Abstract
The role of the inducible costimulatory of T cells (ICOS) has been shown to be important for many different T cell outcomes and is indispensable for follicular helper T cell (TFH) responses. Since its discovery, there have been several studies on the regulation of ICOS at a transcriptional level. However, the post-translational regulation of ICOS has not been well characterized. Here, we demonstrate that ICOS is internalized following ligation. We show that costimulation with CD3 results in differential internalization and fate than stimulation of ICOS alone. Additionally, we show that ICOS internalization is PI3K and clathrin mediated. The studies presented here not only increase the mechanistic understanding of ICOS post-translational regulation but also give insight into the potential mechanisms by which T cells expressing high affinity receptors may be preferentially chosen to become TFH cells with increased ICOS levels.
Keywords: ICOS, ICOSL, Follicular helper T cells, TFH, CD3, Costimulation
Highlights
-
•
ICOS is internalized following receptor ligation.
-
•
CD3 + ICOS costimulation alters the fate of ICOS to be recycled rather than degraded in lysosomes.
-
•
ICOS internalization is PI3K and clathrin mediated.
1. Introduction
The seminal model of two signal lymphocyte activation states that lymphocytes require not just an antigen receptor signal, but also a second (costimulatory) signal [[1], [2], [3]]. The most well characterized costimulatory families are the B7 and CD28 families. The B7 family includes the ligand of Inducible Costimulator of T cells (ICOSL), PD-L1, PD-L2, B7–H3, and B7–H4. These ligands are primarily found on APCs, but can also be found on non-hematopoietic cells. The CD28 family members include CD28, ICOS, PD-1, BTLA and CTLA-4. Interaction between B7 and CD28 family members have key roles in regulating T cell responses. While several of these family members are necessary for proper T cell activation in response to T dependent antigens, ICOS and ICOSL have been shown to be essential for both TFH development and correspondingly productive GC responses [4].
ICOS was first discovered in humans as being a homodimeric protein with a molecular mass of 55–60 kDa [5]. In contrast to CD28 which is able to bind to both CD80 and CD86, ICOS binds only one ligand, ICOSL [[6], [7], [8]]. ICOS lacks the MYPPPY motif present in CD28 and CTLA-4 which is necessary for interaction with CD80 and CD86 [5,9]. Early studies indicated that downstream signaling was similar between ICOS and CD28. Both CD28 and ICOS ligation causes phosphatidylinositol 3-kinase (PI3K) activation which ultimately results in the activation of AKT to promote cell proliferation and survival [[10], [11], [12], [13]]. ICOS crosslinking, similar to CD28 crosslinking, can lead to the recruitment of both p50α and p85α regulatory subunits of PI3K [12,14]. However, ICOS crosslinking has been shown to result in stronger AKT activation than CD28 crosslinking in activated CD4+ T cells. ICOS crosslinking preferentially recruits p50α for AKT activity, but p85α recruitment by ICOS stimulation has recently been shown to result in TFH differentiation of CD4+ T cells [14]. Additionally, ICOSL deficient mice have a similar phenotype to ICOS deficient mice, suggesting the importance of their cognate interaction [15].
Since its discovery by Hutloff et al. [5], there have been many studies showing the importance of ICOS in T cell function. ICOS is lowly expressed on resting T cells but is quickly upregulated in response to TCR cross-linking and/or CD28 stimulation [5,6]. The primary mechanism of transcriptional regulation of Icos is thought to be through T cell activation via TCR signaling. However, there have been several studies showing the importance of Icos mRNA regulation through miRNAs and other proteins. Vinuesa et al. showed that a loss of function mutation in Roquin-1 led to increased Icos mRNA levels and humoral autoimmunity [16]. This lab later showed the importance of Roquin-1 in limiting Icos mRNA levels through the 3’ UTR of Icos mRNA [20]. However, complete loss of Roquin-1 in mice did not have the same increase in Icos mRNA levels as Sanroque mice suggesting a redundant pathway in Roquin-1 mediated regulation of Icos mRNA [21]. This group showed that Roquin-2 was able to function in the absence of Roquin-1 to limit Icos mRNA levels. However, with a loss of function mutation in Roquin-1, Roquin-2 did not function to limit Icos mRNA [17]. These studies showed that there is some redundancy in Icos mRNA regulation. Roquin mediated Icos mRNA regulation requires mir146a [18]. Mir146a KO mice had a similar phenotype to Sanroque mice due to elevated Icos mRNA levels [18].
Similar to the internalization of CD28 following cognate interaction, it has also been shown that crosslinking of ICOS results in ICOS internalization on T cells [19]. Mice that lack the sheddase for ICOSL (ADAM10) result in an overexpression of surface ICOSL on APCs [19]. Interestingly, this increased ICOSL expression caused a compensatory decrease in ICOS surface levels on T cells. This was not due to decreased transcriptional levels of Icos but rather internalization of the protein. However, the fate of internalized ICOS was not determined in that study but it was hypothesized that it may be degraded upon internalization as total ICOS (surface + intracellular) was decreased in mice with elevated ICOSL levels [19].
The findings in this study give insight into the post-transcriptional regulation of ICOS, particularly after internalization following receptor ligation. We show that ICOS is preferentially shuttled to recycling endosomes following internalization when CD3 costimulation is present. In the absence of CD3 costimulation, ICOS is shuttled to the lysosomal pathway. These studies not only give a mechanistic picture of ICOS post-transcriptional regulation, but may also explain why antigen specific T cells are preferentially chosen to become TFH cells which rely on high ICOS levels.
2. Materials and Methods
2.1. Mice
C57BL6/J mice were purchased from Jackson (000664) and maintained at Virginia Commonwealth University (VCU) in a barrier vivarium facility in accordance with the humane treatment of laboratory animals sets forth by the National Institutes of Health and the American Association for the Accreditation of Laboratory Animal Care. All mouse protocols were conducted with the permission and oversight of the VCU Institutional Animal Care and Use Committee. Sex was randomized between groups by including equal numbers of male and female mice mixed in each pooled sample. Mice ages ranged between 8 and 16 weeks for all experiments.
2.2. T cell isolation
Spleens were homogenized and passed through a 40 μm mesh. Erythrocytes were lysed using ammonium-chloride-potassium lysing buffer (Quality Biologic). CD4+ T cell isolation was conducted using negative selection via MojoSort Mouse CD4 T cell isolation kit (BioLegend). Single cell suspensions were incubated with CD4 T cell isolation antibody cocktail for 30 min on ice. Cells were washed 3 times with PBS and magnetic beads were incubated with cells for 10 min at room temperature. Magnetic separation was used and non-bound cells were collected and cultured as below.
2.3. Cell culture
Isolated mouse T cells were cultured in cRPMI 1640 containing 10% FBS, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 mM HEPES, 1 mM sodium pyruvate and 2 mM 2-mercaptoethanol.
2.4. ICOS internalization assays
Isolated WT CD4+ T cells were incubated with anti-CD3 (2 μg/mL) for 16 h to increase surface ICOS levels. Cells were rested for 2 h on ice and then incubated with either 5 μg/mL biotinylated anti-CD3 (145-2C11; BioLegend), 5 μg/mL biotinylated anti-ICOS (C398.4A; BioLegend), or both antibodies for 30 min on ice. Cells were then washed two times with cold PBS to remove unbound, excess antibody. Prewarmed media with 20 μg/mL purified streptavidin was then added to cells to crosslink biotinylated antibodies. Cells were fixed at indicated time points and cells were permeabilized and stained for ICOS, Tfr, and LAMP1 and analyzed by Amnis ImageStream. For studies with inhibitors, cells were pretreated for 60 min with indicated inhibitors prior to addition of biotinylated antibodies. Because of the reversible nature of some of the inhibitors, inhibitors were included during incubation steps included antibody binding and crosslinking. Wortmannin was used at 1 μM ammonium chloride was used at 10 mM, dynasore was used at 40 μM, and cycloheximide was used at 20 μg/mL.
2.5. Flow cytometry and image cytometry
For flow cytometry cells were washed with FACs buffer (5% FBS in PBS with 2 mM EDTA). Fc receptors were blocked with 5 μg 2.4g2 [20] for 10 min at 4 °C. Antibodies were added at indicated concentrations 45 min at 4 °C. Cells were washed two times with FACs buffer and fixed in Fixation Buffer (BioLegend, 420801), or secondaries were added and incubated for 30 min at 4 °C followed by fixation. For intracellular staining, following fixation, cells were permeabilized using Intracellular Stain Permeabilization Buffer (BioLegend, 421002) according to manufacturer's protocol. Cells were stained for intracellular markers for 60 min at room temperature and washed and fixed. Flow cytometry data was collected on an LSR FortessaX-20 (BD) and analyzed in FCS Express 5. For image cytometry, stimulation was quenched using 2X fixative and incubated on ice for at least 10 min. Cells were then stained for surface and intracellular proteins, as described above and as in Ref. [19]. Data was acquired using ImageStreamX Mark II (Luminex) with a 40X objective lens. Internalization index and bright similarity detailed were calculated using IDEAS 6.0 software (Luminex) as in Ref. [19].
3. Results and discussion
3.1. ICOS stimulation in the presence of CD3 stimulation alters ICOS internalization and degradation
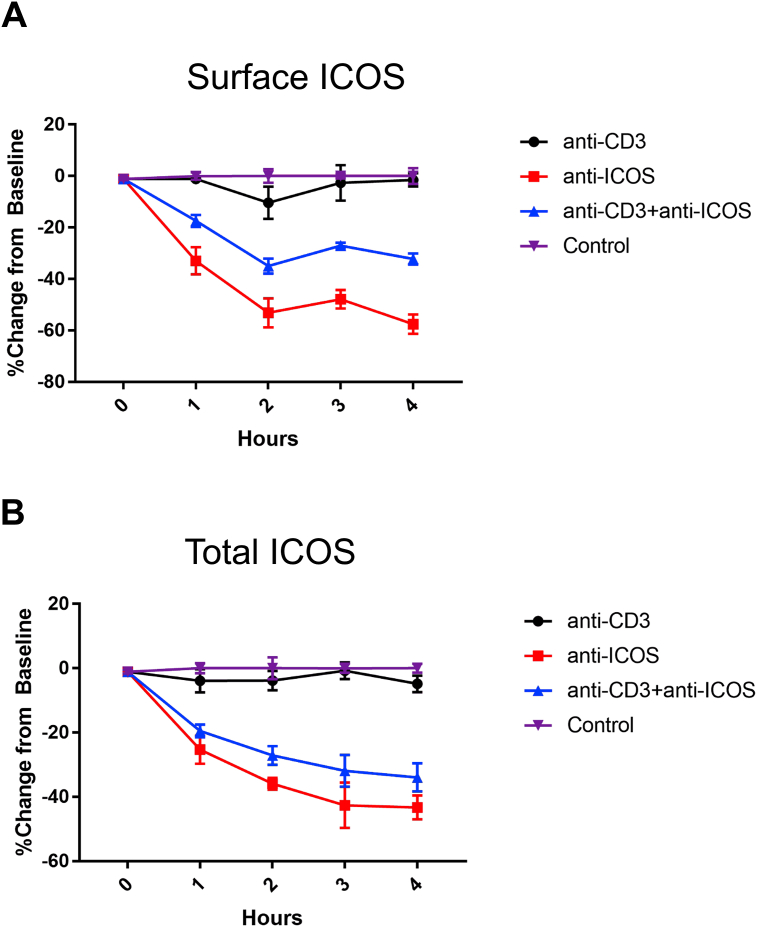
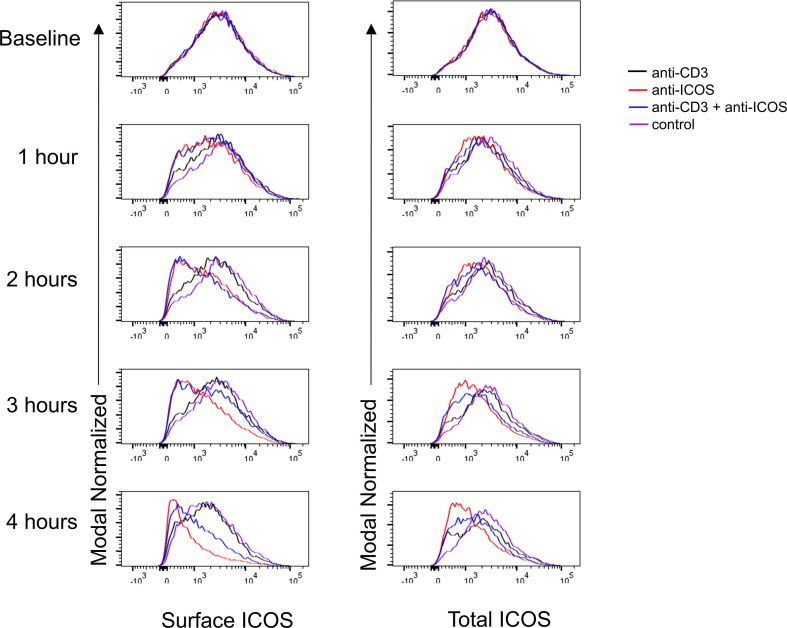
With the finding that ICOS is internalized in the presence of elevated ICOSL levels [23], we examined ICOS internalization in an in vitro system. We wanted to determine whether there was a difference in total ICOS levels following anti-CD3 + anti-ICOS stimulation together vs. anti-ICOS alone. To test this, we stimulated T cells with either no simulation, anti-CD3 alone, anti-ICOS alone, or anti-CD3 + ICOS for times from 0 to 4 h. We observed that T cells stimulated with CD3 alone did not have an effect on surface or total ICOS levels, suggesting that CD3 signaling alone does not alter ICOS levels (Fig. 1A–B). Additionally, we observed that ICOS stimulation by itself resulted in a further reduction in ICOS levels than anti-CD3 + anti-ICOS stimulation, suggesting that more ICOS may be destined for degradation pathways (Fig. 1A–B). This was observed at both the surface and total levels, supporting our data of increased ICOS recycling to the cell surface upon CD3 costimulation. These findings are particularly important in the context of TFH and effector T cell development, which both rely heavily on ICOS stimulation [4,21]. These data suggest that high affinity T cells would have ICOS shuttled back to the cell surface, thus increasing the amount of ICOS available for costimulation and thus further increasing their propensity for TFH or effector T cell commitment.
Fig. 1.
ICOS undergoes receptor mediated endocytosis and is degraded. (A–B) CD4+ T cells were stimulated as in Materials and Methods. Surface (A) and total (B) ICOS levels were determined at indicated time points by flow cytometry. Mean ± SD with n = 4 for each time point and treatment group. Data is representative of two individual experiments.
ICOS enters the lysosomal pathway following internalization and can recycle to the cell surface with CD3 stimulation.
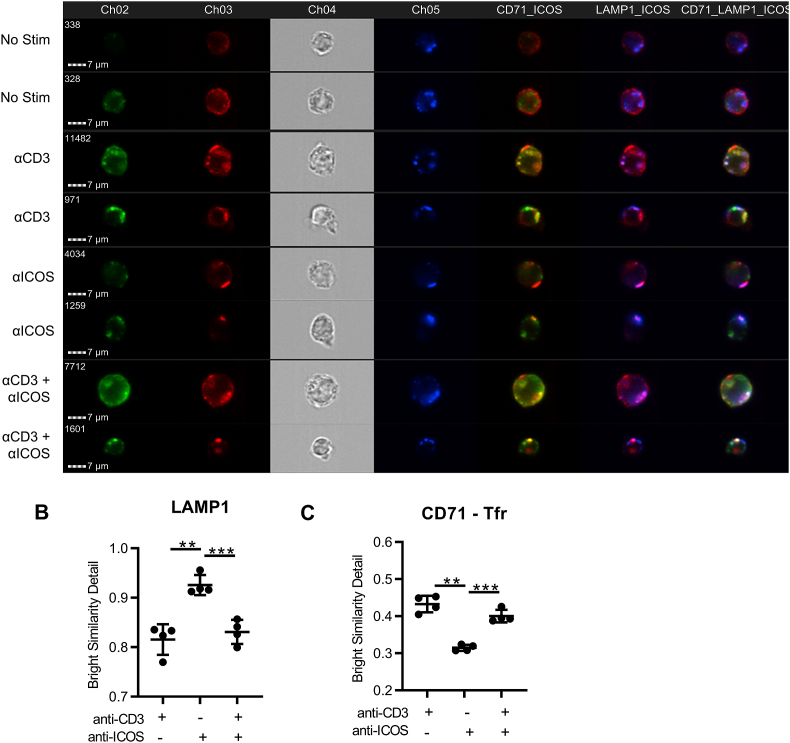
With our above findings, we next wanted to examine which endosomal pathway ICOS enters in response to various stimulations. To determine which signals drive ICOS lysosomal degradation, we isolated T cells and stimulated them for 48 h with anti-CD3 coated plates to increase ICOS surface levels. The T cells were then rested for 2 h and biotinylated anti-CD3, anti-ICOS, or a combination was added to the cells. Excess antibody was removed and cells were incubated with purified streptavidin to crosslink the anti-CD3, mimicking ligand interaction [25]. Colocalization of ICOS with LAMP1 or the recycling endosome marker transferrin receptor (TfR) was assessed by ImageStream analysis at various time points (Fig. 2A). Interestingly, stimulation with anti-CD3 and anti-ICOS together led to significantly less colocalization with LAMP1 but rather a significantly greater degree of colocalization with TfR compared to anti-ICOS stimulation alone, indicating ICOS is entering a recycling pathway (Fig. 2B–C). Together, these results suggest novel regulation of ICOS by internalization followed by rapid recycling or lysosomal degradation depending on the presence of TCR co-stimulation. This divergent shuttling pathway is very reminiscent of CTLA-4 shuttling, which can either enter recycling or degradation pathways upon ligand interaction [[22], [23], [24]].
Fig. 2.
Type of stimulation dictates ICOS lysosomal pathways. CD4+ T cells were isolated from spleens of mice and stimulated as indicated for indicated amount of time. Cells were then fixed and permeabilized and stained for ICOS, LAMP1 and TfR and examined by Amnis ImageStream. (A) Representative images of cells with indicated stimulations for 15 min. (B) Quantitative analysis of LAMP1 and ICOS colocalization at 15 min. (C) Quantitative analysis of ICOS and TfR colocalization at 15 min. One-way analysis of variance with Tukey's post-test (B & C; mean ± SD). Data are pooled from two experiments.
3.2. ICOS internalization is mediated through PI3K
With the finding that ICOS costimulation with CD3 stimulation resulted in increased colocalization with recycling endosomes, we hypothesized that ICOS stimulation alone would result in increased levels of degradation of ICOS and hence decreased total ICOS levels.
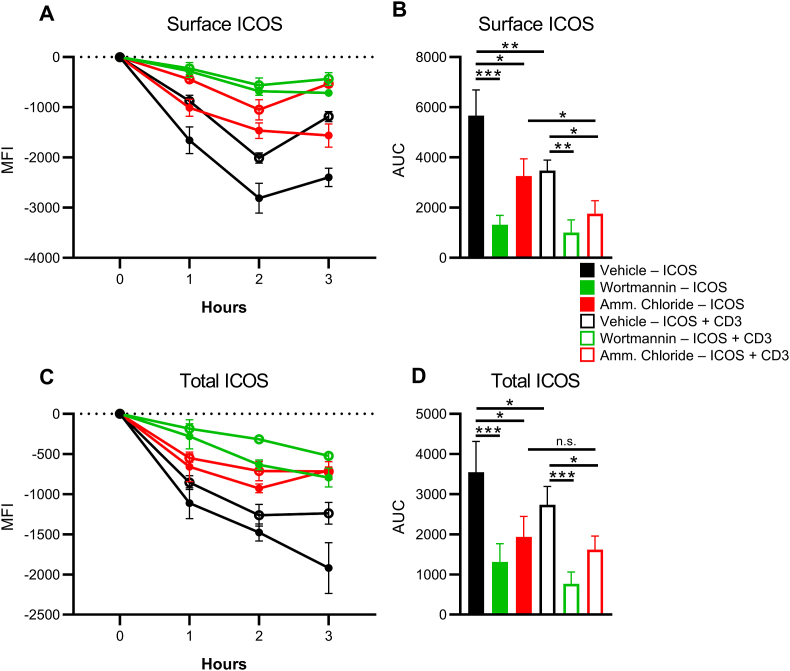
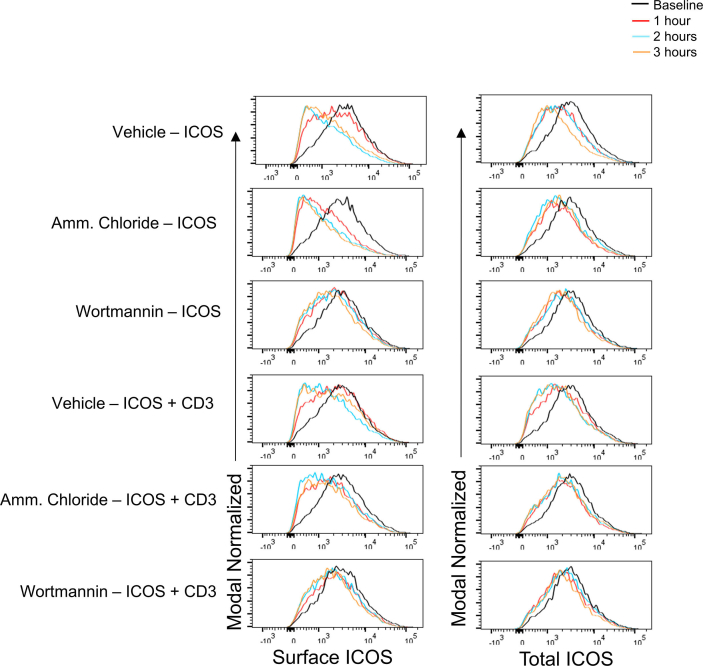
It has been well established that ICOS and CD3 both signal through PI3K and that ICOS costimulation with CD3 enhances CD3-mediated PI3K activation [12,25,26]. With these findings, we sought to examine whether the internalization of ICOS was dependent on this PI3K signaling. To do this, we used thePI3K inhibitor wortmannin [27] and repeated the stimulations described above. Additionally, we pretreated cells with the lysosomal inhibitor ammonium chloride [28]. Upon ICOS stimulation alone, we saw that wortmannin was able to decrease ICOS loss from the cell surface as well as total ICOS levels, suggesting that ICOS internalization and degradation are both PI3K dependent (Fig. 3A–D). Additionally, when we neutralized lysosomes with ammonium chloride, we observed decreased loss of surface and total ICOS levels further confirming that ICOS degradation and internalization was dependent on the lysosomal pathway (Fig. 3A–D).
Fig. 3.
ICOS internalization and degradation requires lysosomes and PI3K. (A–B) CD4+ T cells were stimulated as in Materials and Methods. Cells were pretreated with indicated treatments for 60 min before stimulation with anti-ICOS or anti-CD3. (A) Surface ICOS levels were determined at indicated time points by flow cytometry following stimulation with ICOS crosslinking or ICOS + CD3 crosslinking. (B) Area under the curve analysis of A. (C) Total ICOS levels were determined at indicated time points by flow cytometry following stimulation with ICOS crosslinking or ICOS + CD3 crosslinking. (D) Area under the curve analysis of C. One-way analysis of variance with Tukey's post-test (B & D; mean ± SD). Data are pooled from two experiments.
3.3. ICOS internalization is clathrin mediated
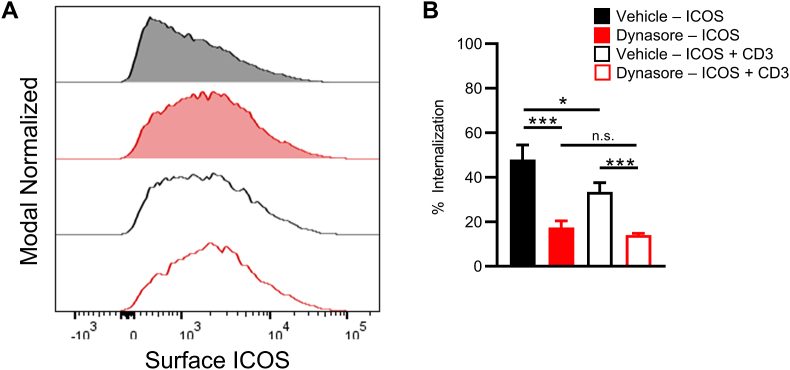
Endocytosis of receptors is a well characterized phenomenon. One of the mechanisms of receptor mediated endocytosis is through clathrin-mediated endocytosis (CME). One of the critical components of CME is dynamin. To determine whether CME was involved in ICOS internalization, we used the dynamin inhibitor, dynasore [29]. We stimulated T cells for 60 min that had been pretreated with either wortmannin, dynasore, or a vehicle control. We saw that dynamin inhibition by dynasore blocked ICOS internalization compared to the vehicle alone (Fig. 4). These findings suggest that in addition to being PI3K dependent, ICOS internalization occurs through CME.
Fig. 4.
ICOS internalization can be blocked with dynamin inhibitors. (A) Representative flow cytometry histograms of CD4+ T cells as stimulated as in Materials and Methods. Cells were pretreated with Dynasore for 60 min prior to stimulation with anti-ICOS or anti-CD3. (B) Quantitative analysis of percent internalization as determined by examining MFI of ICOS on the cell surface before and after crosslinking. One-way analysis of variance with Tukey's post-test (A; mean ± SD). Data are pooled from two experiments.
It will also be important to determine whether ICOS internalization is used by the cell as a way to cease signaling through ICOS or whether signaling is enhanced by internalization into microanatomical clusters. Inhibiting internalization of other receptors such as TGFβ receptors actually leads to enhancement of downstream signaling [30]. There have been several studies examining the effects of dynamin-2 deficiency in T cells. These findings suggest that internalization of the TCR signaling complex is necessary for long term signaling and that absence of this internalization results in defective survival and proliferation of T cells [31]. With ICOS having several signaling pathways such as PI3K and calcium signaling [25,26,32,33] it is important to determine whether these individual signaling pathways are differentially affected by ICOS internalization.
Fundimg
This work was funded by NIH/NIAID R01AI18697A1to D.H.C. and R.K.M. Flow cytometry utilized at the VCU Flow Cytometry Shared Resource. Supported, in part, by funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
CRediT authorship contribution statement
Joseph C. Lownik: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft, Writing - review & editing. Daniel H. Conrad: Conceptualization, Funding acquisition, Resources, Supervision, Visualization, Writing - review & editing. Rebecca K. Martin: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Project administration, Visualization, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank Krishna Patel for her help with experiments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100803.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
References
- 1.Bretscher P., Cohn M. A theory of self-nonself discrimination. Science. 1970 Sep;169(3950):1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham A.J., Lafferty K.J. A simple conservative explanation of the H-2 restriction of interactions between lymphocytes. Scand. J. Immunol. 1977;6(1–2):1–6. doi: 10.1111/j.1365-3083.1977.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 3.Lafferty K.J., Cunningham A.J. A new analysis of allogeneic interactions. Aust. J. Exp. Biol. Med. Sci. 1975 Feb;53(1):27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 4.Crotty S.T. Follicular helper cell differentiation, function, and roles in disease. Immunity. 2014 Oct;41(4):529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutloff A., Dittrich A.M., Beier K.C., Eljaschewitsch B., Kraft R., Anagnostopoulos I. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999 Dec;402:21–24. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 6.Yoshinaga S.K., Whoriskey J.S., Khare S.D., Sarmiento U., Guo J., Horan T. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999 Dec;402(6763):827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 7.Aicher A., Hayden-Ledbetter M., Brady W.A., Pezzutto A., Richter G., Magaletti D. Characterization of human inducible costimulator ligand expression and function. J. Immunol. 2000 May;164(9):4689–4696. doi: 10.4049/jimmunol.164.9.4689. [DOI] [PubMed] [Google Scholar]
- 8.Ling V., Wu P.W., Finnerty H.F., Bean K.M., Spaulding V., Fouser L.A. Cutting edge: identification of GL50, a novel B7-like protein that functionally binds to ICOS receptor. J Immunol (Baltimore, Md 1950) 2000 Feb;164(4):1653–1657. doi: 10.4049/jimmunol.164.4.1653. [DOI] [PubMed] [Google Scholar]
- 9.Peach R.J., Bajorath J., Brady W., Leytze G., Greene J., Naemura J. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7-1. J. Exp. Med. 1994 Dec;180(6):2049–2058. doi: 10.1084/jem.180.6.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slomovitz B.M., Coleman R.L. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin Cancer Res An Off J Am Assoc Cancer Res. 2012 Nov;18(21):5856–5864. doi: 10.1158/1078-0432.CCR-12-0662. [DOI] [PubMed] [Google Scholar]
- 11.Coyle A.J., Lehar S., Lloyd C., Tian J., Delaney T., Manning S. The CD28-related molecule ICOS is required for effective T cell–dependent immune responses. Immunity. 2000 Jul;13(1):95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- 12.Fos C., Salles A., Lang V., Carrette F., Audebert S., Pastor S. ICOS ligation recruits the p50α PI3K regulatory subunit to the immunological synapse. J. Immunol. 2008 Aug;181(3):1969–1977. doi: 10.4049/jimmunol.181.3.1969. [DOI] [PubMed] [Google Scholar]
- 13.Riley J.L., Mao M., Kobayashi S., Biery M., Burchard J., Cavet G. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc. Natl. Acad. Sci. U. S. A. 2002 Sep;99(18):11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leavenworth J.W., Verbinnen B., Yin J., Huang H., Cantor H. A p85α-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat. Immunol. 2015 Jan;16(1):96–106. doi: 10.1038/ni.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mak T.W., Shahinian A., Yoshinaga S.K., Wakeham A., Boucher L.-M., Pintilie M. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell–dependent B cell responses. Nat. Immunol. 2003 Aug;4(8):765–772. doi: 10.1038/ni947. [DOI] [PubMed] [Google Scholar]
- 16.Vinuesa C.G., Cook M.C., Angelucci C., Athanasopoulos V., Rui L., Hill K.M. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005 May;435(7041):452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 17.Vogel K.U., Edelmann S.L., Jeltsch K.M., Bertossi A., Heger K., Heinz G.A. Roquin paralogs 1 and 2 redundantly repress the Icos and Ox40 costimulator mRNAs and control follicular helper T cell differentiation. Immunity. 2013 Apr;38(4):655–668. doi: 10.1016/j.immuni.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Pratama A., Srivastava M., Williams N.J., Papa I., Lee S.K., Dinh X.T. MicroRNA-146a regulates ICOS-ICOSL signalling to limit accumulation of T follicular helper cells and germinal centres. Nat. Commun. 2015;6:6436. doi: 10.1038/ncomms7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lownik J.C., Luker A.J., Damle S.R., Cooley L.F., El Sayed R., Hutloff A. ADAM10-mediated ICOS ligand shedding on b cells is necessary for proper T Cell ICOS regulation and T follicular helper responses. J. Immunol. 2017;199(7) doi: 10.4049/jimmunol.1700833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Unkeless J.C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J. Exp. Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burmeister Y., Lischke T., Dahler A.C., Mages H.W., Lam K.-P., Coyle A.J. ICOS controls the pool size of effector-memory and regulatory T cells. J. Immunol. 2008 Jan;180(2):774–782. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 22.Egen J.G., Allison J.P. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002 Jan;16(1):23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 23.Qureshi O.S., Kaur S., Hou T.Z., Jeffery L.E., Poulter N.S., Briggs Z. Constitutive clathrin-mediated endocytosis of CTLA-4 persists during T cell activation. J. Biol. Chem. 2012 Mar;287(12):9429–9440. doi: 10.1074/jbc.M111.304329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur S., Qureshi O.S., Sansom D.M. Comparison of the intracellular trafficking itinerary of CTLA-4 orthologues. PloS One. 2013 Apr;8(4) doi: 10.1371/journal.pone.0060903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J., Heinrichs J., Leconte J., Haarberg K., Semple K., Liu C. Phosphatidylinositol 3-kinase–independent signaling pathways contribute to ICOS-mediated T cell costimulation in acute graft-versus-host disease in mice. J. Immunol. 2013 Jul;191(1):200–207. doi: 10.4049/jimmunol.1203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gigoux M., Shang J., Pak Y., Xu M., Choe J., Mak T.W. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. Unit. States Am. 2009 Dec;106(48):20371–20376. doi: 10.1073/pnas.0911573106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arcaro A., Wymann M.P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem. J. 1993 Dec;296(Pt 2):297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hart P.D., Young M.R. Ammonium chloride, an inhibitor of phagosome-lysosome fusion in macrophages, concurrently induces phagosome-endosome fusion, and opens a novel pathway: studies of a pathogenic mycobacterium and a nonpathogenic yeast. J. Exp. Med. 1991 Oct;174(4):881–889. doi: 10.1084/jem.174.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill T.A., Gordon C.P., McGeachie A.B., Venn-Brown B., Odell L.R., Chau N. Inhibition of dynamin mediated endocytosis by the dynoles—synthesis and functional activity of a family of indoles. J. Med. Chem. 2009 Jun;52(12):3762–3773. doi: 10.1021/jm900036m. [DOI] [PubMed] [Google Scholar]
- 30.Chen C.-L., Hou W.-H., Liu I.-H., Hsiao G., Huang S.S., Huang J.S. Inhibitors of clathrin-dependent endocytosis enhance TGFβ signaling and responses. J. Cell Sci. 2009 Jun;122(11):1863–1871. doi: 10.1242/jcs.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willinger T., Staron M., Ferguson S.M., Camilli P De, Flavell R.A. Dynamin 2-dependent endocytosis sustains T-cell receptor signaling and drives metabolic reprogramming in T lymphocytes. Proc. Natl. Acad. Sci. Unit. States Am. 2015 Apr;112(14):4423–4428. doi: 10.1073/pnas.1504279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feito M.J., Vaschetto R., Criado G., Sánchez A., Chiocchetti A., Jiménez-Periáñez A. Mechanisms of H4/ICOS costimulation: effects on proximal TCR signals and MAP kinase pathways. Eur. J. Immunol. 2003 Jan;33(1):204–214. doi: 10.1002/immu.200390023. [DOI] [PubMed] [Google Scholar]
- 33.Leconte J., Bagherzadeh Yazdchi S., Panneton V., Suh W.-K. Inducible costimulator (ICOS) potentiates TCR-induced calcium flux by augmenting PLCγ1 activation and actin remodeling. Mol. Immunol. 2016 Nov;79:38–46. doi: 10.1016/j.molimm.2016.09.022. [DOI] [PubMed] [Google Scholar]