Abstract
Background:
General anesthetics influence mitochondrial homeostasis, placing individuals with mitochondrial disorders and possibly carriers of recessive mitochondrial mutations at increased risk of perioperative complications. In Drosophila, mutations in the ND23 subunit of Complex I of the mitochondrial electron transport chain – analogous to mammalian NDUFS8 – replicate key characteristics of Leigh syndrome, an inherited mitochondrial disorder. We used the ND23 mutant for testing the hypothesis that anesthetics have toxic potential in carriers of mitochondrial mutations.
Methods:
We exposed wild-type flies and ND23 mutant flies to behaviorally equivalent doses of isoflurane or sevoflurane in 5%, 21%, or 75% oxygen. We used percent mortality (mean±SD, n≥3) at 24 h after exposure as a readout of toxicity and changes in gene expression to investigate toxicity mechanisms.
Results:
Exposure of 10–13-day-old male ND23 flies to isoflurane in 5%, 21%, or 75% oxygen resulted in 16.0±14.9% (n=10), 48.2±16.1% (n=9), and 99.2±2.0% (n=10) mortality, respectively. Comparable mortality was observed in females. In contrast, under the same conditions, mortality was <5% for all male and female groups exposed to sevoflurane, except 10–13-day-old male ND23 flies with 9.6±8.9% (n=16) mortality. The mortality of 10–13-day-old ND23 flies exposed to isoflurane was rescued by neuron- or glia-specific expression of wild-type ND23. Isoflurane and sevoflurane differentially affected expression of antioxidant genes in 10–13-day-old ND23 flies. ND23 flies had elevated mortality from paraquat-induced oxidative stress compared to wild-type flies. The mortality of heterozygous ND23 flies exposed to isoflurane in 75% oxygen increased with age, resulting in 54.0±19.6% (n=4) mortality at 33–39 days old, and the percent mortality varied in different genetic backgrounds.
Conclusions:
Mutations in the mitochondrial Complex I subunit ND23 increase susceptibility to isoflurane-induced toxicity and to oxidative stress in Drosophila. Asymptomatic flies that carry ND23 mutations are sensitized to hyperoxic isoflurane toxicity by age and genetic background.
Introduction
Exposure to general anesthetics is very common but not without risks, and mutations in some anesthetic target proteins increase the risk of toxicity.1 Of particular interest are mutations affecting mitochondria because mitochondrial diseases are the most frequently inherited metabolic disorders, and mitochondrial proteins are targets of general anesthetics.2,3 Since volatile general anesthetics inhibit the function of Complex I of the mitochondrial electron transport chain,4 they may increase the risk of perioperative complications for patients with disorders caused by mutations in Complex I such as Leigh syndrome and other similar neurodegenerative disorders.5 Indeed, Leigh syndrome patients are hypersensitive to volatile general anesthetics6 and are challenging to manage in the perioperative setting.7 Most of these disorders are caused by mutations in the nuclear genome rather than the mitochondrial genome and follow a Mendelian pattern of inheritance.8 Leigh syndrome is rare, diagnosed in only 1:20,000 live births, but the prevalence of heterozygosity in the general population is substantially higher at 1–2:100. The extent to which heterozygous carriers of pathogenic mutations are at increased risk of adverse reactions from anesthetics is unknown. Furthermore, it is not known how age, sex, and environmental factors modulate the penetrance of the underlying pathophysiology,9 as they do for other more common diseases caused by Complex I mutations.10 The risk of Complex I mutations can be explored in model organisms to generate testable hypotheses. Recently, two Drosophila melanogaster models of Leigh syndrome have been characterized in flies carrying mutations in the mitochondrially encoded ND2 subunit11 or the nuclearly encoded ND23 subunit12 of Complex I. ND23 is homologous to mammalian NDUFS8 (NADH:Ubiquinone Oxidoreductase Subunit S8), which is commonly mutated in mitochondrial diseases.13
We examined homozygous and heterozygous Drosophila ND23 mutants. For most of the studies, we used the ND2360114 allele, which contains a point mutation that results in a glycine-to-aspartic acid substitution at position 199 of the 217 amino acid protein.12 ND2360114 flies exhibit age-dependent neurodegeneration and a reduced lifespan. Neurodegeneration becomes apparent as spongiform lesions form in the neuropil at 10–12 days old and increase in severity with age. Mimicking children with Leigh syndrome, young ND2360114 flies are more sensitive than wild-type flies to behavioral effects of isoflurane and sevoflurane.14 Therefore, we tested whether ND2360114 flies could serve as a sensitized model for anesthetic-induced toxicity to address whether heterozygous carriers are at risk and whether genetic background influences the risk of toxicity.
Materials and Methods
Approval from the institutional animal care and use committee was waived. We followed ARRIVE guidelines to the degree applicable to invertebrate organisms.15,16
Fly Lines and Culturing
All flies were cultured at 25°C on standard cornmeal-molasses food. The ND2360114 and UAS-ND23 lines are described in Loewen et al.12 ND23G14097, which is homozygous lethal, contains a P-element insertion 259 bp downstream of the transcription start site for ND23. ND23Del (i.e., Df(3R)Exel8162), which is homozygous lethal, deletes an ~125 kb region that contains 19 genes, including ND23. Canton S, w1118, Tub-Gal4, C155-Gal4, Repo-Gal4, RAL352, and RAL774 lines were obtained from the Bloomington Drosophila Stock Center. Canton S flies are referred to as wild-type throughout the paper because the ND2360114 mutation was generated in the Canton S genetic background. Genotypes shown in Figure 3 were generated by crossing female ND2360114 flies containing the UAS-ND23 transgene to male ND23G14097 flies containing the driver transgene. Heterozygous lines in Figures 7–9 were generated by crossing ND23 females to males of the other genotype.
Figure 3. Expression of ND23 in the nervous system rescues the isoflurane toxicity of ND2360114/ND23G14097 flies.
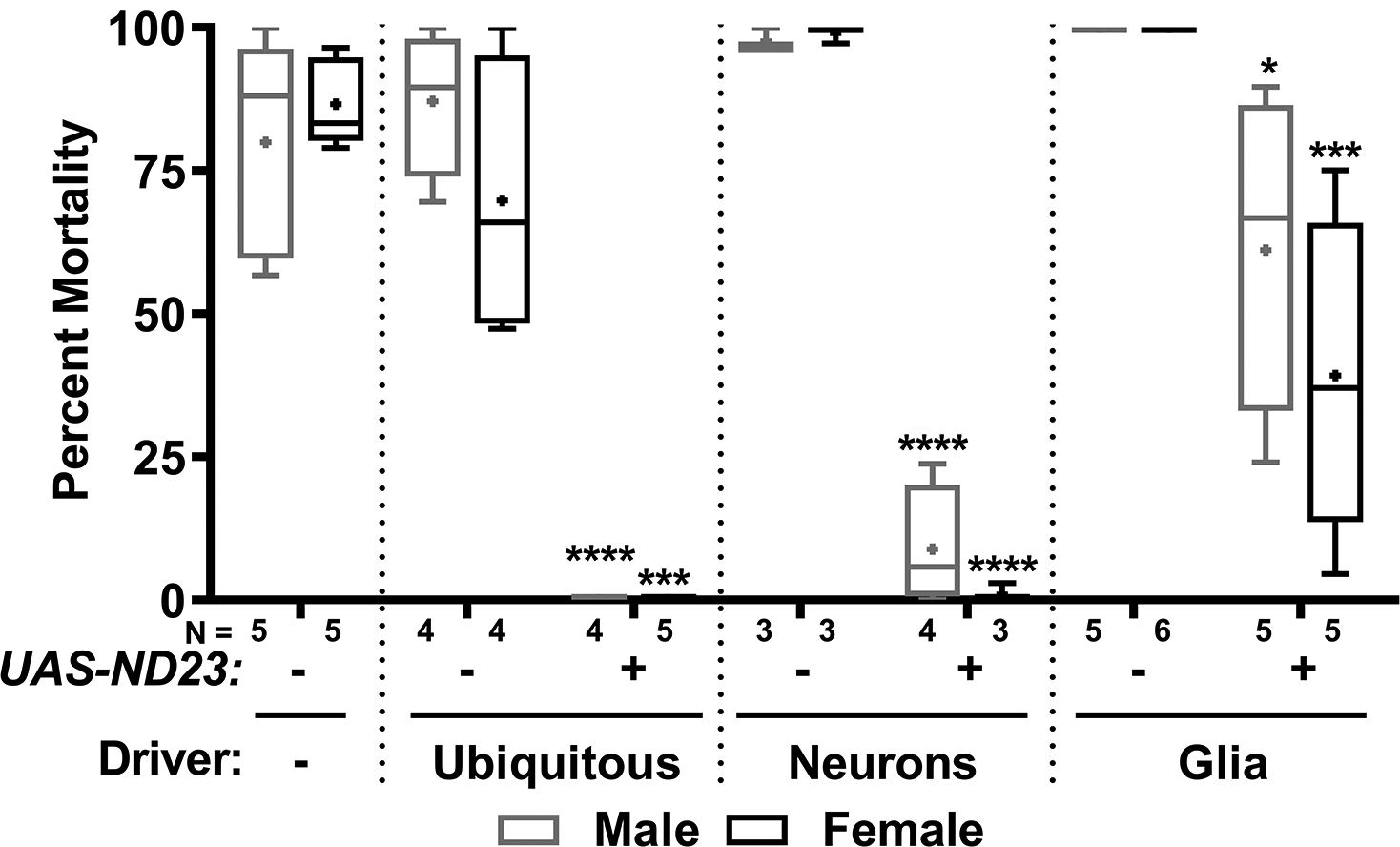
The percent mortality at 24 h following exposure to 2h of 2% isoflurane in 21% O2 was determined for 10–15-day-old, male and female ND2360114/ND23G14097 flies without (−) or with (+) a UAS-ND23 transgene and without (−) or with (transgene) a Gal4 driver transgene: Tub-Gal4 (ubiquitous), C155-Gal4 (neurons), or Repo-Gal4 (glia). Note the high control mortality (close to 100%) in C155-Gal4 and Repo-Gal4 crosses possibly due to a more isoflurane-sensitive genetic background. N=number of biological replicates. Symbols indicate the following: box (Q2-Q3), + (mean), horizontal bar (median), and whiskers (minimum and maximum). Significance between control and experimental data was determined using the unpaired equal-variance two-tail Student’s t-test. *, P<0.05; ***, P<0.001; ****, P<0.0001.
Figure 7. Mortality after exposure to isoflurane increases with age in heterozygous ND2360114 flies.
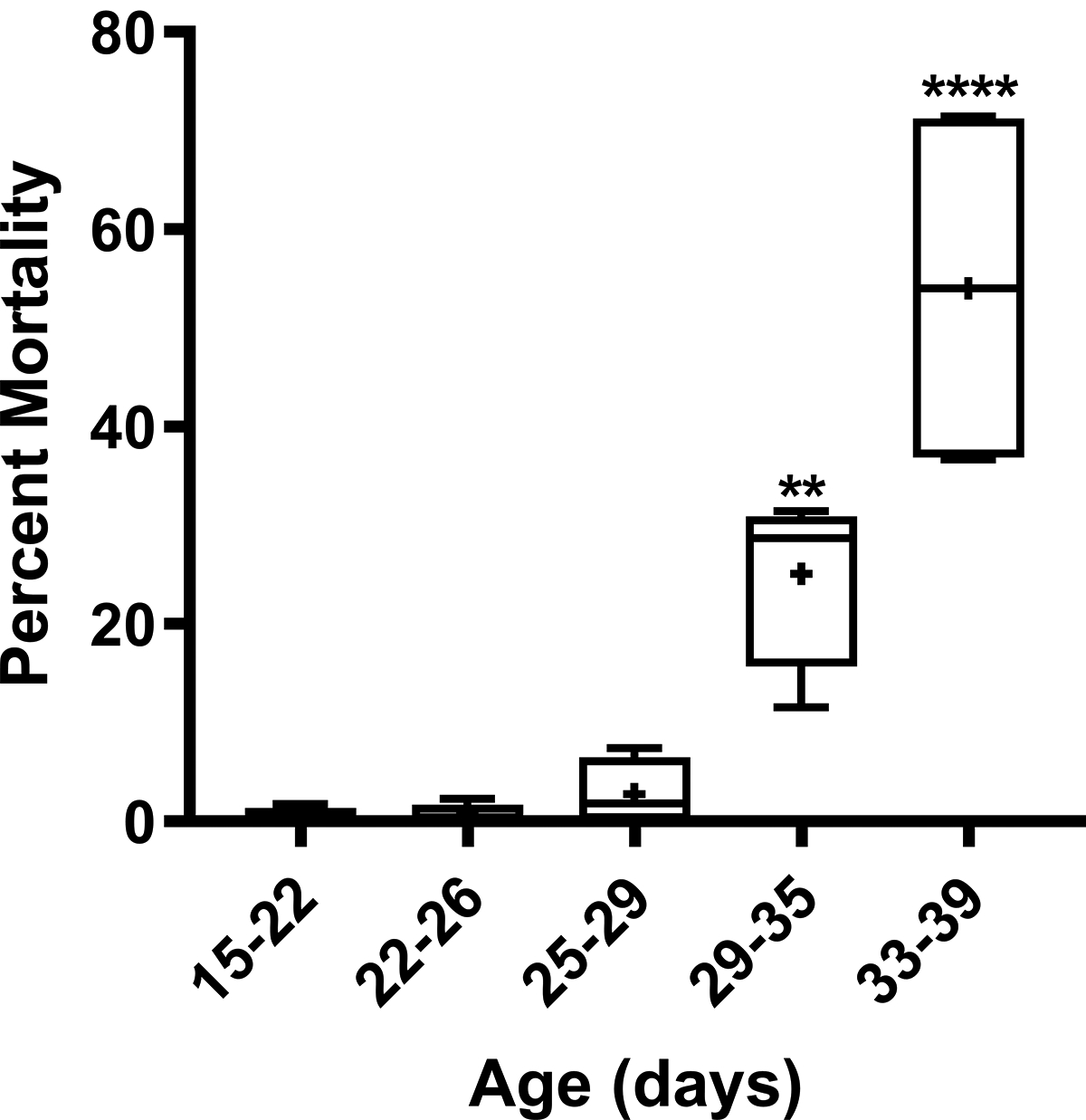
ND2360114/wild-type flies of the indicated ages were exposed to 2h of 2% isoflurane in 75% O2, and the percent mortality at 24 h was determined. Each experiment consisted of four biological replicates. Symbols indicate the following: box (Q2-Q3), + (mean), horizontal bar (median), and whiskers (minimum and maximum). Significance was determined using an ordinary one-way ANOVA followed by the Dunnett post-hoc test for multiple comparisons. **, P<0.01; ****, P<0.0001.
Figure 9. Genetic background alters the sensitivity of heterozygous ND23 mutants to mortality from isoflurane and hyperoxia.
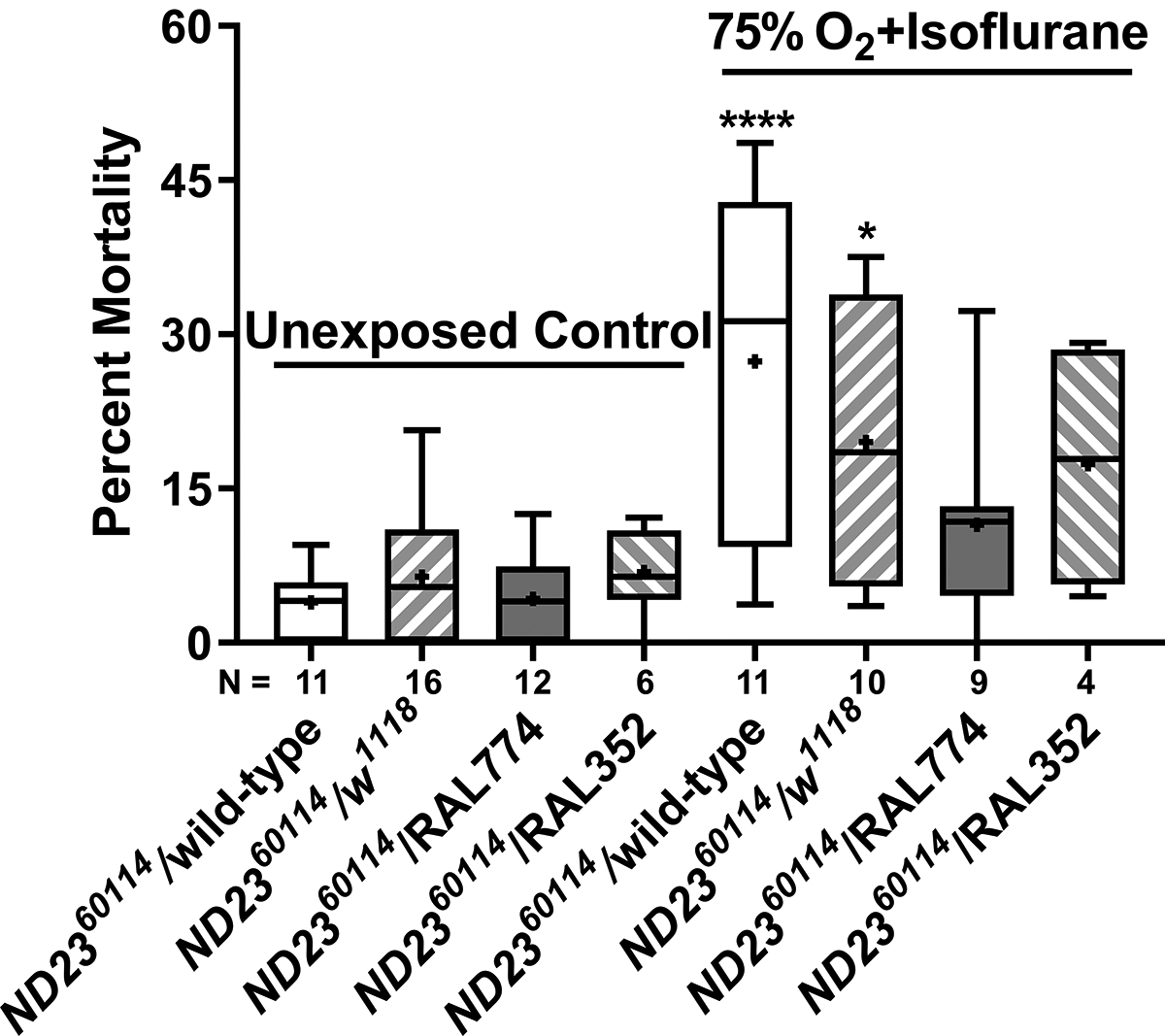
30–39-day-old females of the indicated genotypes were not exposed (Unexposed Control) or exposed to 2% isoflurane and 75% O2 for 2 h (75% O2 + isoflurane) and the percent mortality was determined at 24 h. Percent mortality in exposed flies increased for ND2360114/wild-type (P<0.0001) and ND2360114/w1118 (P=0.021) flies but not for ND2360114/RAL 774 (P=0.667) and ND2360114/RAL352 (P=0.671) flies. There was a significant effect of drug/genotype interaction (P=0.0435, two-way ANOVA). Males were not examined because not enough survived for biological replicates. Significance was determined using a two-way ANOVA followed by the Tukey post-hoc test for multiple comparisons. *, P<0.05; ****, P<0.0001.
Anesthesia and Oxygen Exposure
On the day of the experiment, flies of different sex, age, and genotype were allocated to vials ensuring equal numbers for the different treatment groups. Flies were exposed to volatile general anesthetics and oxygen at specific concentrations using a device that permits simultaneous exposure of up to eight groups of flies.14 Volatile general anesthetics were administered using a Datex-Ohmeda Aestiva/5 anesthesia machine equipped with commercial agent-specific vaporizers (Datex-Ohmeda Inc., Madison, WI). Compressed gas cylinders (Airgas USA, LLC, Radnor, PA) containing 100% O2, 100% N2, or air (21%O2/79%N2) provided carrier gas of the desired composition. Anesthetic exposure consisted of either 2% isoflurane or 3.5% sevoflurane for 2 h at room temperature. Isoflurane and sevoflurane were obtained from Piramal Enterprises Ltd. (Maharashtra, India). To control for effects of circadian rhythm, flies were anesthetized at a similar time of day (between 10:00 AM and 2:00 PM).
Percent Mortality Assay
Percent mortality was determined by counting the number of dead flies 24 h after the end of an anesthetic exposure and dividing by the total number of flies per vial. Mortality is a binary readout and was assessed by an unblinded observer. We interpret the observed mortality as due to anesthetic and/or O2 toxicity. Each experiment was conducted in at least triplicate with 25–50 flies in each sample. The mortality time course in Figure 2B was composed of independent experiments covering three timeframes 0–8, 8–16, and 16–24 h. All experiments followed the same basic structure. All flies were cultured at 25°C until and switched to 29°C at 0–2 days post eclosion when they were also separated by sex under CO2 narcosis. Flies remained at 29°C for the remainder of their life except during exposure to anesthetics, with food changes every other day. For older heterozygous lines, food changing was paired with CO2 narcosis.
Figure 2. Age of onset and time course of mortality in ND2360114 flies.
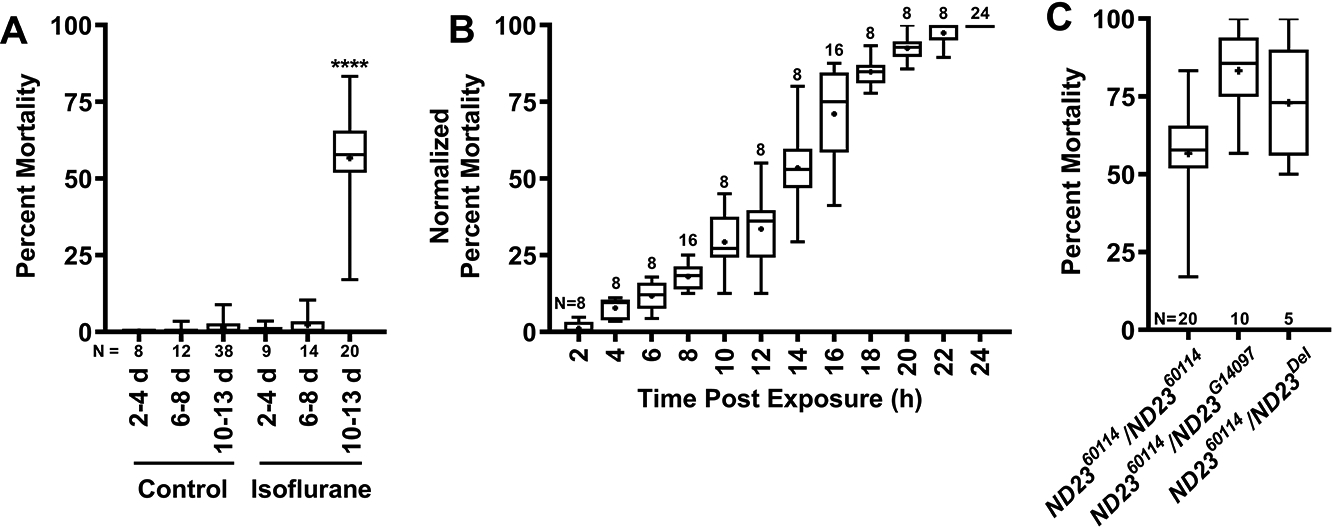
(A) Percent mortality was determined for mixed sex ND2360114 flies not exposed (Control) or exposed to 2h of 2% isoflurane and 21% O2 at the indicated ages. Significance between control and experimental data was determined using the unpaired equal-variance two-tail Student’s t-test. ****, P<0.0001. (B) The time at which 10–13-day-old, mixed-sex ND2360114 flies died during the 24 h following exposure to 2h of 2% isoflurane and 21% O2. At 2 h intervals the percent of dead flies was normalized to the percent of flies that were dead at 24 h. (C) 10–15-day-old, mixed-sex ND2360114, ND2360114/ND23G14097, and ND2360114/ND23Del flies were exposed to 2h of 2% isoflurane and 21% O2, and the percent mortality at 24 h was determined. N=number of biological replicates. Symbols indicate the following: box (Q2-Q3), + (mean), horizontal bar (median), and whiskers (minimum and maximum).
Gene expression
We used quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) to measure the expression of select genes known to respond to oxidative stress. For each treatment, ND2360114 flies were collected 30 min after the end of exposure to anesthesia. RNA isolation and processing have been described previously.17 In brief, 25 flies per treatment were transferred to 1.5 mL tubes and frozen in liquid N2, tubes were shaken vigorously by vortexing to shear heads from bodies, and heads were isolated from other body parts by sequential use of US Standard no. 25 (710 μm) and no. 40 (425 μm) sieves. Total RNA was isolated from heads using TRI reagent (Sigma). The resulting RNA was resuspended in 40 μL diethylpyrocarbonate (DEPC)-treated water and subjected to DNase treatment using the TURBO DNA-free kit (Invitrogen, ThermoFischer Scientific, Waltham, MA). The resulting RNA was ammonium acetate precipitated and resuspended in 20 μL of DEPC-treated water. cDNA was generated using the iScript cDNA Synthesis Kit (Bio-Rad). Real-time reverse transcription-polymerase chain reaction was carried out using a Bio-Rad CFX96 Real-Time System C1000 Touch™ Thermal Cycler (Bio-Rad laboratories, Hercules, CA). Primer sequences are provided in Supplemental Table 1.
Data Analysis
No a priori power calculation was conducted in this discovery-driven project. The unit for data analysis (replicate) was one vial containing at least 20 flies. The number of flies in a vial varied because of uneven fertility among mutant and wild-type strains. Independently tested vials were considered biological replicates. Data analysis is based on biological replicates and visualized using GraphPad Prism 6 software (La Jolla, CA).
Statistical Analysis
The Box plots used to describe the data include the second and third quartiles of data (Q2 and Q3). The mean (+) and the median (horizontal bar) are indicated, and whiskers extend to the minimum and maximum data point. We used parametric descriptive statistics because mortality rates for male and female mutants were normally distributed and mean and median values were similar in all experiments. Statistical significance between two data points (control and experimental condition) was tested using an unpaired equal-variance two-tail Student’s t-test. Multiple groups were compared using one-way ANOVA unless otherwise indicated in the figure legend followed by the Dunnett post-hoc test for multiple comparisons. Significance level was set at 0.05. Outliers were included in all analyses.
Results
As ND2360114 flies age, they become susceptible to isoflurane but not sevoflurane toxicity
To examine whether the ND2360114 mutation sensitizes flies to toxic effects of volatile general anesthetics, we exposed wild-type and ND2360114 flies to equipotent doses of isoflurane and sevoflurane (2% and 3.5% for 2 h, i.e., 4%h and 7%h, respectively) in 21% O2 (normoxia) and determined the percent mortality at 24 h after the exposure. We examined male and female flies at two age ranges, 2–4 and 10–13 days old, which for simplicity we refer to as young and old flies, respectively. For wild-type flies, no significant mortality was observed under any of the conditions (Fig. 1A). In contrast, for old male and female ND2360114 flies, isoflurane caused about 50% mortality (Fig. 1B). No significant mortality was observed for young ND2360114 flies exposed to isoflurane or for ND2360114 flies exposed to sevoflurane at either age. These data indicate that the underlying age-associated isoflurane toxicity mechanism is not shared by sevoflurane, despite the fact that sevoflurane induces a state of anesthesia indistinguishable from that of isoflurane.14
Figure 1. Isoflurane, but not sevoflurane, is toxic to male and female ND2360114 flies in an age-dependent manner.
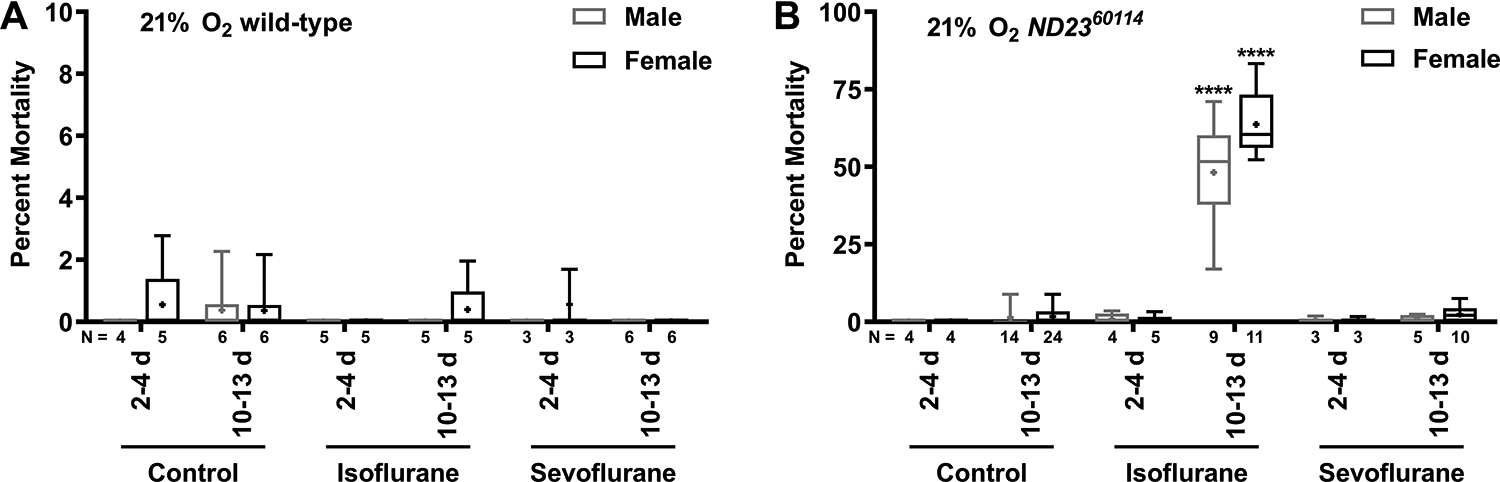
(A) wild-type and (B) ND2360114 male and female flies at different ages were either not exposed (Control) or exposed to 4%h isoflurane or 7%h sevoflurane in room air (21% O2) at either 2–4 days old (2–4 d) or 10–13 days old (10–13 d) and the percent mortality was determined after 24 h. N=number of biological replicates. Symbols indicate the following: box (Q2-Q3), + (mean), horizontal bar (median), and whiskers (minimum and maximum). Significance between control and experimental data was determined using the unpaired equal-variance two-tail Student’s t-test. ****, P<0.0001. Note the difference in y-axis scale between (A) and (B).
To test the age-dependence, the time-course, and the ND23 allele-dependence of isoflurane toxicity, we conducted the experiments shown in Figure 2. First, to narrow the age window for the onset of the toxic effect of isoflurane, we tested 6–8-day-old male and female flies. We observed no mortality in this age group (Fig. 2A). We conclude that sensitization to isoflurane mortality develops in a narrow time window between 8 and 10 days of age. To examine whether mortality was due to an acutely lethal dose of isoflurane or a delayed effect, we determined when 10–13-day-old ND2360114 flies died during the 24 h period following exposure. All flies initially regained mobility after exposure to isoflurane, and most flies that died did so between 12 and 24 h after exposure (Fig. 2B). Therefore, the mechanism underlying isoflurane toxicity in ND2360114 flies is likely to involve cellular and molecular signaling events that take several hours to build up. Finally, to test whether the age-dependent isoflurane toxicity of ND2360114 flies was due to the ND23 mutation, we repeated the mortality assay with other ND23 alleles. We tested flies that were heteroallelic for ND2360114 and ND23G14097, which contains a P-element insertion in ND23, or ND23Del, which deletes ND23. We found that similar to 10–13-day-old ND2360114 flies, exposure to isoflurane caused >50% mortality in 10–15-day-old ND2360114/ND23G14097 and ND2360114/ND23Del flies (Fig. 2C). Thus, mutations in ND23 are a risk factor for age-dependent isoflurane toxicity.
Isoflurane mortality is due to loss of ND23 function in neurons and glia
To determine whether specific cell types control vulnerability to isoflurane toxicity, we attempted to rescue mortality in ND23 mutants using the GAL4-UAS system to express wild-type ND23 in various subsets of cells.18 We found that ubiquitous expression of UAS-ND23 (using the Tubulin-Gal4 driver) completely rescued the mortality of old male and female ND2360114/ND23G14097 flies exposed to isoflurane (Fig. 3). This result provides additional evidence that isoflurane-induced mortality was due to the loss of ND23, as opposed to loss of another gene. Neuron-specific expression of UAS-ND23 (using the C155-Gal4 driver) also resulted in almost complete rescue of the mortality from 97.6±2.1% to 8.8±10.7% and 99.1±1.60% to 0.9±1.7% in males and females, respectively. In addition, glia-specific expression of UAS-ND23 (using the Repo-Gal4 driver) partially rescued the mortality from isoflurane exposure from 100.0±0.0% to 61.2±27.7% and 100.0±0.0% to 39.2±27.7% in males and females, respectively. Taken together, these results indicate that isoflurane-induced mortality in ND23 mutants is largely due to cell non-autonomous effects of loss of ND23 activity in the nervous system. Cell non-autonomous effects may reflect the close interconnection between glial and neuronal energetic homeostasis in neurodegenerative conditions.19
The severity of isoflurane-induced mortality of ND2360114 flies is oxygen-dependent
Because volatile general anesthetics are typically administered under hyperoxic conditions, we explored whether changes in oxygen concentration influenced the vulnerability of ND2360114 flies to isoflurane and sevoflurane toxicity. Male and female wild-type and ND2360114 flies were maintained in room air (~21% O2) before and after a 2 h exposure to volatile general anesthetic in 5% O2 (hypoxia) or 75% O2 (hyperoxia). As with normoxia (Fig. 1), no significant mortality was observed in young or old wild-type flies exposed to isoflurane or sevoflurane and hyperoxia or hypoxia (Figs. 4A and C). In contrast, old male and female ND2360114 flies exposed to isoflurane and hyperoxia had significantly increased mortality compared with normoxia, while exposure to isoflurane and hypoxia had significantly reduced mortality compared with normoxia (Figs. 4B and D). The mortality from sevoflurane and hyperoxia or hypoxia was low, but male ND2360114 flies exposed to sevoflurane and hyperoxia had significantly higher mortality than equivalently treated wildtype flies (9.63±8.87% and 0.0±0.0% for ND2360114 and wild-type flies, respectively. P=0.0473). Therefore, oxygen is an obligate co-factor for the manifestation of isoflurane toxicity in old ND2360114 flies and susceptibility to inhalational agent toxicity may be greater in males than females. Rescue of isoflurane-induced mortality by hypoxia and aggravation by hyperoxia in ND2360114 flies is comparable to the oxygen responsiveness of disease progression in Ndufs4 mutant mice which is also a model of Leigh syndrome.20 The Ndufs4 subunit (NADH:Ubiquinone Oxidoreductase Subunit S4) is, like ND23 (NADH:Ubiquinone Oxidoreductase Subunit S8), part of the Core of Complex I of the mitochondrial electron transport chain. Our results are consistent with a potential role of therapeutic hypoxia in certain mitochondrial diseases.21
Figure 4. Isoflurane toxicity in male and female ND2360114 flies is oxygen-dependent.
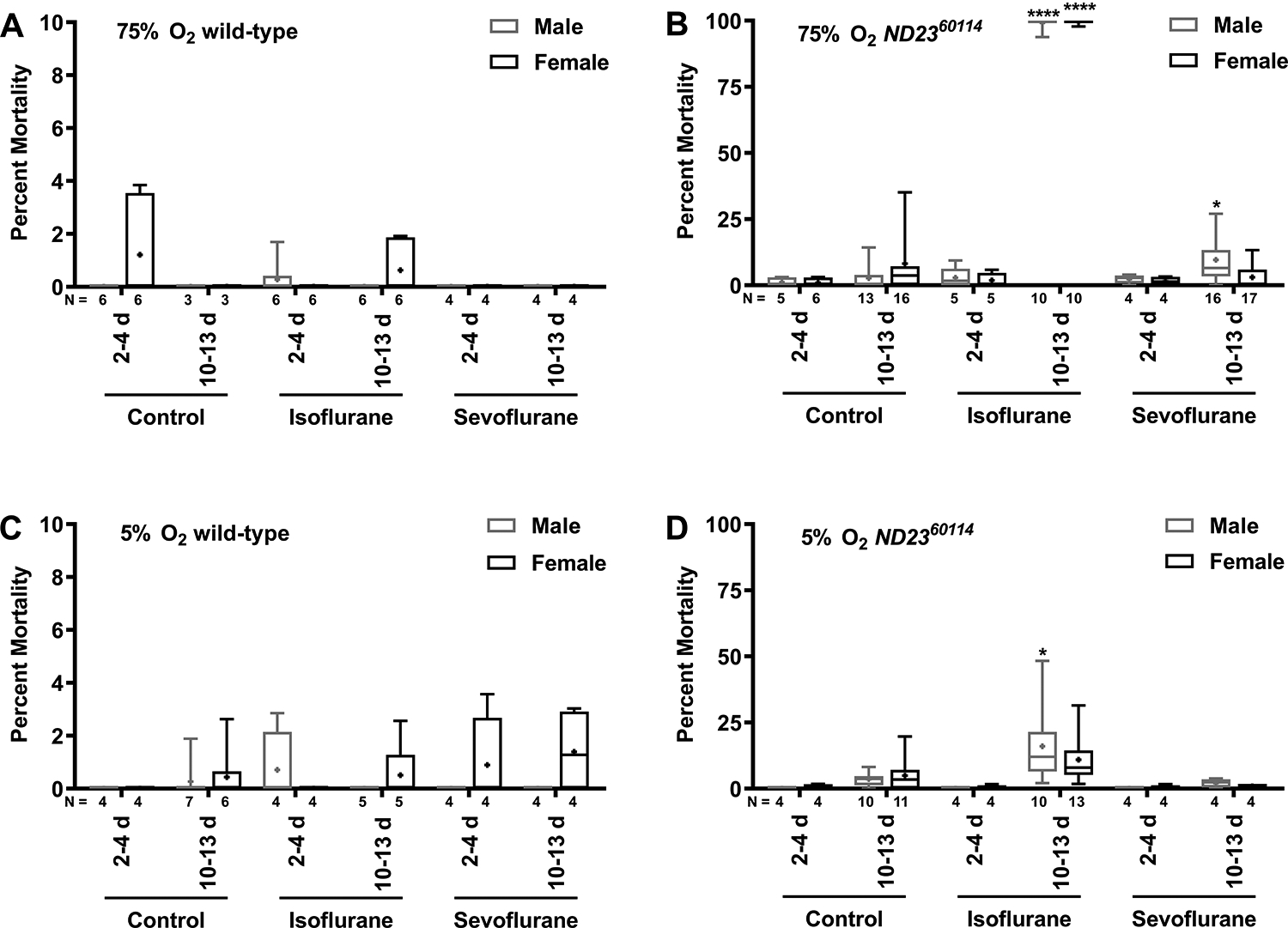
Male and female wild-type (A and C) and ND2360114 (B and D) flies were either not exposed (Control) or exposed to 2h of 2% isoflurane or 2h of 3.5% sevoflurane at either 2–4 days old (2–4 d) or 10–13 days old (10–13 d), and the percent mortality was determined after 24 h. Flies were exposed to 75% (A and B) or 5% (C and D) O2 for 2 h, concurrent with the anesthetic. Note the difference in y-axis scale between (A and C) and (B and D). Also, note almost 100% mortality in panel B for isoflurane in old animals. N=number of biological replicates. Symbols indicate the following: box (Q2-Q3), + (mean), horizontal bar (median), and whiskers (minimum and maximum). Significance between control and experimental data was determined using the unpaired equal-variance two-tail Student’s t-test. *, P<0.05.
Oxidative stress may contribute to isoflurane toxicity
A parsimonious explanation of these data is that an excessive increase in reactive oxygen species induced by a combination of isoflurane and hyperoxia22 tilts the balance from redox homeostasis to oxidative stress23 in the brains of old ND2360114 flies. To test whether ND2360114 flies have increased susceptibility to reactive oxygen species toxicity, we fed 10–13-day-old wild-type and ND2360114 flies paraquat, which increases superoxide generation by Complex I.24 For the first 24 h of the experiment, following a 2 h starvation period, flies were fed paraquat in 5% sucrose, and for the second 24 h, they were fed standard cornmeal-molasses food. At 48 h after exposure to 0.05 or 0.5 mM paraquat, ND2360114 flies had a significantly higher percent mortality than wild-type flies (Fig. 5), indicating that the ND23 mutation reduces resilience to oxidative stress.
Figure 5. ND2360114 flies are more sensitive to paraquat than wild-type flies.
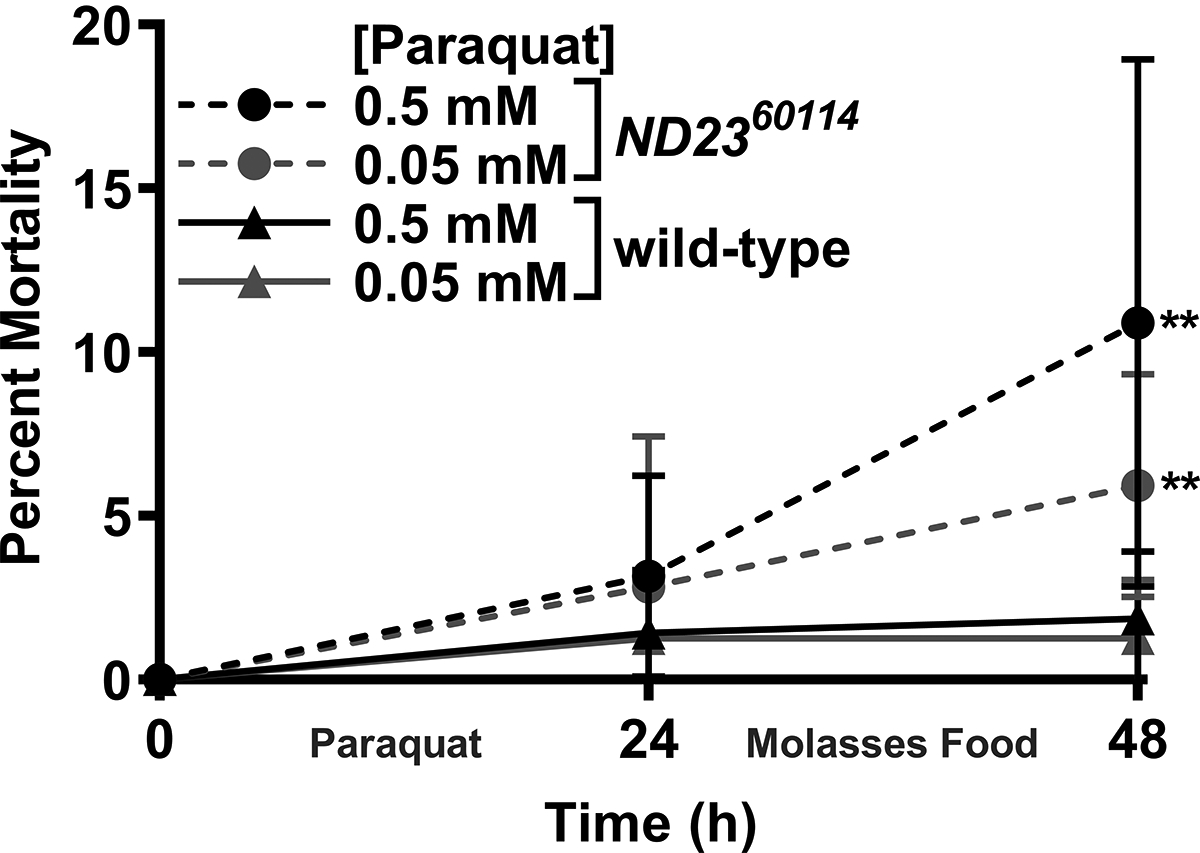
10–13-day-old, mixed-sex ND2360114 and wild-type flies were fed different concentrations of paraquat in 5% sucrose for 24 h and then cornmeal-molasses food for the next 24 h. The percent mortality was determined at 24 and 48 h. Shown are the mean of 8–13 biological replicates with error bars representing the standard deviations. For each dose and time point, comparisons were made between ND2360114 and wild-type. Significance was determined using the unpaired, Student’s t-test assuming equal variances. ** P<0.01; ****, P<0.0001.
To investigate the molecular pathways mediating the differential mortality of 10–13-day-old ND2360114 flies to isoflurane and hyperoxia relative to sevoflurane and hyperoxia, we examined the expression of genes that are transcriptionally induced by oxidative stress. We examined the expression of GstD1 and GstD2 (encoding Glutathione-S-transferase D1 and D2, respectively, homologous to mammalian Glutathione-S-transferases), Jafrac1 and Jafrac2 (encoding Thioredoxin peroxidase 1 and 2, respectively, homologous to mammalian Peroxiredoxins 1 and 4),25 SOD1 and SOD2 (encoding Superoxide dismutase 1 and 2, respectively, homologous to mammalian superoxide dismutases), Cat (encoding Catalase, homologous to mammalian catalases), and IRC (encoding Immune-regulated catalase, homologous to mammalian COX2). We used real-time reverse transcription-polymerase chain reaction to measure mRNA amounts in heads of 10–13-day-old ND2360114 flies 30 min after a 2 h exposure to isoflurane or sevoflurane in hyperoxia or hypoxia. We examined this time point, which is prior to significant mortality (Fig. 2B), because gene expression changes relevant to death should occur prior to its onset and because others have found that changes in gene expression become detectable as early as 30 min after initiation of isoflurane exposure.26
For almost all of the genes, the transcript amount was altered by exposure to either agent under both hyperoxic and hypoxic conditions, and the fold-change was smaller in females than males (Figs. 6A and B). Moreover, in males, isoflurane and hyperoxia or hypoxia increased GstD2 mRNA 5.6±0.6 and 4.8±0.3 fold, respectively (Fig. 6A), whereas, sevoflurane elicited a substantially larger increase in expression, 22.1±0.4 and 25.2±0.6 fold, respectively. Comparable differences between isoflurane and sevoflurane were observed in females (Fig. 6B). Likewise, in both males and females, sevoflurane and hyperoxia or hypoxia lead to a greater reduction in Jafrac2 expression than isoflurane and hyperoxia or hypoxia (Fig. 6). In contrast, in both males and females, isoflurane or sevoflurane and hyperoxia or hypoxia caused equivalent increases in Sod1 and Sod2 expression. Unexpectedly, oxygen concentration did not affect the expression of any of the genes. These data suggest that both isoflurane and sevoflurane activate reactive oxygen species-protective pathways but they do so to different extents, which may contribute to the differences in mortality.
Figure 6. Anesthetics modulate gene expression in reactive oxygen species-responsive pathways.
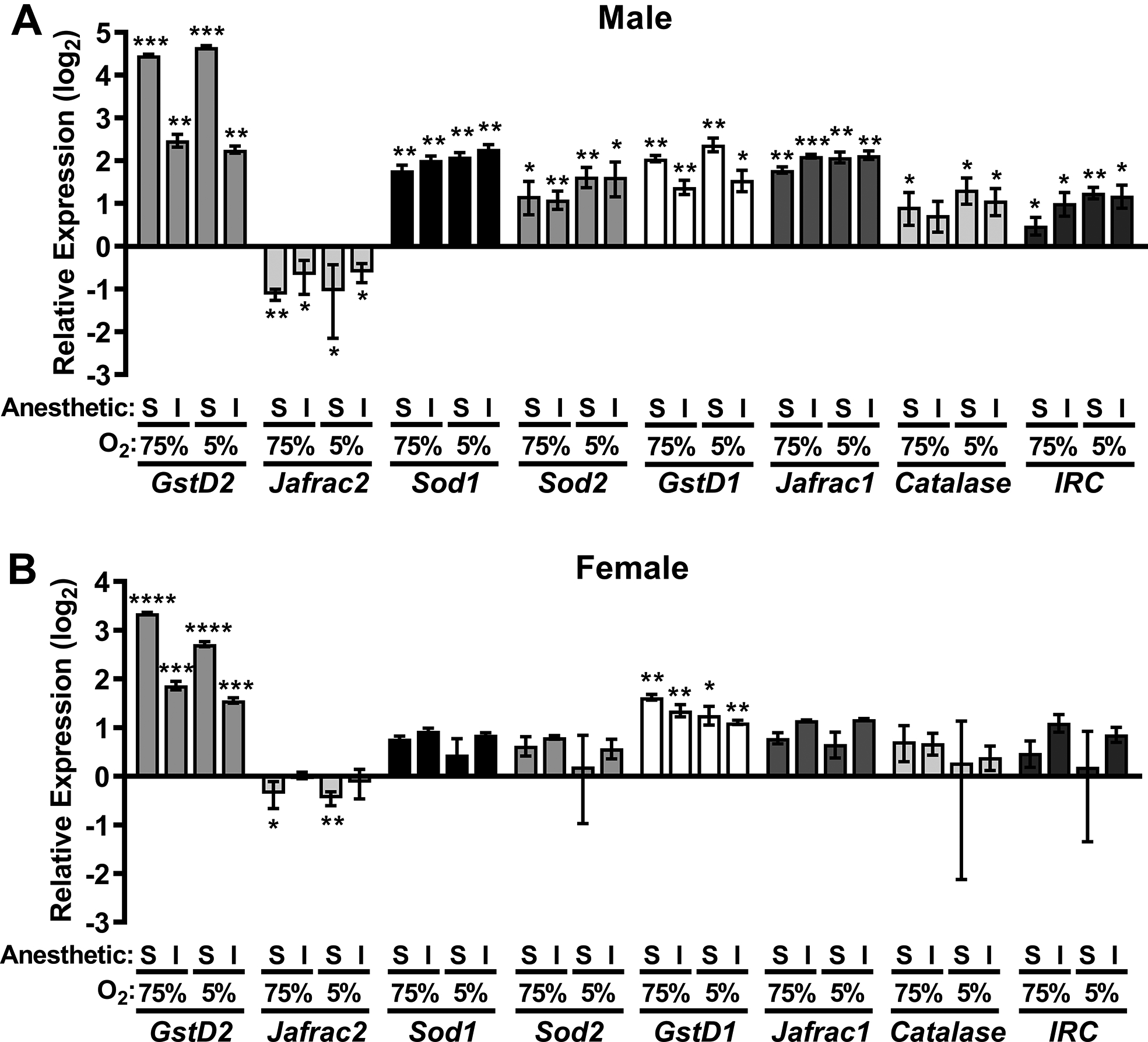
Real-time reverse transcription-polymerase chain reaction was performed on heads of 10–13 day old male (A) and female (B) ND2360114 flies collected 30 min after 2 h of 2% isoflurane or 2h of 3.5% sevoflurane in either 5% or 75% O2. Expression of the indicated genes was normalized to expression of ribosomal protein L32 (RpL32) and to expression of the genes in heads of unexposed, 10–13 day old ND2360114 flies. Each experiment consisted of three biological replicates. Significance was determined using the unpaired, Student’s t-test assuming equal variances. *, P<0.05; ** P<0.01; ***, P<0.001; ****, P<0.0001. Error bars represent standard deviations.
Aging sensitizes heterozygous ND23 mutants to toxicity from isoflurane and hyperoxia
To examine the dose-dependence of ND23 for isoflurane toxicity, we repeated the mortality assay with heterozygous ND2360114 flies that have approximately half the amount of ND23 protein and a slightly longer lifespan compared with wild-type flies.12 At 15–22 days old, ND2360114/CS flies (derived by crossing female ND2360114 flies to male CS flies) were not vulnerable to mortality from exposure to isoflurane and hyperoxia (2h of 2% isoflurane in 75% O2) (Fig. 7). As aging is associated with deteriorating mitochondrial function,27 we also tested older ND2360114/wild-type flies. We did not observe substantial mortality for ND2360114/wild-type flies until >30 days old. At 33–39 days old, exposure to isoflurane and hyperoxia resulted in 54.05±19.59% mortality in mixed-sex ND2360114/wild-type flies. Thus, aging sensitizes old heterozygous ND23 mutant flies to toxicity from isoflurane and hyperoxia.
In studies of 30–39-day-old flies, isoflurane and hyperoxia did not increase mortality in wild-type flies beyond natural attrition (Fig. 8A); however, it significantly increased the mortality of female ND2360114/wild-type from 3.9±3.1% to 27.4±15.9% (Fig. 8B). Mortality was also increased in ND2360114/wild-type male flies, but only the increase in isoflurane and normoxia from 25.7±8.6% to 53.5±24.5% was significant, probably due to the high rate of natural mortality. Similar results were observed with another ND23 allele, ND23Del/wild-type flies (Fig. 8C). Because of their short lifespan, ND23Del/wild-type flies were tested at 21–26 days old. Finally, we found that isoflurane and hyperoxia increased the mortality of 30–39-day-old ND23G14097/wild-type flies, but the difference did not reach the significance cutoff (Supplemental Fig. 1). This was unexpected because ND23G14097 is a stronger allele than ND2360114 in other contexts.12 Thus, advanced age renders heterozygous carriers of some ND23 alleles susceptible to toxicity from exposure to isoflurane and hyperoxia.
Figure 8. Aging and hyperoxia sensitize heterozygous ND23 mutants to isoflurane toxicity.
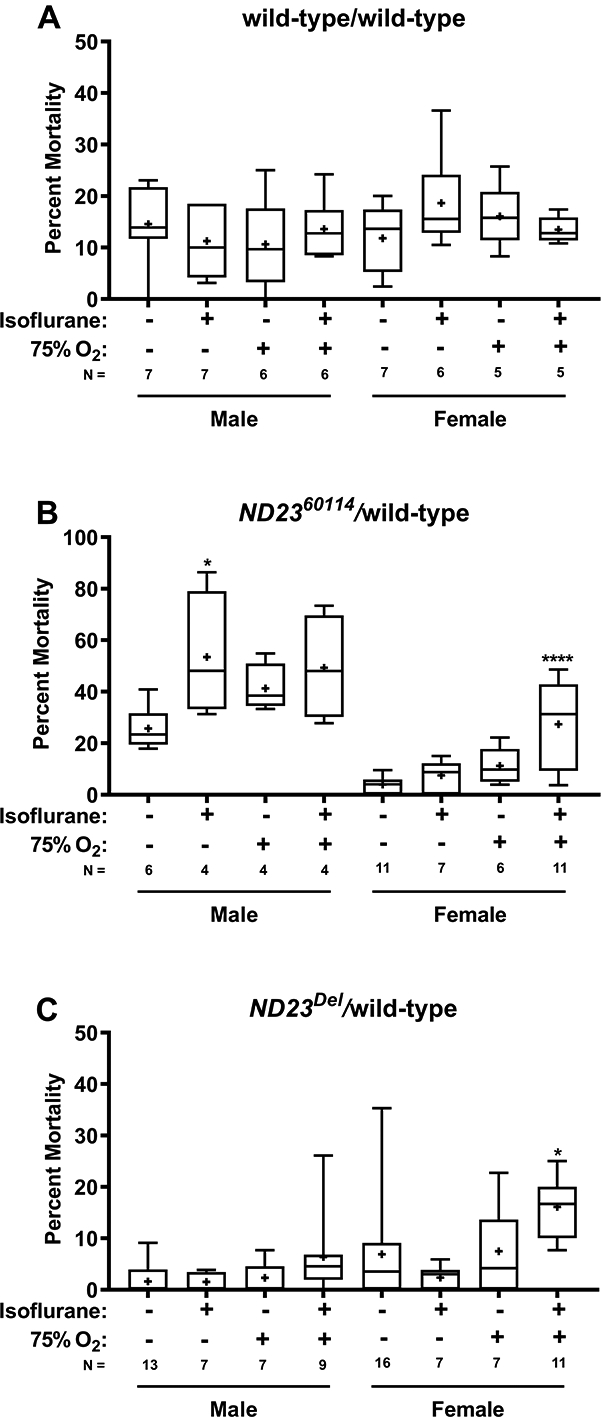
The percent mortality at 24 h was determined for 30–39-day-old, males and females (A) wild-type/wild-type, (B) ND2360114/wild-type, and (C) 21–26-day-old ND23Del/wild-type exposed (+) or not exposed (−) to 2% isoflurane and 75% O2 for 2 h. Symbols indicate the following: box (Q2-Q3), + (mean), horizontal bar (median), and whiskers (minimum and maximum). Significance between control and experimental data was determined using an ordinary one-way ANOVA followed by the Dunnett post-hoc test for multiple comparisons. *, P<0.05; ****, P<0.0001.
Genetic background modulates the toxicity of isoflurane and hyperoxia in aged heterozygous ND23 mutants
To test whether genetic background influences mortality from isoflurane and hyperoxia, we crossed ND2360114 flies to w1118 flies (a standard laboratory line) and to RAL774 and RAL352 flies, which are part of the Drosophila Genetic Reference Panel collection of inbred lines from a natural population.28 RAL774 and RAL352 were selected for analysis because they are behaviorally more sensitive to isoflurane than w1118 and wild-type and less sensitive than ND2360114.14 Exposure to isoflurane in hyperoxia significantly increased the mortality of ND2360114/wild-type flies (p<0.0001) and ND2360114/w1118 flies (p<0.05). Interestingly, ND2360114/RAL774 flies (p=0.667) and ND2360114/RAL352 flies (p=0.671) (Fig. 9) were not significantly affected. We conclude that polymorphisms in the genetic background modulate the toxicity of isoflurane and hyperoxia in aged heterozygous ND23 flies and that the tested RAL lines contain genetic polymorphisms in their background that apparently counteract the increased mortality that would otherwise be expected under the conditions tested. The nature of these polymorphisms could be explored with a genome-wide association study analysis of the Drosophila Genetic Reference Panel collection.
Discussion
It is not known how age, sex, and environmental factors modulate the penetrance of the pathophysiology underlying Leigh syndrome,9 as they do for other more common diseases caused by Complex I mutations, such as Leber’s hereditary optic neuropathy.10 Obtaining information to answer these questions would thus be an important advance for the mitochondrial disease community and their medical teams. We used an invertebrate model of Leigh syndrome and of asymptomatic carriers to explore interactions with age, sex, volatile general anesthetics, and oxygen.
Behavioral sensitivity and toxicity of isoflurane and sevoflurane are dissociated in ND23 mutants.
Young, 2–4-day-old ND2360114 flies are equally hypersensitive to isoflurane and sevoflurane, compared with six inbred strains, including the wild-type.14 Notably, the relative potencies of isoflurane and sevoflurane (expressed as the ratio of EC50 isoflurane/EC50 sevoflurane) are nearly identical (0.42 and 0.40 for ND2360114 and wild-type flies, respectively), indicating a proportional increase in the sensitivity of behavioral circuits to both agents without toxicity in animals lacking morphological signs of neurodegeneration.12 Similarly, studies in mammals also report increased sensitivity to vapor anesthetics in young (23–27-day-old) mice harboring mutations in Complex I at an age when the animals do not yet exhibit signs of neurodegeneration.29 Whether advancing age uncovers toxic phenotypes in rodents is unknown. Furthermore, in the developing chemotactic system of worms, isoflurane neurotoxicity is linked to inhibition of mitochondrial function.30
In contrast with the similar behavioral sensitivity of ND23 mutant flies to isoflurane and sevoflurane, ND23 mutants exposed to isoflurane but not to sevoflurane abruptly developed a mortality phenotype between 8 and 10 days of age (Figs. 1 and 2A). In this context, it is important to note that we do not expect the toxic phenotype (i.e. mortality) in the fly model to translate as mortality in higher animals. Hyperoxia (99% O2), for example, dramatically shortens lifespan in fruit flies31,32 but causes only impaired cognition without lethality in rats.33 Analogously, mortality in flies serves as an indicator of interference with brain mitochondrial homeostasis that may present with cognitive (‘neurotoxic’) phenotypes in higher animals.
soflurane-induced mortality of ND23 mutants is not the result of an acute anesthetic overdose because: (i) mortality increased over many hours after initial recovery from the behavioral effect of anesthesia (Fig. 2B), (ii) no death occurred in flies up to eight days of age despite behavioral hypersensitivity (Fig. 2A and Ref. 14), and (iii) ND2360114 flies were equally hypersensitive to isoflurane and sevoflurane but only isoflurane caused high mortality. We interpret these results as indicating that anesthesia and mortality are dissociable: molecular targets mediating the conventional behavioral effects of vapor anesthetics (‘anesthesia’) are largely shared between isoflurane and sevoflurane, but targets contributing to mortality in mutant flies (and possibly to adverse neurocognitive outcomes in higher animals) may differ. The finding that isoflurane is more toxic than sevoflurane in cultured neuroblastoma cells injured by oxygen-glucose deprivation supports this notion.34 However, only a few studies have directly compared toxic effects of isoflurane and sevoflurane administered in vivo. No differences were observed in the induction of apoptotic cell death by isoflurane and sevoflurane in the brains of neonatal mice,35 but with a different experimental paradigm that used behavioral outcomes, isoflurane resulted in a more extensive memory impairment phenotype than sevoflurane.36 Moreover, a clear difference between isoflurane and sevoflurane was found in their propensity to activate members of the transient receptor potential (TRP) superfamily TRPA1 and TRPV1, resulting in an in vivo inflammatory response after exposure to isoflurane but not to sevoflurane,37 thereby demonstrating that biologically significant differences between these interchangeably used agents exist.
Production of reactive oxygen species in the nervous system may mediate the toxicity of isoflurane in ND23 mutants.
Our finding that isoflurane-induced mortality of ND2360114 flies was rescued by expression of wild-type ND23 in neurons or in glia indicates that mortality is driven by cell non-autonomous consequences of ND23 activity in the brain (Fig. 3). Notably, selective expression of ND23. in neurons rescued mortality to a greater extent than expression in glia. Given that glial cells constitute only 10% of cells in the fly brain, the disproportionally high degree of rescue observed with glia-specific expression of ND23 may be due to a higher rate of reactive oxygen species production by glia and/or to the energy support of neurons by glia.38 Lopez-Fabuel et al.39 found that Complex I in neurons is mainly assembled into supercomplexes that produce reactive oxygen species at a lower rate than free Complex I, which is more abundant in astrocytes. Interestingly, mice carrying a mutation of Complex I only in astrocytes exhibit a subtle behavioral phenotype under isoflurane anesthesia.40 Thus, isoflurane-induced production of reactive oxygen species in neurons and glia may mediate toxicity in ND23 mutants.
In C. elegans, suppression of Complex I function by isoflurane increases production of reactive oxygen species in wild-type and mutant mitochondria,4 while Complex I mutations confer increased sensitivity to oxidizing agents such as paraquat.41 Further data from rodents42 and from a mammalian cell culture model43 suggest a role for reactive oxygen species in the toxicity of isoflurane. Our data regarding the effects of oxygen concentration on isoflurane toxicity (Figs. 1 and 4), the increased sensitivity of ND23 mutants to paraquat compared with wild-type flies (Fig. 5), are also consistent with a role for reactive oxygen species in mortality. Additional support for the reactive oxygen species hypothesis of isoflurane toxicity is provided by and our gene expression analysis showing that isoflurane and sevoflurane elicit qualitatively similar but quantitatively different patterns of transcriptional changes in some gene target of reactive oxygen species response pathways (GstD2 and Jafrac2 Fig. 6) are compatible with the reactive oxygen species hypothesis. GstD2 expression in flies increases in response to hyperoxic stress44 and to stress caused by blue light that results in neurodegeneration.45 Jafrac2 has both antioxidant and signaling activity that regulates intracellular hydrogen peroxide (H2O2) levels.25 Suppression of Jafrac2 expression results in shortened lifespan in the face of oxidative stress with H2O2 and paraquat.25 Reactive oxygen species are natural byproducts of electron transfer in mitochondria functioning as signaling molecules responsive to environmental cues.46,47 Mitochondrial dysfunction and reactive oxygen species-induced damage accumulate with aging32,48,49 and may sensitize flies to the acute oxidative stress-like insult from isoflurane. Therefore, while it is evident that exposure to both isoflurane and sevoflurane triggers responses in oxidative stress pathways, our data do not allow us to infer which changes are protective (e.g., strong upregulation of GstD2), which are signs of a failure to adapt (e.g., weak upregulation of GstD2) or how these changes in gene expression relate to mortality.
A toxicity mechanism involving reactive oxygen species pathways is also consistent with the age dependence of isoflurane-induced mortality in ND23 mutant flies (Figs. 1, 2A, 4, and 7).
Aging and genetic background shape the phenotypes of isoflurane in heterozygous ND23 mutants.
About 5.9 in 100,000 individuals are at risk of nuclear DNA-related mitochondrial diseases,8 the majority of which are due to recessive mutations. We found that age renders heterozygous carriers of ND23 mutations vulnerable to the stress of isoflurane under normoxic and hyperoxic conditions (Figs. 7 and 8). Polymorphisms in the nuclear genome, introduced either by other alleles of ND23 (Fig. 8) or by crosses into other fly lines (Fig. 9), modified the genetic environment of the ND23 mutation and resulted in varying degrees of vulnerability to the toxicity of isoflurane and hyperoxia. These results suggest that heterozygous mutations in Complex I genes that underlie Leigh syndrome, the most common mitochondrial encephalomyelopathy,5 can increase the risk for deleterious manifestations under the combined stresses of advanced age, anesthesia, and hyperoxia with the degree of risk dependent on genetic background. Importantly, our data from heterozygous flies indicate that under conditions of advanced age and environmental stress (e.g., surgery under anesthesia with isoflurane and hyperoxia) an otherwise silent reduction in Complex I function may translate into adverse outcomes. In the clinical scenario, the association of cardiopulmonary bypass with a high incidence of temporary CNS dysfunction50 and with mitochondrial dysfunction and oxidative damage51 may be not accidental.
In summary, our data raise testable predictions. Other mutations affecting Complex I and mutations affecting other components of oxidative metabolism should result in a toxic phenotype under anesthesia. Furthermore, strategies aimed at mitigating oxidative stress should reduce anesthetic toxicity. A limitation of our data is that we have not yet established a direct causal relationship between oxidative stress and mortality. Other tests such as genome-wide transcriptome analysis are necessary to provide a more comprehensive overview of pathways involved in isoflurane toxicity. A better understanding of these pathways will reveal genetic risk factors and therapeutic approaches to anesthetic neurotoxicity. Our experiments demonstrate the value of Drosophila for understanding the multifaceted interactions of anesthetics, aging, genetic background, and mitochondrial function.
Supplementary Material
Figure 1. The ND23G14097 does not sensitize heterozygous ND23 mutants to isoflurane toxicity. The percent mortality at 24 h was determined for 30-39-day-old, male and female ND23G14097/wild-type flies. N= number of biological replicates. Symbols indicate the following: box (Q2-Q3), + (mean), horizontal bar (median), and whiskers (minimum and maximum). Significance between control and experimental data was determined using an ordinary one-way ANOVA followed by the Dunnett post-hoc test for multiple comparisons.
Acknowledgements:
We thank the anonymous reviewers whose comments and suggestions have substantially improved the manuscript. We also Carin Loewen, PhD , University of Wisconsin-Madison for fly stocks and we thank Grace Boekhoff-Falk, PhD, Department of Cell and Regenerative Biology, University of Wisconsin-Madison, Madison, WI, USA for helpful discussions throughout the project and Mary Roth1 for excellent assistance with proofreading.
Funding Statement: We gratefully acknowledge support by a seed grant from the University of Wisconsin-Madison Department of Anesthesiology, Madison, WI, to MP and R01GM134107 to MP and DW.
Footnotes
Clinical trial number and registry URL: Not applicable.
Prior Presentations: This work has been presented in part at the Annual Meeting of the ASA in Orlando, FL, on October 21, 2019 within the framework of the ‘Best of basic science abstracts’ session.
Conflicts of Interest: The authors declare no competing interests.
References
- 1.Mungunsukh O, Deuster P, Muldoon S, O’Connor F, Sambuughin N: Estimating prevalence of malignant hyperthermia susceptibility through population genomics data. Br J Anaesth 2019; 123: e461–e463 [DOI] [PubMed] [Google Scholar]
- 2.Smeitink J, van den Heuvel L, DiMauro S: The genetics and pathology of oxidative phosphorylation. Nat Rev Genet 2001; 2: 342–52 [DOI] [PubMed] [Google Scholar]
- 3.Hanley PJ, Ray J, Brandt U, Daut J: Halothane, isoflurane and sevoflurane inhibit NADH:ubiquinone oxidoreductase (complex I) of cardiac mitochondria. J Physiol 2002; 544: 687–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kayser EB, Suthammarak W, Morgan PG, Sedensky MM: Isoflurane selectively inhibits distal mitochondrial complex I in Caenorhabditis elegans. Anesth Analg 2011; 112: 1321–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finsterer J: Leigh and Leigh-like syndrome in children and adults. Pediatr Neurol 2008; 39: 223–35 [DOI] [PubMed] [Google Scholar]
- 6.Morgan PG, Hoppel CL, Sedensky MM: Mitochondrial defects and anesthetic sensitivity. Anesthesiology 2002; 96: 1268–70 [DOI] [PubMed] [Google Scholar]
- 7.Niezgoda J, Morgan PG: Anesthetic considerations in patients with mitochondrial defects. Paediatr Anaesth 2013; 23: 785–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorman GS, Schaefer AM, Ng Y, Gomez N, Blakely EL, Alston CL, Feeney C, Horvath R, Yu-Wai-Man P, Chinnery PF, Taylor RW, Turnbull DM, McFarland R: Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol 2015; 77: 753–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKelvie P, Infeld B, Marotta R, Chin J, Thorburn D, Collins S: Late-adult onset Leigh syndrome. J Clin Neurosci 2012; 19: 195–202 [DOI] [PubMed] [Google Scholar]
- 10.Kirkman MA, Yu-Wai-Man P, Korsten A, Leonhardt M, Dimitriadis K, De Coo IF, Klopstock T, Chinnery PF: Gene-environment interactions in Leber hereditary optic neuropathy. Brain 2009; 132: 2317–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burman JL, Itsara LS, Kayser EB, Suthammarak W, Wang AM, Kaeberlein M, Sedensky MM, Morgan PG, Pallanck LJ: A Drosophila model of mitochondrial disease caused by a complex I mutation that uncouples proton pumping from electron transfer. Dis Model Mech 2014; 7: 1165–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loewen CA, Ganetzky B: Mito-Nuclear Interactions Affecting Lifespan and Neurodegeneration in a Drosophila Model of Leigh Syndrome. Genetics 2018; 208: 1535–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marina AD, Schara U, Pyle A, Moller-Hartmann C, Holinski-Feder E, Abicht A, Czermin B, Lochmuller H, Griffin H, Santibanez-Koref M, Chinnery PF, Horvath R: NDUFS8-related Complex I Deficiency Extends Phenotype from “PEO Plus” to Leigh Syndrome. JIMD Rep 2013; 10: 17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olufs ZPG, Loewen CA, Ganetzky B, Wassarman DA, Perouansky M: Genetic variability affects absolute and relative potencies and kinetics of the anesthetics isoflurane and sevoflurane in Drosophila melanogaster. Sci Rep 2018; 8: 2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews PL: Introduction: laboratory invertebrates: only spineless, or spineless and painless? ILAR J 2011; 52: 121–5 [DOI] [PubMed] [Google Scholar]
- 16.Harvey-Clark C: IACUC Challenges in Invertebrate Research. ILAR J 2011; 52: 213–20 [DOI] [PubMed] [Google Scholar]
- 17.Petersen AJ, Rimkus SA, Wassarman DA: ATM kinase inhibition in glial cells activates the innate immune response and causes neurodegeneration in Drosophila. Proc Natl Acad Sci U S A 2012; 109: E656–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brand AH, Perrimon N: Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993; 118: 401–15 [DOI] [PubMed] [Google Scholar]
- 19.Liu L, Zhang K, Sandoval H, Yamamoto S, Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A, Bellen HJ: Glial lipid droplets and ROS induced by mitochondrial defects promote neurodegeneration. Cell 2015; 160: 177–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari M, Jain IH, Goldberger O, Rezoagli E, Thoonen R, Cheng KH, Sosnovik DE, Scherrer-Crosbie M, Mootha VK, Zapol WM: Hypoxia treatment reverses neurodegenerative disease in a mouse model of Leigh syndrome. Proc Natl Acad Sci U S A 2017; 114: E4241–E4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, Goldberger O, Peng J, Shalem O, Sanjana NE, Zhang F, Goessling W, Zapol WM, Mootha VK: Hypoxia as a therapy for mitochondrial disease. Science 2016; 352: 54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman BA, Crapo JD: Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem 1981; 256: 10986–92 [PubMed] [Google Scholar]
- 23.Schieber M, Chandel NS: ROS function in redox signaling and oxidative stress. Curr Biol 2014; 24: R453–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cocheme HM, Murphy MP: Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem 2008; 283: 1786–98 [DOI] [PubMed] [Google Scholar]
- 25.Radyuk SN, Klichko VI, Michalak K, Orr WC: The effect of peroxiredoxin 4 on fly physiology is a complex interplay of antioxidant and signaling functions. FASEB J 2013; 27: 1426–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamaya Y, Takeda T, Dohi S, Nakashima S, Nozawa Y: The effects of pentobarbital, isoflurane, and propofol on immediate-early gene expression in the vital organs of the rat. Anesthesia Analgesia 2000; 90: 1177–1183 [DOI] [PubMed] [Google Scholar]
- 27.Kauppila TES, Kauppila JHK, Larsson NG: Mammalian Mitochondria and Aging: An Update. Cell Metab 2017; 25: 57–71 [DOI] [PubMed] [Google Scholar]
- 28.Mackay TF, Richards S, Stone EA, Barbadilla A, Ayroles JF, Zhu D, Casillas S, Han Y, Magwire MM, Cridland JM, Richardson MF, Anholt RR, Barron M, Bess C, Blankenburg KP, Carbone MA, Castellano D, Chaboub L, Duncan L, Harris Z, Javaid M, Jayaseelan JC, Jhangiani SN, Jordan KW, Lara F, Lawrence F, Lee SL, Librado P, Linheiro RS, Lyman RF, Mackey AJ, Munidasa M, Muzny DM, Nazareth L, Newsham I, Perales L, Pu LL, Qu C, Ramia M, Reid JG, Rollmann SM, Rozas J, Saada N, Turlapati L, Worley KC, Wu YQ, Yamamoto A, Zhu Y, Bergman CM, Thornton KR, Mittelman D, Gibbs RA: The Drosophila melanogaster Genetic Reference Panel. Nature 2012; 482: 173–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintana A, Kruse SE, Kapur RP, Sanz E, Palmiter RD: Complex I deficiency due to loss of Ndufs4 in the brain results in progressive encephalopathy resembling Leigh syndrome. Proc Natl Acad Sci U S A 2010; 107: 10996–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Na HS, Brockway NL, Gentry KR, Opheim E, Sedensky MM, Morgan PG: The genetics of isoflurane-induced developmental neurotoxicity. Neurotoxicol Teratol 2017; 60: 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philpott DE, Bensch KG, Miquel J: Life span and fine structural changes in oxygen-poisoned Drosophila melanogaster. Aerosp Med 1974; 45: 283–9 [PubMed] [Google Scholar]
- 32.Walker DW, Benzer S: Mitochondrial “swirls” induced by oxygen stress and in the Drosophila mutant hyperswirl. Proc Natl Acad Sci U S A 2004; 101: 10290–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukui K, Onodera K, Shinkai T, Suzuki S, Urano S: Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Ann N Y Acad Sci 2001; 928: 168–75 [DOI] [PubMed] [Google Scholar]
- 34.Schallner N, Ulbrich F, Engelstaedter H, Biermann J, Auwaerter V, Loop T, Goebel U: Isoflurane but not sevoflurane or desflurane aggravates injury to neurons in vitro and in vivo via p75NTR-NF-kB activation. Anesth Analg 2014; 119: 1429–41 [DOI] [PubMed] [Google Scholar]
- 35.Istaphanous GK, Howard J, Nan X, Hughes EA, McCann JC, McAuliffe JJ, Danzer SC, Loepke AW: Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology 2011; 114: 578–87 [DOI] [PubMed] [Google Scholar]
- 36.Ramage TM, Chang FL, Shih J, Alvi RS, Quitoriano GR, Rau V, Barbour KC, Elphick SA, Kong CL, Tantoco NK, Ben-Tzur D, Kang H, McCreery MS, Huang P, Park A, Uy J, Rossi MJ, Zhao C, Di Geronimo RT, Stratmann G, Sall JW: Distinct long-term neurocognitive outcomes after equipotent sevoflurane or isoflurane anaesthesia in immature rats. Br J Anaesth 2013; 110 Suppl 1: i39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matta JA, Cornett PM, Miyares RL, Abe K, Sahibzada N, Ahern GP: General anesthetics activate a nociceptive ion channel to enhance pain and inflammation. Proc Natl Acad Sci U S A 2008; 105: 8784–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkenhoff A, Weiler A, Letzel M, Stehling M, Klambt C, Schirmeier S: Glial Glycolysis Is Essential for Neuronal Survival in Drosophila. Cell Metab 2015; 22: 437–47 [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Fabuel I, Le Douce J, Logan A, James AM, Bonvento G, Murphy MP, Almeida A, Bolanos JP: Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc Natl Acad Sci U S A 2016; 113: 13063–13068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramadasan-Nair R, Hui J, Itsara LS, Morgan PG, Sedensky MM: Mitochondrial Function in Astrocytes Is Essential for Normal Emergence from Anesthesia in Mice. Anesthesiology 2019; 130: 423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hartman PS, Ishii N, Kayser EB, Morgan PG, Sedensky MM: Mitochondrial mutations differentially affect aging, mutability and anesthetic sensitivity in Caenorhabditis elegans. Mech Ageing Dev 2001; 122: 1187–201 [DOI] [PubMed] [Google Scholar]
- 42.Boscolo A, Starr JA, Sanchez V, Lunardi N, DiGruccio MR, Ori C, Erisir A, Trimmer P, Bennett J, Jevtovic-Todorovic V: The abolishment of anesthesia-induced cognitive impairment by timely protection of mitochondria in the developing rat brain: the importance of free oxygen radicals and mitochondrial integrity. Neurobiol Dis 2012; 45: 1031–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ni C, Li C, Dong Y, Guo X, Zhang Y, Xie Z: Anesthetic Isoflurane Induces DNA Damage Through Oxidative Stress and p53 Pathway. Mol Neurobiol 2017; 54: 3591–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landis G, Shen J, Tower J: Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging (Albany NY) 2012; 4: 768–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nash TR, Chow ES, Law AD, Fu SD, Fuszara E, Bilska A, Bebas P, Kretzschmar D, Giebultowicz JM: Daily blue-light exposure shortens lifespan and causes brain neurodegeneration in Drosophila. NPJ Aging Mech Dis 2019; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M, Flaherty JT: Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 1993; 268: 18532–41 [PubMed] [Google Scholar]
- 47.Zorov DB, Juhaszova M, Sollott SJ: Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 2014; 94: 909–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shigenaga MK, Hagen TM, Ames BN: Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A 1994; 91: 10771–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Landis GN, Abdueva D, Skvortsov D, Yang J, Rabin BE, Carrick J, Tavare S, Tower J: Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc Natl Acad Sci U S A 2004; 101: 7663–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez MG, Hughes CG, DeMatteo A, O’Neal JB, McNeil JB, Shotwell MS, Morse J, Petracek MR, Shah AS, Brown NJ, Billings FTt: Intraoperative Oxidative Damage and Delirium after Cardiac Surgery. Anesthesiology 2020; 132: 551–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenfeldt F, Marasco S, Lyon W, Wowk M, Sheeran F, Bailey M, Esmore D, Davis B, Pick A, Rabinov M, Smith J, Nagley P, Pepe S: Coenzyme Q10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J Thorac Cardiovasc Surg 2005; 129: 25–32 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1. The ND23G14097 does not sensitize heterozygous ND23 mutants to isoflurane toxicity. The percent mortality at 24 h was determined for 30-39-day-old, male and female ND23G14097/wild-type flies. N= number of biological replicates. Symbols indicate the following: box (Q2-Q3), + (mean), horizontal bar (median), and whiskers (minimum and maximum). Significance between control and experimental data was determined using an ordinary one-way ANOVA followed by the Dunnett post-hoc test for multiple comparisons.


