Dear Editor,
Swallowing is a complex sensorimotor process, which involves precise temporal coordination of the upper and lower lips, tongue, and pharyngeal and esophageal musculatures. This integrated process is controlled by neural interplay across cortical and subcortical networks. Although numerous functional brain imaging studies have suggested means to measure post-stroke pathological changes in the cortical control region of swallowing [1], identifying the precise functional localization of the motor area for swallowing has been challenging. This complexity is partly due to the extensive cortical network that executes the swallowing function [2]. Transcranial magnetic stimulation (TMS) has been proposed and used as a powerful tool for functional localization of swallowing function [3]. However, because pharyngeal motor cortex (M1) representation of the intrinsic swallowing muscles is overlaid by those of the tongue and jaw in the somatotopic organization of the motor cortex, localization of swallowing area is substantially more challenging than the standard motor mapping of the hand area. This makes the administering of the non-invasive stimulation to relevant swallowing areas as well as the recording of the elicited muscle responses particularly challenging.
As a solution, we propose that surface electromyography (sEMG) recordings of lip orbicularis oris (OO) muscle activities elicited by a functional MRI (fMRI)-guided neuronavigated TMS (nTMS) technique would aid in the detection of the submental complex (SMC) muscles' motor evoked potentials (MEPs) for localization of the SMC target in pharyngeal M1. We hypothesized that the lip OO target as a control target, could readily assist us in determining the SMC target (the candidate target). This hypothesis was made based on the previous studies in which the OO muscle was localized in the motor cortex during lip-pursing tasks using the rTMS procedure [4]. The OO muscle is an important orofacial muscle for initiating the swallowing function by creating and maintaining an adequate labial seal that prevents foods or liquids from leaking out of the mouth during mastication and the swallowing reflex. In addition, it plays critical roles in speech articulation and facial expressions. Therefore, mapping the lip motor area would not only provide an efficient means to precisely determine the SMC targeting intervention for rehabilitation of dysphagia, but also gives insight into therapeutic techniques for restoring lip functions in speech articulation and other lip muscle activities.
Three healthy adult volunteer subjects (age = 32.0 years; 1 female and 2 males) participated in this pilot study. Each subject participated in three study visits including the fMIR scan session, the TMS MEPs session, and the continuous theta burst transcranial magnetic stimulation (cTBS) session (please see Supplemental Materials for details) at the Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital (MGH). For the validation of the central swallowing control, fMRI scans were obtained from each subject during a lip-pursing task and swallowing movement. According to the TMS standard safety guidelines, participants with a history of seizures were not recruited for the study. In addition, none of the participants had intracranial metal clips, cardiac pacemakers, metal implants, claustrophobia, or were pregnant. The Institutional Review Board at MGH approved the study and all subjects gave written informed consent.
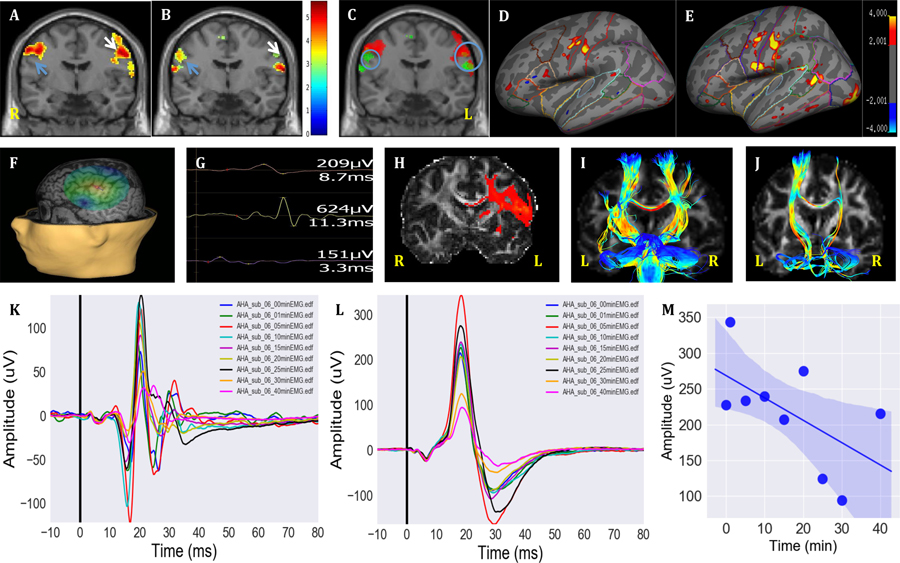
Figures 1A–B represent the localization of the lip and swallowing activation areas with a group analysis of three subjects. Figure 1C illustrates the overlay spots of both localizations. Figures 1D–E represent the lip and swallowing activation surface maps of one subject’s surface brain in which the ‘virtual lesion’ model was applied. For this subject, the individual SMC target was identified by fMRI-guided nTMS (Figures 1F and 1G). Following cTBS to the swallowing representation in the left M1, the inhibitory effect was investigated through a cortical excitability assessment at multiple time points (Figures 1K and 1L). Multiple comparisons indicated that the lip OO and SMC MEP amplitudes were significantly decreased at 15 min (Figures 1K and 1L, p = 0.005, in reference to baseline). The OO and SMC MEP amplitudes were also significantly different across time points (p < 0.001). The OO and SMC MEP amplitudes showed significant changes at 40 min (p < 0.001) compared to the baseline (Figures 1M). The precise swallowing target of central structural connectivity was confirmed by fiber tractography (Figure 1H). Fiber tracts (corpus callosum) in the left pharyngeal M1 pass through the right pharyngeal M1 to bilateral pons (Figure 1I). Fiber tracts (corticobulbar tract) in the left pharyngeal M1 pass through the ipsilateral pons (Figure 1J). We further applied the left overlaid activations of lip pursing and swallowing movement as regions of interest (ROIs) to trace to the contralateral pons. Tractography results demonstrate the overlap between the descending lip corticofugal and pharyngeal corticofugal pathways (Supplemental Materials, Figure 2). There were no significant differences in the latencies of the OO or SMC MEPs across time points. The subjects did not report side effects during or after the experiment.
Figure 1.
(A) Representative lip pursing fMRI activations from a three-subject group analysis. Lip motion-related changes in BOLD signals were found in the precentral gyrus (M1) (blue arrow indicates right hemisphere M1 and white arrow indicates left hemisphere M1). (B) Swallowing fMRI activations from the same three subject group analysis. BOLD activations displayed swallowing motion-related activity in the precentral gyrus (M1) (blue arrow indicates right hemisphere M1 and white arrow indicates left hemisphere M1). (C) The overlaid activations of lips motion (red) vs. swallowing movement (green) in different M1 regions (small blue circle indicates right hemisphere overlaying spot and big blue circle indicates left hemisphere overlaying spot). (D) Representative activation of the lip pursing task shown on an inflated representation of the left cerebral cortex from one subject. (E) Representative activations of the swallowing task shown on an inflated representation of the left cerebral cortex from the same subject in D. (F) The swallowing task fMRI-guided target transformed to the Nexstim navigation system with the same MRI coordinates (−56, −8, 44,) in the left hemisphere representing the same subject shown in E. The magnitude of the E-field is shown on a color scale on top of the cortical surface with the same subject. The surface rendering of the Nexstim NBS navigation system, in which the activations are computed onto a spherical surface that is painted on the “peeled” MRI. (G) The surface EMG recording of lip OO MEPs and SMC MEP when searching around OO ‘hot spot’ to determine SMC ‘hot spot’ from the same representative subject. The upper wave indicates indirect lip OO MEP; the middle wave indicates indirect swallowing SMC MEP; and the lower wave indicates direct lip OO MEP. (H) Coronal view of diffusion tractography of the left pharyngeal M1 to ipsilateral pons. (I) Bilateral fiber tracts (corticobulbar tract, CBT) in the pharyngeal M1 passing thought the pons. (J) CBT in the left pharyngeal M1 passing through the left pons. Representative fMRI-guided nTMS ‘virtual lesion’ model of lip OO MEPs (K) and fMRI-guided nTMS ‘virtual lesion’ model of SMC MEPs (L) obtained from the ssame subject. Cortical excitability at baseline (pre-cTBS) and post-cTBS at 1, 5, 10, 15, 20, 25, 30, and 40 min. Average of 15 trials at each time point with average values plotted on a single graph. Suppression was not induced immediately after cTBS; however, the significant inhibitory effect occurred at 40 min post-cTBS (p = 0.03739, paired t- test). The latency of MEPs around 9.99 ± 3.9 ms in each time point. (M) Linear regression showing significant decline before and after cTBS over time (r = −0.66, p = 0.005).
In the proposed approach, we targeted the pharyngeal motor cortex with the assistance of individual fMRI-guided nTMS and control targeting of lips. Our results provided supporting evidence to establish a causal relationship between the predominant fMRI guided nTMS identified central swallowing activation and inhibitory effect of cTBS on SMC targeting using a ‘virtual lesion’ model.
Our first goal was to combine multi-modal non-invasive neuroimaging techniques to detect the central swallowing localization considering the SMC as the candidate targeting muscle and the lip OO as the control targeting muscle. In contrast to Kothari’s study [5] who did not obtain reliable measures of the surface EMG to assess SMC, our results demonstrated potential benefit of using surface EMG to assess swallowing musculature function under the premise of identifying the precise targeting muscle. We suggest that concurrent application of task-based fMRI and the navigation system rather than the traditional TMS, can particularly guide the precise detection of the candidate musculature. The fMRI and nTMS cortical mapping typically show slight discrepancies in the exact loci of motor areas [6]. One reason pertains to the fact that the BOLD fMRI signal reflects the hemodynamic responses elicited by relatively complex (voluntary) movements of the muscles and therefore tends to produce more widespread patterns of activity. However, TMS directly activates the cortical muscle representations, and the location of the ‘hot spot’ is inferred through the analysis of the EMG responses. Therefore, a combined fMRI and nTMS approach would provide a more comprehensive and reliable mapping of motor functions in terms of spatial localization and functional specificity.
Our findings suggest that the control targeting of lips OO could readily assist detection of the SMC target with surface electrodes. To limit the anatomical search volume, we first mapped the motor area for a robustly represented nearby muscle group (i.e., the lip OO muscle) during a lip-pursing task. We then used this information to assist our identification of the SMC target. Therefore, our proposed technique provides a novel and non-invasive approach to detect a deep muscle complex through targeting a superficial single musculature. Currently, the intraluminal pharyngeal electromyographic measurements are popularly used to detect the central swallowing excitability due to the potential reliability [7]. A recent study reported that eliciting pharyngeal MEPs is more difficult than those of other muscles [8]. Our pilot study, however, suggested that sEMG recordings of lip OO muscle activities through fMRI-guided nTMS could aid in the detection of the SMC muscles’ MEPs for localization of the SMC target in M1. This finding is consistent with fMRI task activations since both lip pursing and swallowing motion have overlapping activations in M1. OO muscles as the control target, might also promote the identification of deep masseter or tongue muscle targets. Nevertheless, further studies with larger sample size are warranted to substantiate these findings.
Finally, our assumption was verified by ‘virtual lesion’ model and further confirmed by central structural connectivity using fiber tractography. Repetitive TMS (rTMS) is commonly used to investigate causal relationships between regional brain function and behavior by inducing ‘virtual lesions’ when normal brain function is temporarily and reversibly disrupted in focal regions [9]. The ‘virtual lesion’ method provides a unique and safe approach to measure causal relationships between behavior or cortical responses in both healthy and disease states. In previous studies, cTBS rapidly suppressed human primary motor cortex excitability for a maximum of 60 min [10]. The effect of direct cortical stimulation on OO muscles has been studied using TMS. In accordance with previous studies, the current findings indicated that cTBS can effectively inhibit lip and pharyngeal motor cortex excitability. Our lip-pursing/swallowing task fMRI identified anatomic regions that are active during lip pursing/swallowing, including the primary sensory and motor cortex, and the supplementary motor area. We speculate that after suppression of OO muscle/SMC motor cortex excitability, the balance between excitatory and inhibitory influences was broken. Thus, the precise cortical lip OO target (the control target) and SMC target (the candidate target) were further verified temporally through the changes in the inhibitory effects. A causal relationship was established after a reversible, short-term ‘virtual lesion’ that produced detectable changes near the 40 min break in our study. Interestingly, through the lip OO target control, simultaneous suprahyoid EMG responses were detected, and vice versa. Based on this observation, once we confirmed the lip OO target, we searched nearby areas and readily determined the SMC hotspot without an invasive administration. Using a combination of multi-modal non-invasive approaches, we mapped the precise cortical location for central swallowing. The findings revealed that the surface EMG is a safe and non-invasive technique with the capability to define the pharyngeal motor cortex. One of the strength points of this pilot study lies in the fact that we verified the localizer in combination with DTI, which allowed us to examine all aspects of functional and structural component of swallowing musculature.
Limitations associated with this preliminary report include small sample size and lack of swallowing assessment before and after cTBS. More studies with larger sample sizes are needed to examine the reproducibility and feasibility of the proposed approach.
In summary, our findings provide supporting evidence for the precise and reliable targeting of the pharyngeal motor cortex through a combined approach of individual fMRI-guided nTMS, lip control targeting, and diffusion tensor image (DTI). The functional patterns of swallowing, including localization of central activation and interconnectivity of the regions in each hemisphere, are demonstrated using multi-modal neuroimaging of different motion tasks (i.e., lip pursing and swallowing). This multi-modal neuroimaging could enable visualization of the neural biomarker specific to central swallowing, and thus help the development of effective clinical treatment strategies for neurogenic swallowing disorders.
Supplementary Material
Acknowledgments
Funding sources
Funding support for Dr. Shasha Li was received from the American Heart Association Postdoctoral Fellowship Award (17POST32530004) and the National Institute On Deafness and Other Communication Disorders of the National Institutes of Health (NIH) under award number K23DC018022. This work was also supported by NIH awards R01MH111829 and R01DC016915. Dr. Joutsa was funded by the Academy of Finland, Finnish Medical Foundation, and Orion Research Foundation.
Footnotes
Declarations of conflicts of interest
The authors report no conflicts of interest.
Contributor Information
Shasha Li, Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown MA, USA, Harvard Medical School, Boston, MA, USA.
Marziye Eshghi, Speech and Feeding Disorders Lab, MGH Institute of Health Professions, Charlestown, MA, USA.
Sheraz Khan, Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown MA, USA, Harvard Medical School, Boston, MA, USA.
Qiyuan Tian, Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown MA, USA, Harvard Medical School, Boston, MA, USA.
Juho Joutsa, Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown MA, USA, Harvard Medical School, Boston, MA, USA, Turku Brain and Mind Center and Clinical Neurosciences, University of Turku, Turku, Finland, Division of Clinical Neurosciences and Turku PET Centre, Turku University Hospital, Turku, Finland.
Yangming Ou, Department of Radiology and Pediatrics, Boston Children’s Hospitalm, Boston, MA, USA, Harvard Medical School, Boston, MA, USA, Computational Health Informatics Program (CHIP), Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA.
Qing Mei Wang, Stroke Biological Recovery Laboratory, Spaulding Rehabilitation Hospital, the teaching affiliate of Harvard Medical School, Charlestown, MA, USA.
Jian Kong, Department of Psychiatry, Massachusetts General Hospital, Charlestown MA, USA, Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown MA, USA, Harvard Medical School, Boston, MA, USA.
Bruce Robert Rosen, Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown MA, USA, Harvard Medical School, Boston, MA, USA, Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, USA.
Jyrki Ahveninen, Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown MA, USA, Harvard Medical School, Boston, MA, USA.
Aapo Nummenmaa, Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown MA, USA, Harvard Medical School, Boston, MA, USA.
References
- 1.Lima MS de, Mangilli LD, Sassi FC, Andrade CRF de. Functional magnetic resonance and swallowing: critical literature review. Braz J Otorhinolaryngol. 2015. December;81(6):671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kern MK, Birn R, Jaradeh S, Jesmanowicz A, Cox R, Hyde J, et al. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol. 2001. April;280(4):G531–538. [DOI] [PubMed] [Google Scholar]
- 3.Macrae PR, Jones RD, Huckabee M-L. The effect of swallowing treatments on corticobulbar excitability: A review of transcranial magnetic stimulation induced motor evoked potentials. J Neurosci Methods. 2014. August;233:89–98. [DOI] [PubMed] [Google Scholar]
- 4.Möttönen R, Rogers J, Watkins KE. Stimulating the lip motor cortex with transcranial magnetic stimulation. J Vis Exp JoVE. 2014. June 14;(88). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kothari M, Stubbs PW, Pedersen AR, Jensen J, Nielsen JF. Reliability of surface electromyography measurements from the suprahyoid muscle complex. J Oral Rehabil. 2017. September;44(9):683–90. [DOI] [PubMed] [Google Scholar]
- 6.Najib U, Bashir S, Edwards D, Rotenberg A, Pascual-Leone A. Transcranial brain stimulation: Clinical applications and future directions. Neurosurg Clin N Am. 2011. April;22(2):233–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michou E, Mistry S, Jefferson S, Singh S, Rothwell J, Hamdy S. Targeting unlesioned pharyngeal motor cortex improves swallowing in healthy individuals and after dysphagic stroke. Gastroenterology. 2012. January;142(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Lin T, Li X, Jing Y, Wu C, Li M, et al. TMS brain mapping of the pharyngeal cortical representation in healthy subjects. Brain Stimulat. 2020. May;13(3):891–9. [DOI] [PubMed] [Google Scholar]
- 9.Silvanto J, Cattaneo Z. Common framework for “virtual lesion” and state-dependent TMS: The facilitatory/suppressive range model of online TMS effects on behavior. Brain Cogn. 2017. December;119:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y-Z, Sommer M, Thickbroom G, Hamada M, Pascual-Leonne A, Paulus W, et al. Consensus: New methodologies for brain stimulation. Brain Stimulat. 2009. January;2(1):2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.