The COVID-19 pandemic has led to widespread disruption in the delivery of health care with a significant impact on patients, healthcare professionals and health systems.1 It is unknown how cardiovascular clinical research has been disrupted. To understand how COVID-19 has affected site-based heart failure (HF) clinical research operations, we queried sites participating in the CONNECT-HF clinical trial.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Leveraging the infrastructure of the CONNECT-HF trial, a 12-question electronic survey designed to assess changes in HF-related clinical research activities was developed by expert consensus and sent to all CONNECT-HF site clinical research coordinators (CRCs). CONNECT-HF is a prospective, cluster-randomized trial of 151 HF programs in 36 states across the US, and designed to assess an in-hospital and post-discharge quality improvement intervention, compared to usual care, on HF outcomes and quality of care.2 Survey results were compiled and analyzed using descriptive statistics and content analysis was performed for free text. This study was approved by the Duke University Institutional Review Board.
Results
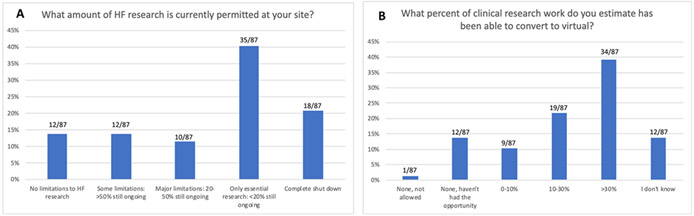
Between 5/11/2020 – 5/18/2020 surveys were sent to 131 currently active sites. 87 CRCs (66%) responded from 25 academic health systems (29%), 14 large tertiary hospitals (16%), 44 community hospitals (51%), and 4 public health centers (4.6%). Figure 1 shows the proportions of ongoing HF research at CONNECT-HF sites. Only 11.5% of sites with active research activities designated CONNECT-HF as essential. Half of responders reported that 30% or less of their responsibilities were converted to remote work.
Figure 1.
Bar graphs representing answers to survey questions. HF=Heart failure.
As of May 2020, 32% of sites had resumed clinical research activities, 27% planned to loosen restrictions in the next 2-3 months, and 41% had no plans to resume. There was considerable variation in where sites obtained guidance when determining how to resume clinical research activities: 5.2% followed federal guidance, 28% used state guidelines, and 7.2% used city/county guidance. Over half of reporting sites used a source not listed or were not aware of specific guidance.
A wide spectrum of comfort-level with respect to clinical research staff returning to the hospital was reported. 42% of responders reported at least some degree of fear associated with returning, 32% expressed little to no fear, and 26% reported feeling neutral. 59% reported not having clinical research staff furloughed and 96% of sites had not experienced permanent staff termination.
Discussion
The results of this survey demonstrate the extent to which COVID-19 has obstructed various aspects of HF site-based research. Over half of CONNECT-HF sites have had major disruptions or complete cessation of all HF clinical research activity with substantial research coordinator work unable to be completed virtually. Furthermore, plans and guidance used to reopen HF research activities were variable.
To our knowledge, this survey represents the first attempt to quantify the impact of COVID-19 on site-based clinical research. This survey demonstrates how COVID-19 has identified inefficiencies in the clinical trial ecosystem. During the COVID-19 pandemic, regulatory agencies have advocated for operating clinical research programs with flexibility; ensuring the safety of trial participants, maintaining compliance with good clinical practice, and minimizing risk to trial integrity.3,4 Unfortunately, these recommendations do not provide tangible ways to ensure continuation of research activity. By reducing in-person visits to keep participants and staff safe, it is unclear how slowed enrollment, delayed follow-up visits, and not having ways for reliable safety assessment will affect future outcomes actively enrolling clinical trials. Because CONNECT-HF is a cluster-randomized trial that promotes guideline-based care and thus is low risk to patients, it is suited to converting to virtual enrollment and follow-up. Trials of novel therapeutics or for new therapeutic indications may not have this same flexibility given higher stakes in potential direct patient effects.
Regulatory and professional society recommendations also lack guidance on when or how to resume in-person clinical research operations.3,4 This is reflected in variable responses about timing of reopening and guidance for reopening. A substantial number of survey responders expressed significant fear about returning to work. We did not assess whether fear of COVID-19 exposure would make research staff less likely to approach patients for research participation, though this would provide further justification for creation of robust digital platforms to conduct important research related activity. We also did not assess how patients will perceive enrollment in clinical trials as COVID-19 restrictions are lifted. Finally, this survey was created to ascertain changes to HF research activities in general; however, these findings may not be generalizable to broader cardiovascular research in general.
Conclusions
The COVID-19 pandemic has led to widespread disruption in the daily delivery of health care with a significant impact on HF clinical trials. This study demonstrated that COVID-19 has significantly interrupted HF site-based research activities across the US, staff has limited access to avenues for virtual work, and plans to resume activities are varied. Conversion to virtual platforms, when feasible, would potentially help alleviate many of these issues.
Acknowledgments
Sources of Funding: The CONNECT-HF study is funded by Novartis Pharmaceuticals Corporation (East Hanover, NJ) through an investigator-initiated trial program (CLCZ696BUS05T).
Footnotes
Conflict of Interest Disclosures: Dr. Samsky’s salary is supported by an NIH T32 training grant (HL069749). Dr. Samsky also reports receiving research funding from Boston Scientific. Adam DeVore reports research funding through his institution from the American Heart Association, Amgen, AstraZeneca, Bayer, Intra-Cellular Therapies, American Regent, Inc, the NHLBI, Novartis and PCORI. He also provides consulting services for Amgen, AstraZeneca, Bayer, CareDx, InnaMed, LivaNova, Mardil Medical, Novartis, Procyrion, scPharmaceuticals, and Zoll. Dr. Felker has received research grants from NHLBI, American Heart Association, Amgen, Bayer Merck, Cytokinetics, Myokardia, Bayer, and Roche Diagnostics; he has acted as a consultant to Novartis, Amgen, BMS, Cytokinetics, Medtronic, Cardionomic, V-Wave, Myokardia, Innolife, EBR Systems, Arena, Abbott, Roche Diagnostics, Alnylam, LivaNova, Eidos Therapuetics, Rocket Pharma, Reprieve, and SC Pharma. Dr. Fonarow reports consulting for Abbott, Amgen, AstraZeneca, Bayer, Janssen, Medtronic, Merck, and Novartis. Dr. Allen has received grant funding from AHA, NIH, and PCORI, and consulting fees from Abbott, ACI Clinical, Amgen, Boston Scientific, Cytokinetics, and Novartis. Dr. Albert reports consulting for Novartis.
Footnote: This manuscript was sent to Barry A. Borlaug, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
References
- 1.Solomon MD, McNulty EJ, Rana JS, Leong TK, Lee C, Sung S-H, Ambrosy AP, Sidney S and Go AS. The Covid-19 Pandemic and the Incidence of Acute Myocardial Infarction. N Engl J Med. 2020. May 19. doi: 10.1056/NEJMc2015630. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.DeVore AD, Granger BB, Fonarow GC, Al-Khalidi HR, Albert NM, Lewis EF, Butler J, Piña IL, Heidenreich PA and Allen LA. Care Optimization Through Patient and Hospital Engagement Clinical Trial for Heart Failure: Rationale and design of CONNECT-HF. Am Heart J. 2020;220:41–50. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. FDA guidance on conduct of clinical trials of medical products during COVID-19 pandemic: guidance for industry, investigators, and institutional review boards. Available at: https://www.fda.gov/media/136238/download. Accessed March 28, 2020.
- 4.Bagiella E, Bhatt DL, Gaudino M. The consequences of the COVID-19 pandemic on non-COVID-19 clinical trials. J Am Coll Cardiol. 2020;76:342–345. doi: 10.1016/j.jacc.2020.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]