Abstract
Background:
Electroconvulsive therapy (ECT) technique is often changed after insufficient improvement, yet there has been little research on switching strategies.
Objective:
To document clinical outcome in ECT nonresponders who were received a second course using high dose, brief pulse, bifrontotemporal (HD BP BL) ECT, and compare relapse rates and cognitive effects relative to patients who received only one ECT course and as a function of the type of ECT first received.
Methods:
Patients were classified as receiving Weak, Strong, or HD BP BL ECT during three randomized trials at Columbia University. Nonresponders received HD BP BL ECT. In a separate multi-site trial, Optimization of ECT, patients were randomized to right unilateral or BL ECT and nonresponders also received further treatment with HD BP BL ECT.
Results:
Remission rates with a second course of HD BP BL ECT were high in ECT nonresponders, approximately 60% and 40% in the Columbia University and Optimization of ECT studies, respectively. Clinical outcome was independent of the type of ECT first received. A second course with HD BP BL ECT resulted in greater retrograde amnesia immediately, two months, and six months following ECT.
Conclusions:
In the largest samples of ECT nonresponders studied to date, a second course of ECT had marked antidepressant effects. Since the therapeutic effects were independent of the technique first administered, it is possible that many patients may benefit simply from longer courses of ECT. Randomized trials are needed to determine whether, when, and how to change treatment technique in ECT.
Keywords: electroconvulsive therapy (ECT), switching strategy, relapse, retrograde amnesia
Remarkable progress has been made in modifying ECT treatment technique to maintain antidepressant efficacy while reducing adverse cognitive effects [1]. For several decades after its inception, treatment with ECT involved sine wave stimulation, typically at maximum device output, using the bifrontotemporal (bilateral, BL) electrode placement [2, 3]. In contrast, current practice may use BL, bifrontal (BF), or the right unilateral (RUL) electrode placements [4]. Sine wave stimulation has been largely replaced by brief pulse (BP) or ultrabrief pulse (UB) stimulation. High fixed dose treatment is discouraged in professional guidelines, and empirical dose titration is recognized as the most precise method for quantifying seizure threshold (ST) and determining subsequent dosing [5, 6]. It is now well established that electrical dose relative to ST impacts cognitive effects for all electrode placements, while also strongly determining the efficacy of RUL ECT [7–10].
As an example, in current practice high dose (HD) (6 x ST), UB (0.25–0.30 ms), RUL ECT is often started for many patients in a major depressive episode [11, 12]. This treatment has shown strong efficacy and is the form of ECT with the mildest cognitive side effects [13–15]. However, if improvement is insufficient, it is common to change treatment technique to increase treatment response. In this example, the ECT practitioner has the option of continuing with UB RUL ECT, but increasing the electrical dose relative to threshold (e.g., 9 x ST), switching from UB to BP stimulation (e.g., from 0.3 to 1.0 ms pulse width) while continuing with RUL ECT, or switching to the BF or BL placement at either a near UB (0.5 ms) or BP width (1.0 ms). Determining whether, when, and how to switch treatment technique is a critical and common clinical issue in ECT practice, yet systematic research on this topic has rarely been conducted [16].
The presumption when switching technique is that the new method is likely to be more effective than continuing treatment with the prior technique, but often with increased risk of more severe cognitive effects [17, 18]. For example, HD BP BL ECT is widely considered the “gold standard,” more effective than any other form of ECT [5, 19]. However, recent randomized trials [15, 20, 21], including a large non-inferiority study [22], and a comprehensive meta-analysis [23] have established that HD (6 x ST) BP RUL ECT is as effective as low dose (LD) (1.5 x ST) or HD (2.5 x ST) BP BL ECT when used as the initial ECT technique. Furthermore, this research has demonstrated that the RUL intervention results in less severe and less persistent retrograde amnesia for autobiographical information [23–25]. Given these findings, the rationale for switching from RUL ECT to HD BP BL ECT can be questioned. The lack of difference in initial efficacy suggests that a switch to BL ECT may not result in improved clinical outcome compared to continuing with the same RUL ECT technique. On the other hand, such a switch is likely to intensify cognitive side effects.
The relative benefits and costs of switching strategies can be best addressed with double-blind, randomized controlled trials (RCTs) in which patients, in the context of insufficient improvement, are randomized to stay on the same treatment compared to switching to a new strategy. Such an ECT trial has never been conducted. Indeed, the only RCT of ECT switching strategies randomized 24 patients to either HD (5.5 x ST) BP RUL ECT or HD (2.5 x ST) BP BL ECT, in the context of insufficient improvement after 5–8 treatments with moderate dose (2.5 x ST) BP RUL ECT [26]. While the difference in antidepressant efficacy was not statistically significant, the numerical difference between the two switch strategies favored the RUL group, and the RUL group had significantly less severe cognitive effects at ECT completion.
In each of three consecutive RCTs, conducted at the New York State Psychiatric Institute (NYSPI)/Columbia University, patients were randomized to four ECT techniques that differed in factors such as electrode placement (BL vs. RUL), electrical dosage, and stimulus waveform (UB vs. BP) [8, 15, 20]. In each study, one of randomized interventions was HD (2.5 x ST), BP (1.5 ms), BL ECT. Patients who did not meet response criteria following the randomized treatment course (Mode 1) were offered a second course of ECT (Mode 2). All such patients were treated with the same HD BP BL ECT technique as had been used as one of the randomized techniques in Mode 1. The blind for Mode 1 treatment assignment was maintained for all patients through study completion. Thus, outcome assessors were masked to the initial (Mode 1) treatment assignment when evaluating clinical progress during and following Mode 2. The form of ECT used in Mode 2 was not masked.
These three RCTs used ECT techniques in Mode 1 that differed markedly in efficacy. It is now well established that low dose (1 x ST) and moderate dose (2.5 x ST) RUL ECT have inferior antidepressant effects compared to HD (6 x ST) RUL ECT or BL ECT [8, 20, 27]. In contrast, HD RUL ECT (BP or UB), and low dose (1.5 x ST) BL ECT are each highly effective treatments. In these and other studies, they did not differ in efficacy compared to HD BP BL ECT [8, 15, 20–22, 28]. Thus, if a change to an intrinsically more effective treatment technique underlies the efficacy of switch strategies, one might expect the response and remission rates following Mode 2 treatment with HD BP BL ECT to be highest for patients who received a relatively ineffective form of ECT in Mode 1 (e.g., LD BP RUL ECT), lower in patients who received an effective form of ECT in Mode 1 (e.g., LD BP BL ECT), and especially lower in those who received the same treatment in Mode 2 as the one to which they had not responded in Mode 1, HD BP BL ECT. In contrast, if simply receiving more treatment is associated with strong efficacy, the response and remission rates should be high in patients who received HD BP BL ECT in both Mode 1 and Mode 2 and not differ substantially from the other groups.
Neurocognitive evaluations were conducted at preECT baseline, following the randomized Mode 1, after Mode 2, and at two-month and six-month follow-up intervals. This provided descriptive information on the cognitive effects of receiving a second course of ECT with HD BP BL treatment. Patients who responded to ECT either in Mode 1 or Mode 2 were closely monitored for relapse over a one-year period. Relapse rates have never been compared in patients who respond to an initial course of ECT relative to those who respond after a switch in treatment technique.
We also addressed similar questions in a separate, large, multi-site randomized controlled trial (RCT), the Optimization of ECT (OPT-ECT) study [21, 29]. In this study, patients were double randomized during Mode 1 to either HD (6 x ST) BP (1 ms) RUL ECT or LD (1.5 x ST) BP (1 ms), BL ECT, as well as to concurrent placebo, nortriptyline or venlafaxine pharmacotherapy. Patients who did not respond in Mode 1 could be switched to HD BP BL ECT. Mode 2 of this study was not as highly structured as in the Columbia RCTs, but again, clinical outcome was evaluated without unmasking the Mode 1 treatment conditions. Neurocognitive testing was conducted at preECT baseline, following termination of Mode 1 ECT, following termination of Mode 2, and at two-month and six-month follow-up.
Methods
Columbia University Studies
Four consecutive, single-site, RCTs were conducted at NYSPI/Columbia University, whose Institutional Review Board approved each study. The first study (Study 1) did not include a formal Mode 2 for ECT nonresponders and is not included here [30]. The remaining three studies, each compared four techniques of ECT in a randomized Mode 1 and included a protocol-defined Mode 2 (Table 1). In Study 2 [8], 96 patients were randomized to treatment with BP (1.5 ms) RUL or BL ECT, with dosage either just above seizure threshold (1 x ST) or moderately above threshold (2.5 x ST). In Study 3 [20], 80 patients were randomized to three techniques of BP (1.5 ms) RUL ECT,−1.5 x ST, 2.5 x ST, or 6 x ST,- or to BP BL ECT (2.5 x ST). In Study 4 [15], 90 patients were randomized to RUL (6 x ST) or BL ECT (2.5 x ST), using either an UB (0.3 ms) or BP (1.5 ms) width. After completion of evaluations following the randomized Mode 1, in all three studies, ECT nonresponders were eligible to receive a full second course of ECT, using HD (2.5 x ST) BP (1.5 ms) BL treatment.
Table 1.
Classification and Sample Size of the Randomized Treatment Conditions in the Columbia University Studies
| Weak ECT | Strong ECT | High Dose, BP, BL ECT | |
|---|---|---|---|
| Study 2 | 1.0 x ST, BP, RUL ECT N=23 |
1.0 x ST, BP, BL ECT N=23 |
N=27 |
| 2.5 x ST, BP, RUL ECT N=23 |
|||
| Study 3 | 1.5 x ST, BP, RUL ECT N=20 |
6.0 x ST, BP, RUL ECT N=20 |
N=20 |
| 2.5 x ST, BP, RUL ECT N=20 |
|||
| Study 4 | 2.5 x ST, UB, BL ECT N=23 |
6.0 x ST, UB, RUL ECT N=22 |
N= 23 |
| 6.0 x ST, BP, RUL ECT N=22 |
|||
| Total | N=109 | N=87 | N=70 |
ST = seizure threshold; BP = brief pulse; UB = ultrabrief pulse; BL = bifrontotemporal or bilateral; RUL = right unilateral.
Patients
The same inclusion/exclusion criteria were applied across the Columbia RCTs. Patients met Research Diagnostic Criteria [31] and Diagnostic and Statistical Manual (DSM-IIIR or DMS-IV) criteria [32, 33] for major depressive episode (unipolar or bipolar), had a pretreatment score of 18 or greater on the Hamilton Rating Scale for Depression (HRSD, 24-item) [34], clinical indication for ECT, and provided written informed consent. Patients were excluded with a history of schizophrenia, schizoaffective disorder, other functional psychosis, rapid-cycling bipolar disorder, neurologic illness or insult, alcohol or other drug abuse within the past year, ECT within the past 6 months, or severe medical illness (Clinical Trials: NCT00487500).
Except for lorazepam (up to 3 mg/d), psychotropic medications were discontinued in all patients at least 5 days before starting preECT research evaluations and until 1 week after completing Mode 1 and Mode 2. The medication washout before starting ECT averaged 15.5 ± 7.4 days (30-day upper limit) across the three studies. The average dose of lorazepam during ECT was approximately 1 mg/d in each study.
Electroconvulsive Therapy
Atropine (0.4 mg), methohexital (1.0 mg/kg), and succinylcholine (0.75 mg/kg) were the anesthetic medications. ECT was administered three times per week with bidirectional, rectangular pulse, constant current devices (MECTA Corporation, Tualatin, Oregon, USA). The standard bifrontotemporal and d’Elia electrode placements were used for BL and RUL ECT, respectively [5]. ST was quantified at the first and last treatments using the empirical titration procedure [35]. A masked clinical evaluation team informed the treatment team as to when the last treatment would likely occur. At all other treatments electrical dosage was a specific multiple of the initial ST, as indicated in Table 1. Duration of motor convulsive movements, using the “cuff” technique [36], and two channels of prefrontal electroencephalogram (EEG) (left and right frontopolar-mastoid montage) were recorded. The criterion for an adequate seizure was at least 20 s of tonic-clonic movement or 25 s of EEG seizure activity.
Clinical Evaluations
The patients and the clinical evaluation team (research psychiatrist and social worker) were masked to Mode 1 randomized treatment assignment. The team completed HRSD24 ratings before the first treatment, twice weekly during the treatment course, and within three days and one week after the end of Mode 1. Patients were considered initial responders if their HRSD24 scores decreased at least 60 percent from pretreatment to immediately (24–72 hours) after the final treatment and had a post-treatment score of 16 or lower. Final responders maintained this level of improvement for at least one week after ECT while medication free. Immediate and final remission status were defined as a HRSD24 score of 10 or less at these same time points. The same senior psychiatrist (JP) assessed all patients in both Mode 1 and Mode 2 in each of the three studies.
The number of ECT treatments administered in Mode 1 was determined by the masked clinical evaluation team and based on clinical progress. Patients who showed benefit continued to receive ECT until they were asymptomatic or had no further symptomatic improvement over two treatments. Patients received at least 10 treatments before classification as nonresponders. This minimum was lowered to 8 treatments for those who had HRSD24 reductions of 20% or less.
Regardless of the Mode 1 randomized treatment assignment, nonresponders could receive a second course of ECT, Mode 2, using BP (1.5 ms), BL stimulation, with HD (2.5 times the re-determined ST). Treatment and assessment procedures in Mode 2 were identical to those used in Mode 1, except that Mode 2 treatment was open label. For this report, Mode 2 completers were defined as patients who met initial response or remission criteria or as nonresponders who had at least 6 treatments. The interval between Mode 1 and Mode 2 averaged 6.12 (SD = 2.63) days (range = 2–12 days).
Patients who met final response criteria after Mode 1 or Mode 2 were followed until relapse or for one year. HRSD24 interviews were conducted every two weeks for the first two months after ECT and monthly thereafter. During follow-up, all patients received continuation pharmacotherapy provided by psychiatrists in the community. There was no indication that continuation pharmacotherapy differed for the patients who responded to Mode 1 or Mode 2. As defined previously [8, 9, 37], relapse was declared when HRSD24 scores increased over two consecutive interviews at least one week apart by 10 points or more relative to the score at ECT termination, with a minimum score of at least 16, or if the patient was hospitalized for symptomatic worsening, or presented with psychotic features or suicidal intent.
Cognitive Evaluations
The Columbia studies included extensive neuropsychological testing. For this report, only two measures were examined. The modified Mini Mental State Exam (mMMS) [38] was used as a measure of global cognitive status, while the Columbia University Autobiographical Memory Interview (CUAMI) (full version) [39, 40] served as the measure of retrograde amnesia for autobiographical information. It was hypothesized that receipt of Mode 2 would result in more severe and persistent retrograde amnesia, whereas a deleterious effect on global cognitive status following Mode 2 would only be seen transiently, immediately following this second treatment course. The mMMS was not administered at a six-month follow-up in Study 2. Only Study 4 provided CUAMI data at the post Mode 2 time point and the six-month follow-up.
Classification of Treatment Conditions
As seen in Table 1, the Mode 1 randomized ECT conditions were classified as ”Weak ECT”, “Strong ECT”, or “HD BP BL ECT”. The weak forms of ECT all had final response rates less than 50% and all had significantly inferior antidepressant effects relative to the HD BP BL ECT condition in the same study. The strong forms of ECT all had final response rate greater than 50% and did not differ in antidepressant effects from the HD BP BL ECT condition in the same study.
Optimization of ECT Study
The OPT-ECT study [21, 29] was conducted at Wake Forest University Health Sciences, Washington University in St. Louis, and the Western Psychiatric Institute and Clinic, with Columbia University serving as the coordinating center. The institutional review boards at each site approved the study and all patients provided informed consent. Patients met the same inclusion/exclusion as in the Columbia University studies except that the baseline HRSD24 score was 21 or greater, and patients with a known allergy or contraindication to nortriptyline or venlafaxine were excluded (Clinical Trials: NCT00045916).
Patients were withdrawn from psychotropic medications before starting ECT, other than lorazepam (up to 3 mg/d). Patients were randomized to receive either HD (6 x ST), BP (1 ms), RUL ECT or LD (1.5 x ST), BP (1 mS), BL ECT (Mode 1). They were also randomized to receive nortriptyline, venlafaxine, or placebo using a double-dummy technique, starting the afternoon of the first ECT treatment. ECT methods and clinical evaluation and outcome classification followed the methods described for the Columbia University studies. Patients were evaluated with the HRSD24 by a masked outcome assessor within 72 hours of the end of Mode 1. Preliminary responders and remitters were re-evaluated 4–8 days later to establish a sustained response/remitter status. Patients who were nonresponders after 8 or more treatments in Mode 1 could crossover to Mode 2 and receive HD (2.5 x ST), BP (1 ms), BL ECT, without any change in randomized medication status until the completion of Mode 2. Patients were re-evaluated with the HRSD24 within 72 hours of the end of Mode 2. After completion of ECT, responders could enter either a six-month naturalistic follow-up or a RCT contrasting pharmacotherapy strategies in relapse prevention [29]. However, too few patients who responded to Mode 2 were followed for relapse (n=18) to conduct meaningful analyses of durability of benefit. The neurocognitive battery in the OPT-ECT study also included the mMMS and the short form of the CUAMI (CUAMI-SF). For the mMMS, patients were tested at baseline, following the randomized Mode 1, and at two-month and six-month follow-up. The same schedule was used for the CUAMI-SF, except that as a matter of convenience some patients who received Mode 2 were tested immediately following Mode 1 (n= 18) and some were tested immediately following Mode 2 (n=25).
Statistics
Comparisons of two groups (e.g., Mode 1 only vs. Mode 2) used independent sample t-tests for continuous measures and chi-square analyses for dichotomous variables. For three group comparisons, one-way analyses of variance (ANOVA) were conducted. Prior to conducting t-tests and one-way ANOVAs, the O’Brien [41] test for homogeneity of variance was performed, and Welch’s [42] test was used for comparison of means if inequality was found. HRSD24 scores at baseline, during, and following both Mode 1 and Mode 2 were contrasted using repeated measures analysis of variance, with treatment group classification as a between-subjects term and time point of assessment as the repeated measures factor. The Huynh-Feldt correction was used to adjust degrees of freedom for sphericity [43]. Nonparametric and parametric estimates of the survival distribution were used to contrast treatment groups in rates and timing of relapse. For nonparametric models, the survival distribution functions for each group were computed using the Kaplan-Meier method and contrasted with the log-rank (Mantel-Cox) test [44]. For parametric analyses, simultaneous regression models, with treatment group as a covariate, were fit to the right-censored relapse-time data using the Weibull distribution [37, 45]. All statistical tests were two-tailed (without correction for multiple comparisons) and the alpha level was 0.05.
Results
Columbia University Studies
Mode 2 Participants
Table 2 presents the Mode 1 response rates for the treatment classifications. The response rates among patients randomized to the Weak ECT group were half that of either the Strong or HD BL ECT groups (P<0.0001). Of the 134 Mode 1 final nonresponders, 97 (72.4%) participated in Mode 2. The Weak ECT and Strong ECT groups each had high Mode 2 participation rates (> 75%). However, only 11 of 27 (40.7%) eligible patients treated with HD BP BL ECT in Mode 1 participated in Mode 2 (P=0.0002).
Table 2.
Demographic and Clinical Characteristics of the Mode 2 Samples in the Columbia University Studies
| Variable | Total Sample | Weak ECT | Strong ECT | HD BP BL ECT | P-Value* | |
|---|---|---|---|---|---|---|
| Received Mode 1 | N | 266 | 109 | 87 | 70 | |
| Final Responders Mode 1 | 132 (49.62) | 35 (32.11) | 54 (62.07) | 43 (61.43) | <.0001 | |
| Received Mode 2 | N (% eligible) | 97 (72.39) | 58 (78.38) | 28 (84.85) | 11 (40.74) | 0.0002 |
| Age | Mean±SD | 57.37±16.38 | 60.13±14.86 | 52.30±16.90 | 55.67±11.09 | 0.08 |
| Gender (female) | N (%) | 65 (67.01) | 40 (68.97) | 19 (67.86) | 6 (54.55) | 0.64 |
| Education, yrs | Mean±SD | 14.44±3.43 | 14.36±3.50 | 14.29±3.36 | 15.27±3.41 | 0.70 |
| Full Scale IQ | Mean±SD | 100.71±15.40 | 98.06±15.90 | 102.22±14.50 | 111.88±9.33 | 0.053 |
| Familial socioeconomic status† | Mean±SD | 2.23±1.13 | 2.31±1.23 | 2.21±1.03 | 1.82±0.75 | 0.42 |
| PreECT HRSD | Mean±SD | 33.16±8.26 | 33.78±8.25 | 33.11±8.87 | 30.09±6.12 | 0.40 |
| PreECT BDI | Mean±SD | 36.76±11.94 | 36.27±11.15 | 35.00±13.57 | 35.30±12.18 | 0.90 |
| Bipolar Depression | N (%) | 25 (25.77) | 17 (29.31) | 6 (21.43) | 2 (18.18) | 0.61 |
| Psychotic Depression | N (%) | 35 (36.08) | 25 (43.10) | 7 (25.00) | 3 (27.27) | 0.21 |
| Age at Onset of Mood Disorder | Mean±SD | 27.49±19.10 | 27.62±17.67 | 28.57±22.60 | 24.09±18.10 | 0.81 |
| Length of Current Episode. mo‡¶ | Mean±SD | 19.67±24.29 | 14.06±14.57 | 29.14±33.66 | 25.14±30.46 | 0.07 |
| Number of Previous Episodes‡ | Mean±SD | 3.22±2.84 | 3.21±2.71 | 3.22±3.19 | 3.27±2.87 | 0.99 |
| Number of Previous Hospitalizations‡ | Mean±SD | 1.99±2.03 | 1.74±1.82 | 2.46±2.40 | 2.09±2.02 | 0.30 |
| History of Previous ECT | N (%) | 39 (40.21) | 26 (44.83) | 9 (32.14) | 4 (36.36) | 0.51 |
| Total Number of Medication Trials, Current Episode§ | Mean±SD | 4.83±3.40 | 4.38±3.36 | 5.59±3.60 | 5.14±2.95 | 0.30 |
| Potency of “Best” Antidepressant Trial§ | Mean±SD | 3.11±1.39 | 2.89±1.35 | 3.45±1.51 | 3.45±1.13 | 0.15 |
| Medication Resistant§ | N (%) | 64 (65.98) | 36 (65.98) | 19 (62.07) | 9 (81.82) | 0.43 |
ECT, Electroconvulsive therapy; IQ, intelligence quotient; HRSD, Hamilton Rating Scale for Depression; BDI, Beck Depression Inventory, HD BP BL, High Dosage, Brief Pulse, Bilateral
P-values reflect results of one-way analyses of variance (ANOVA) comparing the three groups for continuous variables and chi-square analyses for categorical variables.
Socioeconomic status was assessed with the Hollingshead Four-Factor Index [62] (1, highest socioeconomic status; 5 lowest socioeconomic status).
Current episode was limited to 120 months; Number of previous episodes and hospitalizations were each limited to 10.
Number of antidepressant medication trials, potency of each trial (scored 1= low, 5 = high), and medication resistance categorization pertain to the current major depressive episode and were evaluated using the Antidepressant Treatment History Form (ATHF) [63].
Welch’s test used to compare means given unequal variances.
Table 2 also presents demographic and clinical characteristics of the Mode 2 sample as a whole and as a function of the Mode 1 treatment classification. The treatment groups tended to differ in current episode duration, with the Weak ECT group having shorter episode length. The Weak ECT group also had numerical advantages in other variables predictive of superior ECT outcome, including age, psychotic subtype, and indices of treatment resistance. These differences presumably reflected the use of ineffective forms of ECT in Mode 1, enriching this group in the characteristics associated with positive ECT response.
Clinical Outcomes Following Mode 1 and Mode 2
Figure 1 presents average HRSD24 scores at preECT baseline, during and following Mode 1, and following Mode 2, for each treatment group. The repeated measures ANOVA yielded a main effect of time, F(2.96, 278.14) = 110.11, P < 0.0001. Across the three groups, HRSD24 scores decreased modestly during and following Mode 1, and then decreased markedly following Mode 2. There was also a significant interaction between treatment group and time, F(5.92, 278.14) = 2.77, P = 0.01. As seen in Figure 1, each of the three treatment groups had a marked and equivalent reduction in HRSD24 scores following Mode 2. The significant interaction was due to the poorer symptomatic response in the Weak ECT group during and following Mode 1. While the Weak ECT group was defined by low rates of Mode 1 response, it was also the case that, among nonresponders, the Weak ECT group had less symptomatic improvement in Mode 1 than the Strong ECT and HD BL ECT groups.
Figure 1.
Patient flow through Mode 1 and Mode 2 treatment with ECT and one-year monitoring for relapse in the Columbia University studies.
Table 3 presents the response and remission rates for the treatment conditions following Mode 2, as well as the number of treatments administered in Mode 1 and Mode 2. Mode 2 resulted in marked and widespread clinical improvement, with 80.4% of the total intent-to-treat sample classified as initial responders, and nearly 60% classified as final remitters. This marked efficacy was observed in each of the treatment groups, who did not differ in response or remission rates or in the number of treatments administered in Mode 1 or Mode 2.
Table 3.
Response and Remission Rates and Number of Treatments in Mode 2 in the Columbia University Studies
| Total Sample | Weak ECT | Strong ECT | High Dose BL ECT | P-Value* | ||
|---|---|---|---|---|---|---|
| Mode 2 Participants | N | 97 | 58 | 28 | 11 | |
| Initial Responder Mode 2 | N (%) | 78 (80.41) | 48 (82.76) | 21 (75.00) | 9 (81.82) | 0.69 |
| Final Responder Mode 2 | N (%) | 67 (69.07) | 40 (68.97) | 20 (71.43) | 7 (63.64) | 0.89 |
| Initial Remitter Mode 2 | N (%) | 74 (76.29) | 46 (79.31) | 19 (67.68) | 9 (81.82) | 0.45 |
| Final Remitter Mode 2 | N (%) | 57 (58.76) | 34 (58.62) | 16 (57.14) | 7 (63.64) | 0.93 |
| Number of Treatments | ||||||
| Mode 1 | Mean±SD | 9.57±2.31 | 9.62±2.58 | 9.79±1.81 | 8.73±1.85 | 0.42 |
| Mode 2 | Mean±SD | 8.07±2.82 | 8.22±3.00 | 7.96±2.65 | 7.55±2.38 | 0.75 |
P-values reflect results of one-way analyses of variance (ANOVA) comparing the three groups for continuous variables and chi-square analyses for categorical variables.
On average, patients received nearly 10 treatments in Mode 1 and an additional 8 treatments in Mode 2. Only 2 patients were excluded from the completer sample since they were classified as initial nonresponders and received fewer than 6 Mode 2 treatments. Thus, all the data reported here pertain to the intent-to-treat sample. Patients who were initial nonresponders following Mode 2 received more Mode 2 treatments (n=19, 9.32±3.14) than Mode 2 initial responders (n=78, 7.77±2.67), t(95) = 2.18, P = 0.03.
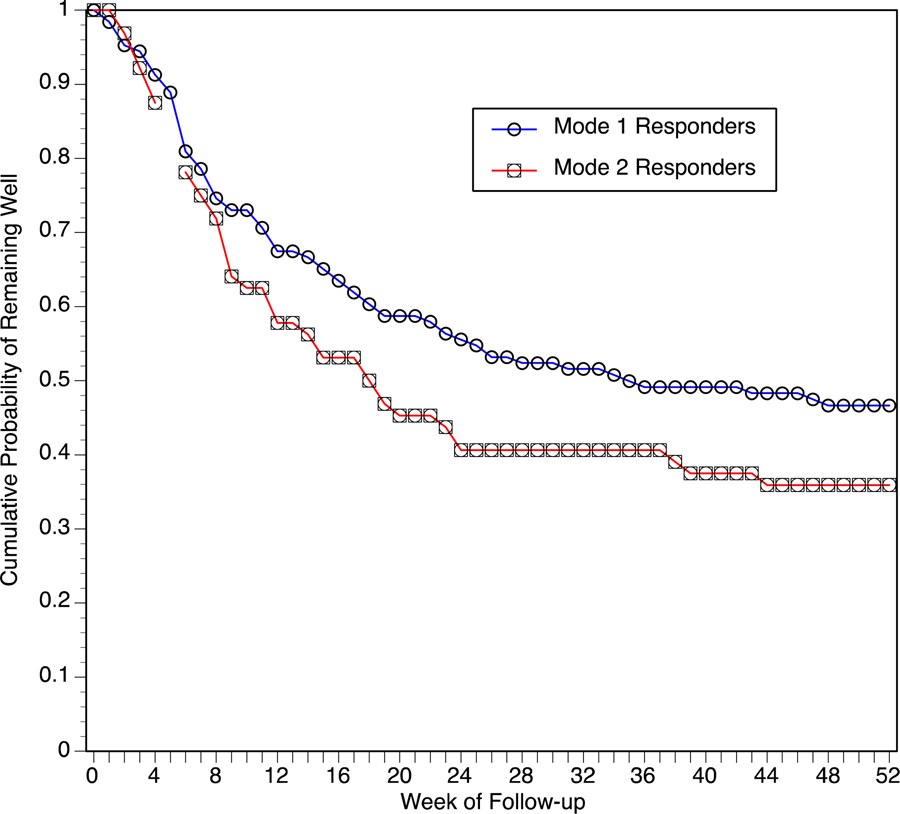
Relapse Following Mode 1 and Mode 2
Of 132 Mode 1 final responders, 126 (95.45%) participated in the one-year clinical monitoring. Of 67 Mode 2 final responders, 64 (95.52%) participated in the one-year clinical monitoring. The Kaplan-Meier survival functions for Mode 1 and Mode 2 samples are presented in Figure 2. Both the parametric (P=0.04) and nonparametric (P=0.06) analyses indicated that survival time was greater in Mode 1 final responders than in Mode 2 final responders. Of the 126 monitored Mode 1 responders, 67 patients (53.2%) met relapse criteria within the year, 56 patients (44.4%) completed without relapse, and 3 patients (2.34%) dropped out during monitoring. Of those who did relapse, the average time to the event was 13.7 ± 11.3 weeks. Of the 64 monitored Mode 2 responders, 41 patients (64.1%) met relapse criteria within the year and 23 patients (35.9%) completed without relapse. Of those who did relapse, the average time to the event was 12.5 ± 10.1 weeks. The three Mode 1 treatment groups did not differ in the likelihood or timing of relapse following response in Mode 2 (data not shown).
Figure 2.
Hamilton Rating Scale for Depression Scores (mean ± SE) before, during, and after Mode 1 (Randomized Phase) and after Mode 2 treatment with ECT, for groups that received weak or strong forms of ECT or high dose bilateral ECT in Mode 1. Asterisks indicate that the three Mode 1 treatment groups differed significantly (P<0.05) in scores at that time point. Mode 1 data are only from patients that participated in Mode 2.
Cognitive Effects of Mode 1 and Mode 2
As seen in Table 4, patients who received only Mode 1 or who also received Mode 2 did not differ in preECT mMMS scores. Across all patients, mMMS scores decreased significantly following Mode 1, paired t(233) = 5.21, P<0.0001. Following Mode 2, there was a substantial and further decrease in mMMS scores relative to performance following Mode 1, paired t(76) = 4.07, P<0.0001. However, at the two-month follow-up patients who received Mode 1 only and those who received Mode 2 did not differ in mMMS scores, and the total sample showed significant improvement relative to the preECT baseline, paired t(163) = 4.56, P<0.0001. There was a considerably smaller sample with six-month follow-up mMMS scores. At this time point, patients who received Mode 2 had lower mMMS scores than patients who received Mode 1 only, F(1, 61) = 5.47, P=0.02. As also seen in Table 4, among patients who received Mode 2, mMMS scores did not differ at any time point as a function of the three classifications of Mode 1 ECT.
Table 4.
Scores on the Modified Mini-Mental State Exam (mMMS) and the Columbia University Autobiographical Memory Interview (CUAMI) for the Mode 1 Only and Mode 2 Samples Across Assessment Time Points in the Columbia University Studies
| Mode 2 Sample |
||||||||
|---|---|---|---|---|---|---|---|---|
| Mode 1 Only | Mode 2 Sample | P-Value* | Weak ECT | Strong ECT | High Dose BL ECT | P-Value* | ||
| mMMS | ||||||||
| Pre-ECT | Mean | 49.72 | 49.58 | 0.86 | 48.83 | 50.21 | 51.55 | 0.42 |
| SD | 5.98 | 6.89 | 7.18 | 6.45 | 6.55 | |||
| N | 159 | 92 | 53 | 28 | 11 | |||
| Post Mode 1 | Mean | 47.5 | 48.87 | 0.07 | 49.10 | 48.70 | 48.27 | 0.13 |
| SD | 7.57 | 6.76 | 7.58 | 5.01 | 7.21 | |||
| N | 149 | 86 | 48 | 27 | 11 | |||
| Post Mode 2 | Mean | 45.37 | 44.00 | 47.56 | 46.50 | 0.43 | ||
| SD | 6.99 | 7.42 | 5.83 | 6.49 | ||||
| N | 83 | 48 | 25 | 10 | ||||
| Two-Month Follow-up | Mean | 51.05 | 50.6 | 0.29 | 50.14 | 50.88 | 52.00 | 0.82 |
| SD | 5.42 | 5.45 | 5.53 | 5.84 | 4.54 | |||
| N | 104 | 60 | 35 | 17 | 8 | |||
| Six-Month Follow-up† | Mean | 52.26 | 51.33 | 0.02 | 48.30 | 54.43 | 53.50 | 0.12 |
| SD | 4.76 | 4.95 | 5.52 | 1.72 | 2.65 | |||
| N | 43 | 21 | 10 | 7 | 4 | |||
| CUAMI Consistency Score | ||||||||
| Post Mode 1 | Mean | 66.6 | 69.91 | 0.21 | 72.78 | 67.24 | 60.77 | 0.03 |
| SD | 14.14 | 12.07 | 10.43 | 12.18 | 15.66 | |||
| N | 135 | 76 | 46 | 22 | 8 | |||
| Post Mode 2‡ | Mean | 50.09 | 41.95 | 55.30 | 51.38 | 0.34 | ||
| SD | 19.41 | 16.10 | 18.60 | 37.92 | ||||
| N | 22 | 8 | 12 | 2 | ||||
| Two-Month Follow-up | Mean | 71.51 | 61.52 | 0.0002 | 62.87 | 58.17 | 63.35 | 0.67 |
| SD | 11.24 | 19.02 | 17.34 | 24.32 | 11.04 | |||
| N | 87 | 57 | 34 | 17 | 6 | |||
| Six-Month Follow-up‡ | Mean | 72.03 | 59.62 | 0.01 | 50.24 | 70.75 | 52.63 | 0.07 |
| SD | 11.55 | 13.71 | 9.12 | 10.92 | -- | |||
| N | 26 | 9 | 4 | 4 | 1 | |||
P-values reflect independent t-tests comparing the Mode 1 and Mode 2 groups or one-way analyses of variance (ANOVAs) comparing the three Mode 1 ECT classifications.
mMMS assessment at six-month follow-up was not conducted in Study 2.
CUAMI data were available only for Study 4 at the post Mode 2 and six-month follow-up assessments.
As seen in Table 4, patients who did or did not receive Mode 2 did not differ in CUAMI consistency scores following Mode 1. There was a marked drop in these scores following Mode 2, t(21) = 5.87, P<0.0001. At two-month follow-up, consistency scores significantly improved in the Mode 1 only group relative to post Mode 1, t(83) = 3.39, P<0.0001, while these scores deteriorated in the Mode 2 group, t(51) = 4.13, P<0.0001. Consequently, the two groups differed in two-month follow-up consistency scores, F(1, 141) = 14.87, P=0.0002. While a considerably smaller sample had six-month follow-up testing, the same pattern emerged. The Mode 1 only group had improved scores relative to post Mode 1, t(24) = 2.50, P=0.02, while the Mode 2 group tended to show deterioration, t(8) = 1.96, P=0.09. The two groups differed in these six-month follow-up scores, F (1, 32) = 6.72, P=0.01.
As indicated in Table 4, CUAMI consistency scores following Mode 1 differed among the three ECT groups who went on to receive Mode 2, F(2, 72) = 3.80, P=0.007. Post hoc tests indicated that the Weak ECT group had higher scores than the HD BP BL ECT group, while the Strong ECT group was intermediate and did not differ from the other two groups. At the two-month follow-up, after receiving Mode 2, the Weak ECT group, paired t(31) = 3.52, P=0.001, and the Strong ECT group, paired t(14) = 2.38, P=0.03, showed significant deterioration, and the three ECT groups did not differ from each other.
Optimization of ECT Study
Table 5 presents the response rates for the two ECT treatment groups in Mode 1 and rates of participation in Mode 2. Of 156 Mode 1 nonresponders, 59 patients (37.8%) received Mode 2, with no difference between the Mode 1 treatment groups in rates of Mode 2 participation. Among Mode 2 participants, there were no significant differences between the Mode 1 treatment groups in baseline demographic and clinical features (Table 5).
Table 5.
Demographic and Clinical Characteristics of the Mode 2 Samples in the Optimization of ECT (OPT-ECT) Study
| Variable | Total Sample | 6 x ST RUL | 1.5 x ST BL | P-Value* | |
|---|---|---|---|---|---|
| Received Mode 1 | N | 319 | 155 | 164 | |
| Responders Mode 1 | N (%) | 163 (51.10) | 85 (54.84) | 78 (47.56) | 0.19 |
| Received Mode 2 | N (% eligible) | 59 (37.82) | 30 (42.86) | 29 (33.72) | 0.24 |
| Age | Mean±SD | 49.56±15.93 | 49.77±16.67 | 49.34±15.42 | 0.92 |
| Gender (female) | N (%) | 34 (57.63) | 19 (63.33) | 15 (51.72) | 0.37 |
| Education, yr | Mean±SD | 14.24±2.66 | 14.60±2.58 | 13.86±2.74 | 0.29 |
| PreECT HRSD | Mean±SD | 30.80±5.83 | 30.13±6.30 | 31.48±5.32 | 0.38 |
| PreECT BDI | Mean±SD | 38.56±9.02 | 39.12±8.88 | 38.38±9.98 | 0.78 |
| Bipolar Depression | N (%) | 13 (22.03) | 6 (20.00) | 7 (24.14) | 0.70 |
| Psychotic Depression | N (%) | 13 (22.03) | 8 (26.67) | 5 (17.24) | 0.38 |
| Length of Current Episode, mo † | Mean±SD | 14.26±17.91 | 15.53±16.19 | 12.89±19.79 | 0.58 |
| Total Number of Medication Trials§ | Mean±SD | 5.66±2.85 | 5.47±2.83 | 5.86±2.91 | 0.60 |
| Potency of “Best” Antidepressant Trial§ | Mean±SD | 3.27±1.17 | 3.20±1.13 | 3.35±1.23 | 0.65 |
| Medication Resistant§ | N (%) | 45 (76.27) | 23 (76.67) | 22 (75.86) | 0.94 |
| Number of Failed Adequate Trials§ | Mean±SD | 1.47±1.29 | 1.23±1.23 | 1.72±1.39 | 0.15 |
HRSD, Hamilton Rating Scale for Depression; BDI, Beck Depression Inventory,
P-values reflect results of independent t-tests or chi-square tests comparing the two Mode 1 randomized groups
Current episode duration was limited to 120 months;
Number of antidepressant medication trials, potency of each trial (scored 1= low, 5 = high), medication resistance categorization and number of failed adequate trials pertain to the current major depressive episode and were evaluated using the Antidepressant Treatment History Form (ATHF) [63]
Mode 2 participants had average HRSD24 scores of 30.80 ± 5.83 at preECT baseline, 22.20 ± 6.58 after the randomized Mode 1 phase, and 16.64 ± 11.40 after Mode 2. The repeated measures ANOVA yielded a main effect of time, F (1.64, 93.44) = 70.16, P<0.0001, but no effects involving Mode 1 treatment group. As seen in Table 6, 45.8% of participants were responders and 42.4% were remitters following Mode 2, also with no significant differences between the Mode 1 treatment groups. Mode 2 participants averaged nearly 9.5 treatments in Mode 1 and 5 treatments in Mode 2.
Table 6.
Response and Remission Rates and Number of Treatments in Mode 2 in the Optimization of ECT Study
| Total Sample | 6 x ST RUL | 1.5 x ST BL | P-Value* | ||
|---|---|---|---|---|---|
| Mode 2 Participants | N | 59 | 30 | 29 | |
| Responder Mode 2 | N (%) | 27 (45.76) | 15 (50.00) | 12 (41.38) | 0.51 |
| Remitter Mode 2 | N (%) | 25 (42.37) | 14 (46.67) | 11 (37.93) | 0.50 |
| Number of Treatments | |||||
| Mode 1 | Mean±SD | 9.47±1.75 | 9.50±1.48 | 9.45±2.01 | 0.91 |
| Mode 2 | Mean±SD | 4.90±2.97 | 5.37±3.15 | 4.41±2.75 | 0.22 |
BL, Bilateral; RUL, Right Unilateral; ST, seizure threshold
P-values reflect results of independent t-tests or chi-square tests comparing the two Mode 1 randomized groups
Of the 59 Mode 2 participants, 22 nonresponders received fewer than 6 Mode 2 treatments. The remaining 37 patients were considered completers, with 27 (73.0%) patients classified as Mode 2 responders and 25 (67.6%) patients classified as Mode 2 remitters.
There were no effects of receiving Mode 2 on mMMS scores at any time point, and the randomized Mode 1 ECT groups who received Mode 2 did not also differ in these scores at any time point (Table 7). In contrast, patients who received Mode 2 had significantly lower CUAMI-SF scores at two-month (P=0.005) and six-month (P=0.02) follow-ups compared to patients treated with Mode 1 only. In the Mode 2 sample, the form of ECT received in Mode 1 had no impact on CUAMI-SF scores at any time point.
Table 7.
Scores on the Modified Mini-Mental State Exam (mMMS) and the Columbia University Autobiographical Memory Interview-Short Form (CUAMI-SF) for the Mode 1 Only and Mode 2 Samples Across Assessment Time Points in the Optimization of ECT (OPT-ECT) Study
| Mode 2 Sample |
|||||||
|---|---|---|---|---|---|---|---|
| Mode 1 Only | Mode 2 Sample | P-Value* | 6 x ST RUL | 1.5 x ST BL | P-Value* | ||
| mMMS | |||||||
| Pre-ECT | Mean | 50.10 | 48,31 | 0.11 | 47.00 | 49.62 | 0.29 |
| SD | 6.79 | 8,87 | 8.88 | 8.84 | |||
| N | 226 | 52 | 26 | 26 | |||
| Post Mode 1 | Mean | 47.29 | 47.77 | 0.63 | 46.86 | 48.97 | 0.22 |
| SD | 7.10 | 6.10 | 6.65 | 5.24 | |||
| N | 181 | 52 | 29 | 23 | |||
| Two-Month Follow-up | Mean | 51.54 | 51.38 | 0.89 | 50.00 | 52.75 | 0.19 |
| SD | 5.50 | 5.01 | 5.56 | 4.18 | |||
| N | 112 | 24 | 12 | 12 | |||
| Six-Month Follow-up | Mean | 51.69 | 52.63 | 0.35 | 51.55 | 53.53 | 0.29 |
| SD | 4.48 | 4.33 | 5.30 | 3.26 | |||
| N | 98 | 24 | 11 | 13 | |||
| CUAMI Consistency Score | |||||||
| Post Mode 1 | Mean | 59.27 | 56.86 | 0.46 | 56.28 | 55.22 | 0.90 |
| SD | 20.16 | 18.29 | 20.33 | 16.06 | |||
| N | 178 | 18 | 11 | 7 | |||
| Post Mode 2 | Mean | 49.43 | 51.24 | 47.75 | 0.60 | ||
| SD | 16.16 | 15.08 | 17.54 | ||||
| N | 25 | 12 | 13 | ||||
| Two-Month Follow-up | Mean | 67.09 | 54.75 | 0.005 | 53.01 | 57.03 | 0.60 |
| SD | 15.41 | 18.18 | 20.43 | 15.52 | |||
| N | 105 | 23 | 13 | 10 | |||
| Six-Month Follow-up | Mean | 68.44 | 59.40 | 0.02 | 57.57 | 61.23 | 0.59 |
| SD | 15.96 | 15.26 | 18.92 | 11.13 | |||
| N | 97 | 22 | 11 | 11 | |||
BL, Bilateral; RUL, Right Unilateral; ST, seizure threshold
P-values reflect independent t-tests comparing the Mode 1 only and Mode 2 samples or the Mode 2 sample as a function of the randomized Mode 1 treatment assignment.
Discussion
This report contains the largest samples of ECT nonresponders that were prospectively followed through subsequent treatment. Clinical outcome in ECT research is usually assessed within a few days of ECT completion, corresponding to the initial outcome assessment in the Columbia University studies. The Columbia University studies and the OPT-ECT trial used more conservative criteria, with final response and remission based on evaluations one week following treatment termination. Nonetheless, in the Columbia University studies, among patients who were nonresponders after completing a first course of ECT (Mode 1), approximately 70% met final response criteria and 60% met final remission criteria after a second course of ECT (Mode 2). In the OPT-ECT study, rates of Mode 2 participation were lower, and patients averaged considerably fewer Mode 2 treatments (4.90 ± 2.97) than in the Columbia University studies (8.07 ± 2.82). Nonetheless, in the OPT-ECT study, more than 40% of Mode 2 participants were still classified as remitters. Furthermore, the OPT-ECT sample was restricted to completers, patients who received at least six Mode 2 treatments before nonresponder classification, the response and remission rates were comparable to those in the Columbia University studies.
Patients who do not benefit from ECT present a treatment dilemma. ECT is widely considered the most effective of all antidepressant treatments, and patients are typically referred for ECT due to resistance to several courses of pharmacotherapy. Indeed, the literature on optimal treatment of ECT nonresponders is largely limited to case reports, with little empirical support for particular strategies [46, 47]. This report provides compelling evidence that administration of additional ECT can produce remission in a substantial percentage of initial ECT non-responders, and perhaps the majority of them. We also found a suggestion that patients who responded to Mode 2 treatment were more likely to relapse during the subsequent year than patients who had responded to their initial ECT course. However, this was a relatively small effect, amounting to an approximate 10% difference in relapse rates. Thus, not only did Mode 2 result in frequent and substantial clinical improvement. its durability of benefit was nearly the same as in patients who responded to their initial ECT course.
In this report, high dosage, brief pulse, bilateral (HD BP BL) ECT was used for Mode 2 treatment. Undoubtedly, some will interpret our findings as indicating that this form of ECT is the preferred technique for additional treatment in ECT nonresponders. We note, however, that the clinical benefits of Mode 2 treatment were independent of the type of ECT administered in Mode 1, including receipt of the very same technique or receipt of other forms of ECT with established efficacy. This underscores the possibility that a critical factor subserving the efficacy of Mode 2 was the provision of additional ECT treatment, and not necessarily the specific technique used. Indeed, for patients who were deemed nonresponders after HD BP BL ECT in Mode 1, the most likely explanation for their improvement after receiving the very same treatment in Mode 2 is that simply administering more sessions of ECT can have salutary impact. While it is possible that patients who did not benefit from HD BP BL ECT in Mode 1 differed from the other patients in factors predictive of ECT outcome, this was not evident when the groups that received Mode 2 were contrasted in an extensive set of clinical features (Table 2).
This perspective suggests that in current practice a significant proportion of patients may be deemed as not benefiting from ECT because treatment is terminated prematurely. The earliest observations on the efficacy of ECT in mood disorders reported that the modal ECT course comprised 4–6 treatments [48]. Over the ensuing decades, this range increased to 6–8 treatments [49], and then to 8–10 treatments [13, 25]. Similarly, rates of relapse following ECT have progressively increased from the 1960’s until the present [50]. We suggest that these secular changes reflect the increasing representation and extent of treatment resistance in ECT samples. Previous research has suggested that treatment resistance may both decrease the likelihood of benefiting from ECT [51–53] and increase the likelihood of relapse, if clinical benefit is achieved [8, 15, 20, 29, 37, 54]. It is also probable that treatment resistance impacts on the length of the treatment needed to achieve response and remission. At a practical level, these data suggest that a substantial number of depressed patients would benefit from longer courses of treatment than had previously been considered, perhaps on the order of 15–20 sessions.
The findings from the Columbia University studies and the OPT-ECT RCT were consistent in indicating that provision of Mode 2 had only a short-term impact on mMMS scores, with no long-term sequalae. In contrast, scores on the CUAMI, a measure of retrograde amnesia for autobiographical information, were more impaired both two months and six months following ECT completion in patients treated with Mode 2 than patients who only received Mode 1. This pattern is line with observations that most cognitive functions, including global cognitive status, return to baseline or better within a few days of ECT [25, 55], but retrograde amnesia for autobiographical information is often persistent, as well as sensitive to treatment parameters [24]. Thus, the provision of Mode 2 clearly had a cost, greater persistent retrograde amnesia for aspects of one’s life.
This report demonstrates that the provision of additional ECT with the HD BP BL technique is often markedly effective in patients identified as ECT nonresponders. The extent of benefit was greater in the Columbia studies than the OPT-ECT trial. The stronger efficacy in the Columbia studies might be attributable to the use of longer courses of Mode 2 treatment. However, the Columbia and OPT-ECT samples were differed in other important respects. The OPT-ECT sample contained both inpatients and outpatients who were recruited from individuals routinely scheduled for ECT at the three academic hospitals. The Columbia University samples were inpatients who had consented to participate in ECT research as a requirement for their hospitalization. The OPT-ECT study was conducted under the constraints imposed by the patients’ personal medical insurance, including number of treatments and length of stay, while the Columbia University studies were conducted in a dedicated research unit where care was provided without charge and with no limit on length of stay. These and other differences make comparison of the clinical outcomes tenuous.
This report also documents that the form of Mode 2 treatment used in these studies exerted a cost in terms of more persistent and severe retrograde amnesia. This is the type of memory deficit that patients often report as distressing [56–59]. Consequently, an urgent unanswered issue in ECT practice is identification of an optimal switching strategy that maintains the level of efficacy observed in Mode 2 with HD BP BL ECT, yet avoids the negative cognitive consequences of this intervention. This report provides strong justification for future RCTs that address whether, when, and how to best alter ECT treatment technique given inadequate benefit.
This study has several limitations. First, Mode 2 was open label, and only the form of treatment received in Mode 1 was kept masked. The strong efficacy seen with Mode 2 treatment might be attributed to a bias in outcome assessors. This is unlikely since the same clinical evaluation teams conducted assessments in both Mode 1 and Mode 2, and in the Columbia studies, the masked assessments were highly sensitive to the form of ECT administered. In addition, the major outcomes reported here based on observer ratings were consistent with the patients’ self-ratings of depression severity following Mode 1 and Mode 2 (data not shown).
Another limitation in the Columbia University studies was the relatively low rate of Mode 2 participation by patients randomized to HD BP BL ECT in Mode 1. In the Columbia studies, only 40.7% of such patients participated in Mode 2, whereas 80.4% of “Weak ECT” and “Strong ECT” Mode 1 nonresponders participated. The relatively small sample of patients treated with HD BP BL ECT in both Mode 1 and Mode 2 limits confidence in the overall outcomes of this particular subgroup. Unfortunately, we did not track why specific patients did not participate in Mode 2, but it is likely that short-term cognitive effects were a major contributing factor. The fact that a substantial number of patients ultimately benefit from longer than usual courses of ECT is another reason practitioners might consider initiating ECT with a technique that results in minimal cognitive impairment in order to limit premature dropout
Conclusions
In the largest samples of ECT nonresponders ever prospectively followed, we found that provision of a second course of ECT resulted in marked clinical benefit, with remission rates as high as commonly reported for patients receiving their initial course of ECT, and likely much higher than would be achieved with psychopharmacological interventions [60, 61]. The second course used HD BP BL treatment, a form of ECT often considered the “gold standard” for efficacy. However, we suggest that the remarkable efficacy obtained in Mode 2 was at least in part due to the provision of additional ECT treatments and not necessarily the switch to this specific treatment technique. This issue is especially critical to resolve since we also demonstrated that the second course of ECT using this technique resulted in increased and persistent retrograde amnesia. Thus, while we have shown that many ECT nonresponders can achieve remission when given additional ECT, specifically high dose bilateral ECT, whether treatment technique should be changed, and the nature and timing of the optimal switch strategy, need replication and further investigation.
Figure 3.
Kaplan-Meier estimates of the proportion of patients who remained well during the year following ECT as a function of responding to Mode 1 or Mode 2.
Highlights.
In two samples, depressed patients who received a second course of ECT after not responding to a full first course had remarkably high response and remission rates.
Clinical outcome following the second course, using high dose bilateral ECT, was independent of the form of ECT used in the first course.
Patients who received a second course of ECT had more severe retrograde amnesia for autobiographical information immediately, two months and six months after ECT completion.
Many patients may need longer ECT courses to show clinical benefit.
Randomized trials are needed to test optimal methods for when and how to change ECT treatment technique
Acknowledgments
Funding
The three studies at Columbia University were each supported by NIMH R01 MH35636:01-27 (Dr. Sackeim). The OPT-ECT study was supported by NIMH R01 MH61609 for the coordinating center (Dr. Sackeim), and NIMH RO1 MH61594 for the Wake Forest University recruiting site (Dr. McCall), NIMH RO1 MH61621 for the Washington University in St. Louis recruiting site (Dr. Isenberg), and NIMH R01 MH61591 for the Western Psychiatric Institute and Clinic recruiting site (Dr. Haskett). The OPT-ECT study was also supported by a grant from Wyeth Pharmaceuticals for medication supplies. The MECTA Corporation provided the ECT devices used in the Columbia and OPT-ECT studies.
Dr. Sackeim serves as a scientific adviser to LivaNova PLC, MECTA Corporation, and Neuronetics Inc. He receives honoraria and royalties from Elsevier, Inc. and Oxford University Press. He is the inventor on non-remunerative US patents for Focal Electrically-Administered Seizure Therapy (FEAST), titration in the current domain in ECT, and the adjustment of current in ECT devices, each held by the MECTA Corporation. He is also the originator of magnetic seizure therapy (MST).
Dr. Devanand has served as a scientific adviser to Acadia, Eisai, Genentech, Grifols, Neuronix, and BXcel Therapeutics.
Dr. Mulsant currently receives research support from Brain Canada, the Canadian Institutes of Health Research, the CAMH Foundation, the Patient-Centered Outcomes Research Institute (PCORI), the US National Institute of Health (NIH), Capital Solution Design LLC (software used in a study funded by CAMH Foundation), and HAPPYneuron (software used in a study funded by Brain Canada). Within the past five years, he has also received research support from Bristol-Myers Squibb (medications for a NIH-funded clinical trial), Eli Lilly (medications for a NIH-funded clinical trial), and Pfizer (medications for a NIH-funded clinical trial). He directly own stocks of General Electric (less than $5,000).
Dr. McCall has been an advisor for Sage, Janssen, Sunovion, and Jazz, receives honoraria from Wolters Kluwer, and has research supported by Vistagen, PCORI, MECTA Corporation, and Merck.
Abbreviations:
- BF
bifrontal
- BL
bifrontotemporal or bilateral
- BP
brief pulse
- ECT
electroconvulsive therapy
- EEG
electroencephalogram
- HD
high dose
- HRSD
Hamilton Rating Scale for Depression
- RCT
randomized controlled trial (RCT)
- RUL
right unilateral
- ST
seizure threshold
- UB
ultrabrief pulse
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflicts of Interest
Drs. Prudic, Nobler, Haskett, and Rosenquist have no conflicts of interest to declare.
References
- 1.Sackeim HA. Modern Electroconvulsive Therapy: Vastly Improved yet Greatly Underused. JAMA Psychiatry. 2017;74(8):779–80. [DOI] [PubMed] [Google Scholar]
- 2.Weiner RD. ECT and seizure threshold: effects of stimulus wave form and electrode placement. Biol Psychiatry. 1980;15(2):225–41. [PubMed] [Google Scholar]
- 3.Scott AI, Rodger CR, Stocks RH, Shering AP. Is old-fashioned electroconvulsive therapy more efficacious? A randomised comparative study of bilateral brief-pulse and bilateral sine-wave treatments. Br J Psychiatry. 1992;160:360–4. [DOI] [PubMed] [Google Scholar]
- 4.Kellner CH, Knapp R, Husain MM, Rasmussen K, Sampson S, Cullum M, et al. Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. British Journal of Psychiatry.196:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. The Practice of ECT: Recommendations for Treatment, Training and Privileging. Second Edition. Washington, D.C.: American Psychiatric Press; 2001. [Google Scholar]
- 6.Weiss A, Hussain S, Ng B, Sarma S, Tiller J, Waite S, et al. Royal Australian and New Zealand College of Psychiatrists professional practice guidelines for the administration of electroconvulsive therapy. Aust N Z J Psychiatry. 2019:4867419839139. [DOI] [PubMed]
- 7.Sackeim HA. The convulsant and anticonvulsant properties of electroconvulsive therapy: towards a focal form of brain stimulation. Clinical Neuroscience Review. 2004;4:39–57. [Google Scholar]
- 8.Sackeim HA, Prudic J, Devanand DP, Kiersky JE, Fitzsimons L, Moody BJ, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993;328(12):839–46. [DOI] [PubMed] [Google Scholar]
- 9.Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Archives of General Psychiatry. 2000;57(5):425–34. [DOI] [PubMed] [Google Scholar]
- 10.McCall WV, Reboussin DM, Weiner RD, Sackeim HA. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: acute antidepressant and cognitive effects. Archives of General Psychiatry. 2000;57(5):438–44. [DOI] [PubMed] [Google Scholar]
- 11.Galletly C, Clarke P, Paterson T, Rigby A, Gill S. Practical considerations in the use of ultrabrief ECT in clinical practice. J ECT. 2014;30(1):10–4. [DOI] [PubMed] [Google Scholar]
- 12.Loo CK, Katalinic N, Martin D, Schweitzer I. A review of ultrabrief pulse width electroconvulsive therapy. Ther Adv Chronic Dis. 2012;3(2):69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellner CH, Husain MM, Knapp RG, McCall WV, Petrides G, Rudorfer MV, et al. Right Unilateral Ultrabrief Pulse ECT in Geriatric Depression: Phase 1 of the PRIDE Study. Am J Psychiatry. 2016;173(11):1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tor PC, Bautovich A, Wang MJ, Martin D, Harvey SB, Loo C. A systematic review and meta-analysis of brief versus ultrabrief right unilateral electroconvulsive therapy for depression. J Clin Psychiatry. 2015. [DOI] [PubMed]
- 15.Sackeim HA, Prudic J, Nobler MS, Fitzsimons L, Lisanby SH, Payne N, et al. Effects of pulse width and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(2):71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapidus KA, Kellner CH. When to switch from unilateral to bilateral electroconvulsive therapy. Journal of ECT. 2011;27(3):244–6. [DOI] [PubMed] [Google Scholar]
- 17.Kellner CH, Farber KG. The Role of Bilateral ECT When Right Unilateral ECT Is Inferior. Am J Psychiatry. 2016;173(7):731. [DOI] [PubMed] [Google Scholar]
- 18.McLoughlin DM. Response to Kellner and Farber: Addressing Crossover of High-Dose Right Unilateral ECT to Bitemporal ECT. Am J Psychiatry. 2016;173(7):731–2. [DOI] [PubMed] [Google Scholar]
- 19.Abrams R Electroconvulsive Therapy. Fourth ed. New York: Oxford University Press; 2002. [Google Scholar]
- 20.Sackeim HA, Prudic J, Devanand DP, Nobler MS, Lisanby SH, Peyser S, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57(5):425–34. [DOI] [PubMed] [Google Scholar]
- 21.Sackeim HA, Dillingham EM, Prudic J, Cooper T, McCall WV, Rosenquist P, et al. Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes: short-term efficacy and adverse effects. Archives of General Psychiatry. 2009;66(7):729–37. [DOI] [PubMed] [Google Scholar]
- 22.Semkovska M, Landau S, Dunne R, Kolshus E, Kavanagh A, Jelovac A, et al. Bitemporal Versus High-Dose Unilateral Twice-Weekly Electroconvulsive Therapy for Depression (EFFECT-Dep): A Pragmatic, Randomized, Non-Inferiority Trial. Am J Psychiatry. 2016;173(4):408–17. [DOI] [PubMed] [Google Scholar]
- 23.Kolshus E, Jelovac A, McLoughlin DM. Bitemporal v. high-dose right unilateral electroconvulsive therapy for depression: a systematic review and meta-analysis of randomized controlled trials. Psychol Med. 2017;47(3):518–30. [DOI] [PubMed] [Google Scholar]
- 24.Sackeim HA. Autobiographical Memory and Electroconvulsive Therapy: Do Not Throw Out the Baby. J ECT. 2014;30:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sackeim HA, Prudic J, Fuller R, Keilp J, Lavori PW, Olfson M. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology. 2007;32(1):244–54. [DOI] [PubMed] [Google Scholar]
- 26.Tew JD Jr., Mulsant BH, Haskett RF, Dolata D, Hixson L, Mann JJ. A randomized comparison of high-charge right unilateral electroconvulsive therapy and bilateral electroconvulsive therapy in older depressed patients who failed to respond to 5 to 8 moderate-charge right unilateral treatments. J Clin Psychiatry. 2002;63(12):1102–5. [DOI] [PubMed] [Google Scholar]
- 27.McCall WV, Reboussin DM, Weiner RD, Sackeim HA. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: acute antidepressant and cognitive effects. Arch Gen Psychiatry. 2000;57(5):438–44. [DOI] [PubMed] [Google Scholar]
- 28.McCall WV, Dunn A, Rosenquist PB, Hughes D. Markedly suprathreshold right unilateral ECT versus minimally suprathreshold bilateral ECT: antidepressant and memory effects. J Ect. 2002;18(3):126–9. [DOI] [PubMed] [Google Scholar]
- 29.Prudic J, Haskett RF, McCall WV, Isenberg K, Cooper T, Rosenquist PB, et al. Pharmacological strategies in the prevention of relapse after electroconvulsive therapy. Journal of ECT. 2013;29(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sackeim HA, Decina P, Kanzler M, Kerr B, Malitz S. Effects of electrode placement on the efficacy of titrated, low-dose ECT. Am J Psychiatry. 1987;144(11):1449–55. [DOI] [PubMed] [Google Scholar]
- 31.Spitzer R, L, Endicott J, Robins E. Research diagnostic criteria: Rationale and reliability. Arch Gen Psychiatry. 1978;35:773–82. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association. Diagnositc and Statistical Manual of Mental Disorders, Third Edition - Revised. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, D. C.: American Psychiatric Association; 1994. [Google Scholar]
- 34.Hamilton M Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–96. [DOI] [PubMed] [Google Scholar]
- 35.Sackeim HA, Decina P, Prohovnik I, Malitz S. Seizure threshold in electroconvulsive therapy. Effects of sex, age, electrode placement, and number of treatments. Arch Gen Psychiatry. 1987;44(4):355–60. [DOI] [PubMed] [Google Scholar]
- 36.Fink M, Johnson L. Monitoring the duration of electroconvulsive therapy seizures: ‘cuff’ and EEG methods compared. Arch Gen Psychiatry. 1982;39(10):1189–91. [DOI] [PubMed] [Google Scholar]
- 37.Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA. 2001;285(10):1299–307. [DOI] [PubMed] [Google Scholar]
- 38.Stern Y, Sano M, Pauson J, Mayeux R. Modified mini-mental status examination: validity and reliability. Neurology. 1987;37 (suppl 1):179.3808297 [Google Scholar]
- 39.McElhiney MC, Moody BJ, Steif BL, Prudic J, Devanand DP, Nobler MS, et al. Autobiographical memory and mood: Effects of electroconvulsive therapy. Neuropsychology. 1995;9:501–17. [Google Scholar]
- 40.Sackeim HA. Autobiographical memory and electroconvulsive therapy: do not throw out the baby. J ECT. 2014;30(3):177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien RG. A general ANOVA method for robust test of additive models for variance. Journal of the American Statistical Association. 1979;74:877–80. [Google Scholar]
- 42.Welch BL. The generalization of “Student’s” problem when several different population variances are involved. Biometrika. 1947;34(1–2):28–35. [DOI] [PubMed] [Google Scholar]
- 43.Huynh HS, Feldt LS. Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs. J Educ Statistics. 1976;1:69–82. [Google Scholar]
- 44.Peto R, Peto J. Asymptomatically efficient rank invariant procedure. J R Statist Soc Series A. 1972;135:185–207. [Google Scholar]
- 45.Kalbfleisch JD, Prentice RL. Survival Models and Data Analysis. New York: John Wiley; 1980. [Google Scholar]
- 46.Sackeim HA, Prudic J, Devanand DP . Treatment of medication-resistant depression with electroconvulsive therapy In: Tasman A, Goldfinger SM, Kaufmann CA, editors. Annual Review of Psychiatry, Volume 9 Washington, D.C.: American Psychiatric Press; 1990. p. 91–115. [Google Scholar]
- 47.Zimmerman M, Coryell W, Pfohl B, Stangl D. What happens when ECT does not work? A prospective follow-up study of ECT failures. Ann Clin Psychiatry. 1990;2:47–51. [Google Scholar]
- 48.Kalinowsky L, Hoch PH. Shock treatments and other somatic procedures in psychiatry. Grune & Straton,New York: 1946;0:−. [Google Scholar]
- 49.Fink M Convulsive Therapy: Theory and Practice. New York: Raven Press; 1979. [Google Scholar]
- 50.Jelovac A, Kolshus E, McLoughlin DM. Relapse following successful electroconvulsive therapy for major depression: a meta-analysis. Neuropsychopharmacology. 2013;38(12):2467–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prudic J, Sackeim HA, Devanand DP. Medication resistance and clinical response to electroconvulsive therapy. Psychiatry Res. 1990;31(3):287–96. [DOI] [PubMed] [Google Scholar]
- 52.Prudic J, Haskett RF, Mulsant B, Malone KM, Pettinati HM, Stephens S, et al. Resistance to antidepressant medications and short-term clinical response to ECT. Am J Psychiatry. 1996;153(8):985–92. [DOI] [PubMed] [Google Scholar]
- 53.Heijnen WT, Birkenhager TK, Wierdsma AI, van den Broek WW. Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol. 2010;30(5):616–9. [DOI] [PubMed] [Google Scholar]
- 54.Sackeim HA, Prudic J, Devanand DP, Decina P, Kerr B, Malitz S. The impact of medication resistance and continuation pharmacotherapy on relapse following response to electroconvulsive therapy in major depression. J Clin Psychopharmacol. 1990;10(2):96–104. [DOI] [PubMed] [Google Scholar]
- 55.Semkovska M, McLoughlin DM. Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biological Psychiatry. 2010;68(6):568–77. [DOI] [PubMed] [Google Scholar]
- 56.Freeman C, Weeks D, Kendell R. ECT: II: patients who complain. Br J Psychiatry. 1980;137:17–25. [DOI] [PubMed] [Google Scholar]
- 57.Donahue AB. Electroconvulsive therapy and memory loss: a personal journey. J ECT. 2000;16(2):133–43. [DOI] [PubMed] [Google Scholar]
- 58.Berman RM, Prudic J, Brakemeier EL, Olfson M, Sackeim HA. Subjective evaluation of the therapeutic and cognitive effects of electroconvulsive therapy. Brain Stimul. 2008;1(1):16–26. [DOI] [PubMed] [Google Scholar]
- 59.Brakemeier EL, Berman R, Prudic J, Zwillenberg K, Sackeim HA. Self-evaluation of the cognitive effects of electroconvulsive therapy. Journal of ECT. 2011;27(1):59–66. [DOI] [PubMed] [Google Scholar]
- 60.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163(11):1905–17. [DOI] [PubMed] [Google Scholar]
- 61.Conway CR, George MS, Sackeim HA. Towards an evidence-based, operational definition of treatment-resistant depression: When enough is enough. JAMA Psychaitry. 2017;74(1):9–10. [DOI] [PubMed] [Google Scholar]
- 62.Hollingshead AB. Four factor index of social status. New Haven: Yale University; 1975. [Google Scholar]
- 63.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62 Suppl 16:10–7. [PubMed] [Google Scholar]