Abstract
An increasing number of patients are able to survive traumatic brain injuries (TBIs) with advanced resuscitation. However, the role of their pre-injury health status in mortality in the following years is not known. Here, we followed 77,088 consecutive patients (59% male) who survived the TBI event in Ontario, Canada for more than a decade, and examined the relationships between their pre-injury health status and mortality rates in excess to the expected mortality calculated using sex- and age-specific life tables. There were 5,792 deaths over the studied period, 3,163 (6.95%) deaths in male and 2,629 (8.33%) in female patients. The average excess mortality rate over the follow-up period of 14 years was 1.81 (95% confidence interval =1.76–1.86). Analyses of follow-up time windows showed different patterns for the average excess rate of mortality following TBI, with the greatest rates observed in year one after injury. Among identified pre-injury comorbidity factors, 33 were associated with excess mortality rates. These rates were comparable between sexes. Additional analyses in the validation dataset confirmed that these findings were unlikely a result of TBI misclassification or unmeasured confounding. Thus, detection and subsequent management of pre-injury health status should be an integral component of any strategy to reduce excess mortality in TBI patients. The complexity of pre-injury comorbidity calls for integration of multidisciplinary health services to meet TBI patients’ needs and prevent adverse outcomes.
Keywords: Age, Comorbidity, Environmental exposures, Injury severity, Health services, Mortality, Life tables, Risk, Sex differences, Traumatic brain injury
Introduction
Traumatic brain injury (TBI), defined ‘as an alteration in brain function or other evidence of brain pathology caused by an external force’1, has long intrigued researchers and clinicians because of its multifaceted presentation and, more recently, because of the multiple additional clinically-relevant entities (i.e., comorbid conditions), previously existing, adding complexity and complication to the study and management of TBI2,3. These entities are widespread in TBI and have been shown to encompass not only aspects of a person’s health, both mental and physical, but also factors external to the human body, the environment, which people often have little to no control3.
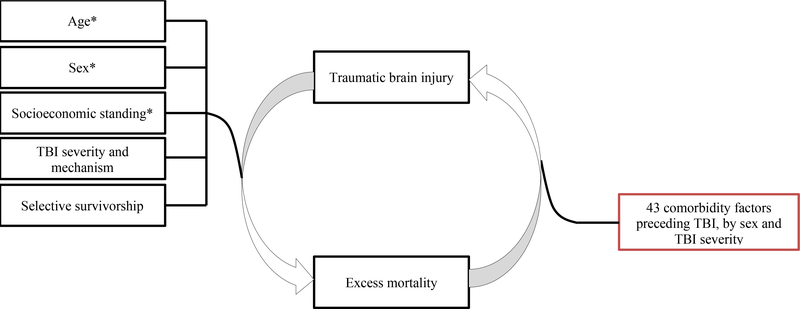
Although evidence exists for relationships between certain pre-existing comorbid disorders and both in-hospital and all-cause mortality4,5, uncertainty surrounds the magnitude of these associations, and the contributions of different comorbidities to the development of long-term adverse outcomes6. Chronic medical disorders, mental health disorders and alcohol and drug use, and a range of comorbid conditions through comorbidity indices are the most commonly reported predictors of all-cause mortality (supplementary table 1)6–15; however, a variety of non-diagnostically specific ways were used to study and categorize these comorbidities and/or comorbidity indices6. By focusing almost exclusively on selective comorbid disorders within an individual, both mental and physical, that are collected via self-report measures or chart abstractions from clinical files6, clinicians and researchers may miss opportunities to risk-stratify patients with less common comorbidities, and those emerging from environmental adversities. Likewise, the burden of different comorbid disorders differs between male and female patients with TBI7, and it is possible that certain comorbidities may be more relevant and offer better predictive ability when studying mortality risk in the sexes, bringing forth another objective for the study of mortality in TBI – sex-specific analysis. Finally, temporal separation of the comorbidity and TBI event, to protect against reverse causality bias, has received little attention in the scientific literature6–15, but is highly relevant in the context of TBI-mortality prevention research. The present study addresses the gaps outlined above by following a large cohort of persons across the spectrum of TBI severity over more than a decade, evaluating the relationship between a comprehensive set of comorbidity factors preceding TBI and all-cause mortality. We hypothesized that TBI-preceding systemic comorbid disorders that require continuous treatment and disorders that arise from adverse environmental conditions that are difficult to modulate would increase the mortality risk in male and female persons across TBI severities beyond the expected mortality based on the person’s age and sex. We also hypothesized that TBI severity and biological sex will have an impact on the strength of the association between pre-existing comorbid disorders and all-cause mortality (Figure 1).
Figure 1.
Hypothesized relationships related to excess mortality outcome after TBI. Red colour indicates primary hypothesis. Black colour indicates covariates, some (*) previously described. TBI=traumatic brain injury.
Methods
The study protocol was approved by the ethics committees at the clinical and academic institutions affiliated with the authors. The findings were reported in compliance with the STROBE guidelines for cohort studies16.
Study design and data sources
Data for this retrospective cohort study were obtained from the Institute for Clinical Evaluation Sciences (ICES)17, which houses high-quality health administrative data on various publicly funded services to residents, including individual-level information on emergency department (ED) and acute care hospitalisations within Ontario, Canada. Diagnoses are indicated by the ICD-10 Canadian Enhancement (ICD-10-CA) 18. All residents diagnosed with TBIs (ICD-10-CA codes S02.0, S02.1, S02.3, S02.7, S02.8, S02.9, S04.0, S07.1, and S06; supplementary table 2) of any severity and by any mechanism in an ED or acute care hospital between April 1, 2002 and March 31, 2016 were considered in this study (supplementary figure 1). To protect against overfitting and for internal validation, the data was split into three datasets, i.e. training, validation, and testing, with an allocation of 50%, 25%, and 25%, respectively19. Frequencies were calculated for the complete and testing datasets, and the training and validation datasets were used for model building and internal validation.
Predictors
Health status preceding TBI was evaluated as a predictor in our statistical models. From all possible ICD-10 codes classifying patients’ health conditions, a previous data mining and validation study identified 43 factors (supplementary table 3) that differentiated patients with TBI from those with other diagnoses, individually matched based on age, sex, income level, and place of residence3. These comorbidity factors were significantly overrepresented in TBI patients compared to the reference patients in the five years preceding their TBI event. Each factor was considered a predictor and was studied individually.
Outcome
The primary outcome was excess mortality rates attributable to each of the 43 comorbidity factors preceding TBI, by sex and injury severity. Age- and sex-specific mortality rates were derived from a cause-elimination life table of the general population generated by Statistics Canada18 and calculated excess mortality rates by differentiating the age- and sex-specific mortality rates (background mortality) from the reported mortality for each TBI patient, by each year interval, depending on whether the patient survived their TBI.
Covariates
Age, sex, socioeconomic standing, TBI severity and mechanism, and selective survivorship are important in the study of mortality. Injury severity was defined according to a previously published severity classifications 20,21. Injury mechanism was determined using major external cause of injury group codes (E codes)22, divided into falls, struck by/against object, motor vehicle accidents, other transport injury, intent (intentional or unintentional), and sports-related injury.
Statistical approach
Analysis of patients' first TBI
Due to ICES confidentiality policies, we were unable to obtain the exact dates for TBI-related hospital visits. Instead, two variables were attributed to each hospital visit: number of days from index date (i.e., first TBI event) to admission date and fiscal year of the admission date. To calculate the number of days to the censor date, the TBI event date was set as the first day of the fiscal year. Previous research considered mortality within a 30-day window to be TBI-related mortality23. A histogram representing the death distribution by day after the TBI event was constructed for all TBI patients. This histogram peaked around the index date, with little change after 100 days, and dropping drastically 30 days after the index date. The 30-day window, therefore, was determined as a TBI-related mortality window, and all patients alive 31 days after their first TBI were studied.
Discrete-time survival analysis
To calculate the excess mortality rate, the discrete-time method was used 24. This required conversion from a person-record to a person-year dataset, which was achieved by creating a new entry for every year the patient remained in the cohort; the number of records in the new person-year dataset equalled the total sum of patient-years spent in the cohort. The patient’s age was modified for each year he/she remained in the cohort, and the binary variable ‘death’ was set to zero for all person-year records except the last year. At that point, the patient was either censored or would have died in that year (assigned the value of zero or one, respectively). Subsequently, the sex-, age-, and calendar year-specific death rates in the general population, extracted from life tables18, were age- and sex-matched to each surviving TBI patient of that year and used as an offset term to calculate excess mortality rate25.
Poisson distribution
To determine the mortality rate per year for each TBI patient, a Poisson distribution was used to convert the binary variable ‘death’ to the mortality rate in that particular year26. A general linear model with a log-link function was used 27, where the outcome variable was the excess mortality rate in that year for a TBI patient versus that for someone from the general population with the same sex and age in that fiscal year of death, and the predictor variable was the TBI-preceding health status. The results were adjusted for age, income quintile, place of residence (urban vs. rural), injury mechanism, and fiscal year since the first TBI, for each comorbidity factor preceding TBI, and reported for male and female patients separately, by injury severity.
Age effects
Next, we accounted for possible effects of age. Since the offset term is based on the age-specific hazard for that particular year, it is already controlled for any increase in risk caused by patient age. However, age might have an additional effect in TBI patients compared to the general population. Thus, linear, quadratic, cubic, and quartic age effects were modelled. The results highlighted wide confidence intervals (CIs) for the cubic and quartic models. Follow-up likelihood-ratio tests revealed the quadratic age effect was optimal, which was used in the modelling process.
Effects of follow-up year on mortality rate
The assumption that the mortality rate after the first TBI (supplementary figures 2 and 3) remained unchanged throughout subsequent years and that any observed changes between years would only derive from age increases was tested. Our results highlighted that this was not the case; the percentages of patient deaths decreased with time post-TBI.
To better understand this phenomenon, least-square means were used to compare excess mortality rates in all follow-up years (supplementary figure 4). Excess mortality rates for each subsequent year were further tested in a categorical and binary effect of ≥ 2 years post-TBI. The Akaike28 and Bayesian29 information criteria were considered (supplementary table 4); the categorical effect was shown to be optimal. All statistical analyses were conducted using SAS software (version 9.410, SAS Inc., Cary, NC).
Results
Among the Ontario population of 12 and 14 million people in 2002 and 2016, respectively30, 319,700 patients had their first TBI-related visit in either an emergency department (ED) or acute care hospital between the fiscal years 2002/03 and 2015/16. Of these, 11,347 (3.55%) patients died within 30 days following their TBIs and were excluded from analysis. The final sample was randomly split into training (50%; n = 154,177), validation (25%; n = 77,088), and testing (25%; n = 77,088) datasets (supplementary figure 1).
The 77,088 patients in the testing dataset comprised of 59% male and 41% female patients. The most common causes of TBI were falls (n = 33,800 [43.85%]; male: n = 17,518 [38.48%]; female: n = 16,282 [51.59%]) and being struck by/against an object (n = 27,407 [35.55%]; male: n = 18,383 [40.38%]; female: n = 9024 [28.59%]). Injury severity was not established in 12,629 (16.38%) male and 11,011 (14.28%) female patients; among these, most cases were recorded as concussion without a specified length of unconsciousness (ICD-10-CA code S06.0). Accidents and intentional injury accounted for 92% and 8% of TBIs, respectively. Of all injuries, 26% were sports-related, and 13% were related to motor vehicle accidents (Table 1 and supplementary table 5).
Table 1.
Characteristics of patients with traumatic brain injury overall, and by sex and outcome by year after injury event
| VARIABLES | Overall N (%) 77,088 (100) |
Female patients (N, %) 31,560 (40.94) |
Male patients (N, %) 45,528 (59.06) |
|---|---|---|---|
| Socio-demographic characteristics | |||
| Age at first TBI, years old | |||
| Mean (SD) | 33.96 (24.26) | 37.70 (25.71) | 31.37 (22.85) |
| Median (IQR) | 25.00 (15.00 – 51.00) | 31.00 (16.00 – 57.00) | 23.00 (14.00 – 46.00) |
| Income Q1 (poorest) | 15,124 (19.62) | 6,073 (19.24) | 9,051 (19.88) |
| Income Q2 | 14,839 (19.25) | 6,025 (19.09) | 8,814 (19.36) |
| Income Q3 | 15,203 (19.72) | 6,302 (19.97) | 8,901 (19.55) |
| Income Q4 | 16,166 (20.97) | 6,678 (21.16) | 9,488 (20.84) |
| Income Q5 (wealthiest) | 15,756 (20.44) | 6,482 (20.54) | 9,274 (20.37) |
| Rural residence | 12,779 (16.58) | 5,069 (16.06) | 7,710 (16.93) |
| TBI-related characteristics | |||
| Cause: Falls | 33,800 (43.85) | 16,282 (51.59) | 17,518 (38.48) |
| Cause: Struck by/against object | 27,407 (35.55) | 9,024 (28.59) | 18,383 (40.38) |
| Cause: Motor vehicle accident | 9,667 (12.54) | 3,916 (12.41) | 5,751 (12.63) |
| Cause: Other transport injury | 3,304 (4.29) | 1,304 (4.13) | 2,000 (4.39) |
| Cause: Other cause | 4,383 (5.69) | 1,526 (4.84) | 2,857 (6.28) |
| Unintentional injury | 71,040 (92.15) | 30,433 (96.43) | 40,607 (89.19) |
| Intentional injury | 6,111 (7.93) | 1,120 (3.55) | 4,991 (10.96) |
| Sport-related injury | 19,882 (25.79) | 5,978 (18.94) | 13,904 (30.54) |
| Number of deaths during the study period | |||
| 1st year after TBI event | 1,648 (2.14) | 704 (2.23) | 944 (2.07) |
| 2nd year after TBI event | 914 (1.38) | 401 (1.51) | 513 (1.29) |
| 3rd year after TBI event | 726 (1.26) | 356 (1.58) | 370 (1.05) |
| 4th year after TBI event | 572 (1.15) | 266 (1.41) | 306 (0.99) |
| 5th year after TBI event | 455 (1.07) | 221 (1.39) | 234 (0.87) |
| 6th year after TBI event | 375 (1.04) | 183 (1.38) | 192 (0.84) |
| 7th year after TBI event | 330 (1.07) | 159 (1.43) | 171 (0.87) |
| 8th year after TBI event | 224 (0.87) | 103 (1.13) | 121 (0.72) |
| 9th year after TBI event | 186 (0.86) | 86 (1.15) | 100 (0.71) |
| 10th year after TBI event | 136 (0.77) | 59 (0.97 | 77 (0.67) |
| 11th year after TBI event | 115 (0.83) | 48 (1.02) | 67 (0.74) |
| 12th year after TBI event | 70 (0.69) | 29 (0.84) | 41 (0.61) |
| 13th year after TBI event | 32 (0.49) | NR | NR |
| 14th year after TBI event | 9 (0.27) | < 6 | < 6 |
Abbreviations: IQR, interquartile range; TBI, traumatic brain injury. NR, not reportable due to residual disclosure.
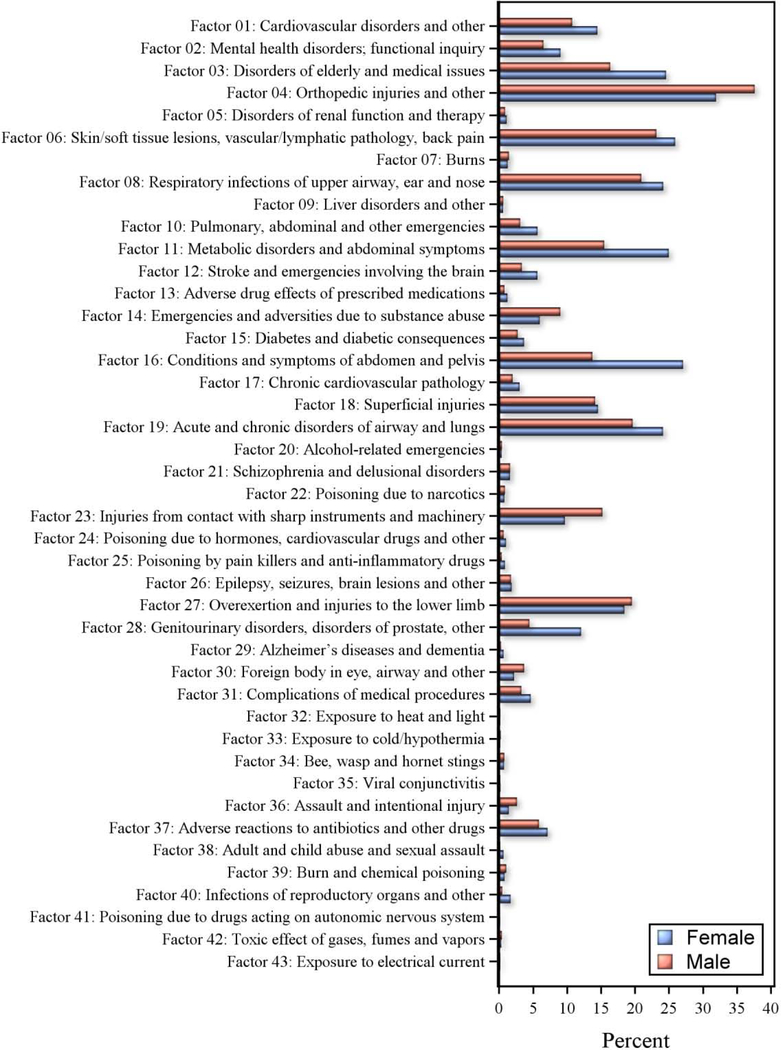
Among the 43 pre-injury comorbidity factors, orthopaedic injuries (Factor 4 [41%]), disorders of elderly (Factor 3 [26%]), airway/lung disorders (Factor 19 [26%]), metabolic disorders (Factor 11 [24%]), and cardiovascular disorders (Factor 1 [17%]) had the highest occurrence. Factors related to medical procedure complications (Factor 31 [5%]), pharmacology emergencies (Factors 24 [0.9%], 25 [0.6%], and 41 [0.1%]), toxicology (Factor 20 [0.4%]), and environmental exposure (Factors 43 [0.12%], 32 [0.2%], and 42 [0.34%]) occurred less frequently (Figure 2).
Figure 2.
Comorbidity factors preceding injury in males and females with TBI in Ontario, Canada 2002–2016.
There were 5792 (7.51%) deaths over the study period, including 3163 (6.95%) deaths in male and 2629 (8.33%) in female patients. Most deaths (n = 1648; 28.45%) occurred within 1-year post-TBI diagnosis, after which these percentages decreased for the next 6 years, within the range of 15.78–5.70%, reaching a minimum of 0.27% at year 14. This was true for both sexes, although the frequencies were higher in female than in male patients across most years and injury severities (Table 1).
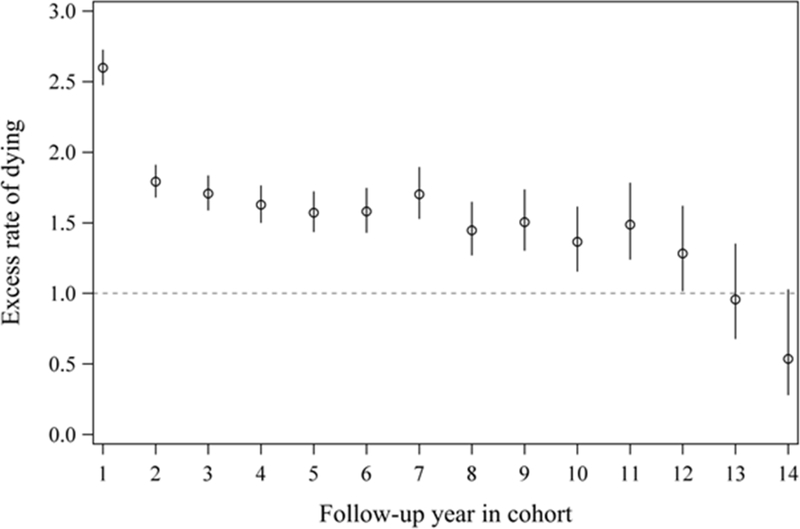
The average excess mortality rate over the entire follow-up period was 1.81 (95% CI=1.76−1.86). Analyses of follow-up time windows showed different patterns for the average excess mortality rate post-TBI, with the greatest difference observed in year one post-injury (Figure 3 and supplementary figure 2).
Figure 3.
Excess mortality rate for patients with TBI versus general population (individually matched by age and sex), by fiscal year remained in cohort.
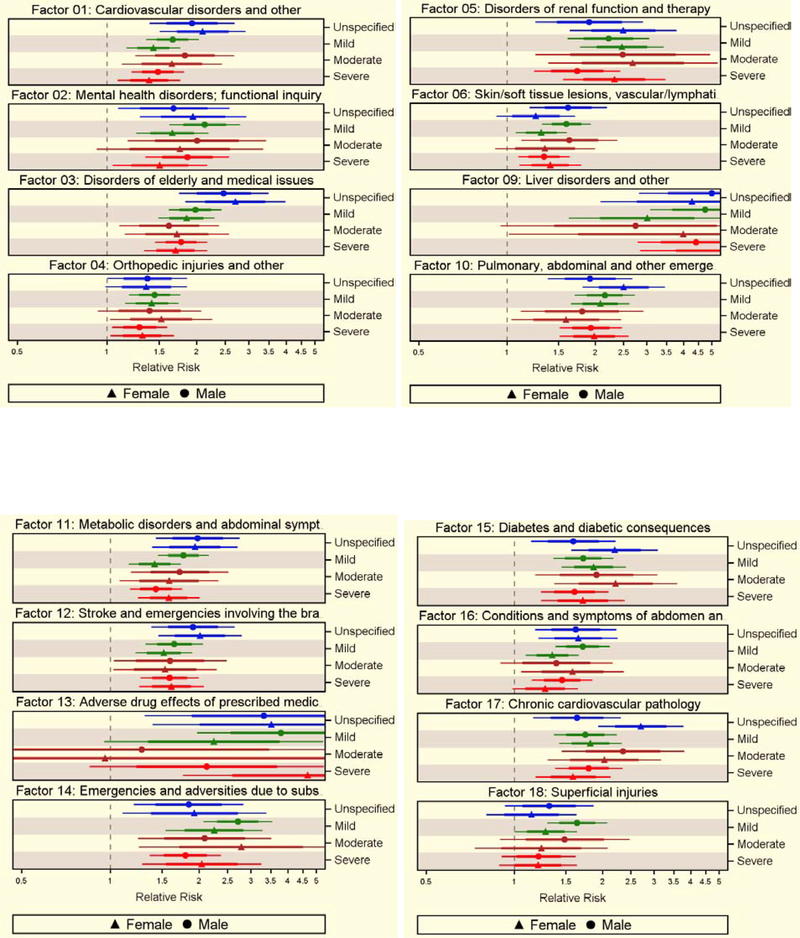
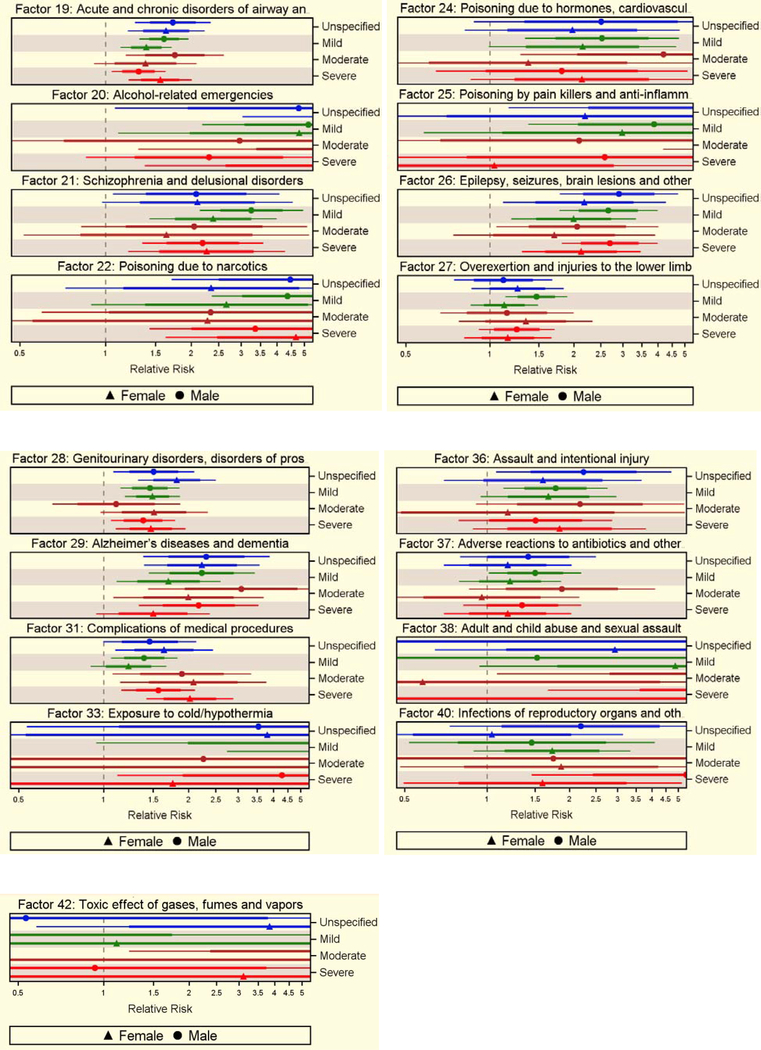
Older age, urban residence, and lowest income quintile were associated with excess mortality rates (supplementary table 5). Among the 43 factors preceding TBI, 33 were associated with excess mortality rate after adjusting for relevant covariates. Among the high-frequency factors, Factors 1 (cardiovascular disorders and other), 3 (disorders of elderly and medical issues), 4 (orthopaedic injuries and other), 11 (metabolic disorders and abdominal symptoms), and 19 (acute and chronic disorders of airways and lungs) were associated with excess risk of dying at 1.56 (95% CI=1.42–1.70), 1.88 (95% CI=1.72–2.03), 1.36 (95% CI=1.24–1.49), 1.57 (95% CI=1.43–1.71), and 1.45 (95% CI=1.33–1.59), respectively. Among low-frequency factors, Factors 9 (liver disorders, others), 20 (alcohol-related emergencies), 22 (narcotic poisoning), and 33 (cold exposure, hypothermia) were associated with greater risks of excess mortality at 4.17 (95% CI=3.36–5.17), 4.45 (95% CI=2.78–7.12), 4.48 (95% CI=2.46–4.92), and 4.51 (95% CI=2.09–9.73), respectively (supplementary tables 6 and 7). The estimates varied by severity, but the results were comparable between sexes, although marginal factor-related differences were observed (Figure 4).
Figure 4.
Excess rate of death due to 33 health status factors preceding TBI, with 95% CI, without (denser line) and with (thinner line) Bonferroni adjustment, by sex and injury severity, controlling for potential confounders of excess rate of death.
All detected associations, except for Factors 38 (adult, child abuse, sexual assault), 40 (reproductive organ infections, other), and 42 (toxic effects of gases, fumes, vapours), were internally validated via retesting with the validation dataset (supplementary figure 5).
Discussion
In this large representative cohort of persons with universal healthcare insurance (N=77, 088 patients, 57% males) who survived a TBI event, almost eight percent died over a period of 14 years following injury. When sex- and age-specific general population mortality rates were considered, the mortality risk for the TBI cohort remained elevated for most of the examined years. Among comorbidity factors preceding injury, 33 were strongly associated with excess mortality rates; the ten with the largest effects, irrespective of injury severity and sex, were (in no specific order): (1) alcohol related emergencies and emergencies and adversities due to substance use (Factors 20, 14), (2) liver disorders and others (Factor 9), (3) schizophrenia, delusional disorder (Factor 21), (4) epilepsy, seizures, brain lesions (Factor 26), (5) adult and child abuse, sexual assault (Factor 28), (6) poisoning due to narcotics, hormones and cardiovascular drugs, pain killers and anti-inflammatory drugs (Factors 22, 24, 25), and (7) exposure to the cold/hypothermia (Factor 33). These results suggest that pre-injury comorbidities with underlying pathophysiological processes due to systemic disorders, neuropharmacology, lifestyle, environment, and healthcare practices31 could be mechanistic links in the relationship between TBI and excess mortality.
There is growing evidence from human studies on the effect of alcohol in persons with TBI on mortality, given the metabolic and neurological effects of alcohol33. Several population-based cohort studies reported that alcohol intoxication at the time of injury was not associated with in-hospital or long-term mortality or improved survivorship, taking into account TBI severity, age and sex34,35,36; one study37 reported the opposite – that alcohol intoxication increases risk of mortality. Such discrepancies may be due to the numerous dose-related effects of alcohol, the time of exposure in relation to the injury, temporary versus continuous effects38, or genetic susceptibility39. It has also been noted that alcohol intoxication at the time of injury affects the accuracy of injury severity assessment40. Our findings highlighted the effect of alcohol-related emergencies and adverse effects of alcohol in the time preceding TBI on mortality in persons who survived the injury event; we also observed wide confidence intervals pertaining to the effect and differences in the magnitude of the effect based on TBI severity and biological sex. These results can reasonably be explained by relative and not absolute elimination of compensatory responses and disruption of physiological regulation that protects the living organism against severe threats and sustained life in response to TBI long after the injury. Future studies looking at genetic vulnerability to the metabolic and neurological effects of alcohol would provide further insight into this relationship in TBI.
Our findings on neurological (epilepsy, seizures, brain lesions 41,42,43) and gastrointestinal disorders (systemic hepato-renal disorders42,44,45) increasing mortality risk are consistent with the findings of previous studies, pointing to potentially disrupted immune-to-brain communication pathways46, a complex array of defensive mechanisms aimed at tissue repair after injury, which is crucial for survival. Future studies should look at immune response to a TBI event in persons with systemic disorders.
A past study described links between abuse and sexual assault and TBI events, with women significantly more likely to die from a head injury due to assault as compared to men47. In this study such events were rare, however in both the complete and testing datasets, men who visited ED or acute care as a result of abuse or assault in the five years preceding their TBI had increased risk of mortality years after moderate and severe TBI. This is significant and may point to a less thorough approach to surveillance of abuse and assault in men.
Knowledge about factors infrequently co-occurring with TBI, described here and previously3, is essential for preventive medicine. Exposure to cold/hypothermia, which was present in 0.15% of the sample, increased the overall excess mortality rate by 4.51 (95% 0=2.09–9.73). This has clinical implications, as several other episodes of excess mortality associated with cold exposure have been described recently, tightly linked to older age, male sex, mental illness, and homelessness48,49.
Perhaps the most unique finding of this study is the magnitude of excess mortality due to adverse effects of psychoactive drugs and other medications preceding TBI. Drug therapy are frequently cited as the largest cause of adverse events post–hospital discharge, and it has been estimated that 13% of adverse drug events after discharge from the hospital result in an ED visit or rehospitalization50,51. Other research reported that narcotic analgesics frequently cause opioid induced respiratory depression, a combination of lowered level of consciousness, decreased respiratory drive, and upper airway obstruction, and are implicated in cerebral hypoxia and falls with or without loss of consciousness.52 The link between adverse effects of medications and excess mortality in our study is noteworthy, and endorses attention to principles of quaternary prevention53.
Aside from the ten comorbidities described above, diabetes mellitus and stroke, along with cardiovascular and respiratory disorders (previously reported in the literature in relation to mortality in TBI3,6–14) were shown to be associated with excess mortality across injury severities and in both biological sexes. These disorders have been reported to impact surgical care and rehabilitation outcomes in TBI54,55, adding to the challenge of balancing the risk-benefit ratio of feasible therapies 56, limiting TBI-related treatment options57. Drug management of the comorbid disorders described above often requires multi-drug prescriptions58. Polypharmacy may indirectly increase risks related to TBI3, which are often preventable by accounting for pharmacological interactions. There has been a recent call to utilise a minimally disruptive approach59 when deciding on pharmacological management of conditions to reduce the likelihood of drug interactions and medical errors post-injury53.
Our finding on the association between history of Alzheimer’s disease and dementia and excess mortality across injury severities and sexes, are novel and could be used to inform care of patients with these conditions. Dementia and other TBI-preceding comorbidities are linked to reduced tolerance to rehabilitation interventions; for instance, elderly patients and patients with gait or balance deficits, often associated with orthopaedic injuries of elderly, may be excluded from rehabilitation interventions post-TBI 60,61, which may negatively influence long-term survivorship, independent of TBI-related processes. The present research calls for greater focus on comorbidity in the coordination of care and rehabilitation, to prevent long-term adverse effects in persons who survived their injury.
Our research addresses many limitations of previously published observational studies. The study includes over 77,000 patients from a population-based cohort and consequently has a large number of death events, allowing us to control for all available confounders. The use of population-based sex- and age-specific life tables is particularly important because these variables could confound associations between TBI-preceding comorbidity and mortality. Further, we stratified our results by sex accounting for differential help-seeking behaviours and contact with healthcare providers62. Finally, we used rigorous methods for dealing with age and selective survivorship effects.
Our study has limitations. Despite being internally validated in a large cohort, the 43 factors preceding TBI were not medical diagnoses with well-defined criteria5. Therefore, excess mortality rates may incorporate some imprecision, similar to that of other parameters, e.g., differences between physiological and chronological age 63,64 or frailty being a relative vs. absolute state. However, herein, health status, depicted by our pre-TBI comorbidity factors, incorporated environmental adversities, and therefore more accurately represents clinical situations by reflecting the complex personal and social factors presenting in TBI patients65. By limiting our sample to patients presenting to emergency and acute care facilities, mortality rates may have been overestimated because these individuals may represent more severe TBI cases, injury-preceding comorbidities, or both 66. However, this approach has the advantage of investigating patients who access services in healthcare settings that are non-differential and free at the point of care. Although validated algorithms were used to define TBI severity, patient misclassification is possible, resulting in miscalculated TBI-related predictors. Finally, while health-related information routinely collected for administrative purposes provides a unique resource to study pre-TBI comorbidity and long-term outcomes in TBI patients, this approach is limited in its ability to identify continued unhealthy exposures after TBI.
Conclusion
We observed that comorbidities preceding TBI substantially increased excess mortality across injury severities and in both sexes, even after adjusting for other proxies of mortality. Early attention to comorbidity preceding TBI diagnosis, as an opportunity to intervene to prevent adverse TBI outcome, cannot be underestimated.
Supplementary Material
Highlights.
Pre-injury health status and mortality after traumatic brain injury were studied
Age and sex-specific life tables were used to calculate excess mortality rates
The excess mortality rates over the follow-up period were comparable between sexes
33 pre-injury comorbidity factors were associated with excess mortality rates
Attention to pre-injury health status is needed to reduce excess mortality.
Acknowledgements
This study made use of de-identified data from the ICES Data Repository, which is managed by the Institute for Clinical Evaluative Sciences with support from its funders and partners: Canada’s Strategy for Patient-Oriented Research (SPOR), the Ontario SPOR Support Unit, the Canadian Institutes of Health Research and the Government of Ontario. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). The opinions, results, and conclusions reported are those of the authors. No endorsement by ICES or any of its funders or partners, nor CIHI, is intended or should be inferred.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R21HD089106. AC was funded by the Gender and Health grant #CGW-126580. TM was supported by the postdoctoral research grant from the Alzheimer’s Association (AARF-16-442937). The funders had no role in study design, data collection, decision to publish, or preparation of the manuscript.
Footnotes
Competing Interests
The authors declare no financial and non-financial competing interests.
Ethical approval and informed consent
Approval: The study protocol was approved by the ethics committees at the clinical (Toronto Rehabilitation Institute-University Health Network) and academic (Institute for Clinical Evaluative Sciences) institutions. All methods were carried out in accordance with the relevant guidelines and regulations. Informed consent: This research utilised encrypted administrative health data with no access to personal information.
Availability of materials and data
The datasets generated during and/or analysed during the current study are available in the ICES repository, [www.ices.on.ca/DAS<http://www.ices.on.ca/DAS], under accession DAS 2016–257(2018 0970 084 000). Data sharing agreements prohibit ICES from making the datasets publicly available; however, access may be granted to those who meet pre-specified criteria for confidential access. The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Quaglio G, Gallucci M, Brand H, Dawood A, Cobello F Traumatic brain injury: a priority for public health policy. Lancet Neurol. 16(12), 951–952 (2017). 10.1016/S1474-4422(17)30370-8 [DOI] [PubMed] [Google Scholar]
- [2].Feinstein AR The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. 23(7), 455–468 (1970). 10.1016/0021-9681(70)90054-8 [DOI] [PubMed] [Google Scholar]
- [3].Mollayeva T, Sutton M, Chan V, Colantonio A, Jana S, Escobar M Data mining to understand health status preceding traumatic brain injury. Sci Rep. 9(1), 5574 (2019). 10.1038/s41598-019-41916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stein SC, Georgoff P, Meghan S, Mizra K, Sonnad SS 150 years of treating severe traumatic brain injury: a systematic review of progress in mortality. J Neurotrauma. 27(7), 1343–1353 (2010). 10.1089/neu.2009.1206 [DOI] [PubMed] [Google Scholar]
- [5].Mushkudiani NA, Hukkelhoven CW, Hernández AV, Murray GD, Choi SC, Maas AI, Steyerberg EW A systematic review finds methodological improvements necessary for prognostic models in determining traumatic brain injury outcomes. J Clin Epidemiol. 61(4),331–343 (2008). 10.1016/j.jclinepi.2007.06.011 [DOI] [PubMed] [Google Scholar]
- [6].Xiong C, Hanafy S, Chan V, Hu ZJ, Sutton M, Escobar M, Colantonio A, Mollayeva T Comorbidity in adults with traumatic brain injury and all-cause mortality: A systematic review. BMJ Open. 9(11), e029072 (2019). doi: bmjopen-2019-029072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chan V, Mollayeva T, Ottenbacher KJ, Colantonio A Clinical profile and comorbidity of traumatic brain injury among younger and older men and women: a brief research notes. BMC Res Notes. 10(1), 371 (2017). 10.1186/s13104-017-2682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fuller GW, Ransom J, Mandrekar J, Brown AW Long-term survival following traumatic brain injury: A Population-Based Parametric Survival Analysis. Neuroepidemiology. 47(1),1–10 (2016). 10.1159/000445997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fu TS, Jing R, McFaull SR, Cusimano MD Recent trends in hospitalization & in-hospital mortality associated with traumatic brain injury in Canada: A nationwide, population-based study. J Trauma Acute Care Surg. 79(3), 449–454 (2015). 10.1097/ta.0000000000000733 [DOI] [PubMed] [Google Scholar]
- [10].Jonsdottir GM,Lund SH,Snorradottir B,Karason S,Olafsson IH,Reynisson K, Mogensen B, Sigvaldason K A population-based study on epidemiology of intensive care unit treated traumatic brain injury in Iceland. Acta Anaesthesiol Scand. 61(4), 408–417 (2017). 10.1111/aas.12869 [DOI] [PubMed] [Google Scholar]
- [11].Catapano JS, Chapman AJ, Horner LP, Lu M, Fraser DR, Fildes JJ Preinjury polypharmacy predicts mortality in isolated severe traumatic brain injury patients. Am J Surg. 213(6),1104–1108 (2017). 10.1016/j.amjsurg.2016.07.010 [DOI] [PubMed] [Google Scholar]
- [12].Harrison-Felix C, Kolakowsky-Hayner SA, Hammond FM, Wang R, Englander J, Dams-O'Connor K, Kreider SE, Novack TA, Diaz-Arrastia R Mortality after surviving traumatic brain injury: risks based on age groups. J Head Trauma Rehabil. 27(6),E45–56 (2012). 10.1097/HTR.0b013e31827340ba [DOI] [PubMed] [Google Scholar]
- [13].vanderPloeg T, Nieboer D,Steyerberg EW Modernmodeling techniqueshad limited external validity in predicting mortality from traumatic brain injury. J Clin Epidemiol. 78, 83–89 (2016). 10.1016/j.jclinepi.2016.03.002 [DOI] [PubMed] [Google Scholar]
- [14].Rickels E,von Wild K, Wenzlaff P Head injury in Germany: A population-based prospective study on epidemiology, causes, treatment & outcome of all degrees of head-injury severity in two distinct areas. Brain Inj. 24(12), 1491–1504 (2010). 10.3109/02699052.2010.498006 [DOI] [PubMed] [Google Scholar]
- [15].Ventura T, Harrison-Felix C, Carlson N, Diguiseppi C, Gabella B, Brown A, Devivo M, Whiteneck G Mortality after discharge from acute care hospitalization with traumatic brain injury: a population-based study. Arch Phys Med Rehabil. 91(1),20–29 (2010). 10.1016/j.apmr.2009.08.151 [DOI] [PubMed] [Google Scholar]
- [16].von Elm E, Altman DG, Egger M,Pocock SJ,Gøtzsche PC,Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Prev Med 45(4), 247–251 (2007). 10.1016/j.ypmed.2007.08.012 [DOI] [PubMed] [Google Scholar]
- [17].Institute for Clinical Evaluation Sciences. Privacy Code–Protecting Personal Health Information at ICES. Toronto: Institute for Clinical Evaluation Sciences; https://www.ices.on.ca/Data-and-Privacy/Privacy-at-ICES [Google Scholar]
- [18].Public Health Agency of Canada Steering Committee on Health-Adjusted Life Expectancy. Public Health Agency of Canada; 2012. [Catalogue No. Ottawa (ON): Health-adjusted life expectancy in Canada: 2012 report by the Public Health Agency of Canada. HP35-32/2012E]. [PubMed]
- [19].Hastie T, Tibshirani R, Friedman J Model Assessment and Selection The Elements of Statistical Learning: Data Mining, Inference, and Prediction. New York, NY, Springer, 219–223 (2009). [Google Scholar]
- [20].Gedeborg R, Warner M, Chen LH, Gulliver P, Cryer C, Robitaille Y, Bauer R, Ubeda C, Lauritsen J, Harrison J, Henley G, Langley J Internationally comparable diagnosis-specific survival probabilities for calculation of the ICD-10-based Injury Severity Score. J Trauma Acute Care Surg. 76(2),358–365 (2014). 10.1097/TA.0b013e3182a9cd31 [DOI] [PubMed] [Google Scholar]
- [21].Gagné M, Moore L, Sirois MJ, Simard M, Beaudoin C, Kuimi BL Performance of International Classification of Diseases-based injury severity measures used to predict in-hospital mortality and intensive care admission among traumatic brain-injured patients. J Trauma Acute Care Surg. 82(2),374–382 (2017). 10.1097/TA.0000000000001319 [DOI] [PubMed] [Google Scholar]
- [22].Association of Public Health Epidemiologists in Ontario. Recommended ICD-10-CA Codes for Injury Indicators. http://core.apheo.ca/index.php?pid=306
- [23].Marincowitz C, Lecky F, Allgar V, Sheldon T Evaluation of the impact of the NICE head injury guidelines on inpatient mortality from traumatic brain injury: an interrupted time series analysis. BMJ Open. 9(6):e028912 (2019). 10.1136/bmjopen-2019-028912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Allison PD. Discrete-time methods for the analysis of event histories. American Sociological Association. Sociol Methodol 13, 61–98 (1982). doi: doi:10.2307/270718 [Google Scholar]
- [25].Baan CA, Nusselder WJ, Barendregt JJ, Ruwaard D, Bonneux L, Feskens EJ The burden of mortality of diabetes mellitus in The Netherlands. Epidemiology. 10(2),184–187 (1999). www.jstor.org/stable/3703096. [PubMed] [Google Scholar]
- [26].Colantonio A, Escobar MD, Chipman M, McLellan B, Austin PC, Mirabella G, Ratcliff G Predictors of postacute mortality following traumatic brain injury in a seriously injured population. J Trauma. 64(4),876–882 (2008). 10.1097/TA.0b013e31804d493e [DOI] [PubMed] [Google Scholar]
- [27].Laird N, Olivier D Covariance analysis of censored survival data using log-linear analysis techniques. Am Stat Assoc. 76, 231–240 (1981). doi: doi:10.2307/2287816 [Google Scholar]
- [28].Roberts S, Martin MA The question of non linearity in the dose-response relation between particulate matter air pollution and mortality: can Akaike's Information Criterion be trusted to take the right turn? Am J Epidemiol. 164(12), 1242–1250 (2006). 10.1093/aje/kwj335 [DOI] [PubMed] [Google Scholar]
- [29].Spiegelhalter DJ,Best N, Carlin BP, van der Linde A Bayesian measures of model complexity and fit (with discussion). J R Stat Soc B. 64(4),583–639 (2002). 10.1111/1467-9868.00353 [DOI] [Google Scholar]
- [30].Census Profile, 2017. Census. Ontario and Canada: https://www12.statcan.gc.ca/census-recensement/2017/dp-pd/prof/details/Page.cfm?Lang=E&Geo1=PR&Code1=35&Geo2=&Code2=&Data=Count&SearchText=Ontario&SearchType=Begins&SearchPR=01&B1=All&GeoLevel=PR&GeoCode=35 [Google Scholar]
- [31].Lalonde M A New Perspective on the Health of Canadians. Ottawa: Ministry of Supply and Services, 1974. https://www.phac-aspc.gc.ca/ph-sp/pdf/perspect-eng.pdf [Google Scholar]
- [32].Duric V, Clayton S, Leong M, Yuan LL Comorbidity Factors and Brain Mechanisms Linking Chronic Stress and Systemic Illness. Neural Plast. 2016,5460732 (2016). 10.1155/2016/5460732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Raj R, Mikkonen E,D, Siironen J, Hernesniemi J, Lappalainen J, Skrifvars MB Alcohol and mortality after moderate to severe traumatic brain injury: a metaanalysis of observational studies. J Neurosurg. 124(6), 1684–1692 (2016). 10.3171/2015.4.JNS141746. [DOI] [PubMed] [Google Scholar]
- [34].Leijdesdorff HA, Legué J, Krijnen P, Rhemrev S, Kleinveld S, Schipper IB Traumatic brain injury and alcohol intoxication: effects on injury patterns and short-term outcome. Eur J Trauma Emerg Surg. (2020). 10.1007/s00068-020-01381-6 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Raj R, Skrifvars MB, Kivisaari R, Hernesniemi J, Lappalainen J, Siironen J Acute alcohol intoxication and long-term outcome in patients with traumatic brain injury. J Neurotrauma. 32(2), 95–100 (2015). 10.1089/neu.2014.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Albrecht JS, Afshar M, Stein DM, Smithm GS.Association of Alcohol With Mortality After Traumatic Brain Injury. Am J Epidemiol. 187(2),233–241 (2018). 10.1093/aje/kwx254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pandit V, Patel N, Rhee P, Kulvatunyou N, Aziz H, Green DJ., O'Keeffe T, Zangbar B, Tang A, Gries L, Friese RS, Joseph B Effect of alcohol in traumatic brain injury: is it really protective? J Surg Res. 190(2), 634–639 (2014). 10.1016/j.jss.2014.04.039. [DOI] [PubMed] [Google Scholar]
- [38].Taylor AN, Sutton RL Evidence for Beneficial and Adverse Effects of Alcohol in Animal Models and Clinical Studies of Traumatic Brain Injury In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 48. Frontiers in Neuroengineering. [PubMed] [Google Scholar]
- [39].Kiiskinen T, Mars NJ, Palviainen T, Koskela J, Rämö JT, Ripatti P, Ruotsalainen S; FinnGen, GSCAN Consortium, Palotie A, Madden PAF, Rose RJ, Kaprio J, Salomaa V, Mäkelä P, Havulinna AS, Ripatti S Genomic prediction of alcohol-related morbidity and mortality. Transl Psychiatry. 10(1), 23 (2020). 10.1038/s41398-019-0676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Uccella L, Bongetta D, Fumagalli L, Raffa G, Zoia C Acute alcohol intoxication as a confounding factor for mild traumatic brain injury. Neurol Sci. (2020). 10.1007/s10072-020-04313-9 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [41].Dams-O'Connor K, Gibbons LE, Landau A, Larson EB, Crane PK Health problems precede traumatic brain injury in older adults. J Am Geriatr Soc. 64,844–848 (2016).doi:10.1111/jgs.14014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lin WJ, Harnod T, Lin CL, Kao CH Mortality Risk and Risk Factors in Patients with Posttraumatic Epilepsy: A Population-Based Cohort Study. Int J Environ Res Public Health. 16(4)(2019).doi:10.3390/ijerph16040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Spitz G, Downing MG, McKenzie D, Ponsford JL Mortality following traumatic brain injury inpatient rehabilitation. J Neurotrauma. 32,1272–1280(2015). 10.1089/neu.2014.3814 [DOI] [PubMed] [Google Scholar]
- [44].Cheng CY, Ho CH, Wang CC, Wang JJ, Chio CC, Chang CH, Kuo JR One-Year mortality after traumatic brain injury in liver cirrhosis Patients—Aten-year population-based study. Medicine. 94, e1468 (2015). 10.1097/MD.0000000000001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Shibahashi K, Sugiyama K, Okura Y, Hoda H, Hamabe Y Multicenter Retrospective Cohort Study of "Talk and Die" After Traumatic Brain Injury. World Neurosurg. 107,82–86 (2017). 10.1016/j.wneu.2017.07.117 [DOI] [PubMed] [Google Scholar]
- [46].Muccigrosso MM, Ford J, Benner B, Moussa D, Burnsides C, Fenn AM, Popovich PG, Lifshitz J, Walker FR, Eiferman DS, Godbout JP Cognitive deficits develop 1 month after diffuse brain injury and are exaggerated by microglia-associated reactivity to peripheral immune challenge. Brain Behav Immun. 54,95–109 (2016). 10.1016/j.bbi.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gilthorpe MS, Wilson RC, Moles DR, Bedi R Variations in admissions to hospital for head injury and assault to the head. Part 1: Age and gender. Br J Oral Maxillofac Surg. 37(4), 294–300 (1999). 10.1054/bjom.1998.0039 [DOI] [PubMed] [Google Scholar]
- [48].Lane K, Ito K, Johnson S, Gibson EA, Tang A, Matte T Burden and Risk Factors for Cold-Related Illness and Death in New York City. Int J Environ Res Public Health. 15,4 (2018). 10.3390/ijerph15040632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fazel S, Geddes JR, Kushel M The health of homeless people in high-income countries: descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet. 384(9953),1529–1540 (2014). 10.1016/S0140-6736(14)61132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Forster AJ, Clark HD, Menard A Adverse events among medical patients after discharge from hospital. CMAJ. 170, 345–349 (2004). www.cmaj.ca/cgi/content/full/170/3/345/DC1 [PMC free article] [PubMed] [Google Scholar]
- [51].Isetts BJ, Brummel AR, Ramalho, de. Oliveira D, Moen DW Managing drug-related morbidity and mortality in the patient-centered medical home. Med Care. 50, 997–1001 (2012). doi:10.1097/MLR.0b013e31826ecf9a [DOI] [PubMed] [Google Scholar]
- [52].Wolff ML, Kewley R, Hassett M, Collins J, Brodeur MR, Nokes S Falls in skilled nursing facilities associated with opioid use. J Am Geriatr Soc. 60(5), 987 (2012). 10.1111/j.1532-5415.2012.03913.x. [DOI] [PubMed] [Google Scholar]
- [53].Jamoulle M Quaternary prevention, an answer of family doctors to overmedicalization. Int J Health Policy Manag. 4(2):61–64 (2015). 10.15171/ijhpm.2015.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Beck A, Salem K, Krischak G, Kinzl L, Bischoff M, Schmelz A Nonsteroidal anti-inflammatory drugs (NSAIDs) in the perioperative phase in traumatology and orthopedics effects on bone healing. Oper Orthop Traumatol.17(6),569–578 (2005). 10.1007/s00064-005-1152-0. [DOI] [PubMed] [Google Scholar]
- [55].Bisson EJ, Fakolade A, Pétrin J, Lamarre J, Finlayson M Exercise interventions in multiple sclerosis rehabilitation need better reporting on comorbidities: a systematic scoping review. Clin Rehabil. 31(10),1305–1312 (2017). 10.1177/0269215517698734. [DOI] [PubMed] [Google Scholar]
- [56].Lewandowski-Romps L, Schroeder HM, Berglund PA, Colpe LJ, Cox K, Hauret K, Hay JD, Jones B, Little RJA, Mitchell C, Schoenbaum M, Schulz P, Stein MB, Ursano RJ, Heeringa SG; Army STARRS Collaborators. Medical-encounter mental health diagnoses, non-fatal injury and polypharmacy indicators of risk for accident death in the US Army enlisted soldiers, 2004–2009. Prev Med 111,299–306 (2018). 10.1016/j.ypmed.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nicholson K, Makovski TT, Griffith LE, Raina P, Stranges S,van den Akker M Multimorbidity and comorbidity revisited: refining the concepts for international health research. J Clin Epidemiol. 105,142–146 (2019). 10.1016/j.jclinepi.2018.09.008. [DOI] [PubMed] [Google Scholar]
- [58].Collett GA, Song K, Jaramillo CA, Potter JS, Finley EP, Pugh MJ Prevalence of Central Nervous System Polypharmacy and Associations with Overdose and Suicide-Related Behaviors in Iraq and Afghanistan War Veterans in VA Care 2010–2011. Drugs Real World Outcomes. 3(1),45–52 (2016). 10.1007/s40801-015-0055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Leppin AL, Montori VM, Gionfriddo MR Minimally Disruptive Medicine:A Pragmatically Comprehensive Model for Delivering Care to Patients with Multiple Chronic Conditions. Healthcare (Basel). 3(1),50–63 (2015). 10.3390/healthcare3010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Spaite DW, Bobrow BJ, Keim SM, Barnhart B, Chikani V, Gaither JB, Sherrill D, Denninghoff KR, Mullins T, Adelson PD, Rice AD, Viscusi C, Hu C Association of Statewide Implementation of the Prehospital Traumatic Brain Injury Treatment Guidelines With Patient Survival Following Traumatic Brain Injury: The Excellence in Prehospital Injury Care (EPIC) Study. JAMA Surg. 154(7),e191152 (2019). 10.1001/jamasurg.2019.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Leo P, McCrea M Epidemiology. In: Laskowitz D, Grant G, editors. Translational Research in Traumatic Brain Injury. Boca Raton: (FL): CRC Press/Taylor and Francis Group; 2016. Chapter 1. Frontiers in Neuroscience. [PubMed] [Google Scholar]
- [62].Mollayeva T, Mollayeva S, Colantonio A Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat Rev Neurol 14(12),711–722 (2018). 10.1038/s41582-018-0091-y. [DOI] [PubMed] [Google Scholar]
- [63].Cole JH, Leech R, Sharp DJ; Alzheimer's Disease Neuroimaging Initiative. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol. 77(4),571–81 (2015). 10.1002/ana.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang Z, Li L, Glicksberg BS, Israel A, Dudley JT, Ma'ayan A Predicting age by mining electronic medical records with deep learning characterizes differences between chronological and physiological age. J Biomed Inform. 76,59–68 (2017). 10.1016/j.jbi.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wilson L, Stewart W, Dams-O'Connor K, Diaz-Arrastia R, Horton L, Menon DK, Polinder S The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 16(10), 813–825 (2017). 10.1016/S1474-4422(17)30279-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Nelson LD, Temkin NR, Dikmen S, Barber J, Giacino JT, Yuh E, Levin HS, McCrea MA, Stein MB, Mukherjee P, Okonkwo DO, Diaz-Arrastia R, Manley GT; and the TRACK-TBI Investigators. Recovery After Mild Traumatic Brain Injury in Patients Presenting to US Level I Trauma Centers: A Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Study. JAMA Neurol (2019). 10.1001/jamaneurol.2019.1313 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.