Abstract
Background
The pathogenesis of arterial and venous thrombosis is in large part interlaced. How much platelet phenotype relates to acute venous thromboembolism (VTE) independent of the underlying cardiovascular profile is presently poorly investigated.
Methods
Platelet count and mean platelet volume (MPV), platelet aggregation in whole blood and platelet rich plasma (PRP), platelet-dependent thrombin generation (TG) and platelet surface activation markers were measured under standardized conditions. Machine learning was applied to identify the most relevant characteristics associated with VTE from a large array (N = 58) of clinical and platelet-related variables.
Findings
VTE cases (N = 159) presented with lower platelet count and MPV vs controls (N = 140). Whole blood aggregation showed shorter collagen/Epinephrine closure times in cases, particularly within acetylsalicylic acid (ASA) users. Within ASA users, higher PRP aggregation after adenosine diphosphate (ADP), epinephrine, collagen and arachidonic acid was observed in cases vs controls. Within non-ASA and/or subjects on anticoagulants, cases presented with lower aggregation after ADP and collagen vs controls. Lower platelet-dependent TG, higher CD63 on resting and lower PAC-1 expression after collagen/ADP in-vitro stimulated platelets further characterized VTE cases vs controls, independent of therapy. Lasso regression analysis identified 26 variables associated with VTE of which 69% were platelet-related.
Interpretation
Comprehensive phenotyping of platelet function identified a large proportion of low responders to ASA in VTE cases. Lower platelet-dependent TG and lower platelet reactivity after ex-vivo stimulation characterized the “platelet exhausted syndrome” in cases. Finally, from a large array of covariates including clinical risk factors, platelet biomarkers comprised 69% of all selected variables differentiating VTE cases vs controls.
Funding
German Federal Ministry of Education and Research, CTH-Mainz and Bayer AG.
Research in context.
Evidence before this study
The role of platelets in atherogenesis, atherosclerosis and ultimately atherothrombosis is well acknowledged. Recent evidence from animal models demonstrated an important role of platelets in development of venous thrombosis; however, the clinical evidence is rather limited. In humans, the pathogenesis of arterial and venous thrombosis is interlaced; individuals with venous thromboembolism (VTE) are shown to have an increased risk of atherosclerotic disease as well as individuals with atherosclerotic disease are at increased risk of subsequent VTE.
Added value of this study
This is the largest study comprehensively investigating platelet function in subjects with acute VTE to date. We demonstrated that platelet phenotype substantially differs between VTE cases and those with a VTE ruled out independent of the underlying cardiovascular risk profile. This study provides evidence for the occurrence of acetylsalicylic acid low-responsiveness in acute VTE which might explain the failure of acetylsalicylic acid to better protect subjects at risk, but also provides evidence for “exhausted” platelets in this disease. Using a machine learning technic including large array of the most relevant clinical and laboratory characteristics, platelet biomarkers comprised 69% of all selected variables differentiating VTE cases vs VTE controls.
Implications of all the available evidence
The available evidence substantiates the relevance of platelet function for the presence of an acute VTE event and gives insights into the role of platelets in the pathophysiology of this disease. Whether platelet phenotype characterization could improve identification of individuals at risk for recurrent VTE and/or worse outcome remains to be determined.
Alt-text: Unlabelled box
1. Introduction
Platelets are complex cells, without nucleus but rich in granules, which promptly respond to signals from circulating cells, vessel wall and other blood components. The role of platelets in atherogenesis, atherosclerosis and ultimately atherothrombosis is well acknowledged [1]. Activated platelets are key players in arterial thrombosis, the principal cause of e.g. myocardial infarction and ischemic stroke. Antiplatelet agents interfere with platelet aggregation and represent a cornerstone for treatment and secondary prevention of atherothrombosis [2]. Conventional cardiovascular risk factors (CVRFs) are strongly related to development of cardiovascular adverse events and have been consistently associated with platelet activation [3,4]. Hence, there is solid evidence linking platelet activation and arterial prothrombotic conditions.
Most recent evidence from animal models demonstrated also an important role of platelets in development of venous thrombosis [5,6]; however, the clinical evidence is rather limited. In a small group of subjects with acute VTE, increased platelet activation with increased expression of platelet surface P-selectin and increased percentage of platelet-leukocyte conjugates, has been reported [7]. The most often investigated platelet parameter is mean platelet volume (MPV), a potential marker of platelet activation [8]. In a prospective population-based study, higher MPV was associated with higher risk of incident unprovoked VTE [9]. Differently, in patients presenting at the emergency department, lower MPV was associated with higher risk for objectively confirmed VTE diagnosis [10]. Likewise, in cancer patients, lower MPV was linked with higher VTE risk and worse survival [11]. The results from clinical trials assessing the effect of low-dose acetylsalicylic acid (ASA) against placebo showed a modest effect with around 40% reduction of recurrent VTE events, and at the same time reduction in major arterial events as well [12].
The pathogenesis of arterial and venous thrombosis is to some extend interlaced and individuals with VTE are shown to have an increased risk of atherosclerotic disease [13,14]. Similarly, it has been suggested that individuals with atherosclerotic disease are at increased risk of subsequent venous disease [15]. Whether arterial and venous thrombosis is the same disease at different vascular beds is still controversial considering the knowledge that traditional atherosclerotic risk factors are not entirely shared by both conditions [16].
To what extent platelet pathophysiology is involved in the acute VTE process, independent of the subjects’ underlying cardiovascular profile, is unknown. In the present work we undertook a comprehensive exploration of platelet function including platelet aggregation, platelet-dependent thrombin generation and platelet surface activation status in subjects presenting with signs and symptoms of deep vein thrombosis (DVT) and/or pulmonary embolism (PE) in an acute care setting. To better understand the role of platelets in the pathophysiology of VTE independent of underlying CVRFs and VTE risk factors, we used machine learning technic including large array of the most relevant clinical and laboratory characteristics predictive for VTE.
2. Methods
2.1. Study sample
Participants from the VTEval and FOCUS BioSeq studies at the Johannes Gutenberg University Medical Center Mainz in Germany, randomly selected at their baseline examination, were included in the platelet function study. The VTEval (NCT02156401) is a prospective observational single-center study including individuals with clinical suspicion for DVT and/or PE, recruited in the acute care setting such as emergency rooms, chest pain units and outpatient clinics, as described in detail before [17]. FOCUS BioSeq is a multi-center prospective study, conducted nationwide in Germany, enrolling individuals with objectively confirmed acute symptomatic PE [18]. In the platelet function study, confirmed subjects with DVT and/or PE were enrolled from both VTEval and FOCUS BioSeq study at the University Medical Center in Mainz. Controls were subjects with excluded VTE diagnosis and were selected from the VTEval study. Subjects with active cancer (n = 35) were excluded, leaving 294 individuals with platelet function measurements for the analysis. The VTEval and FOCUS BoSeq studies were implemented in accordance to the General Data Protection Regulation (EU 2016/679) and the Declaration of Helsinki (2013, 7th Revision). All participants provided written informed consent for participation.
Blood sampling and plasma preparation
Venous blood sampling was performed at inclusion of the subjects in the study when presenting at the University Medical Center in Mainz with signs and symptoms of VTE. Venous blood sampling was performed using tubes containing trisodium citrate (3.2%, 0.109 M, 1:9 vol:vol) and a PFA monovette (3.8%, 0.129 M) for the Innovance Platelet Function Analyzer (PFA)-200 (Siemens Healthcare, Marburg, Germany). Fresh citrated whole blood for platelet function analysis was hand delivered in the platelet epidemiology laboratory within 30 min after blood withdrawal at time of enrolment. Whole blood, platelet-rich plasma (PRP), platelet-poor plasma (PPP) and platelet-free plasma (PFP) were prepared as follows: PRP was isolated by centrifugation of whole blood at 200 × g for 10 min at room temperature (RT); after collecting the top 2/3rd of PRP, PPP was obtained by further centrifugation of the sample for 15 min at 2,000 × g at RT; PFP was obtained by centrifugation of whole blood for 5 min at 2,000 x g at RT. The collected PPP was further centrifuged for 10 min at 11,000 x g. The isolated PFP was stored at -80°C until further laboratory testing.
Platelet function assays
Platelet function testing was performed at the day of study subject's inclusion in a highly standardized manner with time tracking directly after blood withdrawal at the Platelet Epidemiology laboratory of the Johannes Gutenberg University Medical Center Mainz. In addition to standard platelet parameters available from blood count analysis, four platelet function assays were performed for assessing platelet aggregation, platelet-dependent thrombin generation and platelet surface activation. The flow of platelet function measurements has been described in detail before [19].
Standard platelet parameters
Platelet indices, namely platelet count and mean platelet volume (MPV) were automatically assessed on an ADVIA 120 Hematology System (Siemens, Erlangen, Germany) between 30 and 90 min after blood sampling as described in detail before [20].
Platelet aggregation
Platelet aggregation was investigated by two methods, in whole blood by PFA-200 and in PRP by Light Transmission Aggregometry (LTA).
PFA-200 (Siemens Healthcare, Marburg, Germany) is an automated assay using provided cartridges (Siemens Healthcare, Marburg, Germany) coated with platelet agonists (collagen/adenosinediphosphate [ADP] and collagen/epinephrine [EPI]) to assess primary hemostasis at high shear rates. In the aperture of the cartridges 800 mL of whole blood was pipetted. The occlusion time until complete central aperture sealing is automatically reported as closure time (CT), expressed in seconds (sec). The maximum measurable CT was 300 s.
LTA was performed using APACT 4S Plus aggregometer (LABiTec, Ahrensburg, Germany). Parameters for response were adjusted to 100% using PPP and 0% using PRP from each participant. Agonists were added to the PRP (unadjusted for platelet count), and platelet aggregation was monitored for 10 min by aggregometer tracings. Final test concentrations of agonists were 0.5 μmol/L and 2 μmol/L for ADP, 0.5 μmol/L and 5 μmol/L for EPI, 2 μg/mL for collagen, 1 mmol/L for arachidonic acid and 10 μmol/L for thrombin receptor activated peptide-6 (TRAP-6). In addition, no-trigger aggregation without addition of platelet agonists was assessed. The maximum percent aggregation (%) and velocity aggregation (%/min) were the recorded parameters of interest.
Thrombin generation
Thrombin generation (TG) was measured by the Calibrated automated thrombogram (CAT) assay (Thrombinoscope BV, Maastricht, The Netherlands) according to standardized protocols as previously reported [21]. TG in PRP was assessed in fresh material, whereas TG in PFP was assessed in frozen material. For TG measurements in PRP, 20 μl of the exogenous PRP reagent (1pM TF) were added to 80 μl PRP (with adjusted platelet concentration of 150,000 platelets/μl using autologous PPP), whereas for the measurements in PFP, 20 μl of the exogenous low PPP reagent (1pM TF together with 4 μM phospholipids) were added to 80 μl plasma. Following 10 min incubation at 37°C in the fluorimeter, 20 μl of low-affinity fluorogenic substrate for thrombin (Z-Gly-Gly-Arg-AMC) and calcium chloride mixture (FluCa), were automatically dispensed into each well. To correct for inner filter effects and substrate consumption, TG measurements were calibrated against a signal from the calibration well obtained in a sample from the same plasma (80 μL PRP or PFP, respectively), supplemented with a fixed amount of thrombin–α2-macroglobulin complex (20 μL of Thrombin Calibrator) and 20 μL of FluCa by means of Thrombinoscope software (Thrombinoscope BV). All CAT reagents were obtained from Stago Deutschland GmbH (Düsseldorf, Germany). After 120 min, the resulting TG curve was analysed for the following parameters: lag time, as the time for minimum thrombin formed (min), peak height, as the maximum concentration of thrombin formed (nM thrombin), endogenous thrombin potential (ETP), as the total amount of thrombin formed over time (nM*min), time to peak, as the time until the peak height (min) and velocity index defined with the formula: peak height/(time to peak − lag time), as a measure of the speed of TG (nM/min).
Flow cytometric analysis of platelets
Platelet surface activation antigens were assessed in citrated whole blood on Accuri C6 (BD, Heidelberg, Germany). Briefly, 5 µl of whole blood at resting condition was double stained with a platelet identifying monoclonal antibody CD42a-PerCP (Becton Dickinson, Heidelberg, Germany) for gating plus one of the following antibodies: CD41-PE (Beckman Coultier, Krefeld, Germany), CD62p-FITC (Becton Dickinson, Heidelberg, Germany), CD63-FITC (Beckman Coultier, Krefeld, Germany), anti-human Fibrinogen-FITC (DAKO, Glostrup, Denmark), anti-human Tissue Factor-FITC (Sekusui Diagnostics, Stamford, Connecticut, US). In addition, 5µl of whole blood after Collagen/ADP test with the PFA-200 assay was double stained with CD42a-PerCP and PAC-1-FITC (Becton Dickinson, Heidelberg, Germany). The samples were incubated for 20 min at room temperature in dark after which the reaction was stopped with addition of 500 µl phosphate-buffered saline. The samples were then immediately analyzed and percentages (%) of platelets as well as the mean fluorescence intensity (MFI) expressing the specific antigens were recorded.
Determination of levels of thromboxane B2 (TXB2) by ELISA
Levels of TXB2, a stable metabolite of thromboxane A2 (TXA2), were determined colorimetrically in citrated plasma by competitive ELISA (ab133022; Abcam, Cambridge, UK), according to the instructions of the manufacturer. The generated color intensity was determined at 405 nm with correction at 580 nm using a Tecan Infinite M200 Pro microplate reader (Tecan Group Ltd., Männedorf, Switzerland). The results were expressed as nanogramm per milliliter (ng/ml).
Data management and statistical analysis
All data underwent quality control ensuring completeness and plausibility by using predefined algorithms and criteria by a central data management team. Definitions of tradition cardiovascular risk factors (CVRFs), VTE risk factors and categorization of medications are presented in supplemental material (part A).
Data are reported as mean (standard deviation) when normally distributed or median (interquartile range) when with skewed distribution. Categorical variables are presented as absolute numbers and percentages (%). Differences between groups have been calculated using the t test and/or Mann-Whitney U test, if needed. Because of the explorative character of the analysis, a threshold of significance was not defined for P values. P values should rather be interpreted as a continuous measure of statistical evidence.
Least absolute shrinkage and selection operator (LASSO) regularized logistic regression, a common technique for supervised machine learning, was selected for the ability to deal with p > n situations, when number of variables (p) exceeds the number of observations (n) [22]. This method was used to select variables from a comprehensive panel of clinical markers and platelet function markers that most relevantly distinguish between VTE cases and controls. The optimal regularization parameter, λ, was selected using 3-fold cross-validation, minimizing the binomial deviance. Fractional polynomial transformations were applied to all variables included in the regression models to account for non-linear relationships. K-fold cross-validation is the main established method of lambda selection in lasso regression, whereas fractional polynomials were introduced in response to our concern that the chosen method might leave substantial nonlinear relationships uncovered. To enable a wide range of nonlinear relationships, continuous predictors were entered to the power of 1 and to the powers of -2, -1, -0.5, 0.5, 2, and 3 and as log of the original variable. Missing values were imputed using random forest imputation, implemented in the R package ‘missForest’ [23]. Variables included in the LASSO regression are reported in the supplemental material (part B). Selected variables were ordered by Lambda Ratio (LR), a scale-invariant measure of predictive robustness of each variable. LR was calculated by computing the ratio of the λ at which a variable was omitted from the model to the optimal λ selected by cross-validation.
Data Statement
Data are not made available for the scientific community outside the established and controlled workflows and algorithms. To meet the general idea of verification and reproducibility of scientific findings, we offer access to data at the local database in accordance with the ethics vote upon request at any time. Interested researchers make their requests to the coordinating principal investigator of the VTE studies (Dr. Philipp S. Wild; philipp.wild@unimedizin-mainz.de)."
Results
Characteristics of the study subjects
Descriptive data are presented in Table 1 for 159 individuals with confirmed acute VTE (case group) and 140 individuals with objectively excluded VTE (control group). Females were 20% less frequent in the VTE group compared to the control group. Age and body mass index did not differ between groups whereas, as expected, D-Dimer levels were higher in cases vs controls. Compared to individuals without VTE, cases were more likely to have a history of VTE, including history of DVT and PE. Recent immobilization and trauma (in the last 30 days) were also more frequently reported in VTE cases. Information on VTE phenotype was available in 154 VTE cases from which 35 (22.7%) were individuals with isolated DVT, 28 (18.2%) with isolated PE and 91 (59.1%) presented with combine PE+DVT phenotype. Presence of conventional CVRFs was not notably different between groups, whereas atrial fibrillation and coronary artery disease were more likely reported by individuals with an exclusion of VTE. Regarding antithrombotic agents, no differences were observed for the reported use of acetylsalicylic acid (ASA) regimen in the past 10 days, prior to inclusion in the study and the blood draw. Use of Clopidogrel (ATC code: B01AC04) was reported in N = 4 cases and N = 6 controls. The use of any anticoagulant agent was reported in 81% of cases and 44% of controls. Most frequently used were heparin with 67% in cases and 36% in controls. The use of direct Factor Xa inhibitors was reported in 25% of cases and 4% of controls. Furthermore, cases had 18% more frequently cardiovascular therapy (ATC codes: C01-C10) compared to the control group.
Table 1.
Study subject characteristics
| VTE case | VTE ruled out | |
|---|---|---|
| Number | 159 | 140 |
| Females, % (N) | 41% (65) | 61% (86) |
| Age (years) | 60 (50/73) | 59 (45/72) |
| Body Mass Index (kg/m2) | 28 (25/32) | 28 (24/32) |
| D-Dimer (mg/l) | 5 (2/11) | 0•8 (0•5/1) |
| VTE risk factors | ||
| VTE (history) | 35% (51/148) | 19% (27/139) |
| DVT (history) | 31% (46/148) | 18% (25/139) |
| PE (history) | 15% (22/148) | 7% (9/138) |
| Immobilization (last 30 days) | 16% (24/149) | 5% (7/138) |
| Long-distance flight/travel | 14% (21/148) | 10% (14/137) |
| Pregnancy (current) | 0•6% (1/158) | 1% (2/140) |
| Surgery (last 30 days) | 3% (5/148) | 2% (3/136) |
| Thrombophilia | 4% (5/126) | 5% (7/138) |
| Trauma (last 30 days) | 8% (11/147) | 1% (2/138) |
| Cardiovascular risk factors | ||
| Arterial Hypertension | 57% (81/143) | 48% (67/139) |
| Diabetes | 17% (25/144) | 12% (16/139) |
| Smoking | 19% (27/142) | 16% (22/138) |
| Obesity | 37% (58/158) | 37% (51/137) |
| Cardiovascular diseases | ||
| Atrial fibrillation | 6% (8/144) | 11% (15/137) |
| Congestive heart failure | 6% (8/143) | 8% (11/137) |
| Coronary artery disease | 9% (13/145) | 16% (22/136) |
| Stroke | 6% (8/145) | 7% (9/138) |
| Peripheral artery disease | 5% (6/114) | 4% (5/138) |
| Therapy | ||
| Antithrombotic agents (B01A) | 93% (148/159) | 63% (88/140) |
| Acetylsalicylic acid (B01AC06) | 35% (55/159) | 29% (41/140) |
| Clopidogrel (B01AC04) | 3% (4/159) | 4% (6/140) |
| Anticoagulant agents* | 81% (128/159) | 44% (61/140) |
| Vitamin K antagonists (B01AA) | 5% (8/159) | 4% (6/140) |
| Heparin group (B01AB) | 67% (107/159) | 36% (50/140) |
| Direct FXa inhibitors (B01AF) | 25% (39/159) | 4% (6/140) |
| Cardiovascular agents⁎⁎ | 60% (95/159) | 42% (59/140) |
Presented are demographic and clinical characteristics as relative frequencies in percentage (%), rounded to one informative digit, for the venous thromboembolism (VTE) cases and VTE ruled outs. Age and body mass index are presented as median (25% quartile and 75% quartile). Anatomical Therapeutic Chemical Classification System code:
B01AA, B01AB, B01 AF.
C01-C10.
Platelet count and MPV
VTE cases presented with lower platelet count (222 [179/268] x 109/L) and lower MPV (7.8 [7.3/8.4] fL) compared to VTE controls (platelet count: 245 [211/273] x 109/L; MPV: 8.1 [7.6/8.7] fL). According to therapy, as presented in supplemental Table S1, the differences for the platelet count remained lower in VTE cases compared to controls across all subgroups, except for the subgroup of combined ASA and anticoagulant use where no differences were observed. MPV remained lower for VTE cases vs controls only within the subgroup on anticoagulant therapy.
Platelet aggregation
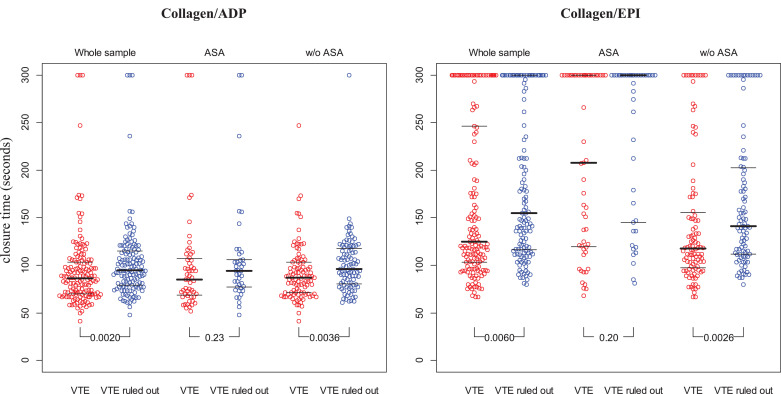
The results of platelet aggregometry on collagen/adenosinediphosphate (collagen/ADP) and collagen/epinephrine (collagen/EPI) assessed by PFA-200 are presented in Fig. 1. VTE cases showed shorter closure time for collagen/ADP (86.5 [70.0/104.2] sec.) and collagen/EPI (125.0 [102.3/251.7] s.) compared to VTE controls (collagen/ADP: 94.5 [79.0/115.0] s.; collagen/EPI: 155.0 [115.3/300.0] s.). Within the subgroup of subjects taking ASA, the differences between cases and controls remained with diminished statistical power, for both collagen/ADP and collagen/EPI. In subjects without ASA, VTE cases had shorter CTs than the control group for both triggers, collagen/ADP and collagen/EPI.
Fig. 1.
Distribution of platelet aggregation with PFA-200 in VTE cases vs VTE ruled out individuals.
Data are presented as individual plots with mean ± standard deviation; ADP, adenosine diphosphate; EPI, epinephrine; ASA, acetylsalicylic acid; VTE, venous thromboembolism.
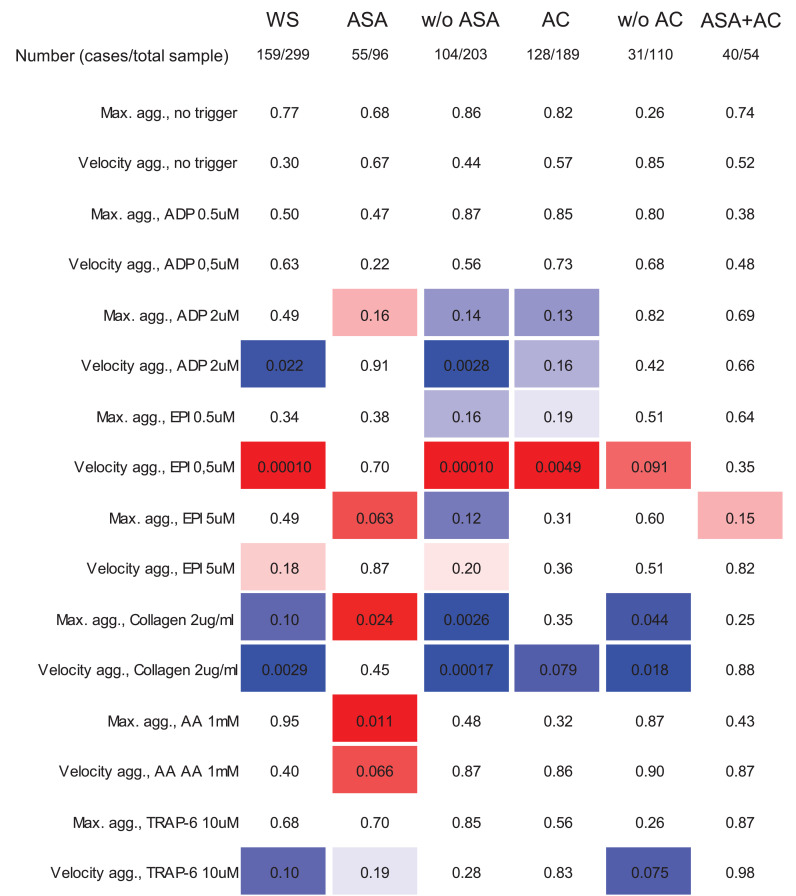
The results from LTA stratified for presence of VTE is presented in the whole sample and in subgroups according to intake of antithrombotic therapy in Supplemental Table S2. The strength of evidence for the difference (reflected by the p-value for testing) in maximum aggregation and/or velocity aggregation between cases and controls according to therapy is reported in Fig. 2. Aggregation velocity after low EPI (0.5 µM) was higher and after ADP (2 µM) and collagen lower in VTE cases not only in the whole sample, but also in the subgroups of ASA non-users and in subjects on anticoagulation. Furthermore, within subjects taking ASA, VTE cases had higher maximum aggregation particularly after EPI (5 µM), collagen and arachidonic acid compared to controls.
Fig. 2.
Platelet aggregation in platelet rich plasma according to intake of antithrombotic medication in individuals with suspected venous thromboembolism. Presented data indicate the evidence for a difference of LTA aggregation in VTE cases vs individuals with a rule out of VTE by p-values. The color indicates the direction of the difference with red if aggregation is higher and with blue if aggregation is lower in VTE cases. The intensity of the color indicates the strength of evidence for the difference between groups, ranging from p < 0•001 to p = 0•2. Abbreviations: WS, whole sample; ASA, acetylsalicylic acid; AC, anticoagulant; ADP, adenosine diphosphate; EPI, epinephrine; TRAP-6, thrombin receptor activating peptide-6.
Thromboxane B2
To confirm the low response on ASA, observed as higher platelets aggregation in cases vs controls, levels of Thromboxane B in citrated plasma were assessed. As shown in Figure S1, VTE cases presented with higher Thromboxane B2 levels compared to VTE controls in the whole sample (cases: 3.4 [2.2/6.1]; controls: 1.7 [1.0/3.9]), within subgroup of ASA users (cases: 3.0 [1.4/4.3]; controls: 1.0 [0.7/1.5] and in the subgroup of subjects not on ASA (cases: 3.6 [2.5/6.3]; controls: 2.6 [1.4/4.9]).
Thrombin generation in presence and absence of platelets
In presence of platelets, TG from VTE cases presented with longer lag time and time to peak, lower ETP and peak height and lower velocity compared to the control group (Table 2). As anticoagulation therapy has a substantial impact on TG parameters, the analysis was stratified according to intake of anticoagulant agents. Within subjects on anticoagulants, differences between groups were not observed for most of TG parameters, except for lower velocity index and a tendency to lower peak height in VTE cases. In subjects not on anticoagulation, peak height and velocity were also lower in VTE cases (though with a clearer difference between groups), but additionally lag time and time to peak were longer in the cases. Interestingly, ETP did not differ with presence or absence of VTE in individuals not taking anticoagulants.
Table 2.
Thrombin generation in presence and absence of platelets and according to intake of anticoagulants
| whole sample |
with anticoagulants |
w/o anticoagulants |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VTE case (N: 159) | VTE ruled out (N: 140) | P-value | VTE case (N: 128) | VTE ruled out (N: 61) | P-value | VTE case (N: 31) | VTE ruled out (N: 79) | P-value | ||
| Platelet Rich Plasma | Lag time (min) | 10.17 (6.29/17.67) | 8.00 (5.72/11.35) | 0.0099 | 10.50 (6.24/18.14) | 9.54 (6.86/13.73) | 0.49 | 9.70 (7.07/14.51) | 6.86 (5.38/9.70) | 0.023 |
| ETP (nM*min) | 1140.26 (336.36/1608.99) | 1346.50 (813.16/1655.27) | 0.026 | 1088.99 (108.47/1608.77) | 1236.08 (602.35/1619.23) | 0.46 | 1209.07 (722.06/1618.03) | 1382.85 (1185.14/1690.29) | 0.14 | |
| Peak height (nM) | 48.31 (9.46/86.20) | 86.61 (33.01/122.10) | <0.0001 | 48.08 (1.38/82.53) | 52.59 (25.57/95.25) | 0.17 | 55.44 (23.63/92.35) | 98.25 (74.11/131.61) | 0.0043 | |
| Time to peak (min) | 20.11 (11.46/39.44) | 15.83 (12.61/23.92) | 0.064 | 20.58 (9.90/41.01) | 20.25 (14.47/29.86) | 0.84 | 19.50 (12.65/28.09) | 14.44 (11.83/20.00) | 0.072 | |
| Velocity index (nM/min) | 2.84 (0.37/8.64) | 10.01 (2.71/16.93) | <0.0001 | 2.64 (0.07/7.71) | 4.71 (1.21/11.25) | 0.025 | 5.87 (1.70/12.31) | 13.27 (5.89/20.62) | 0.033 | |
| Platelet Free Plasma | Lag time (min) | 2.03 (0/11.97) | 7.91 (5.57/10.47) | 0.047 | N.A (N.A/14.23) | 8.67 (0/15.05) | 0.037 | 7.12 (0/9.47) | 7.59 (5.68/9.17) | 0.66 |
| ETP (nM*min) | 25.79 (0/774.62) | 779.35 (78.68/1132.33) | <0.0001 | 0 (0/571.13) | 322.86 (0/955.08) | 0.0068 | 827.80 (0/1195.22) | 984.25 (465.27/1199.88) | 0.20 | |
| Peak height (nM) | 1.52 (0/76.34) | 74.12 (3.40/120.36) | <0.0001 | 0 (0/36.04) | 26.59 (0/93.36) | 0.0063 | 119.01 (0/147.45) | 103.82 (47.00/140.78) | 0.73 | |
| Time to peak (min) | 5.09 (0/18.15) | 13.08 (10.29/15.28) | 0.034 | N.A (N.A/20.03) | 14.33 (0/18.50) | 0.050 | 11.75 (0/13.42) | 12.50 (10.60/14.33) | 0.28 | |
| Velocity index (nM/min) | 0.09 (0/15.09) | 13.12 (0.22/23.95) | <0.0001 | 0 (0/6.48) | 3.69 (0/18.57) | 0.0070 | 12.01 (0/35.50) | 19.47 (6.72/27.50) | 0.73 | |
Presented data are median (25th and 75th percentile); Subjects on anticoagulants were taking one of the following agents: low molecular weight heparin, factor Xa inhibitor or Vitamin K antagonist; VTE, venous thromboembolism; ETP, endogenous thrombin potential; w/o, without; N.A., non applicable (within 120 min thrombin generation curve was not generated).
In absence of platelets, similar findings as in presence of platelets were observed with strongly diminished TG parameters in VTE cases compared to controls. Within those on anticoagulant agents, in more than 50% of VTE cases TG presented as flat line without thrombin formation (e.g. for lag time: 25th and 75th percentile were non applicable and 14.23, respectively). In subjects without anticoagulants TG parameters were comparable between cases and controls.
Platelet surface activation
Flow cytometric results of platelet surface activation markers between groups according to presence of VTE are displayed in supplemental Table S3. At resting platelet conditions, a higher percentage of platelets expressing P-selectin, CD63 and fibrinogen were found in VTE cases compared to controls. No relevant differences were observed for MFI of these activation markers. Differently, the percentage of platelets expressing PAC-1, after collagen/ADP trigger with the PFA-200 system, showed trend for lower expression in cases (20.8 [9.7/38.0] vs. 27.6 [11.0/46.0]). Within ASA users the differences remained unchanged for most of the activation markers. The difference for percentage of platelets expressing P-selectin was not present anymore, however an important difference for P-selectin MFI was observed between VTE cases (2580 [2092/4486]) and controls (3612 [2327/9272]). Within subjects on anticoagulants, CD63 expression remained higher and PAC-1 remained lower in cases vs controls. Within the combined anticoagulant and ASA therapy, cases presented with lower percentage of platelets expressing PAC-1 compared to controls.
Variables predicting VTE by applying machine learning
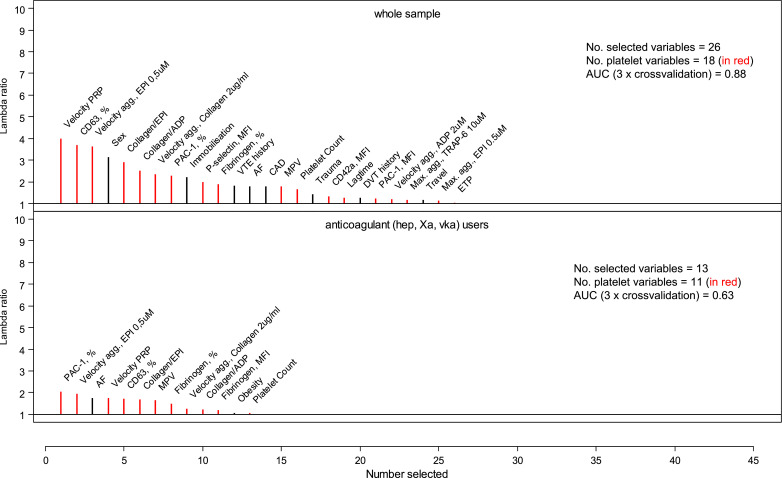
Least absolute shrinkage and selection operator (LASSO)-regularized logistic regression including a large variable set of 58 covariates including age, sex, clinical information on traditional CVRFs and VTE risk factors, and platelet biomarkers as predictive variable was applied to distinguish VTE cases from individuals without VTE. The variables selected with this approach are presented in supplemental Table S4 with 3 fold cross-validated AUC= 0.88. Fig. 3 summarizes the selected variables in the whole sample and in the subsample taking anticoagulants (without presenting the same variable – if selected – in several transformations). When analyzing the whole sample, 26 variables were identified as predictors or presence of VTE. Of these 26 variables, 69% (18 variables) were platelet-related biomarkers. The largest effect for presence of a VTE event was the velocity index in presence of platelets with a lambda ratio of 3.97 and a negative association (being lower in VTE cases) followed by a positively associated expression of CD63 (% of platelets), as a platelet dense granule protein, with lambda ratio of 3.65. Within the subgroup of subjects taking anticoagulant agents, in total 13 variables predictive of VTE were selected of which even greater percentage, 84.6% (11 variables), were platelet-related biomarkers. The highest lambda ratio of 3.18, negatively associated with VTE, was attributed to expression of PAC-1 (% of platelets) followed by the positively associated velocity of aggregation after low EPI with lambda ratio of 3.02. The selection of variables predictive of VTE in the smaller subgroups of ASA users is presented in supplemental Figure S1.
Figure 3.
Selection of variables predictive for VTE cases compared to VTE ruled outs. Model A present results in the whole sample, No of subjects = 294; and model B within subjects on anticoagulants, No of subjects = 219, No of covariates = 58 for both models. The full list of covariates is available in the supplemental material part B. Abbreviations: PRP, platelet rich plasma; EPI, epinephrine; ADP, adenosine diphosphate; MPV, mean platelet volume; AF, atrial fibrillation; CAD, coronary artery disease; DVT, deep vein thrombosis; agg., aggregation; MFI, mean fluorescence intensity; ETP, endogenous thrombin potential; AA, arachidonic acid.
Discussion
This comprehensive investigation including platelet aggregation, platelet-dependent and –independent thrombin generation and platelet-surface activation reports important evidence for distinct platelet characteristics in individuals with acute VTE independent of the underlying cardiovascular profile.
Individuals with VTE presented with different platelet aggregation, compared to subjects without VTE, also under intake of ASA: platelet aggregation in whole blood showed shorter CT after Collagen/ADP and Collagen/EPI in cases and shorter Collagen/EPI CT was also observed in the subgroup taking low dose ASA. The findings were confirmed by LTA using the agonists EPI and arachidonic acid, both agents sensitive for assessing inhibition of the acetylic acid pathway, as well as by the thromboxane B2 measurements as a gold standard for measuring ASA response [24]. Furthermore, Collagen/EPI, Collagen/ADP and velocity aggregation after low EPI were in the top 5 selected platelet variables differentiating between presence and absence of VTE independent of potential clinical confounders. Low-responsiveness to ASA has been largely debated in the scientific community: the variability in response to ASA also described as “suboptimal response to ASA”, “ASA resistance” or “high on treatment platelet reactivity” has been extensively studied in the arterial setting where up to 60% of patients were reported to exhibit a certain degree of low response to ASA [25]. Noncompliance to ASA use is one of the most frequently stated causes of laboratory defined low-responsiveness to ASA [26]. Under pathological conditions, multiple agonists activating platelets simultaneously could overcome the effect of ASA inhibition on the thromboxane A2 pathway [27]. Furthermore, increased oxidative stress may promote platelet activation and reduce ASA response by producing increased amounts of 8-isoprostagladine F2α [28]. In addition chronic aspirin use has been suggested to cause increased platelet response to thromboxane A2-independent stimuli, such as ADP, thrombin, epinephrine and collagen [29]. In patients presenting with acute myocardial infarction while on chronic ASA treatment, administration of a loading dose of ASA has been related to greater reduction of platelet thromboxane synthesis [30]. Uninhibited cyclooxygenase (COX)-2 due to increased platelet production and release of new reticulated platelets is another proposed mechanism in the case of myeloproliferative neoplasm patients and in perioperative patients at risk for thrombosis [31,32]. Despite the lower platelet count and lower MPV in VTE individuals compared to those without VTE, increased platelet production and turnover could not be excluded in the acute phase of the disease. Lower MPV has already been reported in acute VTE patients, particularly in those with active cancer, and has been related to worse prognosis and survival [11]. A recent study including more than 6,900 individuals taking ASA, clopidogrel or non-steroidal anti-inflammatory drugs suggested that malignancy may contribute to high on treatment platelet reactivity when using the PFA-100 system [33]. In the present study cancer patients were excluded and therefore prevalent cancer was unlikely a contributor to shorter CTs in the PFA-200 analysis. The findings of low responders to ASA could explain the lower protection from ASA for incident VTE in high risk patients compared to anticoagulant strategies, as repeatedly reported [34]. Whether ASA low-responsiveness in the acute phase remain in the chronic post-VTE period is for now unknown. This is particularly relevant in light of the reported inadequate protection by low dose ASA in subjects at risk for recurrent VTE, as observed in the low dose ASA clinical trials [12]. For patients requiring ASA after discontinuation of the standard anticoagulant treatment, it is important to reassess the response to ASA and in case of low responders to consider alternative prophylactic measures. It has been recently demonstrated that twice daily low dose ASA significantly improves the antiplatelet response in patients with Essential Thrombocythemia, recognized as low-responders to once daily low dose ASA [35].
Whereas platelet aggregation was increased in cases vs controls for the ASA sensitive assays, differently LTA after high ADP, TRAP-6 and particularly collagen agonism was lower in cases vs controls. Similarly, the results on platelet-dependent thrombin generation showed longer lag time, slower velocity and lower peak height in the VTE group compared to the controls. The difference was particularly evident for platelet-dependent TG within the subgroup of subjects not on anticoagulant therapy. Moreover, in the same subgroup and in absence of platelets, no differences in TG profile were observed between cases and controls. These findings of lower platelet-dependent TG in conjunction to the increased expression of platelet surface activation markers and lower expression of PAC-1 after ex vivo collagen/ADP agonism could indicate the presence of an “exhausted platelet syndrome” in individuals with VTE. This concept has been already reported in acute medical conditions such as subjects with cancer at risk for VTE as well as subjects with trauma-induced coagulopathy [36]. In addition, coagulation factor consumption at the time of the acute thrombus formation could further contribute to the observed reduction of TG in cases compared to controls. Decreased plasma Factor VII levels, lower fibrinogen concentration and Factor XIII levels have been frequently reported in patients with acute thrombosis, such as DVT and PE [37,38].
Our study has limitations. VTE is an acute disease that encompasses two different clinical manifestations of PE and DVT, frequently presenting with different severity and duration of the clinical signs and symptoms. These aspects including the different timing and duration of intake for both anticoagulant and antiplatelet agents could affect the results on platelet function.
This study has several strengths: It is the largest study comprehensively investigating platelet function in subjects with acute VTE to date. The highly standardized assessment of platelet function minimized pre-analytical variability that might have affected results. LTA measurements without trigger support the absence of in-vitro platelet activation. On the other side, certain limitations merit consideration: data on ASA compliance were not available, as ASA effectiveness was not the aim of this explorative study. However, in conclusion from the present analysis, low response to ASA will be subject of future investigations. The sample size was unique for the comprehensive assessment, however was still comparably small for analyzing the relation of platelet phenotype to clinical outcome in an acute VTE. It has been previously reported that higher TG is associated with higher risk for incident or recurrent VTE. This is the first study that reports the TG profile in presence and absence of platelets at the time of the acute venous thrombus formation [39,40].
The application of machine learning technic identified the most relevant differentiating factors between confirmed and objectively excluded VTE. Our finding that 69% of the selected variables were platelet-related puts special emphasis on the relevance of platelets and platelet function testing in venous thrombosis. It has been reported that hemodynamics affects the proportion of activated platelets, e.g at low shear rate, such as in the venous system, larger proportion of platelets get activated and release their granule content, unlike in the arterial system at high shear hemodynamics [41]. This study supports the value of platelet function testing to explore the degree of platelet activation in the thrombotic process.
In conclusion, this study characterizes the platelet phenotype in individuals with acute VTE and proves platelet function to substantially differ between subjects with VTE and those with a VTE ruled out independent of the present clinical profile and risk factors. The data further provide evidence for the occurrence of ASA low-responsiveness in acute VTE which might explain the failure of ASA to better protect subjects at risk, but also provides evidence for “exhausted” platelets in this disease. Whether the platelet phenotype could improve identification of individuals at risk for recurrent VTE and/or worse outcome remains to be determined.
Authorship Contributions
MP-N, SH, VL, SK, KL and PSW designed the study. MP-N, SH, IM, CG, VL, KL and PSW designed this analysis. MN performed the statistical analysis. MP-N, BW, VtC, JHP, HMS, TK, TM and KJL performed research and contributed to the interpretation of results. MP-N, HtC and PSW drafted the manuscript. All authors contributed to critical review and the composition of the final manuscript.
Disclosures of Conflict of Interest
The VTEval and FOCUS BioSeq studies were funded by the German Federal Ministry of Education and Research (BMBF 01EO1003 and 01EO1503), internal funds of the Clinical Epidemiology and Systems Medicine (Center for Thrombosis and Hemostasis, Mainz, Germany), and a grant from Bayer AG. PS Wild is PI of the German Center for Cardiovascular Research (DZHK) and he is funded by the Federal Ministry of Education and Research (BMBF 01EO1503). He received honoraria for lectures or consulting from Boehringer Ingelheim, Bayer AG, Sanofi-Aventis, Bayer Vital, AstraZeneca, DiaSorin and Evonik and received non-financial support from DiaSorin and I.E.M. S. Konstantinides reports grants and personal fees from Bayer AG, grants from Boehringer Ingelheim, personal fees from MSD, grants from Servier, grants and personal fees from Actelion - Janssen, grants and personal fees from Daiichi-Sankyo, personal fees from Pfizer - Bristol-Myers Squibb, outside the submitted work. JH. Prochaska reports grants from Federal Ministry of Education and Research, Germany, personal fees from Bayer AG, personal fees from Boehringer Ingelheim, non-financial support from German Center for Cardiovascular Research, outside the submitted work. T. Koeck reports personal fees from Bayer AG, outside the submitted work. The remaining authors declare no competing interests.
Acknowledgments
We are very grateful to all study participants of the GMP-VTE study and VTEval Study, Heidrun Lamparter, Bianca Zäpf and Dr. Heidrun Dorsch for project management.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102978.
Appendix. Supplementary materials
References
- 1.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 2.Collins R, Peto R, Hennekens C. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. LancetLancet. 2009;373(9678):1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santilli F, Vazzana N, Liani R, Guagnano MT, Davi G. Platelet activation in obesity and metabolic syndrome. Obes Rev: Off J Int Assoc Study of Obes. 2012;13(1):27–42. doi: 10.1111/j.1467-789X.2011.00930.x. [DOI] [PubMed] [Google Scholar]
- 4.Ferroni P, Basili S, Falco A, Davi G. Platelet activation in type 2 diabetes mellitus. J Thromb Haemost: JTH. 2004;2(8):1282–1291. doi: 10.1111/j.1538-7836.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 5.Brill A, Fuchs TA, Chauhan AK. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. BloodBlood. 2011;117(4):1400–1407. doi: 10.1182/blood-2010-05-287623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heestermans M, Salloum-Asfar S, Streef T. Mouse venous thrombosis upon silencing of anticoagulants depends on tissue factor and platelets, not FXII or neutrophils. BloodBlood. 2019;133(19):2090–2099. doi: 10.1182/blood-2018-06-853762. [DOI] [PubMed] [Google Scholar]
- 7.Chirinos JA, Heresi GA, Velasquez H. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45(9):1467–1471. doi: 10.1016/j.jacc.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 8.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17(1):47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 9.Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Mean platelet volume is a risk factor for venous thromboembolism: the Tromso Study, Tromso, Norway. J Thromb Haemost: JTH. 2010;8(1):157–162. doi: 10.1111/j.1538-7836.2009.03498.x. [DOI] [PubMed] [Google Scholar]
- 10.Lippi G, Buonocore R, Cervellin G. The mean platelet volume is decreased in patients diagnosed with Venous Thromboembolism in the emergency department. Semin. Thromb. Hemost. 2016;42(6):632–635. doi: 10.1055/s-0036-1571335. [DOI] [PubMed] [Google Scholar]
- 11.Riedl J, Kaider A, Reitter EM. Association of mean platelet volume with risk of venous thromboembolism and mortality in patients with cancer. results from the Vienna Cancer and Thrombosis Study (CATS) Thromb. Haemost. 2014;111(4):670–678. doi: 10.1160/TH13-07-0603. [DOI] [PubMed] [Google Scholar]
- 12.Simes J, Becattini C, Agnelli G. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014;130(13):1062–1071. doi: 10.1161/CIRCULATIONAHA.114.008828. [DOI] [PubMed] [Google Scholar]
- 13.Madridano O, del Toro J, Lorenzo A. Subsequent arterial ischemic events in patients receiving anticoagulant therapy for venous thromboembolism. J. Vasc. Surg. Venous Lymphat. Disord. 2015;3(2):135–141. doi: 10.1016/j.jvsv.2014.11.002. e1. [DOI] [PubMed] [Google Scholar]
- 14.Lind C, Flinterman LE, Enga KF. Impact of incident venous thromboembolism on risk of arterial thrombotic diseases. Circulation. 2014;129(8):855–863. doi: 10.1161/CIRCULATIONAHA.113.004168. [DOI] [PubMed] [Google Scholar]
- 15.Reich LM, Folsom AR, Key NS. Prospective study of subclinical atherosclerosis as a risk factor for venous thromboembolism. J Thromb Haemost: JTH. 2006;4(9):1909–1913. doi: 10.1111/j.1538-7836.2006.02121.x. [DOI] [PubMed] [Google Scholar]
- 16.Prandoni P. Venous and arterial thrombosis: two aspects of the same disease. Eur J Intern Med. 2009;20(6):660–661. doi: 10.1016/j.ejim.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Frank B, Ariza L, Lamparter H. Rationale and design of three observational, prospective cohort studies including biobanking to evaluate and improve diagnostics, management strategies and risk stratification in venous thromboembolism: the VTEval Project. BMJ Open. 2015;5(7) doi: 10.1136/bmjopen-2015-008157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konstantinides SV, Barco S, Rosenkranz S. Late outcomes after acute pulmonary embolism: rationale and design of FOCUS, a prospective observational multicenter cohort study. J. Thromb. Thrombolysis. 2016;42(4):600–609. doi: 10.1007/s11239-016-1415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ten Cate V, Koeck T, Panova-Noeva M. A prospective cohort study to identify and evaluate endotypes of venous thromboembolism: Rationale and design of the Genotyping and Molecular Phenotyping in Venous ThromboEmbolism project (GMP-VTE) Thromb Res. 2019;181:84–91. doi: 10.1016/j.thromres.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Panova-Noeva M, Schulz A, Hermanns MI. Sex-specific differences in genetic and nongenetic determinants of mean platelet volume: results from the Gutenberg Health Study. BloodBlood. 2016;127(2):251–259. doi: 10.1182/blood-2015-07-660308. [DOI] [PubMed] [Google Scholar]
- 21.Panova-Noeva M, Schulz A, Spronk HM. Clinical Determinants of Thrombin Generation Measured in Presence and Absence of Platelets-Results from the Gutenberg Health Study. Thromb Haemost. 2018;118(5):873–882. doi: 10.1055/s-0038-1641565. [DOI] [PubMed] [Google Scholar]
- 22.Crown WH. Potential application of machine learning in health outcomes research and some statistical cautions. Value in Health: J Int Soc Pharmacoecon Outcomes Res. 2015;18(2):137–140. doi: 10.1016/j.jval.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Stekhoven DJ, Buhlmann P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–118. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 24.Blann AD, Kuzniatsova N, Lip GY. Vascular and platelet responses to aspirin in patients with coronary artery disease. Eur J Clin Invest. 2013;43(1):91–99. doi: 10.1111/eci.12021. [DOI] [PubMed] [Google Scholar]
- 25.Gasparyan AY, Watson T, Lip GY. The role of aspirin in cardiovascular prevention: implications of aspirin resistance. J Am Coll Cardiol. 2008;51(19):1829–1843. doi: 10.1016/j.jacc.2007.11.080. [DOI] [PubMed] [Google Scholar]
- 26.Biondi-Zoccai GG, Lotrionte M, Agostoni P. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J. 2006;27(22):2667–2674. doi: 10.1093/eurheartj/ehl334. [DOI] [PubMed] [Google Scholar]
- 27.Yan Y, Phillips DR. Aspirin response and failure in diabetic patients with cardiovascular disease. Curr Opin Pharmacol. 2005;5(2):190–197. doi: 10.1016/j.coph.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, Wang J, Feng J. Aspirin resistance mediated by oxidative stress-induced 8-Isoprostaglandin F2. J Clin Pharm Ther. 2019;44(5):823–828. doi: 10.1111/jcpt.12838. [DOI] [PubMed] [Google Scholar]
- 29.Valles J, Lago A, Moscardo A, Tembl J, Parkhutik V, Santos MT. TXA2 synthesis and COX1-independent platelet reactivity in aspirin-treated patients soon after acute cerebral stroke or transient ischaemic attack. Thromb Res. 2013;132(2):211–216. doi: 10.1016/j.thromres.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Santos MT, Madrid I, Moscardo A. The administration of a loading dose of aspirin to patients presenting with acute myocardial infarction while receiving chronic aspirin treatment reduces thromboxane A2-dependent platelet reactivity. PlateletsPlatelets. 2014;25(4):268–273. doi: 10.3109/09537104.2013.816671. [DOI] [PubMed] [Google Scholar]
- 31.Pascale S, Petrucci G, Dragani A. Aspirin-insensitive thromboxane biosynthesis in essential thrombocythemia is explained by accelerated renewal of the drug target. BloodBlood. 2012;119(15):3595–3603. doi: 10.1182/blood-2011-06-359224. [DOI] [PubMed] [Google Scholar]
- 32.Gong X, Wang X, Xu Z. Over-expression of cyclooxygenase-2 in increased reticulated platelets leads to aspirin resistance after elective off-pump coronary artery bypass surgery. Thromb Res. 2017;160:114–118. doi: 10.1016/j.thromres.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Kweon OJ, Lim YK, Kim B, Lee MK, Kim HR. Effectiveness of platelet function analyzer-100 for Laboratory Detection of Anti-Platelet Drug-Induced Platelet Dysfunction. Ann. Lab Med. 2019;39(1):23–30. doi: 10.3343/alm.2019.39.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geerts WH, Bergqvist D, Pineo GF. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 Suppl):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 35.Rocca B, Tosetto A, Betti S. A randomized, double-blind trial of three aspirin regimens to optimize antiplatelet therapy in essential thrombocythemia. Blood. 2020 doi: 10.1182/blood.2019004596. [DOI] [PubMed] [Google Scholar]
- 36.Riedl J, Kaider A, Marosi C. Decreased platelet reactivity in patients with cancer is associated with high risk of venous thromboembolism and poor prognosis. Thromb Haemost. 2017;117(1):90–98. doi: 10.1160/TH16-02-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kucher N, Schroeder V, Kohler HP. Role of blood coagulation factor XIII in patients with acute pulmonary embolism. Correlation of factor XIII antigen levels with pulmonary occlusion rate, fibrinogen, D-dimer, and clot firmness. Thromb Haemost. 2003;90(3):434–438. doi: 10.1160/TH03-07-0031. [DOI] [PubMed] [Google Scholar]
- 38.Schut AM, Meijers JC, Lisman-van Leeuwen Y. Decreased plasma levels of activated factor VII in patients with deep vein thrombosis. J Thromb Haemost: JTH. 2015;13(7):1320–1324. doi: 10.1111/jth.12980. [DOI] [PubMed] [Google Scholar]
- 39.Tripodi A, Martinelli I, Chantarangkul V, Battaglioli T, Clerici M, Mannucci PM. The endogenous thrombin potential and the risk of venous thromboembolism. Thromb Res. 2007;121(3):353–359. doi: 10.1016/j.thromres.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 40.van Hylckama Vlieg A, Christiansen SC, Luddington R, Cannegieter SC, Rosendaal FR, Baglin TP. Elevated endogenous thrombin potential is associated with an increased risk of a first deep venous thrombosis but not with the risk of recurrence. Br J Haematol. 2007;138(6):769–774. doi: 10.1111/j.1365-2141.2007.06738.x. [DOI] [PubMed] [Google Scholar]
- 41.Welsh JD, Poventud-Fuentes I, Sampietro S, Diamond SL, Stalker TJ, Brass LF. Hierarchical organization of the hemostatic response to penetrating injuries in the mouse macrovasculature. J Thromb Haemost: JTH. 2017;15(3):526–537. doi: 10.1111/jth.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.