Abstract
Current anabolic drugs to treat osteoporosis and other disorders of low bone mass all have important limitations in terms of toxicity, contraindications, or poor efficacy in certain contexts. Addressing these limitations will require a better understanding of the molecular pathways, such as the mitogen activated protein kinase (MAPK) pathways, that govern osteoblast differentiation and, thereby, skeletal mineralization. Whereas MAP3Ks functioning in the extracellular signal-regulated kinases (ERK) and p38 pathways have been identified in osteoblasts, MAP3Ks mediating proximal activation of the c-Jun N-terminal kinase (JNK) pathway have yet to be identified. Here, we demonstrate that thousand-and-one kinase 3 (TAOK3, MAP3K18) functions as an upstream activator of the JNK pathway in osteoblasts both in vitro and in vivo. Taok3-deficient osteoblasts displayed defective JNK pathway activation and a marked decrease in osteoblast differentiation markers and defective mineralization, which was also confirmed using TAOK3 deficient osteoblasts derived from human MSCs. Additionally, reduced expression of Taok3 in a murine model resulted in osteopenia that phenocopies aspects of the Jnk1-associated skeletal phenotype such as occipital hypomineralization. Thus, in vitro and in vivo evidence supports TAOK3 as a proximal activator of the JNK pathway in osteoblasts that plays a critical role in skeletal mineralization.
Keywords: TAOK3, JNK, MAPK, osteoblasts, bone
Introduction
Mitogen activated protein kinases (MAPKs) have emerged as one of the most critical regulators of osteoblast differentiation and skeletal mineralization with relevance to both skeletal dysplasia and regulation of adult bone mass [1,2]. For instance, overactivation of ERK and other pathways downstream of neurofibromatosis type 1 (NF1) loss-of-function contributes to skeletal fragility and impaired fracture healing in NF1 [3-5]. MAPKs, including the three best studied pathways, the ERK, JNK and p38 pathways, overall, largely play an essential role in promoting osteoblast differentiation. Both ERK and p38 are required for activation of Runx2, a master transcription factor involved in osteoblast specification and differentiation, and defects in the ERK or p38 pathways result in a blockade in early osteoblast differentiation and hypomineralization [6-9]. The JNK pathway is critical in late-stage osteoblast differentiation, and defects in the JNK pathway in mice lead to a signature occipital hypomineralization and osteopenia that is particularly prominent early in life [10,11]. Thus, MAPK pathways are key regulators of osteoblast differentiation and clarifying the molecular basis of MAPK activation is likely to reveal both therapeutic and pathologic means of regulating osteoblast activity.
Three cascade-connected kinases, MAP kinase kinase kinases (MAP3K), MAP kinase kinases (MAP2K) and MAPK, comprise the major components of the MAPK signaling pathways [12]. Of these, identifying the proximal MAP3Ks involved with pathway activation is particularly of interest. Whereas MAP2K activation of MAPKs tend to be wired in a somewhat tissue invariant manner, the connection between MAP3Ks and both their upstream activators and downstream MAP2Ks appears to be highly cell type and stimulus specific, thereby conferring context specificity to signaling. Previously we had identified upstream MAP3K regulators of ERK and p38 in osteoblasts [7,13]. However, the MAP3K functioning to activate the JNK pathway in osteoblasts remains to be elucidated.
TAOK3 is a MAP3K belonging to the serine/threonine-kinase STE20 family[14]. Similar to many other kinases in STE20 family, TAOK3 functions as a critical mediator in response to various stimuli. Through inhibition of p38 activation, TAOK3 reacts to genotoxic stimuli as a DNA damage response mediator [15]. In the neural system, TAOK3 controls downstream JNK activation and modulates ethanol sensitivity [16]. However, the function of TAOK3 in different other physiologic contexts remains largely unknown. In addition, previous in vitro findings offer conflicting data about whether TAOK3 activates or suppresses downstream MAPK pathway activation [14,17,18].
Here, we examined osteoblasts and the overall skeletal phenotype of a Taok3-deficient murine model (hereafter referred to Taok3−/− mice). In vitro, TAOK3 functions as a MAP3K mediating JNK activation during osteoblast differentiation. Consistent with this, Taok3−/− mice display signature defects also seen in Jnk1−/− mice, including early onset osteopenia and occipital hypomineralization. These findings nominate TAOK3 as a critical proximal mediator of JNK activation in osteoblasts that contributes to both osteoblast differentiation and skeletal mineralization.
Materials and methods
Animals
Taok3−/− mice were produced as previously described[19]. A genetrap cassette containing a strong acceptor site was inserted into the first exon of the Taok3 gene, leading to premature transplational termination and absence of TAOK3 protein. This splice acceptor site is flanked by loxP sites to permit reversal of the genetrap allele in the presence of cre recombinase, restoring normal TAOK3 protein expression. Mice displaying germline transmission were backcrossed with C57BL/6 mice for 10 generations and maintained on a C57BL/6 background. Mice were housed, bred in a barrier facility, fed ad libitum chow, and followed a standard day-night lighting cycle. All animal experiments were conducted in compliance with approved protocols by the Weill Cornell Medical College animal care committee (IACUC).
Isolation of mouse calvarial osteoblasts, cell culture and differentiation assays
Mouse calvarial osteoblast (COBs) isolation, in vitro COB and human mesenchymal stromal cells (hMSCs, LONZA) culture, and Von Kossa staining were performed as previously described [7,10]. hMSCs used for differentiation assay were transfected with a vector control or human TAOK3 (hTAOK3) shRNA-bearing lentivirus to silence TAOK3. The lentiviral vector encoding hTAOK3 shRNA was purchased from Sigma-Aldrich using the Mission shRNA library.
In vitro osteoblast gene trap allele reversal
The gene trap allele was reversed through cre-mediated excision of the gene trap cassette. Briefly, Taok3−/− COB precursors were transduced with cre-recombinase or vector expressing lentivirus and were then treated with puromycin after 48 hours of infection to screen for successfully transduced cells. Cells expressing cre then underwent excision of the gene trap cassette.
Quantitative PCR and Western Blot analysis
Quantitative polymerase chain reaction (qPCR) and Western Blotting (WB) were performed as previously described[6,20]. Primary antibodies specific to Taok3 (Millipore), Gapdh (Affinity Bioreagents), Hsp90 (Millipore), Phospho-Sapk/Jnk (Cell Signaling) and Phospho-Erk (Cell Signaling) were used for immunoblotting. Ultraviolet light was used for stimulation. The primers used for qPCR are listed in Table. S1. Samples were assessed for differences in the transcript levels of osteoblast markers.
Micro-CT imaging and data analysis
Micro-computed tomography (μCT) was conducted using a Scanco Medical Micro-CT 35 system (Scanco Medical, Switzerland). Femurs and skulls were harvested and scanned at 7 and 20 micrometer resolution, respectively. For analysis of femoral bone mass, a region of trabecular bone 2.1mm wide was contoured, starting 280 microns from the proximal end of the distal femoral growth plate. Femoral trabecular bone was thresholded at 211 permille. Femoral cortical bone was thresholded at 350 permille. Calvarium was thresholded at 260 permille. A Gaussian noise filter optimized for murine bone was applied to reduce noise in the thresholded 2-dimensional image. Cortical and trabecular thickness (Cort. Th, Tb. Th), trabecular number (Tb. N), trabecular spacing (Tb. Sp) and bone volume/total volume (BV/TV) were calculated by contouring the corresponding regions of the scan slices. 3D reconstruction images were generated by stacking the contours of 2D images. μCT analysis was conducted by an individual blinded to the genotype of each mouse.
Statistical analysis
All statistical calculations and graphs were created using GraphPad Prism. The results shown were reported as mean ± standard deviation (SD). When applicable, Student’s t-test or One-Way analysis of variance (ANOVA), followed by Tukey’s test, were used to evaluate the statistical significance. Statistical significance was accepted as a p-value less than 0.05.
Results
Taok3 is required for osteoblast differentiation in vitro.
To evaluate the function of TAOK3 in osteoblasts, mice bearing a homozygous gene trap-mediated loss-of-function Taok3 allele were examined [21]. To validate the efficacy of the Taok3 gene trap allele, calvarial osteoblasts (COBs) from homozygous Taok3 gene trap mice (hereafter, Taok3−/− mice) were subjected to qPCR. COBs from Taok3−/− mice showed a marked reduction in Taok3 expression after either 7 or 14 days of culture (Fig. 1A). Taok3-deficient COBs displayed impaired mineralization capacity as shown by Von Kossa staining (Fig. 1B). qPCR indicated that expression of characteristic late-stage markers such as type 1 collagen α1 (Col1a1), osteocalcin (Bglap) and bone sialoprotein (Ibsp) were substantially reduced in Taok3-deficient osteoblasts in vitro (Fig. 1C). Additionally, expression of transcripts upregulated during early stages of osteoblast differentiation, Runt-related transcription factor 2 (Runx2), tissue non-specific alkaline phosphatase (Alpl) and Osterix (Sp7), were also significantly reduced (Fig. 1C). Thus, osteoblast differentiation is impaired in the absence of Taok3.
Figure 1. Taok3 is required for osteoblast differentiation in vitro.
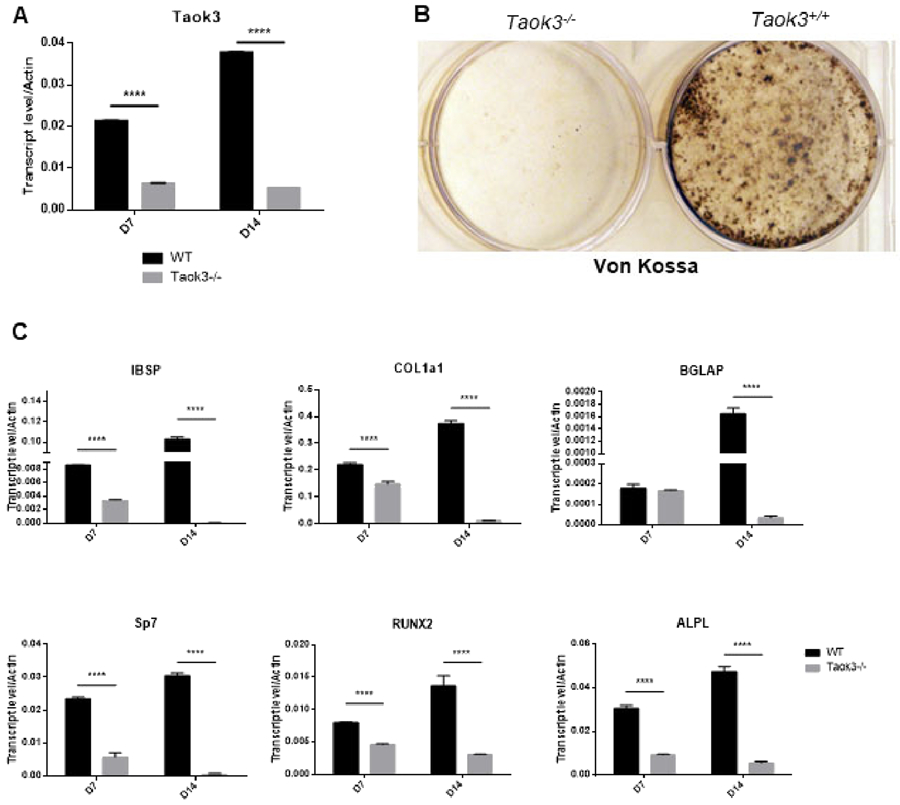
(A) RNA expression of Taok3 in day 7 and day 14 osteoblasts after differentiation cultivation (n=3 per group).
(B) Primary COBs were isolated from Taok3+/+ and Taok3−/− mice and cultured under differentiation conditions. Representative Von Kossa staining of differentiated COBs after cultivating for 20 days.
(C) Primary COBs were isolated from Taok3+/+ and Taok3−/− mice and cultured under differentiation conditions. qPCR analysis for indicated genes was performed after 7-day and 14-day culture, respectively (n=3 per group). **** p<0.001.
TAOK3 regulates late-stage osteoblast differentiation through the JNK MAPK pathway.
We have previously sought to identify the MAP3Ks upstream of JNK1 in osteoblasts. Osteoblasts from Tak1−/−, Mlk3−/−, and Mekk2−/− − mice were examined and found to display intact JNK activation [7,13,22]. Here, JNK activation was examined in Taok3−/− osteoblasts (Fig. 2A). Analysis of ERK and JNK activation showed markedly reduced phospho-JNK levels and a more modest decrease in ERK activation at day 7 of in vitro osteoblast differentiation (Fig. 2A). This decrease did not reflect a global inability of TAOK3-deficient osteoblasts to activate the JNK pathway, as application of classic JNK activating stimuli like ultraviolet light (UV) showed intact JNK activation (Fig. 2B). Thus, TAOK3 selectively mediates JNK pathway activation under osteoblast differentiation conditions but not in response to the model genotoxic stressor applied here.
Figure 2. TAOK3 regulates late-stage osteoblast differentiation through JNK/MAPK pathway.
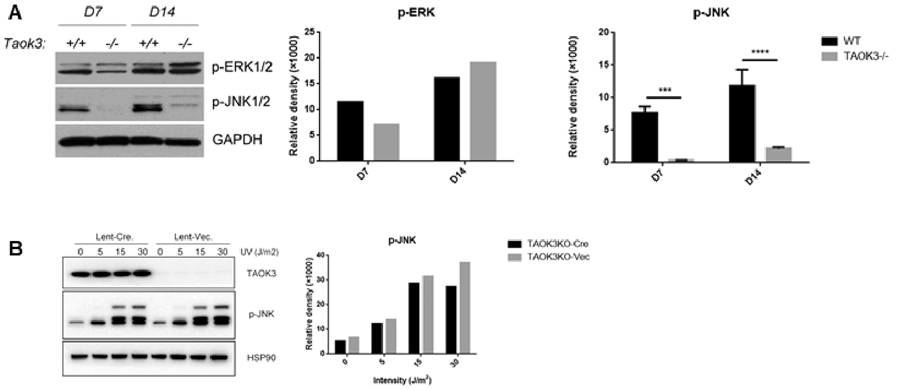
Primary COBs were isolated from Taok3+/+ and Taok3−/− mice and cultured under differentiation conditions. To address the upstream regulator of TAOK3, COBs were infected with vector or cre-recombinase expressing lentivirus followed by serial dosage of indicated stimuli.
(A) COB lysates were immunoblotted with indicated antibodies on either day 7 or day 14. Quantification of phospho-ERK and phospho-JNK levels were performed using ImageJ. ***p<0.001, **** p<0.001.
(B) COBs were cultured with 0, 10, 30, 60 micro mole (μM) As2O3 under differentiation conditions and cell lysates were immunoblotted with indicated antibodies followed by quantification.
(C) COBs were cultured with 0, 5, 15, 30 Joule per square meter (J/m2) UV under differentiation conditions and cell lysates were immunoblotted with indicated antibodies followed by quantification.
TAOK3 is required for differentiation of osteoblasts from human MSCs in vitro.
To confirm if the importance of Taok3 observed in murine COB holds true in human cells, primary human mesenchymal stromal cells (hMSCs) were subjected to TAOK3 knock down using a shRNA lentiviral-mediated knockdown system in vitro. First, the knockdown efficiency of various TAOK3-targeting shRNAs was validated with robust TAOK3 knockdown observed (Fig. 3A). Similar to findings in murine osteoblasts, TAOK3 knockdown blocked mineralization activity as shown by Von Kossa staining (Fig. 3B). Additionally, IBSP, ALPL expression levels and ALP activity were significantly decreased in hTAOK3-deficient hMSCs (Fig. 3C). Thus, TAOK3 is also required for early stage differentiation and mineralization activity in a human osteoblast differentiation system.
Figure 3. TAOK3 is required for differentiation of osteoblasts from human MSCs in vitro.
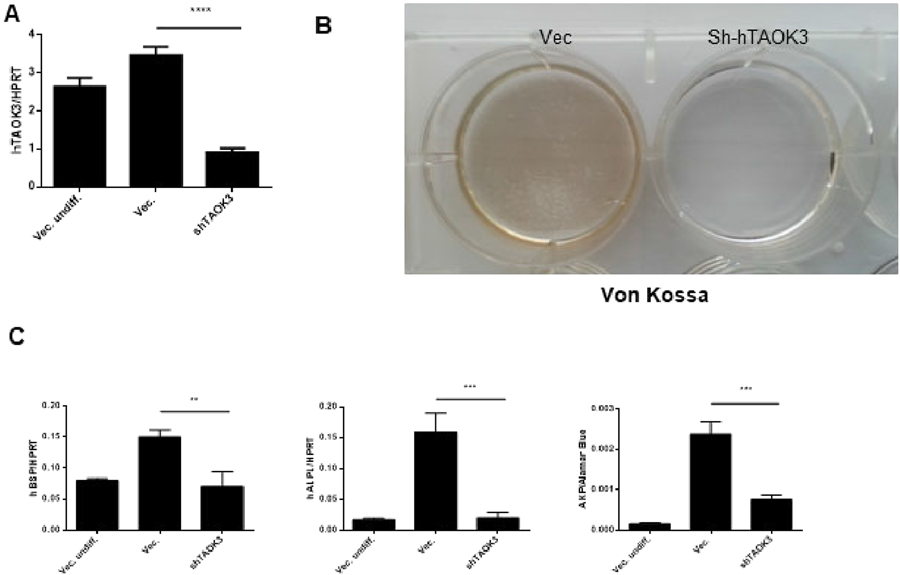
(A) hMSCs were cultured under undifferentiated or osteogenic differentiation conditions. hMSCs were infected with a vector control or human TAOK3 shRNA bearing lentivirus to silence TAOK3 gene in vitro. TAOK3 mRNA levels were measured by qPCR analysis (n=3 per group). **** p<0.001.
(B) Von Kossa staining of hMSCs infected with vector or shRNA bearing lentivirus was performed 21 days after osteogenic differentiation.
(C) aPCR analysis was performed to measure mRNA levels of IBSP and ALPL in hMSCs 7 days after osteogenic differentiation (n=3 per group). ALP assay was performed to quantify ALP activity (n=3 per group). ** p<0.01, ***p<0.001.
Phenotypic similarity between Taok3−/− mice and Jnk1−/− mice.
The skeletal mineralization of Taok3−/− mice was examined via micro-computed tomography μT) to identify the role of Taok3 in osteoblasts in vivo. 4-week-old male Taok3−/− mice showed a significant decrease in trabecular bone volume/total volume (BV/TV), cortical thickness (Cort. Th), trabecular number (Tb. N), and trabecular thickness (Tb. Th) (Fig. 4A, B). Taok3−/− mice also exhibited a distinct occipital hypomineralization, an enlarged anterior fontanelle, and delayed closure of the sagittal suture (Fig. 4C). These characteristics mimicked the phenotype reported in Jnk1−/− mice, providing physiological evidence supporting the observation that JNK pathway activation is reduced in Taok3−/− osteoblasts in vitro.
Figure 4. Phenotypic similarity between Taok3−/− mice and Jnk1−/− mice.
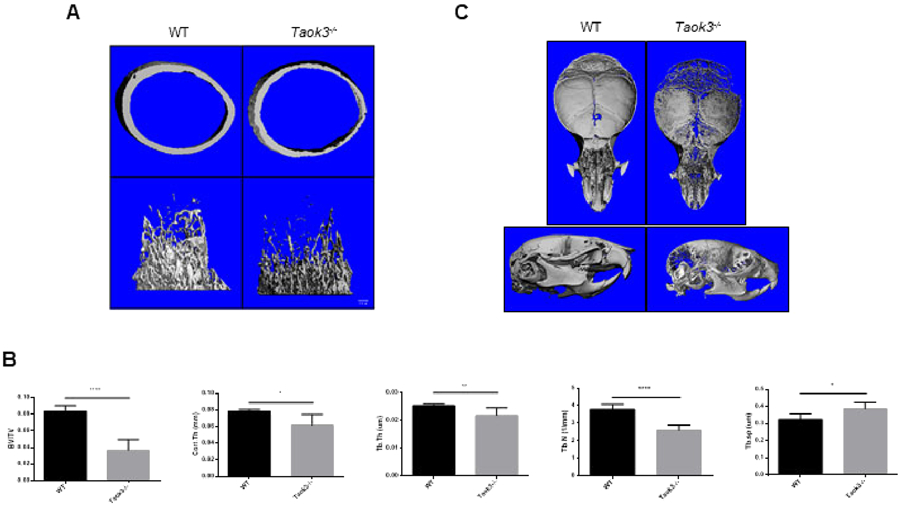
(A) 3-dimensional reconstructions of μCT analysis showing the femurs of 4-week-old male mice (n=6 per group). Displayed are cortical bone (top) and trabecular bone (bottom).
(B) Quantification of femoral bone mass of 4-week-old male mice using μCT analysis (n=6 per group). Bone volume/total volume (BV/TV), cortical thickness (Cort. Th), and trabecular thickness (Tb. Th.) were all significantly reduced. *p<0.05, ** p<0.01, ***p<0.001.
(C) 3-dimensional reconstructions of μCT analysis showing the skulls of 4-week-old male mice (n=6 per group). Displayed are vi(top) and lateral view (bottom).
Discussion
Osteoblast dysfunction is a critical etiology for both skeletal developmental diseases, such as osteogenesis imperfecta, and adult disorders of low bone mass, such as postmenopausal osteoporosis [23]. In this study, the Taok3-deficient murine model displayed enlarged fontanelles, occipital hypomineralization and low bone mass in long bones, all of which are consistent with the observed defects in osteoblast differentiation observed in TAOK3-deficient osteoblasts in vitro. These phenotypes were also observed in osteoblasts derived from hMSCs. Thus, a TAOK3-JNK axis contributes to osteoblast differentiation, raising the possibility that this pathway will offer therapeutic opportunities or will display specific contributions to craniofacial or bone mass disorders. Regarding the latter, it is likely that identifying patients with occipital hypomineralization will be a key to ultimately identifying the clinical relevance of this pathway
Examining specific, differentiating features of skeletal phenotypes can provide compelling evidence linking specific genes in vivo. For example, mice with deletion of the MAP3K TAK1 in osteoblast lineage cells display impaired clavicle mineralization and an open anterior fontanelle, features of human cleidocranial dysplasia caused by haploinsufficiency for RUNX2 [7, 24]. Similarly, previous research on Mlk3-deficient mice revealed a skeletal phenotype with characteristics of the human disorder faciogenital dysplasia, commonly known as Aarskog-Scott Syndrome, which similarly corresponded to MAP3K mixed-lineage kinase 3 (MLK3, MAP3K11) mediating signaling downstream of the faciogenital dysplasia associated gene FGD1 [13]. In this study, by comparing the skeletal phenotype in Jnk1−/− mice and Taok3−/− mice, we found both phenotypic and biochemical evidence linking TAOK3 to the JNK MAPK pathway.
Our previous study of Jnk1−/− osteoblasts showed a selective reduction in differentiation markers, such as Bglap and Ibsp, and a corresponding blockade in late-stage differentiation [10]. In contrast, both ERK and p38 loss-of-function induced a blockade in early-stage osteoblast differentiation [6,7,9,25]. Our findings show evidence of TAOK3 acting as a critical late-stage regulator in osteoblast function consistent with its contributions to JNK activation. However, both late-stage and early-stage differentiation markers are diminished in Taok3−/− COBs, corresponding to a moderate decrease in ERK activation levels. This is consistent with the known role of ERK1/2 as critical mediators of early-stage osteoblast differentiation, in part via the ability to phosphorylate and activate RUNX2 [6-8]. TAOK3 has been reported to be also able to activate the p38 MAPK pathway in other tissues, and p38 has a similar ability to ERK to activate RUNX2 and promote early osteoblast differentiation[7,15]. TAOK3 may additionally participate in non-MAPK signaling pathways relevant to osteoblasts, such as the notch pathway, required for early osteoblast differentiation [19]. Taken together, this suggested that downstream of TAOK3, the JNK pathway is possibly not the only MAPK member affected, and these other, non-JNK pathways may also function downstream of TAOK3 and contribute to the phenotype observed here.
This study also offers additional data relevant to conflicting results on whether TAOK3 activates or inhibits JNK activation. Previous studies reported that TAOK3 is an inhibitor of JNK pathway activation in non-osteoblast cell lines and in Drosophila [14,16]. However, other studies found that TAOK3 can potentially activate the JNK pathway under specific conditions. For instance, activation of JNK was seen in NIH-3T3 cells when exogenous TAOK3 was overexpressed [17], and TAOK3 is able to activate JNK in response to stress stimuli [18]. Here, we conclude that in osteoblasts TAOK3 is a positive mediator of the JNK pathway activation accompanying differentiation conditions in osteoblasts, but not in response to genotoxic stimuli. This is similar to findings that while MLK3 is essential for JNK activation downstream of TNF activation in some contexts, in osteoblasts MLK3 primarily contributes to p38 and ERK activation [13,26,27]. This emphasizes that MAP3Ks are a point where context specificity is conferred during signaling, and therefore an attractive point for therapeutic intervention.
Supplementary Material
TAOK3 is an upstream activator of the JNK pathway in osteoblasts.
TAOK3-deficient osteoblasts display defective differentiation in vitro
TAOK3 deficient mice display skeletal hypomineralization and low bone mass.
Acknowledgements
This project was supported by a Career Award for Medical Scientists from the Burroughs Wellcome Fund, the NIH under award DP5OD021351 and R01AR075585. This publication is based on research supported by the Pershing Square Sohn Cancer Research Alliance via an award to MBG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Long F, Building strong bones: Molecular regulation of the osteoblast lineage, Nat. Rev. Mol. Cell Biol 13 (2012) 27–38. 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- [2].Marie PJ, Signaling pathways affecting skeletal health, Curr. Osteoporos. Rep 10 (2012) 190–198. 10.1007/s11914-012-0109-0. [DOI] [PubMed] [Google Scholar]
- [3].Sharma R, Wu X, Rhodes SD, Chen S, He Y, Yuan J, Li J, Yang X, Li X, Jiang L, Kim ET, Stevenson DA, Viskochil D, Xu M, Yang FC, Hyperactive Ras/MAPK signaling is critical for tibial nonunion fracture in neurobromin-decient mice, Hum. Mol. Genet 22 (2013) 4818–4828. 10.1093/hmg/ddt333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Elefteriou F, Benson MD, Sowa H, Starbuck M, Liu X, Ron D, Parada LFF, Karsenty G, ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae, Cell Metab. 4 (2006) 441–451. 10.1016/j.cmet.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Elefteriou F, Kolanczyk M, Schindeler A, Viskochil DH, Hock JM, Schorry EK, Crawford AH, Friedman JM, Little D, Peltonen J, Carey JC, Feldman D, Yu X, Armstrong L, Birch P, Kendler DL, Mundlos S, Yang FC, Agiostratidou G, Hunter-Schaedle K, Stevenson DA, Skeletal abnormalities in neurofibromatosis type 1: Approaches to therapeutic options, Am. J. Med. Genet. Part A 149 (2009) 2327–2338. 10.1002/ajmg.a.33045. [DOI] [PubMed] [Google Scholar]
- [6].Kim JM, Yang YS, Park KH, Oh H, Greenblatt MB, Shim JH, The ERK MAPK pathway is essential for skeletal development and homeostasis, Int. J. Mol. Sci 20 (2019). 10.3390/ijms20081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Greenblatt MB, Shim J-H, Zou W, Sitara D, Schweitzer M, Hu D, Lotinun S, Sano Y, Baron R, Park JM, Arthur S, Xie M, Schneider MD, Zhai B, Gygi S, Davis R, Glimcher LH, The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice, J. Clin. Invest 120 (2010) 2457–2473. 10.1172/JCI42285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ge C, Yang Q, Zhao G, Yu H, Kirkwood KL, Franceschi RT, Interactions between extracellular signal-regulated kinase 1/2 and P38 Map kinase pathways in the control of RUNX2 phosphorylation and transcriptional activity, J. Bone Miner. Res 27 (2012) 538–551. 10.1002/jbmr.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ge C, Xiao G, Jiang D, Yang Q, Hatch NE, Roca H, Franceschi RT, Identification and functional characterization of ERK/MAPK phosphorylation sites in the Runx2 transcription factor, J. Biol. Chem 284 (2009) 32533–32543. 10.1074/jbc.M109.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu R, Zhang C, Shin DY, Kim JM, Lalani S, Li N, Yang YS, Liu Y, Eiseman M, Davis RJ, Shim JH, Greenblatt MB, c-Jun N-Terminal Kinases (JNKs) Are Critical Mediators of Osteoblast Activity In Vivo, J. Bone Miner. Res 32 (2017) 1811–1815. 10.1002/jbmr.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Matsuguchi T, Chiba N, Bandow K, Kakimoto K, Masuda A, Ohnishi T, JNK activity is essential for Atf4 expression and late-stage osteoblast differentiation, J. Bone Miner. Res 24 (2009) 398–410. 10.1359/jbmr.081107. [DOI] [PubMed] [Google Scholar]
- [12].Plotnikov A, Zehorai E, Procaccia S, Seger R, The MAPK cascades: Signaling components, nuclear roles and mechanisms of nuclear translocation, Biochim. Biophys. Acta - Mol. Cell Res 1813 (2011) 1619–1633. 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- [13].Zou W, Greenblatt MB, Shim JH, Kant S, Zhai B, Lotinun S, Brady N, Hu DZ, Gygi SP, Baron R, Davis RJ, Jones D, Glimcher LH, MLK3 regulates bone development downstream of the faciogenital dysplasia protein FGD1 in mice, J. Clin. Invest 121 (2011) 4383–4392. 10.1172/JCI59041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tassi E, Biesova Z, Di Fiore PP, Gutkind JS, Wong WT, Human JIK, a novel member of the STE20 kinase family that inhibits JNK and is negatively regulated by epidermal growth factor., J. Biol. Chem 274 (1999) 33287–33295. 10.1074/jbc.274.47.33287. [DOI] [PubMed] [Google Scholar]
- [15].Raman M, Earnest S, Zhang K, Zhao Y, Cobb MH, TAO kinases mediate activation of p38 in response to DNA damage, EMBO J. 26 (2007) 2005–2014. 10.1038/sj.emboj.7601668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kapfhamer D, King I, Zou ME, Lim JP, Heberlein U, Wolf FW, JNK pathway activation is controlled by Tao/TAOK3 to modulate ethanol sensitivity., PLoS One. 7 (2012) e50594 10.1371/journal.pone.0050594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang W, Chen T, Wan T, He L, Li N, Yuan Z, Cao X, Cloning of DPK, a novel dendritic cell-derived protein kinase activating the ERK1/ERK2 and JNK/SAPK pathways., Biochem. Biophys. Res. Commun 274 (2000) 872–879. 10.1006/bbrc.2000.3244. [DOI] [PubMed] [Google Scholar]
- [18].Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M, Activation of Caspase-12, an Endoplastic Reticulum (ER) Resident Caspase, through Tumor Necrosis Factor Receptor-associated Factor 2-dependent Mechanism in Response to the ER Stress, J. Biol. Chem 276 (2001) 13935–13940. 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- [19].Hammad H, Vanderkerken M, Pouliot P, Deswarte K, Toussaint W, Vergote K, Vandersarren L, Janssens S, Ramou I, Savvides SN, Haigh JJ, Hendriks R, Kopf M, Craessaerts K, De Strooper B, Kearney JF, Conrad DH, Lambrecht BN, Transitional B cells commit to marginal zone B cell fate by Taok3-mediated surface expression of ADAM10, Nat. Immunol 18 (2017) 313–320. 10.1038/ni.3657. [DOI] [PubMed] [Google Scholar]
- [20].Greenblatt MB, Park K.H. wa., Oh H, Kim JM, Shin D.Y. eo., Lee J.M. yu., Lee J.W. o., Singh A, Lee K. young, Hu D, Xiao C, Charles JF, Penninger JM, Lotinun S, Baron R, Ghosh S, Shim JH, CHMP5 controls bone turnover rates by dampening NF-κB activity in osteoclasts, J. Exp. Med. 212 (2015) 1283–1301. 10.1084/jem.20150407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Friedel RH, Soriano P, Gene trap mutagenesis in the mouse, Methods Enzymol. 477 (2010) 243–269. 10.1016/S0076-6879(10)77013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Greenblatt MB, Shina DY, Oh H, Lee KY, Zhai B, Gygi SP, Lotinun S, Baron R, Liu D, Su B, Glimcher LH, Shim JH, MEKK2 mediates an alternative β-catenin pathway that promotes bone formation, Proc. Natl. Acad. Sci. U. S. A 113 (2016) E1226–E1235. 10.1073/pnas.1600813113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Glorieux FH, A disease of the osteoblast, Lancet. 358 (2001) S45 10.1016/s0140-6736(01)07058-1. [DOI] [PubMed] [Google Scholar]
- [24].Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GWH, Beddington RSP, Mundlos S, Olsen BR, Selby PB, Owen MJ, Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development, Cell. 89 (1997) 765–771. 10.1016/S0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- [25].Suzuki A, Guicheux J, Palmer G, Miura Y, Oiso Y, Bonjour JPP, Caverzasio J, Evidence for a role of p38 MAP kinase in expression of alkaline phosphatase during osteoblastic cell differentiation, Bone. 30 (2002) 91–98. 10.1016/S8756-3282(01)00660-3. [DOI] [PubMed] [Google Scholar]
- [26].Gallo KA, Johnson GL, Mixed-lineage kinase control of JNK and p38 MAPK pathways, Nat. Rev. Mol. Cell Biol 3 (2002) 663–672. 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- [27].Brancho D, Ventura J-J, Jaeschke A, Doran B, Flavell RA, Davis RJ, Role of MLK3 in the Regulation of Mitogen-Activated Protein Kinase Signaling Cascades, Mol. Cell. Biol 25 (2005) 3670–3681. 10.1128/mcb.25.9.3670-3681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


