Abstract
Background:
Although physical activity (PA) has been shown in helping prevent and treat obesity, current PA interventions are still not effective in ameliorating the obesity epidemic. Additional forms of PA need to be investigated to improve PA engagement and outcomes. We hypothesize that pairing a narrative (i.e., story) with an active video game (AVG), a less traditional form of PA, will increase participant engagement in PA. This paper presents the rationale, implementation, and pilot results of a study assessing the effect of narrative’s impact on PA and a series of other health outcomes.
Objective:
This paper presents the rationale, implementation, and pilot results of a study assessing the effect of narrative’s impact on PA and a series of other health outcomes.
Methods/design:
The Active Video Game Study is a six-month randomized controlled single-blind trial projected to include 210 participants. The intervention strategy will pair a narrative to an active video game (AVG). Participants will be randomized into 3 groups: condition A [Narrative + AVG], condition B [AVG Only], and condition C [Control]. Participants will undergo three in-person data collection visits over the course of six months. Inclusion criteria are that children are between the ages of 8–12 and have a BMI ≥ 85%. The primary outcome is change in moderate to vigorous physical activity (MVPA). Secondary outcome measures include change in BMI percentile, fasting insulin and glucose, lipid panel, C-reactive protein, and cognitive function. A pilot trial of n = 6 was conducted to help develop procedures and address problems that could arise in the main trial.
Discussion:
Successful completion of this study will provide the empirical basis for novel intervention and design strategies to enhance the impact of AVGs on long-term MVPA.
1. Introduction
In the United States 37–42% of children are considered overweight or obese [35]. This trend has continued to increase linearly among all ages 2–19 [38]. Childhood obesity can have lifelong implications, including increased risk for cardiovascular disorders, diabetes, and cancer [13]. Interventions need to be developed and tested to reduce or prevent this epidemic.
Physical activity (PA) helps prevent childhood obesity [39]. However, most PA interventions have not achieved long-term effects, with lack of access and motivation being identified as key challenges [31]. Thus, additional forms of PA need to be identified.
One alternative form of PA is Active Video Games (AVGs), or “interactive video or electronic games that feature player movement, such as would occur in ‘real-life’ exercise participation” ([2], p. 597), have been shown to promote moderate-to-vigorous physical activity (MVPA) [43] and prevent health-related risks [34].
While AVGs have been proven to elicit MVPA, previous studies demonstrated mixed results in reducing obesity rates due to lack of habitual AVG use and continued use of sedentary videogames [22]. Furthermore, children’s motivation to play AVGs decreases quickly, ultimately reducing the long-term beneficial effects of AVGs [11]. Thus, in order to increase the sustained efficacy of AVGs, game engagement must also be increased to be on par with sedentary videogames.
An engaging aspect of many sedentary videogames that is often lacking in AVGs is a story line or narrative aspect [e.g., the Halo (Microsoft, Redmond, WA, USA), Assassin’s Creed (Ubisoft, Montreuil, France), and Zelda (Nintendo, Kyoto, Japan) series] [28,29]. Narratives possess unique motivational properties that may encourage increased AVG play [3,24]. In many video game genres, narratives are integral to the overall user rated experience [4].
A previous exploratory study that connected a story to an existing AVG that did not contain a narrative found that 8–12-year-old children in the narrative condition had 40% more measured steps in a single gaming session than their counterparts [25]. Among college students, narratives produced 58% more MVPA than the no narrative condition [17]. AVGs delivered to children have positive effects on PA and relative weight [41] and cognitive functions [5].
The purpose of the proposed research therefore tests the long-term effect of narratives on players’ long-term MVPA levels as the primary outcome. We hypothesize that adding narratives will increase time spent playing AVGs and therefore, increase MVPA. Since PA has a positive influence on children’s cognition as well as their brain structure and function [9] and there is preliminary evidence for the acute effect from AVG on cognition among young adults [17], cognitive functions have been added as the secondary outcome.
2. Methods
Children will be recruited from pediatric obesity and primary care clinics to participate in a 6-month AVG intervention. They will be randomly assigned, with equal probability, to one of three conditions [A: Narrative + AVG] or [B: AVG Only] or [C: wait-list Control] at the first visit using permuted-block randomization Our hypothesis is that Condition (A) will produce more MVPA, better health outcomes, better cognitive function, and better affective evaluation of AVG than condition (B), which is better than condition (C).
2.1. Study design and population
A three-arm intervention study will be implemented to determine if the addition of narrative will increase MVPA. The Narrative+ AVG group will receive an XBox with the intervention narrative animation, Ataraxia (72 episodes in six seasons), and six AVGs during the six-month participation. At the end of each Ataraxia episode, there is a “call to game” screen prompting the child to play the AVG in accordance with the episode’s content. We provide an Xbox with three episodes preloaded for the participants and later will provide additional episodes in batches of threes to them for the rest of the study. The AVG only condition will receive access to the same XBox model and games; however, this group will not receive any Ataraxia episodes. Our control condition will not receive an XBox or the game until after the conclusion of their participation (wait-list control). All three groups will receive usual obesity care from their care providers. After the six-month interventional period, an additional six-month follow-up will be conducted to record the playing time of the participants in all three conditions (Fig. 1). More details about the Ethical approval was obtained from the Northeastern University institutional review board.
Fig. 1.
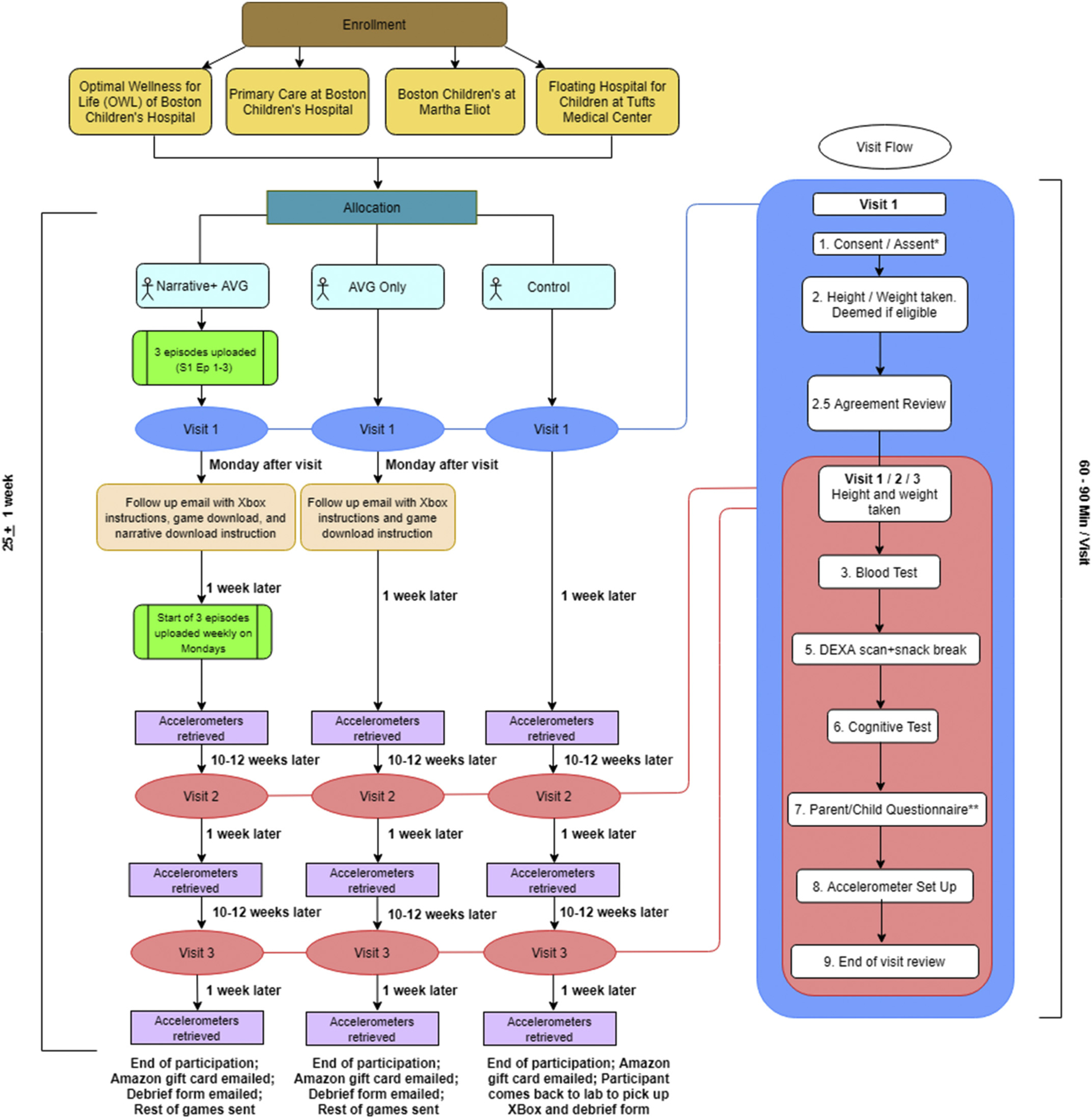
Presents an overview of the overall timeline of participation and an overview of the individual visit components. Participants will undergo three visits in ~25 weeks, depending on schedule adherence. Condition A will receive three episodes uploaded weekly in between visits, and three active video games in between each visit. Condition B will receive three active video games in between each visit. Condition C will receive the games and XBox at the end of their participation. Each of the three visits follow similar procedures; however, visit 1 has the addition of a consent/assent form review and an agreement review.
2.2. Narrative production
Fiction has been recognized as the most popular reading choice among elementary-school-aged children, with around 95% reading fiction [7]. Children have also reported that fiction was easier and more fun to read than other types of stories [32]. Because of the popularity of fiction, it has been integrated with games; however, when a story is undesirable, the amount of game play is diminished [23]. Previous research in our lab identified elements of stories appealing to overweight children, culminating in the creation of Ataraxia, currently paired with our AVG games. The story takes place in a dystopian future, where a twin brother and sister, with the ability to absorb and take away people’s pain, have been kidnapped by an evil dictator to create an army of indestructible soldiers.
Preference tests with 8–12-year-old children rated a dystopian science fiction story as their favorite across all weight, race, and gender groups [27]. Virtuous characters, extraordinary character actions, interesting plots, superpowers, and engaging cliffhangers are narrative characteristics that would help children be more physically active [26]. Using these guidelines, a professional media production company, FableVision Studios, created a six-season animated story called Ataraxia that incorporates six selected AVGs in the plot line. The narrative features a second-person perspective by addressing the children players directly as “you”. The “you” character is created as a gender-neutral character presented as a silhouette to appeal to children of all backgrounds. For example, specific game-related moves, or physical activity requirements, are discussed by the characters as the required actions to advance the plot.
Ataraxia is a science fiction narrative with the backdrop of a post-apocalyptic future. “Your” mother adopted and raised twin babies and later finds out that the twins have special powers. The family tries to keep this secret to protect the twins, but word gets out. An evil dictator abducts the twins to use their genes to develop a super army. “You” must build up physical strength to save your siblings. Throughout the course of the narrative, “you” also start developing superpowers and must train, using AVGs, to hone “your” powers to aid in not only saving the twins, but also saving your planet from various villains throughout the seasons. Throughout the story, various plot points are included to keep children engaged. Each episode is around three minutes long and ends with an instruction to play the AVG in order to physically train for “your” mission.
A total of six AVGs have been selected for this project. They feature upper, whole, and lower body movement, have been rated to be family friendly, and also have been found to be engaging and able to induce MVPA among 8–12-year-old children [16]. Many game-related visual and audio elements have been incorporated by integrating the AVGs into our child preferred story arc.
2.3. Xbox set up
Each participant will receive an XBox One S, a Kinect, and a Kinect adaptor either in the beginning or end of participation depending on their conditions. All Xboxes will be assigned a serial code number, username, and password, unique to each participant. The participants will be informed of their assigned username and password upon reception of the Xbox and will be instructed to only play the active video game using the provided username/password.
The setting on the Xbox will be arranged by the lab to automatically sign users in and remain signed in, in order to ensure the child will play the AVG with the account the lab has access to. Each Xbox also comes with a OneDrive app downloaded by the lab, in which participants in the narrative condition will access the narrative episodes.
2.4. Recruitment
Children will be recruited from the Optimal Wellness for Life (OWL) clinic at Boston Children’s Hospital, primary care at Boston Children’s Hospital, the Martha Eliot Center at Boston Children’s Hospital and the Floating Hospital for Children in Boston at the Tufts Medical Center. Upon reception of recruitment materials, parents will fill out an online questionnaire with contact information and preliminary information regarding their child. Verbal report of child height and weight will be conducted as an initial screening process. (See Fig. 2.)
Fig. 2.
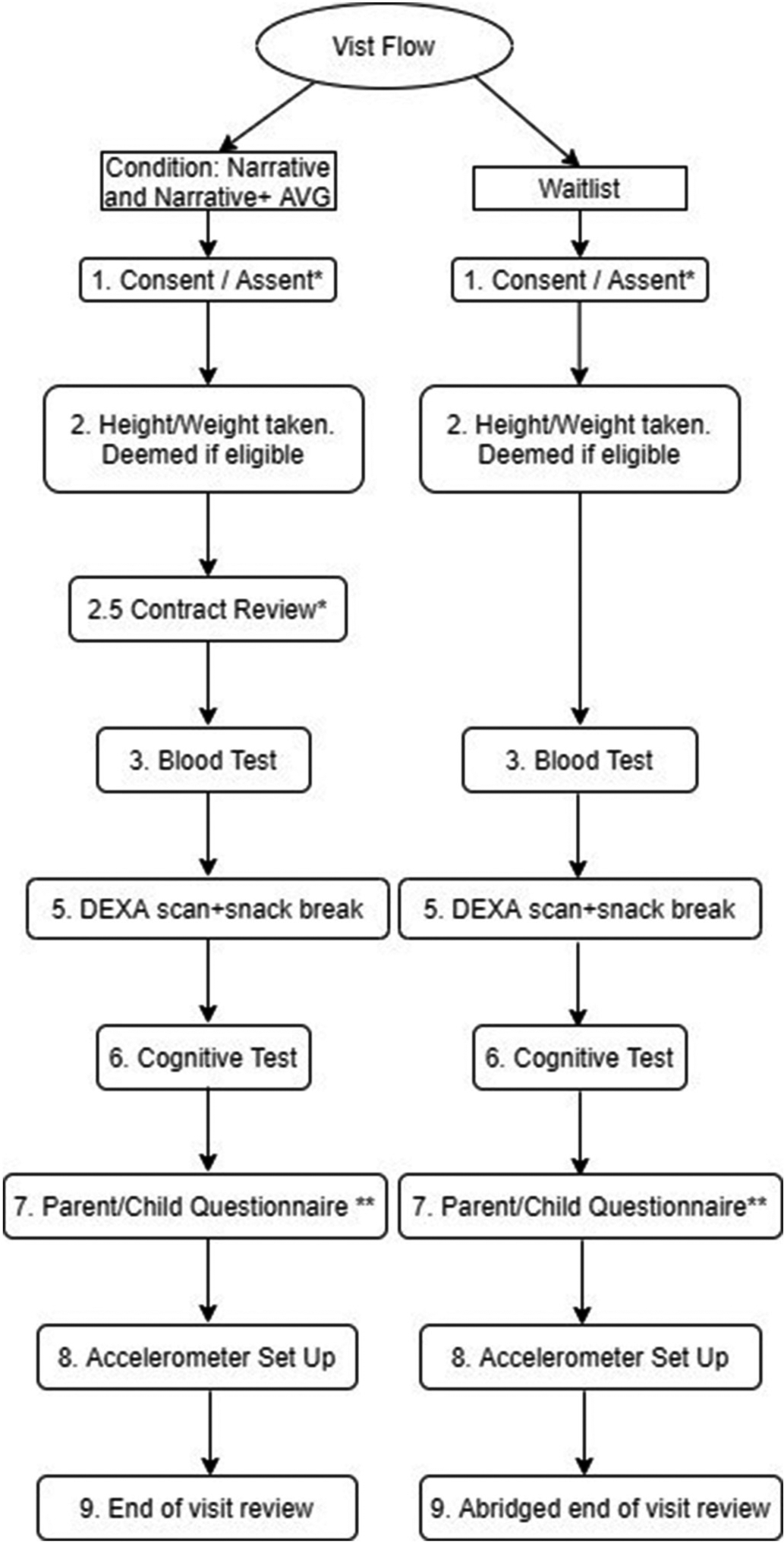
Presents an overview of the first visit flow. A * indicates a step exclusive to visit 1, and a ** indicates a step that is modified for future visits.
2.5. First visit protocol
2.5.1. Initial Intake
A visit record form will be created on RedCap [14] on an iPad using the given participant ID. Before the visit, participants are instructed to fast for 8–12 h, which will be assessed verbally upon entry to the assessment. Children reporting non-fasting will be marked, and their fasting blood result will not be included in analysis. Upon arrival, each participant is assigned to two research assistants (RAs), with at least one gender matched. After both consent and assent forms are reviewed and signed, the RA measures the participant’s height (to the nearest 0.1 cm) using a ShorrBoard (Weight and Measure, LLC, Olney, MD, USA) and weight (to 0.1 kg) using a SECA scale (SECA Inc., Chino, CA, USA) twice and then computes body mass index using the mean (BMI; kg/m2). A third measurement will be taken if there is > 0.2 cm or 0.2 kg difference between the first two measurements. Their height and weight are validated using the Dual-energy X-ray absorptiometry (DEXA) scanner later. BMI-for-age percentiles for both boys and girls are obtained using the CDC growth charts [21]. If the participants’ BMI is 85%tile or higher, speaks and understands English, and is willing and able to complete all measures, they are deemed eligible to continue the study. The exclusion criteria include children who do not speak and understand English, have already played the selected AVG, have vision and hearing problems, have any intellectual disability that will prevent them from providing assent or complete tasks, have medical or physical problems that prevent them from playing AVGs, such as epilepsy or severe asthma or paralysis, or who use orthopedic devices. If the participants do not meet the requirements, they are told they are not eligible to continue and provided with an age-appropriate Lego toy set ($15) as compensation for their time.
2.5.2. Agreement review for compliance
A family agreement will be reviewed with the parent/legal guardian of participants in all conditions. For the Narrative + AVG and AVG Only condition, this agreement will outline Xbox requirements, such as keeping the Xbox in the home for the duration of the trial and only having the participant use the provided account. The child is asked to also sign that he/she will play the provided games for a minimum of 60 min/week. The research team will track gameplay weekly and will send an email reminder to participants who are not meeting game play for 60 min. Participants are also provided with a fridge magnet detailing the time requirements, serving as a reminder to play the active video game. For the control condition, participants are asked to sign an agreement indicating that they will exercise for a minimum of 60 min/week in order to control for the accountability factor the agreement adds. The lab will also send reminder emails to participants in condition C to exercise a minimum of 60 min/week. The number of reminder emails sent to condition C will be the weekly average of the reminder emails sent to Conditions A and B. The lab will send reminder emails to the condition C participants whose BMI% is closest to the participants who received the weekly reminder email in the A and B groups. The purpose of the agreement is to ensure a minimum compliance among the groups.
2.5.3. Blood testing
Participants fast for 8–12 h before the visit, and any reported consumption is documented on the RedCap form. Blood is drawn via venipuncture of a child’s forearm by a certified pediatric phlebotomist. Two tubes, one EDTA tube for plasma and one tube for serum (8.5–10 mL/tube), are collected per child. The EDTA tubes are centrifuged at 4 °C at 2300 RCF (g) for 15 min. For the serum tube, the serum is allowed to clot for 30 min at room temperature prior to centrifugation at 3000 RCF (g) for 15 min. Serum and plasma samples are aliquoted and stored at −80 °C. Insulin and C-reactive protein will be measured by ELISA. Total cholesterol, HDL, LDL, triglycerides and glucose will be measured using standard biochemical assays.
2.5.4. DEXA test
After the blood test, the participant is taken to the Dual-energy X-ray absorptiometry (DEXA) room, where a certified DEXA technician assessed their body mass and composition (G.E. Lunar Corp. Madison, WI, USA) The participants lie on the bed in a supine position on the table centered in the scan field with their arms at their sides, palms down, and thighs separated. Scans are analyzed using the whole-body fan beam method to determine regional (arms, trunk, and legs) and total % body fat, fat mass, fat free mass, and bone mass. Participants are then invited to help themselves to a snack and a cup of water directly outside the lab space.
2.5.5. Cognitive test
Participants are told they are going to play a computer game, and escorted into a secluded room with one RA, where a cognitive test is administered via The Psychology Experiment Building Language (PEBL) [33], consisting of a total of three tests. The three tests assess Sustained Attention (measured by PVT: Perceptual Vigilance Task) (6 min), Working Memory (measured by Match-to-Sample Task)(7 min) and Inhibitory control (measured by Victoria Stroop Test) (5min).
During this time, another RA is informally asking the guardian questions regarding how they heard about the study and if the child has any restrictions preventing him/her from playing the AVGs for at least 60 min per week. Responses are only recorded if they affect the child’s ability to participate in the study (e.g. if the parent reports the child has a lot of after school activities and may not be able to meet the 60-min minimum every week).
2.6. Questionnaires
Both participants and guardians are asked to complete questionnaires electronically, which they do at the same time. The guardian’s questionnaire involves basic background questions, demographic information and the SNAP-IV Children Behavioral Scale [6]. The questionnaire for the child for the first visit involves assessing the child’s physical activity level [20], reward responsiveness [44] and social desirability [37], which may impact the evaluative responses of AVGs, thereby affecting the validity of the study.
2.6.1. Accelerometer setup
After finishing the questionnaires, participants are taken to the accelerometer station, where using the ActiLife software, an ActiGraph GTX3+ waist tracker and an ActiGraph LINK wristwatch are initialized for seven days starting at midnight (00:00 am) of the day of the visit, thus starting around 12 h post visit. Previous studies showed that the selected AVGs induce PA from both upper body and lower body, therefore both wrist and waist measures were adopted [15]. Furthermore, studies have shown a 76% compliance with the waistband, above the NHANES standard, and therefore the additional monitoring should not present a problem in terms of compliance [42]. Both devices will be set to record at a 80 hertz rate [40]. The ActiGraph accelerometers are among the smallest, lightest, and least intrusive in providing accurate PA measurement [1].
Participants are instructed to wear both devices for the full week on their nondominant side, except during water activities, and then mail the accelerometers to the lab in a prepaid package. Participants are given a sheet with information regarding the wear instructions and a sleep log (time they go to and get out of bed, to assist in the accelerometer data processing) and when to return the worn accelerometers. The lab also keeps a copy of the time of expected return and a tracking code associated with the given prepaid package. Participants and guardians are informed they have up to two weeks to mail the accelerometers from the last day worn for them to receive the benefits, $15 Amazon gift card, of returning the accelerometers. The participants are then instructed to choose their own color bands for the accelerometers and instructed to put them on before leaving the lab, to ensure they are properly wearing the devices.
2.6.2. End of visit review
Once the participants are wearing the accelerometers, and if they are in conditions A or B, they are given an XBox, with the associated log-in username and password printed on the box. Condition A is provided with instructions regarding the XBox set up and downloading the provided games, and instructions on accessing the narratives from OneDrive. Condition B is only given Xbox set up and game downloading instructions. A benefit sheet detailing the compensation associated with the trial is reviewed. For conditions A and B, the benefits are demonstrated as a grid with the breakdown of the potential $100 Amazon gift card benefit, should the participants meet the detailed requirements. If the participant is in condition C, they still have the opportunity to earn up to $100 in Amazon gift cards; however, their requirements differ slightly in that they will get the $100 Amazon gift cards as long as they have completed the required visits and measurement protocol.
2.6.3. Post visit follow up
Within two days of their visit, an email detailing the XBox username, password, and playtime instructions is sent to participants in condition A or B to ensure that they have the necessary information if participants cannot locate the username/passcode sticker attached to the package box. Furthermore, the set-up instructions provided on paper in the visit are sent electronically as well.
Once a week on Monday, the lab downloads play time information from the XBox website. If participants have yet to play or download the games, emails, phone calls, and video conferencing will be enlisted to ensure that the participant is not experiencing technical problems. Participants are next contacted on the Tuesday before their following appointment, to schedule that weekend’s visit and confirmed again the Friday before their next visit.
Once data accelerometers are received by the lab, the data will be downloaded using the ActiLife software and will be processed using established protocols that are detailed elsewhere [19]. In short, for the waist-worn device, data will be downloaded in 60 and 15 s epochs using the low filter extension. Time between in bed and out of bed will be removed from the analysis. First, using the 60 s epoch files, wake-nonwear will be identified (20 consecutive minutes of zero counts) [30]. Sleep/wake times are determined based on a sleep log that the participants’ parents will mail back along with the accelerometers. We date the collection for a week starting from the participant’s weekend visit; therefore, a weekend day will always be included in the selection. While analyzing the data, we will choose to purposefully include at least 1 weekend day.
The rest of the time will be analyzed with the 15 s epoch files. Using the Evenson cut points [10], time spent sedentary, in light intensity, moderate intensity, vigorous intensity, and moderate-to vigorous physical activity will be calculated in order to determine a correlational effect between increased MVPA via AVGs, light activity, and sedentary activity outside of the intervention alone. Physical activity data will be considered valid if participants have at least 4 days with ≥10 h of wake-wear. For the wrist-worn device the information from the sleep will be used to determine in bed and out of bed times. The ActiLife software will be used to download the data in 60 s epochs and to process the data. Sleep time, wake after sleep onset, and sleep efficiency will be estimated using the Cole-Kripke algorithm [36].
2.7. Second and third visit protocol
All three visits have similar protocols with some steps excluded from the second and third visit.
2.7.1. Initial Intake
The height and weight of the participant is measured first.
2.7.2. Questionnaires
The parent questionnaire for visits 2 and 3 concerns future travel plans in order to best accommodate future visits and contact information updates should the phone number or address of the parent have changed during the 6-month main trial. All children (A, B and C) are given questionnaires about physical activity and reward responsiveness; those in groups A and B answer a game experience questionnaire [18]; and those in Group A answer a narrative immersion questionnaire [12] that assesses their immersion into the animated story.
2.7.3. End of visit review
The participant is reminded of playtime if they are in condition A or B. If they are in condition C, they are reminded to exercise for 60 min per week if it is their second visit, or if it is their third visit, a time is arranged for the guardian to come in the lab in order to retrieve their XBox after the conclusion of their trial.
2.8. Study completion
Participation is considered complete after the third visit accelerometers are received by the lab. Once a trial is completed, for all conditions, the participants are emailed an Amazon gift card as incentive with the amount they have earned. Gift card eligibility is determined using a Microsoft Excel file detailing dates of the three visits, dates of accelerometer return, amount of valid PA data, and for condition A and B, a “warning agreement” page detailing the number of weeks the participant did not play for at least 60 min per week. If any reminder to play the game is given to a participant, it indicates that the participant did not play the 60 min for that week and is therefore ineligible for the $25 Amazon gift card. Condition C does not have the minimum game play requirement and will therefore receive the $25 gift card if they complete the study. All three conditions will be awarded an additional $15 gift card for every accelerometer returned within two weeks of the expected return date. Also, participants are given $10 Amazon gift card for every in-person visit completed. Participants in conditions A and B will be emailed the debrief form along with the gift card. Condition C will have on extra in person visit to pick up the Xbox console, and will therefore be debriefed in person and the gift card will be emailed. In addition, conditions B and C participants will gain access to all six seasons of Ataraxia for all conditions to receive the same number of benefits at the end of the trial. Thus, all conditions will have access to the same resources as condition A at the end of the six-month period. See Table. 1 for a detailed layout of the Gift Card distribution.
Table 1.
Presents a break-down of the Gift Card distribution for Conditions A, B, and C.
| Visit Number | Coming in for visit | Returning Accelerometers | A/B: 60+ minute game play per week C: Meet the minimum requirements |
|---|---|---|---|
| 1 | $10 Amazon Gift Card | $15 Amazon Gift Card | |
| 2 | $10 Amazon Gift Card | $15 Amazon Gift Card | $25 Amazon Gift Card |
| 3 | $10 Amazon Gift Card | $15 Amazon Gift Card | |
| Total | Up to $100 Amazon Gift Card |
2.9. Statistical analysis
Data will be assessed for normality and homogeneity by Kolmogorov-Smirnov and Levene’s test, respectively. For the main outcomes in this RCT an intention-to-treat analysis will be conducted. Thus, available data on dropouts and participants not compliant to the treatment (AVG or narrative) from all conditions will be analyzed according to the condition they were originally assigned through randomization.
Statistical methods for within- (visit 1, visit 2 and visit 3) and between-groups (conditions A, B and C) comparisons will be applied to test the main hypothesis. Specifically, linear mixed effects models with fixed effects for time, group, and time*group interaction and subject-specific random intercepts and slopes will be fitted. A likelihood ratio test (LRT) will be used to test whether changes in MVPA, BMI percentile, fasting insulin and glucose, lipid panel, C-reactive protein, and cognitive function are linear or nonlinear over time. If the null hypothesis of the LRT is not rejected, indicating that linear time is adequate, time will be modeled using a continuous covariate; if it is rejected, time will be modeled using a categorical covariate.
2.9.1. Sample size and power calculation
Based on the following assumptions, a power and sample size calculation (G*Power 3.1.9.6) was conducted. In our pilot test the dropout rate was between 20% and 33%. Based on a small effect size (d = 0.3) [8] and statistical significance of 0.05 to apply a within-between interaction ANOVA model (three measurements and three groups), we need 171 participants for a power of 95%. Using a 20–30% drop rate, 210 children will be enrolled in the study and assigned to one of the 3 groups via stratified randomization. In this case, the primary goal of the study is to compare the change across time in MVPA in the three groups. Therefore, we are expecting to have between 143 and 170 children for analysis at the end of the intervention (1-β > 90%).
3. Challenges and solutions learned from a pilot trial
The feasibility of the above protocol was tested using a pilot trial of six participants. The steps regarding visit flow were identical; however, participation involved a six-week trial. Participants in condition A and B received one AVG during the trial, and the condition A received one season of 12 Ataraxia episodes, with three episodes uploaded weekly.
Of the six initially recruited pilot participants, one has unexpectedly moved away from the Greater Boston area after the first visit and therefore has been removed from the subsequent report. Four of the remaining five participants completed all three visits and returned all visit accelerometers by either mail or in person. The fifth participant completed two visits and returned both visit accelerometers, but then stopped responding to both phone call and email attempts to schedule a third visit.
While conducting the pilot study, we noted potential challenges and identified potential solutions.
3.1. Challenge one: no shows/cancelations
Participant scheduling proved to be challenging, as some participants would schedule appointments, confirm, then not show up on appointment day. In addition, some participants did not respond to both email and phone attempts to reschedule. The agreement was used to discourage participants from scheduling appointments then not coming in on appointment day. In the agreement, there is a clause stating that dismissal from the study could be due to missing appointments or not responding to our attempts to schedule follow up appointments.
For the main trial, our lab would like to use a standardized scheduling window. of ± 2 weeks 12 weeks after the previous appointment. Participants will be contacted 10 weeks after their previous appointment. If we cannot reach them for the next visit within the next four weeks, they will be dismissed from the study and all materials would need to be returned to the lab.
A sequence of three emails will be sent to the participant’s guardian should the participant book an appointment but does not show up for a given appointment. If the participant writes back that they are interested, we would still reschedule them assuming they are within the ± 2-week timeframe. If the participant is still unresponsive after the third email, no further attempt at communication is made.
3.2. Challenge two: Xbox setup
The at home Xbox set up proved to be technologically difficult for some families. Most issues revolved around not setting up a Wi Fi connection, or confusion regarding game download. To address this, participants are given both electronic and print out instructions with images regarding Xbox set up, game download, and, if in condition A, narrative download. For conditions A and B, the first game is installed by the research team. They are also given the lab email and phone number to contact should they run into technological issues not addressed in the directions.
All technological assistance will be given through email or video conferencing and participants will be instructed to send pictures of their error screen and console setup so the lab could best assist them in addressing the problem.
3.3. Challenge three: game play initiation / adherence
Initiating participant game play proved challenging. Participants presented with either no game play time, or minimal game play time upon the first few days of participation, and then no game play time afterwards. In order to encourage participants across both A and B groups to play the active video games, a bare minimum game play time of 60 min per week was established. Participants and their guardians will be given an agreement stating the bare minimum participation time, and the consequence of not meeting that time being potential termination of participation in the study. The participant will sign that they will play for 60 min/week. In addition, participants will be given a visual reminder in the form of a fridge magnet detailing the ideal daily play time and minimum play time. All visual cues will have similar themes and elements in accordance with the integrated marketing communication principles.
In order to minimize measuring the motivational effect of the agreement instead of the narrative, the lab will consider the difference of mean play time between A and B groups, as opposed to raw mean, thus accounting for any artificial mean increase the 60-min minimum may add. Furthermore, the control condition will also sign an agreement, indicating a 60-min exercise minimum/week. Thus, the motivational effects of the agreement will be accounted for across all groups. An average number of the weekly emails sent to the A and B condition groups will be sent to the C condition groups. The C participants receiving the reminder email will be those that have the closest BMI% to the contacted A and B participants.
3.4. Challenge four: narrative delivery/viewing tracking
Regarding narrative download specifically, the lab cannot remotely check if the condition A participant is watching the uploaded episodes on OneDrive. To address this concern, the participant answers questions about the narrative in a questionnaire in visits two and three, and can self-report “never” for questions regarding story involvement, signaling a lack of engagement with the story.
Participants will also be sent an online questionnaire via parent email at the end of season one, three, and five. The questionnaires have two basic multiple-choice questions to ensure the participant is watching the narrative.
To encourage participants to watch the episodes, a flyer is made with a brief summary regarding the narrative plot and included pictures of the characters and animations.
3.5. Challenge five: accelerometer retrieval
Accelerometer return was originally severely delayed in the pilot. Participants were instructed to mail back the accelerometers in a prepaid UPS box, which has a tracking code sticker on it that the lab also had access to. Out of the six participants, only two successfully mailed back all three accelerometers, whereas the other participants chose to return at least one visit’s accelerometers during a following visit, as opposed to mailing the accelerometers.
To encourage participants to mail back the accelerometers and to reduce the perceived burden, a sentence was added to the accelerometer return sheet highlighting that participants do not need to go to the post office, but rather return the package in a blue mailbox. Furthermore, a qualification was added to the benefits sheet stating that the accelerometers must be returned by mail to the lab within two weeks of the end of wear date in order to receive the associated $15 Amazon gift card.
4. Discussion
Childhood obesity continues to present an increasing problem as the effects result in lifelong implications; however, physical exercise can help mitigate the rising childhood obesity rate and therefore diminish the effects. While traditional forms of exercise have been studied, further investigation is needed to research alternative forms of exercise, such as active video gaming. While active video gaming has been demonstrated to be an effective form of physical exercise [34], children’s engagement with AVGs decreases rapidly [27].
Despite the efficacy of AVGs, there are still challenges that are faced when using such devices. In a constantly developing digital age, the devices that will be used during interventions may be modified during prolonged trial periods. Furthermore, because of the ever-adapting nature of the technological world, the technological literacy of families can present a challenge in self-operating newer devices at home during the trial. In addition, working with families presents an added challenge of accommodating both the parents’ and children’s schedules as the research is reliant on both parties attending every appointment and adhering to the appointment schedule.
Limitations of the current study include limited ability to track physical activity outside of the AVG play time and the potentially PA encouraging effect of the agreement. The accelerometers, measuring steps and therefore physical activity, are only worn for three weeks during the six-month trial; thus, the data may not be representative of the child’s overall physical activity. For example, if the child did increase overall physical activity since the intervention, but fell sick during the week wearing the accelerometer, the child would display reduced physical activity. To address this, the lab will also collect physical activity affinity, which will demonstrate if the child’s desire to participate in physical activity increased but will still not show if the child does participate in more physical activity. In addition, the influence and implementation of the agreement adds an extra motivational aspect that we are not testing. To combat this, the agreement will be implemented across all groups, including the control; however, because the agreement is slightly different between group C and groups A and B, there still may be an agreement motivational effect.
We intend this study to be the first in a series of rigorous systematic inquiries into the behavioral potential of narratives via AVGs for combating childhood obesity and Type 2 Diabetes. Successful completion of this study will provide the empirical basis for novel design strategies to enhance the impact of AVGs on long-term MVPA.
Acknowledgements
This study was supported in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (1R01DK109316, PI: A.S.L.), and by the College of Arts, Media, and Design and Bouvé College of Health Sciences at Northeastern University, Boston, MA.
References
- [1].ActiGraph, ActiGraph Research Database, Retrieved from, 2010. http://www.theactigraph.com/research-database/.
- [2].Bailey BW, McInnis K, Energy cost of exergaming: a comparison of the energy cost of 6 forms of exergaming, Archives of pediatrics & adolescent medicine 165 (7) (2011) 597–602. [DOI] [PubMed] [Google Scholar]
- [3].Baranowski MT, Lu AS, Buday R, Lyons EJ, Schell J, Russoniello C, Stories in games for health: more pros or cons? Games for health journal 2 (5) (2013) 256–263, 10.1089/g4h.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bateman C, Game Writing: Narrative Skills for Videogames, Charles River Media, Boston, MA, 2006. [Google Scholar]
- [5].Best JR, Exergaming in youth: effects on physical and cognitive health, Z. Psychol 221 (2) (2013) 72–78, 10.1027/2151-2604/a000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bussing R, Fernandez M, Harwood M, Hou W, Garvan CW, Eyberg SM, Swanson JM, Parent and teacher SNAP-IV ratings of attention deficit hyperactivity disorder symptoms: psychometric properties and normative ratings from a school district sample, Assessment 15 (3) (2008) 317–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Clark C, Rumbold K, Reading for Pleasure: A Research Overview, National Literacy Trust, 2006. [Google Scholar]
- [8].Cohen J, Statistical Power Analysis for the Behavioral Sciences, Routledge, 2013. [Google Scholar]
- [9].Donnelly JE, Hillman CH, Castelli D, Etnier JL, Lee S, Tomporowski P, Szabo-Reed AN, Physical activity, fitness, cognitive function, and academic achievement in children: a systematic review, Med. Sci. Sports Exerc 48 (6) (2016) 1197–1222, 10.1249/mss.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG, Calibration of two objective measures of physical activity for children, J. Sports Sci 26 (14) (2008) 1557–1565, 10.1080/02640410802334196. [DOI] [PubMed] [Google Scholar]
- [11].Graves LE, Ridgers ND, Atkinson G, Stratton G, The effect of active video gaming on children’s physical activity, behavior preferences and body composition, Pediatr. Exerc. Sci 22 (4) (2010) 535–546. [DOI] [PubMed] [Google Scholar]
- [12].Green MC, Brock TC, The role of transportation in the persuasiveness of public narratives, J. Pers. Soc. Psychol 79 (5) (2000) 701–721. [DOI] [PubMed] [Google Scholar]
- [13].Han JC, Lawlor DA, Kimm SY, Childhood obesity, Lancet 375 (9727) (2010) 1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform 42 (2) (2009) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hwang J, Fernandez A, Lu A, Application and validation of activity Monitors’ epoch lengths and placement sites for physical activity assessment in Exergaming, J. Clin. Med 7 (9) (2018) 268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hwang J, Lee IM, Fernandez AM, Hillman CH, Lu AS, Exploring energy expenditure and body movement of exergaming in children of different weight status, Pediatr. Exerc. Sci (2019) 1–10, 10.1123/pes.2019-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hwang J, Lu AS, Narrative and active video game in separate and additive effects of physical activity and cognitive function among young adults, Sci. Rep 8 (1) (2018) 11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].IJsselsteijn W, De Kort Y, Poels K, The Game Experience Questionnaire, Technische Universiteit Eindhoven, Eindhoven, 2013, pp. 3–9. [Google Scholar]
- [19].Katzmarzyk PT, Barreira TV, Broyles ST, Champagne CM, Chaput J-P, Fogelholm M, Church TS, The international study of childhood obesity, lifestyle and the environment (ISCOLE): design and methods, BMC Public Health 13 (1) (2013) 900, , 10.1186/1471-2458-13-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kowalski KC, Crocker PR, Donen RM, The Physical Activity Questionnaire for Older Children (PAQ-C) and Adolescents (PAQ-A) Manual College of Kinesiology, vol. 87, University of Saskatchewan, 2004, pp. 1), 1–38. [Google Scholar]
- [21].Kuczmarski RJ, CDC Growth Charts, (2000) (United States: ). [PubMed] [Google Scholar]
- [22].LeBlanc AG, Chaput J-P, McFarlane A, Colley RC, Thivel D, Biddle SJ, Tremblay MS, Active video games and health indicators in children and youth: a systematic review, PLoS One 8 (6) (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lebowitz J, Klug C, Interactive Storytelling for Video Games: A Player-Centered Approach to Creating Memorable Characters and Stories, Taylor & Francis, 2011. [Google Scholar]
- [24].Lu AS, Narrative in exergames: thoughts on procedure, mechanism, and others, Games for health journal 4 (1) (2015) 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lu AS, Baranowski T, Hong SL, Buday R, Thompson D, Beltran A, … Chen T-A, The narrative impact of active video games on physical activity among children: a feasibility study, J. Med. Internet Res 18 (10) (2016) e272, 10.2196/jmir.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lu AS, Buday R, Thompson D, Baranowski T, What type of narrative do children prefer in active video games? An exploratory study of cognitive and emotional responses, in: Tettegah S, Huang W-H (Eds.), Emotions, Technology, and Digital Games, Elsevier Publications, London, 2016. [Google Scholar]
- [27].Lu AS, Green MC, Thompson D, Using narrative game design to increase Children’s physical activity: exploratory thematic analysis, JMIR serious games 7 (4) (2019) e16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lu AS, Kharrazi H, A state-of-the-art systematic content analysis of games for health, Games for health journal 7 (1) (2018) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lu AS, Kharrazi H, Gharghabi F, Thompson D, A systematic review of health videogames on childhood obesity prevention and intervention, Games for health journal 2 (3) (2013) 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Mark AE, Janssen I, Dose-response relation between physical activity and blood pressure in youth, Med. Sci. Sports Exerc 40 (6) (2008) 1007–1012, 10.1249/MSS.0b013e318169032d. [DOI] [PubMed] [Google Scholar]
- [31].Metcalf B, Henley W, Wilkin T, Effectiveness of intervention on physical activity of children: systematic review and meta-analysis of controlled trials with objectively measured outcomes (EarlyBird 54), BMJ : British Medical Journal 345 (2012). [DOI] [PubMed] [Google Scholar]
- [32].Moyer JE, Learning from leisure reading, Ref. User Serv. Q 46 (4) (2007) 66–79. [Google Scholar]
- [33].Mueller ST, Piper BJ, The psychology experiment building language (PEBL) and PEBL test battery, J. Neurosci. Methods 222 (2014) 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].O’Donovan C, Hussey J, Active video games as a form of exercise and the effect of gaming experience: a preliminary study in healthy young adults, Physiotherapy 98 (3) (2012) 205–210, 10.1016/j.physio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- [35].Ogden CL, Carroll MD, Kit BK, Flegal KM, Prevalence of childhood and adult obesity in the United States, 2011–2012, JAMA 311 (8) (2014) 806–814, 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Quante M, Kaplan ER, Cailler M, Rueschman M, Wang R, Weng J, Redline S, Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms, Nat Sci Sleep 10 (2018) 13–20, 10.2147/nss.S151085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Reynolds CR, Paget KD, National normative and reliability data for the revised Children’s manifest anxiety scale, Sch. Psychol. Rev 12 (1983) 324–336. [Google Scholar]
- [38].Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC, Prevalence of obesity and severe obesity in US children, 1999–2016, Pediatrics 141 (3) (2018), 10.1542/peds.2017-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Thivel D, O’Malley G, Aucouturier J, Exercise and Childhood Obesity Pediatric Obesity, Springer, 2018, pp. 569–587. [Google Scholar]
- [40].Troiano RP, McClain JJ, Brychta RJ, Chen KY, Evolution of accelerometer methods for physical activity research, Br. J. Sports Med 48 (13) (2014) 1019–1023, 10.1136/bjsports-2014-093546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Trost SG, Sundal D, Foster GD, Lent MR, Vojta D, Effects of a pediatric weight management program with and without active video games: a randomized trial, JAMA Pediatr. 168 (5) (2014) 407–413. [DOI] [PubMed] [Google Scholar]
- [42].Tudor-Locke C, Barreira TV, Schuna JM, Mire EF, Chaput J-P, Fogelholm M, Lambert EV, Improving wear time compliance with a 24-hour waist-worn accelerometer protocol in the international study of childhood obesity, lifestyle and the environment (ISCOLE), Int. J. Behav. Nutr. Phys. Act 12 (2015) 1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].van’t Riet J, Crutzen R, Lu AS, How effective are active videogames among the young and the old? Adding meta-analyses to two recent systematic reviews, Games for health journal 3 (5) (2014) 311–318, 10.1089/g4h.2014.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Van den Berg I, Franken IH, Muris P, A new scale for measuring reward responsiveness, Front. Psychol 1 (2010) 239. [DOI] [PMC free article] [PubMed] [Google Scholar]


