Abstract
Epigallocatechin-3-gallate (EGCG), a major polyphenol component of green tea, presents anticancer efficacy. However, its exact mechanism of action is not known. In this study, we evaluated the effect of EGCG alone or in combination with current chemotherapeutics [gemcitabine, 5-flourouracil (5-FU), and doxorubicin] on pancreatic, colon, and lung cancer cell growth, as well as the mechanisms involved in the combined action. EGCG reduced pancreatic, colon, and lung cancer cell growth in a concentration and time-dependent manner. EGCG strongly induced apoptosis and blocked cell cycle progression. Moreover, EGCG enhanced the growth inhibitory effect of 5-FU and doxorubicin. Of note, EGCG enhanced 5-FU’s and doxorubicin’s effect on apoptosis, but not on cell cycle. Mechanistically, EGCG reduced ERK phosphorylation concentration-dependently, and sensitized gemcitabine, 5-FU, and doxorubicin to further suppress ERK phosphorylation in multiple cancer cell lines. In conclusion, EGCG presents a strong anticancer effect in pancreatic, colon, and lung cancer cells and is a robust combination partner for multiple chemotherapeutics as evidenced by reducing cancer cell growth, in part, by inhibiting the ERK pathway.
Keywords: pancreatic cancer, colon cancer, lung cancer, epigallocatechin-3-gallate, gemcitabine, 5-FU, doxorubicin, ERK
Introduction
Pancreatic, colon, and lung malignancies have the highest cancer morbidity and mortality for both genders, in the United States [1]. Besides surgery and radiation, the use of chemotherapy, either alone or in combination, is one of the most common ways to treat cancer. Unfortunately, conventional drug therapies have obvious limitations due to chemoresistance, as well as undesirable systemic side effects, which are often severe. For example, gastrointestinal tumors treated with the chemotherapy 5-fluorouracil (5-FU), can easily acquire resistance. Furthermore, 5-FU is associated with health risks, ranging from nausea and diarrhea to neurological disorders and myelosuppression [2, 3]. For these reasons, it is imperative to search for safer treatment strategies.
Over the last two decades, there has been a growing interest in identifying bioactives with anticancer effects. Due to chemotherapy’s significant side effects, combining chemotherapeutic drugs with other agents, such as bioactives, is a promising approach to reduce toxicity while maintaining (or enhancing) the desired efficacy. Among several bioactives under investigation, many phytochemicals have been shown to possess anticancer effects, suppressing cancer growth at various steps. Epigallocatechin-3-gallate (EGCG), a major bioactive component in green tea, is one of these phytochemicals with anticancer activity [4]. Indeed, we have recently shown that EGCG synergized with gemcitabine to suppress pancreatic cancer cell growth [5, 6]. However, the ability of EGCG to enhance the effect of chemotherapeutic drugs in other cancer types is not completely understood.
Raf/MEK/ERK pathway is frequently activated in various malignancies, correlating to cell growth, cell cycle, and even apoptosis prevention [7]. Notably, activation of Raf/MEK/ERK pathway is also correlated to drug resistance [8]. Thus, inhibitors of Raf, MEK, ERK or some downstream effectors could be the target for therapeutic intervention. However, though the Raf/MEK/ERK pathway plays a vital role in controlling tumor growth and drug resistance, the regulation effect of EGCG remains unclear.
In this study, we evaluated the efficacy and mechanisms of EGCG in combination with chemotherapeutics (gemcitabine, 5-FU, and doxorubicin) active against pancreatic, colon, and lung cancers to elucidate whether EGCG is a potential adjuvant agent for cancer treatment. We observed that EGCG enhanced gemcitabine, 5-FU, and doxorubicin cell growth inhibition and induced apoptotic cell death in pancreatic, colon, and lung cancer cells, and this effect was associated, in part, with the suppression of the Raf/MEK/ERK pathway.
Materials and Methods
Chemicals and Reagents
EGCG (≥98%) was purchased from Tocris (Minneapolis, MN) and a stock solution (100 mM) was prepared in sterile DMSO. Doxorubicin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (≥97.5%), RIPA lysis buffer, Halt Protease Inhibitor Cocktail, and Phosphatase Inhibitor Cocktail were purchased from MilliporeSigma (St. Louis, MO). SuperSignal™ West Dura Extended Duration Substrate were purchased from ThermoFisher Scientific (Waltham, MA). Gemcitabine was purchased from BIOTANG (Waltham, MA, USA). 5-FU (≥99%) was purchased from Alfa Aesar (Haverhill, MA, USA). Bradford protein assay reagent, 30% (w/v) Acrylamide/Bis Solution, 4×Laemmli sample buffer, Immun-Blot Polyvinylidene difluoride (PVDF) Membranes and were purchased from Bio-Rad (Hercules, CA).
Cell Culture
Human pancreatic cancer cell lines (Panc-1, MIA PaCa-2, and BxPC-3), human colon cancer cell lines (SW480, HCT15, and HT29), and human lung cancer cell lines (HT1975, H358, and A549) were purchased from the American Type Culture Collection (Manassas, VA). All cell lines were grown as monolayers in the specific medium suggested by the vendor. Although these cells lines were not authenticated in our lab, they were characterized by cell morphology and growth rate, and cultured in our laboratory less than six months after being received.
Cell Viability
After treating cells with EGCG alone or together with specific chemotherapeutic drugs for 24, 48 and 72 h, the reduction of MTT dye was determined according to the manufacture’s protocol (MilliporeSigma, St. Louis, MO).
Clonogenic Assay
This was performed as previously described [9]. Briefly, HCT15 colon cancer cells were plated in 6-well plates (1,000 cells per well), and treated with 5-FU alone or in combination with EGCG for 24 h. Following treatment, cells were then incubated with fresh media for 20 days. Media was replaced once weekly during the incubation. On the last day, colonies were fixed with methanol and stained with 0.1% (w/v) crystal violet in phosphate buffered saline (PBS) (pH 7.4). Cells were then rinsed with distilled water, air-dried, and colonies were counted and analyzed using ImageJ software (V1.46, NIH, Bethesda, MD, USA).
Cell Apoptosis
Cells were seeded in 100 mm plates at a density of 1.5 million cells per plate. The following day, cells were treated with EGCG, chemotherapy drugs, or a combination. After 48 h treatment, cells were trypsinized and stained with Annexin V-fluorescein isothiocyanate (FITC) (100× dilution) and propidium iodide (PI) (0.5 μg/mL) for 15 min. Annexin V-FITC and PI fluorescence intensities were analyzed by FACScan (Becton Dickinson, San Jose, CA, USA). Annexin V (+)/PI (−) cells are apoptotic cells, Annexin V (+)/ PI (+) cells have undergone secondary necrosis, and Annexin V (−)/ PI (+) cells are necrotic cells. Results were analyzed by using FlowJo software.
Cell Cycle Analysis
Cells were seeded in 6-well plates and treated the following day with EGCG, chemotherapy drugs, or a combination for 24 h. After each treatment, cells were trypsinized and fixed in 70% ethanol overnight at −20°C, stained with PI (50 μg/ml) and RNase A (10 mg/ml) for 15 min and subjected to flow cytometric analysis by FACScan (Becton Dickinson; San Jose, CA).
Western Blot
Following treatment with EGCG, chemotherapy drugs, or a combination, cells were lysed, and total cell fractions were obtained as previously described [10]. Aliquots of total fractions containing 10–30 μg protein were separated by using 10–12% (w/v) polyacrylamide gel electrophoresis and electroblotted to PVDF membranes. After blocking with 5% (w/v) non-fat milk for 1 h, membranes were probed overnight with the following primary antibodies (1:1000 dilution) from Cell Signaling Technology (Danvers, MA): Caspase-3 (Cat #14220), Caspase-7 (Cat #12827), Caspase-9 (Cat #9508), PARP (Cat #9542), phospho-Chk1 (Ser345) (Cat #2348), phospho-p53 (Ser15) (Cat #9286), p53 (Cat #2527), p21 Waf1/Cip1 (Cat #2947), cdc2 (Cat #28439), Cyclin B1 (Cat #12231), Bcl-xL (Cat #2802), Bad (Cat #9239), XIAP (Cat # 14334), survivin (Cat # 2808), p-ERK1/2 (Cat #4370), and ERK1/2 (Cat #9102). β-Actin (Cat #8457) was used at the same time as a loading control. After incubation for 60 min at room temperature in the presence of the secondary antibody (HRP-conjugated; 1:5,000 dilution), the conjugates were developed and visualized using a Molecular Imager FX™ System (BioRad; Hercules, CA) and analyzed using ImageJ software(V1.46, NIH, Bethesda, MD, USA).
Immunohistochemistry
Immunohistochemistry was performed using tumor samples from a previous efficacy study that evaluated the effect of EGCG and gemcitabine on murine pancreatic cancer xenografts [5]. Briefly, immunohistochemical staining for p-ERK1/2 (Cat #4370; Cell Signaling Technology, Danvers, MA, USA) was performed as previously described [33]. Briefly, paraffin-embedded sections (5 μm thick) were deparaffinized and rehydrated, followed by antigen retrieval performed by microwave-heating in 0.01 M citrate buffer (pH 6.0). H2O2 3% was used to block endogenous peroxidase activity for 10 min at room temperature. Slides were blocked for 60 min with serum, and incubated with primary antibody overnight at 4 °C. The following morning, slides were washed thrice with PBS, and then incubated with the biotinylated secondary antibody and the streptavidin-biotin complex (Invitrogen, Carlsbad, CA, USA) for 1 h each at room temperature. After washing with PBS three times, slides were stained with 3,3′-Diaminobenzidine tetrahydrochloride hydrate (DAB) solution, and then counterstained with hematoxylin. Images were captured at 100× magnification. At least five fields per sample were scored and analyzed using Image J software (V1.46, NIH, Bethesda, MD, USA).
Statistical Analysis
The data, obtained from at least three independent experiments, were expressed as mean ± standard deviation (SD). Statistical evaluation was performed using one-factor analysis of variance (ANOVA) followed by the Duncan test for multiple comparisons. T-tests were used to analyze the difference between two groups. A P value<0.05 was regarded as statistically significant.
Results
EGCG reduces cancer cell growth in multiple cancer cell lines
To test the anticancer effect of EGCG on cancer cell growth, we included nine human cancer cell lines from pancreatic (Panc-1, MIA PaCa-2, and BxPC-3), colon (HCT15, SW480, and HT29) and lung cancer (A549, H358, and HT1975) and treated them with increasing concentrations of EGCG (20–100 μM) for 24, 48, and 72 h. In all nine cell lines, EGCG reduced cancer cell growth in a time- and concentration-dependent manner. However, different cell lines displayed varying sensitivity to EGCG, as BxPC-3, HT1975, and HCT15 were relatively more sensitive to EGCG, while Panc-1, H358, and HT29 showed more resistance (Fig 1). The Inhibitory Concentration at 48 h (48 h-IC50) for EGCG in each cell line is summarized in Figure 1D. Given the high prevalence of Kras mutations in pancreatic, colon, and lung cancer, we chose Panc-1, MIA PaCa-2, HCT15 and A549 cell lines, which are Kras mutant cell lines, for the subsequent studies.
Figure 1: EGCG reduces pancreatic, colon, and lung cancer cell growth in vitro.
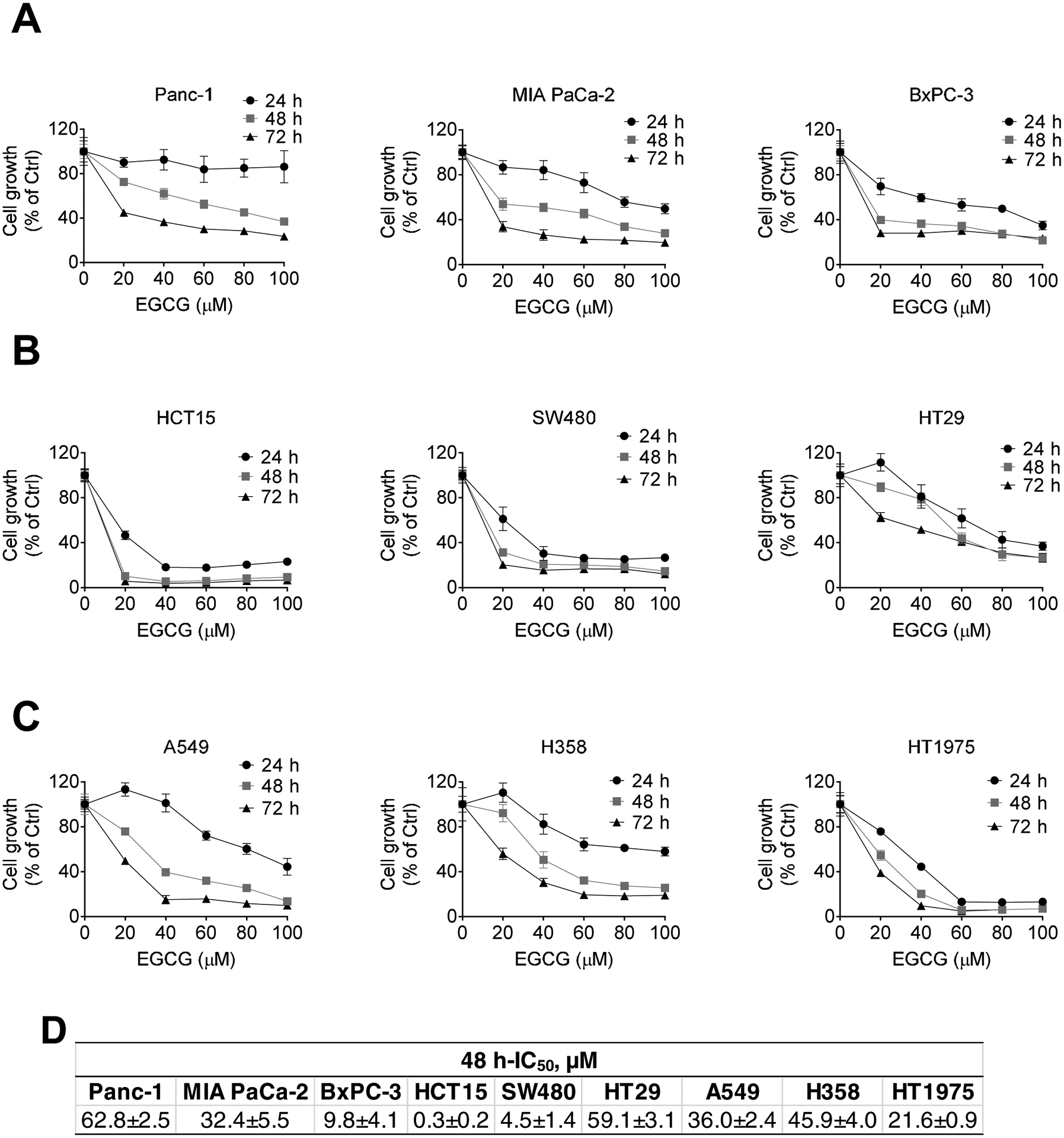
EGCG inhibits pancreatic, colon, and lung cancer cell growth in a concentration- and time-dependent manner. Cell growth was determined in: A: pancreatic Panc-1, MIA PaCa-2, BxPC-3, B: colon HCT15, SW480, HT-29, and C: lung A549, H358, and HT1975 cancer cells, after treatment with escalating concentrations of EGCG for 24, 48 or 72 h. Results (mean ± SD) are expressed as percentage of control. D: Inhibitory Concentration 50 (IC50) values for pancreatic, colon, and lung cancer cells treated with EGCG for 48 h (mean ± SD).
EGCG reduces cancer cell growth through a strong cytokinetic effect
EGCG inhibited tumor growth through a potent cytokinetic effect. Treatment of Panc-1 and MIA PaCa-2 cells with EGCG for 48 h led to a concentration-dependent induction of apoptosis (Fig. 2A). EGCG at 1xIC50 for 48 h induced apoptosis by 3.5 and 2.1-fold over control in Panc-1 and MIA PaCa-2 cells, respectively (p<0.01). Notably, EGCG predominantly induced apoptotic cell death, with no significant induction of cell necrosis [Annexin V(−) but PI (+)].
Figure 2: EGCG induces cell death by apoptosis in pancreatic, colon, and lung cancer cells.
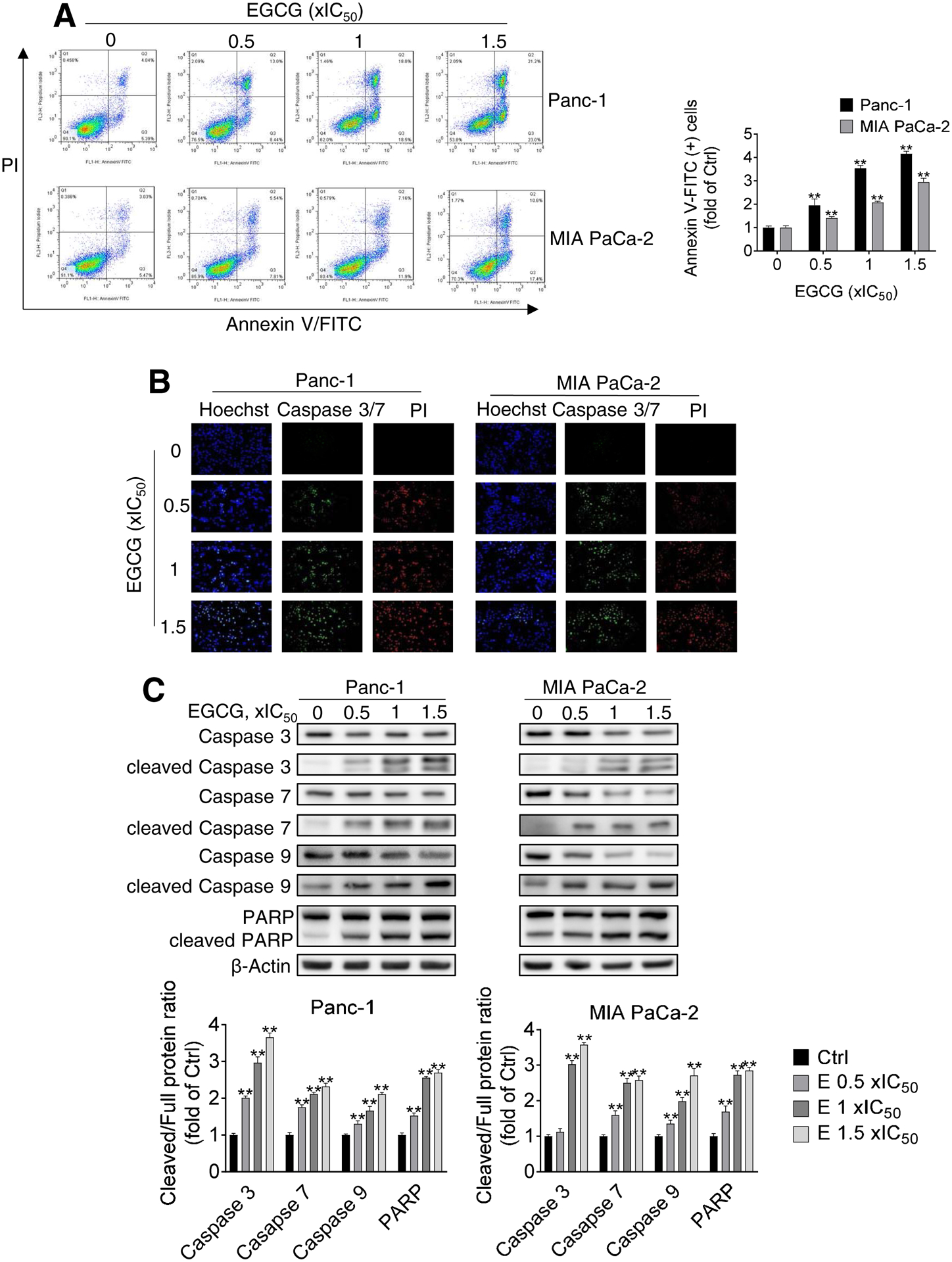
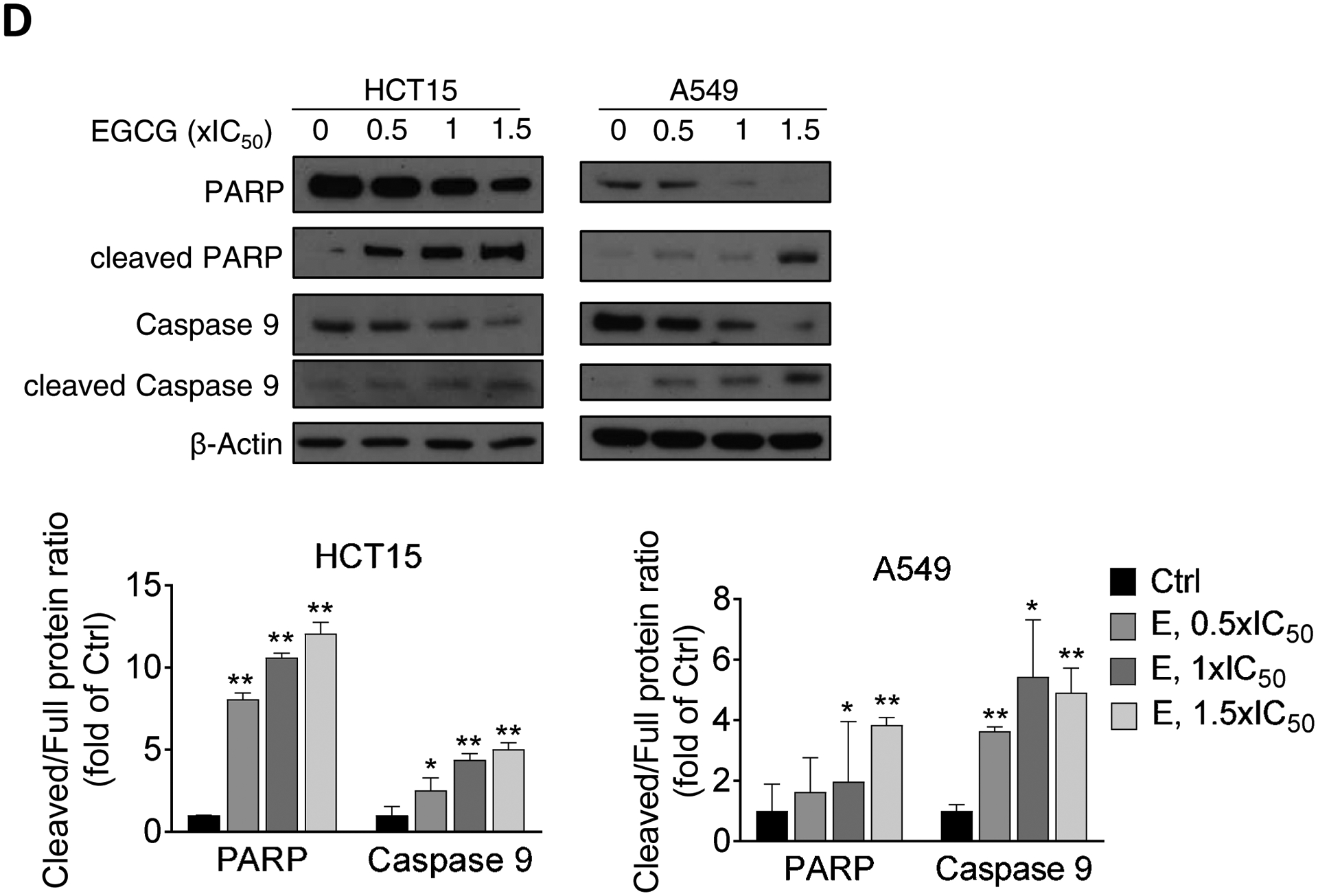
A: Panc-1 and MIA PaCa-2 cells, treated with EGCG for 48 h, were stained with Annexin V/propidium iodide (PI), and the percentage of apoptotic cells was determined by flow cytometry. Results are expressed as fold change of control. *p<0.05, **p<0.01 vs. control. B: Caspase 3/7 activation was examined by immunofluorescence, by triple-staining control and EGCG-treated cells with Hoechst, PI and Caspase 3/7 green detection reagents. Representative images are shown (200x). C: Immunoblots for full length and cleaved caspases 3,7, and 9 as well as full length and cleaved PARP in total cell protein extracts from Panc-1 and MIA PaCa-2 cells treated with escalating concentrations of EGCG (0.5x, 1x and 1.5x IC50) for 48 h. Loading control: β-Actin. Bands were quantified and results are shown as the ratio between the cleaved/full length protein; *p<0.05, **p<0.01 vs. control. D: Immunoblots for full length and cleaved caspase 9 as well as full length and cleaved PARP in total cell protein extracts from HCT15 colon cancer and A549 lung cancer cells treated with escalating concentrations of EGCG (0.5x, 1x and 1.5x IC50) for 48 h. The control sample labeled as “0” refers to untreated control. Loading control: β-Actin. Bands were quantified and results are shown as the ratio between the cleaved/full length protein; *p<0.05, **p<0.01 vs. control.
These findings were validated by determining the activation and levels of apoptotic-related Caspases by microscopy and Western blot (Fig. 2B–C). In Panc-1 and MIA PaCa-2 cells, EGCG treatment induced the activation of Caspase 9, 7, and 3 in a concentration-dependent manner. For example, EGCG at 1xIC50 activated Caspase 3 levels by 2.9 and 3.0-fold in Panc-1 and MIA PaCa-2 cells, respectively, compared to the control group (p<0.01 for both). As a consequence of Caspase 3 activation, levels of cleaved poly(ADP-ribose) polymerase (PARP) increased in all EGCG treatments (Fig. 3C).
Figure 3. Effect of EGCG on apoptosis-related protein expression and ERK phosphorylation in multiple human cancer cells.
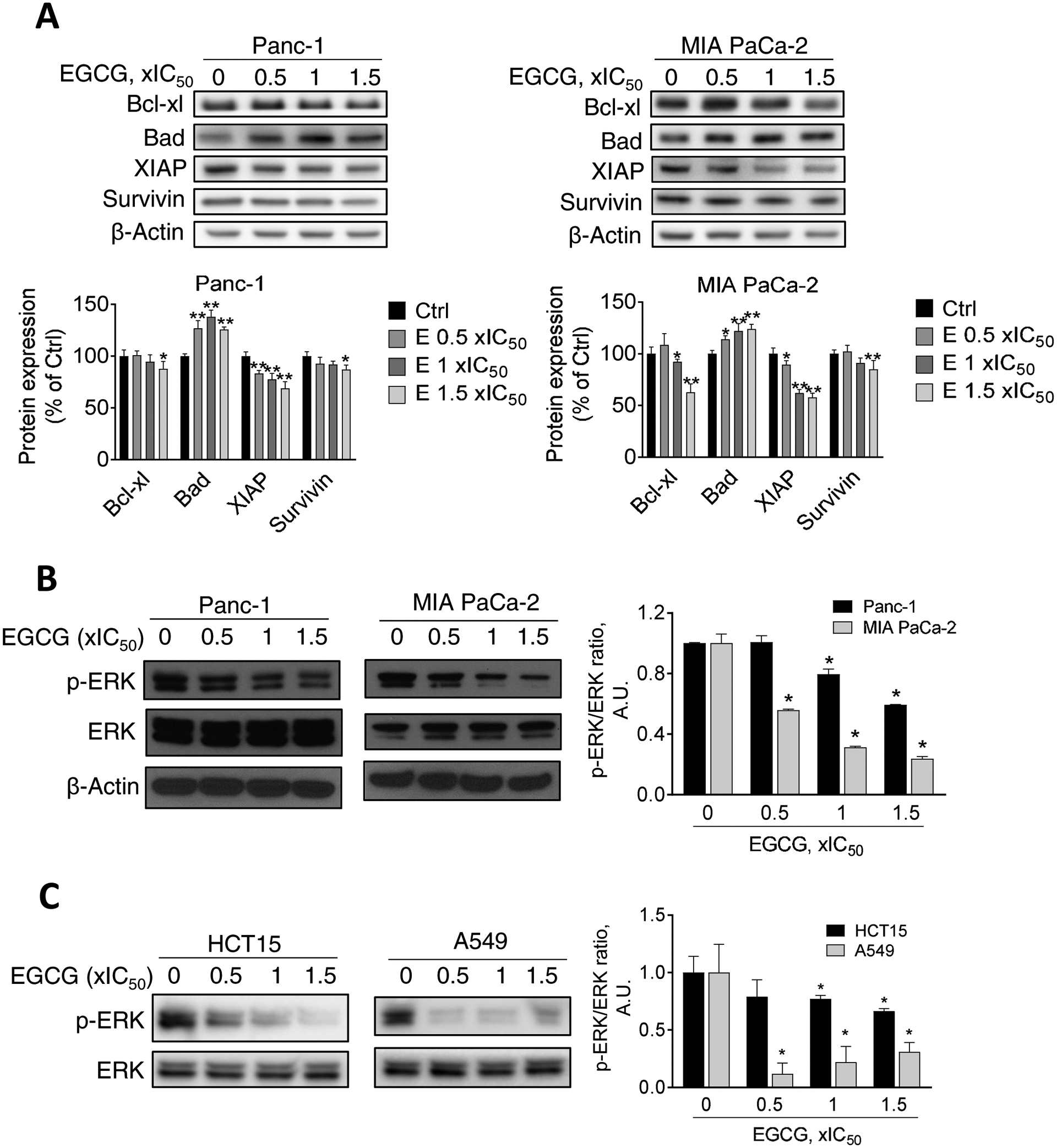
A: EGCG modulates the Bcl-2 family and XIAP family protein expression. Immunoblots for Bcl-xL, Bad, XIAP, and survivin in total cell protein extracts from Panc-1 and MIA PaCa-2 cells treated with escalating concentrations of EGCG, as indicated, for 24 h. Loading control: β-Actin. Bands were quantified and results are expressed as percentage of control. *p<0.05, **p<0.01 vs. control. B: EGCG reduces ERK phosphorylation in Panc-1 and MIA PaCa-2 cells, in a concentration-dependent manner. Results are expressed as the p-ERK/ERK ratio, normalized to control. The sample labeled as “0” refers to an untreated control. *p<0.05, vs. control. C: EGCG reduces ERK phosphorylation in HCT15 and A549 cells. Immunoblots for p-ERK, and ERK in total cell protein extracts from HCT15 and A549 cells treated with escalating concentrations of EGCG (0.5x, 1x and 1.5x IC50) for 24 h. Results are expressed as the p-ERK/ERK ratio, normalized to control. The sample labeled as “0” refers to an untreated control. *p<0.05, vs. control.
Next, we evaluated whether EGCG can also induce apoptosis in colon and lung cancer cells. For this purpose, we treated HCT15 colon cancer and A549 lung cancer cell lines with increasing concentration of EGCG for 48 h, and determined apoptotic-related caspases by Western blot. EGCG treatment induced the activation of Caspase 9, and PARP in a concentration-dependent manner in both HCT15 and A549 cells (Fig. 2D).
To explore the apoptosis mechanism induced by EGCG, we determined, in Panc-1 and MIA PaCa-2 cells, the expression levels of multiple proteins that regulate apoptosis, including proteins in the inhibitor of apoptosis protein (XIAP) and Bcl-2 family. As shown in Figure 3, while EGCG reduced Bcl-xl, XIAP, and survivin levels, it increased the levels of the proapoptotic protein Bad concentration-dependently.
Because ERK1/2 has been shown to modulate cell survival through the regulation of Bcl-2 protein family [11], we next evaluated the effect of EGCG on ERK phosphorylation. In Panc-1 and MIA PaCa-2 cells, EGCG treatment for 24 h reduced ERK1/2 phosphorylation in a concentration-dependent manner (Fig. 3B).
Next, we evaluated whether EGCG can also modulate the ERK pathway in colon and lung cancer cells. For this purpose, we treated HCT15 colon cancer and A549 lung cancer cell lines with increasing concentration of EGCG for 24 h, and determined ERK phosphorylation by Western blot. Consistent with our findings in pancreatic cancer cells, EGCG treatment strongly reduced ERK phosphorylation in both HCT15 and A549 cells (Fig. 3C).
To examine whether EGCG can affect cell cycle progression, we performed flow cytometry to test cell cycle distribution and determined the levels of cell cycle regulators by Western blot. EGCG induced an S/G2 arrest in Panc-1 cells. However, under the same experimental conditions, a lesser effect was observed in MIA PaCa-2 cells (Fig. 4A). Given the effect of EGCG on the S/G2 transition, we examined the expression levels of G2 phase checkpoint proteins. In Panc-1 and MIA PaCa-2 cells, EGCG treatment increased the expression of p-Chk1, p-p53, and p21 Waf1/Cip1, whereas it reduced the levels of cdc2 and Cyclin B1 (Fig. 4B).
Figure 4. EGCG induces S/G2 cell cycle arrest in pancreatic cancer cells.
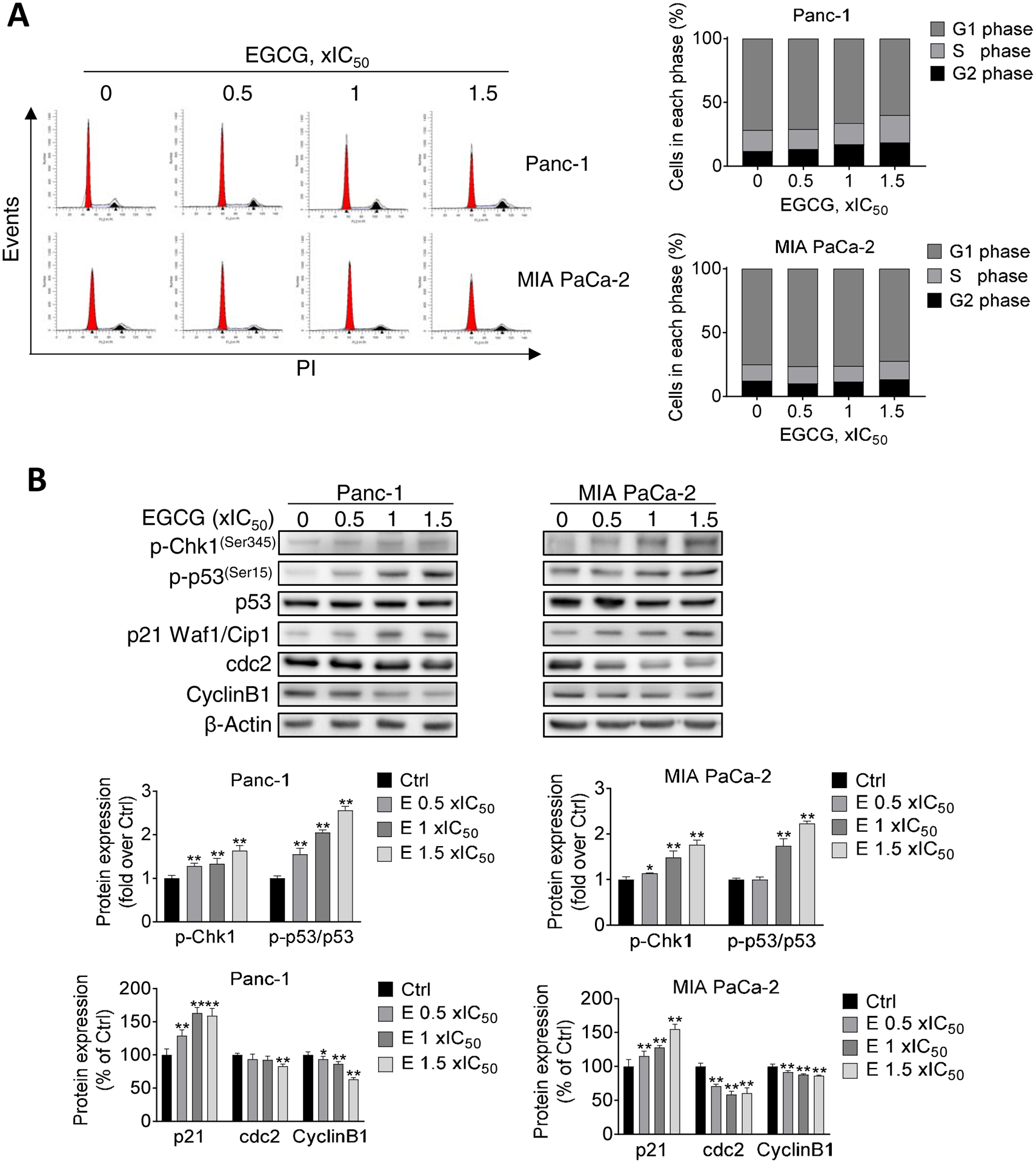
A: Panc-1 and MIA PaCa-2 cells were treated with EGCG for 24 h, as indicated, and the cell cycle phase transition was determined by flow cytometry following PI staining. B: EGCG modulated S/G2 phases arrest protein expression. Immunoblots for phosphorylated Chk1 (p-Chk1), phosphorylated p53 (p-p53), p53, p21, cdc2, and cyclin B1 in total cell protein extracts from Panc-1 and MIA PaCa-2 cells treated with escalating concentrations of EGCG, as indicated, for 24 h. Loading control: β-Actin. Bands were quantified and results are expressed as percentage of control. *p<0.05, **p<0.01 vs. control.
EGCG enhances the cytotoxicity of chemotherapeutics in colon and lung cancer cells
The use of drugs in combination to treat cancer patients is a common practice. We have recently documented that EGCG enhances the chemotherapeutic efficacy of gemcitabine in pancreatic cancer cells and xenografts [5, 6]. Here, we evaluated whether EGCG can enhance the efficacy of chemotherapeutic drugs in colon and lung cancer cells. For this purpose, we treated cells with EGCG together with 5-FU or doxorubicin, two chemotherapeutics commonly used clinically and experimentally in colon and lung cancer. As shown in Figure 5A, EGCG increased the cytotoxicity of 5-FU in HCT15 cells. Compared with the control group, 20 μM 5-FU decreased cell growth to 62.7%, while the cell growth was further reduced to 29.4% after treatment together with EGCG at 1xIC50 (p<0.01). In A549, after co-treating cells with EGCG and doxorubicin, cell growth decreased to about 40%, lower than the doxorubicin alone treated groups, while kept a similar level as EGCG alone group (Fig. 5A).
Figure 5. EGCG enhances the cell growth inhibitory effect of 5-FU and Doxorubicin in colon and lung cancer cells.
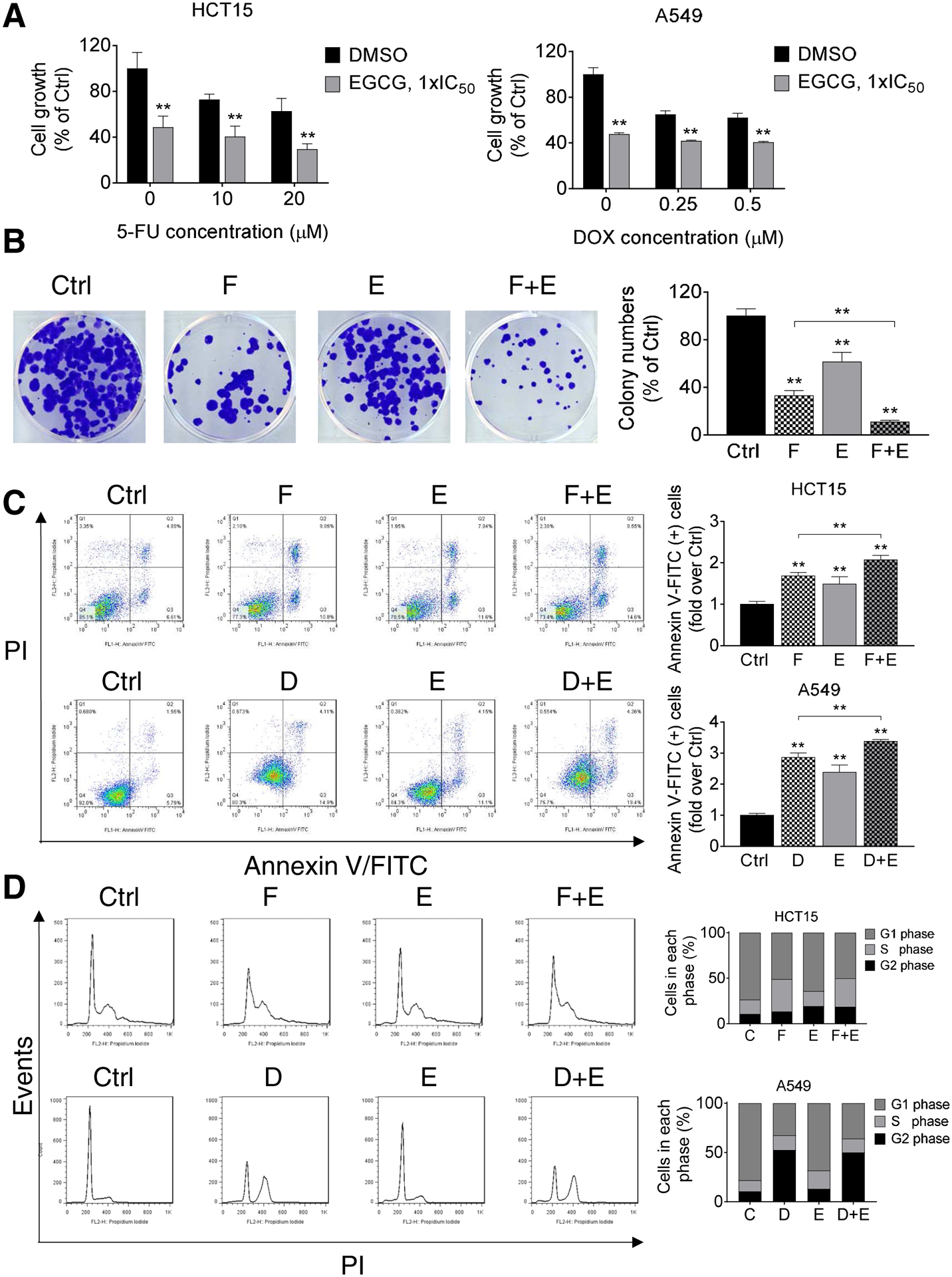
A: Cell growth was determined in HCT15 and A549 cells following treatment with EGCG (1xIC50), 5-FU, doxorubicin (DOX), or combinations of EGCG + 5-FU or EGCG + DOX for 72 h. Results are expressed as a percentage of control. ** p<0.01 vs. control. B: HCT15 cells were treated with EGCG (E), 5-FU (F), or both (F + E) for 48 h, and the colony formation capacity was determined. Results are expressed as a percentage of control. * p<0.05, ** p<0.01 vs. control. C: HCT15 cells (top) were treated with 1xIC50 EGCG (E), 10 μM 5-FU (F), or both (F+E) for 48 h, while A549 cells (bottom) were treated with 1xIC50 EGCG (E), 0.25 μM doxorubicin (D), or both (D+E) for 48 h. The percentage of apoptotic cells were determined by flow cytometry using dual staining (Annexin V and propidium iodide(PI)). The percentages of Annexin V (+) cells was calculated, and results are expressed as the fold-increase over control. Co-treatment with EGCG (E) further increased the apoptosis rate induced by 5-FU (F) in HCT15 cells and that of doxorubicin (D) in A549 cells, after 48 h. Results are expressed as percentage of control. *p<0.05, **p<0.01 vs. control. D: EGCG (E) does not enhance the effect of 5-FU or doxorubicin on the cell cycle. Following treatment with 1xIC50 EGCG (E), 10 μM 5-FU (F), 0.25 μM doxorubicin (D), or combinations of these agents for 24 h, cells were stained with PI and the number of cells in each phase of the cell cycle was measured by flow cytometry.
In agreement with the growth inhibitory results, EGCG and 5-FU together also effectively inhibited the colony formation in HCT15 cells (Fig 5B). For example, 5-FU alone reduced the formation rate by 66.9%, and the inhibitory effect was enhanced to 90.1% when combined with EGCG (p<0.01).
EGCG and chemotherapy drugs together also induced more apoptosis compared with each treatment alone. In HCT15 and A549 cells, compared to control, treatment with 5-FU or doxorubicin for 48 h resulted in a 1.7 and 2.8 fold increase in apoptosis, respectively. The effect was further enhanced to 2.1 and 3.4 fold after treatment together with EGCG (p<0.01, Fig. 5C). In contrast, EGCG treatment did not enhance the cell cycle arrest inhibitory effect of 5-FU or doxorubicin on cell cycle progression (Fig. 5D).
EGCG enhances drug sensitivity by the inhibition of Raf/MEK/ERK pathway
We next investigated the potential mechanisms by which EGCG plus chemotherapeutics reduced cell growth and induced cell death by apoptosis. Because the ERK pathway plays a critical role in controlling tumor growth and drug resistance [12], we evaluated the effect of EGCG in combination with gemcitabine, 5-FU, and doxorubicin on the ERK pathway.
We first explored the effect of EGCG in combination with gemcitabine on ERK activation in pancreatic cancer cells. As shown in Figure 6A, EGCG 1xIC50 reduced the levels of ERK phosphorylation in Panc-1 and MIA PaCa-2 cells by 53% and 37.5% (p<0.05 for both), respectively, and this effect was enhanced in both cell lines, when combined with gemcitabine (70% and 49% in Panc-1 and MIA PaCa-2 cells, respectively). Of note, gemcitabine alone did not affect ERK phosphorylation in Panc-1 and MIA PaCa-2 cells (Fig. 6A). Consistent with the in vitro results, EGCG plus gemcitabine had an additive effect reducing the levels of p-ERK (p < 0.05) in pancreatic tumor xenografts [5] by 91%, compared to control, and the effect was stronger than either treatment alone (Fig. 6B).
Figure 6: EGCG enhances the effect of gemcitabine on the ERK pathway inhibition in pancreatic cancer.
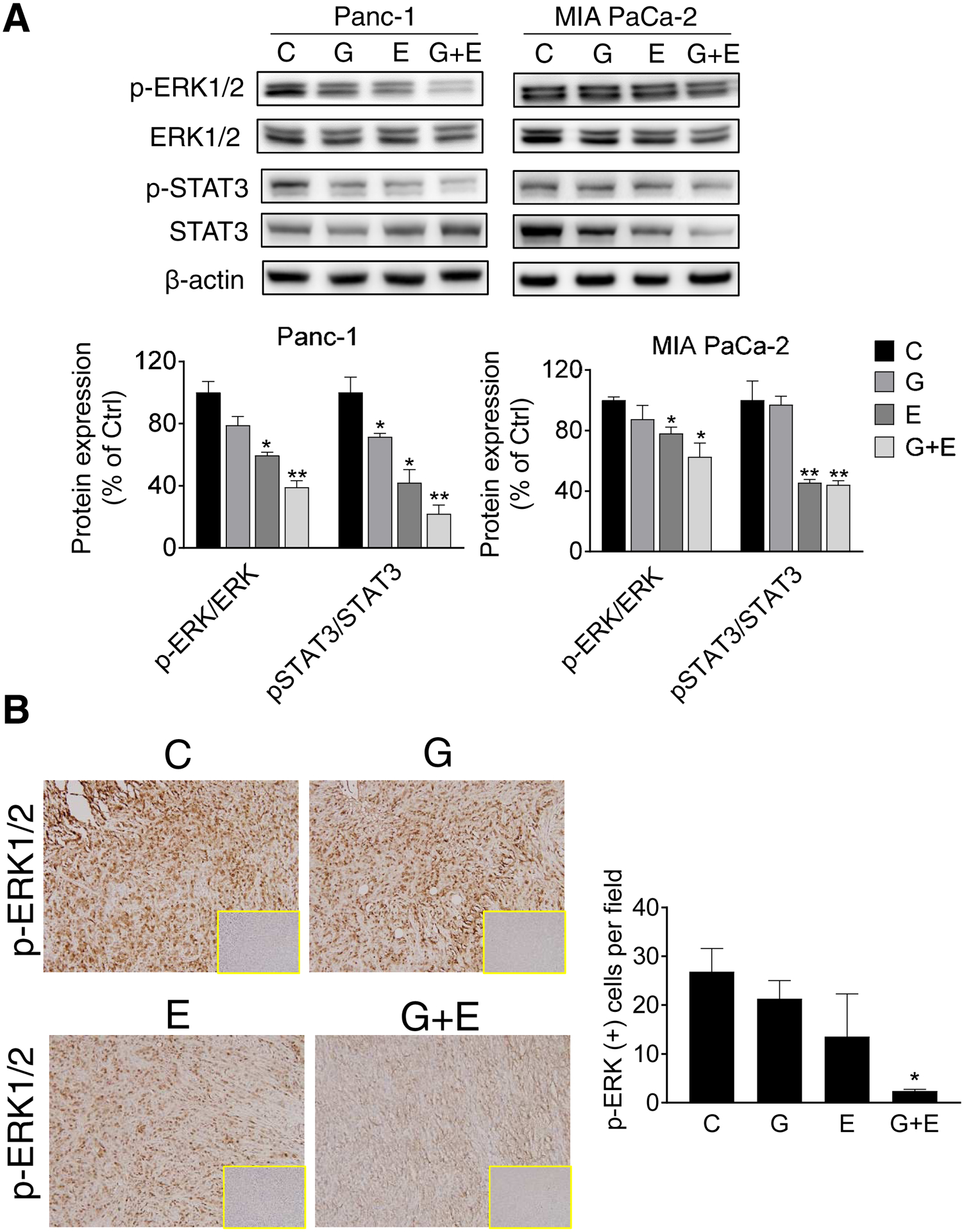
A: Immunoblots for phosphorylated/total ERK and phosphorylated and total ERK in whole cell protein extracts from Panc-1 and MIA PaCa-2 cells treated with EGCG (E), gemcitabine (G), or both (G + E) for 24 h. Loading control: β-Actin. Bands were quantified and results expressed as the p-ERK/ERK ratio, normalized to control. * p<0.05, ** p<0.01 vs. control. B: Immunohistochemistry analysis performed on mouse pancreatic tumor xenograft samples. p-ERK immunostainings were performed on tumor sections and photographs were taken at 20x magnification. Representative images are shown. Results were expressed as percent of p-ERK(+) cells ± SD per 20x field. *Significant compared to control group; p<0.05.
Because ERK is known to phosphorylate STAT3 at the serine 727 residue [13], we also tested the effect of EGCG alone, gemcitabine alone, and EGCG and gemcitabine in combination on STAT3 phosphorylation. EGCG alone reduced STAT3 phosphorylation in Panc-1 and MIA PaCa-2 cell lines. While gemcitabine alone only reduced p-STAT3 levels in Panc-1 cells lines, the combination of EGCG plus gemcitabine had an additive effect and reduced STAT3 phosphorylation. In MIA PaCa-2 cells, p-STAT3 expression in the EGCG plus gemcitabine group was similar to that of EGCG alone (Fig. 6A).
We then evaluated whether EGCG enhanced the effect of 5-FU and doxorubicin on ERK phosphorylation in colon and lung cancer cells. In HCT15 cells, EGCG reduced ERK phosphorylation by 80% and no additional effect was observed when combined with 5-FU (Fig. 7A). In contrast, EGCG treatment reduced ERK phosphorylation in A549 by 27% and this was significantly enhanced when combined with doxorubicin (75% reduction vs. control; Fig. 7B).
Figure 7: EGCG enhances sensitivity of 5-FU and doxorubicin on ERK inhibition in colon and lung cancer cells.
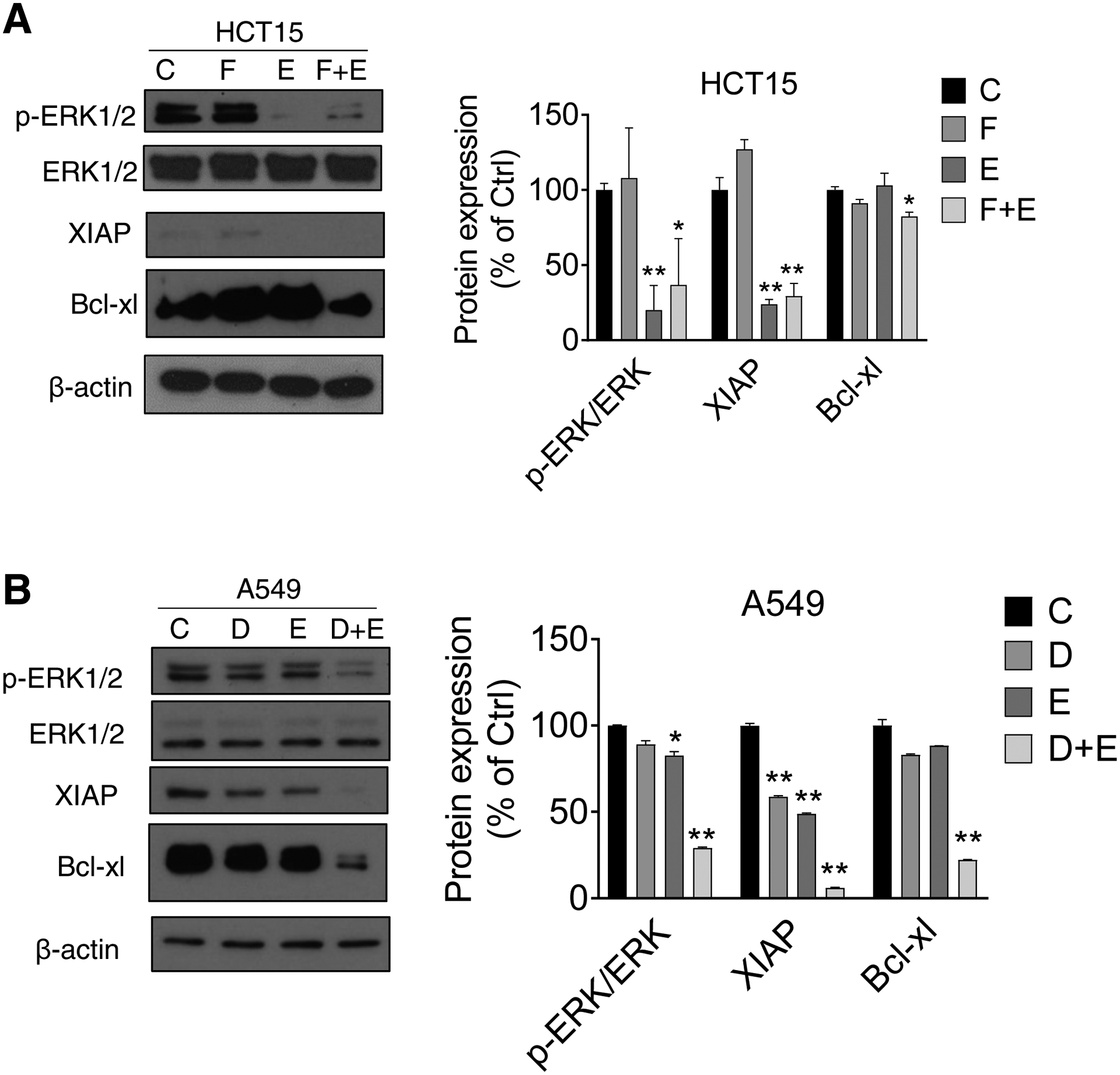
A: HCT15 cells were treated with 1xIC50 EGCG (E), 10 μM 5-FU (F), or both (F+E) for 24 h. Immunoblots for phosphorylated/total ERK, Bcl-xL, and XIAP in whole cell protein extracts from HCT15 cells are shown. Loading control: β-Actin. Bands were quantified and results are expressed as a percentage of control. * p<0.05, ** p<0.01 vs. control. B: A549 cells were treated with 1xIC50 EGCG (E), 0.25 μM doxorubicin (D), or both (D+E) for 24 h. Immunoblots for phosphorylated/total ERK, Bcl-xL, and XIAP in whole cell protein extracts from A549 cells are shown. Loading control: β-Actin. Bands were quantified and results are expressed as a percentage of control. * p<0.05, ** p<0.01 vs. control.
Finally, in both HCT15 and A549 cells, the expression of Bcl-xl and XIAP decreased in the EGCG plus 5-FU as well as in the EGCG plus doxorubicin groups compared to 5-FU alone or doxorubicin alone (Figure 7A–B).
Discussion
Patients receiving chemotherapy usually experience side effects, many of which are often severe. Therefore, there is an active search for safer and more effective therapeutic approaches. In this study, we show that the polyphenol EGCG, is a successful combination partner of various chemotherapy drugs in pancreatic, colon, and lung cancer cells, and that its anticancer effect is due, in part, through the modulation of the ERK pathway.
EGCG is a major bioactive component in green tea, with strong anticancer activity in multiple types of cancers [6, 14–19]. Indeed, EGCG strongly reduced the growth of pancreatic, colon, and lung cancer cell in a time- and concentration-dependent manner. The anticancer effect of EGCG results from its strong cytokinetic effect: inhibition of proliferation, induction of apoptosis, and block at the S/G2 cell cycle transition [18]. The apoptotic effect of EGCG seems to be the dominant one. For example, EGCG at 1xIC50 induced apoptosis in pancreatic cancer cells by up to 3.5-fold compared to controls, with arrest of the cell cycle showing only a moderate effect. The apoptotic cascade of the pancreatic cancer cells was manifested by the activation of execution caspases [20], and the modulation of Bcl-2 and the inhibitor of apoptosis protein families by EGCG. The apoptotic effect was not restrained to only pancreatic cancer cells, since EGCG also strongly induced apoptosis in colon and lung cancer cells, showing that this effect is observed in multiple cancer types. Our findings are consistent with others, showing that EGCG strongly induces apoptosis in various types of tumors [21–24].
Conventional chemotherapy is commonly associated with both acute and chronic toxicity [25]. Based on the World Health Organization classification, signs of toxicity can be classified in grades 1–4 (ranging from mild (grade 1) to life-threatening (grade 4). Common side effects of gemcitabine, 5-FU, and doxorubicin include anorexia, nausea, vomiting, and fatigue. Other, less common but often severe, unwanted effects of these drugs include hair loss and low white blood cell count. For this reason, combining chemotherapy with safer agents, such as bioactives, is a viable option to potentially reduce side effects while maintaining or enhancing anticancer efficacy.
Over the past decades, there has been increasing interest in exploring the use of phytochemicals that be used as combination partners with chemotherapeutics [26]. For example, curcumin has been shown to enhance the efficacy of multiple chemotherapeutic drugs [27–29]. For example, it potentiates the effect of 5-FU in colon cancer cells by inhibition of NF-κB and Src protein kinase [29], and enhances the anticancer effect of gemcitabine in preclinical models of pancreatic cancer [28].
Besides curcumin, resveratrol, a polyphenol present in grapes and red wine, has strong antitumor effects [30–35]. Resveratrol has also been shown to be an effective combination partner with chemotherapy drugs [36]. For example, resveratrol has also been shown to sensitize pancreatic cancer and colon cancer to gemcitabine and 5-FU [37, 38]. Moreover, resveratrol has been shown to protect against the myotoxicity of doxorubicin in aged mice [39].
Specifically for EGCG, we have recently shown that EGCG is a strong combination partner with gemcitabine in pancreatic cancer cells and xenografts [5]. In addition, EGCG is a strong combination partner for 5-FU and doxorubicin in colon and lung cancer cells, respectively. Consistent with our findings, EGCG is also a great partner for many other drugs, including cisplatin [40], paclitaxel [41] and metformin [42], highlighting its translational potential. Of note, the differential sensitivity that the multiple cancer cell lines had to EGCG and the combination treatment could be due to different genetic mutations present in these cancer cell lines, making them, either, more resistant or more sensitive to treatment. Therefore, implementing high-throughput drug screening and single-cell profiling techniques [43, 44] that can rapidly find effective drug combination for cancers with specific mutations, will likely be instrumental in improving cancer care and facilitating personalized treatment.
Mechanistically, ERK signaling pathway appears to an important pathway modulated by EGCG alone or in combination. The RAS-regulated RAF-MEK-ERK signaling pathway is frequently activated in various malignancies, correlating to cell growth, cell cycle, and even apoptosis prevention [7]. Moreover, activation of Raf/MEK/ERK pathway is also correlated to drug resistance [8]. The importance of this signaling pathway has driven the development of a variety of pharmaceutical agents to inhibit RAF/MEK/ERK axis in cancer and some RAF and MEK inhibitors are already approved and used in the clinic [45].
However, there is now much interest in targeting ERK directly for multiple reasons. A critical one is the development of acquired resistance to RAF or MEK inhibitors (i.e. KRAS or BRAF amplification, MEK mutation, etc.), which involves relief of negative feedback and pathway re-activation with all signaling going through ERK. This validates the search for ERK inhibitors with RAF or MEK inhibitors as an up-front combination. EGCG strongly reduces ERK phosphorylation and its downstream STAT3 activation. Furthermore, its effect on ERK phosphorylation is enhanced when combined with chemotherapy drugs, suggesting a key pathway is affected.
Although EGCG has shown promise in preclinical models of cancer, its use in the clinic has been limited, due, in part, to its poor bioavailability and stability. A few studies have shown a benefit of EGCG clinically in cancer therapy and prevention [46–48], as well as in ameliorating side effect from drugs and radiation [49, 50]. These above mentioned limitations have led to the exploration of multiple approaches, including the formulation of EGCG in nanoparticles, delivering it as a pro-drug, or using it in combination [44, 51–54]. All of these strategies are aimed at improving EGCG’s bioavailability and stability, with the ultimate goal of improving the clinical use of EGCG.
In summary, our study provides new insights into the cellular mechanisms responsible for the antitumor effect of EGCG when combined with chemotherapeutics in multiple cancer types. EGCG has a beneficial effect when combined with chemotherapeutics, in part, through the inhibition of the ERK pathway. Further studies are warranted to precisely assess the in vivo effects of EGCG in combination with chemotherapeutic drugs.
Highlights.
EGCG reduces cell growth and induces apoptosis in multiple cancer cell lines
EGCG potentiates the growth inhibitory effect of chemotherapy drugs
ERK mediates, in part, the anticancer effect of EGCG plus chemotherapy
Acknowledgements:
Grant Support: Supported by funds from the University of California, Davis and NIFA-USDA (CA-D-NTR-2397-H) to GGM. Ran Wei was sponsored by a China Scholarship Council fellowship. Yasmin Esparza was a participant in the UC Davis Continuing Umbrella of Research Experiences (CURE) Program, which is supported by a supplement to the UC Davis Comprehensive Cancer Center NCI P30CA093373. Jazmin Machuca is a participant of the NSF LSAMP/CAMP program at UC Davis, supported by the NSF. Flow cytometry experiments were funded in part by the UC Davis Comprehensive Cancer Center Support Grant (CCSG) (NCI P30CA093373). The study sponsors had no role in the study design, in the collection, analysis, and interpretation of data; in the writing of the manuscript; nor in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure: All authors declare no conflict of interest.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2020, CA Cancer J Clin 70(1) (2020) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Longley DB, Harkin DP, Johnston PG, 5-fluorouracil: mechanisms of action and clinical strategies, Nat Rev Cancer 3(5) (2003) 330–8. [DOI] [PubMed] [Google Scholar]
- [3].Deng J, Wang Y, Lei J, Lei W, Xiong JP, Insights into the involvement of noncoding RNAs in 5-fluorouracil drug resistance, Tumour Biol 39(4) (2017) 1010428317697553. [DOI] [PubMed] [Google Scholar]
- [4].Negri A, Naponelli V, Rizzi F, Bettuzzi S, Molecular Targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer, Nutrients 10(12) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wei R, Hackman RM, Wang Y, Mackenzie GG, Targeting Glycolysis with Epigallocatechin-3-Gallate Enhances the Efficacy of Chemotherapeutics in Pancreatic Cancer Cells and Xenografts, Cancers (Basel) 11(10) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wei R, Penso NEC, Hackman RM, Wang Y, Mackenzie GG, Epigallocatechin-3-Gallate (EGCG) Suppresses Pancreatic Cancer Cell Growth, Invasion, and Migration partly through the Inhibition of Akt Pathway and Epithelial-Mesenchymal Transition: Enhanced Efficacy when Combined with Gemcitabine, Nutrients 11(8) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA, Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance, Biochim Biophys Acta 1773(8) (2007) 1263–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Garnett MJ, Marais R, Guilty as charged: B-RAF is a human oncogene, Cancer Cell 6(4) (2004) 313–9. [DOI] [PubMed] [Google Scholar]
- [9].Mackenzie GG, Keen CL, Oteiza PI, Microtubules are required for NF-kappaB nuclear translocation in neuroblastoma IMR-32 cells: modulation by zinc, J Neurochem 99(2) (2006) 402–15. [DOI] [PubMed] [Google Scholar]
- [10].Mallangada NA, Vargas JM, Thomas S, DiGiovanni MG, Vaeth BM, Nemesure MD, Wang R, LaComb JF, Williams JL, Golub LM, Johnson F, Mackenzie GG, A novel tricarbonylmethane agent (CMC2.24) reduces human pancreatic tumor growth in mice by targeting Ras, Mol Carcinog 57(9) (2018) 1130–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Boucher MJ, Morisset J, Vachon PH, Reed JC, Laine J, Rivard N, MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells, J Cell Biochem 79(3) (2000) 355–69. [PubMed] [Google Scholar]
- [12].Gu J, Yao W, Shi P, Zhang G, Owonikoko TK, Ramalingam SS, Sun SY, MEK or ERK inhibition effectively abrogates emergence of acquired osimertinib resistance in the treatment of epidermal growth factor receptor-mutant lung cancers, Cancer (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gough DJ, Koetz L, Levy DE, The MEK-ERK pathway is necessary for serine phosphorylation of mitochondrial STAT3 and Ras-mediated transformation, PLoS One 8(11) (2013) e83395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen D, Wan SB, Yang H, Yuan J, Chan TH, Dou QP, EGCG, green tea polyphenols and their synthetic analogs and prodrugs for human cancer prevention and treatment, Adv Clin Chem 53 (2011) 155–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Masuda M, Wakasaki T, Toh S, Shimizu M, Adachi S, Chemoprevention of Head and Neck Cancer by Green Tea Extract: EGCG-The Role of EGFR Signaling and “Lipid Raft”, J Oncol 2011 (2011) 540148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tachibana H, Molecular basis for cancer chemoprevention by green tea polyphenol EGCG, Forum Nutr 61 (2009) 156–69. [DOI] [PubMed] [Google Scholar]
- [17].Wang Y, Ren X, Deng C, Yang L, Yan E, Guo T, Li Y, Xu MX, Mechanism of the inhibition of the STAT3 signaling pathway by EGCG, Oncol Rep 30(6) (2013) 2691–6. [DOI] [PubMed] [Google Scholar]
- [18].Wei R, Mao L, Xu P, Zheng X, Hackman RM, Mackenzie GG, Wang Y, Suppressing glucose metabolism with epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth in preclinical models, Food Funct 9(11) (2018) 5682–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shan X, Li Y, Meng X, Wang P, Jiang P, Feng Q, Curcumin and (−)-epigallocatechin-3-gallate attenuate acrylamide-induced proliferation in HepG2 cells, Food Chem Toxicol 66 (2014) 194–202. [DOI] [PubMed] [Google Scholar]
- [20].Shi Y, Mechanisms of caspase activation and inhibition during apoptosis, Mol Cell 9(3) (2002) 459–70. [DOI] [PubMed] [Google Scholar]
- [21].Chen J, Zhang L, Li C, Chen R, Liu C, Chen M, Lipophilized Epigallocatechin Gallate Derivative Exerts Anti-Proliferation Efficacy through Induction of Cell Cycle Arrest and Apoptosis on DU145 Human Prostate Cancer Cells, Nutrients 12(1) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].La X, Zhang L, Li Z, Li H, Yang Y, (−)-Epigallocatechin Gallate (EGCG) Enhances the Sensitivity of Colorectal Cancer Cells to 5-FU by Inhibiting GRP78/NF-kappaB/miR-155–5p/MDR1 Pathway, Journal of agricultural and food chemistry 67(9) (2019) 2510–2518. [DOI] [PubMed] [Google Scholar]
- [23].Sheng J, Shi W, Guo H, Long W, Wang Y, Qi J, Liu J, Xu Y, The Inhibitory Effect of (−)-Epigallocatechin-3-Gallate on Breast Cancer Progression via Reducing SCUBE2 Methylation and DNMT Activity, Molecules 24(16) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu PP, Kuo SC, Huang WW, Yang JS, Lai KC, Chen HJ, Lin KL, Chiu YJ, Huang LJ, Chung JG, (−)-Epigallocatechin gallate induced apoptosis in human adrenal cancer NCI-H295 cells through caspase-dependent and caspase-independent pathway, Anticancer Res 29(4) (2009) 1435–42. [PubMed] [Google Scholar]
- [25].Schirrmacher V, From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review), Int J Oncol 54(2) (2019) 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fantini M, Benvenuto M, Masuelli L, Frajese GV, Tresoldi I, Modesti A, Bei R, In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: perspectives on cancer treatment, Int J Mol Sci 16(5) (2015) 9236–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guorgui J, Wang R, Mattheolabakis G, Mackenzie GG, Curcumin formulated in solid lipid nanoparticles has enhanced efficacy in Hodgkin’s lymphoma in mice, Archives of biochemistry and biophysics 648 (2018) 12–19. [DOI] [PubMed] [Google Scholar]
- [28].Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB, Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products, Cancer Res 67(8) (2007) 3853–61. [DOI] [PubMed] [Google Scholar]
- [29].Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A, Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-kappaB and Src protein kinase signaling pathways, PLoS One 8(2) (2013) e57218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB, Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells, Blood 109(6) (2007) 2293–302. [DOI] [PubMed] [Google Scholar]
- [31].Carter LG, D’Orazio JA, Pearson KJ, Resveratrol and cancer: focus on in vivo evidence, Endocr Relat Cancer 21(3) (2014) R209–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gatouillat G, Balasse E, Joseph-Pietras D, Morjani H, Madoulet C, Resveratrol induces cell-cycle disruption and apoptosis in chemoresistant B16 melanoma, J Cell Biochem 110(4) (2010) 893–902. [DOI] [PubMed] [Google Scholar]
- [33].Harper CE, Patel BB, Wang J, Arabshahi A, Eltoum IA, Lamartiniere CA, Resveratrol suppresses prostate cancer progression in transgenic mice, Carcinogenesis 28(9) (2007) 1946–53. [DOI] [PubMed] [Google Scholar]
- [34].Kim C, Baek SH, Um JY, Shim BS, Ahn KS, Resveratrol attenuates constitutive STAT3 and STAT5 activation through induction of PTPepsilon and SHP-2 tyrosine phosphatases and potentiates sorafenib-induced apoptosis in renal cell carcinoma, BMC Nephrol 17 (2016) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang YP, Chang YL, Huang PI, Chiou GY, Tseng LM, Chiou SH, Chen MH, Chen MT, Shih YH, Chang CH, Hsu CC, Ma HI, Wang CT, Tsai LL, Yu CC, Chang CJ, Resveratrol suppresses tumorigenicity and enhances radiosensitivity in primary glioblastoma tumor initiating cells by inhibiting the STAT3 axis, Journal of cellular physiology 227(3) (2012) 976–93. [DOI] [PubMed] [Google Scholar]
- [36].Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB, Chemosensitization of tumors by resveratrol, Annals of the New York Academy of Sciences 1215 (2011) 150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Chung SS, Dutta P, Austin D, Wang P, Awad A, Vadgama JV, Combination of resveratrol and 5-flurouracil enhanced anti-telomerase activity and apoptosis by inhibiting STAT3 and Akt signaling pathways in human colorectal cancer cells, Oncotarget 9(68) (2018) 32943–32957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Harikumar KB, Kunnumakkara AB, Sethi G, Diagaradjane P, Anand P, Pandey MK, Gelovani J, Krishnan S, Guha S, Aggarwal BB, Resveratrol, a multitargeted agent, can enhance antitumor activity of gemcitabine in vitro and in orthotopic mouse model of human pancreatic cancer, Int J Cancer 127(2) (2010) 257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sin TK, Tam BT, Yu AP, Yip SP, Yung BY, Chan LW, Wong CS, Rudd JA, Siu PM, Acute Treatment of Resveratrol Alleviates Doxorubicin-Induced Myotoxicity in Aged Skeletal Muscle Through SIRT1-Dependent Mechanisms, J Gerontol A Biol Sci Med Sci 71(6) (2016) 730–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Deng P, Hu C, Xiong Z, Li Y, Jiang J, Yang H, Tang Y, Cao L, Lu R, Epigallocatechin-3-gallate-induced vascular normalization in A549-cell xenograft-bearing nude mice: therapeutic efficacy in combination with chemotherapy, Cancer Manag Res 11 (2019) 2425–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ramadass SK, Anantharaman NV, Subramanian S, Sivasubramanian S, Madhan B, Paclitaxel/epigallocatechin gallate coloaded liposome: a synergistic delivery to control the invasiveness of MDA-MB-231 breast cancer cells, Colloids Surf B Biointerfaces 125 (2015) 65–72. [DOI] [PubMed] [Google Scholar]
- [42].Sabry D, Abdelaleem OO, El Amin Ali AM, Mohammed RA, Abdel-Hameed ND, Hassouna A, Khalifa WA, Anti-proliferative and anti-apoptotic potential effects of epigallocatechin-3-gallate and/or metformin on hepatocellular carcinoma cells: in vitro study, Mol Biol Rep 46(2) (2019) 2039–2047. [DOI] [PubMed] [Google Scholar]
- [43].Fior R, Povoa V, Mendes RV, Carvalho T, Gomes A, Figueiredo N, Ferreira MG, Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts, Proc Natl Acad Sci U S A 114(39) (2017) E8234–E8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lim SH, Sa JK, Lee DW, Kim J, Kim ST, Park SH, Ku B, Park JO, Park YS, Lim H, Kang WK, Nam DH, Lee J, Systematic Evaluation of Gastric Tumor Cell Index and Two-Drug Combination Therapy via 3-Dimensional High-Throughput Drug Screening, Frontiers in oncology 9 (2019) 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Croce L, Coperchini F, Magri F, Chiovato L, Rotondi M, The multifaceted anti-cancer effects of BRAF-inhibitors, Oncotarget 10(61) (2019) 6623–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].McLarty J, Bigelow RL, Smith M, Elmajian D, Ankem M, Cardelli JA, Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro, Cancer Prev Res (Phila) 2(7) (2009) 673–82. [DOI] [PubMed] [Google Scholar]
- [47].Henning SM, Wang P, Abgaryan N, Vicinanza R, de Oliveira DM, Zhang Y, Lee RP, Carpenter CL, Aronson WJ, Heber D, Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer, Molecular nutrition & food research 57(3) (2013) 483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ahn WS, Yoo J, Huh SW, Kim CK, Lee JM, Namkoong SE, Bae SM, Lee IP, Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions, Eur J Cancer Prev 12(5) (2003) 383–90. [DOI] [PubMed] [Google Scholar]
- [49].Zhao H, Xie P, Li X, Zhu W, Sun X, Sun X, Chen X, Xing L, Yu J, A prospective phase II trial of EGCG in treatment of acute radiation-induced esophagitis for stage III lung cancer, Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 114(3) (2015) 351–6. [DOI] [PubMed] [Google Scholar]
- [50].Zhao H, Zhu W, Xie P, Li H, Zhang X, Sun X, Yu J, Xing L, A phase I study of concurrent chemotherapy and thoracic radiotherapy with oral epigallocatechin-3-gallate protection in patients with locally advanced stage III non-small-cell lung cancer, Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 110(1) (2014) 132–6. [DOI] [PubMed] [Google Scholar]
- [51].Ahmed RS, Liu G, Renzetti A, Farshi P, Yang H, Soave C, Saed G, El-Ghoneimy AA, El-Banna HA, Foldes R, Chan TH, Dou QP, Biological and Mechanistic Characterization of Novel Prodrugs of Green Tea Polyphenol Epigallocatechin Gallate Analogs in Human Leiomyoma Cell Lines, J Cell Biochem 117(10) (2016) 2357–69. [DOI] [PubMed] [Google Scholar]
- [52].Heyza JR, Arora S, Zhang H, Conner KL, Lei W, Floyd AM, Deshmukh RR, Sarver J, Trabbic CJ, Erhardt P, Chan TH, Dou QP, Patrick SM, Targeting the DNA Repair Endonuclease ERCC1-XPF with Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCG) and Its Prodrug to Enhance Cisplatin Efficacy in Human Cancer Cells, Nutrients 10(11) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lazzeroni M, Guerrieri-Gonzaga A, Gandini S, Johansson H, Serrano D, Cazzaniga M, Aristarco V, Macis D, Mora S, Caldarella P, Pagani G, Pruneri G, Riva A, Petrangolini G, Morazzoni P, DeCensi A, Bonanni B, A Presurgical Study of Lecithin Formulation of Green Tea Extract in Women with Early Breast Cancer, Cancer Prev Res (Phila) 10(6) (2017) 363–370. [DOI] [PubMed] [Google Scholar]
- [54].Wang J, Man GCW, Chan TH, Kwong J, Wang CC, A prodrug of green tea polyphenol (−)-epigallocatechin-3-gallate (Pro-EGCG) serves as a novel angiogenesis inhibitor in endometrial cancer, Cancer letters 412 (2018) 10–20. [DOI] [PubMed] [Google Scholar]


