Abstract
Objective:
Benign Childhood Epilepsy with Centrotemporal Spikes (BECTS) and Childhood Absence Epilepsy (CAE) are the most common childhood epilepsy syndromes and they share a similar agedependence. However, the two syndromes clearly differ in seizures and EEG patterns. The aim of this study is to investigate whether children of the same age with BECTS, CAE and typically-developing children have significant differences in grey matter volume that may underlie the different profiles of these syndromes.
Methods:
Twenty one patients with newly-diagnosed BECTS and 18 newly diagnosed and drug naïve CAE were included and compared to 31 typically-developing children. Voxel-based morphometry was utilized to investigate grey matter volume differences among BECTS, CAE, and controls. We also examined the effect of age on grey matter volume in all three groups. In addition to the whole brain analysis, we chose regions of interest analysis based on previous literature suggesting the involvement of these regions in BECTS or CAE. The group differences of grey matter volume was tested with 2-sample t-test for between two groups’ comparisons and ANOVA for three group comparisons.
Results:
In the whole brain group comparisons, the grey matter volume in CAE was significantly decreased in the areas of right inferior frontal and anterior temporal compared to BECTS and controls (F2,67 =27.53, p<0.001). In the control group, grey matter volume in bifrontal lobes showed a negative correlation with age (r=−0.54, p< 0.05), whereas no correlation was found in either CAE or BECTS. With ROI analyses, the grey matter volume of posterior thalami was increased in CAE compared to other 2 groups (p< 0.05).
Significance:
This study shows that there are grey matter volume differences between CAE and BECTS. Our findings of grey matter volume differences may suggest that there may be localized, specific differences in brain structure between these two types of epilepsy.
Introduction
Benign Childhood Epilepsy with Centrotemporal Spikes (BECTS) and Childhood Absence Epilepsy (CAE) are the two most common childhood epilepsy syndromes. BECTS comprises approximately 15-20 % of epilepsies in children (Camfield and Camfield 2002) and CAE occurs in 10–17% of all childhood onset epilepsy.(Berg et al. 2000, Jallon et al. 2001) They share very similar age characteristics; age of seizure onset is 3 to 12 years (peak 7-8 years for BECTS, 5-6 years for CAE). The conventional clinical evaluation of structural Magnetic Resonance Imaging (MRI) is typically normal for a diagnosis of these childhood epilepsy syndromes. However, the two syndromes clearly differ in several aspects. 1) The frequency of seizures and time of occurrence: seizures in BECTS are very infrequent and mostly nocturnal. Most patients (60–70%) have 2 to 10 total, and 10–20% may have only one. On the other hand, children with CAE have very frequent seizures (multiple daily, often dozens to up to 100 in some patients) throughout a day. 2) The EEG pattern in BECTS involves interictally focal centrotemporal spikes (CTS) or sharp-and-slow wave complexes that activate in drowsiness and sleep. CTS may be unilateral or bilateral. CAE is characterized by generalized 3Hz spike and waves during seizures, rarely with interictal discharges. 3) Seizure semiology in BECTS involves peri-oral sensorimotor symptoms while seizures in CAE manifest as transient impairment of consciousness with or without eye movements and motor automatisms. They are characteristically provoked by hyperventilation.
Subsets of patients in both groups show a wide range of cognitive or behavioral problems even though general intellectual function (Full Scale IQ) is typically in the normal range. However, different domains and profiles of cognitive and behavioral problems have been observed between the two syndromes, i.e., children with BECTS may have more difficulties with language, verbal memory and processing speed, (Filippini et al. 2013, Overvliet et al. 2013, Teixeira and Santos 2018) whereas children with CAE may have cognitive and attention problems. (Barnes and Paolicchi 2008, Caplan et al. 2008, Masur et al. 2013)
Although a normal clinical brain MRI is a clinical criteria for both epilepsy syndromes, there have been reports suggesting structural differences compared to typically developing children when using quantitative MRI analysis methods, such as measures of grey matter volume and cortical thickness analyses. (Betting et al. 2006, Chan et al. 2006, Pardoe et al. 2008, Overvliet et al. 2013, Pardoe et al. 2013, Garcia-Ramos et al. 2015, Kim et al. 2015) . These results have varied between studies and at times have been contradictory in reporting thalamic atrophy/hypertrophy and cortical thinning/thickening, possibly because of heterogenous patient populations or variable MRI scanners. In order to eliminate the potential effects of these factors, our study utilized carefully selected homogeneous populations for both groups and the exact same MRI scanner in order to optimize the possibility of detection of intrinsic structural changes.
We hypothesized that children with BECTS and CAE would exhibit different patterns of grey matter volume increase; for BECTS, in regions where the CTS are localized, and for CAE, in the thalami. The aim of this study was to investigate whether children of the same age range with drug-naïve BECTS and CAE, at the time of their diagnoses, have grey matter volume differences in any brain regions that may underlie the different profiles of these two idiopathic childhood epilepsy syndromes.
Methods
Participants
Children with BECTS
Fifty-one patients (age: 5-13 yrs, mean ± SD: 7.9 ± 2.0 yrs, F/M=26/25) were consecutively recruited from New Onset Seizure and general Neurology Clinics in the Division of Neurology at Cincinnati Children’s Hospital Medical Center according to International League Against Epilepsy (ILAE) criteria (https://www.epilepsydiagnosis.org ) for the diagnosis of BECTS. (International League Against Epilepsy, 1989) This study population was the same study population as previously published.(Fujiwara et al. 2018) Briefly, the mandatory seizure semiology was including “fronto-parietal opercular features with hemifacial (lip, mouth and tongue) clonic movements, laryngeal symptoms, articulation difficulty, swallowing or chewing movements and hyper-salivation”. Seizures were all self-reported or reported by parents. Interictally, the definition of CTS were also based on ILAE criteria (https://www.epilepsydiagnosis.org). This describes CTS as “High amplitude centrotemporal spikes or sharp-and-slow wave complexes that activate in drowsiness and sleep”. These may be unilateral or bilateral and are usually frequent. There may be focal spikes seen outside the centrotemporal region (midline, parietal, frontal, occipital). The centrotemporal spike has a typical morphology with maximum negativity in centrotemporal electrodes (C3/C4 and T3/T4) and maximum positivity frontally. It is mandatory that there must be marked increase in frequency of epileptiform activity in drowsiness and sleep, when spikes or spike-and-slow waves often also have a wilder field and may be bilaterally synchronous.” Patients with CTS plus spikes in other locations were excluded from our study. Additional criteria were that patients were 1) less than one year from their BECTS diagnosis 2) no AEDs prescribed at the time of study enrollment.
Children with CAE
Twenty patients (age: 6.0 – 11.8 yrs, mean ± SD: 8.6 ± 1.8 yrs, F/M=9/11) with newly diagnosed CAE based on ILAE criteria (https://www.epilepsydiagnosis.org) were recruited. Specifically, children with CAE exhibit frequent (multiple daily) and brief (average duration ~ 10 seconds) absence seizures with awareness and responsiveness impaired, but subset of participant may exhibit continuous intentional motion yet altered fashion during ictal phase. Ictal EEG shows the regular 3Hz generalized spike-and-wave associated with absence episodes.
Exclusion criteria included treatment with an antiepileptic medication for more than 7 days; history of afebrile seizures other than absence seizures; history consistent with juvenile absence or juvenile myoclonic epilepsy; and history of major psychiatric disease, autistic-spectrum disorder, or any clinically significant medical condition.
Typically developing children as controls
Fifty-six typically developing children (age: 5-13 yrs, mean ± SD: 8.1 ± 2.2 yrs, F/M=27/29) were recruited. Inclusion criteria for healthy controls were included age between 5 and 14 years old, normal intelligence (clinician judged), and regular academic education plan (no special education class). Participants were excluded if they had a chronic neurological disorder, pervasive developmental disorder, progressive neurological disease, major medical disease, history of neonatal seizures, pregnancy, prior anti-epileptic therapy, taking any psychoactive agent other than a psychostimulant for attention deficit disorder/attention deficit hyperactivity disorder (ADD/ADHD), taking a psychostimulant for ADD/ADHD and not on a stable dose for at least 2 weeks, or special education placement based on ability or behavior.
Participants from the three groups were native speakers of English with no history of neuropsychological or learning disorders. Informed consent was obtained from a parent/ guardian for all participants. Assent was obtained verbally from participants whose age was 10 years or younger at the time of recruitment; written assent was obtained only from children age 11 and older, to assure that the children had a sufficient reading level to comprehend the written consent form. The data from children and BECTS and typically developing children were collected as part of a prospective NIH funded study (NIH R01NS065840) and neuroimaging data from these groups has been previously published.(Fujiwara et al. 2018)
Informed consent was obtained for experimentation from all participants.
Structural Magnetic Resonance Imaging (MRI)
MRI Acquisition
All participants completed high resolution T1-weighted MRI acquisition on a 3-Tesla Philips Acheiva MRI scanner using a 32-channel head coil (Philips Healthcare, Andover, MA, USA). Threedimensional 1mm isotropic T1-weighted anatomical images were obtained with the following parameters: repetition time 8.0 msec, echo time 3.7 msec, matrix 256 x 256). All MRIs were reviewed by pediatric neuroradiologists who verified that they were visually normal under the clinical review.
Voxel-based Morphometry (VBM) Preprocessing
VBM analysis was carried out using FSL version 5.0 software (http://www.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM)(Douaud et al. 2007) and an optimized VBM protocol(Good et al. 2001) carried out with FSL tools. The following pre-processing steps were carried out. 1) T1-weighted images were brain-extracted (skull-stripped) and all results were reviewed. 2) These brain-extracted images were then segmented into white matter, grey matter, and cerebrospinal fluid (CSF) volume probability maps by using local intensity changes before being normalized to the MNI 152 standard space using non-linear registration. 3) The resulting images were averaged and flipped along the x-axis to create a left-right symmetric study-specific grey matter template. 4) This template image was registered to the MNI 152 standard space using non-linear registration and modulated to correct for local expansion due to the non-linear component of the spatial transformation. 5) The modulated grey matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 2 mm.
Cortical Thickness Analysis Preprocessing
Cortical thickness analysis was performed using FreeSurfer image analysis suite (version 5.3) (http://surfer.nmr.mgh.harvard.edu/). Cortical reconstruction and volumetric segmentation detail was as follows: motion correction and averaging,(Reuter et al. 2010) removal of non-brain tissue,(Segonne et al. 2004) segmentation of the subcortical white matter and deep gray matter volumetric structures,(Fischl et al. 2001) intensity normalization, tessellation of the gray matter white matter boundary, automated topology correction,(Fischl et al. 2001) and surface deformation following intensity gradients to obtain the boundaries between brain tissue (CSF, grey and white matter).(Fischl and Dale 2000) This surface was then refined to follow the intensity gradients between the white and grey matter (white surface). The white surface was nudged to follow the intensity gradients between the grey matter and CSF (pial surface). The thickness of cortex was then measured as the distance between the white and the pial surfaces at the paired vertices for each column. The cortex was parcellated into 34 units in each hemisphere with respect to gyral and sulcal structure.(Fischl et al. 2004, Desikan et al. 2006) The results of preprocessing were reviewed to make certain that the segmentation was done without errors.
Post-processing Quality Control
Quality control for both VBM and cortical thickness after preprocessing were performed. The detailed processes were described in supplementary material 1.
Statistical Analysis
For VBM, a voxel-wise general linear model (GLM) was applied to compare voxel-wise differences in grey matter volume among three groups. Non-parametric statistics were performed using a randomization with 5000 permutations with threshold-free cluster enhancement (TFCE) –based analysis (Smith and Nichols 2009) as well as cluster-based thresholding with p < 0.05 and corrected for multiple comparisons. Covariates in the analysis included age, gender and total brain volume (TBV). The TBV was calculated as the demeaned value of the summed tissue segments (grey matter + white matter volume).
In addition to the whole brain analysis, we chose a-priori regions of interest (ROI) analysis based on previous literature and electrophysiological evidence, in order to investigate, with greater sensitivity, whether structural changes would be observed. Specifically, for BECTS, we hypothesized that there would be an association between centrotemporal spikes and grey matter volume. Based on that, the ROIs were centrotemporal areas, including bilateral primary motor, primary sensory, and supramarginal gyri. For CAE, we chose ROIs based on the functional networks identified in functional MRI studies, mostly seizure-related networks, including thalami, bilateral inferior frontal gyrus and precuneous. The Jiilich histological (cyto- and myelo-architectonic) atlas(Eickhoff et al. 2005, Eickhoff et al. 2007) and Harvard-Oxford Cortical Structural Atlas(Makris et al. 2006, Goldstein et al. 2007) were utilized to define these ROIs.
Group differences in grey matter volume were tested with 2-sample t-test for between two groups comparisons and ANOVA for three group comparisons. The multiple comparisons were corrected by using the null distribution of the max (across the image) voxel-wise test statistic. For cortical thickness, GLM analysis was also carried out with whole brain cluster-wise correction for multiple comparisons.(Hagler et al. 2006) The cluster-forming threshold was set at uncorrected p < 0.001 and cluster-wise p-value (cwp) at corrected p < 0.05. We performed three groups comparison and included age, gender and total brain volume (TBV) as covariates.
All study procedures were approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
Results
Participants
A total of 18 children with CAE, 23 children with BECTS and 32 typically developing children were included for grey matter volume analyses (Table 1) after processes undertaken to meet inclusion and exclusion criteria (supplementary material 2) . All participants were right handed, except 4 (BECTS=3, controls=1). The time since BECTS diagnosis ranged 1 - 7 months (median: 2 months). History of recognized seizures ranged from 1 - 9 at time of participation. Groups did not differ significantly in age.
Table 1.
Demographics of CAE, BECTS and Controls.
| CAE (n=18) | BECTS (n=21) | Controls (n=31) | |
|---|---|---|---|
| Age (mean ± SD, range) | 8.8 ± 1.8, 6.4 – 11.8 | 8.81 ± 2.0, 5 - 12 | 8.3 ± 2.3, 5 - 13 |
| Gender (M/F) | 9/9 | 12/9 | 15/16 |
Grey matter volume differences
Whole Brain Analyses with VBM
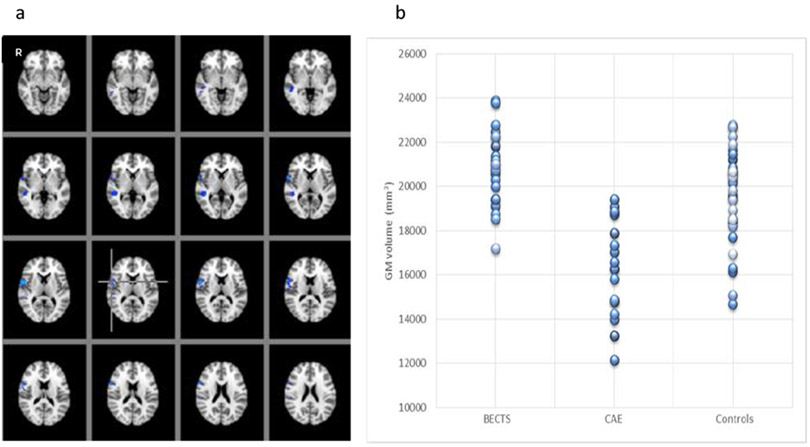
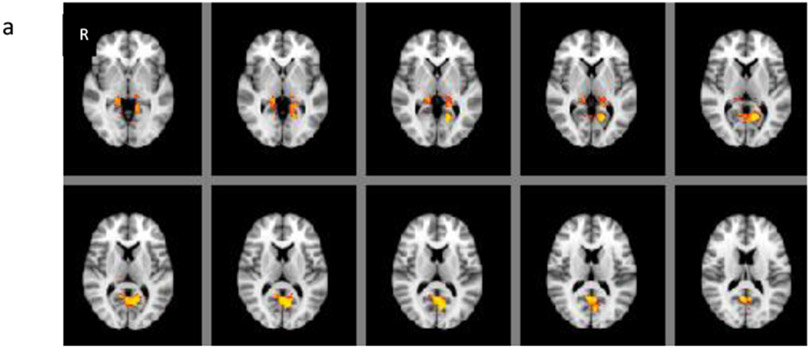
Grey matter volume in CAE was statistically significantly decreased in the areas of right inferior frontal and anterior temporal compared to BECTS and controls (F2, 67 =27.53, p<0.001) (Figures 1a-b). There were no differences between BECTS and controls based on the whole brain voxel-based comparisons. On the other hand, there were statistically significant differences between CAE and controls; grey matter volume was increased in bilateral posterior thalami and in the bilateral inferior precuneus (p < 0.05) (Figure 2a) and decreased in decreased in right inferior anterior frontal, anterior temporal and left posterior medial temporal areas in CAE compared to controls (p < 0.05) (Figure 2b). There was a statistically significant negative correlation with age in various locations in each frontal lobe; superior and anterior frontal in left and inferior lateral frontal in right hemisphere in the control group (r=−0.54, p< 0.05), but not in CAE (r=−0.30) or BECTS (r=−0.12).
Figure 1:
(a) Regions of grey matter volume (blue) including the areas of right inferior frontal and anterior temporal, where statistically significantly decreased in CAE compared to BECTS and controls using ANOVA (F-test). (F 2,67 =27.53, p<0.001). (b) The graph on the right shows each participant (each circle) grey matter volume within all the regions of difference among three groups showing blue in (a).
Figures 2.
(a) Grey matter volume was increased in bilateral posterior thalami and precuneous and (b) decreased in right inferior anterior frontal, anterior temporal and left posterior medial temporal areas in only the CAE group compared to controls (p < 0.05).
Whole Brain Analyses with Cortical Thickness
There were no whole-brain cortical thickness differences observed among the 3 groups.
ROI analyses
Grey matter volume of posterior thalami was increased in CAE group compared to BECTS and controls (p< 0.05) (Figure 3a-b) whereas there was no difference between BECTS and typically developing children. Within the bilateral frontal areas, the CAE group showed decreased grey matter volume in the right inferior anterior frontal region compared to BECTS (p < 0.05) and in bilateral anterior inferior and middle frontal regions compared to the control group (p < 0.05). No difference was found between BECTS and controls in these regions. There were no differences in precentral and postcentral gyri among the three groups.
Figures 3:
ROI analysis: (a) Increased grey matter volume were observed in thalami diffusely in CAE group compared to BECTS and (b) in posterior thalami in CAE compared to typically developing children (b) (p< 0.05)
Discussion
This study aimed to investigate differences in brain morphology in grey matter and subcortical volume between the two most common childhood age-related epilepsies, newly diagnosed drug-naïve BECTS and CAE. Greater grey matter volume was observed in thalami and bilateral inferior precuneous and the decreased grey matter was observed in bilateral inferior frontal regions in CAE compared to BECTS and typically developing children. These findings were consistent in both whole brain and ROI analyses in our study. Even though these two childhood epilepsy syndromes have very similar age profiles and clinical brain MRI is typically read as normal, this new evidence of grey matter volume differences between two childhood epilepsy syndromes may help to explain differences in mechanisms and outcomes.
The strong findings of thalamic and precuneous grey matter volume increases in CAE in our study suggests probable structural pathophysiological evidence of involvement of these two regions in the seizure network in CAE. Furthermore, there have been neuroimaging studies that identified functional connectivity between thalamus and precuneus as well as their involvement in default mode network (DMN) using resting state functional connectivity. (Danielson et al. 2011, Luo et al. 2011) In addition to the functional connectivity evidence between thalamus and precuneus, a recent study by Cunningham et al, (2007) found a high level of structural connectivity among thalamus, precuneus and DMN when measuring fractional anisotropy (FA) using diffusion tensor imaging (DTI). (Cunningham et al. 2017) (Vanhaudenhuyse et al. 2011) The precuneus, which is one of the largest core hubs with in DMN, has been proposed to be involved in self-consciousness, visuo-spatial integration and imaginary as well as episodic memory along with posterior cingulate. (Cavanna and Trimble 2006, Vanhaudenhuyse et al. 2011) This was also shown with cerebral glucose metabolism measured by positron emission tomography (PET), which was at its highest in the precuneus during wakefulness but was most reduced in individuals under general anesthesia. (Hudetz 2012) Further, the thalamus is also involved in the regularization of consciousness along with alertness and sleep. Those regions were also in agreement with previous functional MRI and magnetoencephalography (MEG) studies of CAE, highlighting the importance of these regions as critical components of functionally abnormal networks in children with CAE (Szaflarski et al. 2010, Masterton et al. 2013, Tenney et al. 2014). Furthermore, findings of structural abnormalities with increased volume of grey matter in thalami and decreased in frontal cortex also could explain the evidence of attention deficit comorbidities in children with CAE. A recent animal study suggested that the thalamic reticular nucleus plays a role as a switchboard to control the amount of information the brain receives, limiting and filtering out sensory information. (Wimmer et al. 2015) There is also a well-established role of the prefrontal cortex (PFC) in the seizure network of CAE(Szaflarski et al. 2010, Carney et al. 2012), and in attention and executive function as shown in fMRI studies. Taken together, the fact that children with CAE have very distinct characteristics of seizure semiology with consciousness disruption and alteration of self-awareness is consistent with these structural abnormalities. Furthermore, the pathophysiological observation with EEG that shows generalized epileptiform discharges during seizures can is also consistent with the thalamo-cortico network involvement for CAE.
Children with BECTS did not show any difference in grey matter volume compared to typically developing children. The significant grey matter volume difference was observed between BECTS and CAE with similar to the difference between CAE and controls. This suggests that children with BECTS may have no structural or volumetric abnormalities, or very subtle if any.
The developmental trajectory of grey matter volume and cortical thickness have previously been explored and have showed dynamic changes in the age range during which CAE and BECTS are diagnosed (5 – 17 years of age). A negative correlation with age was observed only in controls and there were no correlation observed in BECTS or CAE. This may be related to a larger sample size of controls compared to BECTS and CAE in this study.
Studies using quantitative analysis of structural imaging on both children with BECTS and with CAE have shown contradicting results. A possible cause of these contradicting findings may be the inhomogeneous sample of each population. Specifically, these previous reports have been confounded by the long term use of AEDs. In fact, some of AEDs which are some of the medications used to treat CAE and less often used for BECTS, such as valproate and levetiracetam have been shown to cause brain remodeling and subsequent VBM abnormalities. (Tang et al. 2015) Moreover, participants in these studies often had imaging completed many years following their diagnosis, when they were either in remission or had had many years of uncontrolled seizures. A review study by Alhusaini et al. discussed the importance of homogeneity of a study population using endophenotypes, occupying the domain between disease phenotype and the genetic variants that influence disease risk and quantifiable measures that are arguably closer to the genetic architecture of a disease.(Alhusaini et al. 2016) This may make it possible to deconstruct phenotypically heterogeneous syndromes into phenomena more reflective of the underlying genetic architecture, improving power to detect risk genes and their functional consequences. In this study, the populations of both groups of children with BECTS and with CAE were carefully evaluated to be as homogenous as possible and the structural images were at or close to the time of diagnosis and at a medication naïve stage in order not to contaminate any potential covariates that affect the grey matter volume measurement so that our results could more accurately reflect the acute epilepsy process. Furthermore, extensive pre- and post- FSL-VBM processing quality controls were performed since the automated segmentation for VBM is very sensitive to the small movement artifacts and inhomogeneity of the original images. (Ducharme et al. 2016)
There are a few limitations in this study. First, we did not perform the white matter quantitative measurement, i.e., fractional anisotropy or radial diffusivity using diffusion tensor imaging. Second, our samples for all three groups are relatively small. Much larger samples are needed to validate our findings. Third, this study was adapted from a larger cohort in an EEG/fMRI-focused study for BECTS as well as with a larger cohort of EEG/fMRI and MEG focused study in CAE. Therefore we did not anticipate the limitation from the artifacts from the EEG cap during structural MRI acquisition. This led to a smaller sample size for our study. Even with these limitations, our findings may help understand the epilepsy mechanism in general using these two homogeneous populations of epilepsy syndromes.
This study showed that there are grey matter volume differences between CAE and BECTS. The two most common types of childhood epilepsy, BECTS and CAE share some similar features, but semiology of seizures and electrophysiological features are very different. Our findings of grey matter volume differences may suggest that there may be localized, specific differences in brain structure between these two types of epilepsy. Our findings of grey matter volume differences may suggest that there may be localized differences in the structure of the developing brain between these two types of epilepsy that may underlie the different profiles of these two idiopathic childhood epilepsy syndromes.
Supplementary Material
Highlights.
Grey matter volume in CAE was significantly decreased in the areas of right inferior frontal and anterior temporal compared to BECTS and controls
Grey matter volume of posterior thalami was increased in CAE group compared to other 2 groups (p< 0.05)
There may be localized, specific differences in brain structure between these two types of childhood epilepsy
Acknowledgements
Portions of this study were funded by NIH R01NS065840 (J.V.) a “Taking Flight” award from Citizens United for Research in Epilepsy (CURE) (J.R.T.) and a Procter Scholar award from Cincinnati Children’s Research Foundation (J.R.T.).
Footnotes
Disclosure of Conflicts of Interest
None of the authors has any conflict of interest to disclose.
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1989). "Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy." Epilepsia 30(4): 389–399. [DOI] [PubMed] [Google Scholar]
- Alhusaini S, Whelan CD, Sisodiya SM and Thompson PM (2016). "Quantitative magnetic resonance imaging traits as endophenotypes for genetic mapping in epilepsy." Neuroimage Clin 12: 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes GN and Paolicchi JM (2008). "Neuropsychiatric comorbidities in childhood absence epilepsy." Nat Clin Pract Neurol 4(12): 650–651. [DOI] [PubMed] [Google Scholar]
- Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S and Beckerman B (2000). "How well can epilepsy syndromes be identified at diagnosis? A reassessment 2 years after initial diagnosis." Epilepsia 41(10): 1269–1275. [DOI] [PubMed] [Google Scholar]
- Betting LE, Mory SB, Lopes-Cendes I, Li LM, Guerreiro MM, Guerreiro CA and Cendes F (2006). "MRI volumetry shows increased anterior thalamic volumes in patients with absence seizures." Epilepsy Behav 8(3): 575–580. [DOI] [PubMed] [Google Scholar]
- Camfield P and Camfield C (2002). "Epileptic syndromes in childhood: clinical features, outcomes, and treatment." Epilepsia 43 Suppl 3: 27–32. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, Koh S, Sankar R and Shields WD (2008). "Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities." Epilepsia 49(11): 1838–1846. [DOI] [PubMed] [Google Scholar]
- Carney PW, Masterton RAJ, Flanagan D, Berkovic SF and Jackson GD (2012). "The frontal lobe in absence epilepsy EEG-fMRI findings." Neurology 78(15): 1157–1165. [DOI] [PubMed] [Google Scholar]
- Cavanna AE and Trimble MR (2006). "The precuneus: a review of its functional anatomy and behavioural correlates." Brain 129(Pt 3): 564–583. [DOI] [PubMed] [Google Scholar]
- Chan CH, Briellmann RS, Pell GS, Scheffer IE, Abbott DF and Jackson GD (2006). "Thalamic atrophy in childhood absence epilepsy." Epilepsia 47(2): 399–405. [DOI] [PubMed] [Google Scholar]
- Cunningham SI, Tomasi D and Volkow ND (2017). "Structural and functional connectivity of the precuneus and thalamus to the default mode network." Hum Brain Mapp 38(2): 938–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson NB, Guo JN and Blumenfeld H (2011). "The default mode network and altered consciousness in epilepsy." Behav Neurol 24(1): 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS and Killiany RJ (2006). "An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest." Neuroimage 31(3): 968–980. [DOI] [PubMed] [Google Scholar]
- Douaud G, Smith S, Jenkinson M, Behrens T, Johansen-Berg H, Vickers J, James S, Voets N, Watkins K, Matthews PM and James A (2007). "Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia." Brain 130(Pt 9): 2375–2386. [DOI] [PubMed] [Google Scholar]
- Ducharme S, Albaugh MD, Nguyen TV, Hudziak JJ, Mateos-Perez JM, Labbe A, Evans AC and Karama S (2016). "Trajectories of cortical thickness maturation in normal brain development--The importance of quality control procedures." Neuroimage 125: 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K and Amunts K (2007). "Assignment of functional activations to probabilistic cytoarchitectonic areas revisited." Neuroimage 36(3): 511–521. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K and Zilles K (2005). "A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data." Neuroimage 25(4): 1325–1335. [DOI] [PubMed] [Google Scholar]
- Filippini M, Boni A, Giannotta M and Gobbi G (2013). "Neuropsychological development in children belonging to BECTS spectrum: long-term effect of epileptiform activity." Epilepsy Behav 28(3): 504–511. [DOI] [PubMed] [Google Scholar]
- Fischl B and Dale AM (2000). "Measuring the thickness of the human cerebral cortex from magnetic resonance images." Proc Natl Acad Sci U S A 97(20): 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A and Dale AM (2001). "Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex." IEEE Trans Med Imaging 20(1): 70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B and Dale AM (2004). "Automatically parcellating the human cerebral cortex." Cereb Cortex 14(1): 11–22. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Tenney J, Kadis DS, Byars A, Altaye M, Spencer C, Glauser T and Vannest J (2018). "Cortical morphology, epileptiform discharges, and neuropsychological performance in BECTS." Acta Neurol Scand. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ramos C, Jackson DC, Lin JJ, Dabbs K, Jones JE, Hsu DA, Stafstrom CE, Zawadzki L, Seidenberg M, Prabhakaran V and Hermann BP (2015). "Cognition and brain development in children with benign epilepsy with centrotemporal spikes." Epilepsia 56(10): 1615–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Makris N, Ahern T, O'Brien LM, Caviness VS Jr., Kennedy DN, Faraone SV and Tsuang MT (2007). "Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability." Biol Psychiatry 61(8): 935–945. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ and Frackowiak RS (2001). "A voxel-based morphometric study of ageing in 465 normal adult human brains." Neuroimage 14(1 Pt 1): 21–36. [DOI] [PubMed] [Google Scholar]
- Hagler DJ Jr., Saygin AP and Sereno MI (2006). "Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data." Neuroimage 33(4): 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG (2012). "General anesthesia and human brain connectivity." Brain Connect 2(6): 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International League Against Epilepsy. from https://www.epilepsydiagnosis.org/.
- Jallon P, Loiseau P and Loiseau J (2001). "Newly diagnosed unprovoked epileptic seizures: presentation at diagnosis in CAROLE study. Coordination Active du Reseau Observatoire Longitudinal de l’ Epilepsie." Epilepsia 42(4): 464–475. [DOI] [PubMed] [Google Scholar]
- Kim EH, Yum MS, Shim WH, Yoon HK, Lee YJ and Ko TS (2015). "Structural abnormalities in benign childhood epilepsy with centrotemporal spikes (BCECTS)." Seizure 27: 40–46. [DOI] [PubMed] [Google Scholar]
- Luo C, Li Q, Lai Y, Xia Y, Qin Y, Liao W, Li S, Zhou D, Yao D and Gong Q (2011). "Altered functional connectivity in default mode network in absence epilepsy: a resting-state fMRI study." Hum Brain Mapp 32(3): 438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT and Seidman LJ (2006). "Decreased volume of left and total anterior insular lobule in schizophrenia." Schizophr Res 83(2-3): 155–171. [DOI] [PubMed] [Google Scholar]
- Masterton RA, Carney PW, Abbott DF and Jackson GD (2013). "Absence epilepsy subnetworks revealed by event-related independent components analysis of functional magnetic resonance imaging." Epilepsia 54(5): 801–808. [DOI] [PubMed] [Google Scholar]
- Masur D, Shinnar S, Cnaan A, Shinnar RC, Clark P, Wang J, Weiss EF, Hirtz DG, Glauser TA and Childhood G Absence Epilepsy Study (2013). "Pretreatment cognitive deficits and treatment effects on attention in childhood absence epilepsy." Neurology 81(18): 1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvliet GM, Besseling RM, Jansen JF, van der Kruijs SJ, Vies JS, Hofman PA, Ebus SC, de Louw A, Aldenkamp AP and Backes WH (2013). "Early onset of cortical thinning in children with rolandic epilepsy." Neuroimage Clin 2: 434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvliet GM, Besseling RM, van der Kruijs SJ, Vles JS, Backes WH, Hendriksen JG, Ebus S, Jansen JF, Hofman PA and Aldenkamp AP (2013). "Clinical evaluation of language fundamentals in Rolandic epilepsy, an assessment with CELF-4." Eur J Paediatr Neurol 17(4): 390–396. [DOI] [PubMed] [Google Scholar]
- Pardoe H, Pell GS, Abbott DF, Berg AT and Jackson GD (2008). "Multi-site voxel-based morphometry: methods and a feasibility demonstration with childhood absence epilepsy." Neuroimage 42(2): 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoe HR, Berg AT, Archer JS, Fulbright RK and Jackson GD (2013). "A neurodevelopmental basis for BECTS: evidence from structural MRI." Epilepsy Res 105(1-2): 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M, Rosas HD and Fischl B (2010). "Highly accurate inverse consistent registration: a robust approach." Neuroimage 53(4): 1181–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK and Fischl B (2004). "A hybrid approach to the skull stripping problem in MRI." Neuroimage 22(3): 1060–1075. [DOI] [PubMed] [Google Scholar]
- Smith SM and Nichols TE (2009). "Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference." Neuroimage 44(1): 83–98. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, DiFrancesco M, Hirschauer T, Banks C, Privitera MD, Gotman J and Holland SK (2010). "Cortical and subcortical contributions to absence seizure onset examined with EEG/fMRI." Epilepsy Behav 18(4): 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Yu X, Zhang X, Xia W, Wu X, Zou X, Li H, Huang X, Stefan H, Chen Q, Gong Q and Zhou D (2015). "Single-dose intravenous administration of antiepileptic drugs induces rapid and reversible remodeling in the brain: Evidence from a voxel-based morphometry evaluation of valproate and levetiracetam in rhesus monkeys." Neuroscience 303: 595–603. [DOI] [PubMed] [Google Scholar]
- Teixeira J and Santos ME (2018). "Language skills in children with benign childhood epilepsy with centrotemporal spikes: A systematic review." Epilepsy Behav 84: 15–21. [DOI] [PubMed] [Google Scholar]
- Tenney JR, Fujiwara H, Horn PS, Vannest J, Xiang J, Glauser TA and Rose DF (2014). "Low- and high-frequency oscillations reveal distinct absence seizure networks." Ann Neurol 76(4): 558–567. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Demertzi A, Schabus M, Noirhomme Q, Bredart S, Boly M, Phillips C, Soddu A, Luxen A, Moonen G and Laureys S (2011). "Two distinct neuronal networks mediate the awareness of environment and of self." J Cogn Neurosci 23(3): 570–578. [DOI] [PubMed] [Google Scholar]
- Wimmer RD, Schmitt LI, Davidson TJ, Nakajima M, Deisseroth K and Halassa MM (2015). "Thalamic control of sensory selection in divided attention." Nature 526(7575): 705–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.