Abstract
Background
During atherogenesis, cholesterol precipitates into cholesterol crystals (CC) in the vessel wall, which trigger plaque inflammation by activating the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome. We investigated the relationship between CC, complement and NLRP3 in patients with cardiovascular disease.
Methods
We analysed plasma, peripheral blood mononuclear cells (PBMC) and carotid plaques from patients with advanced atherosclerosis applying ELISAs, multiplex cytokine assay, qPCR, immunohistochemistry, and gene profiling.
Findings
Transcripts of interleukin (IL)-1beta(β) and NLRP3 were increased and correlated in PBMC from patients with acute coronary syndrome (ACS). Priming of these cells with complement factor 5a (C5a) and tumour necrosis factor (TNF) before incubation with CC resulted in increased IL-1β protein when compared to healthy controls. As opposed to healthy controls, systemic complement was significantly increased in patients with stable angina pectoris or ACS. In carotid plaques, complement C1q and C5b-9 complex accumulated around CC-clefts, and complement receptors C5aR1, C5aR2 and C3aR1 were higher in carotid plaques compared to control arteries. Priming human carotid plaques with C5a followed by CC incubation resulted in pronounced release of IL-1β, IL-18 and IL-1α. Additionally, mRNA profiling demonstrated that C5a and TNF priming followed by CC incubation upregulated plaque expression of NLRP3 inflammasome components.
Interpretation
We demonstrate that CC are important local- and systemic complement activators, and we reveal that the interaction between CC and complement could exert its effect by activating the NLRP3 inflammasome, thus promoting the progression of atherosclerosis.
Keywords: Cholesterol crystals, Complement system, NLRP3 inflammasome, Coronary artery disease, Carotid atherosclerosis, Atherosclerosis, Inflammation
Research context.
Evidence before the study
Atherosclerotic lesions are characterized by accumulation of lipids, cholesterol crystals (CC), and infiltration of immune cells. CC are present at all stages of atherogenesis and constitute primary endogenous danger signals that incite plaque inflammation. CC activate the NLRP3 inflammasome leading to IL-1β release and formation of atherosclerotic lesions. Based on these data a large clinical trial (CANTOS) inhibiting IL-1β by canakinumab was started, and this trial revealed a significant reduced risk of recurrent cardiovascular events. The CANTOS trial clearly demonstrates the important role of inflammation and IL-1β in atherosclerotic disorders. However, the interactions between inflammatory pathways leading to NLRP3 activation and IL-1β release in human clinical atherosclerosis are still not fully understood. We have earlier reported that complement activation is an important part of the inflammatory response induced by CC. However, data on interactions between CC, complement, and NLRP3 in patients with atherosclerotic disease are limited. Thus, we performed a detailed analysis of the relation between CC, complement and NLRP3 signaling pathways in patients with coronary artery disease - and carotid atherosclerosis.
Added value of this study
In in vitro, ex vivo, and using patient's samples, we show that complement contributes to the CC-driven inflammatory responses. Our data imply a positive association between CC-induced complement and NLRP3 activation, both systemically and within the atherosclerotic lesion, with disease severity and instability.
Implications of all the available evidence
The pathogenic loop between complement, CC and NLRP3 inflammasomes may represent a promising target for therapy of atherosclerotic disorders.
Alt-text: Unlabelled box
1. Introduction
Atherosclerosis is a progressive chronic disease with the bidirectional interaction between inflammation and lipids as its hallmark. Indeed, the atherosclerotic lesions are characterized by the accumulation of lipids together with infiltration of immune cells such as monocytes, macrophages, T cells and neutrophils [1]. When cholesterol accumulates in the vessel wall to the level that exceeds macrophage capacity for elimination, precipitation into cholesterol crystals (CC) will occur [2]. Oxidized low-density lipoprotein is endocytosed by CD36 that coordinates the intracellular conversion of this ligand to CC [3,4]. CC are present at all stages of atherogenesis, and constitute the primary endogenous danger signals that incite plaque inflammation, contributing to the development and progression of atherosclerosis and its complications like myocardial infarction and ischemic stroke [[5], [6], [7]]. Phagocytosis of CC causes lysosomal damage and this is sensed by the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome, leading to the release of interleukin (IL)-1β [5]. IL-1β is upregulated in atherosclerotic disorders and is associated with disease severity and outcome [8,9]. More recently, a large clinical trial on IL-1β inhibition by canakinumab, a monoclonal antibody against IL-1β, revealed a significant reduced risk of recurrent cardiovascular events demonstrating the important role of IL-1β in atherosclerotic disorders [10]. However, the mechanisms of enhanced release of IL-1β as well as the role of CC-induced NLRP3 inflammasome activation in clinical atherosclerosis are not fully understood.
Early studies have demonstrated the importance of both systemic and local complement in the progression of atherosclerosis, and depending on the stage of atherogenesis, complement can either promote or attenuate atherogenesis [[11], [12], [13], [14], [15], [16]]. Complement is strongly activated by CC, with all three pathways involved, i.e., the classical (CP), the alternative (AP), and the lectin pathway (LP), leading to complement deposition on the surface and subsequent phagocytosis and stimulation of endothelial cells [[17], [18], [19], [20]]. CC also employs complement to induce coagulation activation [21]. Complement activation leads to release of the anaphylatoxins C3a and C5a which trigger inflammatory signalling through their G-protein-coupled receptors, resulting in increased vascular permeability and chemotaxis [22]. CC generate C5a in whole blood which, in combination with tumour necrosis factor (TNF), works as signal 1 and is necessary for priming of monocytes for NLRP3 activation leading to IL-1β release (mature form 17KDa) [18]. The role of this interaction in clinical atherosclerosis is, however, not clear. Thus, the aim of the present study was to perform a detailed analysis of the relation between complement, NLRP3 signalling pathways and CC in patients with coronary artery disease and carotid atherosclerosis.
2. Materials and methods
2.1. Reagents
PBMC were isolated from whole-blood anticoagulated with lepirudin (Refludan®, Celgene). Recombinant C5a, ultrapure cholesterol, 1-propanol were purchased from Sigma-Aldrich, and TNF from Genentech. The following reagents were used for qRT-PCR analyses: RNeasy Mini kit (Qiagen), DNase and High Capacity RNA-to-cDNA Kit (Applied Biosystems) and PerfeCTa® qPCR FastMix™ (Quanta Biosciences). Assays were from Applied Biosystems: GAPDH (Hs99999905_m1), NLRP3 (Hs00918082_m1), TNF (Hs00174128_m1), and IL-1β (Hs01555410_m1) and primers from SYBR as shown in Supplementary Table I. Antibodies used for immunohistochemistry: polyclonal rabbit anti-human C1q (A0136, Dako), normal rabbit IgG (Ab105-C, R&D systems), goat anti-mouse conjugated to Alexa 647 (Invitrogen), anti-C5b-9 (DIA-011, Antibodyshop), anti-CD68 (Dako Agilent M0814), anti-CD20 (Dako Agilent M0755), anti-CD3 (Dako Agilent A0452), anti-CD15 (BD Pharmingen 559045), control IgG2a (BD Pharmigen). Antibodies used for flow cytometry included anti-CD14 FITC (M5E2), anti-CD4 PerCP-CY 5.5 (RPA-T4), anti-CD8 BV605 (SK1) and anti-CD19 BV711 (SJ25C1) and were purchased from BD Pharmigen, and anti-CD3 APC (UCHT1) were from Invitrogen. Fluorescence-minus-one (FMO) was used as control.
2.2. Preparation of cholesterol crystals (CC)
CC were made as described in Samstad et al. [18] Briefly, Ultrapure cholesterol (100 mg) was dissolved in 1-propanol (50 ml). The solution was mixed with distilled water (1:1.5) and rested for at least ten minutes for monohydrate crystals to stabilize. 1-Propanol was removed by evaporation and CC were resuspended in PBS/0.05% HSA. All steps were performed at room temperature yielding CC with a size range of one–two μm that were stored at 4°C. CC were tested for endotoxin contamination using Limulus amebocyte lysate assay (LAL), and the LPS level was under the detection limit. Furthermore, treating whole blood with anti-CD14 Abs or lipid Iva did not reduce the CC-induced cytokines, whereas the LPS response was completely inhibited with the anti-CD14 Ab (data not shown).
2.3. Patient recruitment
Patients with coronary artery disease: Peripheral mononuclear cells (PBMC) and plasma were collected from a patient population with coronary artery disease (CAD). The study included only patients with CAD verified by coronary angiography (lesions with ≥50% lumen reduction compared to reference segment). Patients were classified into either stable angina pectoris (SAP) referred to elective coronary angiography with at least one significant coronary stenosis or acute coronary syndrome (ACS). SAP was defined as episodes of reversible ischemic chest pain with significant CAD. ACS patients consisted of patients with unstable angina pectoris, defined as angina at rest or crescendo angina with a clinical indication of urgent (within 48 h) coronary angiography or non-ST elevation myocardial infarction. NSTEMI was defined according to the universal definition of MI as typical rise and fall of the cardiac specific biomarker troponin T (TnT) with at least one value above the 99th percentile of the upper reference limit in patients presenting with symptoms of ischemia without any ST elevation on ECG [23]. Mean age of the study population was about 65 years and around 82 % male. Exclusion criteria included clinically unstable patients with cardiac arrest, cardiogenic shock, hypotension or pulmonary congestion, active malignant disease, chronic inflammatory disease (e.g. inflammatory bowel disease, rheumatic arthritis, systemic lupus erythematosus), intercurrent infection or use of glucocorticosteroids. Controls in Figs. 1 and 3 were apparently healthy based on disease history and biochemical markers within normal range, including CRP less than 4.5. Controls in Fig. 2 were healthy controls, age and gender matched. Clinical characteristics are presented in Supplementary Table II and III.
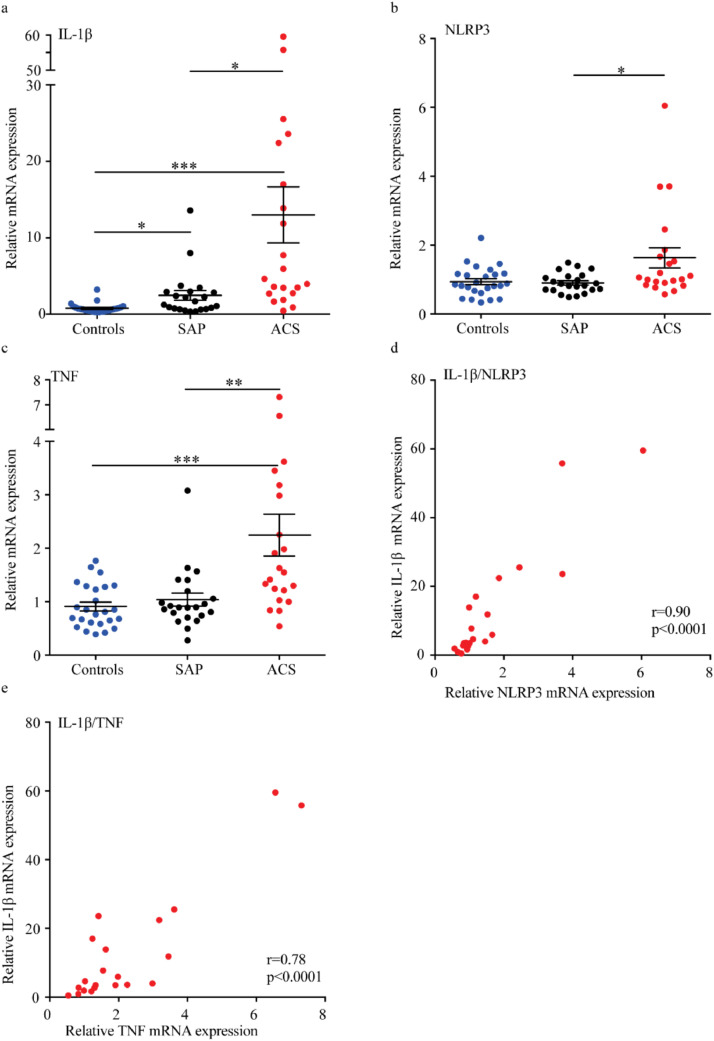
Fig. 1.
Transcripts of IL-1β, NLRP3 and TNF in PBMC from controls and ACS and SAP patients. PBMC were isolated from blood from patients with SAP, ACS and healthy controls, RNA isolated and PCR run. Relative mRNA expression for IL-1β (a), NLRP3 (b) and TNF (c) is presented as mean ± SEM, n=21–25. Furthermore, a correlation between IL-1β (y-axis) and NLRP3 (d) and TNF (e) (x-axes) mRNA expressions in PBMC from patients with ACS were found. ACS, acute coronary syndrome; IL, interleukin; NLRP3, NACHT, LRR and PYD domains-containing protein 3; SAP, Stable angina pectoris; TNF, tumour necrosis factor. *p<0.05, **p<0.01, ***p<0.001. (a-c) Kruskal Wallis and Dunn's multiple comparison test, (d,e) Spearman's correlation.
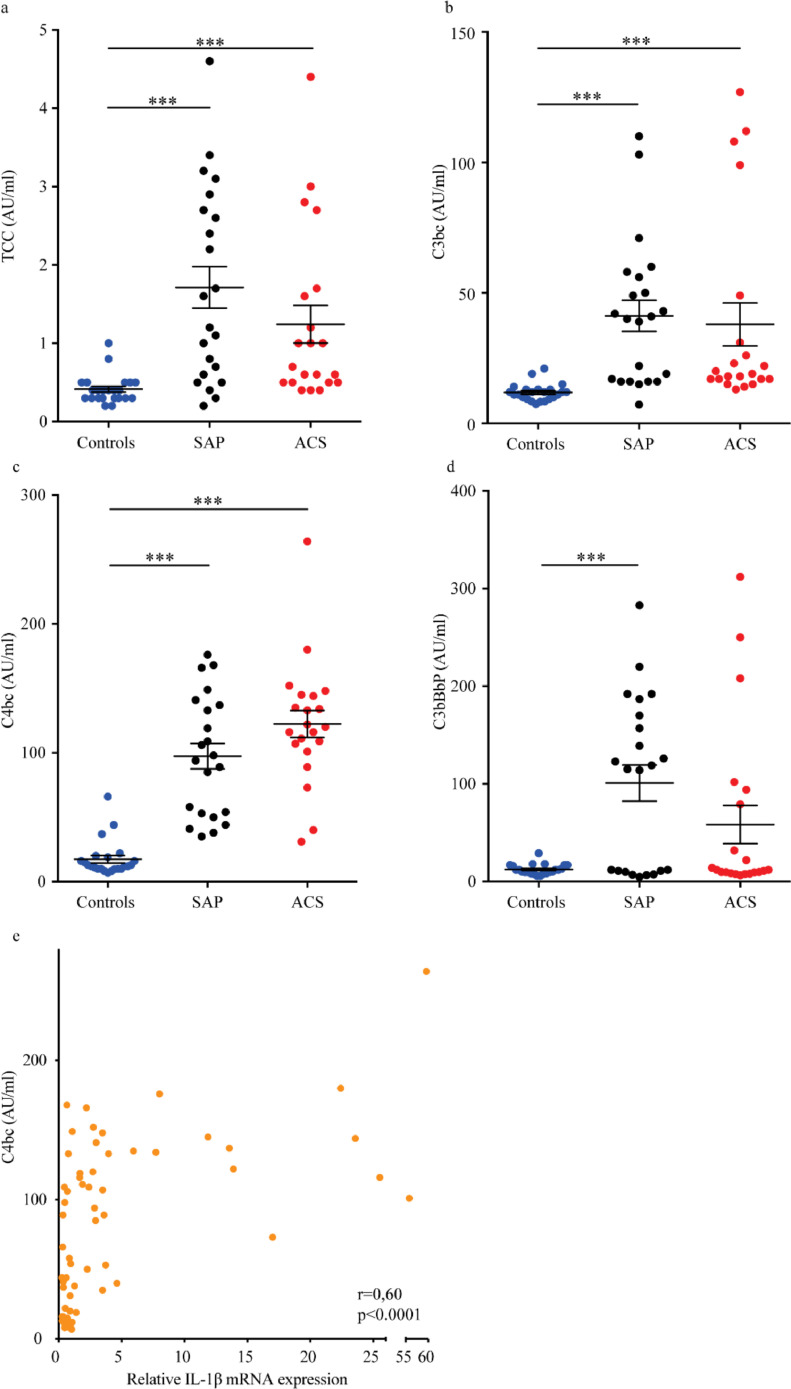
Fig. 3.
Complement activation products in plasma from controls and atherosclerotic patients with SAP and ACS. Presence of the end product of complement activation, TCC (a), the common complement component for the different pathways, C3bc (b), the activation product for both the classical and lectin pathway, C4bc (c), and the activation product for the alternative pathway, C3bBbP (d) in patients with SAP, ACS and healthy controls. Results are presented as mean ±SEM, n=21-23. e) Presence of activation product C4bc was correlated to IL-1β mRNA expression of controls, ACS and SAP combined, n=66. ACS, acute coronary syndrome; AU, arbitrary unit; C: Complement factor; IL, interleukin; SAP, Stable angina pectoris; TCC, terminal complement complex. ***p<0.001, Bartlett's test for equal variance on log-transformed data with Bonferroni post-tests, (e) Spearman's correlation.
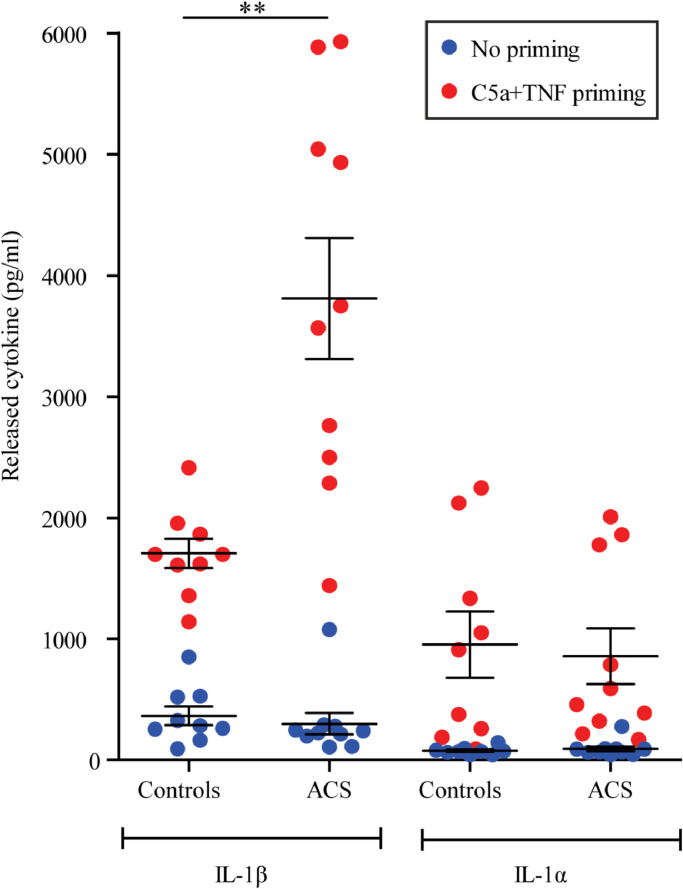
Fig. 2.
Cytokine release from PBMC from patients with ACS and controls primed with combination of C5a and TNF and stimulated with CC. PBMC were isolated from blood from patients with ACS and healthy controls, primed with C5a+TNF or left unprimed prior to incubation with CC and supernatant collected. IL-1β and IL-1α released is presented for the two groups as mean ± SEM, n=9-10. Basal levels and control treatments are presented in Supplementary figure I. ACS, acute coronary syndrome; C, Complement factor; CC, cholesterol crystals; IL, interleukin; TNF, tumour necrosis factor. **p<0.01 between controls and ACS with priming, Mann-Whitney two-tail test.
Patients with carotid plaques: Patients with high-grade internal carotid stenosis (≥70%) or ischemic stroke within the last month were recruited at the Department of Neurology, Oslo University Hospital Rikshospitalet. Patients were classified into either symptomatic group including patients with ischemic stroke within the last month, or asymptomatic group without ischemic stroke the last month. The carotid stenosis was diagnosed and classified by precerebral colour duplex and CT angiography according to consensus criteria. As part of the ultrasound examination, the total extracranial part of the carotid artery was examined with B-mode and Doppler analyses. Ultrasound plaque appearance in terms of echogenicity was classified according to consensus criteria [24]. Mean age of the study population was about 67 years and around 65 % male. Exclusion criteria were heart failure, liver disease, kidney disease, and severe concomitant disease such as infection, connective tissue disease, or malignancy. Distal carotid arteries were disease free segments from the same patients. Normal renal arteries are taken post-mortem with no history of cardiovascular disease. Clinical characteristics are presented in Supplementary Table IV and V.
2.4. Cell isolation and culture
PBMC were separated from fresh heparinized blood by Isopaque-Ficoll gradient centrifugation using Lymphoprep (Nycomed, Oslo, Norway) or BD Vacutainer CPT (ref 362780 Becton, Dickinson and Company, Franklin Lakes). Data in Fig. 1 include patients with stable angina (SAP, N=21), acute coronary syndrome (ACS, N=25), or healthy donors (controls, N=25) and stored in liquid nitrogen until mRNA analyses. For protein analysis (in Fig. 2), ten patients with ACS and nine healthy donors were recruited, and their PBMCs were used immediately for further in vitro experiments. Cells were maintained in RPMI supplemented with 10% heat inactivated pooled human A+ serum (The Blood Bank, St. Olav's Hospital, Trondheim, Norway) unless otherwise noted. Cells were primed with C5a (1µg/ml), TNF (10 ng/ml) a combination of C5a and TNF or PBS for 2hrs prior to stimulation with CC (500 or 1000 μg/ml) for 16 hrs.
2.5. Culturing of human atherosclerotic plaques
Atherosclerotic carotid plaques were collected during carotid endarterectomy. Pieces of plaques used were dissected out as equal as possible in size. Plaques used for RNA analysis were rapidly frozen in liquid nitrogen and stored at -80°C until further analyses, whereas plaques for culture and immunohistochemistry were kept in PBS until further processing. Biopsies from atherosclerotic carotid plaques were placed in Dulbecco's modified Eagle's medium (D-MEM/F12; Gibco) enriched with 30 mg/ml endotoxin-free and fatty acid-free bovine serum albumin (Sigma). The biopsies containing atherosclerotic plaques of each patient were split into macroscopically equal pieces and primed with C5a (1µg/ml), TNF (10 ng/ml), a combination of C5a and TNF or PBS for 2hrs prior to stimulation with CC (1000 μg/ml) for 6 hrs. The plaque biopsies were placed in RNA Later (Qiagen) for RNA analysis and plaque supernatants were collected, centrifuged (1700 g for 5 min, 4°C), and snap frozen before being stored at -80°C until further analysis. Some results were presented as fold changes compared to PBS control, as the biopsies are heterogenous tissues with different cellular composition. The relative mRNA expression was presented when comparing atherosclerotic plaques to a distal area from the plaques or to normal arteries. Published gene expression profiles of 8 human carotid plaques [25] (Human Genome U133A 2.0 Array, Affymetrix) were analysed using CIBERSORT [26] to estimate the relative abundance of 22 infiltrating immune cell types, using the LM22 signature matrix and 1000 permutations (Fig. 8).
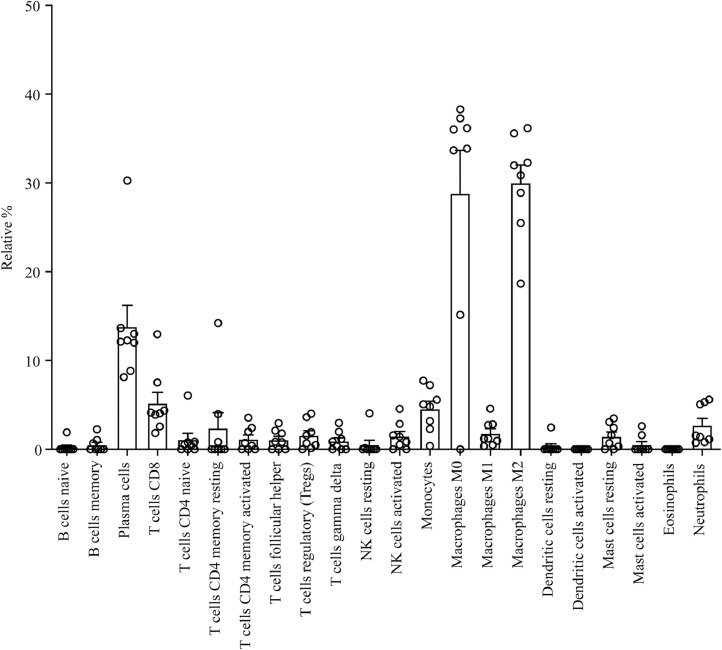
Fig. 8.
Immune cell types detected in carotid plaques. CIBERSORT analysis was done on microarray results performed on RNA isolated from carotid plaques [26]. Presented are different cell types as relative % in relation to the total cell content. Results are shown for 8 different plaques from 8 different patients.
2.6. Quantitative real time PCR
Total RNA was extracted from PBMC or human atherosclerotic plaques using RNeasy Mini kit as recommended by the manufacturer. Cell extracts were DNase treated before being reverse transcribed using High Capacity RNA-to-cDNA Kit and cycled in a StepOnePlus™ Real-Time PCR cycler (Applied Biosystems). Fold changes were calculated by delta Delta Ct methods using human GAPDH or actin as endogenous control for mRNA expression.
2.7. Immunohistochemistry
Immunohistochemistry was performed on human carotid tissue, where 4 μm paraffin embedded sections were cut onto SuperFrost Plus slides, dried over night at 37°C and then 60 minutes at 60°C. The slides were dewaxed in Tissue Clear, and taken through a series of descending ethanol to water in an automatic slide stainer (Sakura Tissue-Tek © Prisma™). The tissues were pre-treated in Target Retrieval Solution, High/Low pH (Dako K8004/5) in PT Link (Dako) for 20 minutes at 97°C to facilitate antigen retrieval. The further staining was performed on Dako Autostainer. Following washes in wash buffer (Dako S3006), endogenous peroxidase activity was quenched by incubation in Peroxydase block (Dako S2023). Tissues were thereafter stained for complement C5-9 (TCC), and their isotype control, as well as for CD68, CD20, CD3 and CD15. The slides were then washed in wash buffer and incubated for 30 minutes in labelled polymer HRP anti-Rabbit (Dako K4003) and with DAB (Dako K3468) to develop the stain. In Sakura Tissue-Tek © Prisma™ the slides were lightly counterstained with Hematoxylin, dehydrated through ascending grades of ethanol, cleared in Tissue Clear and mounted on cover slips. The sections were analysed using EVOS FL auto microscope (Thermo Fisher Scientific).
2.8. Measurements of systemic or local complement
The following complement activation products were measured by ELISA in EDTA plasma as described elsewhere. [27] soluble TCC, C3bc, C4bc and C3bBbP. These assays are based on monoclonal capture antibodies specific for neoepitopes expressed after a component is activated, and thus only the activated form is detected. An antibody (aE11) to a neoepitope expressed in C9 was used for the detection of soluble TCC. When C3 is activated, the C3b and subsequent C3c fragments both express a neoepitope recognized by the monoclonal antibody bH6. Thus, the ELISA detecting C3 activation is termed C3bc. The same principle applies for detection of C4 activation, which is denoted C4bc.
2.9. Gene expression and bioinformatic analyses
Plaques were homogenized using a FastPrep 24 instrument (≈6 m/s, MD Biomedicals) three times for 40 seconds with zirconium oxide beads (Bertin Tech) (six 2.8 mm beads and 0.8 g 1.4 mm beads per sample) in Isol-RNA Lysis Reagent (VWR, 5Prime). The aqueous phase was isolated after adding chloroform and centrifugation (13000 rpm, 15 min, 4°C), and RNA was isolated further with RNeasy microkit (Qiagen). Nanodrop spectrophotometer (ND-1000, Saveen Werner) was used to measure RNA concentration. RNA expression analysis was run on the nCounter ® analysis system, running 12 samples at a time. The procedure was performed according to the manufacturer´s instructions, applying about 100 ng mRNA. The kit used was a fixed code set for mRNA analysis with genes involved in human immunology nCounter GX Human Immunology Kit v2 (Nanostring Technologies). The number of mRNA molecules per gene was accounted for detection level “average (negative control) + 2SD (negative controls)”, normalized against instrument variations (positive controls) and stable endogenous control genes (ABCF1, HPRT1, OAZ1, POLR2A and RPL19) using nSolver analysis software 2.5.34 (NanoString Technologies). The data detected were imported into Partek Genomics Suite 6.6 and Partek Pathway, log2 transformed and the data were batch corrected for patient variations. A cut-off at value >10 normalized mRNA molecules was set and 503 genes were included in the analyses. ANOVA 3-way was performed for the comparisons of interest and p-value <0.05 was considered significantly different between the groups. Finally, pathway enrichment analysis (Fisher's Exact test) was performed. Three gene lists were prepared with genes involved in the “Regulation of inflammatory response” (GO:0050727), “NOD-like receptor signalling pathway” (KEGG: hsa04621) and genes involved in the complement system. Induced genes were presented as Euclidian-clustered heat maps of genes involved in these lists with fold changes from the ANOVA. The expression of genes relevant for the NLRP3 inflammasome pathway were visualized in Partek Pathway and further visualized in Adobe Illustrator.
2.10. Cytokine and chemokine measurements
IL-1β and IL-1α from PBMC cultures were detected using IL-1β and IL-1α ELISAs (BD Biosciences). Supernatants from cultures of human atherosclerotic plaques were analysed using a multiplex cytokine assay (Bio-Plex; Bio-Rad Laboratories Inc.) for IL-1β, IL-1α, IL-18 and tumour necrosis factor (TNF). The analysis was performed according to the manufacturer's instructions.
2.11. Lactate dehydrogenase (LDH) assay
PBMC were cultured as indicated above. Cell death/cytotoxicity, as determined by LDH release, was measured in culture supernatant using a calorimetric LDH Assay Kit (Abcam) according to the manufacturer's instructions. For each assay, 10µl of Triton-x 100 was used as positive control, and the spontaneous release was used for calculation of the % LDH activity.
2.12. Flow cytometry
PBMC from patients with ACS, or healthy donors (controls) were thawed and analyzed for cell composition using flow cytometry. The following controls were added: unstained cells, single-stained cells or dead cells monitored using the LIVE/DEAD fixable Aqua cell stain Assay (Life Technologies). Cells were treated with Fc receptor blocker (Miltenyi Inc.) prior to fixation. For cell surface staining, cells were fixed with 4% paraformaldehyde for 10 min followed by staining for the different cell population for 20 min with antibodies against CD14, CD3, CD4, CD8 and CD20. Results were analyzed by FlowJo 7.6, where cell debris, clustered cells and dead cells were excluded by gating.
2.13. Statistical analysis
GraphPad Prism (GraphPad Software) was used, and p<0.05 was considered statistically significant. Results in Fig. 1 (a-c) was analysed using Kruskal Wallis and Dunn's multiple comparison test. Figs. 1d, 1e and 3e were analysed with Spearman's correlation. Fig. 3 (a-d) was analysed using Bartlett's test for equal variance on log-transformed data with Bonferroni post-tests. Figs. 2, 4a and Supplementary Fig. I were analysed using Mann-Whitney two-tail test (Fig. 4a on log-transformed data). Fig. 5 was analysed with ANOVA and Dunnett's’ multiple comparison test. Multiplex cytokine results in Fig. 6 were log2 transformed, batch corrected against patient variations and two-way ANOVA was performed in Partek Genomics Suite 6.6. Gene expression data in Fig. 7 and Supplementary Fig. IV, V and VI were analysed with Partek Genomics Suite 6.6 using 3-way ANOVA model as explained above.
Fig. 4.
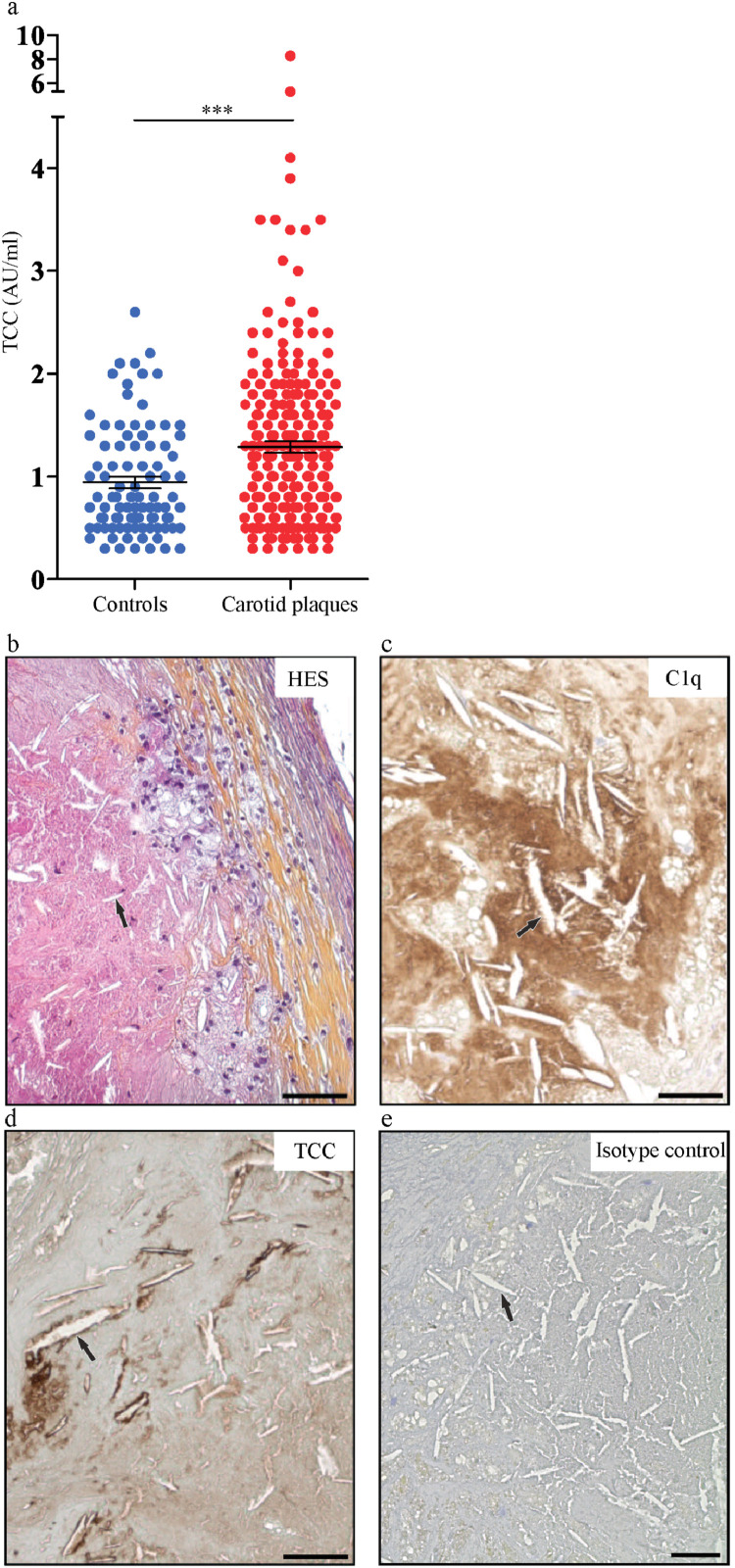
TCC in plasma from controls and patients with carotid plaques and accumulation of C1q and TCC around cholesterol crystal clefts in human carotid plaques. (a) TCC ELISA was performed on plasma collected from patients with carotid plaques and healthy controls. Results are presented as mean ± SEM, n=254 and 92, respectively. Immunohistochemistry was performed on paraffin embedded sectioned plaques which were stained for haematoxylin (HES) (b), C1q (c), TCC (d) and isotype control (e) and imaged using EVOS FL auto microscope. One representative image from 3 plaques from 3 patients. Arrows indicate crystal clefts. Scale bar is 50 μm. AU, arbitrary unit; C, Complement factor; TCC: terminal C5b-9 complement complex. ***p<0.001, Mann-Whitney two-tail test.
Fig. 5.
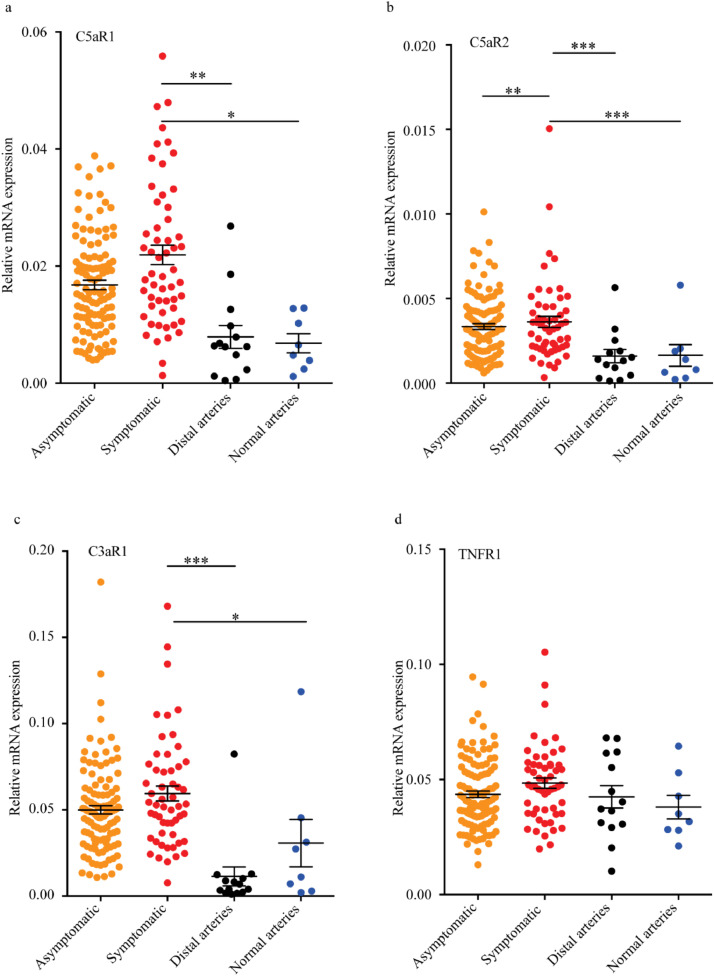
Transcripts of complement anaphylatoxin receptors in control arteries and human carotid plaques. Carotid plaques from asymptomatic, and symptomatic patients (symptoms for more than 2 months), distal area from the plaques and normal renal arteries were isolated from patients, n=115, 55, 14 and 8, respectively. RNA was isolated, and PCR run. mRNA expression for C5aR1 (a), C5aR2 (b), C3aR1 (c) and TNFR1 (d) is presented for the four groups as mean ± SEM. C*R, Complement factor receptor; TNFR, tumour necrosis factor receptor. *p<0.05, **p<0.01, ***p<0.001 vs symptomatic, ANOVA and Dunnett's’ multiple comparison test.
Fig. 6.
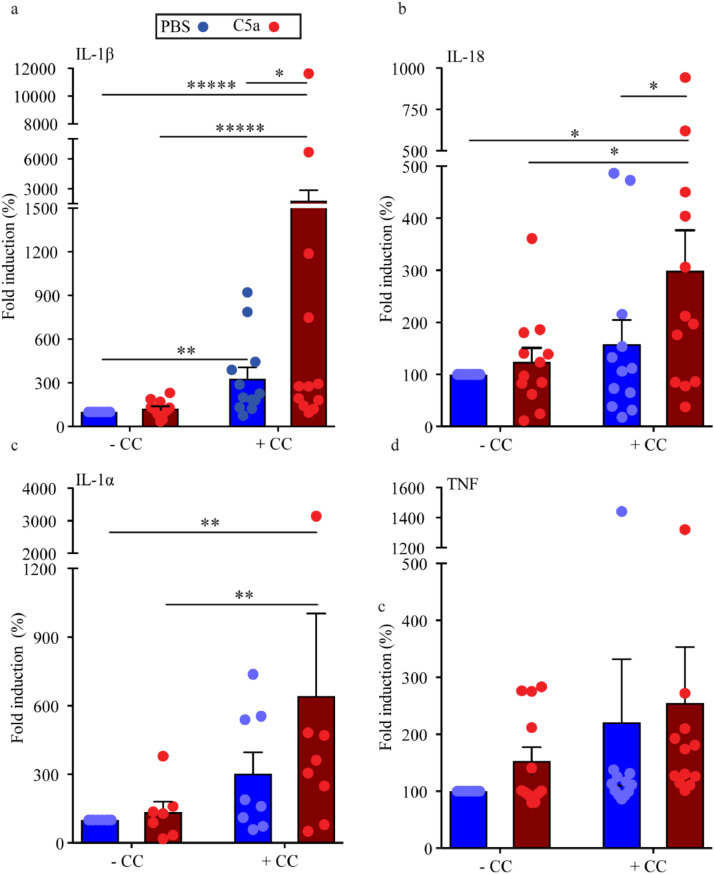
Release of pro-inflammatory cytokines from human carotid plaques upon CC stimulation and C5a priming. Carotid plaques were primed with C5a or left unprimed prior to stimulation with CC ex vivo and supernatants were collected. Multiplex cytokine assay was performed and amount of IL-1β (a), IL-18 (b), IL-1α (b) and TNF (d) is presented as mean fold induction compared to PBS, mean ± SEM, n=12 (for IL-1α n=8). Basal levels with exposure to PBS as mean ± SEM in pg/μl: IL-1β: 20±6.1, IL-18 11±4.1, IL-1α 1.8±0.62, TNF 224±66. CC, cholesterol crystals; IL, interleukin; TNF, tumour necrosis factor. *p<0.05, **p<0.01, *****p<0.00001, 2-way ANOVA.
Fig. 7.
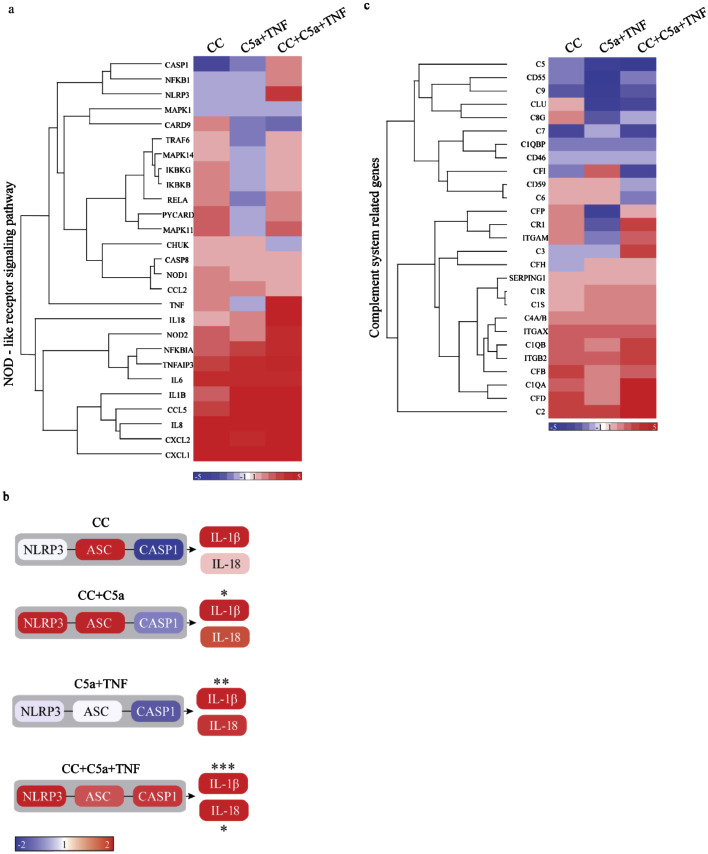
Gene expression profiles from primed human carotid plaques incubated with CC. Carotid plaques were primed with C5a, C5a+TNF or left unprimed prior to incubation with CC or unstimulated ex vivo, before RNA isolation, Nanostring Technologies and Partek Genomics Suite analysis. Genes in the Kegg pathway “NOD-like receptor signalling pathway” are presented in a heat map (a) and genes linked to NLRP3 are presented in a graphical presentation (b). Basal levels in plaques treated with PBS as mean ± SEM normalized number of mRNAs: NLRP3 78±34, ASC 101±50, CASP1 125±52, IL-1β 2601±1186, IL-18 133±66. Genes involved in the complement cascade are presented in a heatmap (c). Colour scale bar indicates mean fold change compared to PBS (red is higher and blue is lower), n=8. ASC/PYCARD, apoptotic speck protein containing a caspase recruitment domain; C, Complement factor; CASP1, Caspase-1; CC, cholesterol crystals; CLU, clusterin; ITGAM/CD11b, integrin alpha M; ITGAX/CD11c, integrin alpha X; ITGB2/CD18, integrin beta 2; IL, interleukin; NLRP3, NACHT, LRR and PYD domains-containing protein 3; NOD, nucleotide-binding oligomerization domain; SERPING1, complement 1 inhibitor; TNF, tumour necrosis factor. *p<0.05, **p<0.01, ***p<0.001, 3-way ANOVA.
2.14. Ethics statement
Written informed consent was obtained from all participants and the study protocols were approved by the Regional Committees for Medical and Health Research Ethics in South-Eastern- and Western Norway. These committees approved the study protocols for human carotid plaques (REK No: 2014/2078), and the protocols for PBMC and plasma from patients with ACS, SAP and healthy controls (No: S-04114). The authors state that the study complies with the Declaration of Helsinki.
3. Results
3.1. PBMC from ACS patients showed increased transcripts of IL-1β and TNF
We first aimed to determine the relationships between NLRP3, IL-1β and TNF transcripts in PBMC from patients with verified CAD with stable (SAP) and unstable (ACS) disease. In accordance with previous reports [28] we observed that cells from patients with SAP and ACS had a marked increase in mRNA levels of IL-1β compared to the healthy controls (p<0.001 and p<0.05, respectively, Kruskal Wallis and Dunn's multiple comparison test, Fig. 1a). Also, ACS patients had significant elevated IL-1β mRNA compared to the SAP group (Fig. 1a). Furthermore, ACS patients had higher levels of NLRP3 transcripts as compared with SAP patients (p<0.05, Kruskal Wallis and Dunn's multiple comparison test, Fig. 1b). Moreover, ACS patients had significantly upregulated TNF transcript compared to both healthy controls (p<0.001) and SAP (p<0.01) (Kruskal Wallis and Dunn's multiple comparison test, Fig. 1c). The upregulated TNF transcripts are also reflected by increase in plasma TNF in the ACS group[28]. Finally, in the ACS group there was a significant correlation between IL-1β and NLRP3 transcripts (r= 0.90, p<0.0001; Fig. 1d) and between IL-1β and TNF transcripts (r= 0.78, p<0.0001; Fig. 1e) (Spearman's correlation). These findings suggest a systemic inflammatory state occurring in ACS patients, potentially linked to NLRP3 inflammasomes.
3.2. PBMC from patients with ACS were highly responsive for priming with combination of C5a and TNF
We next examined if addition of CC with or without priming resulted in elevated IL-1β release in ACS patients compared to healthy controls. We have previously reported that C5a acts in synergy with TNF to induce IL-1β mRNA in human monocytes from healthy controls [18]. We found that when primed with a combination of C5a and TNF prior to CC stimulation, PBMC from ACS patients showed a higher IL-1β protein release (>2-fold increase, p<0.01, Mann-Whitney two-tail test) compared to PBMC from healthy controls (Fig. 2). It has been demonstrated that in a hyperlipidemic condition, CC will stimulate macrophages to produce IL-1α, contributing to atherosclerosis [29]. However, in our experiment, PBMC from healthy donors and ACS patients produced similar amount of IL-1α in response to CC with or without priming (Fig. 2). Moreover, the cell viability was similar for all conditions applied (Supplementary Fig II). All control treatments are presented in Supplementary Fig. I. Correlations between the experimental results and clinical characteristics have been performed and no relevant observations were made (data not shown). The increase in transcripts in PBMC from the ACS patients compared to controls, as well as increase in responsiveness to C5a and TNF priming, were not due to differences in immune cell populations between the groups as the distributions of monocytes, T- and B-cells were similar in PBMC from ACS and controls (Supplementary Fig. III).
3.3. Higher complement activation was detected in plasma from patients with SAP and ACS
We have previously demonstrated that CC are potent activators of complement, both through the CP, LP and AP [18,30]. Thus, we next investigated the presence of complement activation products in plasma from patients with SAP and ACS. The end product of the three pathways, terminal C5b-9 complement complex (TCC) (p<0.001, Fig. 3a), and the common step product, C3bc (p<0.001, Fig. 3b), were significantly higher in the SAP and ACS groups compared to healthy controls (Bartlett's test for equal variance on log-transformed data with Bonferroni post-tests). Moreover, we also observed a significant increase in C4bc (p<0.001, Bartlett's test for equal variance on log-transformed data with Bonferroni post-tests, Fig. 3c), a marker of both the CP and the LP activation, in both SAP and ACS compared to healthy controls, suggesting that the CP and/or LP are involved in the CAD-related initial complement activation. The AP convertase C3bBbP was also significantly (p<0.001, Bartlett's test for equal variance on log-transformed data with Bonferroni post-tests) increased in plasma from SAP patients, but not in ACS patients, compared to healthy controls (Fig. 3d). In addition, C4bc correlated to IL-1β mRNA levels when controls, SAP and ACS patients were combined (Fig. 3e). Our ex vivo experiments showed that C5a and TNF primed PBMC from ACS patients had a marked increase in IL-1β release upon subsequent CC exposure. Hence, the upregulation of the different components of this inflammatory loop i.e., TNF, NLRP3, IL-1β and complement in freshly isolated PBMC and plasma, suggest that these inflammatory interactions also could be operating in vivo.
3.4. Complement factor C1q and TCC were localized around CC clefts in carotid plaques
Complement products have been observed in atherosclerotic lesions [12]. To further elucidate the interaction between CC and complement activation in atherogenesis we next examined the expression of complement components in human atherosclerotic carotid plaques. Importantly, as for the ACS group (Fig. 3a), plasma from patients with carotid atherosclerosis had also significantly higher (p<0.001, Mann-Whitney two-tail test on log-transformed data, Fig. 4a) TCC than plasma from healthy controls. Moreover, light microscopy of rehydrated carotid arteries showed “CC clefts” in proximity to “foam-like” cells and in the necrotic core (Fig. 4b). Importantly, C1q (Fig. 4c) and TCC (Fig. 4d) localized around the CC-clefts. As complement activation is intended to propagate inflammatory responses, it is likely that complement activation at the surface of CC may facilitate the growth of the lesions by recruiting more immune cells to CC.
3.5. High expression of anaphylatoxin receptors were found in advanced human carotid plaques
We next determined whether patients with symptomatic or asymptomatic carotid atherosclerotic plaques expressed different local complement receptor profiles. Distal carotid samples, representing an early stage of atherosclerosis without macroscopically sign of atherosclerosis, and non-atherosclerotic common renal arteries of organ donors were used as controls. Symptomatic plaques had significantly higher transcripts of C5aR1 (p<0.001), C5aR2 (p<0.01) and C3aR1 (p<0.001) than non-atherosclerotic control vessel or distal carotid samples suggesting that these anaphylatoxin receptors are associated with plaque severity of the disease (ANOVA and Dunnett's’ multiple comparison test, Fig. 5a–c). Of interest, minor, but significant difference between plaques from symptomatic- and asymptomatic patients were found for C5aR2 (Fig. 5b). In contrast, no significant differences were observed for TNFR1 mRNA between patients with symptomatic and asymptomatic carotid plaques or between carotid plaques and the two control groups (Fig. 5d). These findings suggest an important role for local complement and anaphylatoxin receptors in generating an inflammatory milieu in carotid atherosclerosis.
3.6. Carotid plaques showed enhanced sensitivity to C5a priming
Given that advanced carotid lesions are in an activated state, the cells present in the plaques may respond to stimulation by CC. Using freshly isolated carotid plaques, we and others have previously shown that incubation of carotid plaques with CC leads to high amount of IL-1β, confirming that the environment in plaques contains mediators (i.e. genes relevant to the NLRP3 inflammasome pathway) that may prime immune cells for CC-induced inflammation [31]. The spontaneously produced TNF is very high, which suggests that it might be present in high enough amounts for priming (224±66 pg/ml). Hence, we addressed the effect of C5a as a priming signal in freshly isolated carotid plaques. In Fig. 6 we have normalized the stimulated plaques against their own controls and present fold changes rather than absolute values. In line with Varghese et al, [31] we found that IL-1β release was significantly increased in this ex vivo model of plaque stimulation during CC incubation (p<0.01, 2-way ANOVA on log-transformed data, Fig. 6a). Interestingly, priming with C5a prior to CC incubation significantly (p<0.05, 2-way ANOVA on log-transformed data) increased both IL-1β and IL-18 release, the two major inflammatory products from NLRP3 activation (Fig. 6a,b), whereas TNF did not change significantly (Fig. 6d). This demonstrates the link between complement and CC-induced NLRP3 activation in atherosclerotic plaques. In murine models, CC have been shown to induce IL-1α by a mechanism involving fatty acid-induced mitochondrial uncoupling [29]. Likewise, addition of CC to human carotid plaques led to a significant (p<0.01, 2-way ANOVA on log-transformed data) increase in IL-1α release in the presence of priming, illustrating the importance of a lipid rich and inflammatory microenvironment for IL-1α release (Fig. 6c). Thus, these data suggest that C5a in atherosclerotic plaques may have an impact on the magnitude of the CC-induced inflammatory response with NLRP3 as a major target.
3.7. Combining C5a and TNF primed carotid plaques for NLRP3 and complement pathway genes
We next performed gene expression profiling on biopsies obtained from carotid endarterectomies. Gene expression profiling of a panel of human immune-related genes was performed in untreated plaques or plaques treated with CC alone, CC+C5a, C5a+TNF and CC+C5a+TNF. We found that the treatment affected many key genes in the GO term “Regulation of inflammatory response” (Supplementary Fig. IV). Of interest, pathway enrichment analysis revealed that the Kegg pathway “NOD-like receptor-signalling pathway” was significantly regulated in the data set (Fig. 7a, Supplementary Fig. V). Incubating carotid plaques with CC increased many inflammatory genes including TNF receptor TNFRSF1B, the inflammatory chemokines CCL5, CCL3, CXCL2, CXCL1 as well as TLR2 and several other inflammatory genes such as IL-6, IL-8 and Cox-2 (Fig. 7a, Supplementary Fig. IV and V). When biopsies were primed with C5a, CC induced upregulation of NLRP3 and apoptotic speck protein containing a caspase recruitment domain (ASC) with a corresponding significant increase of IL-1β gene expression (p<0.05, 3-way ANOVA, Fig. 7b). CC+C5a+TNF condition significantly increased both NLRP3, CASP1, IL-1β and IL-18 gene expression (p<0.05, 3-way ANOVA, Fig. 7b). Interestingly, mRNA expression of the anti-inflammatory cytokine IL-10 was markedly upregulated in C5a+TNF and CC-stimulated carotid plaques (Supplementary Fig. IV). Altogether, these data show that priming with the combination of C5a and TNF prior to stimulation with CC activates inflammation by inducing NLRP3-transcriptional programs in human plaques, as well as several other genes that are relevant to the inflammatory pathogenesis of atherosclerosis.
We also found that priming and stimuli with CC affected many key genes in the complement system (Fig. 7c). Priming with C5a and TNF followed by CC stimulation lead to an increased expression of “upstream genes” of the complement activation cascade, such as C1q alpha- and beta chains (C1QA, C1QB), complement factor D (CFD), C2 and C3. Moreover, expression of phagocytic receptors CR3 (CD11b/CD18) and CR4 (CD11c/CD18) were increased (Fig. 7c and Supplementary Fig. VI). In contrast, “downstream activation genes” like C5, C6, C7 and C9 were reduced by this treatment (Fig. 7c and Supplementary Fig. IV). In addition, complement regulatory genes such as clusterin (CLU), CD59, CD46, CD55 and complement factor I (CFI) were also diminished by priming and CC treatment (Fig. 7c and Supplementary Fig. VI). This indicates that priming and stimulation affects the gene expression of complement genes which again may reflect a shift in the balance that contributes to disease progression.
We identified immune cell types in 8 carotid plaques by using a CIBERSORT analysis [26] on microarray data previously published by our group [25]. Although the amount of different cell types varied between individual plaques, macrophages and monocytes were dominating immune cells (Fig. 8). Also, neutrophils, T-cells and plasma cells were detected in the plaques. Immunohistochemistry of serial sections revealed that neutrophils (CD15+) were present in the necrotic zone of the plaque close to crystal clefts, whereas macrophages were located just outside the necrotic zone (Supplementary Fig. VII). T-cells (CD3+) and B-cells (CD20+) were also present in the immunohistochemistry sections of plaques, but these immune cells were less abundant compared to macrophages and neutrophils (Supplementary Fig. VII).
4. Discussion
In the present study, we propose a molecular mechanism that integrates systemic and local complement- and NLRP3 activation with CC-mediated inflammatory responses in patients with coronary- and carotid atherosclerosis. We show that complement contributes to the CC-driven inflammatory responses both in PBMC from ACS patients and within the atherosclerotic lesion in patients with carotid atherosclerosis. Our data imply a positive association between CC-induced complement and NLRP3 activation, both systemically and within the atherosclerotic lesion, with disease severity and instability.
Whereas much focus has been directed towards IL-1β, data on IL-1α in atherosclerotic disorders are scarce. Herein we show that CC induced marked IL-1α release in carotid plaques in the presence of C5a, while TNF and C5a priming increased IL-1α release in presence of CC in PBMC from ACS patients and controls. Baumer et al. recently suggested that TNF may also play an earlier role in CC formation [32]. We observed that neutrophils were present in lesions and these cells are potential sources for CC-induced IL-1α release. It is interesting that neutrophils were present in areas were CC clefts were detected. As CC are potent activators of complement, neutrophils may be attracted to this area. It has been reported that CC can trigger neutrophils to release neutrophil extracellular traps (NETs) [33]. Moreover, NETs are involved in production of mature IL-1α, which has been implicated in thrombosis caused by superficial erosion [34]. On the other side, Jiang et al. [9] recently found that inhibiting the NLRP3 inflammasome using MCC950 reduced LPS- or ATP-induced IL-1α release in plaque-derived tissue. The exact role of IL-1α in atherogenesis is still not clear, however, it seems to be of importance for metabolic-induced inflammation, as it was recently shown to promote vascular remodelling [35].
In the current study we show that ex vivo priming of carotid plaques by C5a+TNF followed by CC activation induced mRNA expression of wide range of inflammatory genes. This was accompanied by enhanced expression of NLRP3, ASC and CASP1 with a corresponding significant increase of IL-1β and IL-18 gene expression and protein release, suggesting that these broad inflammatory responses within carotid plaques are driven by CC and complement activation. We found that monocytes, macrophages and T-cells were detected in plaques, and these cell types are likely to be major contributors to the inflammasome activation. Notably, also within CAD patients, we found an inflammatory response to C5a+ TNF and CC. PMBC from patients with ACS primed with C5a+TNF, and incubated with CC, showed a >2-fold increase in IL-1β release as compared with PBMC from healthy controls. This result is in line with our data showing that PBMC from ACS patients also had upregulated NLRP3 and IL-1β mRNA. In accordance to our earlier findings [18], these results suggest that both in carotid plaques and in PBMC from ACS patients, the proteins that are needed for NLRP3 complex assembly are upregulated, and when plaques and PBMC are exposed to C5a+TNF and CC, a marked enhanced inflammatory response occurs that may contribute to plaque progression and instability. Disparate results have emerged on the role of IL-1β in atherosclerosis when human and murine studies are compared. While a single cell study analysis of cells in human plaques recently showed that IL1β might be highly upregulated in plaque macrophages from asymptomatic than symptomatic patients [36]. The CANTOS trial [37], demonstrated the benefits of IL-1β neutralization in patients post-myocardial infarction, thus, supporting the findings in murine studies showing the importance of IL-1β in driving inflammation and plaque formation in atherosclerosis [5,38].
Complement activation is highly induced in advanced atherosclerotic lesions, and studies have shown that cells in plaques express complement both at the mRNA and the protein level [16]. This fits well with our findings showing significantly higher levels of systemic TCC in plasma from patients with carotid plaques. Moreover, we show that carotid plaques had higher expression of C5aR1, C5aR2 and C3aR1 than control arteries. Hence, these results provide strong support for the hypothesis that the amplitude of local complement activation has a causal link to the disease progression. A recent study reported a higher expression of NLRP3 in symptomatic plaques compared to asymptomatic [9]. The prevalence of also a higher anaphylatoxin receptor expression in symptomatic compared to asymptomatic carotid arteries further underlines the relevance of local complement factors that reflect processes associated with atherosclerotic disease progression particularly in this group of patients with the highest “inflammatory load”. Although markers of inflammasome activation were different between SAP and ACS, the complement activation was equally high in these two groups. This might be due to the fact that complement activation is a quick response that is upstream the inflammasome activation. Recent studies have shown that complement can also be activated on intracellular compartments, and that this activation might exert functions that are different from systemic serum complement [39,40]. Future studies are needed to understand how this new location of complement affects the biology of atherosclerosis.
Interestingly, IL-10 expression was increased in the C5a+TNF and CC stimulated plaques. IL-10 is a potent anti-inflammatory cytokine with atheroprotective effects [28,41]. Moreover, IL-10 has been suggested to be a mediator in the resolution phase of an inflammatory response. The resolution phase of inflammation is characterized by efferocytosis, which is removal of dead cells. Important in this respect is our observation that stimulation of carotid plaques with C5a+TNF and CC resulted in upregulation of “upstream” complement genes such as C1Q and C3 as well as phagocytic receptors. These genes response may enhance phagocytosis of dead cells and debris in the plaque. Thus, both pro- and anti-inflammatory processes occur in carotid plaque, and the balance between these two pathways may be essential for disease development, and the CC-complement-NLRP3 axis seems to be a key regulator of these processes. Both our studies and previous work by others [36] have revealed the complex immune cell landscape of atherosclerotic plaques. We find a considerable amount of M2 macrophages that may contribute to IL-10 secretion and anti-inflammatory responses. Also, plasma cells are detected which can secrete antibodies which may enhance complement activation in the plaque. Future studies will be needed to clarify the important question as to which plaque-containing leucocyte group induces the observed cross-talk between complement and CC-induced NLRP3 in atherosclerosis.
Nevertheless, this study demonstrates a close relationship between complement and CC-induced NLRP3 inflammasome activation in the inflammatory processes both systemically and within the atherosclerotic lesion. Patients with atherosclerosis have increased levels of C3 and C4 in their plasma, which is a risk factor of cardiovascular events [42]. Most complement proteins, including C3 and C4 are acute phase proteins produced in the liver, supporting the idea that atherosclerosis is a systemic low-grade inflammatory disease. The increase in activation products, like C5a, has also been shown to predict cardiovascular events, particularly in patients with advanced atherosclerosis [43]. This increase in activation products, as we showed for components from the whole cascade, is most likely due to release of activation products from the continuously activated atherosclerotic arterial endothelium. Using ex vivo human atherosclerotic plaques together with plasma and PBMC isolated from whole blood of patients with SAP and ACS, we show that this cross talk plays a mechanistic role in the development of atherosclerosis. The pathogenic loop between complement, CC and NLRP3 inflammasomes may represent a promising target for therapy of atherosclerotic disorders.
Funding sources
This work was supported by the Research Council of Norway [223255/F50, and 251255/F20], and the Liaison Committee in Central Norway [50052400]. The funders had no role in study design, data collection, data analysis, interpretation or writing of the manuscript.
Declaration of Competing Interest
Dr. Latz is scientific co-founder and consultant to IFM Therapeutics, a biotech company that develops small molecule modulators of innate immune pathways. The other authors declare no competing interests with relevance to this study.
Data sharing
The data, analytical methods, and study materials for the purposes of reproducing the results or replicating procedures can be made available on request to the corresponding author.
Author contributions
NN, SSB, TE: conception and design of the study, literature search, figures, data collection, data analysis, writing of the article, final approval of the submitted version. IG, SH, ØS, HLO, LR, AMR, AY, XYK, TBD: data collection, editing, drafting of the article, final approval of the submitted version. BS: data collection, figures, editing, drafting of the article, final approval of the submitted version. MS, EL, LG, GØA, JKD: conception and design of the study, data collection, editing, drafting of the article, final approval of the submitted version. PA, TEM, BH: conception and design of the study, data collection, data interpretation, editing, drafting of the article, editing, final approval of the submitted version.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102985.
Appendix. Supplementary materials
References
- 1.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12(3):204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 2.Katz SS, Shipley GG, Small DM. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976;58(1):200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheedy FJ, Grebe A, Rayner KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14(8):812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart CR, Stuart LM, Wilkinson K. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11(2):155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duewell P, Kono H, Rayner KJ. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiyoshi K, Minami Y, Ishida K. Incidence, factors, and clinical significance of cholesterol crystals in coronary plaque: An optical coherence tomography study. Atherosclerosis. 2019;283:79–84. doi: 10.1016/j.atherosclerosis.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Kataoka Y, Puri R, Hammadah M. Cholesterol crystals associate with coronary plaque vulnerability in vivo. J Am Coll Cardiol. 2015;65(6):630–632. doi: 10.1016/j.jacc.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 8.Kirii H, Niwa T, Yamada Y. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23(4):656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 9.Jiang X, Wang F, Wang Y. Inflammasome-Driven Interleukin-1alpha and Interleukin-1beta Production in Atherosclerotic Plaques Relates to Hyperlipidemia and Plaque Complexity. JACC Basic Transl Sci. 2019;4(3):304–317. doi: 10.1016/j.jacbts.2019.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridker PM, Everett BM, Thuren T. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 11.Lewis RD, Jackson CL, Morgan BP, Hughes TR. The membrane attack complex of complement drives the progression of atherosclerosis in apolipoprotein E knockout mice. Mol Immunol. 2010;47(5):1098–1105. doi: 10.1016/j.molimm.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meuwissen M, van der Wal AC, Niessen HW. Colocalisation of intraplaque C reactive protein, complement, oxidised low density lipoprotein, and macrophages in stable and unstable angina and acute myocardial infarction. J Clin Pathol. 2006;59(2):196–201. doi: 10.1136/jcp.2005.027235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oksjoki R, Kovanen PT, Pentikainen MO. Role of complement activation in atherosclerosis. Curr Opin Lipidol. 2003;14(5):477–482. doi: 10.1097/00041433-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Seifert PS, Kazatchkine MD. Generation of complement anaphylatoxins and C5b-9 by crystalline cholesterol oxidation derivatives depends on hydroxyl group number and position. Mol Immunol. 1987;24(12):1303–1308. doi: 10.1016/0161-5890(87)90125-8. [DOI] [PubMed] [Google Scholar]
- 15.Speidl WS, Kastl SP, Hutter R. The complement component C5a is present in human coronary lesions in vivo and induces the expression of MMP-1 and MMP-9 in human macrophages in vitro. FASEB J. 2011;25(1):35–44. doi: 10.1096/fj.10-156083. [DOI] [PubMed] [Google Scholar]
- 16.Yasojima K, Schwab C, McGeer EG, McGeer PL. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol. 2001;158(3):1039–1051. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilely K, Rosbjerg A, Genster N. Cholesterol Crystals Activate the Lectin Complement Pathway via Ficolin-2 and Mannose-Binding Lectin: Implications for the Progression of Atherosclerosis. 2016;196(12):5064-74. [DOI] [PubMed]
- 18.Samstad EO, Niyonzima N, Nymo S. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J Immunol. 2014;192(6):2837–2845. doi: 10.4049/jimmunol.1302484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niyonzima N, Halvorsen B, Sporsheim B. Complement activation by cholesterol crystals triggers a subsequent cytokine response. Mol Immunol. 2017;84:43–50. doi: 10.1016/j.molimm.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Nymo S, Niyonzima N, Espevik T, Mollnes TE. Cholesterol crystal-induced endothelial cell activation is complement-dependent and mediated by TNF. Immunobiology. 2014;219(10):786–792. doi: 10.1016/j.imbio.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Gravastrand CS, Steinkjer B, Halvorsen B. Cholesterol Crystals Induce Coagulation Activation through Complement-Dependent Expression of Monocytic Tissue Factor. J Immunol. 2019 doi: 10.4049/jimmunol.1900503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Glob Heart. 2012;7(4):275–295. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Brott TG, Halperin JL, Abbara S. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. J Neurointerv Surg. 2011;3(2):100–130. doi: 10.1136/jnis.2011.004762. [DOI] [PubMed] [Google Scholar]
- 25.Dahl TB, Yndestad A, Skjelland M. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115(8):972–980. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- 26.Newman AM, Liu CL, Green MR. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergseth G, Ludviksen JK, Kirschfink M, Giclas PC, Nilsson B, Mollnes TE. An international serum standard for application in assays to detect human complement activation products. Mol Immunol. 2013;56(3):232–239. doi: 10.1016/j.molimm.2013.05.221. [DOI] [PubMed] [Google Scholar]
- 28.Waehre T, Yndestad A, Smith C. Increased expression of interleukin-1 in coronary artery disease with downregulatory effects of HMG-CoA reductase inhibitors. Circulation. 2004;109(16):1966–1972. doi: 10.1161/01.CIR.0000125700.33637.B1. [DOI] [PubMed] [Google Scholar]
- 29.Freigang S, Ampenberger F, Weiss A. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14(10):1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 30.Pilely K, Rosbjerg A, Genster N. Cholesterol crystals activate the Lectin complement pathway via ficolin-2 and mannose-binding lectin: implications for the progression of atherosclerosis. J Immunol. 2016;196(12):5064–5074. doi: 10.4049/jimmunol.1502595. [DOI] [PubMed] [Google Scholar]
- 31.Paramel Varghese G, Folkersen L, Strawbridge RJ. NLRP3 Inflammasome Expression and Activation in Human Atherosclerosis. J Am Heart Assoc. 2016;5(5) doi: 10.1161/JAHA.115.003031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baumer Y, Dey AK, Gutierrez-Huerta CA. Hyperlipidaemia and IFNgamma/TNFalpha synergism are associated with cholesterol crystal formation in endothelial cells partly through modulation of Lysosomal ph and cholesterol homeostasis. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349(6245):316–320. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folco EJ, Mawson TL, Vromman A. Neutrophil Extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1alpha and cathepsin G. Arterioscler Thromb Vasc Biol. 2018;38(8):1901–1912. doi: 10.1161/ATVBAHA.118.311150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vromman A, Ruvkun V, Shvartz E. Stage-dependent differential effects of interleukin-1 isoforms on experimental atherosclerosis. Eur Heart J. 2019;40(30):2482–2491. doi: 10.1093/eurheartj/ehz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez DM, Rahman AH, Fernandez NF. Single-cell immune landscape of human atherosclerotic plaques. Nat Med. 2019;25(10):1576–1588. doi: 10.1038/s41591-019-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridker PM, Everett BM, Thuren T. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 38.Alexander MR, Moehle CW, Johnson JL. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122(1):70–79. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolev M, Le Friec G, Kemper C. Complement–tapping into new sites and effector systems. Nat Rev Immunol. 2014;14(12):811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 40.Liszewski MK, Kolev M, Le Friec G. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39(6):1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 42.Engström G, Hedblad B, Janzon L, Lindgärde F. Complement C3 and C4 in plasma and incidence of myocardial infarction and stroke: a population-based cohort study. Eur J Cardiovasc Prev Rehabil. 2007;14(3):392–397. doi: 10.1097/01.hjr.0000244582.30421.b2. [DOI] [PubMed] [Google Scholar]
- 43.Speidl WS, Exner M, Amighi J. Complement component C5a predicts future cardiovascular events in patients with advanced atherosclerosis. Eur Heart J. 2005;26(21):2294–2299. doi: 10.1093/eurheartj/ehi339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.