Structured Abstract
Background Context:
Non-physiological mechanical loading and inflammation are both critically involved in intervertebral disc (IVD) degeneration, which is characterized by an increase in cytokines and matrix metalloproteases (MMPs) in the nucleus pulposus (NP). This process is known to be mediated by the NF-κB pathway.
Clinical Significance:
Current clinical treatments for IVD degeneration focus on the alleviation of symptoms rather than targeting the underlying mechanism. Injection of an NF-κB inhibitor may attenuate the progression of IVD degeneration.
Purpose:
To investigate the ability of the NF-κB inhibitor, NEMO binding domain peptide (NBD), to alter IVD degeneration processes by reducing IL-1β- and mechanically-induced cytokine and MMP levels in human nucleus pulposus cells (hNPCs) in vitro, and by attenuating IVD degeneration in an in vivo rat model for disc degeneration.
Study Design:
Experimental in vitro and animal model.
Patient Sample:
Discarded specimens of lumbar disc from 21 patients, and 12 Sprague Dawley rats.
Outcome Measures:
Gene and protein expression, cell viability, μMRI and histology.
Methods:
IL-1β-prestimulated hNPCs embedded into fibrin constructs were loaded in the Flexcell FX-5000 compression system at 5kPa and 1Hz for 48 hours in the presence and absence of NBD. Unloaded hNPC/fibrin constructs served as controls. Cell viability in loaded and unloaded constructs was quantified, and gene and protein expression levels determined. For in vivo testing, a rat needle disc puncture model was employed. Experimental groups included injured discs with and without NBD injection and uninjured controls. Levels of disc degeneration were determined via μMRI, qPCR and histology. Funding sources include $48,874 NASS Young Investigator Research Grant and $119,174 NIH 5K01AR071512–02. There were no applicable financial relationships or conflicts of interest.
Results:
Mechanical compression of hNPC/fibrin constructs resulted in upregulation of MMP-3 and IL-8. Supplementation of media with 10μM NBD during loading increased cell viability, and decreased MMP-3 gene and protein levels. IVD injury in rat resulted in an increase in MMP-3, IL-1β and IL-6 gene expression. Injections of 250μg of NBD during disc injury resulted in decreased IL-6 gene expression. μMRI analysis demonstrated a reduction of disc hydration in response to disc needle injury, which was attenuated in NBD-treated IVDs. Histological evaluation showed NP and AF lesion in injured discs, which was attenuated by NBD injection.
Conclusion:
The results of this study show NBD peptide’s capacity to reduce IL-1β- and loading-induced MMP-3 levels in hNPC/fibrin constructs while increasing the cells’ viability, and to attenuate IVD degeneration in rat, involving downregulation of IL-6. Therefore, NBD may be a potential therapeutic agent to treat IVD degeneration.
Keywords: IVD degeneration, mechanical compression, inflammation, matrix metalloproteases, NF-κB, rat disc puncture model
Introduction
As many as 80% of the adult population will experience low back pain (LBP) at some point in their lifetime[1], which has huge economic costs on western societies in terms of lost productivity, increased disability benefits costs, medical and insurance costs with estimated annual health costs of approximately $100-$200 billion[2]. Imaging studies have indicated a link between degenerative disc disease and LBP, with intervertebral disc (IVD) degeneration recognized as a cause of chronic LBP in at least 40% of patients[3]. Most current treatments for disc degeneration, nonsurgical and surgical, address symptoms, but do not treat the underlying process of IVD degeneration. Nonsurgical treatments include physiotherapy and analgesia. Failure of the nonsurgical treatment can lead to surgical options, such as spinal decompression surgery (e.g. discectomy or laminectomy), spinal fusion or disc replacement that can provide satisfactory results in alleviating pain, but are not devoid of complications and poor long-term clinical outcomes[4–8].
The IVD is a complex structure that allows movement between adjacent vertebrae and sustains the weight applied through the spine. The central compartment, nucleus pulposus (NP) contains collagen fibers, which are embedded in a highly hydrated aggrecan-containing matrix. Interspersed at a low density are NP cells. The NP is surrounded with annulus fibrosus (AF). During degeneration, a cell-driven imbalance between matrix synthesis and degradation, particularly within the NP, is thought to be associated with decreased hydration[9] and to result in a loss of IVD function[10]. As a consequence of these degeneration-related alterations in structure and composition of disc tissues, changes occur in material and structural properties of the disc[11] and have direct biomechanical consequences. The multifactorial pathology of IVD degeneration makes it difficult to develop an effective treatment addressed to a specific phenomenon. However, inflammation and its effect on NP cells is considered one of the main factors involved in disk degeneration[12, 13].
The levels of inflammatory and catabolic proteins were shown to be enhanced during IVD degeneration[14–17]. This increased expression of catabolic proteins is thought to be mediated by soluble factors, such as IL-1β and TNF-α[18]. Moreover, the expression of a number of MMPs, including MMP-3 has been shown to increase with degeneration[19, 20]. An upregulation of pro-inflammatory cytokines with the grade of IVD degeneration has been shown for IL-1β, IL-6 and TNFα[14, 15, 21, 22]. IL-8 was also demonstrated to be upregulated in IVD degeneration and low back pain[23, 24], and recently suggested as therapeutic target for treatment of chronic low back pain[25].
The mechanical environment of the IVD determines at least in part the rate of degeneration[11]. While simulated-physiological loading conditions are known to be beneficial to the regulation of cellular metabolism and tissue homeostasis in the IVD[26], mechanical overload is widely assumed to promote degeneration[11, 27]. Dynamic overloading has been shown to result in detectable alterations in gene and protein expression[11]. An increase in inflammatory and catabolic gene expression, including MMP-13 and IL-8, in response to dynamic and static overloading of caprine lumbar IVDs was shown in a compression bioreactor system[27]. In a recent publication, mechanical overloading of human NP cells for 20h was shown to result in cell apoptosis and upregulation of MMP-3 gene expression[28].
Activation of the inducible transcription factor NF-κB was demonstrated to be important in inflammatory and degenerative diseases including rheumatoid arthritis and degenerative disc disease[29, 30]. It is induced by a variety of molecules, including TNF-α and IL-1β[31], and by high mechanical stimulation[28, 32]. Activation of NF-κB results in the transcription of target genes, including the TNFα, IL-1β and MMP-3[30, 33].
Pro-inflammatory cytokine-induced NF-κB activation can be inhibited by blocking NF-κB essential modulator (NEMO) a regulatory subunit with NEMO binding domain (NBD) peptide without affecting the basal activity of IκB kinase (IKK)[34, 35]. This inhibitor has been shown to disrupt NEMO-IKK interactions, thereby preventing the phosphorylation of IκB and subsequent NF-κB nuclear translocation[34, 36]. The selective blockade of NF-κB induction may help to reduce possible side effects, such as the induction of undesired apoptosis and the toxicity issues that can be associated with direct IKK inhibition[37]. NBD’s anti-inflammatory function has been demonstrated in vitro and in vivo[35, 38–40]. For example, continuous administration of NBD was demonstrated to prevent inflammatory bone resorption in vivo without showing signs of toxicity[39]. A recent study demonstated that local treatment of NBD reduces edema and stimulates rhBMP-2 induced bone formation in spinal fusion[40]. Moreover, NBD peptide has been suggested as a therapeutic target for mitigating degenerative disc disease that is associated with aging, since systemic inhibition of NF-κB was demonstrated to increase disc proteoglycan synthesis and to ameliorate loss of disc cellularity in a mouse model of accelerated aging[41]. To evaluate the potential of new therapeutic concepts for attenuation of IVD degeneration, various animal models are being used[42, 43]. These include spontaneous IVD degeneration models, such as genetically modified, disc compression and endplate damage models. Each model system has its advantages and disadvantages. For example, genetically modified mice do not require disc lesion for induction of IVD degeneration. However, mouse disc space is very narrow compared to larger models, such as rat, rabbit and sheep[44–46]. Although animal models are essential to increase our understanding, they still differ significantly from human IVDs (e.g. spine anatomy or cell and tissue composition)[42]. An alternative is the investigation of human derived IVD cells in vitro. Mechanical compression of human and animal IVD cells in 3D culture systems has been demonstrated to be valuable to study the cell responses to different biological and mechanical conditions, known to occur in the IVD[28, 47]. However, in vitro assays are not capable of simulating the biological environment in its full complexity. Therefore, the different model systems are ideally employed in a complementary way.
In the present study, we aimed to (1) demonstrate that NBD can inhibit cytokine- and loading-induced inflammatory and catabolic processes in human NP cells (hNPCs) cultured in a 3D culture system, and to (2) investigate the ability of NBD to reduce in vivo disc degeneration in a rat model.
Materials and Methods
Study specimens, and cell isolation and culture
Institutional Review Board approval was obtained for this study (#Pro00020562, #Pro00036051). The patients’ informed consents were obtained prior to collection and use of their surgical discards. Discarded tissue samples were placed in a dry sterile specimen container (without addition of saline) and collected from operating room by laboratory staff immediately after completion of surgery. On the day of collection, NPCs were isolated from human IVD tissues using sequential enzymatic treatment with 0.2% pronase/0.025% collagenase[48]. Afterwards, isolated cells were passed through a 70μm filter and cultured in DMEM/F12 medium containing 10% fetal bovine serum, pen/strep, 2mM L-glutamine and 50μg/ml ascorbate. Cell culture passages 1–4 were used for experiments.
Cell responses to IL-1β and mechanical loading
Cells were exposed to 2.5ng/ml IL-1β for 24h in a cell culture flask to activate the NF-kB pathway[49]. Then, cells were lifted from the cell culture flask using Trypsin:EDTA Solution – 0.25% (Gembio, USA) and 1×106 cells were immediately embedded in 3D fibrin constructs (450μl each, Tisseel, Baxter, USA). After polymerization for 1h at 37°C, cell constructs were placed in the BioPress™ Compression Plates (Flexcell International Corporation, USA) supplemented with 3ml of DMEM/F12 medium. To apply mechanical loading to hNPCs, the Flexcell FX-5000 compression system (Flexcell International Corporation, USA) was employed. Mechanical compression was applied 2 times per day for 2h at 1Hz and 5kPa for a duration of 48h, which resulted in a reduced elastic stiffness of the hNPC/fibrin construct by ~10–20%, comparable to observations in rat models for IVD degeneration in vivo[50]. The loading frequency of 1Hz was chosen, since this frequency has been shown to induce catabolic gene expression in the NP in response to dynamic compression[47]. To investigate the ability of NBD (Anaspec, Inc) to inhibit the expression of IL-1β and mechanically induced cytokines and metalloproteases in hNPCs, 10μM (hereafter referred to as low dose NBD) and 50μM of NBD (hereafter referred to as high dose NBD) were added to the constructs and medium prior to loading. Dosing of NBD in vitro was chosen based on a study showing blockage of IL-1β induced IL-6 protein expression in fibroblast-like synoviocytes[38]. Pre-stimulated hNPC constructs, without loading and NBD treatment, and pre-stimulated and loaded hNPC constructs, without NBD treatment served as controls. After loading, cell constructs were immediately collected for cell viability testing and RNA isolation. The conditioned medium was collected and frozen for subsequent protein analysis.
Cell viability analysis
For quantification of cell viability, the quantitative CellTiter-Glo 3D assay kit (Promega Madison, WI). For the quantitative cell viability assay, constructs were quartered, opposing segments were selected, macerated, placed separately in 1.5 ml conical tubes, and tested as technical replicates. 112.5μl of CellTiter-Glo 3D (Promega Madison, WI) was added to each tube, 50μl of PBS was added to increase volume to allow for duplicates readout in a 96 well plate. The macerated constructs were shaken for 5 min and spun down at 16,000g for 7 min. 100μl of supernatant was pipetted in duplicate wells of a black bottom 96 well Costar plate (Corning Inc. Corning, NY). Luminescence was measured with a 1 sec integration time on a SpectraMax M3 (Molecular Devices Sunnyvale, CA).
Quantification of gene and protein expression
Total RNA was isolated from 3D constructs using TRIzol®Reagent (Thermo Fisher Scientific, USA) and subsequent homogenization. The quality and quantity of the RNA were determined by spectrophotometry. RNA was transcribed to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). Levels of gene expression were detected using commercially available TaqMan® Expression Assays per manufacturer’s instructions and Bio-Rad CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA). For amplification, the TaqMan™ Universal PCR Master Mix (4304437, Applied Biosystems, USA) and the following assays were used: human MMP-3: HS00968305_m1, human IL-6: HS00985639_m1, human IL-8: HS00174103_M1, rat MMP-3: RN00591740_m1, rat IL-1β: RN00580432_m1, rat IL-6: RN01410330_m1, rat TNFα: RN01525859_g1 (Applied Biosystems LifeTechnologies, USA). Samples were tested in triplicates. Expression levels of mRNA were normalized to 18S rRNA Endogenous Control (4333760T, Applied Biosystems, USA). For data analysis, the 2−ΔΔCq (Livak) method was used. Protein expression levels of human MMP-3, IL-6 and IL-8 in response to mechanical overloading were determined with Quantikine ELISAs according to manufacturer’s instructions (R&D Systems, USA).
Animal surgery
To investigate the effect of NBD peptide on disc degeneration in vivo, a rat needle disc puncture model was employed as previously reported by our group[44] and others[44, 51, 52]. Animal experiments were performed in accordance to the Institutional Animal Care and Use Committee-approved protocol (#003364). Briefly, under 2% of isoflurane inhalation anesthesia and after incision, the lumbar spine was exposed through a retroperitoneal approach. To initiate IVD degeneration, an 18-gauge needle (1.27mm diameter) was inserted into the NP of any two non-contiguous lumbar discs of L2/3, L3/4, L4/5 and L5/6 (experimental vs.. control treatment conditions were rotated between discs for each animal to achieve a balanced study design) of 12 healthy, male CD® Sprague Dawley IGS rats (Charles River, MA), 10 weeks of age, with an average weight of 350g at time of surgery. The needle inserted at a depth of 2mm into each disc. It was held in the NP for 5 seconds, rotated and removed. During the same procedure, 250μg of NBD for in vivo use in animal research (Anaspec, Inc) reconstituted in 10μl distilled water (according to Manufacturer’s instruction) was injected into the center of the NP of one of the punctured discs using a 26-gauge needle. NBD dosing was selected based on previous studies of our research team[40] and others[35] showing NBD injections in the range of 100–500μg to be safe and efficient for reduction of inflammation in rodents. According to the practice guideline for dose conversion between animals and human[53], the employed dose of 714g/kg would correspond to approximately 3mg/kg in humans. Punctured discs injected with 10μl of distilled water only served as controls. After treatment, the incision was closed, warm fluids and pain medication (0.05mg/kg buprenorphine, SC) were administered. Twelve hours after surgery, application of pain medication (0.05mg/kg buprenorphine, SC) was repeated. Rats were single housed after surgery to minimize risk of injury through companions. The rats’ welfare was assessed daily. No prophylactic antibiotics were given. No infections, poor conditions or drug related adverse events were detected. All 12 rats survived and recovered well. MRI scans were performed on all animals. After sacrifice, 4 spines were used for histology. All remaining spines were dedicated for qPCR analysis of the IVD tissue (NP and AF, without endplates). Exclusion criteria were poor MRI image quality and poor RNA quality.
Micro-magnetic resonance imaging
To visualize the level of disc degeneration, disc hydration was measured (pre-surgery and at 4 weeks post-surgery) with micro-magnetic resonance imaging (μMRI), as previously reported[44]. The follow-up time was chosen based on prior literature demonstrating significant changes in MRI values at 4 weeks post-surgery in rat IVD degeneration models[44, 54, 55]. Micro-MRI was performed at the Imaging Core facility under the Imaging Core’s approved IACUC protocol (#007376, Core protocol: μMRI Imaging for Rat Research). Each rat had one μMRI pre-operatively to needle puncture and injection of treatments, and one month postoperatively. Briefly, anesthetized rats were placed on the examining bed in the prone position. First, a series of axial, coronal and sagittal pilot proton density scans (TR: 50ms, TE: 1.7ms) were performed to ascertain the optimal angle for sagittal slice scanning. After obtaining satisfactory sagittal midsection proton density scans for outlining the disc location and size, sagittal T2-weighted scans with exact same imaging geometries were performed (TR: 5000ms, TE: 30ms). The level of disc hydration was quantitatively measured using MIPAV computer imaging software (Medical Image Processing, Analysis, and Visualization, NIH, Bethesda, MD). Utilizing the iliac crest as the anatomical landmark for each scan, regions of interest (ROIs) of 5 consecutive lumbar IVDs were manually contoured for measurements of high signal area values of the NP. To account for differences in scan intensity between MRI images, signal intensities of the experimental disc levels were normalized to the uninjured level L6-S1 within the same MRI scan. In the figure, relative high signal area values of injured and uninjured IVDs post-surgery compared to pre-surgery are shown.
Histology
After sacrifice (at 4 weeks post-surgery), lumbar spines underwent histological evaluation, as previously performed[44]. Therefore, spines were explanted, fixed in 10% neutral-buffered formalin and decalcified in EDTA solution for 30 days for histological testing. After decalcification, the spine was cut in the middle of the vertebral bodies from lumbar spine L2 until S1, resulting in single IVD segments. Vertebral bodies were bisected in the mid-sagittal plane. Subsequently, the samples were rinsed, dehydrated, paraffin-embedded, and sectioned to a thickness of 5μm. The sections were stained with hematoxylin and eosin (H&E). The stained slides were scanned with an Aperio R slide scanner (Leica Microsystems, USA). Histological grading was performed on all slides. For assessment, the semi-quantitative IVD degeneration grading scale described by Evashwick-Rogler et al was performed[56]. Five categories were graded: 1) AF, 2) AF and NP interphase, 3) cellularity of NP, (4) matrix of NP, and 5) endplate. Each category received a score of 0–2 with score 0 for normal morphology and score 2 for characteristic severe degeneration. The maximum degenerative score per section was 10. All sections were assessed by two researchers blinded to the experimental groups.
Statistics
All statistical analyses were performed using Prism 7 (GraphPad Software, Inc., La Jolla, CA); p<0.05 was considered significant. The outcome measurements were 1) luminescence (RLU), 2) levels of gene expression, 3) levels of protein expression and 4) relaxation time on μMRI (T2-RT). Separately for each outcome measure, a one-way ANOVA with Tukey’s test as post hoc comparison was performed using mean values. In figures, the results are presented as mean values with standard deviations.
Results
In vitro
Description of the specimens
Discarded specimens of lumbar disc were collected from 21 patients. Human NPCs were isolated from discs having had an average Pfirrmann Grade[57] of 5±2; these tissues were obtained from males (38%) and female (62%) patients, with an average age of 53±3.
IL-1β pre-stimulation and intermitted cyclic compression result in an upregulation of MMP-3 and IL-8 protein levels
To evaluate the cell response to inflammatory and mechanical stimuli in a 3D construct, the conditioned media of non-stimulated, IL-1β stimulated, and IL-1β and mechanically stimulated hNPCs were investigated for MMP-3 and cytokine regulation. IL-1β pre-stimulation resulted in an increase in MMP-3 and IL-8 protein levels compared to non-stimulated controls (MMP-3IL-1β vs. none: p<0.01; IL-8IL-1β vs. none: p<0.05, Suppl. Fig. 1). Mechanical loading of IL-1β pre-stimulated hNPCs twice per day at 5kPa and 1Hz for 48 hours resulted in further upregulation of MMP-3 and IL-8 protein levels compared to controls (MMP-3IL-1β, loaded vs. IL-1β, unloaded: p<0.05, MMP-3IL-1β, loaded vs. none, unloaded: p<0.05, IL-8IL-1β, loaded vs. IL-1β, unloaded: p<0.05, IL-8IL-1β, loaded vs. none, unloaded: p<0.01). Since the described experiments resulted in an increase of MMPs and cytokines, IL-1β pre-stimulation and loading were applied to all remaining experiments to simulate a degenerative cell environment in vitro.
Low dose NBD increases cell viability when combined with cell loading and inhibits upregulation of MMP-3 levels in response to mechanical loading
A quantitative cell viability assay in the presence and absence of different does of NBD showed an increase in viability of loaded hNPCs in the presence of low dose NBD (RLUunloaded: 337±184, RLUloaded+low dose NBD: 553±234, p<0.05, Fig. 2A). To investigate the effect of NBD on cytokine and MMP expression of loaded and unloaded hNPCs, levels of MMP-3, IL-6 and IL-8 gene expression and protein secretion were analyzed in the different experimental groups. Gene expression analysis showed a reduction of loading-induced MMP-3 levels in the presence of low dose, but not high dose of NBD compared to loaded samples without NBD (MMP-3unloaded: 1.0±0.02, MMP-3loaded: 2.2±0.3, MMP-3loaded+low dose NBD: 89.8±21.4, unloaded vs. loaded: p<0.05, loaded vs. loaded+low dose NBD: p<0.05, Fig. 2B). Analysis of secreted proteins’ levels in the conditioned media detected a reduction of loading-induced MMP-3 levels in the presence of low dose NBD compared to loaded samples without NBD, which was further reduced when high dose NBD was added (MMP-3unloaded: 99.8%±17.5%, MMP-3loaded: 145.6%±29.8%, MMP-3loaded+low dose NBD: 134.4%±26.9%, MMP-3loaded+high dose NBD: 87.8%±27.3%, unloaded vs. loaded: p<0.05, loaded vs. loaded+low dose NBD: p<0.05, loaded+low dose NBD vs. loaded+high dose NBD: p<0.05, Fig. 2E). IL-6 and IL-8 gene and protein levels were not affected by NBD (Fig. 2 C–D and F–G).
Fig. 2. NBD increases cell viability when combined with cell loading and reduces MMP-3 levels in IL-1β pre-stimulated, mechanically loaded hNPCs.
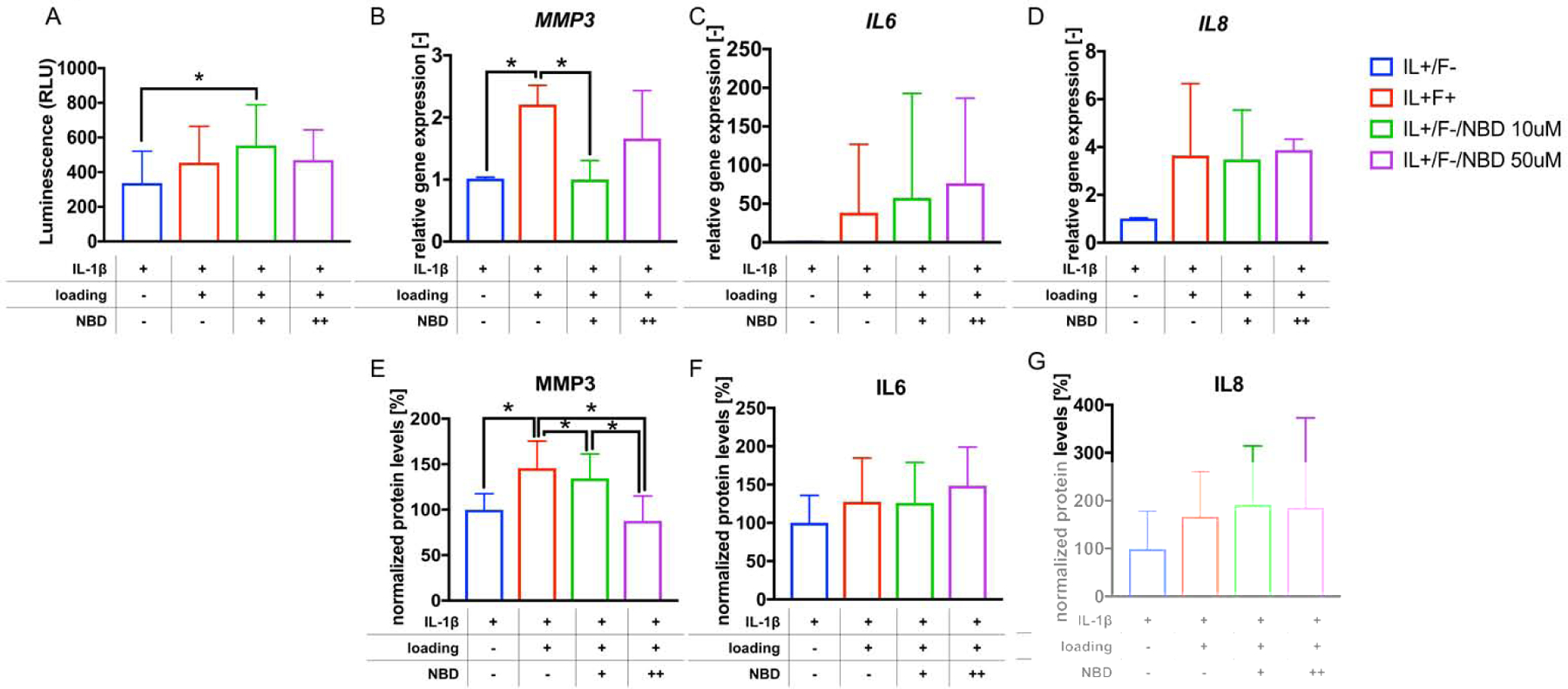
A) Shown are levels of luminescence detected as part of a cell viability assay performed with hNPCs from the different experimental conditions. B-D) relative levels of gene expression of MMP-3, IL-6 and IL-8 are shown. E-G) relative levels of MMP-3, IL-6 and IL-8 protein in the CM from the different experimental groups are shown. *p<0.05. nviability assay≥4, PCR≥4, nELISA≥5. NBD +: low dose, NBD++: high dose.
In vivo
NBD blocks upregulation of IL-6 in degenerated discs
Increased levels in relative gene expression of MMP-3, IL-1β and IL-6 were found in the injured discs compared to uninjured controls (MMP-3uninjured: 0.9±0.4, MMP-3injured: 4.2±3.7, p<0.05; IL-1βuninjured: 1.0±0.5, IL-1βinjured: 2.6±2.0, p<0.01; IL-6uninjured: 1.0±0.3, IL-6injured: 5.6±4.3, p<0.001, Fig. 3 A–C). In injured NBD injected discs, relative IL-6 gene levels were lower, and relative IL-1β and MMP-3 levels unchanged compared to IVD injury only (IL-6injured+NBD: 2.1±1.6, p<0.05, Fig. 3C). No significant differences were detected in TNFα gene expression (Fig. 3D).
Fig. 3. Discs of rats treated with NBD demonstrated reduced levels of IL-6.
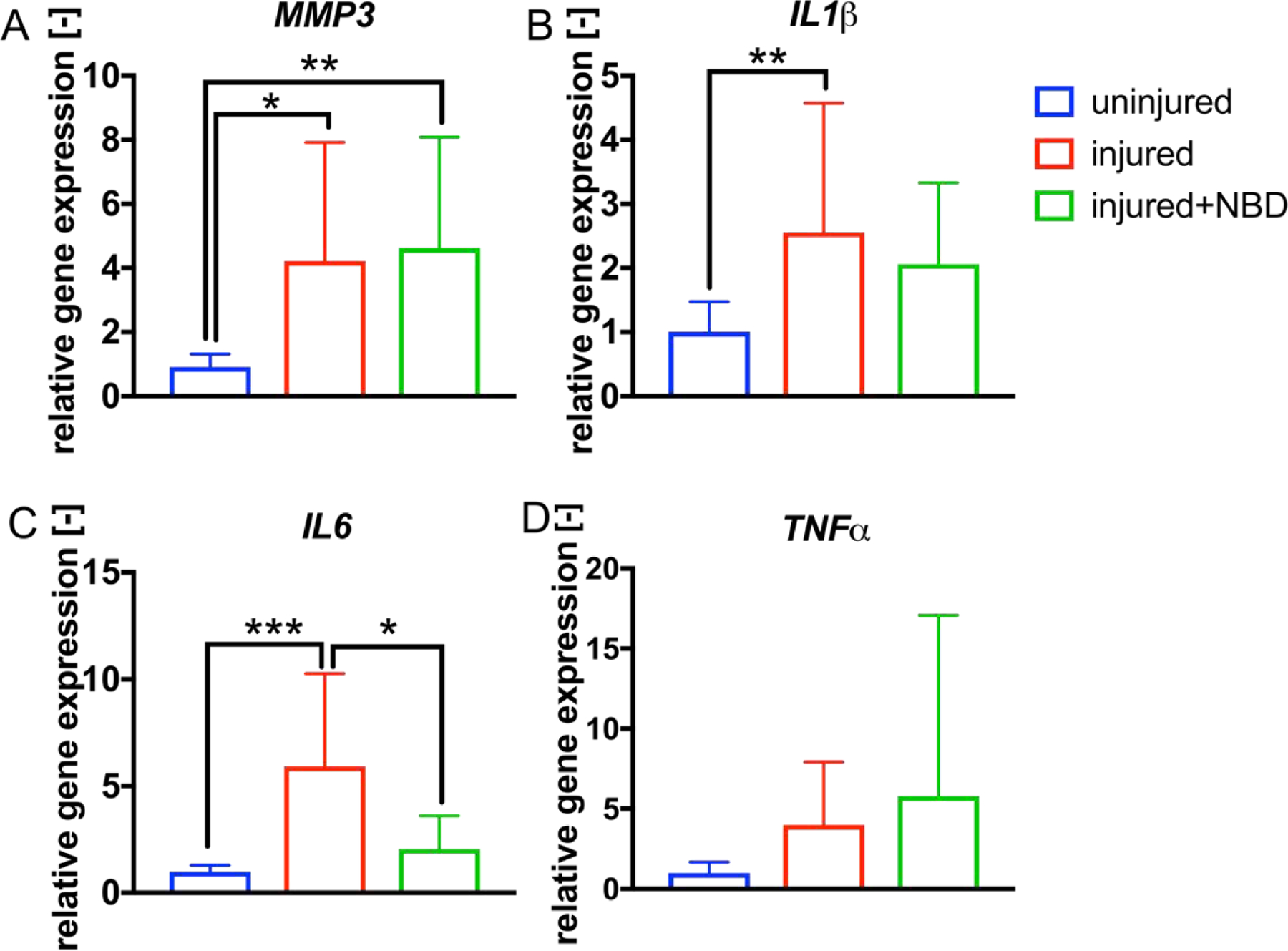
A-D) Shown are the relative gene expression levels of MMP-3, IL-1β, IL-6 and TNFα detected in uninjured, injured (injected with 10μl distilled water) and injured+NBD (injected with NBD in 10μl distilled water) treated IVDs. *p<0.05, **p<0.01, ***p<0.001, n≥5.
NBD attenuates disc dehydration in response to IVD needle injury
Micro-MRI data analysis was performed pre-surgery and at 4 weeks post-surgery to evaluate levels of disc hydration in the presence and absence of NBD injections. T2 values of the different experimental groups were normalized to pre-surgical values. In injured discs, intensities were reduced compared to pre-surgical samples (T2pre-surgery: 90.4±12.0, T2injured: 52.1±13.3, p<0.01) as well as compared to uninjured controls (T2uninjured: 93.7±19.1, p<0.001). Injection of NBD into injured discs resulted in higher T2 values compared to the injury only group (T2injured+NBD: 97.5.8±26.7, p<0.001) (Fig. 4A–B).
Fig. 4. NBD injections attenuate injury-induced disc dehydration in rat IVD.
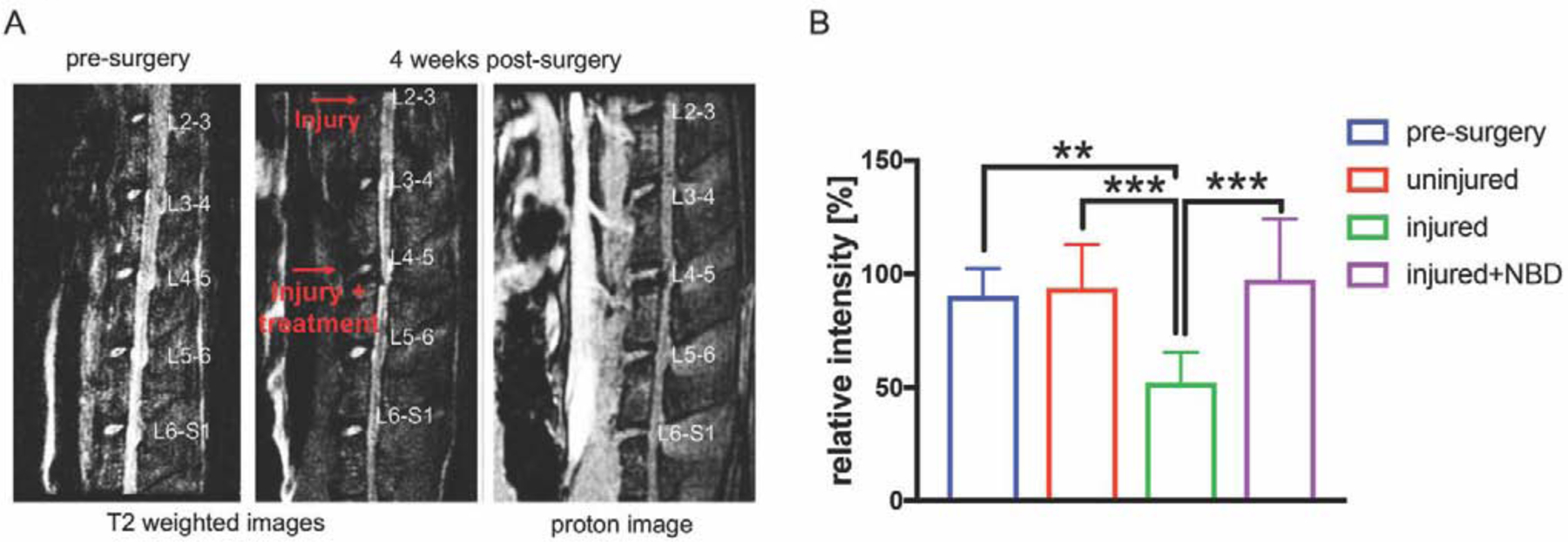
A) Representative T2-weighted and proton density images of a rat spine that underwent needle injury (injected with 10μl distilled water), needle injury+NBD (injected with NBD in 10μl distilled water) treatment pre- and 4 weeks post-surgery. B) Shown are relative high signal NP area values of T2-weighted images of the different experimental groups. Data were normalized to pre-surgery scans. N≥8.
NBD attenuates histological signs of IVD degeneration
Semi-quantitative histological analysis of lumbar disc segments of the different experimental groups at 4 weeks post-surgery showed an intact NP surrounded by intact AF lamellae in uninjured samples. Needle injury of IVDs resulted in morphological changes, which included complete destruction of the NP/AF interface, changes in the NP matrix, strongly reduced NP cellularity as well as destruction of the AF lamellae. In the injured+NBD group, the NP/AF interface was slightly altered in most of the samples. The NP matrix and cellularity were reduced compared to intact IVD, but not as severe as in the injury group. AF lamellae were mostly intact in all injured+NBD group samples investigated (Fig. 5A,B).
Fig. 5. NBD injections attenuate NP and AF destruction in response to needle injury of rat IVD.
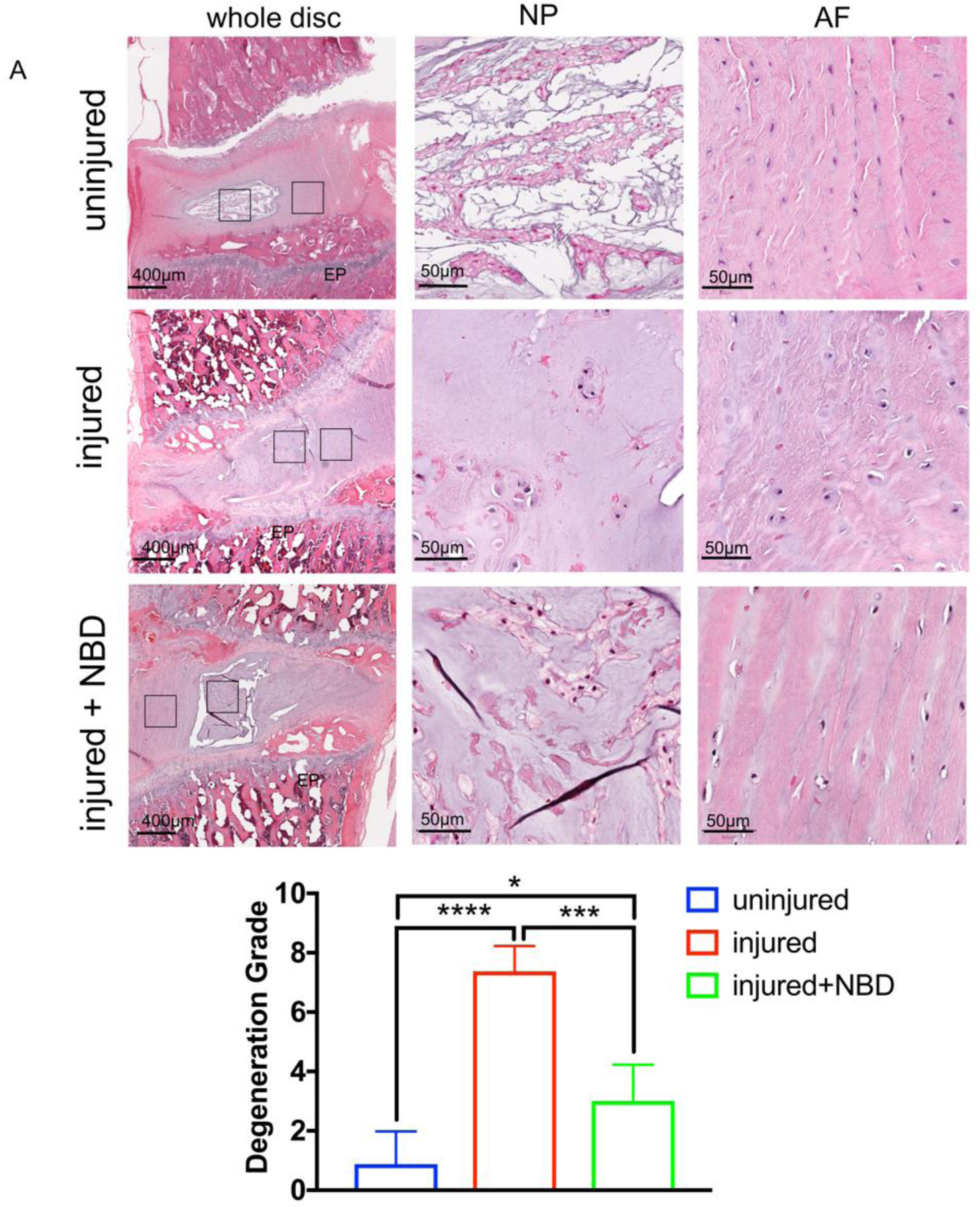
A) Shown are representative H&E stained histology slides of rat lumbar discs with no injury, injury (injected with 10μl distilled water), and injury+NBD (injected with NBD in 10μl distilled water) treatment. EP: end plate, NP, nucleus pulposus, AF: annulus fibrosus. B) Shown is the histological grading of all discs. *p<0.05, ***p<0.001, ****p<0.0001, n=4.
Discussion
This study demonstrates the potential of NBD peptide treatment to reduce IL-1β and loading-induced MMP-3 expression in hNPCs and to attenuate IVD degeneration in a rat model. In vitro, we exposed IL-1β pre-stimulated hNPCs and embedded in a 3D constructs to loading conditions that resulted in an upregulation of IL-8 and MMP-3 on both gene and protein levels. Addition of NBD during loading reduced the observed upregulation of MMP-3. To simulate IVD degeneration in vivo, rat lumbar IVDs were injured via needle puncture[51], which led to upregulation of MMP-3, IL-1β and IL-6 genes, disc dehydration, alteration in the NP-AF interface and lesion of NP. Local NBD injections to the IVDs reduced IL-6 levels, and attenuated the disc dehydration as well as histological signs of IVD degeneration.
Our study showed MMP-3 and IL-8 levels to be increased in hNPC/fibrin constructs in response to IL-1β pre-stimulation. Our results are consistent with the literature showing that MMP and cytokine levels in human disc cells and tissue are induced by cytokines[14, 17, 18]. For example, an IL-1β-induced expression of MMP-3 gene and protein in the human NP was previously shown[17]. Furthermore, IL-1β pre-stimulation induced MMP-3 and IL-8 gene and protein expression in hNPCs cultured in a monolayer[49, 58], as well as in alginate pellets[18].
Cyclic mechanical compression of the hNPC/fibrin constructs resulted in an upregulation of IL-1β induced MMP-3 and IL-8 levels, but not IL-6 levels. The observed cytokine upregulation is in line with the findings by Paul et al showing an upregulation of IL-8, but not IL-6 in response to intermitted high dynamic loading (0.4–0.8MPa at 1Hz for 16 hours, 3 times per day for 30 minutes each) versus no loading in caprine lumbar disc explants[27]. Dynamic loading of rat caudal discs (1MPa, 1Hz) in vivo increased MMP-3 gene expression, whereas low loading (0.2 MPa, 0.2 Hz) had no significant effect[47]. Mechanical overloading but not moderate loading of hNPCs was shown to result in an upregulation of MMP-3 and NF-κB[28]. While the loading forces applied to whole discs are not comparable with hydrogel embedded cells that were used in our study (5kPa applied on hNPC/fibrin constructs) due to differences in loads that were transferred to the NP cells, the achieved response to the compression appears to be similar, since an induction of inflammatory cytokines and MMPs was detected in both cases[28].
Our study demonstrated the ability of NBD to inhibit IL-1β and loading induced upregulation of MMP-3 expression in hNPCs/fibrin constructs and to inhibit upregulation of IL-6 levels in rat degenerated IVDs, but not MMP-3 or IL-1β in rat degenerated disc. While our study did not provide proof of a direct response of NF-κB signaling to the NBD inhibitor, the inhibition of MMP-3 and IL-6 is in line with the literature showing the association of NF-κB pathway activation with an elevation of NF-κB downstream target genes, including MMP-3, IL-1β, IL-6, IL-8 in degenerated IVDs[21, 30, 41]. For example, in human IVD tissue and NPCs an upregulation of p65 along with IL-6 and MMP-3 was observed with increasing degrees of IVD degeneration[61]. Furthermore, mechanical overloading of hNPCs has been shown to result in an upregulation of the NF-κB pathway along with an increase in MMP-3 levels[28]. Differences in the cell response between our two systems may be due to the different species compared, cell surrounding matrices, the loading modes, or time of investigation.
The results of our study demonstrated a less effective attenuation of NF-κB downstream targets in response to higher NBD doses compared to lower doses. This might be due to a transactivation of other pathways, resulting from the higher disruption the protein-protein interactions within the IKK complex[62]. While the IKK complex is the master regulator of the NF-κB signaling pathway, it has also been shown to transactivate other pathways, including MAPK[63]. Further research is needed to evaluate whether other pathways are activated in response to higher NBD doses.
In contrast to other studies, we did not detect a reduced cell viability in response to mechanical overloading of hNPC/fibrin constructs. While overloading often induces cell death, the in this study employed loading magnitudes of 5kPa induced inflammatory and catabolic factors in hNPC/fibrin constructs without induction of cell apoptosis within 48 hours. An explanation may the induction of intrinsic and extrinsic apoptosis pathways in other studies using other stimuli, such as higher loading magnitudes [30, 64, 65]. Comparable to our in vitro results, acute IVD degeneration is mainly characterized by a loss in hydration and proteoglycan.
The potential of NF-κB inhibition to reduce IVD degeneration has been previously demonstrated[66]. For example, the potential of simvastatin to attenuate IL-1β induced expression and MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 activities and NF-κB activation by inhibiting p65 phosphorylation and translocation and blocking inhibitor κB-α degradation was previously shown in 2D cultured hNPCs[66]. To the best of our knowledge, this is the first study to attenuate NF-κB activity by selectively blocking the activation of the IκB kinase (IKK) complex, using two different IVD degeneration models. While broad-spectrum inhibitors may target a wider range of factors, a suppression of NF‐κB’s basal activity or nonspecific activities may occur and be associated with cell toxicity and unwanted side effects[35]. Interestingly, our study demonstrated an increase in cell viability in the presence of low dose NBD in IL-1β pre-stimulated and loaded hNPC/fibrin constructs. NF-κB has been shown to have dual roles in cell death and survival by regulating the expression of a broad array of genes involved in these processes[67]. Therefore, it is likely that the attenuation of NF-κB activity in hNPCs in response to dose NBD results in suppresion cell apoptosis or stimulation of cell survival.
In addition to the alterations in gene and protein expression, we detected an attenuation of disc dehydration and histological signs of IVD degeneration in response to an NBD treatment. Comparable with our findings, NBD was shown to ameliorate age-associated disc proteoglycan loss and of histopathologic changes in a mouse model of accelerated aging (Ercc1-/Δ mice)[41]. Based on our results, it is likely that the inhibition of MMP-3 and IL-6 by NBD affects IVD degeneration, which may happen in multiple ways. Activated MMP-3 is not only known to degenerate collagens, including collagen II, but also to activate further MMPs, such as MMP-7, 8, −9 and −13 and to regulate the bioavailability of growth factors, including TNF-alpha in the IVD[17]. IL-6 is a key player in IVD degeneration and has been shown to potentiate the catabolic actions of IL-1 and TNF-α as well as to contribute an discogenic pain, as summarized by Risbud and Shapiro[21]. Moreover, other factors may be targeted by NBD that we did not include in our study.
In conclusion, this study identified NBD as a potential peptide to reduce inflammatory cytokine-and mechanical loading-induced catabolic factor expression in hNPCs in vitro, and to attenuate IVD degeneration in rat in vivo. While the employed rat disc injury model provided comprehensive insight of cell responses to NBD treatments in a complex biological system, the accompanying in vitro model system allowed us to investigate hNPCs. Understanding the mode of action of this peptide under different disc degeneration simulating conditions will help to determine the potential of NBD to attenuate the progression of disc degeneration in patients and to reduce the need of conventional surgical interventions.
Our study is not without limitations. Our in vitro data focus on the regulation of NF-kB downstream targets in response to NBD treatment of IL-1β pre-stimulated and mechanically loaded hNPCs. In future research, direct changes in the NF-kB pathway (e.g. p65 phosphorylation) and other mechanosensitive pathways in response to IL-1β and mechanical loading in the presence and absence of NBD should be investigated. Also, the effect of NBD on hNPCs in response to either IL-1β pre-stimulation or mechanical loading should be evaluated separately. In a similar study, changes in the p65 phosphorylation levels in IL-1β pre-stimulated hNPCs in response to the IKK inhibitor, BAY11–7082, were shown in a 2D environment[49]. Finally, it would be of interest to evaluate the lack of regulation of IL-6 in response to NBD treatment in our in vitro system. Our in vivo study focusses on the effect of NBD injection on early IVD degeneration, since the follow-up time of 4 weeks is within the acute phase of IVD degeneration[68]. To study the long term effect of NBD on IVD degeneration and chronic back pain, a follow-up of 16–20 weeks should be considered for future research[68]. Prior to testing this drug in humans, the efficacy and optimal dosing of NBD for attenuation of IVD degeneration should to be further investigated in larger cohorts and in large animal models, as employed in studying other pathologies associated with increased NF-κB signaling [69, 70].
Supplementary Material
Fig. 1. Study overview to analyze the effect of NBD on disc degeneration. Top: In vitro study to investigate the effect of NBD on mechano-loaded human NPC/fibrin constructs.
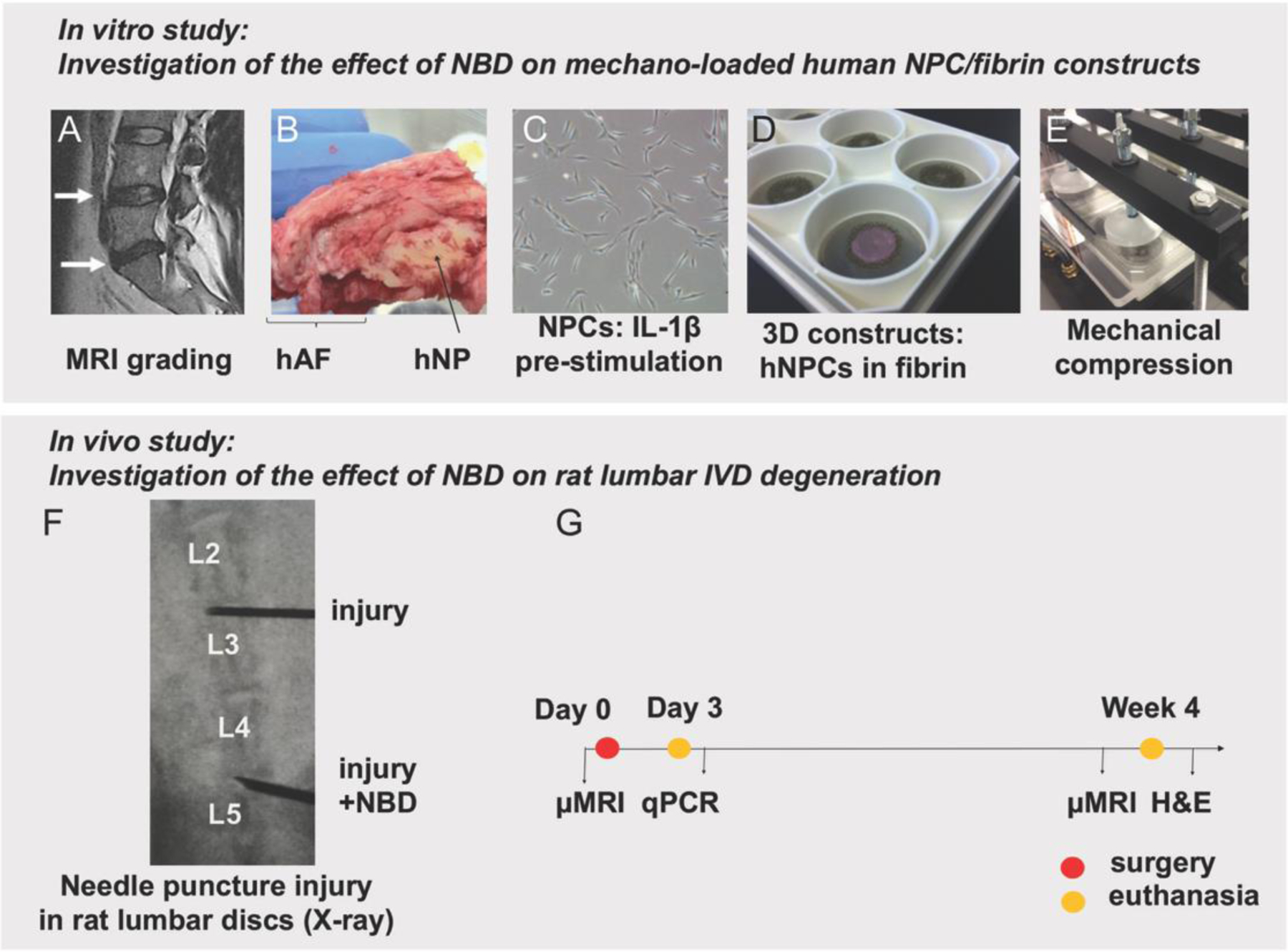
A) Pfirrmann grading (by surgeon) of pre-operative MRI was used to identify eligible human discs for IVD isolation. B) hNP tissue was separated from human IVDs. C) hNPCs were isolated from hNP tissue, expanded and pre-stimulated with IL-1β. D) hNPCs were embedded into 3D fibrin constructs. E) cells underwent mechanical compression. Cells and CM from loaded (+/− NBD) and unloaded cell constructs underwent further analysis. Bottom: In vivo study to investigate the effect of NBD on rat lumbar IVD degeneration. F) per rat, two non-contiguous lumbar discs underwent needle puncture injury in the presence and absence of NBD injections. G) Experimental design: Efficiency of NBD on the attenuation of IVD degeneration was assessed using μMRI, qPCR and histology.
Acknowledgements
The authors wish to acknowledge Cedars-Sinai Imaging Core facility and specifically to Dr. Shawn Wagner for the help with performing the μMRI scans and analysis and Biobank and Translational Research Core for performing the histological analysis and scanning the slides.
Funding disclosure statement
This study has been supported by the NASS Young Investigator Research Grant to JG and partially supported by NIH/NIAMS K01AR071512 to DS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no competing financial interests in relation to this work.
References
- 1.Macfarlane GJ, Thomas E, Croft PR, Papageorgiou AC, Jayson MI, Silman AJ. Predictors of early improvement in low back pain amongst consulters to general practice: the influence of pre-morbid and episode-related factors. Pain. 1999;80(1–2):113–9. [DOI] [PubMed] [Google Scholar]
- 2.Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luoma K, Riihimaki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976). 2000;25(4):487–92. [DOI] [PubMed] [Google Scholar]
- 4.Kao FC, Hsu YC, Wang CB, Tu YK, Liu PH. Short-term and long-term revision rates after lumbar spine discectomy versus laminectomy: a population-based cohort study. BMJ Open. 2018;8(7):e021028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim T, Tleyjeh IM, Gabbar O. Surgical versus non-surgical treatment of chronic low back pain: a meta-analysis of randomised trials. Int Orthop. 2008;32(1):107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976). 2012;37(1):67–76. [DOI] [PubMed] [Google Scholar]
- 7.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–5. [DOI] [PubMed] [Google Scholar]
- 8.Salzmann SN, Plais N, Shue J, Girardi FP. Lumbar disc replacement surgery-successes and obstacles to widespread adoption. Curr Rev Musculoskelet Med. 2017;10(2):153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. The Journal of clinical investigation. 1996;98(4):996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodgkinson T, Shen B, Diwan A, Hoyland JA, Richardson SM. Therapeutic potential of growth differentiation factors in the treatment of degenerative disc diseases. JOR Spine. 2019;2(1):e1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine. 2004;29(23):2724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber KT, Alipui DO, Sison CP, et al. Serum levels of the proinflammatory cytokine interleukin-6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis research & therapy. 2016;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Y, Lv FJ. Symptomatic versus Asymptomatic Intervertebral Disc Degeneration: Is Inflammation the Key? Critical reviews in eukaryotic gene expression. 2015;25(1):13–21. [DOI] [PubMed] [Google Scholar]
- 14.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7(4):R732–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine (Phila Pa 1976). 2005;30(1):44–53; discussion 4. [DOI] [PubMed] [Google Scholar]
- 16.Shamji MF, Setton LA, Jarvis W, et al. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 2010;62(7):1974–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vo NV, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, Kang JD. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13(3):331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millward-Sadler SJ, Costello PW, Freemont AJ, Hoyland JA. Regulation of catabolic gene expression in normal and degenerate human intervertebral disc cells: implications for the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2009;11(3):R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204(1):47–54. [DOI] [PubMed] [Google Scholar]
- 20.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11(4):308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng X, Zhao F, Kang B, Zhang X. Elevated interleukin-6 expression levels are associated with intervertebral disc degeneration. Exp Ther Med. 2016;11(4):1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84(2):196–201. [DOI] [PubMed] [Google Scholar]
- 24.Molinos M, Almeida CR, Caldeira J, Cunha C, Goncalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12(108):20150429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krock E, Millecamps M, Anderson KM, et al. Interleukin-8 as a therapeutic target for chronic low back pain: Upregulation in human cerebrospinal fluid and pre-clinical validation with chronic reparixin in the SPARC-null mouse model. EBioMedicine. 2019;43:487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul CP, Zuiderbaan HA, Zandieh Doulabi B, et al. Simulated-physiological loading conditions preserve biological and mechanical properties of caprine lumbar intervertebral discs in ex vivo culture. PloS one. 2012;7(3):e33147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul CP, Schoorl T, Zuiderbaan HA, et al. Dynamic and static overloading induce early degenerative processes in caprine lumbar intervertebral discs. PloS one. 2013;8(4):e62411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang K, Xue C, Lu N, et al. Mechanical loading mediates human nucleus pulposus cell viability and extracellular matrix metabolism by activating of NF-kappaB. Exp Ther Med. 2019;18(3):1587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baldwin AS Jr. Series introduction: the transcription factor NF-kappaB and human disease. The Journal of clinical investigation. 2001;107(1):3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wuertz K, Vo N, Kletsas D, Boos N. Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-kappaB and MAP kinases. European cells & materials. 2012;23:103–19; discussion 19–20. [DOI] [PubMed] [Google Scholar]
- 31.Wang YF, Xu X, Fan X, et al. A cell-penetrating peptide suppresses inflammation by inhibiting NF-kappaB signaling. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19(10):1849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knapik DM, Perera P, Nam J, et al. Mechanosignaling in bone health, trauma and inflammation. Antioxidants & redox signaling. 2014;20(6):970–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6853–66. [DOI] [PubMed] [Google Scholar]
- 34.May MJ, D’Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289(5484):1550–4. [DOI] [PubMed] [Google Scholar]
- 35.di Meglio P, Ianaro A, Ghosh S. Amelioration of acute inflammation by systemic administration of a cell-permeable peptide inhibitor of NF-kappaB activation. Arthritis and rheumatism. 2005;52(3):951–8. [DOI] [PubMed] [Google Scholar]
- 36.Baima ET, Guzova JA, Mathialagan S, et al. Novel insights into the cellular mechanisms of the anti-inflammatory effects of NF-kappaB essential modulator binding domain peptides. J Biol Chem. 2010;285(18):13498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrington FD, Carmody RJ, Goodyear CS. Modulation of NF-kappaB Signaling as a Therapeutic Target in Autoimmunity. J Biomol Screen. 2016;21(3):223–42. [DOI] [PubMed] [Google Scholar]
- 38.Tas SW, Vervoordeldonk MJ, Hajji N, May MJ, Ghosh S, Tak PP. Local treatment with the selective IkappaB kinase beta inhibitor NEMO-binding domain peptide ameliorates synovial inflammation. Arthritis research & therapy. 2006;8(4):R86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jimi E, Aoki K, Saito H, et al. Selective inhibition of NF-kappa B blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nature medicine. 2004;10(6):617–24. [DOI] [PubMed] [Google Scholar]
- 40.Glaeser JD, Salehi K, Kanim LEA, et al. Anti-Inflammatory Peptide Attenuates Edema and Promotes BMP-2-Induced Bone Formation in Spine Fusion. Tissue Eng Part A. 2018;24(21–22):1641–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nasto LA, Seo HY, Robinson AR, et al. ISSLS prize winner: inhibition of NF-kappaB activity ameliorates age-associated disc degeneration in a mouse model of accelerated aging. Spine. 2012;37(21):1819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin L, Balian G, Li XJ. Animal models for disc degeneration-an update. Histol Histopathol. 2018;33(6):543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NaPier Z, Kanim LEA, Arabi Y, et al. Omega-3 Fatty Acid Supplementation Reduces Intervertebral Disc Degeneration. Med Sci Monit. 2019;25:9531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976). 2005;30(1):5–14. [DOI] [PubMed] [Google Scholar]
- 46.Oehme D, Ghosh P, Shimmon S, et al. Mesenchymal progenitor cells combined with pentosan polysulfate mediating disc regeneration at the time of microdiscectomy: a preliminary study in an ovine model. J Neurosurg Spine. 2014;20(6):657–69. [DOI] [PubMed] [Google Scholar]
- 47.Maclean JJ, Lee CR, Alini M, Iatridis JC. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22(6):1193–200. [DOI] [PubMed] [Google Scholar]
- 48.Li X, An HS, Ellman M, et al. Action of fibroblast growth factor-2 on the intervertebral disc. Arthritis Res Ther. 2008;10(2):R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhongyi S, Sai Z, Chao L, Jiwei T. Effects of nuclear factor kappa B signaling pathway in human intervertebral disc degeneration. Spine (Phila Pa 1976). 2015;40(4):224–32. [DOI] [PubMed] [Google Scholar]
- 50.Michalek AJ, Funabashi KL, Iatridis JC. Needle puncture injury of the rat intervertebral disc affects torsional and compressive biomechanics differently. Eur Spine J. 2010;19(12):2110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, La Marca F, Hollister SJ, Goldstein SA, Lin CY. Developing consistently reproducible intervertebral disc degeneration at rat caudal spine by using needle puncture. Journal of neurosurgery Spine. 2009;10(6):522–30. [DOI] [PubMed] [Google Scholar]
- 52.Than KD, Rahman SU, Wang L, et al. Intradiscal injection of simvastatin results in radiologic, histologic, and genetic evidence of disc regeneration in a rat model of degenerative disc disease. The spine journal : official journal of the North American Spine Society. 2014;14(6):1017–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Li Z, Chen F, et al. TGF-beta1 suppresses CCL3/4 expression through the ERK signaling pathway and inhibits intervertebral disc degeneration and inflammation-related pain in a rat model. Exp Mol Med. 2017;49(9):e379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu MH, Yang KC, Chen YJ, Sun YH, Lin FH, Yang SH. Optimization of puncture injury to rat caudal disc for mimicking early degeneration of intervertebral disc. J Orthop Res. 2018;36(1):202–11. [DOI] [PubMed] [Google Scholar]
- 56.Evashwick-Rogler TW, Lai A, Watanabe H, et al. Inhibiting tumor necrosis factor-alpha at time of induced intervertebral disc injury limits long-term pain and degeneration in a rat model. JOR Spine. 2018;1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu LP, Qian WW, Yin GY, Ren YX, Hu ZY. MRI assessment of lumbar intervertebral disc degeneration with lumbar degenerative disease using the Pfirrmann grading systems. PloS one. 2012;7(12):e48074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Chee A, Shi P, et al. Intervertebral Disc Cells Produce Interleukins Found in Patients with Back Pain. Am J Phys Med Rehabil. 2016;95(6):407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976). 2000;25(23):3005–13. [DOI] [PubMed] [Google Scholar]
- 60.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis research & therapy. 2007;9(4):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Z, Yin Z, Liu C, Tian J. The Changes in the Expression of NF-KB in a Degenerative Human Intervertebral Disc model. Cell Biochem Biophys. 2015;72(1):115–22. [DOI] [PubMed] [Google Scholar]
- 62.Gamble C, McIntosh K, Scott R, Ho KH, Plevin R, Paul A. Inhibitory kappa B Kinases as targets for pharmacological regulation. Br J Pharmacol. 2012;165(4):802–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu F, Xia Y, Parker AS, Verma IM. IKK biology. Immunol Rev. 2012;246(1):239–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang F, Zhao X, Shen H, Zhang C. Molecular mechanisms of cell death in intervertebral disc degeneration (Review). Int J Mol Med. 2016;37(6):1439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao CQ, Jiang LS, Dai LY. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11(12):2079–88. [DOI] [PubMed] [Google Scholar]
- 66.Tu J, Li W, Zhang Y, et al. Simvastatin Inhibits IL-1beta-Induced Apoptosis and Extracellular Matrix Degradation by Suppressing the NF-kB and MAPK Pathways in Nucleus Pulposus Cells. Inflammation. 2017;40(3):725–34. [DOI] [PubMed] [Google Scholar]
- 67.Qin ZH, Tao LY, Chen X. Dual roles of NF-kappaB in cell survival and implications of NF-kappaB inhibitors in neuroprotective therapy. Acta Pharmacol Sin. 2007;28(12):1859–72. [DOI] [PubMed] [Google Scholar]
- 68.Leimer EM, Gayoso MG, Jing L, Tang SY, Gupta MC, Setton LA. Behavioral Compensations and Neuronal Remodeling in a Rodent Model of Chronic Intervertebral Disc Degeneration. Sci Rep. 2019;9(1):3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kornegay JN, Peterson JM, Bogan DJ, et al. NBD delivery improves the disease phenotype of the golden retriever model of Duchenne muscular dystrophy. Skelet Muscle. 2014;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Habineza Ndikuyeze G, Gaurnier-Hausser A, Patel R, et al. A phase I clinical trial of systemically delivered NEMO binding domain peptide in dogs with spontaneous activated B-cell like diffuse large B-cell lymphoma. PLoS One. 2014;9(5):e95404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


