Highlights
-
•
The expression of HERP is increased after intracerebral hemorrhage in vivo and in vitro.
-
•
Intracerebral hemorrhage can induce endoplasmic reticulum stress.
-
•
Herp is an endoplasmic reticulum stress associated protein.
-
•
HERP protects hemin-exposed primary cortical neurons to a certain extent.
Abbreviations: ER, endoplasmic reticulum; ICH, intracerebral hemorrhage; HERP, homocysteine-induced endoplasmic reticulum protein; Herpud1, homocysteine-inducible and ER-stress-inducible, ubiquitin-like domain member 1; ERAD, endoplasmic reticulum-associated protein degradation; TM, tunicamycin; TUDCA, tauroursodeoxycholic acid
Keywords: Apoptosis, Autophagy, Endoplasmic reticulum stress, Intracerebral hemorrhage, Homocysteine-induced endoplasmic reticulum protein, Mice
Abstract
Intracerebral hemorrhage (ICH) is defined as bleeding into the brain parenchyma with a high mortality and morbidity rate. Unfortunately, it remains an unresolved medical problem. Therefore, it is necessary to find ways to reduce cellular apoptosis after ICH. Homocysteine-induced endoplasmic reticulum protein (HERP), a 54 kD transmembrane protein, is an early stress response protein encoded by ubiquitin-like domain member 1 (Herpud1) gene. In the present work, our group investigated the role of HERP after ICH and hemin stimulation, HERP expression was examined in mouse and primary cortical neurons after ICH and hemin stimulation by western blot and Immunofluorescent labeling. Using shRNA-HERP plasmid and recombinant adenovirus, we also investigated how HERP affected neuronal apoptosis after ICH and hemin stimulation. In addition, behavioral evaluation was used to ensure our models’ success. In vivo and vitro studies, the expression of HERP was increased following ICH and hemin-exposed primary cortical neurons. HERP depletion activated the endoplasmic reticulum (ER) stress pathway and apoptosis in hemin-exposed primary cortical neurons, but inhibited autophagy in hemin-exposed primary cortical neurons. Overexpression of HERP inhibited the ER stress pathway and apoptosis, but activated autophagy in hemin-exposed primary cortical neurons. Consequently, we confirm that HERP plays a protective role in ICH model.
1. Introduction
With a high mortality and morbidity rate around 10–15 % of all strokes worldwide, intracerebral hemorrhage (ICH) is defined as bleeding in the brain parenchyma, which is the second most common subtype of stroke worldwide and the percentage is even larger in Asia (Ikram et al., 2012; Weimar and Kleine-Borgmann, 2017). In spite of its destructively social and economic burden on individuals and society, and many clinical and basic studies on exploring the pathogenesis of ICH, ICH remains a medical problem with little improvement in patient outcomes which we would better solve in the past 20 years. As yet, there still lacks of effective medical and surgical strategies to reduce the morbidity and mortality of ICH. A number of works in recent years indicate that the pathological process of ICH is complex, including brain edema, hematoma formation, and the activation of cytotoxic, inflammatory, oxidative, autophagy and endoplasmic reticulum (ER) stress pathways. Therefore, it is necessary to elucidate these cellular and molecular mechanisms of ICH (Li et al., 2020; Mohammed Thangameeran et al., 2020).
Homocysteine-induced endoplasmic reticulum protein (HERP), a 54 kD transmembrane protein, is an early stress response protein encoded by homocysteine-inducible and ER-stress-inducible, ubiquitin-like domain member 1 (Herpud1) gene. It is dramatically induced by ER stress in a variety of pathological conditions, including ICH (Cao et al., 2019; George et al., 2018; Kokame et al., 2000; Maeda et al., 2018). HERP also plays a significant role in ER-associated protein degradation (ERAD), which is the principal mechanism of protein degradation that misfolding unfortunately. HERP degrades the unfolded and misfolded proteins via the ERAD pathway and its knockout causes the accumulation of ERAD substrates (Torres et al., 2020).
Autophagy, a lysosomal degradation pathway, involves the degradation of long-lived, misfolded, or damaged proteins and cytoplasmic organelles. The previous studies indicate that HERP is related to the level of autophagy closely and autophagy regulates neural apoptosis induced by ICH (Durocher et al., 2019; Quiroga et al., 2013). Furthermore, it has been reported that in ICH autophagy also plays an essential role, however, it is not yet clear how HERP plays the role between ER stress and autophagy in ICH and whether HERP plays a pro-death or a pro-survival mechanism in ICH. In this work, recombinant adenovirus vectors for HERP shRNA expression and HERP overexpression were constructed to investigate the roles of HERPs’ different expressions between autophagy and ER stress after ICH and its effects on the apoptosis of neurons. All in above, exploring the mechanisms and effects of HERP in ICH models is our study’s purpose.
2. Experimental procedures
2.1. Experimental animals
According to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, which was published in 1996 by the National Research Council, we conducted all animal care and surgical procedures. The protocols were approved by the Animal Care and Use Committee in Nantong University (ethical approval number: 20160906‐001). Sixty male C57BL/6 mice weighing 20−25 g (five mice in each cage) were fed with adequate food and drinking water, in a circumstance of 12 -h light-dark cycle with constant temperature 24 °C and relative humidity 55–60 %. 10 female, pregnant, C57BL/6 mice were placed under the same condition. We have a 10 percent mortality rate. In addition, our experimental results were repeatedly validated.
2.2. Mice ICH mode
The mice were injected with sodium pentobarbital (50 mg/kg) intraperitoneally, and a skull hole (1 mm in diameter) was drilled certain 3.5 mm outside the middle near the right coronal suture in a stereotaxic frame. Autologous whole blood (15 μl) was collected from the tail tip into a sterile syringe (Shi et al., 2020; Yan et al., 2020). The sterile syringe was tactically inserted into the right basal ganglia (coordinates: 0.2 mm anterior, 3.5 mm ventral, and 2.5 mm lateral to the bregma) (Ng et al., 2020). Then, we injected the blood with the rate of 1 μl /min. After 10 min, the needle was removed and the skin incision was closed to allow the animals to recover. The sham group mice were only inserted a needle. Experimental mice (n = 6/time point) were sacrificed at 6 h, 12 h, 1d, 3d and 5d, separately. The sham mice were sacrificed on 7d. Animal experiments were carried out in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory and approved by the Chinese National Committee to Use of Experimental Animals for Medical Purposes, Jiangsu Branch. Every effort had been made to minimize the number of animals used and their suffering.
2.3. Behavioral evaluation
The forelimb placing test was performed blindly as previously described (Krafft et al., 2014; Zan et al., 2017). Held by the torso, each mouse was free to hang the forelimb. The movements of each forelimb were caused by brushing the opposite tentacles at the corner of the table. The healthy and sham-operated mice quickly placed their contralateral forelimbs on the countertop after a stimulation on their tentacles. Due to the degree of injury, the ICH mice may not be able to place the left paralyzed forelimb which was contralateral to the impairment. The test was designed for 6 units (on 30 min intervals). The percentage of trials in which the mice placed the left forelimb was calculated.
Corner turn test was also carried out blindly. The mice were allowed to enter a 30-degree corner, then they would turn left or right to leave the corner. Only turns involving complete rearing along the wall were calculated. Depending on the degree of injury, mice which had ICH had the tendency towards turning to the ipsilateral side of impairment. The percentage of trials in which the mice turned right was calculated. A unit was consisted of 10 times and the mice would be tested for a total of 6 units (Krafft et al., 2014; Nakamura et al., 2004; Zan et al., 2017).
The mice were also tested on a rotarod treadmill (RAD Life Science, Shenzhen, China). After training, they were evaluated 24 h before surgery and at 6 h, 12 h, 1d, 3d and 5d after surgery. The rotarod was programmed the acceleration mode of 5–35 revolutions per minutes for more than 5 min. The maximum time the animal stayed on the rotarod (up to 5 min) was recorded for each performance. A mean of 3 trials was used to represent performance. Values are reported as a percentage of the baseline evaluation (Alonso-Castro et al., 2020; Shiotsuki et al., 2010; Zan et al., 2017). In behavioral experiments, treated animals were assessed prior to control animals. Behavioral experiments were performed between 8 and 12 a.m..
2.4. Primary cortical neurons cultures and treatments
As described in previous studies, primary cortical neurons were isolated from fetuses of 16‐day pregnant mice according to descriptions of previous studies. The isolated neurons were seeded into plates pre-coated with 100 mg/L poly‐L‐lysine, at a density of 1 × 106 cells/well in six‐well plates, 3 × 105 cells/well in 24‐well plates or 1 × 105 cells/well in 96‐well plates. After incubating for 4 h at 37 °C with 5% carbon dioxide, the medium was replaced with NEUROBASALTM medium supplemented with 2% B27. The neurons were used for experiments after 7‐d culture (Croci et al., 2020; Yan et al., 2020).
2.5. Protein extraction and western blot analysis
After an injection of excess sodium pentobarbital (50 mg/kg), the mice were sacrificed at different time points after surgery, and the brain tissue around the hematoma (extending 2 mm to the incision) as well as the equivalent parts of the normal, sham, and contralateral cortex were collected and stored at −80 °C until use. To prepare the lysates, frozen samples were weighed and chopped on ice. The samples were then placed evenly in lysis buffer (1% NP-40, 50 mmol/l Tris, pH = 7.5, 5 mmol/l EDTA, 1% SDS, 1% sodium deoxycholate, 1% Triton X-100, 1 mmol/l PMSF, 10 μg/mL aprotinin, and 1 μg/mL leupeptin) and centrifuged at 12,000 rpm, for 20 min at 4 °C to collect the supernatant. After determining the protein concentration by the Brad-ford assay (Bio-Rad, Richmond, CA, USA), protein samples were separated by SDS-PAGE and transferred to PVDF membranes with the transfer device at 250 mA for 2 h. The membranes were blocked with 5% non-fat milk for 2 h and incubated with primary antibodies against HERP (rabbit, 1:1000; Abcam, Cambridge, MA, USA), caspase-12 (rabbit, 1:1000, Abcam, Cambridge, MA, USA), β-tubulin (rabbit, 1:2000, Abcam, Cambridge, MA, USA), LC3B (rabbit, 1:1000, Abcam, Cambridge, MA, USA), and GRP78(mouse, 1:1000, Abcam, Cambridge, MA, USA) overnight at 4 °C. Eventually, the membrane was incubated with HRP-conjugated secondary antibodies for 2 h and visualized using an enhanced chemiluminescence system (ECL; Invitrogen, Carlsbad, CA, USA).
2.6. Sections
Mice were deeply anesthetized for 2 days after surgical, perfused with 500 ml 0.9 % saline, and continuously administered 4% paraformaldehyde (PFA) through the heart. Immediately, the whole brain tissues were removed, soaked PFA for 24 h, then immersed in 20 % for 2–3 days and transferred to 30 % sucrose for another 2–3 days at 4 °C. 6μm-thick continuous coronal frozen sections were cut on a freezing microtome and stored at −20 °C until use.
2.7. Immunofluorescent labeling
After air-dried for 1 h, sections were initially blocked with 10 % normal donkey serum blocking solution for 2 h at RT, which was containing 3% (w/v) bovine serum albumin (BSA), 0.1 % Triton X-100 and 0.05 % Tween. Then the primary antibodies against HERP (rabbit, 1:1000; Abcam, Cambridge, MA, USA), NeuN (mouse, 1:300; Chemicon, Temecula, CA, USA), caspase-12 (rabbit, 1:1000, Abcam, Cambridge, MA, USA) and GRP78 (rabbit, 1:1000, Abcam, Cambridge, MA, USA) were incubated in the sections at 4 °C overnight, followed in the incubation of a mixture of FITC- and TRITC-conjugated secondary antibodies (1:1000; Thermo Fisher Scientific, Grand Island, NY, USA) at 4 °C for 2 h. The Zeiss fluorescence microscope (Germany) was used to examine the stained sections.
2.8. Cell counting kit‐8 (CCK‐8) assay
The viability of neurons after treatments of hemin was determined by CCK‐8 according to the manufacturer's protocol. Cells were seeded in the 96‐well plates at 1 × 105 cells/well. After incubation overnight, neurons were exposed to hemin (0−200 μmol/L) for 1 day. CCK‐8 solution was added to each well at a final concentration of 10 % (v/v) and the neurons were incubated at 37 °C for 2 h. Absorbance at 490/630 nm was measured by a microplate reader (Bio‐Rad, Hercules, CA).
2.9. Construction of shRNA-HERP plasmid
The shRNA with vector was purchased from Hanbio (Hanbio, shanghai, China). The shRNA1-HERP (sequences were listed in the Table 1) and vectors from Hanbio (Hanbio, shanghai, China), the latter of which contained an ineffective shRNA cassette against EGFP, were used to knockdown the expression of HERP.
Table 1.
Sequences of shRNA-HERP.
| Strand | Sequences | |
|---|---|---|
| siRNA | TTCTCCGAACGTGTCACGTAA | |
| shRNA | Top strand | GATCCGTTCTCCGAACGTGTCACGTAATTCAAGAGATT ACGTGACACGTTCGGAGAATTTTTTC |
| Bottom strand | AATTGAAAAAATTCTCCGAACGTGTCACGTAATCTCTT GAATTACGTGACACGTTCGGAGAACG |
|
| siRNA1 | CGGACAACTCTAATCAGACACAGCA | |
| shRNA1 | Top strand | AATTCGCGGACAACTCTAATCAGACACAGCATTCAAGA GATGCTGTGTCTGATTAGAGTTGTCCGTTTTTTG |
| Bottom strand | GATCCAAAAAACGGACAACTCTAATCAGACACAGCATC TCTTGAATGCTGTGTCTGATTAGAGTTGTCCGCG |
|
| siRNA2 | CAGCAGATCTATGCAAGGCAGTACT | |
| shRNA2 | Top strand | AATTCGCAGCAGATCTATGCAAGGCAGTACTTTCAAGAG AAGTACTGCCTTGCATAGATCTGCTGTTTTTTG |
| Bottom strand | GATCCAAAAAACAGCAGATCTATGCAAGGCAGTACTTCT CTTGAAAGTACTGCCTTGCATAGATCTGCTGCG |
|
| siRNA3 | GAGCCAATCAGAACTTGCGGATGAA | |
| shRNA3 | Top strand | AATTCGAGCCAATCAGAACTTGCGGATGAATTCAAGAGA TTCATCCGCAAGTTCTGATTGGCTCTTTTTTG |
| Bottom strand | GATCCAAAAAAGAGCCAATCAGAACTTGCGGATGAATCT CTTGAATTCATCCGCAAGTTCTGATTGGCTCG |
We use shRNA to knockdown the expression of HERP. There are three shRNAs (shRNA1, shRNA2, shRNA3) available. We will choose the most effective one for our research.
2.10. Adenoviral infection
The primary cortical neurons were seeded into six‐well plates for 1 × 106 cells/well, 3 × 105 cells/well in 24‐well plates or 1 × 105 cells/well in 96‐well plates. Cultured overnight, the primary cortical neurons were infected with serially diluted concentrations of recombinant adenovirus. After incubation for 1 day, the previous growth medium was removed and fresh NEUROBASALTM medium was added, and treated with adenovirus.
2.11. Statistical analysis
Data are presented as mean ± SD. Statistical difference was determined by one-way ANOVA, followed by all pairwise multiple-comparison procedures with Bonferroni’s test. Bar chart values were analyzed by Student’s t-test. P < 0.05, p < 0.01, p < 0.001 and p < 0.0001 was considered statistically significant.
3. Results
3.1. In vivo study, the expression of HERP was increased following ICH
In this study, an experimental ICH model was established as described above and the mice were assessed with behavioral test comprising of rotarod test, forelimb placing and corner turn test at different time points after ICH (Fig. 1) (Krafft et al., 2014). As shown in the Fig. 1B, the rotarod latency of ICH group was significantly reduced compared with the sham group. Moreover, as shown in Fig. 1C and D, the mice were assessed with behavioral test comprising of forelimb placing and corner turn test. These results indicated that animal model of ICH was constructed successfully. To identify the changes of HERP after ICH, the protein level of HERP was detected in perihematomal tissues of mice in 6 h, 12 h, 1 day, 3 days, 5 days after ICH using western blot (Fig. 2A). The results showed that, compared with the normal group and sham group, the protein level of HERP was significantly increased respectively after ICH, and continued to increase in 1 day and lasted 5 days (Fig. 2B). According to the western blot, ipsilateral mice brain tissues in 1 day after ICH have been chosen to detect in immunofluorescent staining. To further study HERP localization and expression in neurons after ICH, immunofluorescent staining was performed with cells specific marker: NeuN (a marker of neuron). As described previously (Cao et al., 2019), we found that HERP co-located with neurons in the cytoplasm. As shown in Fig. 2C and D, semi-quantitative analysis of HERP-positive cells indicated that the sham group expressed weaker HERP-positive signals than the ICH group. These results all indicated that the expression of HERP in perihematomal tissues of mice following ICH in 1 day was significantly up-regulated.
Fig. 1.
Model construction of ICH and evaluation of neurological deficits following ICH. (A) The model of ICH was constructed using stereotactic frame. (B) Rotarod, (C) forelimb-placing and (D) corner turn tests were performed at 6 h, 12 h, 1day, 3days, 5days post-ICH (infusion of 15 μL autologous blood into the right striatum) and in the sham control. Values are presented as the mean ± standard deviation (n = 6/group), *P < 0.05, **p < 0.01, ***p < 0.001 vs. sham group.
Fig. 2.
In vivo study, the expression of HERP was increased following ICH. (A) Western blot analysis showed the protein levels of HERP surrounding tissues of cerebral hematoma after intracerebral hemorrhage at different survival times. The expression of HERP was relatively low in the sham-operated group, while up-regulated gradually following ICH, peaked at day 1 and declined thereafter (n = 6/group). (B) The relative density of HERP versus β-Tubulin was shown in the bar graph. ***p < 0.001 versus the normal group. (C) Immunofluorescence staining showed the HERP with neuron marker (NeuN). Sections were labeled with HERP (green, a, d), neuronal marker NeuN (red, b, e). The merged images represented the colocalization of HERP and NeuN (c, f) (n = 6/group). Arrows point to the colocalization of HERP and NeuN. (D) The number of HERP positive cells which co-localized with neurons was significantly increased in the ICH group compared with the sham group. **p < 0.01. Scale bars 50 μm (a–f). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.2. In vitro, the expression of HERP was increased in hemin-exposed primary cortical neurons
To further explore the roles of HERP after ICH, hemin-induced primary cortical neurons were applied to mimic ICH in vitro. After incubation of 25,50,100,200 μmol/L hemin for 24 h, the cell viability rate of primary cortical neurons was examined by cell counting kit-8 (CCK-8) and HERP protein level was also measured by western blot (Fig. 3A∼C). These data demonstrated that Hemin at the concentration of 50 μmol/L induced the expression of HERP in primary cortical neurons for 24 h. To find out the expression and location of HERP in hemin-exposed primary cortical neurons, we chose the double immunofluorescent staining by co-labeling HERP with Map2, which is the maker of neuron. As predicted, compared with the DMSO control group, the expression of HERP increased in the primary cortical neurons induced by 50 μmol/L hemin, while the expression in the normal and DMSO group showed no statistical significance. Immunofluorescence results showed that HERP co-located with neurons after hemin stimulation (Fig. 3D) and the percentage of HERP-positive neurons were obviously larger in hemin-exposed condition than that in normal condition (Fifure.3E). The fluorescence intensity signals were faint in untreated cells. These data showed the expressions of HERP in hemin-treated primary cortical neurons is up-regulated.
Fig. 3.
In vitro, the expression of HERP was increased in hemin-exposed primary cortical neurons. (A) The CCK‐8 assay was operated to examine the cell viability of primary cortical neurons, after 24‐hour exposure to different concentrations of hemin. ****p < 0.0001 versus the dmso group (n = 3/group). (B) Western blot analysis showed the protein levels of HERP at different concentrations of hemin (n = 6/group). The expression of HERP was significantly up-regulated with the concentration of 50 μM.(C)The relative density of HERP versus β-Tubulin was shown in the bar graph. *p < 0.05, **p < 0.01 versus the dmso group. (D)In immunofluorescent staining, the primary cortical neurons after 24‐hr exposure of hemin were labeled with HERP and Map2 (n = 6/group). The yellow color signified the colocalization of HERP (green, a, e) and Map2 (red, b, f). The nuclei of the primary cortical neurons were labeled by hoechst33342 (blue, c, g). The merged images represented the colocalization of HERP, NeuN and hoechst33342 (d, h). Scale bars 50 μm (a–h). (E)The number of HERP- positive primary cortical neurons was significantly increased in the Hemin group compared with the sham group. **p < 0.01. Scale bars 50 μm (a–h). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.3. ER stress activator TM increased the expression of HERP and ER stress inhibitor TUDCA decreased the expression of HERP in hemin-induced primary cortical neurons
Recent studies have shown that Glucose-regulated protein of 78 kD (GRP78), which was also referred to as a marker of ER stress, plays an important role in ER stress (Shimizu et al., 2017). Its expression is regulated by the ER stress (Americo-Da-Silva et al., 2018). To investigate whether HERP is regulated by the ER stress and whether HERP content and HERP production by hemin in ICH models correlate with ER stress, we stimulated the hemin-treated primary cortical neurons with tunicamycin (TM) and tauroursodeoxycholic acid (TUDCA) (Choi et al., 2020; Kokame et al., 2000; Min et al., 2018; Pauly et al., 2017). GRP78 and HERP expression was up-regulated in hemin group, thus it was reasonable to investigate whether HERP was correlated with neuronal ER stress. Exposing the hemin-treated primary cortical neurons to 5μM of TM or 100μM of TUDCA, the expression of HERP was significantly induced or reduced after incubation for 24 h, consistent with the expression of GRP78, as expected. In addition, immunofluorescent labeling showed that HERP co-localized with GRP78 in hemin-treated primary cortical neurons (Fig. 4A–C). Then, we observed the intensity of anti-HERP staining in hemin-treated primary cortical neurons. The fluorescence intensity signals of HERP was faint in TUDCA-treated neurons, and in TM-treated neurons was intense, consistent with the protein levels observed by western blot (Fig. 4D). In double-immunofluorescent labeling, we observed that overexpression and colocalization of HERP and GRP78 was found in hemin-induced primary cortical neurons (Fig. 4E). These results all demonstrated that HERP is an ER stress associated protein. Its expression is up-regulated when ER stress was induced and down-regulated when ER stress was inhibited.
Fig. 4.
Changes in the protein levels of GRP78, HERP in primary cortical neurons. (A) GRP78 and HERP protein levels in primary cortical neurons determined by Western blots (n = 6/group). (B) Western blot analysis showed the relative density of HERP and GRP78 versus β-Tubulin. *p < 0.05, **p < 0.01 versus the normal group. #p < 0.05, ##p < 0.01 versus the hemin group. (C) The expression trends for HERP and GRP78 were similar in the three groups. (D) In immunofluorescent staining, the expression trends for HERP and GRP78 were similar in the three groups (n = 6/group). The yellow color signified the colocalization of HERP (green, a, e, i) and Map2 (red, b, f, j). The nuclei of the primary cortical neurons were labeled by hoechst33342 (blue, c, g, k). The merged images represented the colocalization of HERP, NeuN and hoechst33342 (d, h, l). Arrows point to HERP-positive primary cortical neurons. (E) In double‐immunofluorescent staining, the hemin-induced primary cortical neurons were labeled with HERP (green, a), GRP78 (red, b), and hoechst33342 (blue, c). The yellow color, in the merged images (d), signified the colocalization of HERP with GRP78 (n = 6/group). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
3.4. Effects of HERP-shRNA adenoviral vector on hemin-exposed primary cortical neurons in vitro
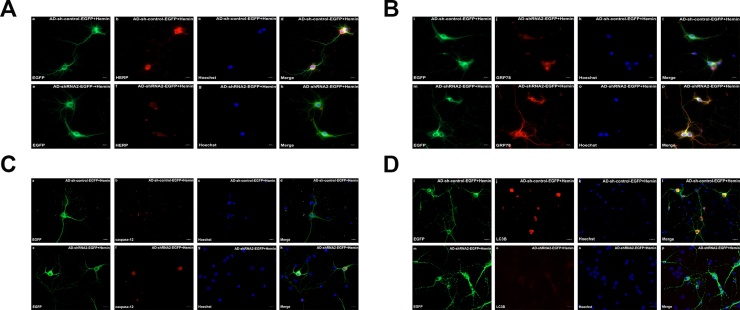
As noted before, hemin induced a dramatic increase in HERP protein expression in primary cortical neurons. To obtain more evidence supporting the role of HERP in vitro during hemin injury, hemin-exposed primary cortical neurons were infected with recombinant adenovirus (rAD) with EGFP (enhanced green fluorescence protein) vector containing the shRNA of HERP to knockdown the expression of HERP. Three independent HERP siRNA sequences were adopted to generate a high titer of AD-HERP-shRNA-EGFP. As shown in the Fig. 5A, when multiplicity of infection (MOI) was 30, EGFP-positive neurons were significantly higher than that in any other group. Intense EGFP-positive neurons were readily detected in primary cortical neurons at as early as 12 h after transfection and became apparent at day 1, whereas the EGFP-positive neurons clusters were less pronounced till around day 2 (Fig. 5B). Therefore, the optimum conditions for rAD infected primary cortical neurons in vitro were 30 MOI and culturing for 1 day. As shown in Fig. 5D, the AD-HERP-shRNA2-EGFP infected primary cortical neurons showed a marked decrease compared with AD-control-shRNA-EGFP, AD-HERP-shRNA1-EGFP and AD-HERP-shRNA3-EGFP infected neurons. Therefore, AD-HERP-shRNA2 was transfected into primary cortical neurons after hemin pre-treatment (50 μmol/L, 24 h). Using western blot and immunofluorescent staining, we examined the expression of HERP in AD-HERP-shRNA2-EGFP versus AD-control-shRNA-EGFP in hemin-exposed primary cortical neurons in vitro and HERP was significantly down -regulated in the AD-HERP-shRNA2-EGFP group (Fig. 6A and B). Furthermore, HERP depletion increased expression of GRP78 and caspase-12, but decreased the expression of LC3BII/I in hemin-exposed primary cortical neurons (Fig. 6). Utilizing immunofluorescent staining to detect the expression of HERP, it gave the same result as the western blot experiment (Fig. 7). These data provided that HERP depletion activated the ER stress pathway and apoptosis in hemin-exposed primary cortical neurons and inhibited autophagy in hemin-exposed primary cortical neurons.
Fig. 5.
The primary cortical neurons were infected with AD-EGFP-vector containing the shRNA to knockdown the expression of HERP.(A)Recombinant adenovirus infected the primary cortical neurons at different MOI for 24 h. Scale bars 20 μm. (B) Recombinant adenovirus infected the primary cortical neurons at different times in the MOI of 30. Scale bars 20 μm. (C) The primary cortical neurons infected with AD-EGFP (green, a, e, i, m) was stained with the marker of neurons Map2 (red, b, f, j, n) in immunofluorescent staining. The transfection efficiency of AD-sh-control-EGFP, AD-shRNA1-EGFP, AD-shRNA2-EGFP, AD-shRNA3-EGFP were 89.69 %, 97.41 %, 94.52 % and 93.37 % respectively when MOI was 30 for 24 h. Scale bars 100 μm. (D) Western blot analysis showed the protein levels of HERP in different groups (n = 6/group). The relative density of HERP versus β-Tubulin was shown in the bar graph. *p < 0.05, **p < 0.01, ***p < 0.001 versus the normal group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Fig. 6.
The expressions of HERP, GRP78, caspase-12, LC3B were changed in the primary cortical neurons infected with AD-shRNA2-EGFP + hemin in western blot. (A) Western blot analysis showed the protein levels of HERP, GRP78, caspase-12, LC3B in the groups of AD-sh-control-EGFP, AD-sh-control-EGFP + hemin, AD-shRNA2-EGFP and AD-shRNA2-EGFP + hemin (n = 3/group). (B), (C), (D) Quantification of the western blot in (A). *p < 0.05, **p < 0.01, ***p < 0.001 versus the AD-sh-control-EGFP group. #p < 0.05, ##p < 0.01 versus the AD-sh-control-EGFP + hemin.
Fig. 7.
The expressions of HERP, GRP78, caspase-12, LC3B were changed in the primary cortical neurons infected with AD-shRNA2-EGFP + hemin in immunofluorescent staining. (A) The expression of HERP was down-regulated in AD-shRNA2-EGFP + hemin group versus the AD-sh-control-EGFP + hemin group. (B) The expression of GRP78 was up-regulated in AD-shRNA2-EGFP + hemin group versus the AD-sh-control-EGFP + hemin group. (C) The expression of caspase-12 was up-regulated in AD-shRNA2-EGFP + hemin group versus the AD-sh-control-EGFP + hemin group. (D) The expression of LC3B was down-regulated in AD-shRNA2-EGFP + hemin group versus the AD-sh-control-EGFP + hemin group.
3.5. Effects of recombinant adenovirus carrying HERP gene on hemin-exposed primary cortical neurons in vitro
In this experiment, we tested whether the overexpression of HERP was effective in hemin-exposed primary cortical neurons. To assess this, recombinant adenovirus vector expression HERP was constructed and transfected into primary cortical neurons. It was found that the optimum conditions of the AD-HERP-EGFP and AD-control-EGFP in primary cortical neurons were infected for 24 h at 10 MOI. By western blot in primary cortical neurons, AD-HERP-EGFP significantly enhanced the expression of HERP compared to AD-control-EGFP group (Fig. 8). To investigate whether adenovirus expressing HERP could affect the levels of autophagy and apoptosis in hemin-exposed primary cortical neurons, recombinant adenovirus carrying HERP gene was transfected into primary cortical neurons after hemin pre-treatment (50 μmol/L, 24 h). As shown in the Fig. 9, Fig. 10, overexpression of HERP decreased expression of GRP78 and caspase-12, but increased the expression of LC3BII/I in hemin-exposed primary cortical neurons. These results verified that overexpression of HERP inhibited the ER stress pathway and apoptosis, but activated autophagy in hemin-exposed primary cortical neurons.
Fig. 8.
The primary cortical neurons were infected with AD-EGFP vector to overexpress the expression of HERP. (A)Recombinant adenovirus infected the primary cortical neurons at different MOI for 24 h. Scale bars 20 μm. (B) Recombinant adenovirus infected the primary cortical neurons at different times in the MOI of 10. Scale bars 20 μm. (C) The primary cortical neurons infected with AD-EGFP (green, a, e) was stained with the marker of neurons Map2 (red, b, f) in immunofluorescent staining. The transfection efficiency of AD-HERP-control-EGFP, AD-HERP-EGFP were 88.34 % and 90.46 % respectively when MOI was 10 for 24 h. Scale bars 100 μm. (D) Western blot analysis showed the protein levels of HERP in different groups (n = 6/group). The relative density of HERP versus β-Tubulin was shown in the bar graph. **p < 0.01 versus the normal group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Fig. 9.
The expressions of HERP, GRP78, caspase-12, LC3B were changed in the primary cortical neurons infected with AD-HERP-EGFP + hemin in western blot. (A) Western blot analysis showed the protein levels of HERP, GRP78, caspase-12, LC3B in the groups of AD-HERP-control-EGFP, AD-HERP-control-EGFP + hemin, AD-HERP-EGFP, AD-HERP-EGFP + hemin (n = 6/group). (B), (`C), (D) Quantification of the western blot in (A). *p < 0.05, **p < 0.01 versus the AD-HERP-control-EGFP group. #p <0.05, ##p < 0.01 versus the AD-HERP-control-EGFP + hemin.
Fig. 10.
The expressions of HERP, GRP78, caspase-12, LC3B were changed in the primary cortical neurons infected with AD-HERP-EGFP + hemin in immunofluorescent staining. (A) The expression of HERP was up-regulated in AD-HERP-EGFP + hemin group versus the AD-HERP-control-EGFP + hemin group. (B) The expression of GRP78 was down-regulated in AD-HERP-EGFP + hemin group versus the AD-HERP-control-EGFP + hemin group. (C) The expression of caspase-12 was down-regulated in in AD-HERP-EGFP + hemin group versus the AD-HERP-control-EGFP + hemin group. (D) The expression of LC3B was up-regulated in AD-HERP-EGFP + hemin group versus the AD-HERP-control-EGFP + hemin group.
4. Discussion
Previous studies had indicated that autophagy, ER stress and apoptosis were involved in various physiological and pathological conditions in stroke, especially in intracerebral hemorrhage (Durocher et al., 2019; Mohammed Thangameeran et al., 2020; Yang et al., 2018). HERP has been described as a ubiquitin-like membrane protein induced by endoplasmic reticulum stress, however, whether HERP plays a positive or negative role in the intracerebral hemorrhage remains controversial and the role of HERP in the autophagy, ER stress, apoptosis is largely unknown. Apoptosis and ER stress caused by intracerebral hemorrhage damage can lead to neuronal cell death(Duan et al., 2017). HERP serves as an essential regulator of the endoplasmic reticulum (ER) stress response. However, the specific mechanism of HERP between autophagy and ER stress has not been well identified. In this experiment, we provide the following evidence: (1) In vivo study, the expression of HERP is increased following ICH; (2) In vitro, the expression of HERP is increased in hemin-exposed primary cortical neurons; (3) ER stress activator TM increases the expression of HERP and ER stress inhibitors TUDCA decreases the expression of HERP in hemin-induced primary cortical neurons; (4) HERP depletion activates the ER stress pathway and apoptosis in hemin-exposed primary cortical neurons, but inhibits autophagy in hemin-exposed primary cortical neurons; (5) overexpression of HERP inhibits the ER stress pathway and apoptosis, but activates autophagy in hemin-exposed primary cortical neurons. Consequently, we confirm that HERP plays a protective role in ICH model.
ICH models in vivo and in vitro are established to simulate clinical ICH. In vivo, the mice model of ICH was built by a nigrostriatal injection of autologous blood which is carried out easily to mimic human ICH (Zhang et al., 2018). In vitro, we use hemin to treat primary cortical neurons, which can also mimic human ICH. In 2016, a research reported that increased expression of HERPUD1 involved in neuronal apoptosis after intracerebral hemorrhage. However, how HERP plays its role in ICH is still unclear. In this work, we find that HERP is up-regulated surrounding the hematoma after ICH or in the hemin-exposed primary cortical neurons, using western blot and immunofluorescence. In addition, the expression of ER stress associated with protein GRP78 and HERP are detected after TM and TUDCA through western blot. By comparing the expressive trends and correlation analysis of these two molecules, we demonstrate that the expressions of GRP78 and HERP, after ER stress agonist or inhibitor treats, are strongly identical. Meanwhile, accumulating evidences show that activation of ER stress induces HERP accumulation and inhibition of ER stress alleviates HERP expression.
However, it is unclear whether activation of HERP protects cells from hemin-induced apoptosis or leads to cell death in human neurons after ICH. To explore the effect of HERP in hemin-induced primary cortical neurons, we establish the recombinant adenovirus vectors for HERP shRNA expression and HERP overexpression. It is found that recombinant adenovirus vectors for HERP shRNA expression inhibits autophagic promotion and induces apoptosis promotion, recombinant adenovirus vectors for HERP overexpression induces autophagic promotion and inhibits apoptosis promotion. Various studies have shown that activation of autophagy protects cells from apoptosis, allowing cells to alleviate ER stress (Shi et al., 2016). After the infection of recombinant adenovirus vectors in hemin-exposed primary cortical neurons, autophagy level is determined by the expression of LC3B and apoptosis level is determined by the expression of caspase-12 in western blot and immunofluorescent staining. Our results show that HERP inhibits hemin-exposed primary cortical neurons apoptosis by triggering autophagy pathway and HERP’s knockout increased hemin-exposed primary cortical neurons apoptosis by autophagy reduction. These data indicate that HERP plays a protective effect and is involved in autophagy reduction in hemin-exposed primary cortical neurons.
It should be noted that this study has examined only the effects of HERP in vitro after ICH. We have to point out that we do not detect the effects of HERP in vivo. Therefore, we are preparing another experience in mice after ICH to analyze whether HERP protects the neurons on perihematomal tissues following intracerebral hemorrhage.
In summary, it is firstly found that HERP can induce autophagy and deduce ER stress and apoptosis in primary cortical neurons. Furthermore, HERP is involved in the survival mechanism of hemin-exposed primary cortical neurons. Depletion of HERP can further decrease the autophagy induced by hemin and increase the apoptosis induced by hemin. Therefore, these results suggest that HERP plays a protective role in hemin-exposed primary cortical neurons.
5. Conclusion
Our results demonstrated that HERP played a protective role after intracerebral hemorrhage. HERP inhibited hemin-exposed primary cortical neurons apoptosis by triggering autophagy pathway. Furthermore, the effects of HERP in vivo should be detected to further confirm its role after intracerebral hemorrhage.
Conflicts of interest
All authors have declared that no conflicts of interest.
Author contributions
Wu Hui: conceived the study and performed the experiments. Wang Jinglei: participated in its design, performed the experiment and wrote the essay. Cao Maohong: designed the experiment. Liang Jingjing: performed the western blot. Wu Dan: performed the statistical analysis. Gu Xingxing: approved the manuscript. Ke Kaifu: approved the manuscript and revised the essay.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.81873742, 81671135) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX19_0864).
Contributor Information
Xingxing Gu, Email: Guxingxing@ntu.edu.cn.
Kaifu Ke, Email: kekaifu_nt@126.com.
References
- Alonso-Castro A.J., Arana-Argaez V., Yanez-Barrientos E., Ramirez-Camacho M.A., Wrobel K., Torres-Romero J.C. Antinociceptive and anti-inflammatory effects of Cuphea aequipetala Cav (Lythraceae) Inflammopharmacology. 2020 doi: 10.1007/s10787-020-00709-3. [DOI] [PubMed] [Google Scholar]
- Americo-Da-Silva L., Diaz J., Bustamante M., Mancilla G., Oyarzun I., Verdejo H.E., Quiroga C. A new role for HERPUD1 and ERAD activation in osteoblast differentiation and mineralization. FASEB J. 2018;32(9):4681–4695. doi: 10.1096/fj.201701229RR. [DOI] [PubMed] [Google Scholar]
- Cao W., Gao W., Zheng P., Sun X., Wang L. Medroxyprogesterone acetate causes the alterations of endoplasmic reticulum related mRNAs and lncRNAs in endometrial cancer cells. BMC Med. Genomics. 2019;12(1):163. doi: 10.1186/s12920-019-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S.S., Lee S.K., Kim J.K., Park H.K., Lee E., Jang J. Flightless-1 inhibits ER stress-induced apoptosis in colorectal cancer cells by regulating Ca(2+) homeostasis. Exp. Mol. Med. 2020 doi: 10.1038/s12276-020-0448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croci S., Carriero M.L., Capitani K., Daga S., Donati F., Papa F.T. AAV-mediated FOXG1 gene editing in human Rett primary cells. Eur. J. Hum. Genet. 2020 doi: 10.1038/s41431-020-0652-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X.C., Wang W., Feng D.X., Yin J., Zuo G., Chen D.D. Roles of autophagy and endoplasmic reticulum stress in intracerebral hemorrhage-induced secondary brain injury in rats. CNS Neurosci. Ther. 2017;23(7):554–566. doi: 10.1111/cns.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher M., Ander B.P., Jickling G., Hamade F., Hull H., Knepp B. Inflammatory, regulatory, and autophagy co-expression modules and hub genes underlie the peripheral immune response to human intracerebral hemorrhage. J. Neuroinflammation. 2019;16(1):56. doi: 10.1186/s12974-019-1433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A.K., Behera J., Kelly K.E., Mondal N.K., Richardson K.P., Tyagi N. Exercise mitigates alcohol induced endoplasmic reticulum stress mediated cognitive impairment through ATF6-Herp signaling. Sci. Rep. 2018;8(1):5158. doi: 10.1038/s41598-018-23568-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikram M.A., Wieberdink R.G., Koudstaal P.J. International epidemiology of intracerebral hemorrhage. Curr. Atheroscler. Rep. 2012;14(4):300–306. doi: 10.1007/s11883-012-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokame K., Agarwala K.L., Kato H., Miyata T. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J. Biol. Chem. 2000;275(42):32846–32853. doi: 10.1074/jbc.M002063200. [DOI] [PubMed] [Google Scholar]
- Krafft P.R., McBride D.W., Lekic T., Rolland W.B., Mansell C.E., Ma Q. Correlation between subacute sensorimotor deficits and brain edema in two mouse models of intracerebral hemorrhage. Behav. Brain Res. 2014;264:151–160. doi: 10.1016/j.bbr.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Xu W., Ouyang J., Lu X., Sherchan P., Lenahan C. Orexin A alleviates neuroinflammation via OXR2/CaMKKbeta/AMPK signaling pathway after ICH in mice. J. Neuroinflammation. 2020;17(1):187. doi: 10.1186/s12974-020-01841-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Fujita Y., Tanabe-Fujimura C., Zou K., Liu J., Liu S. An E3 ubiquitin ligase, synoviolin, is involved in the degradation of homocysteine-inducible endoplasmic reticulum protein. Biol. Pharm. Bull. 2018;41(6):915–919. doi: 10.1248/bpb.b18-00015. [DOI] [PubMed] [Google Scholar]
- Min S.Y., Ha D.S., Ha T.S. Puromycin aminonucleoside triggers apoptosis in podocytes by inducing endoplasmic reticulum stress. Kidney Res. Clin. Pract. 2018;37(3):210–221. doi: 10.23876/j.krcp.2018.37.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed Thangameeran S.I., Tsai S.T., Hung H.Y., Hu W.F., Pang C.Y., Chen S.Y., Liew H.K. A role for endoplasmic reticulum stress in intracerebral hemorrhage. Cells. 2020;9(3) doi: 10.3390/cells9030750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Xi G., Hua Y., Schallert T., Hoff J.T., Keep R.F. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J. Cereb. Blood Flow Metab. 2004;24(5):487–494. doi: 10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Ng A.C.K., Yao M., Cheng S.Y., Li J., Huang J.D., Wu W. Protracted morphological changes in the corticospinal tract within the cervical spinal cord after intracerebral hemorrhage in the right striatum of mice. Front. Neurosci. 2020;14:506. doi: 10.3389/fnins.2020.00506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly M., Angebault-Prouteau C., Dridi H., Notarnicola C., Scheuermann V., Lacampagne A. ER stress disturbs SR/ER-mitochondria Ca(2+) transfer: implications in Duchenne muscular dystrophy. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863(9):2229–2239. doi: 10.1016/j.bbadis.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Quiroga C., Gatica D., Paredes F., Bravo R., Troncoso R., Pedrozo Z. Herp depletion protects from protein aggregation by up-regulating autophagy. Biochim. Biophys. Acta. 2013;1833(12):3295–3305. doi: 10.1016/j.bbamcr.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Shi S., Tan P., Yan B., Gao R., Zhao J., Wang J. ER stress and autophagy are involved in the apoptosis induced by cisplatin in human lung cancer cells. Oncol. Rep. 2016;35(5):2606–2614. doi: 10.3892/or.2016.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S.X., Li Y.J., Shi K., Wood K., Ducruet A.F., Liu Q. IL (Interleukin)-15 bridges astrocyte-microglia crosstalk and exacerbates brain injury following intracerebral hemorrhage. Stroke. 2020;51(3):967–974. doi: 10.1161/STROKEAHA.119.028638. [DOI] [PubMed] [Google Scholar]
- Shimizu A., Kaira K., Yasuda M., Asao T., Ishikawa O. Clinical and pathological significance of ER stress marker (BiP/GRP78 and PERK) expression in malignant melanoma. Pathol. Oncol. Res. 2017;23(1):111–116. doi: 10.1007/s12253-016-0099-9. [DOI] [PubMed] [Google Scholar]
- Shiotsuki H., Yoshimi K., Shimo Y., Funayama M., Takamatsu Y., Ikeda K. A rotarod test for evaluation of motor skill learning. J. Neurosci. Methods. 2010;189(2):180–185. doi: 10.1016/j.jneumeth.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Torres M., Akhtar S., McKenzie E.A., Dickson A.J. Temperature down-shift modifies expression of UPR-/ERAD-Related genes and enhances production of a chimeric fusion protein in CHO cells. Biotechnol. J. 2020 doi: 10.1002/biot.202000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimar C., Kleine-Borgmann J. Epidemiology, prognosis and prevention of non-traumatic intracerebral hemorrhage. Curr. Pharm. Des. 2017;23(15):2193–2196. doi: 10.2174/1381612822666161027152234. [DOI] [PubMed] [Google Scholar]
- Yan J., Zuo G., Sherchan P., Huang L., Ocak U., Xu W. CCR1 activation promotes neuroinflammation through CCR1/TPR1/ERK1/2 signaling pathway after intracerebral hemorrhage in mice. Neurotherapeutics. 2020 doi: 10.1007/s13311-019-00821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Liu Q., Shi H., Jiang X., Wang S., Lu Y. Interleukin 17A exacerbates ER-stress-mediated inflammation of macrophages following ICH. Mol. Immunol. 2018;101:38–45. doi: 10.1016/j.molimm.2018.05.020. [DOI] [PubMed] [Google Scholar]
- Zan J., Song L., Wang J., Zou R., Hong F., Zhao J. Role of ghrelin in small intestinal motility following pediatric intracerebral hemorrhage in mice. Mol. Med. Rep. 2017;16(5):6958–6966. doi: 10.3892/mmr.2017.7468. [DOI] [PubMed] [Google Scholar]
- Zhang J., Shi X., Hao N., Chen Z., Wei L., Tan L. Simvastatin reduces neutrophils infiltration into brain parenchyma after intracerebral hemorrhage via regulating peripheral neutrophils apoptosis. Front. Neurosci. 2018;12:977. doi: 10.3389/fnins.2018.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]