Graphical abstract
Abbreviations: ACM, aerosol collected mass; AqE, aqueous aerosol extracts; ALI, air-liquid interface; ANOVA, analysis of variance; ARE, antioxidant response element; AqE, aerosol aqueous extract; CRM81, CORESTA recommended method number 81; DCF, 2′,7′ dichlorodihydrofluorescein; DMSO, dimethyl sulfoxide; DSB, double-strand break; FDA, US Food and Drug Administration; GEF, global evaluation factor; GSH, glutathione (reduced form); HCI, Health Canada Intense; HUVEC, human umbilical vein endothelial cell; ISO, International Organisation for Standardisation; IVMn, in vitro micronucleus; MF, mutant frequency; MLA, mouse lymphoma assay; NASEM, US National Academy of Sciences, Engineering and Medicine; NHBE, normal human bronchial epithelial; NRU, neutral red uptake; NVP, new vapour product; RWD, relative wound density; S9, post-mitochondrial supernatant; TobReg, WHO Study Group on Tobacco Product Regulation; TPA, 12-O-tetradecanoylphorbol-13-acetate; TPM, total particulate matter; WA, whole aerosol
Keywords: Cigarette, Electronic cigarette, In vitro, Aerosol
Abstract
We have developed a novel vaping product (NVP) IS1.0(TT), which utilises a stainless-steel mesh to transfer and vaporise the e-liquid, mitigating some of the potential sources of toxicants that can be generated using the more traditional ‘wick and coil’ approach. The emissions from IS1.0(TT) have previously been found to have lower levels of toxicants overall when directly compared with a commercial wick and coil e-cig. This current study assessed the toxicological responses to aerosols from this NVP. Responses induced by IS1.0(TT)were compared to those from a 3R4F reference cigarette, using in vitro test methods which included regulatory genetic toxicological assays as well as some more contemporary screening approaches. The experimental conditions were designed to facilitate the testing of aerosol from this vaping product at doses that in most cases greatly exceeded those of the 3R4F comparator showed little to no toxicological responses and demonstrated significantly reduced effects in these in vitro assays when compared to 3R4F. Furthermore, the extreme doses tested in the present study indicate that the toxicant profile of this NVP translates to lower biological activity in vitro, and suggests that the absolute risk hazard level associated with electronic cigarettes can be reduced through continuous improvement as the technology evolves.
1. Introduction
Electronic cigarettes (e-cigs) have seen a phenomenal increase in popularity in recent years. The scientific evidence on the effects of e-cig use on individual and public health is growing and evolving to keep pace with the development of this product category. In parallel with this burgeoning field of scientific endeavour, the adoption of e-cigs as an alternative to traditional combustible cigarette smoking has been supported by a number of communities, including public health groups and scientific bodies [1,2]. In June 2014, the Royal College of Physicians in the UK stated that, "On the basis of available evidence, the RCP believes that e-cigarettes could lead to significant falls in the prevalence of smoking in the UK, prevent many deaths and episodes of serious illness, and help to reduce the social inequalities in health that tobacco smoking currently exacerbates" [3]. Similarly, and based on a thorough review of available data, Public Health England have recently concluded that e-cigs are around 95 % less harmful than conventional cigarettes [1]. A report by the US National Academy of Sciences, Engineering and Medicine [4] states that “There is conclusive evidence that completely substituting e-cigarettes for combustible tobacco cigarettes reduces users’ exposure to numerous toxicants and carcinogens present in combustible tobacco cigarettes.”
Long-term epidemiological data will aid in the determination of the absolute risk of e-cig use, as well as the relative risk to conventional smoking. In the interim, data from analytical chemistry of aerosol emissions, as well as preclinical and clinical assessment of the effects of exposure to these aerosols, will greatly enhance our understanding of the potential for these products to be less risky to the consumer. In vitro data suggest that e-cig aerosol exposure has reduced toxicological effects on lung [[5], [6], [7], [8]] and cardiovascular cells [9,10], as well as inducing little to no activity in traditional genetic toxicology assessments [11,12].
Scientific frameworks have been proposed [13,14] for the assessment of e-cigs and other next generation tobacco and nicotine products, to help establish a comprehensive data set to evaluate the reduced risk potential of these products. These frameworks include the analytical evaluation of aerosol emissions and data on the toxicological and biological effects of aerosol exposure at an individual and population level, to enable a weight of evidence-based assessment of the potential risk associated with product use.
The vast majority of e-cigarettes on the market are based on the ‘wick and coil’ technology for generating aerosols, and these can vary widely both in their design in their operating power. When device operating characteristics are combined with the variety of e-liquids available, and different nicotine levels in those liquids, it can lead to a wide spectrum of aerosols that could vary in terms of both nicotine and flavour delivery and potential toxicant levels. Moreover, it has been shown that users of low-nicotine e-liquids may engage in ‘compensatory’ behaviour, such as using high-wattage devices and/or consuming higher levels of e-liquid. This in turn could expose the users to higher levels of toxicants [15,16]. We have developed a vaping product which utilises distiller technology, known as ‘PureTech™’, to produce an inhalable aerosol with a large mass per puff [17]. A stainless-steel mesh provides a single mode of liquid transfer and vapour formation, in contrast to the traditional e-cig which uses a separate wick and coil for aerosol formation and fluid transfer, respectively. This innovation substantially reduces the risk of dry-wicking and overheating, which is associated with the production of formaldehyde and other toxic carbonyls in some traditional e-cigs [18]. Recent studies on a new vapour device, (IS1.0(TT)), have shown significant reductions in levels of key constituents identified by regulatory bodies such as the WHO Study Group on Tobacco Product Regulation (TobReg) and the US FDA [17]. A number of these toxicants (e.g. formaldehyde) were lower in the emissions from ISO1.0(TT) than a comparator ‘coil and wick’ e-cig, Vype ePen. This is accompanied by a reduction in the production of thermal degradants, which helps to maintain better flavour delivery and improved sensorial satisfaction for the user.
Here we present a comprehensive in vitro toxicological evaluation of this new vapour product (IS1.0(TT)), as a preclinical complement to the emissions analysis. Test matrices prepared from aerosols were compared with those generated using a scientific reference combustible cigarette (3R4F). The in vitro test battery included ‘classical’ regulatory genetic toxicology tests that have been used over many years in the assessment of combustible cigarettes (e.g. [19,20]). This was complemented with contemporary high content screening (HCS) approaches to assess targeted endpoints related to cell health and early indicators of biological effects such as cellular and oxidative stress, as previously described [21]. Endothelial wound repair was also assessed following treatment with e-cig aerosol, as this endpoint has previously been found to be inhibited by combustible cigarette smoke [10]. In addition, the cytotoxicity of the whole aerosol itself was assessed by direct exposure of human lung cells at the air-liquid interface using our in vitro exposure systems [22].
2. Materials and methods
2.1. Reference cigarette and new vapour product
Two products were tested in this study on a comparative basis; a traditional reference cigarette and the new vapour product ISO1.0(TT).
The Kentucky 3R4F scientific reference cigarette was used as a comparator across the in vitro tests employed. 3R4F is a US-blended king-sized tobacco product with a cellulose acetate filter and an International Organization for Standardization (ISO) tar yield of 9.4 mg/cigarette in approximately nine puffs. It is one of the most well-characterised reference cigarettes in terms of its blend composition, physical construction and mainstream smoke toxicant (e.g., harmful and potentially harmful constituents [HPHC]) yields [23].
The new vapour product used, IS1.0(TT), consists of a disposable cartridge (containing e-liquid and the stainless-steel mesh plate technology), and a rechargeable device section. The e-liquid tested in this study was ‘Twilight Tobacco’ (5 mg/mL nicotine (62.6 %w/w vegetable glycerine, (VG), 36 %w/w propylene glycol (PG), 0.43 %w/w nicotine, 1 %w/w water), and the device was operated at 10 W power setting.
These two products are detailed further in Table 1.
Table 1.
Summary of test products and their product codes.
| Product type [code] | Details / schematic | Aerosol formation mechanism | References |
|---|---|---|---|
| Test product |  |
Capillary liquid transfer via stainless steel mesh; vapourisation of the liquid by the same heated mesh | (USA Patent No. US20170333650A1, 2014) |
| New Vapour Product (NVP); flavour variant “Twilight Tobacco” | 10 W power, 5 mg/mL nicotine (62.6 %w/w VG, 36 %w/w PG, 0.43 %w/w nicotine, 1 %w/w water) | ||
| code IS1.0(TT) | |||
| Comparator product | Blended cigarette with 9.4 mg tar under ISO 4387 machine-smoking | Pyrolysis and combustion of tobacco | (University of Kentucky website, accessed 10/03/2020). |
| Research scientific reference cigarette – 3R4F | |||
| code 3R4F |
2.2. Machine puffing regimen
The test exposure matrices were generated by using defined machine puffing regimens. For the 3R4F cigarette, mainstream smoke was generated following the Health Canada Intense (HCI) puffing regime: 55 mL puff volume, 2 s puff duration, 30 s puff interval, with a bell-shaped puff profile; 100 % vent blocking [24]. ISO1.0(TT) was puffed according to CORESTA recommended method number 81 (CRM81), which consists of a 55 mL puff volume, 3 s puff duration, 30 s puff interval, with a square-wave puff profile [25,26].
2.3. Generation and characterisation of test matrices for assessment
Three different test matrices were used for in vitro assessments: reference cigarette total particulate matter (TPM)/ e-cig aerosol collected matter (ACM), whole aerosol (WA), and aqueous aerosol extract (AqE). Generation of these test matrices are described below and are summarised in Table 2.
Table 2.
Summary of test matrix production methods.
| Test exposure matrix |
||||
|---|---|---|---|---|
| Total particulate matter | Aqueous extract | Whole aerosol | ||
| Aerosol generator | Borgwaldt RM2001 (3R4F cigarette) | Borgwaldt RM20H1 | Borgwaldt RM20S1 (3R4F cigarette) | Vitrocell® VC 102 |
| Cerulean CETI83 (IS1.0(TT) e-cig) | Borgwald LM4E1 (IS1.0(TT) e-cig) | |||
| Preparation procedure | TPM/ACM was captured on a Cambridge filter pad from 3R4F and IS1.0(TT) e-cig products. | Aerosol from 8 puffs of a 3R4F cigarette, or 24 puffs of IS1.0(TT) e-cig, was bubbled through 20 mL of cell culture medium in a glass impinger. | WA cytotoxicity assay - for 3R4F exposures, WA was diluted with air, while undiluted aerosol was used for IS1.0(TT) e-cig assessment. Aerosols were delivered to purpose-built Perspex chambers for exposure of H292 lung epithelial cells at the air-liquid interface. | Ames test - WA was undiluted and delivered to a Vitrocell® AMES 4 exposure module for exposure of bacteria at the air–agar interface |
| Pads were extracted with DMSO to a concentration of 24 mg/mL (3R4F cigarette), and 60 mg/mL (IS1.0(TT) e-cig). | AqE was made fresh for each individual experiment and diluted in the appropriate culture medium for specific assays | |||
| TPM/ACM was frozen at −80 °C until use. | ||||
| Extracts were diluted with cell culture medium to a range of test concentrations | ||||
Abbreviations: ACM, aerosol collected mass; AqE, aqueous extract; DMSO, dimethyl sulfoxide; TPM, total particulate matter; WA, whole aerosol.
Borgwaldt KC, Hamburg, Germany.
Vitrocell® systems, Waldkirch, Germany.
2.3.1. Total particulate matter (TPM) and aerosol collected matter (ACM)
Reference 3R4F cigarettes were conditioned according to the International Organisation for Standardisation (ISO) guideline 3402:1999 [27] and smoked on a Borgwaldt RM200 machine (Borgwaldt-KC, Hamburg, Germany). IS1.0(TT) e-cig was puffed on a Cerulean CETI8 smoking machine (Cerulean, Milton Keynes, U.K.).
Up to 150 mg of 3R4F TPM was collected onto 44 mm Cambridge filter pads, while approximately 400 mg IS1.0(TT) e-cig ACM was collected per pad. The pads were weighed before and after smoking/puffing to determine the mass of the deposited TPM/ACM.
2.3.2. Nicotine concentrations in stock TPM/ACM solutions
Pads were extracted into dimethyl sulphoxide (DMSO) to a final stock concentration of 24 mg/mL of 3R4F TPM, and 60 mg/mL IS1.0(TT) e-cig ACM.
The extracts were stored in single-use aliquots at −80 °C.
2.3.3. Aqueous aerosol extracts (AqEs)
AqE was generated on a Borgwaldt-KC RM20H (Borgwaldt KC GmbH, Hamburg, Germany) rotary smoking machine. In total, eight 55 mL puffs from a single 3R4F cigarette, or twenty-four puffs from the IS1.0(TT) e-cig, were bubbled through 20 mL of VascuLife® VEGF cell culture medium (with added supplements and 0.1 % foetal bovine serum) using an impinger. This produced a 100 % stock AqE, which was diluted to provide further concentrations. Each AqE was immediately placed in a sealed, brown, smoked-glass tube and stored at 2–8 °C and used within 4 h.
To confirm the capture of aerosol and to ensure batch-to-batch consistency between AqEs, two independent physical quality control measures were used, as previously described [28]. Carbon monoxide gas, generated from incomplete combustion during AqE production, was detected and quantified by a carbon monoxide module incorporated in the RM20H smoking machine; this method was used only for 3R4F-derived extracts, because carbon monoxide was not detectable in IS1.0(TT) e-cig aerosols. The opacity of the extracts was determined by optical density, measured at a wavelength of 320 nm on a multimode spectrophotometer (Spectramax M3, Molecular Devices, Sunnyvale, CA, USA).
2.3.4. Whole aerosol (WA) exposure
For the whole aerosol (WA) Ames assay, undiluted aerosol from the IS1.0(TT) e-cig was generated and delivered to bacteria tester strains using a modified Vitrocell VC10 exposure system (Vitrocell® Systems, Waldkirch, Germany), as described previously [12,29].
For the WA cytotoxicity assessment in human lung cells, the test system was exposed to undiluted WA using Borgwaldt (Borgwaldt KC GmbH, Hamburg, Germany) smoking and puffing systems; RM20D for 3R4F cigarettes and LM4E for IS1.0(TT) e-cigs.
2.3.5. Measurement of nicotine in WA and AqE
The amounts of nicotine in test product AqE and in the basal media of WA-exposed cells were quantified as previously described [30]. Briefly, 990 μL sample was spiked with 10 μL of 1 mg/mL nicotine-d4. The solvent was removed using a rotary concentrator, and residue was resuspended in 1 mL 5% v/v acetonitrile: water, shaken, vortexed and centrifuged. 0.5 mL was then removed for analysis by ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS; [30]).
2.4. In vitro biological assessment of tobacco products
The in vitro experimental methods and exposure conditions used to assess the toxicological and biological profile of the tobacco products are summarised in Table 3. The cell systems used were chosen according to specific requirements for each endpoint, and human cells were used where possible. Primary normal human bronchial epithelial (NHBE) cells, and the NCI-H292 human lung epithelial cell line have been used extensively to assess cytotoxicity and cell stress-related endpoints in the lung. Bhas-42 cells were used to assess tumour promotion as this is a well-established assay and there is no human cell-based cell transformation model currently available. Primary human umbilical vein endothelial cells (HUVEC) were utilised for the endothelial wound repair assay due to their relevance to the endpoint in question.
Table 3.
Summary of assays used for in vitro toxicological and biological assessment.
| Endpoint | Techniques | Biological System | Test matrix | References | |
|---|---|---|---|---|---|
| Classical regulatory toxicology | Cytotoxicity | ||||
| Induction of cell death | Neutral red uptake (NRU) assay | Mouse fibroblast cells (Balb/c 3T3) | TPM/ACM | [29] | |
| Chromosome damage | |||||
| Formation of micronuclei due to chromosomal loss/damage induced by clastogens or aneugens | In vitro micronucleus (IVMn) assay | Chinese hamster V79 cells | TPM/ACM | [31] | |
| Mutation | |||||
| Induction of mutations in mammalian cells by the test substance | Mouse lymphoma assay (MLA) | Mouse lymphoma L5178Y tkþ/e cells | TPM/ACM | [29] | |
| Induction of reverse mutations in bacteria | Ames test | Salmonella typhimurium (strains TA98, TA100, TA1535, TA1537 and TA102) | WA/TPM/ACM | [29], [12] | |
| Cell transformation (tumour promotion) | |||||
| Assessment of tumour-promoting potential by an established rodent cell-based cell transformation assay | Bhas 42 cell transformation assay (promotion protocol) | Bhas 42 mouse embryo fibroblast cells | TPM/ACM | [32]; [33] | |
| Contemporary high content screening | Cell health and oxidative stress | ||||
| Endpoints assessed: cell count, nuclear size, DNA structure, mitochondrial mass, mitochondrial membrane potential, oxidative stress, glutathione content and cellular ATP | Image analysis based HCS analysis of fluorescently stained cellular and molecular targets, using Cellomics Arrayscan VTI platform. | Normal human bronchial epithelial (NHBE) cells | TPM/ACM | [21] | |
| DNA damage and stress kinase | |||||
| p-H2AX (DNA damage), and phospho-cJun (stress kinase) | As above | NHBE cells | TPM/ACM | [21] | |
| Lung cell toxicity assays | Oxidative stress | ||||
| Transcriptional activation of antioxidant response element (ARE) | H292-ARE-Luc2P reporter gene assay | H292-ARE-Luc2P reporter cells | TPM | [21] | |
| Cytotoxicity | |||||
| Evaluation of concentration of the test substance to cause cell death | Neutral red uptake (NRU) assay | NCI-H292 human bronchial epithelial cells | WA | [34] | |
| CVD | Endothelial wound repair | ||||
| Inhibition of wound repair in injured cell monolayers | Scratch wound assay | Human umbilical vein endothelial cells (HUVEC) | AqE | [35] | |
Abbreviations: AqE, aqueous extract; ARE, antioxidant response element; DSB, double-strand break; EC50, smoke concentration that kills 50 % of cells; TPA, TPM, total particulate matter; WA, whole aerosol/smoke.
2.4.1. TPM/ACM assessment
2.4.1.1. Neutral red uptake (NRU) cell viability assay
Mouse fibroblast cells (Balb/c 3T3 Clone A31) were obtained from the European Collection of Cell Cultures (ECCC). All testing was based on published guidance from the Interagency Coordinating Committee on the Validation of Alternative Methods [36].
Specific experimental details on TPM/ACM assessment in this assay have previously been described elsewhere [29]. In this study IS1.0(TT) e-cig ACM was tested to concentrations as high as 600 μg/mL, while the top concentration of 3R4F tested was 240 μg/mL.
2.4.1.2. In vitro micronucleus (IVMn) assay
The IVMn assay was performed in accordance to OECD guideline 487 [37,38] using Chinese hamster V79 cells obtained from BAT, Southampton, UK. Cells were exposed to TPM/ACM for 3 h in the absence and presence of an Aroclor 1254 induced rat liver metabolic activation system (S9) (Moltox™, Boone, NC, USA) and sampled at 24 h after the beginning of treatment (3 + 21). This is equivalent to approximately 1.5–2.0 times the average generation time of the cells to be used. Additionally, as a number of chemicals have been reported as only exerting positive effects following prolonged treatment, a continuous treatment for 24 h in the absence of S9 was also included [37,38].
2.4.1.3. Mouse lymphoma assay (MLA)
The MLA assay was performed in accordance with OECD guideline 490 [39], using mouse lymphoma cells (L5178Y tkþ/e) that were originally sourced from Burroughs Wellcome & Co, Dartford, UK. TPM/ACM from 3R4F and IS1.0(TT) e-cig were assessed under three test conditions; 3 h with or without S9, and 24 h without S9, as previously described [29].
2.4.1.4. Ames assay for TPM/ACM assessment
The mutagenic potential of TPM/ACM was assessed by Ames test following OECD guideline 471 [40] and as described previously [41].
To evaluate TPM/ACM, five tester strains of Salmonella typhimurium (TA98, TA100, TA1535, TA1537 and TA102), were used in the presence and absence of S9. One plate–incorporation test and two independent pre-incubation tests were performed. In TA98 and TA100 assays containing S9, five and four replicate plates, respectively, were used per TPM/ACM concentration, as recommended by Scott et al. (2013). All other assay conditions used three replicate plates.
For an increase in revertant numbers to be considered as a mutagenic response in the Ames test, the increase had to be at least 2-fold greater than the concurrent control (significant at the 5% level by Dunnett’s test), and both concentration-related and reproducible over two or more independent experiments.
2.4.1.5. Bhas 42 cell transformation assay
The potential of TPM/ACM from the products to induce tumour development was evaluated using the Bhas 42 cell transformation assay, promoter protocol [37,38]. TPM/ACM was tested at various concentrations up to a maximum concentration of 120 μg/mL in the preliminary cytotoxicity assay to select test concentrations for the main experiment. Each test product was assessed in 3 independent experiments.
Plates were manually scored, and results evaluated as previously described [12,29,37,38,42].
2.4.1.6. High content image analysis-based assays
10 toxicological endpoints relating to cellular stress events such as oxidative stress, DNA damage and mitochondrial damage were assessed in normal human bronchial epithelial (NHBE) cells following TPM/ACM treatment for 4 or 24 h, see Table 3. Fluorescence intensities were measured in these cells using an image analysis system (Cellomics ArrayScan VTI High Content Screening platform and vHCS software (Thermo-Fisher Scientific)) after introduction of a fluorescent stain or antibody for each endpoint. Data were normalised to the vehicle control and expressed as fold changes in assay signal as previously described [21,43]. Details of the endpoints assessed, and controls used are given in Table 4.
Table 4.
High Content Screening (HCS) assay endpoint overview.
| HCS endpoint | Probe | Cellular implication/cellular event | Assay control |
|---|---|---|---|
| ATP | CellTiter Glo® | Inhibition of metabolism measured via a decrease in ATP production | Rotenone and l-buthionine-sulfoximine |
| Cell count | Hoechst 33342 or Syto11 | Antiproliferative, apoptotic or necrotic effects measured through cell counts | |
| Glutathione Content | Monochlorobimane | Measurement of GSH levels. A decrease in GSH indicates loss due to the presence of ROS or covalent binding. Increases in GSH may result from protective cellular responses to oxidative stress | |
| Mitochondrial Mass | MitoTracker® Deep Red | Measurement of mitochondrial mass post-exposure indicates potential effects due to oxidative stress and associated damage | |
| Mitochondrial Membrane Potential | MitoTracker® Deep Red | Decreased mitochondrial membrane potential indicates an impaired cellular energy production, potentially resulting in mitochondrial toxicity and apoptosis. | |
| Nuclear Size | Hoechst 33342, Syto11 | An increase in nuclear size indicates necrosis or cell cycle arrest. A decrease can indicate apoptosis. | |
| ROS formation | Dihydroethidium | An increase in ROS generation indicates the formation of toxic superoxide intermediates resulting from oxidative stress | |
| DNA structure | Hoechst 33342, Syto11 | An increase in DNA structure indicates fragmentation of DNA or chromosomal instability. | Mitomycin C |
| DNA Damage (p-H2AX) | Anti pH2AX antibody | Increased DNA damage (p-H2AX) indicates the presence of double-strand breaks. | Mitomycin C |
| Stress Kinase (p-c-Jun) | Anti cJun antibody | Increased phosphorylation of c-Jun indicates activation of the stress kinase pathway that activate numerous protective responses. | Colchicine |
2.4.1.7. ARE activation reporter assay
H292-ARE-Luc2P reporter cells (Promega, UK) were prepared in 96-well plates and were treated with TPM/ACM for 6 and 24 h as previously described [28]. Transcriptional activation of the ARE by Nrf2 was determined after exposure of the stably transfected GloResponse™ H292-ARE-Luc2P cells (Promega) to the test product AqE. Luminescence signals were measured with a SpectraMax multimode microplate reader (Molecular Devices, Sunnyvale, CA, USA) with a 1-s integration time, directly after the addition of the ONE-Glo™ reporter substrate (Promega) according to the manufacturer’s instructions. 30 μM D,L-sulforaphane (Sigma-Aldrich), a potent inducer of Nrf2, was used to confirm activation of the ARE.
Cell viability was assessed using the CellTiter-Glo viability assay. Briefly, cells were lysed with CellTiter-Glo Assay reagent (Promega) to enable the generation of a luminescent signal that is proportional to the cellular ATP concentration and directly proportional to the cell number. Relative luminescence units were recorded with a 1 s integration time on a multimode microplate Reader. These units were measured for each treatment condition, up to 200 mg/mL TPM/ACM, and expressed as a percentage of the untreated control.
ARE activation and cytotoxicity assays were repeated on 6 independent occasions.
2.4.2. AqE assessment
2.4.2.1. Endothelial cell wound repair
The scratch wound assay, as previously described [10] was utilised to detect and measure endothelial migration rates in vitro following exposure to AqE from test products. In brief, a scratch wound was made on confluent human umbilical vein endothelial cell (HUVEC) monolayers in 96-well ImageLock plates (Essen Instruments, Ann Arbor, MI, USA) using a WoundMaker apparatus (Essen Instruments). After wounding, the media was replaced with AqE, VascuLife® VEGF medium (0.1 % foetal bovine serum), or positive control (2 μ M cytochalasin D). To compare any inhibition of endothelial migration rates, cells were exposed to test product AqE in duplicate wells. Numerical data were obtained by measuring the % relative wound density every 1 h over a period of 22 h using an IncuCyte™ real-time cell imaging system (Essen Instruments) as previously described [44]. This refers to the spatial cell density in the wound area relative to the spatial cell density outside of the wound area at each measurement time point. Data from a minimum of three experimental repeats were then averaged to provide a mean increase (%) in relative wound density for each AqE concentration.
Dunnett’s test of each concentration against the media control was performed for both products, to determine those concentrations that were significantly different from control.
2.4.3. WA assessment
2.4.3.1. WA cytotoxicity
The cytotoxicity of WA was assessed as previously described [22]. In brief, NCI-H292 human bronchial epithelial cells (American Type Culture Collection, Middlesex, UK) were grown on cell-culture inserts, and transitioned to the air–liquid interface (ALI) immediately prior to exposure. Cells were exposed directly to undiluted 3R4F WA for up to 7 puffs and undiluted e-cig aerosols for up to 1000 puffs. As an air control, cultures were exposed to air in an exposure chamber at the same frequency and volume as WA exposure. ALI and submerged cell control cultures were kept in an incubator during the exposure period.
Following exposure, cell culture inserts were transferred to 12-well culture plates. Supplemented UltraCULTURE™ media (Lonza, Walkersville, MD, USA) were added to the basal and apical compartments, and the cells were incubated for a further 24 h. Each exposure was conducted 6–8 times with 3 culture inserts per experiment. Cell viability was measured by the NRU assay 24 h after aerosol exposure, as described previously [34].
2.4.3.2. Ames assay for WA assessment
Our approach for conducting the scaled-down modification of the standard 85 mm Ames methodology is detailed at length elsewhere [12]. In this study, the 5 tester strains used were TA98, TA100, TA102, TA1535 and TA1537. All were tested in the presence of S9 metabolic activation, each with an independent positive control.
Statistical analysis was performed as described for the TPM/ACM Ames assay above.
2.5. Data analysis software
Data were analysed with SAS software (SAS Institute, Cary, NC, USA), Minitab version 16.1.0 (State College, PA, USA), and Excel (Microsoft, Redmond, WA, USA), according to the statistical analysis described in each section.
3. Results
3.1. TPM/ACM assessment
3.1.1. NRU cell viability assay
Under the test conditions described in this study, 3R4F TPM induced cytotoxicity in Balb/c 3T3 fibroblast cells when tested up to a concentration of 140 μg/mL (the maximum achievable concentration in the test system). A mean IC50 value of 77.9 ± 10.7 μg/mL was obtained from the five independent experiments.
IS1.0(TT) ACM did not induce cytotoxicity in Balb/c 3T3 fibroblast cells when tested up to 600 μg/mL, the maximum achievable concentrations in the test system (Fig. 1).
Fig. 1.
Cell viability assessed by neutral red uptake (NRU) assay, following treatment of Balb c-3T3 cells with TPM from 3R4F reference cigarette and IS1.0(TT) e-cig ACM. Data are means ± S.D. (n = 5).
3.1.2. IVMn assay
3R4F cigarette smoke showed a positive cytotoxic response with a full curve and complete cytotoxicity and a clear increase in MNBN (Fig. 2). In contrast, the IS1.0(TT) e-cig ACM was deemed negative under all conditions assessed, up to 600 ug/mL. 3 h + S9 results shown but e-cig ACM was negative under 3 h -S9 and 24 h -S9 conditions (data not shown).
Fig. 2.
Mean % multinucleated binucleate cells (MNBN) at a) 3 h - S9, b) 3 h + S9, and c) 24 h -S9, following exposure to 3R4F TPM and IS1.0(TT) ACM. Negative (DMSO-treated) control values are indicated by a horizontal dotted line. Data are means of n = 4 cultures.
3.1.3. MLA
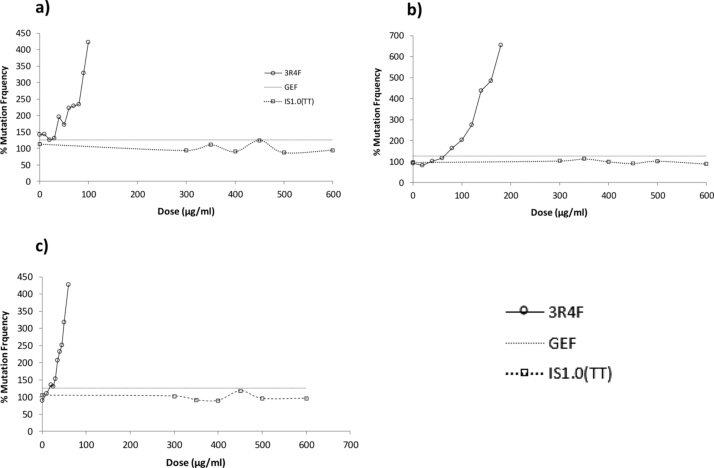
The data from the assessment of mutation in an in vitro mammalian test system (MLA) are shown in Fig. 3. For 3R4F TPM, when tested up to toxic concentrations in the Mutation Experiment, increases in mutant frequency (MF) which exceeded the Global Evaluation Factor (GEF) of 126 mutants per 106 viable cells (compared to concurrent controls) were observed at ≥ 90 μg/mL (3 h -S9), at ≥ 120 μg/mL (3 h + S9), and at ≥ 40 μg/mL (24 h -S9). Statistically significant linear trends (p < 0.001) were observed under all three treatment conditions. These data were indicative of a positive result under each treatment condition.
Fig. 3.
Assessment of mutations induced by reference 3R4F TPM and IS1.0(TT) ACM using the mouse lymphoma assay (MLA), at a) 3 h -S9, b) 3 h + S9, and c) 24 h -S9. Data are means of n = 4 cultures.
When tested up to the maximum practicable concentration of 600 μg/mL in the Mutation Experiment, no increases in MF which exceeded the GEF were observed under any of the three test conditions, and there were no statistically significant linear trends under any treatment condition. These data were indicative of a negative result under each treatment condition.
3.1.4. Ames assay
3R4F TPM induced mutations in the histidine-requiring Salmonella typhimurium strains TA98, TA100 and TA1537 when tested in the presence of S9. This is in line with historic observations for 3R4F TPM [29,45]. These conditions included treatments at concentrations up to 2400 μg/plate using both plate incorporation and pre-incubation treatment methodologies. Smaller increases observed following treatments of strains TA98 and TA100 in the absence of S9 were considered to be further evidence of this mutagenic activity (Fig. 4).
Fig. 4.
Mutant frequencies in tester strains a) TA98, b) TA100, c) TA102, d) TA1535, and e) TA1537, with metabolic activation (3 h + S9), following exposure to 3R4F TPM and IS1.0(TT) ACM. Negative (DMSO-treated) control values are indicated by a horizontal dotted line. Data are means ± S.D. (n = 3).
However, IS1.0(TT) e-cig ACM did not induce mutation in five histidine-requiring Salmonella typhimurium strains when tested up to 6000 μg/plate in the absence and in the presence of S9 using both plate incorporation and pre-incubation treatment methodologies.
3.1.5. Bhas 42 cell transformation assay
Selection of IS1.0(TT) dose levels for the transformation assay was based on reduction in relative cell growth in the preliminary cell growth assay. With the promoter assay method, the relative cell growth ranged from 102.6 % to 113.1 % with respective concentrations ranging from 10 to 120 μg/mL. 3R4F TPM did induce cytotoxicity in the preliminary cell growth assay, with the top concentration (120 μg/mL) resulting in an average cell viability of 42.9 %. Concentrations of 0–120 μg/mL were taken forward to the promotion assay.
No significant increase in the number of transformed foci was observed in the promoter transformation assay with any treatment of IS1.0(TT), up to the maximum concentration tested (120 μg/mL; Fig. 5). Therefore IS1.0(TT) was determined to be negative in this assay. In contrast, 3R4F TPM was positive in the assay, with all concentrations tested giving a significant response above the untreated control, up to the maximum scorable concentration of 50 μg/mL.
Fig. 5.
Assessment of tumour promotion potential induced by TPM from 3R4F reference cigarette and ACM from IS1.0(TT) vapour product using the Bhas 42 cell transformation assay, promotion protocol. Negative (DMSO-treated) control values are indicated by a horizontal dotted line. Data are means ± S.D. (n = 3).
The average transformation frequency induced by the vehicle (solvent) control was less than 12 foci/plate. 12-O-tetradecanoylphorbol-13-acetate (TPA) was used as the positive controls in the promoter assay and induced a statistically significant increase in the transformation frequency over the vehicle control (p ≤ 0.05; T-test).
3.1.6. High content screening image analysis-based assays
The results from the HCS analysis of TPM/ACM from the test products are summarised in Table 5. Positive responses to 3R4F were observed in a number of HCS endpoints at 4 and 24 h, including, nuclear size, DNA structure, mitochondrial membrane potential, cellular ATP, DNA damage and stress kinase endpoints. Compared to 3R4F TPM, IS1.0(TT) ACM treatment, even at the highest concentrations showed little or no responses across all HCS endpoints.
Table 5.
Summary results of image analysis-based HCS assays.
| HCS Endpoint | Exposure time (h) | 3R4F | IS1.0(TT) |
|---|---|---|---|
| Cell count | 4 | – | – |
| 24 | 100↓ | – | |
| Nuclear Size | 4 | – | – |
| 24 | – | – | |
| DNA Structure | 4 | – | – |
| 24 | – | – | |
| Mitochondrial Mass | 4 | – | – |
| 24 | 200↓ | – | |
| Mitochondrial Membrane Potential | 4 | – | – |
| 24 | – | – | |
| Oxidative Stress | 4 | – | – |
| 24 | – | – | |
| Glutathione Content | 4 | 200↓ | – |
| 24 | 50↑ & 200↓ | – | |
| Cellular ATP | 4 | – | – |
| 24 | 100↓ | – | |
| DNA Damage (p-H2AX) | 4 | – | – |
| 24 | 200↑ | – | |
| Stress Kinase (p-cJun) | 4 | – | – |
| 24 | – | – |
Values are the minimum required TPM/ACM concentration (μg/mL) to elicit a ≥ 1.5-fold increase (↑) in assay signal from the 0.5 % DMSO vehicle control or a 30 % decrease (↓).
3.1.7. ARE reporter assay
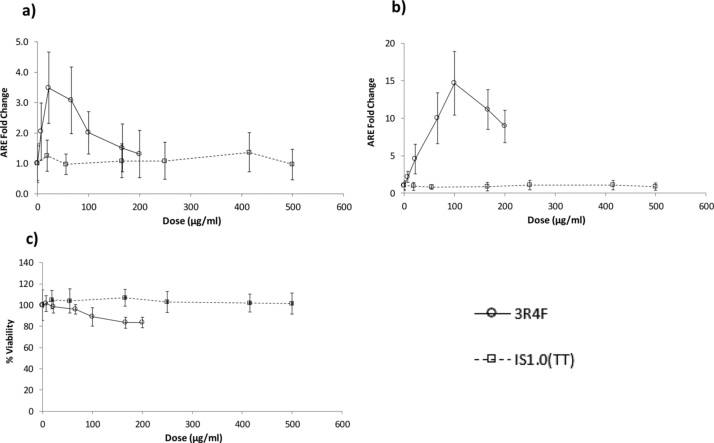
The patterns of H292-ARE-Luc2P assay responses, following exposure (6 h and 24 h) to TPM/ACM derived from 3R4F cigarette smoke or IS1.0(TT) e-cig aerosol are shown in Fig. 6, where the assay signal (RLU) was normalised to the DMSO (0.83 %) control and expressed as a fold-change in relation to this control.
Fig. 6.
Plotted means and 95 % confidence intervals for the fold-changes in RLU (activation of the H292-ARE-Luc2P reporter) from DMSO (0.83 %) control vs. TPM/ACM dose concentration (μg/mL), for 3R4F reference cigarette and ISO1.0(TT) e-cig after a) 6 h, and b) 24 h exposure. Corresponding cell viability data, c) were determined using CellTiter-Glo. Data are means ± S.D. (n = 6).
The highest concentration of 3R4F TPM (200 μg/mL) induced the maximum observed cytotoxicity (<17 %) after 24 h exposure (Fig. 6c). No cytotoxicity was observed following a 6 h exposure to 3R4F TPM (up to 200 μg/mL) or IS1.0(TT) e-cig ACM (up to 600 μg/mL) after 6 h or 24 h. 3R4F TPM induced statistically-significant activation of the ARE (p < 0.001) after 6 h and 24 h, while ISO1.0(TT) ACM was negative in this assay, up to the maximum concentration tested (600 μg/mL).
3.2. AqE assessment
3.2.1. Endothelial wound repair assay
The nicotine concentration of the 100 % stock AqE from IS1.0(TT) was >2-times higher than that of 3R4F (15.18 and 7.01 μg/mL, respectively). In effect, that meant that the top concentration of 3R4F tested (40 % AqE) corresponded to a final nicotine concentration of just 2.8 μg/mL, compared to a top concentration of 15.18 μg/mL in the 100 % top concentration of ISO1.0(TT) AqE tested.
A concentration-dependent inhibition of HUVEC migration was observed following exposure to 3R4F AqE (0–40 %), which confirmed previous findings [10]. Statistical analysis demonstrated that exposure to 3R4F AqE at concentrations ≥15 % significantly (p < 0.05) inhibited the increase in relative wound density (%) in a concentration dependent manner compared to the media control (Fig. 7). There was near complete inhibition of cell migration observed at the top doses (e.g. RWD at 40 % 3R4F AqE = 5%).
Fig. 7.
Mean Increase in Relative Wound Density (%), normalised to media control. Measured in artificial wound generated in HUVEC monolayer following 24 h exposure to a range of dilutions of aqueous extracts of test products 3R4F and IS1.0(TT). Data are means ± S.D. (n = 6).
In contrast, cell migration in IS1.0(TT) AqE treated cells showed a biological response more similar to the media control, demonstrating an increase in RWD in a time-dependent manner, with near complete closure of the wound after 24 h.
3.3. WA assessment
3.3.1. WA cytotoxicity
When the effects of exposure to undiluted whole aerosol from IS1.0(TT) and reference cigarette were assessed on human lung H292 cells, 3R4F was more cytotoxic (Fig. 8). This was true on both a ‘per puff’ basis (Fig. 8a), and when cell viability data were expressed as a function of nicotine dose (Fig. 8b).
Fig. 8.
Effects of undiluted whole aerosol from 3R4F reference cigarette and IS1.0(TT) vaping product on H292 human lung cell viability. Cells were exposed to up to 7 puffs of aerosol from 3R4F reference cigarette, or 1000 puffs from the e-cig (a). Data are also presented as a function of nicotine concentration in basal medium (b). Data are means ± S.D. (n = 3).
3.3.2. Assessment of undiluted whole aerosol using Ames
Following exposure to undiluted IS1.0(TT) whole aerosol, no evidence of toxicity was observed in any experiment in the absence or presence of S9 (Fig. 9). No increases were observed in the revertant number (significant at p ≥ 0.01 using Dunnett’s test), at treatments of up to 900 puffs of undiluted aerosol (Fig. 5).
Fig. 9.
Mutatant frequencies in tester strains a) TA97, b) TA98, c) TA100, d) TA102 and e) TA1535, with metabolic activation (3 h + S9), following exposure to up to 900 puffs of undiluted aerosol from IS1.0(TT). Data are means ± S.D. (n = 3).
4. Discussion
In this study we assessed the in vitro biological effect of IS1.0(TT), a novel e-cig technology, compared to a scientific reference 3R4F cigarette. The IS1.0(TT) e-cig uses a novel distiller plate technology to generate and deliver an e-cig aerosol. Analytical assessment of the respective aerosol emissions has shown that the IS1.0(TT) e-cig aerosol contains significantly reduced levels of known toxicants, and in many cases the toxicant levels were below the levels of detection for the analytical test methods [17]. The same study also showed that IS1.0(TT) emissions contained lower levels of toxicants than those from a comparator e-cig based on coil-and-wick technology. Our findings in this current study have shown that these differences in emissions translate to significant differences in biological activity upon exposure to cells in vitro. The in vitro test battery that was applied included a suite of traditional genetic toxicology tests, which was complemented by more contemporary approaches using human lung and cardiovascular cells. Furthermore, we assessed the effects of aerosols prepared in various ways, enabling a more thorough investigation of potential biological effects. Across each of these test systems, and each of the test matrices (TPM/ACM, WA and AqE), 3R4F reference cigarette aerosol produced a positive response. However,aerosol from the IS1.0(TT) e-cig showed little or no biological activity, even when tested at extreme doses (e.g. undiluted WA).
These findings are consistent with previous assessments of e-cig products by our group and others, where reduced biological activity was reported in comparison to that induced by cigarette smoke [[6], [7], [8],12,29,46]. It is likely that the biological responses that are seen upon exposure to cigarette smoke may be driven by multiple toxic constituents of that aerosol, and that the specific constituents that drive the response may differ from one biological endpoint to the other. That the aerosol from the IS1.0(TT) e-cig had levels of known toxicants that were either substantially reduced or absent is directly reflected in the corresponding lack of biological activity that we observed. Indeed, the distiller plate technology employed by this device delivers even lower levels of toxicants than a conventional coil and wick-based e-cig [17]. IS1.0(TT) was not compared with a coil and wick-based e-cig directly in the current toxicological evaluation study, and so a direct comparison of in vitro data with the aerosol emission data is not possible in this instance. However, the biological responses that we observed indicated that little to no effect was seen even at extremely concentrated exposures to aerosol from this device.
The lower toxicant emissions and the corresponding reduction in biological activity upon exposure to the aerosol from IS1.0(TT) e-cig are also underpinned by the importance of rigorous product stewardship and product design innovation in reducing the risk to the consumer [47]. These product improvements have driven a requirement for increased sensitivity in both the analytical methodologies for emissions testing, and in the biological assays used to assess the exposure. In the current study, we have generated and applied test matrices (TPM/ACM, AqE and WA) wherever possible at higher concentrations than the comparator cigarette test matrix, an approach that is generally applied for most toxicological comparative studies. For example, 3R4F TPM was extracted at 24 mg/mL, whereas a 60 mg/mL ACM stock was prepared from the IS1.0(TT) e-cig. Even with this adjustment, and the resulting increase in the final testing concentration, there was still no response to IS1.0(TT) ACM in any of the assays in which it was assessed.
We took a similar approach for the wound healing assay, where AqE was the test matrix used. The 100 % stock AqE generated from the ISO1.0(TT) had more than double the nicotine content than the respective stock AqE from 3R4F. Nonetheless, even at the highest concentration of 100 % ISO1.0(TT) AqE, there was little to no effect on wound healing compared to cells treated with cell culture medium alone, while complete inhibition of healing was seen at just 40 % of the 3R4F AqE.
With the assays in which whole aerosol from the two comparator products was assessed (Ames assay and WA cytotoxicity assay), we took the approach of using undiluted aerosol. These assays historically have required up to multiple thousand-fold dilution of the test aerosols when assessing traditional combustible cigarettes [12,29], due to the toxicity of these aerosols. However, the lack of any toxicity in diluted e-cig aerosols has led us to develop ways in which to really push the assays in terms of exposing the test system to extremely high doses [12]. This can only be reasonably be achieved by not diluting the aerosol. We have developed this modification to our Ames assay protocol for this purpose as a direct result of the lack of activity we have seen with aerosols from e-cigs in this assay previously. This current study showed that IS1.0(TT) e-cig had no significant effect on mutagenicity when tested at up to 900 puffs of undiluted test aerosol. Where negative effects are seen in genetic toxicology tests in particular, it is important to test at doses or exposure conditions that are as high as feasibly achievable, to increase assay sensitivity and to thereby reduce the potential for a false negative.
For the WA cytotoxicity assay, undiluted aerosol from the IS1.0(TT) e-cig was delivered to human lung epithelial cells at up to 1000 puffs, compared to reference cigarette 3R4F aerosol which could only be tested only up to 7 puffs undiluted before 100 % cytotoxicity was achieved. In contrast, the IS1.0(TT) aerosol only reduced cell viability to approximately 60 % at the top dose of 1000 puffs. Even when the results were compared in the context of nicotine dosimetry, the IS1.0(TT) was tested up to higher levels than those of the 3R4F comparator.
In summary, these data demonstrate that the IS1.0(TT) e-cig demonstrated negative or little responses across all in vitro tests employed. Doses tested often greatly exceeded those of reference 3R4F cigarette smoke tested, where positive responses were observed in all in vitro tests employed. In many cases we also exceeded the dose range we have previously used for coil and wick-based e-cigs. The continuous evolution and improvement in e-cig technologies has led to the development of this device, which has a lower toxicity profile, such that the assays used to detect biological activity had to be adapted to try to improve detection at low levels.
Taken together, the comprehensive suite of in vitro data presented here suggest that the IS1.0(TT) e-cig has the potential to offer a less harmful alternative to the use of traditional combustible cigarettes. Additional longer-term clinical studies and post-market surveillance of users would be required to add further supporting evidence to further understand the risk reduction of these novel products at individual and population levels.
Authors contribution
The study was designed by DT, DB and MG. SB, TJ, SS, MT and AT conducted scratch, whole aerosol neutral red and ARE reporter assays. DT project managed work at Covance. MT project managed work at Cyprotex and DB project managed work at Bioreliance. MG oversaw all testing. DB drafted the manuscript with support from all authors. SB contributed original artwork for the graphical abstract. All authors approved the final version.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Public Health England . 2018. E-Cigarettes and Heated Tobacco Products: Evidence Review.https://www.gov.uk/government/publications/e-cigarettes-and-heated-tobacco-products-evidence-review (Accessed 3 December 2018) [Google Scholar]
- 2.Action on Smoking and Health (ASH) 2018. ASH Welcomes New Public Health England Report on E-Cigarettes. Press Release: 6th February. https://ash.org.uk/media-and-news/press-releases-media-and-news/ash-welcomes-new-public-health-england-report-e-cigarettes/. (Accessed 20 July 2020). [Google Scholar]
- 3.RCP statement on e-cigarettes. Royal College of Physicians. 25 June 2014. https://www.rcplondon.ac.uk/news/rcp-statement-e-cigarettes. (Accessed 20 July 2020).
- 4.National Academies of Sciences Engineering and Medicine . The National Academies Press; Washington, DC: 2018. Public Health Consequences of E-Cigarettes. [PubMed] [Google Scholar]
- 5.Haswell L.E., Corke S., Verrastro I., Baxter A., Banerjee A., Adamson J., Jaunky T., Proctor C., Gaça M., Minet E. Reduced biological effect of e-cigarette aerosol compared to cigarette smoke evaluated in vitro using normalized nicotine dose and RNA-seq-based toxicogenomics. Sci. Rep. 2017;8(1):1145. doi: 10.1038/s41598-017-00852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munakata S., Ishimori K., Kitamura N., Ishikawa S., Takanami Y., Ito S. Oxidative stress responses in human bronchial epithelial cells exposed to cigarette smoke and vapor from tobacco- and nicotine-containing products. Regul. Toxicol. Pharmacol. 2018;99:122–128. doi: 10.1016/j.yrtph.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Czekala L., Simms L., Stevenson M., Trelles-Sticken E., Walker P., Walele T. Toxicological comparison of cigarette smoke and e-cigarette aerosol using a 3D in vitro human respiratory model. Toxicol. In Vitro. 2019;58:86–96. doi: 10.1016/j.yrtph.2019.01.036. [DOI] [PubMed] [Google Scholar]
- 8.Bishop E., Haswell L.E., Adamson J., Costigan S., Thorne D., Gaca M. An approach to testing undiluted e-cigarette aerosol in vitro using 3D reconstituted human airway epithelium. Toxicol. In Vitro. 2019;54:391–401. doi: 10.1016/j.tiv.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Anderson C., Majeste A., Hanus J., Wang S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol. Sci. 2016;154(2):332–340. doi: 10.1093/toxsci/kfw166. [DOI] [PubMed] [Google Scholar]
- 10.Taylor M., Jaunky T., Hewitt K., Breheny D., Lowe F., Fearson I.M., Gaca M. A comparative assessment of e-cigarette aerosols and cigarette smoke on in vitro endothelial cell migration. Toxicol. Lett. 2017;277:123–128. doi: 10.1016/j.toxlet.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Misra M., Leverette R., Cooper B., Bennett M., Brown S. Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: e-liquids, extracts and collected aerosols. Int. J. Environ. Res. Public Health. 2014;11:11325–11347. doi: 10.3390/ijerph111111325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorne D., Hollings M., Seymour A., Adamson J., Dalrymple A., Ballantyne M., Gaca M. Extreme testing of undiluted e-cigarette aerosol in vitro using an Ames air-agar-interface technique. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018;828:46–54. doi: 10.1016/j.mrgentox.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Smith M.R., Clark B., Lüdicke Schaller J.-P., Vanscheeuwijck P., Hoeng J., Peitsch M. Evaluation of the Tobacco Heating System. 2.2. Part 1: description of the system and the scientific assessment program. Regul. Toxicol. Pharmacol. 2016;81(2):S17–S26. doi: 10.1016/j.yrtph.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Murphy J., Gaca M., Lowe F., Minet E., Breheny D., Prasad K., Camacho O., Fearon I.M., Liu C., Wright C., McAdam K., Proctor C. Assessing modified risk tobacco and nicotine products: description of the scientific framework and assessment of a closed modular electronic cigarette. Regul. Toxicol. Pharmacol. 2017;90:342–357. doi: 10.1016/j.yrtph.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Dawkins L., Cox S., Goniewicz M.J., McRobbie H., Kimber C., Doig M., Kośmider L. ‘Real-world’ compensatory behaviour with low nicotine concentration e-liquid: subjective effects and nicotine, acrolein and formaldehyde exposure. Addiction. 2018;113:1874–1882. doi: 10.1111/add.14271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smets J., Baeyens F., Chaumont M., Adriaens K., Van Gucht D. When less is more: vaping low-nicotine vs. High-nicotine e-liquid is compensated by increased wattage and higher liquid consumption. Int. J. Environ. Res. Public Health. 2019;16(5):723. doi: 10.3390/ijerph16050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicol J., Fraser R., Walker L., Liu C., Murphy J., Proctor C. Comprehensive chemical characterization of the aerosol emissions of a vaping product based on a new technology. Chem. Res. Toxicol. 2020 doi: 10.1021/acs.chemrestox.9b00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farsalinos K.E., Voudris V., Spyrou A., Poulas K. E-cigarettes emit very high formaldehyde levels only in conditions that are aversive to users: a replication study under verified realistic use conditions. Food Chem. Toxicol. 2017;109:90–94. doi: 10.1016/j.fct.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 19.DeMarini D.M., Gudi R., Szkudlinska A., Recio L., Kehl M., Kirby P.E., Polzin G., richter P.A. Genotoxicity of 10 cigarette smoke condensates in four test systems: comparisons between assays and condensates. Mutat. Res. 2008;650(1):15–29. doi: 10.1016/j.mrgentox.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi Y., Kanemaru Y., Fukushima T., Eguchi K., Yoshida S., Miller-Holt J., Jones I. Chemical analysis and in vitro toxicological evaluation of aerosol from a novel tobacco vapor product: a comparison with cigarette smoke. Regul. Toxicol. Pharmacol. 2018;92:94–103. doi: 10.1016/j.yrtph.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Taylor M., Thorne D., Carr T., Breheny D., Walker P., Proctor C., Gaça M. Assessment of novel tobacco heating product THP1.0. Part 6: a comparative in vitro study using contemporary screening approaches. Regul. Toxicol. Pharmacol. 2018;93:62–70. doi: 10.1016/j.yrtph.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Jaunky T., Adamson J., Santopietro S., Terry A., Thorne D., Breheny D., Proctor C., Gaça M. Assessment of tobacco heating product THP1.0. Part 5: in vitro dosimetric and cytotoxic assessment. Regul. Toxicol. Pharmacol. 2018;93:52–61. doi: 10.1016/j.yrtph.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 23.University of Kentucky, Centre for Tobacco Reference Products . 2018. Certificate of Analysis, 3R4F Certified Reference Cigarette.https://ctrp.uky.edu/products/gallery/Reference%20Cigarettes/detail/936 [Google Scholar]
- 24.Health Canada . Health Canada; Ottawa: 1999. Official Method T-115. Determination of “Tar”, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke. [Google Scholar]
- 25.CORESTA . 2015. Routine Analytical Machine for CRM No. 81. E-Cigarette Aerosol Generation and Collection - Definitions and Standard Conditions. Cooperation Centre for Scientific Research Relative to Tobacco.https://www.coresta.org/routine-analytical-machine-e-cigarette-aerosol-generation-and-collection-definitions-and-standard [Google Scholar]
- 26.CORESTA . 2015. CRM No. 81 - Routine Analytical Machine for E-cigarette Aerosol Generation and Collection - Definitions and Standard Conditions. Technical Documents.https://www.coresta.org/sites/default/files/technical_documents/main/CRM_81.pdf [Google Scholar]
- 27.International Organization for Standardization Guideline 3402 . 1999. Tobacco and Tobacco Products - Atmosphere for Conditioning and Testing. Standards Catalogue 65.160 Tobacco, Tobacco Products and Related Equipment.https://www.iso.org/standard/28324.html (Accessed 23 May 2019) [Google Scholar]
- 28.Taylor M., Carr T., Oke O., Jaunky T., Breheny D., Lowe F., Gaça M. E-cigarette aerosols induce lower oxidative stress in vitro when compared to tobacco smoke. Toxicol. Mech. Methods. 2016;26(6):465–476. doi: 10.1080/15376516.2016.1222473. [DOI] [PubMed] [Google Scholar]
- 29.Thorne D., Breheny D., Proctor C., Gaca M. Assessment of novel tobacco heating product THP1.0. Part 7: comparative in vitro toxicological evaluation. Regul. Toxicol. Pharmacol. 2018;93:71–83. doi: 10.1016/j.yrtph.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Adamson J., Li X.T., Cui H., Thorne D., Xie F., Gaça M. Nicotine quantification in vitro: a consistent dosimetry marker for e-cigarette aerosol and cigarette smoke generation. Appl. In Vitro Toxicol. 2017;3(1):14–27. [Google Scholar]
- 31.Thorne D., Leverette R., Breheny D., Lloyd M., McEnaney S., Whitwell J., Clements J., Bombick B., Gaca M. Genotoxicity evaluation of tobacco and nicotine delivery products: part two. In vitro micronucleus assay. Food Chem. Toxicol. 2019;132 doi: 10.1016/j.fct.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 32.Han S.G., Pant K., Bruce S.W., Gairola C.G. Bhas 42 cell transformation activity of cigarette smoke condensate is modulated by selenium and arsenic. Environ. Mol. Mutagen. 2016;57:220–228. doi: 10.1002/em.22000. [DOI] [PubMed] [Google Scholar]
- 33.Weisensee D., Poth A., Roemer E., Conroy L.L., Schlage W.K. Cigarette smoke-induced morphological transformation of Bhas 42 cells in vitro. Altern. Lab. Anim. 2013;41:181–189. doi: 10.1177/026119291304100207. [DOI] [PubMed] [Google Scholar]
- 34.Azzopardi D., Patel K., Jaunky T., Santopietro S., Camacho O.M., McAughey J., Gaҫa M. Electronic cigarette aerosol induces significantly less cytotoxicity than tobacco smoke. Tox. Mech. Methods. 2016;26:477–491. doi: 10.1080/15376516.2016.1217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McQuillan K., Carr T., Taylor M., Bishop E., Fearon I.M. Examination of the use of human sera as an exposure agent for in vitro studies investigating the effects of cigarette smoking on cellular cardiovascular disease models. Toxicol. In Vitro. 2015;29:856–863. doi: 10.1016/j.tiv.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 36.ICCVAM . NIH publication No. 07-4519. Research triangle park, NC: National institute for environmental health sciences; 2006. Test Method Evaluation Report (TMER): In Vitro Cytotoxicity Test Methods for Estimating Starting Doses for Acute Oral Systemic Toxicity Tests.https://ntp.niehs.nih.gov/iccvam/docs/acutetox_docs/brd_tmer/at-tmer-complete.pdf (Accessed 23 May 2019) [Google Scholar]
- 37.Organisation for Economic and Cooperative Development . 2016. Test No 231: Guidance Document on the in Vitro Bhas 42 Cell Transformation Assay. Test no: 231. Series on Testing and Assessment. No. 231. Paris. 2016.https://www.oecd.org/env/ehs/testing/ENV_JM_MONO(2016)1.pdf [Google Scholar]
- 38.Organisation for Economic and Cooperative Development . 2016. Test No 487: In Vitro Mammalian Cell Micronucleus Test. OECD Guidelines for the Testing of Chemicals, Section 4: Health Effects. Paris. 2016.https://read.oecd-ilibrary.org/environment/test-no-487-in-vitro-mammalian-cell-micronucleus-test_9789264264861-en#page1# [Google Scholar]
- 39.Organisation for Economic and Cooperative Development . 2015. Test No. 490: in Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene. Test Guideline 490.https://www.oecd-ilibrary.org/environment/test-no-490-in-vitro-mammalian-cell-gene-mutation-tests-using-the-thymidine-kinase-gene_9789264242241-en (Accessed 23 May 2019) [Google Scholar]
- 40.Organisation for Economic and Cooperative Development . 1997. Test No. 471: Bacterial Reverse Mutation Test.https://www.oecd-ilibrary.org/environment/test-no-471-bacterial-reverse-mutation-test_9789264071247-en (Accessed 23 May 2019) [Google Scholar]
- 41.Thorne D., Crooks I., Hollings M., Seymour A., Meredith C., Gaca M. The mutagenic assessment of an electronic-cigarette and reference cigarette smoke using the Ames assay in strains TA98 and TA100. Mutat. Res. 2016;812:29–38. doi: 10.1016/j.mrgentox.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Breheny D., Oke O., Pant K., Gaça M. Comparative tumor promotion assessment of e-cigarette and cigarettes using the in vitro Bhas 42 cell transformation assay. Environ. Mol. Mutagen. 2017;58(4):190–198. doi: 10.1002/em.22091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez-Suarez I., Martin F., Marescotti D., Guedj E., Acali S., Johne S., Dulize R., Baumer K., Peric D., Goedertier D., Frentzel S., Ivanov N.V., Mathis C., Hoeng J., Peitsch M.C. High content screening analysis to evaluate the toxicological effects of harmful and potentially harmful constituents (HPHC) J. Vis. Exp. 2016;10:111. doi: 10.3791/53987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salomon C., Ryan J., Sobrevia L., Kobayashi M., Ashman K., Mitchell M., Rice G.E. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8:e6845. doi: 10.1371/journal.pone.0068451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Combes R., Scott K., Crooks I., Dillon D., Meredith C., McAdam K., Proctor C. The in vitro cytotoxicity and genotoxicity of cigarette smoke particulate matter with reduced toxicant yields. Toxicol. In Vitro. 2013;27(5):1533–1541. doi: 10.1016/j.tiv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Iskandar A.R., Zanetti F., Kondylis A., Martin F., Leroy P., Majeed S., Steiner S., Xiang Y., Ortega Torres L., Trivedi K., Guedj E., Merg C., Frentzel S., Ivanov N.V., Doshi U., Lee K.M., McKinney W.J., Jr., Peitsch M.C., Hoeng J. A lower impact of an acute exposure to electronic cigarette aerosols than to cigarette smoke in human organotypic buccal and small airway cultures was demonstrated using systems toxicology assessment. Intern. Emerg. Med. 2019 doi: 10.1007/s11739-019-02055-x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costigan S., Meredith C. An approach to ingredient screening and toxicological risk assessment of flavours in e-liquids. Regul. Toxicol. Pharmacol. 2015;72:361–369. doi: 10.1016/j.yrtph.2015.05.018. [DOI] [PubMed] [Google Scholar]