left) and conductance (right) (n = 5 neurons). (C-D) Summary data indicate that isoflurane (0.42–0.50 mM, ~1.5 MAC) increases both the holding current (C) and conductance (D) (n = 6 neurons). (E) Summary data indicate that SP (10 μM) increases both the holding current (left) and conductance (right) (n = 6 neurons). (F-G) Summary data show concentration-dependent effects of isoflurane on the holding current and conductance (n = 5 neurons). (H) The effects of isoflurane (0.42–0.50 mM, ~1.5 MAC) on the holding current and conductance were eliminated when extracellular Na+ was replaced with NMDG. (I) Summary data indicate the holding current and conductance after replacement of extracellular Na+ with NMDG (n = 6 neurons), suggesting isoflurane activates leak Na+ conductance. (J) Representative I-V curves recorded from −60 to +80 mV under control (5% CO2) or isoflurane (0.42–0.50 mM, ~1.5 MAC) conditions. (K) I-V relationships are compared under control (5% CO2), isoflurane (0.42–0.50 mM, ~1.5 MAC) and wash (5% CO2) (n = 7 neurons) conditions, indicating isoflurane can increase the cation leak currents. (L-M) In the brain slices after genetic silencing expression of NALCN, NALCN-mediated holding current and conductance are decreased and isoflurane (0.42–0.50 mM, ~1.5 MAC) minimally affects the holding current (n = 10 neurons) (J) and conductance (n = 10 neurons) (M) Data are mean ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001 by one-way repeated measures ANOVA (B, C, D, I, K, J, M), two-tailed paired t-test (E) or independent samples t-test (F, G). NMDG, N-methyl D-glucamine; MAC, minimum alveolar concentration.
Genetic silencing of NALCN in the retrotrapezoid nucleus decreases ventilation and the carbon dioxide response under isoflurane anesthesia
To test the contribution of NALCN in retrotrapezoid nucleus chemoreceptors to isoflurane modulation on breathing, NALCN-specific shRNA in an adeno-associated viral delivery system was bilaterally injected into the medial portion of the retrotrapezoid nucleus region. Approximately 4 weeks after injection, we measured baseline breathing and the ventilatory response to carbon dioxide in NALCN genetically silenced mice and scrambled-shRNA (control) mice (supplementary Fig. 2A–B). Consistent with a previous study,22 mice in which NALCN was genetically silenced in the retrotrapezoid nucleus exhibited normal respiratory activity under control conditions but showed a diminished minute ventilatory response to 5% CO2 (1.9 ± 0.6 vs. 2.9 ± 0.8 ml/min/g, P = 0.026, n = 7, supplementary Fig. 2 B) compared to mice injected with scrambled-shRNA. This respiratory phenotype was primarily mediated by a decrease in respiratory frequency (197.5 ± 48.1 vs 264.4 ± 56.0 bpm, P = 0.033, n = 7, supplementary Fig. 2B). We then applied qRT-PCR (supplementary Fig. 2C) and immunofluorescence staining (supplementary Fig. 3) to confirm expression of NALCN. The mRNA levels of NALCN in the retrotrapezoid nucleus were decreased by 42.0% ± 11.8% (P = 0.007, n = 6 per group, supplementary Fig. 2C) in the mice that received NALCN-shRNA compared to the mice that received scrambled shRNA.
Baseline breathing and the ventilatory response to carbon dioxide under isoflurane were compared between control (scrambled-shRNA) and NALCN genetically silenced mice (n = 7/group). To test concentration-dependent effects of isoflurane on respiratory output, isoflurane at sub-anesthetic (0.8%, ~MAC for loss of righting reflex) and anesthetic (1.5%, ~MAC of immobility)30 concentrations were tested. The sub-anesthetic concentration of isoflurane (0.8%) increased the respiratory output in control mice by increasing both the respiratory frequency (from 166.6 ± 20.7 to 202.0 ± 17.7 bpm, P = 0.002, Fig. 4D) and minute ventilation (from 1.9 ± 0.4 to 2.5 ± 0.6 ml/min/g, P = 0.002, Fig. 4F). Conversely, this same level of isoflurane decreased both the respiratory frequency (P = 0.008, Fig. 4D) and minute ventilation (P = 0.049, Fig. 4F) in NALCN genetically silenced mice. These results are consistent with our cellular data and strongly suggest NALCN in retrotrapezoid nucleus neurons contributes to the excitatory effect of isoflurane on respiration at sub-anesthetic concentrations. Compared to 0.8% isoflurane, 1.5% isoflurane obviously decreased the respiratory output in control mice, including decreasing the respiratory frequency, tidal volume and minute ventilation (Fig. 4 D–F).
Fig. 4. Genetic silencing of NALCN in the retrotrapezoid nucleus decreases ventilation and the carbon dioxide response under isoflurane anesthesia.
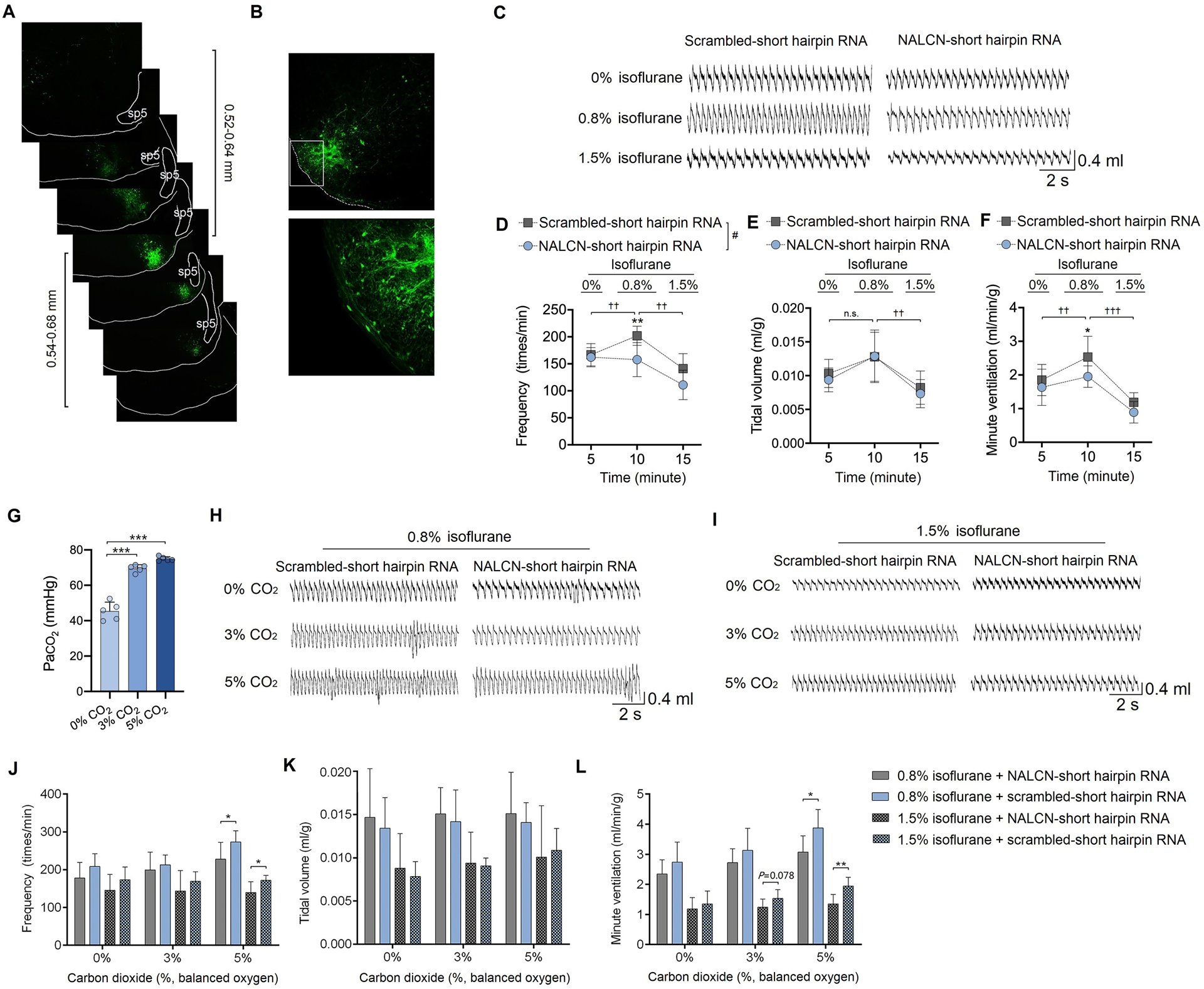
(A) Adeno-associated virus (AAV) was bilaterally injected into the retrotrapezoid nucleus area of mice based on stereotactic coordinates. The injection was centered in the medial region of the caudal retrotrapezoid nucleus and diffused ~1200 μm rostral to caudal. The middle image with the white square is the typical area of the retrotrapezoid nucleus and the distances above or below were analyzed from 3 mice. (B) Enhanced green fluorescent protein (eGFP) was detected in retrotrapezoid nucleus neurons 4 weeks after injection of virus. The lower picture is a zoomed-in image of the white square of the upper picture. (C) Representative traces of respiratory activity during exposure to 0%, 0.8% and 1.5% isoflurane (in 100% O2) in control and NALCN genetically silenced mice. (D-F) Summary data of respiratory frequency (D), tidal volume (normalized with body weight) (E), and minute ventilation (normalized with body weight) (F) are compared after exposure to 0%, 0.8% and 1.5% isoflurane (in 100% O2). (G) Arterial blood gas analysis indicates that PaCO2 increases after exposure to 3% CO2 and 5% CO2. (H-I) Representative traces of respiratory outputs during exposure of 0.8% isoflurane (H) or 1.5% isoflurane (I), and respectively under 100% O2, 3% CO2, 5% CO2. (J-L) Isoflurane-induced change of respiratory frequency, tidal volume and minute ventilation in control and NALCN genetically silenced mice. Summary data of respiratory frequency (J), tidal volume (K) and minute ventilation (L) indicates that NALCN genetic silencing can decrease the CO2 response under both 0.8% and 1.5% isoflurane anesthesia. Data are mean ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001 by two-tailed independent samples t-test (G, J, K, L); # P < 0.001 by two-way repeated measured ANOVA (D); † P < 0.05, †† P < 0.01, ††† P < 0.001 comparison of breathing parameters under 100% O2 or isoflurane anesthesia (0.8% and 1.5%) conditions in control mice by one-way repeated measured ANOVA (D, E, F); n = 7 per group. sp5, spinal trigeminal tract.
To test the ventilatory responses to CO2 under isoflurane anesthesia, control and NALCN genetically silenced mice were successively exposed to 0.8% or 1.5% isoflurane followed by graded increases in hyperoxic hypercapnia of 0%, 3% and 5% CO2, which corresponded to arterial carbon dioxide levels of 45.4 ± 5.1 mmHg, 69.5 ± 2.0 mmHg and 74.9 ± 1.2 mmHg, respectively (Fig. 4G). Compared to control mice, NALCN genetically silenced mice showed a diminished ventilatory response to carbon dioxide under both sub-anesthetic and anesthetic concentrations of isoflurane. This phenotype was primarily due to suppression of respiratory frequency. For example, under 0.8% isoflurane and in 5% CO2, NALCN genetically silenced mice breathed at a rate of 227.7 ± 37.6 bpm, whereas control mice showed a frequency of 278.2 ± 42.7 bpm (P = 0.037, Fig. 4J) and this corresponded with a diminished minute ventilatory output (3.0 ± 0.5 vs. 3.9 ± 0.7 ml/min/g, P = 0.026 by two-tailed independent sample t test, Fig. 4L). Similarly, under 1.5% isoflurane in 5% CO2, NALCN genetically silenced mice also showed a diminished breathing frequency (146.2 ± 29.3 vs. 175.2 ± 11.2 bpm; P = 0.031 by two-tailed independent samples t test vs. control mice, Fig. 4J) and lower minute ventilation (1.4 ± 0.3 vs. 1.9 ± 0.3 ml/min/g; P = 0.007 by two-tailed independent samples t test vs. control mice, Fig. 4L).
Sevoflurane but not propofol enhances NALCN conductance at clinically relevant concentrations in retrotrapezoid nucleus Phox2b neurons
This study originally only investigated the effects of isoflurane. Sevoflurane and propofol were also analyzed in response to peer review. Propofol at 20 μM did not affect the holding currents (from −55.0 ± 16.9 to −53.3 ± 19.5 pA, P = 0.254) or conductance (from 2.7 ± 0.8 to 2.7 ± 0.9 nS, n = 6, P = 0.377) (Fig. 5A–B). Sevoflurane at concentrations of 0.25–0.32 mM (~0.8 MAC, loss of righting reflex) enhanced NALCN-mediated holding currents (from −44.97 ± 12.5 to −87.11 ± 30.5 pA, n = 5, P = 0.002) and conductance (from 1.4 ± 0.6 to 2.9 ± 0.6 nS, n = 5, P = 0.001) (Fig. 5 C–D). Accordingly, the firing rate of retrotrapezoid nucleus Phox2b neurons was suppressed by 20 μM propofol from 1.7 ± 0.7 to 0.6 ± 0.4 Hz (P = 0.013, n = 5, Fig. 5E–F). In contrast, sevoflurane at 0.25–0.32 mM increased the neuronal firing rate from 1.4 ± 0.5 to 2.6 ± 0.6 Hz, P = 0.006, n = 7) (Fig. 5G–H). Bath application of Gd3+ (50 μM) during exposure to sevoflurane suppressed neural activity (Fig. 5G–H). These results indicate that sevoflurane but not propofol can enhance NALCN conductance in retrotrapezoid nucleus Phox2b neurons.
Fig. 5. Sevoflurane but not propofol enhances NALCN conductance and increases the firing rate in retrotrapezoid nucleus Phox2b neurons.
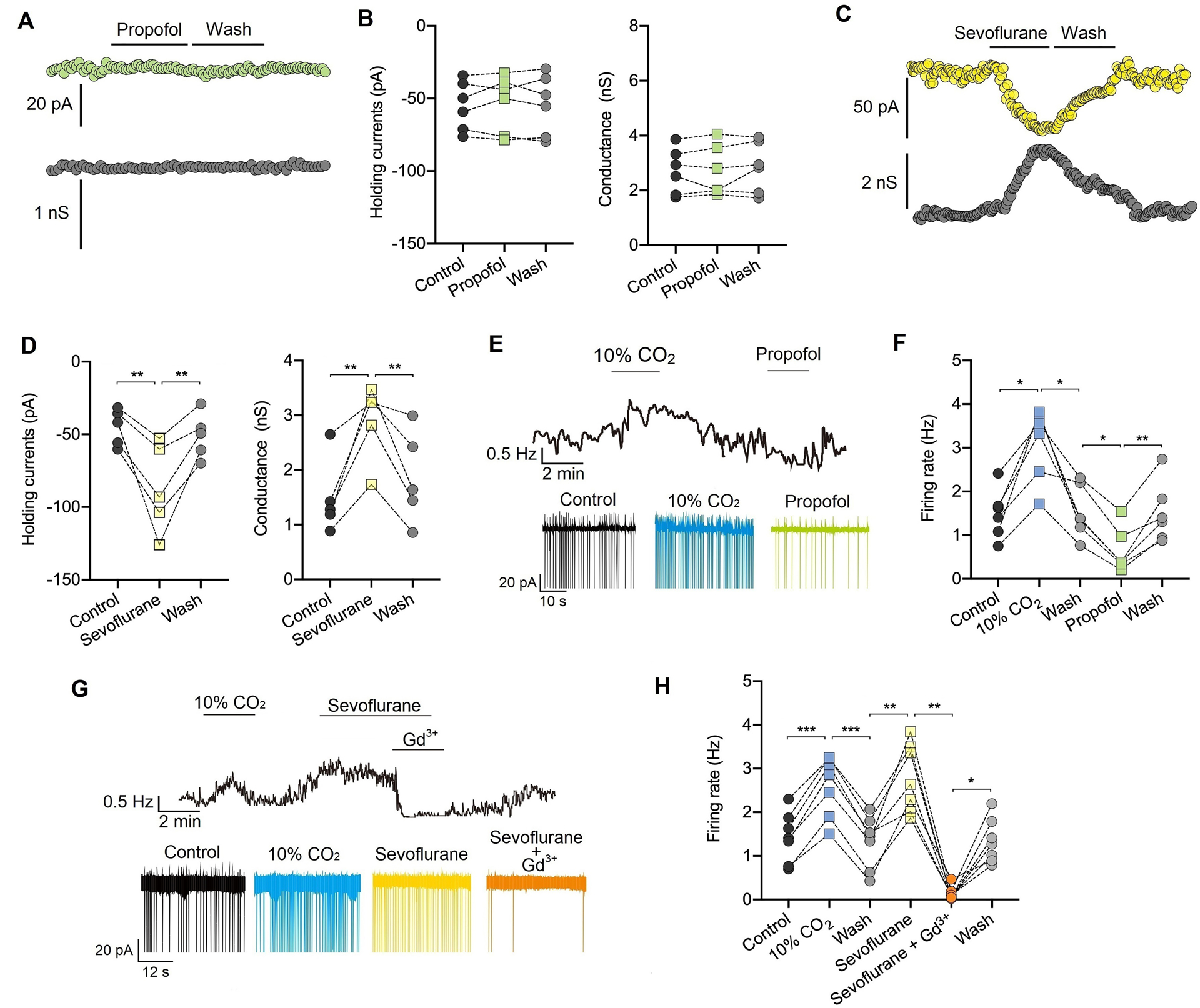
(A) Representative traces of the holding current (upper) and conductance (lower) in retrotrapezoid nucleus Phox2b neurons. Propofol (20 μM) produces no effect on the holding current and conductance. (B) Summary data shows the effect of propofol (20 μM) on NALCN holding current (left) (n = 6 neurons) and conductance (right) (n = 6 neurons). (C) Representative traces of holding current (upper) and conductance (lower) in retrotrapezoid nucleus Phox2b neurons. Sevoflurane at 0.35–0.40 mM (~0.8 MAC, minimum alveolar concentration) enhances the holding current and conductance. (D) Summary data show the effects of sevoflurane 0.35–0.40 mM (~0.8 MAC) on the holding current (left) (n = 5 neurons) and conductance (right) (n = 5 neurons). (E) Trace of the firing rate shows that bath application of propofol (20 μM) suppresses activity of retrotrapezoid nucleus Phox2b neurons. (F) Summary data of the firing rate indicate that 20 μM propofol suppresses neuronal activity (n = 6 neurons). (G) Trace of the firing rate from shows that exposure to sevoflurane at 0.35–0.40 mM (~0.8 MAC) increases the activity of retrotrapezoid nucleus Phox2b neurons while bath application Gd3+ (50 μM) can diminish sevoflurane-evoked neuronal activity. (H) Summary data indicates that sevoflurane at 0.35–0.40 mM (~0.8 MAC) increase the activity of retrotrapezoid nucleus Phox2b neurons and bath application Gd3+ (50 μM) can diminish sevoflurane-evoked neuronal activity (n = 7 neurons). * P < 0.05, ** P < 0.01, *** P < 0.001 by one-way repeated measures ANOVA (B, D, F, H).
Genetic silencing of NALCN in the retrotrapezoid nucleus decreases ventilation and the carbon dioxide response under sevoflurane but not propofol anesthesia
Sub-anesthetic concentrations (~1 MAC for loss of righting reflex) of isoflurane (~0.8%) (Fig. 6A) and sevoflurane (~1.5%) (Fig. 6C) increased the respiratory output in control mice. Compared with control mice, NALCN genetically silenced mice exhibited a lower breathing frequency under isoflurane (209.3 ± 36.6 vs. 150.8 ± 31.55 bpm, P < 0.001, n = 7) (Fig. 6A) or under sevoflurane (214.9 ± 27.4 vs. 176.5 ± 32.7 bpm, P = 0.022, n = 7) (Fig. 6C). NALCN genetically silenced mice exhibited lower minute ventilation under isoflurane (2.7 ± 0.6 vs. 2.1 ± 0.5 ml/min/g, P = 0.047, n = 7) (Fig. 6A) or under sevoflurane (2.1 ± 0.3 vs. 1.4 ± 0.6 ml/min/g, P = 0.014, n = 7) (Fig. 6C). However, propofol at 70 mg/kg (i.p. ED50 of loss of righting reflex) suppressed breathing (Fig. 6E).
Fig. 6. Genetic silencing of NALCN in the retrotrapezoid nucleus decreases ventilation and the carbon dioxide response under anesthesia by volatile anesthetics but not under propofol.
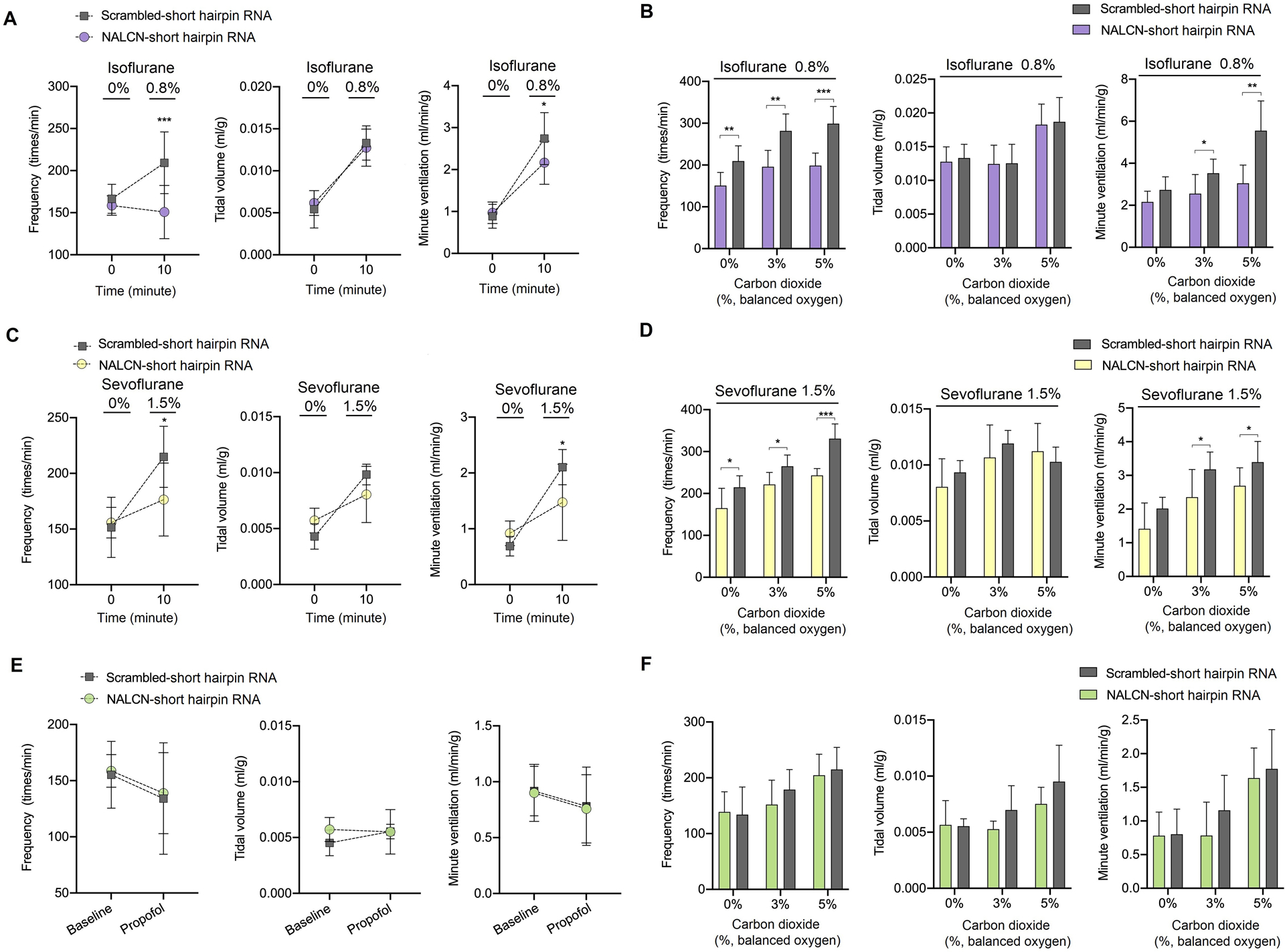
(A) Isoflurane-induced (0.8%, ~0.8 MAC) changes of respiratory frequency (left), tidal volume (middle) and minute ventilation (right) in control and NALCN genetically silenced mice. (B) Compared to control mice, NALCN genetically silenced mice showed a blunted response of respiratory frequency and minute ventilation to 3% CO2 or 5% CO2 under 0.8% isoflurane anesthesia. (C) Sevoflurane-induced (1.5%, ~0.8 MAC) changes of respiratory frequency (left), tidal volume (middle) and minute ventilation (right) in control and NALCN genetically silenced mice. (D) NALCN genetically silenced decreases the carbon dioxide response under anesthesia with 1.5% sevoflurane. (E) Propofol (70 mg/kg, intraperitoneal injection) suppresses respiratory output and there is no difference between control mice and NALCN genetically silenced mice. (F) There is no difference between control mice and NALCN genetically silenced mice in terms of respiratory frequency (left), tidal volume (middle), and minute ventilation (right) response to carbon dioxide under propofol anesthesia. n = 7/group. Data are mean ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001 by two-way repeated measures ANOVA (A, C, E) and two-tailed independent samples t-test (B, D, F). MAC, minimum alveolar concentration.
Ventilatory responses to increased carbon dioxide under 0.8% isoflurane (Fig. 6B) or under 1.5% sevoflurane (Fig. 6D) were diminished in NALCN genetically silenced mice, as revealed by higher breathing frequency and minute ventilation in control mice than in NALCN genetically silenced mice. However, genetic silencing expression of NALCN in the retrotrapezoid nucleus produced no effect on breathing frequency, tidal volume or minute ventilation in response to increased carbon dioxide under propofol anesthesia (Fig. 6F).
For comparisons between sexes, there was no difference in respiratory frequency or minute ventilation responses to carbon dioxide between male (n = 4) and female (n = 3) mice. The following P-values were obtained: for control mice, P = 0.542 for respiratory frequency, and P = 0.262 for minute ventilation (by two-way repeated measures ANOVA); for NALCN genetically silenced mice, P = 0.241 for respiratory frequency, and P = 0.411 for minute ventilation (by two-way repeated measures ANOVA). Under anesthesia by isoflurane, sevoflurane or propofol, there was no difference in respiratory frequency between male and female mice: control mice, P = 0.175 for isoflurane, P = 0.292 for sevoflurane and P = 0.671 for propofol (by two-tailed independent samples t-test); NALCN genetically silenced mice, P = 0.649 for isoflurane, P = 0.825 for sevoflurane and P = 0.555 for propofol (by two-tailed independent samples t-test).
Discussion
This study identified NALCN in chemosensitive retrotrapezoid nucleus neurons as a target of volatile anesthetics to activate respiratory activity. Specifically, we showed at the cellular level that isoflurane and sevoflurane increased excitability of retrotrapezoid nucleus phox2b neurons partly by enhancing NALCN conductance. Further, at the whole-animal level, genetic silencing of NALCN in the retrotrapezoid nucleus decreased the ventilatory responses to carbon dioxide and potentiated volatile anesthetic-induced respiratory depression. These results indicate that NALCN in chemosensitive retrotrapezoid nucleus neurons are important determinants of respiratory activity during anesthesia of volatile anesthetics. In contrast, NALCN-silenced mice showed no difference relative to control mice under propofol anesthesia. Understanding the cellular and molecular mechanisms contributing to maintenance of spontaneous breathing during general anesthesia is clinically relevant, and may facilitate development of novel respiratory stimulants.
The retrotrapezoid nucleus is an important respiratory control center,12,32 and a previous study identified chemosensitive Phox2b neurons in this region as required for maintaining breathing during general anesthesia.15 Neurons in this region that express Phox2b provide the main stimulus for breathing.12,13,33–35 Isoflurane has been shown to stimulate the activity of retrotrapezoid nucleus Phox2b neurons by mechanisms involving inhibition of a THIK-1-like conductance and activation of a yet unidentified leak Na+ current.7 Our previous study indicates that pyramidal neurons expressing NALCN may be activated by isoflurane.24 These results suggest NALCN may contribute to isoflurane activation of retrotrapezoid nucleus neurons and breathing. Consistent with this, here we found that isoflurane and sevoflurane at clinically relevant concentrations increased the excitability of retrotrapezoid nucleus Phox2b neurons in part by enhancing NALCN conductance. Of note, our results cannot exclude involvement of potassium channels such as THIK-1 which are also known to contribute to the excitatory action of isoflurane on retrotrapezoid nucleus Phox2b neurons.7
Compared to intravenous general anesthetics like propofol, volatile anesthetics moderately depress respiratory function.11,36,37 This feature of volatile anesthetics makes them ideally suited for certain medical procedures needing spontaneous breathing such as intubation of a difficult airway and induction of pediatric anesthesia.9–11 Therefore, understanding the mechanisms contributing to the maintenance of breathing during anesthesia of volatile anesthetics is critical for the safety of patients undergoing these types of procedures.
NALCN has been identified as an important modulator of respiratory function.21–23,38 Global loss of NALCN is lethal due to respiratory failure.20,21 Loss of NALCN from key elements of the respiratory circuit, including rhythmogenic neurons in the pre-Bötzinger complex, also results in lethal apnea.23 Diminished expression of NALCN in retrotrapezoid nucleus neurons severely compromises the ventilatory response to carbon dioxide.23,39 Here we confirm the importance of NALCN to retrotrapezoid nucleus function by showing that neuronal activity of the retrotrapezoid nucleus is diminished by the non-selective NALCN blocker Gd3+ or genetic silencing of NALCN by shRNA, and enhanced by substance P, further suggesting that NALCN is a critical modulator of excitability of retrotrapezoid nucleus Phox2b neurons. Although Gd3+ as a pharmacological probe for NALCN has been widely used by previous studies,21,40 its use as a non-specific blocker of NALCN in patch-clamp recordings is a limitation of the present study. To overcome this limitation, we delivered NALCN-shRNA with adenovirus vector to silence NALCN in brain slices, which helps strengthen our conclusions, although the genetic silencing of NALCN is not specific to Phox2b neurons.
We observed two behavioral phenotypes caused by loss of NALCN from retrotrapezoid nucleus neurons. Firstly, consistent with previous work,22 loss of NALCN from retrotrapezoid nucleus neurons minimally affected baseline breathing in awake mice but diminished the ventilatory response to carbon dioxide. Secondly, isoflurane and sevoflurane strongly suppressed respiratory activity in NALCN genetically silenced mice compared to control mice. Meanwhile, propofol produced no effect on NALCN-mediated channel conductance, and genetic silencing of NALCN did not affect the respiratory output under propofol anesthesia. These results indicate that NALCN regulates the excitability of retrotrapezoid nucleus Phox2b neurons and helps maintain breathing during anesthesia by volatile anesthetics but not by propofol.
We used neonatal mice aged of 7–12 days for patch-clamp recordings because it is exceedingly difficult to perform visualized patching on retrotrapezoid nucleus neurons of the animals older than two weeks. We used adult mice for the behavioral tests because of the more stable and better signal to noise ratio obtained in older mice. This age disparity may raise concerns of developmental differences in the respiratory circuits between neonatal and older mice. However, we consider this a minor limitation because retrotrapezoid nucleus neurons in both neonatal and adult mice are intrinsically and strongly activated by CO2/H+ 33,41 and show similar responses to wake-on transmitters, including substance P42, which indicating a similar respiratory circuits between ages by a mechanism involving NALCN.22,23 Furthermore, consistent with a previous study,24 we also found that NALCN is widely expressed in both neonatal and adult mouse brain. Therefore, it is unlikely that fundamental properties of retrotrapezoid nucleus neurons, including NALCN activation by volatile anesthetics, is dramatically different between neonatal and adult mice.
This study originally measured breathing parameters in male adult mice. In response to peer review, we measured respiratory activity in both male and female adult mice in additional experiments. Both sexes of rodents are widely used in whole-body plethysmography and no differences have been found between males and females in this regard.15,22,43 Of note, we repeatedly measured 0.8% isoflurane in both sexes during additional experiments and found similar effects, also indicating that sex may not influence the respiratory measurements.
Although we investigated the contribution of retrotrapezoid nucleus Phox2b neurons here, modulation of respiratory function by volatile anesthetics likely involves multiple levels of the respiratory circuitry, including other chemoreceptor regions or the downstream respiratory rhythm generator.7,8,44 Isoflurane can decrease respiratory output by depression of chemoreceptor regions, including the medullary raphe or the respiratory rhythm generator.8,44,45 Therefore, carbon dioxide-stimulated ventilation is also inhibited by anesthetic concentrations of isoflurane, although isoflurane can still activate retrotrapezoid nucleus central chemoreceptors at this concentration. This inhibition can result from the net actions of isoflurane on the central respiratory rhythmical generator and chemoreceptors outside the retrotrapezoid nucleus while retrotrapezoid nucleus Phox2b neurons are activated by isoflurane.
NALCN also contributes to other non-respiratory pharmacological effects of volatile anesthetics.38,46–48 For example, sub-anesthetic concentrations of isoflurane can activate NALCN in hippocampal pyramidal neurons and our previous study showed that behavioral hyperactivity during isoflurane induction is diminished by forebrain genetic silencing of NALCN.24 Drosophila and nematode unc-79 (component of NALCN complex) mutants are hypersensitive to the immobilizing effects of volatile anesthetics.46 A clinical case reported that a 3-year-old child with a pathological mutation of NALCN showed hypersensitivity to volatile anesthetics, which caused aggravated respiratory depression and cardiac arrest.38
A limitation of this study is that body temperature of mice was not perfectly controlled during plethysmography. General anesthetics slightly decreased body temperature of mice may negatively modulate respiratory function (supplementary Table 1). It is impossible to put a heating pad under the chamber to control body temperature of animals because temperature of the sealed chamber may change the pressure inside and greatly affects accuracy of the results. Instead, room temperature was kept throughout. However, we consider this a minor issue because a robust stable measurement of respiration was found. In addition, anesthetics-induced changes of body temperature were similar between control mice and NALCN genetically silenced mice (supplementary Table 1), indicating the contribution of NALCN did not result from decrease of body temperature. Of note, we may have underestimated the stimulatory effect of volatile anesthetics on breathing because anesthetics including isoflurane can cause a modest reduction in body temperature, and consequently decrease metabolic and respiratory activity.
In summary, volatile anesthetics at clinically relevant concentrations can stimulate activity of Phox2b neurons in the retrotrapezoid nucleus by enhancing NALCN conductance, and help maintain respiratory output during exposure to volatile anesthetics. Likewise, genetic silencing of NALCN in the retrotrapezoid nucleus can aggravate volatile anesthetic-induced respiratory depression.
Supplementary Material
Funding Statement
Supported by grant No. 2018YFC2001800 (to Dr. Zhu and Dr. Ou) from National Key R&D Program of China (Beijing, China), No. 81771486 and No. 81974164 (to Dr. Zhou) from National Natural Science Foundation of China (Beijing, China). This work was also supported in part by grants from the National Institutes of Health Grants HL104101 (to Dr. Mulkey), HL137094 (to Dr. Mulkey), NS099887 (to Dr. Mulkey) (Bethesda, Maryland).
Footnotes
Clinical trail number and registry URL: Not applicable.
Prior Presentations: Not applicable.
Summary Statement: Not applicable.
Conflicts of Interest: The authors declare no competing interests.
References
- 1.Kennedy D, Norman C: What don’t we know? Science 2005; 309: 75. [DOI] [PubMed] [Google Scholar]
- 2.Franks NP: General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci 2008; 9: 370–86. [DOI] [PubMed] [Google Scholar]
- 3.Brown EN, Lydic R, Schiff ND: General anesthesia, sleep, and coma. N Engl J Med 2010; 363: 2638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonhomme V, Staquet C, Montupil J, Defresne A, Kirsch M, Martial C, Vanhaudenhuyse A, Chatelle C, Larroque SK, Raimondo F, Demertzi A, Bodart O, Laureys S, Gosseries O: General Anesthesia: A Probe to Explore Consciousness. Front Syst Neurosci 2019; 13: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teppema LJ, Baby S: Anesthetics and control of breathing. Respir Physiol Neurobiol 2011; 177: 80–92. [DOI] [PubMed] [Google Scholar]
- 6.Sonner JM, Antognini JF, Dutton RC, Flood P, Gray AT, Harris RA, Homanics GE, Kendig J, Orser B, Raines DE, Rampil IJ, Trudell J, Vissel B, Eger EI 2nd: Inhaled anesthetics and immobility: mechanisms, mysteries, and minimum alveolar anesthetic concentration. Anesth Analg 2003; 97: 718–40. [DOI] [PubMed] [Google Scholar]
- 7.Lazarenko RM, Fortuna MG, Shi Y, Mulkey DK, Takakura AC, Moreira TS, Guyenet PG, Bayliss DA: Anesthetic activation of central respiratory chemoreceptor neurons involves inhibition of a THIK-1-like background K(+) current. J Neurosci 2010; 30: 9324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massey CA, Richerson GB: Isoflurane, ketamine-xylazine, and urethane markedly alter breathing even at subtherapeutic doses. J Neurophysiol 2017; 118: 2389–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Datta PK, Sinha R, Ray BR, Jambunathan V, Kundu R: Anesthesia maintenance with ‘induction dose only’ sevoflurane during pediatric ophthalmic examination: comparison with standard low-flow technique through a randomized controlled trial. Paediatr Anaesth 2017; 27: 162–169. [DOI] [PubMed] [Google Scholar]
- 10.Memtsoudis SG, Cozowicz C, Nagappa M, Wong J, Joshi GP, Wong DT, Doufas AG, Yilmaz M, Stein MH, Krajewski ML, Singh M, Pichler L, Ramachandran SK, Chung F: Society of Anesthesia and Sleep Medicine Guideline on Intraoperative Management of Adult Patients With Obstructive Sleep Apnea. Anesth Analg 2018; 127: 967–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Gao X, Wei W, Miao H, Meng H, Tian M: The optimum sevoflurane concentration for supraglottic airway device Blockbuster insertion with spontaneous breathing in obese patients: a prospective observational study. BMC Anesthesiol 2017; 17: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyenet PG, Bayliss DA: Neural Control of Breathing and CO2 Homeostasis. Neuron 2015; 87: 946–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SB, DePuy SD: Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Respir Physiol Neurobiol 2009; 168: 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souza G, Kanbar R, Stornetta DS, Abbott SBG, Stornetta RL, Guyenet PG: Breathing regulation and blood gas homeostasis after near complete lesions of the retrotrapezoid nucleus in adult rats. J Physiol 2018; 596: 2521–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourgeois T, Ringot M, Ramanantsoa N, Matrot B, Dauger S, Delclaux C, Gallego J: Breathing under Anesthesia: A Key Role for the Retrotrapezoid Nucleus Revealed by Conditional Phox2b Mutant Mice. Anesthesiology 2019; 130: 995–1006. [DOI] [PubMed] [Google Scholar]
- 16.Pattinson KT: Opioids and the control of respiration. Br J Anaesth 2008; 100: 747–58. [DOI] [PubMed] [Google Scholar]
- 17.Ford NC, Ren D, Baccei ML: NALCN channels enhance the intrinsic excitability of spinal projection neurons. Pain 2018; 159: 1719–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cochet-Bissuel M, Lory P, Monteil A: The sodium leak channel, NALCN, in health and disease. Front Cell Neurosci 2014; 8: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reinl EL, Zhao P, Wu W, Ma X, Amazu C, Bok R, Hurt KJ, Wang Y, England SK: Na+-Leak Channel, Non-Selective (NALCN) Regulates Myometrial Excitability and Facilitates Successful Parturition. Cell Physiol Biochem 2018; 48: 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren D: Sodium leak channels in neuronal excitability and rhythmic behaviors. Neuron 2011; 72: 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu B, Su Y, Das S, Liu J, Xia J, Ren D: The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell 2007; 129: 371–83. [DOI] [PubMed] [Google Scholar]
- 22.Shi Y, Abe C, Holloway BB, Shu S, Kumar NN, Weaver JL, Sen J, Perez-Reyes E, Stornetta RL, Guyenet PG, Bayliss DA: Nalcn Is a “Leak” Sodium Channel That Regulates Excitability of Brainstem Chemosensory Neurons and Breathing. J Neurosci 2016; 36: 8174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh SY, Huang WH, Wang W, Ward CS, Chao ES, Wu Z, Tang B, Tang J, Sun JJ, Esther van der Heijden M, Gray PA, Xue M, Ray RS, Ren D, Zoghbi HY: Respiratory Network Stability and Modulatory Response to Substance P Require Nalcn. Neuron 2017; 94: 294–303.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ou M, Zhao W, Liu J, Liang P, Huang H, Yu H, Zhu T, Zhou C: The General Anesthetic Isoflurane Bilaterally Modulates Neuronal Excitability. iScience 2020; 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK: Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol 2010; 104: 3042–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ouyang W, Herold KF, Hemmings HC Jr.: Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function. Anesthesiology 2009; 110: 582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franks NP, Lieb WR: Temperature dependence of the potency of volatile general anesthetics: implications for in vitro experiments. Anesthesiology 1996; 84: 716–20. [DOI] [PubMed] [Google Scholar]
- 28.Ren L, Hao X, Min S, Deng J, Chen Q, Chen H, Liu D: Anesthetics alleviate learning and memory impairment induced by electroconvulsive shock by regulation of NMDA receptor-mediated metaplasticity in depressive rats. Neurobiol Learn Mem 2018; 155: 65–77. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Wu Y, Li R, Wang C, Jia N, Zhao C, Wen A, Xiong L: Propofol Regulates the Surface Expression of GABAA Receptors: Implications in Synaptic Inhibition. Anesth Analg 2015; 121: 1176–83. [DOI] [PubMed] [Google Scholar]
- 30.Zhou C, Liang P, Liu J, Ke B, Wang X, Li F, Li T, Bayliss DA, Chen X: HCN1 Channels Contribute to the Effects of Amnesia and Hypnosis but not Immobility of Volatile Anesthetics. Anesth Analg 2015; 121: 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikaido Y, Furukawa T, Shimoyama S, Yamada J, Migita K, Koga K, Kushikata T, Hirota K, Kanematsu T, Hirata M, Ueno S: Propofol Anesthesia Is Reduced in Phospholipase C-Related Inactive Protein Type-1 Knockout Mice. J Pharmacol Exp Ther 2017; 361: 367–374. [DOI] [PubMed] [Google Scholar]
- 32.Holloway BB, Viar KE, Stornetta RL, Guyenet PG: The retrotrapezoid nucleus stimulates breathing by releasing glutamate in adult conscious mice. Eur J Neurosci 2015; 42: 2271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guyenet PG: Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 2014; 4: 1511–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guyenet PG, Mulkey DK: Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol 2010; 173: 244–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon S, Zuccarello M, Rapoport RM: pCO(2) and pH regulation of cerebral blood flow. Front Physiol 2012; 3: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodman NW, Black AM, Carter JA: Some ventilatory effects of propofol as sole anaesthetic agent. Br J Anaesth 1987; 59: 1497–503. [DOI] [PubMed] [Google Scholar]
- 37.Nieuwenhuijs D, Sarton E, Teppema LJ, Kruyt E, Olievier I, van Kleef J, Dahan A: Respiratory sites of action of propofol: absence of depression of peripheral chemoreflex loop by low-dose propofol. Anesthesiology 2001; 95: 889–95. [DOI] [PubMed] [Google Scholar]
- 38.Lozic B, Johansson S, Lovric Kojundzic S, Markic J, Knappskog PM, Hahn AF, Boman H: Novel NALCN variant: altered respiratory and circadian rhythm, anesthetic sensitivity. Ann Clin Transl Neurol 2016; 3: 876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Stornetta RL, Stornetta DS, Onengut-Gumuscu S, Farber EA, Turner SD, Guyenet PG, Bayliss DA: Neuromedin B Expression Defines the Mouse Retrotrapezoid Nucleus. J Neurosci 2017; 37: 11744–11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu B, Su Y, Das S, Wang H, Wang Y, Liu J, Ren D: Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature 2009; 457: 741–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG: Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 2004; 7: 1360–9. [DOI] [PubMed] [Google Scholar]
- 42.Mulkey DK, Rosin DL, West G, Takakura AC, Moreira TS, Bayliss DA, Guyenet PG: Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J Neurosci 2007; 27: 14128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo F-S, Cleary CM, LoTurco JJ, Chen X, Mulkey DK: Disordered breathing in a mouse model of Dravet syndrome. eLife 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johansen SL, Iceman KE, Iceman CR, Taylor BE, Harris MB: Isoflurane causes concentration-dependent inhibition of medullary raphe 5-HT neurons in situ. Auton Neurosci 2015; 193: 51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuribayashi J, Sakuraba S, Kashiwagi M, Hatori E, Tsujita M, Hosokawa Y, Takeda J, Kuwana S: Neural mechanisms of sevoflurane-induced respiratory depression in newborn rats. Anesthesiology 2008; 109: 233–42. [DOI] [PubMed] [Google Scholar]
- 46.Humphrey JA, Hamming KS, Thacker CM, Scott RL, Sedensky MM, Snutch TP, Morgan PG, Nash HA: A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Curr Biol 2007; 17: 624–9. [DOI] [PubMed] [Google Scholar]
- 47.Sedensky MM, Meneely PM: Genetic analysis of halothane sensitivity in Caenorhabditis elegans. Science 1987; 236: 952–4. [DOI] [PubMed] [Google Scholar]
- 48.Speca DJ, Chihara D, Ashique AM, Bowers MS, Pierce-Shimomura JT, Lee J, Rabbee N, Speed TP, Gularte RJ, Chitwood J, Medrano JF, Liao M, Sonner JM, Eger EI 2nd, Peterson AS, McIntire SL: Conserved role of unc-79 in ethanol responses in lightweight mutant mice. PLoS Genet 2010; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


