Abstract
In 2017, the second national reference standard (NRS) for Gloydius snake venom was established to replace the first NRS for Gloydius snake venom. In connection with the second venom NRS, a candidate for the first NRS for Gloydius snake antivenom was produced in 2017. In this study, the qualification of the candidate was estimated and the potency was determined by a collaborative study. The potency (anti-lethal titer and anti-hemorrhagic titer) of the candidate was determined by measuring the capability of the antivenom to neutralize the lethal and hemorrhagic effects of the second NRS for Gloydius snake venom, which was calibrated against the regional reference standard for Gloydius snake antivenom established in 2006. Two Korean facilities contributed data from 20 independent assays. Subsequently, one foreign national control research laboratories participated in this collaborative study. The general common potency of the anti-lethal and anti-hemorrhagic titers was obtained from the results of a total of 25 tests performed at three facilities. According to the results of the present study, the candidate preparation showed good quality and is judged to be suitable to serve as the first NRS for Gloydius snake antivenom with the following potency: an anti-lethal titer of 3100 unit (U) (95% confidence interval 2991–3276 U) and anti-hemorrhagic titer of 3000 U (95% confidence interval 2849–3159 U). In conclusion, the first NRS for Gloydius snake antivenom was established in this study. This reference standard will be used routinely for quality control of a snake antivenom product by manufacturer in Korea, which also can be used for national quality control, including a national lot-release test of the snake antivenom product.
Keywords: National reference standard, Collaborative study, Gloydius snake antivenom, Anti-lethal titer, Anti-hemorrhagic titer
Introduction
The morbidity and mortality associated with venomous snakebites have been a serious public health problem in many countries of the world. The study recently conducted at the global scale estimated that the envenoming incidence/100,000 varies from 0.33 to 15.73, and the death rate/100,000 from 0.033 to 0.347 per year [1]. The snakebites are also heavy burden in South Korea where there are 16 species of snakes including 4 species of venomous snakes, of which the Gloydius brevicaudus, Gloydius intermedius, and Gloydius ussuriensis are responsible for the majority of envenomings and clinical symptoms from snakebites [2]. The genus Gloydius (Serpentes: Crotalinae) is a venomous group of snakes distributed in many regions of Asia, including Korea, Japan, and China [3]. The Gloydius snakebite envenomation can induce local and systemic effects including tissue swelling and necrosis, lethal with cardiac, pulmonary, or renal dysfunction even though the case-fatality rate is very low [4–6]. These clinical manifestations are caused by Gloydius snake venom, which has lethal and hemorrhagic activities [7]. Treatment of envenomations with an antivenom is currently recognized as one of the available therapy methods for venomous snakebites. The antivenom is derived from immunoglobulins, obtained and purified from the plasma of animals immunized with snake venoms, which can neutralize the activities of toxins present in snake venoms [8]. A snake antivenom product also has been used in Korea for the treatment of Gloydius snakebites. The quality of the final antivenom product has been controlled in accordance with the Korean minimum requirements [9]. The quality control of the final product is a key element in the assurance of quality for antivenom. Quality control tests should be performed by the manufacturer and national control laboratory in Korea under its responsibility before the product is released. Among items ensuring the quality of the final product, the potency was traditionally assessed by both the in vivo neutralization of venom lethality in mice and hemorrhagic effect in rabbits in Korea [9]. In other words, the potency of the snake antivenom consists of the anti-lethal and anti-hemorrhagic titers in Korea, which has been calibrated against the reference standard for Gloydius snake antivenom (code no. 011201) established regionally in Japan, China, and Korea [10] using the Korean national reference standard (NRS) for Gloydius snake venom as the test snake venom, which was established individually in 2004 [11]. In 2017, the second NRS for Gloydius snake venom was established to replace the first NRS for Gloydius snake venom [12]. In connection with the second NRS for Gloydius snake venom, a candidate for the reference standard for Gloydius snake antivenom was produced for the first time as the Korean national reference in 2017. In the present study, the potency of the candidate for the first NRS for Gloydius snake antivenom was determined in a manner similar to that of the regional reference standard for Gloydius snake antivenom that had previously been established in 2006 [10] through a collaborative study. Two Korean facilities [1 national control laboratory and 1 manufacturer] and one foreign national control laboratories in Japan contributed to this collaborative study as recommended by the World Health Organization (WHO) that the preparation of national or regional reference standard for antivenom should be undertaken by relevant national control laboratories (NCLs) and regulatory agencies [13]. In addition, the qualities of the candidate were estimated to judge whether it could be suitable to serve as the first Korean NRS for Gloydius snake antivenom.
Materials and methods
Production of a candidate for the first NRS for Gloydius snake antivenom
A candidate for the NRS for Gloydius snake venom (MFDS-B-17-004, 2716 vials) was lyophilized at Korea Vaccine Co., Ltd. The final bulk of Gloydius snake antivenom was supplied by Shanghai Serum Biological Technology (SSBT) in China according to the procedure for the commercial antivenom product with the same formulation. It contained more than 2000 U of G. brevicaudus antivenom, and 1.5 w/v% of glycine per 1 ml vial. To ensure stability of long-term, the candidate was freeze-dried similarly to Korean commercial antivenom product. Korea Vaccine Co., Ltd. also conducted preliminary tests, including an immunodiffusion assay and immune-electrophoresis to estimate the immunological characteristics associated with the snake antivenom as well as quality control tests to verify the quality of the candidate to meet quality control specification.
Estimation of the quality of the lyophilized snake antivenom candidate
The candidate was subjected to quality control tests including identification testing, physical appearance testing, sterility testing, moisture content testing (water determination), mass variation testing (uniformity), foreign insoluble matter testing, and leakage testing to ensure the quality of the lyophilized snake antivenom candidate at Korea Vaccine Co., Ltd. All the tests were examined three times using each of the 10 vials of the candidate preparation. These test items were selected in accordance with the criteria of the Korean minimum requirements for a Korean commercial antivenom product [9]. These test methods were also conducted according to the procedures prescribed in the Korean minimum requirements [9].
Animals
Five mice per group of the ICR strain of specific pathogen free (SPF; female 16 ± 1 g) were used for determination of anti-lethal titer. For determination of anti-hemorrhagic titer, the New Zealand strain of the SPF rabbit (2 rabbits per 1 test; female 2.0 ± 0.2 kg) was used. All animal tests were approved by the NIFDS Ethics Committee on Animal Use (MFDS-18-122) and conducted according to the Korean animal testing guidelines. Animals were housed in an SPF facility at 20–24 ℃ and a relative humidity of 55 ± 10% with 15–20 air changes/hour and 12-h light/darkness cycles. All the animals were acclimatized for one week prior to testing.
Determination of the anti-lethal titer
The anti-lethal titer of the candidate for the first NRS for Gloydius snake antivenom against the lethal activity of test snake venom (the second national reference standard (NRS) for Gloydius snake venom; code no. MFDS-B-17-002, 1 test dose (TD) of lethal titer: 90.1 μg/vial) was determined. The test was performed according to the methods prescribed in Korea [9] using the regional reference standard for Gloydius snake antivenom (code no. 011201, anti-lethal titer: 32,000 U/vial). The regional reference standard for antivenom was dissolved in 0.017 M phosphate buffered sodium chloride solution containing 0.2 w/v% gelatin (pH 7.0) (GPBS) to make a solution of 200 U/ml and serially diluted with GPBS such that 1 ml of each dilution contained 64, 80, 100, 126, or 160 U of antivenom (Table 1). The candidate was dissolved and diluted serially with GPBS in the same way of the regional reference standard for antivenom. The test snake venom was reconstituted and diluted with GPBS to a concentration of 10 test doses/ml. For neutralization, aliquots of 1 ml of the regional reference standard for antivenom diluted appropriately or the candidate were mixed with 1 ml of the test snake venom (Table 1) and kept at room temperature for 1 h, respectively. Then mice were injected intravenously with 0.2 ml of each mixture of solution. The results associated with each mixture solution were observed for 2 days. Each 50% effective dose (ED50) of the regional reference standard for antivenom and the candidate was calculated from the results of the number of dead mice using the Reed–Muench method [14]. The anti-lethal titer of the candidate was determined relative to that of the regional reference standard for antivenom.
Table 1.
Composition of assay mixtures (ml) for Gloydius snake antivenom titer determination
| Preparation | (1) | (2) | (3) | (4) | (5) | |
|---|---|---|---|---|---|---|
| Antivenom |
200 U/ml (anti-lethal titer) or 20 U/ml (anti-hemorrhagic titer) |
0.320 | 0.400 | 0.500 | 0.630 | 0.800 |
| GPBSa | 0.680 | 0.600 | 0.500 | 0.370 | 0.200 | |
| Venom | 10 test dose/ml | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
a0.017 mol/l phosphate buffered sodium chloride solution containing 0.2 w/v% gelatin (pH 7.0)
Determination of the anti-hemorrhagic titer
The capability of the candidate to neutralize the hemorrhagic effect of the test snake venom was examined against the regional reference standard for Gloydius snake antivenom (code no. 011201, anti-hemorrhagic titer: 36,000 U/vial). The second NRS for Gloydius snake venom (code no. MFDS-B-17-002, 1 test dose (TD) of hemorrhagic titer: 10.8 μg/vial) was used as test snake venom.
The regional reference standard for antivenom and the candidate were dissolved in GPBS to a final concentration of 20 U/ml and serially diluted with GPBS. Each the dilution contained 6.4, 8.0, 10.0, 12.6, or 16.0 U per ml of antivenom (Table 1). The test snake venom was also reconstituted and diluted into GPBS to the same concentration as that used in examination for anti-lethal titer, of which was 10 test doses/ml. Aliquots of 1 ml of appropriately diluted the regional reference standard for antivenom or candidate were mixed with 1 ml of the test snake venom (Table 1) and kept at room temperature for 1 h for neutralization. Aliquots of 0.2 ml of these mixtures of solutions were injected intradermally into the shaved backs of two rabbits at two sites in a symmetrically per mixture of solution for each rabbit. After 24 h, the rabbits were euthanized, and the back skin was removed using a surgical instrument. The hemorrhagic spots were measured from the inner side of the skin using Vernier calipers. ED50 of the regional reference standard for antivenom and the candidate were calculated in terms of the size of the hemorrhagic spots. The anti-hemorrhagic titer was calibrated against the regional reference standard for antivenom.
Results
Estimation of the quality of the lyophilized snake antivenom candidate
The optimal conditions for lyophilization of the candidate were found by pilot studies, which could ensure the stability of quality under storage for long periods (Table 2). The results of the quality control tests performed are shown in Table 3. In all the test items, the quality of the candidate was verified to meet quality control standards. In the sterility test, there was no evidence of microbial growth. The results of the moisture content test, foreign insoluble particle matter test, and leakage test also fulfilled the requirements regarding each specification. The homogeneity of the candidate was confirmed by the results of mass variation test performed three times at Korea Vaccine Co., Ltd.
Table 2.
The optimal conditions for freeze-drying the snake antivenom candidate
| Stage | Modes |
|---|---|
| 1 | Pre-cooling, pre-freeze temperature: 5°C |
| 2 |
Freeze mode Temperature: − 50°C, ramp duration: 120 min, soak duration: 420 min |
| 3 |
Condenser mode/vacuum mode Condenser cooling: − 55°C, vacuum start value: 75 mTorr |
| 4 |
Heating mode (primary dry) Step 1. Temperature: − 10°C, ramp duration: 120 min, soak duration: 1120 min, vacuum: 75 mTorr Step 2. Temperature: 10°C, ramp duration: 180 min, soak duration: 1200 min, vacuum: 75 mTorr |
| 5 |
Secondary dry Temperature: 25°C, ramp duration: 120 min, soak duration: 600 min, vacuum: 15 mTorr |
| 6 |
Stoppering Injection N2 gas (> 99.999%) at vacuum break time, stoppering on − 0.4 Bar |
Table 3.
Results of the estimation of quality of the lyophilized antivenom candidate
| Test items | Specification | Test 1 | Test 2 | Test 3 |
|---|---|---|---|---|
| Identification | Detect precipitation line | Pass | Pass | Pass |
| Appearance | White or lemon yellow powder | Pass | Pass | Pass |
| Sterility | No observed microorganism | Pass | Pass | Pass |
| Moisture content (water determination) | < 3.0% | 1.34% | 1.38% | 1.38% |
| Uniformity (mass variation test) | < 10% | 6.1% | 6.0% | 6.4% |
| Foreign insoluble particle matter | No foreign matter in visual (optical particle counting: over 10 μm ≤ 6000 ea./container) | Pass | Pass | Pass |
| Leakage | ∆P < 3.82 | 2.86 | 2.93 | 2.91 |
Determination of the anti-lethal titer
The common potencies of the anti-lethal titers were 2909 (95% confidence interval: 2837–2984) U/vial at NIFDS, 3455 (3195–3735) U/vial at Korea Vaccine Co., Ltd., which were calculated from the geometric mean of the ten results, respectively. NIID in Japan contributed data from five independent assays with the common potency of 2997 (2798–3168) U/vial as shown in Table 4. Intra-laboratory variability was calculated as coefficients of variation (CVs) and are also shown in Table 4. In all three laboratories, the CVs were approximately 10%. The general common potency determined in collaboration with the results of the three facilities was 3131 (2991–3276) U/vial, which was round off to 3100 U/vial.
Table 4.
Results of collaborative study for the anti-lethal titer and anti-hemorrhagic titer determination
| Test | Anti-lethal titer (U/vial) | Anti-hemorrhagic titer (U/vial) | ||||
|---|---|---|---|---|---|---|
| 1a | 2b | 3c | 1a | 2b | 3c | |
| 1 | 2778 | 3678 | 2887 | 2456 | 3111 | 3640 |
| 2 | 3000 | 3537 | 2778 | 2638 | 3515 | 2616 |
| 3 | 3000 | 2900 | 3000 | 2956 | 3111 | 2957 |
| 4 | 2887 | 2900 | 3117 | 2638 | 3111 | 2651 |
| 5 | 2887 | 3152 | 3117 | 2992 | 3500 | 2505 |
| 6 | 2887 | 3609 | 2494 | 3515 | ||
| 7 | 2887 | 3961 | 2962 | 3500 | ||
| 8 | 2778 | 3777 | 2669 | 3111 | ||
| 9 | 3117 | 3678 | 2983 | 3500 | ||
| 10 | 2887 | 3537 | 2937 | 3500 | ||
| Common potency | 2909 | 3455 | 2997 | 2765 | 3342 | 2847 |
| 95% Confidence interval | 2837–2984 | 3195–3735 | 2798–3168 | 2614–2924 | 3198–3492 | 2362–3431 |
| Coefficients of variation | 3.50 | 11.00 | 5.00 | 7.84 | 6.16 | 15.00 |
| General common potency | 3131 | 3000 | ||||
aNational Institute of Food and Drug Safety Evaluation (NIFDS), Korea
bKorea Vaccine co., Korea
cNational Institute of Infectious Diseases (NIID), Japan
Determination of the anti-hemorrhagic titer
The results were 2765 (95% confidence interval: 2614–2924) U/vial at NIFDS, 3342 (3198–3492) U/vial at Korea Vaccine Co., Ltd., and 2847 (2362–3431) U/vial at NIID in the common potencies (Table 4). The CVs of the three facilities were 7.84%, 6.16%, and 15.00%, respectively. The general common potency of the anti-hemorrhagic titer obtained from the results of the twenty-five tests performed at these three facilities was 3000 (2849–3159) U/vial. One of the test results is shown in Fig. 1. The hemorrhagic spots caused by intradermal injection from top to bottom with the 0.2 ml of the mixture of solutions containing the antivenom (in concentrations of 6.4, 8.0, 10.0, 12.6, or 16.0 U per ml) were of varying sizes depending on the concentrations of antivenom. Each ED50 of the regional reference standard for antivenom and the candidate was designated in terms of the concentration of the antivenom inducing the specific size of hemorrhagic spot (average cross-diameter of 10 mm). The anti-hemorrhagic titers were presented by relative potencies to the regional reference standard for antivenom.
Fig. 1.
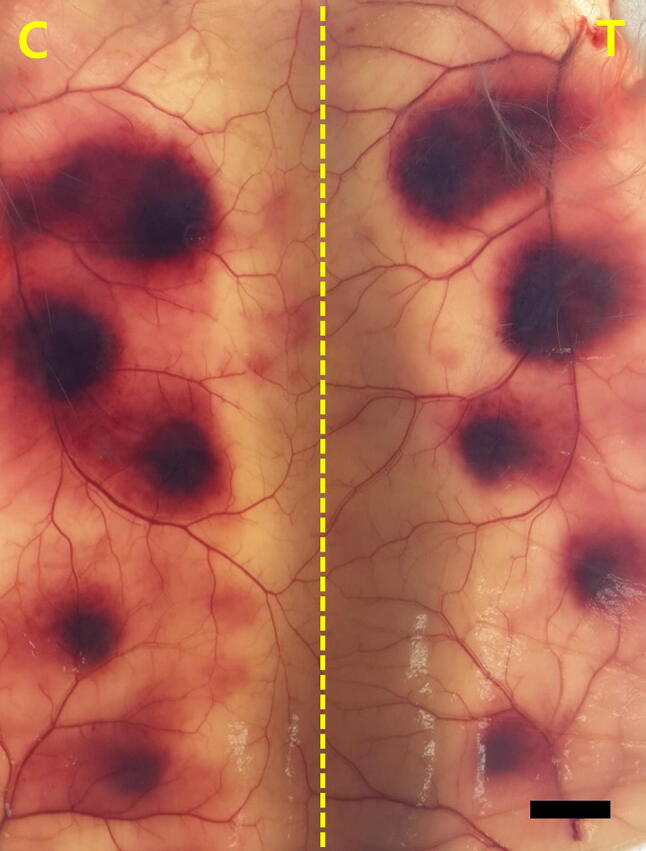
Hemorrhagic spots with different sizes are shown in a symmetry. The hemorrhagic spots resulted from intradermal injections of aliquots of 0.2 ml of mixtures of the test snake venom and the regional reference containing 0.64 U, 0.80 U, 1.00 U, 1.26 U, and 1.60 U of the antivenom (C), whereas the results were from injections with the antivenom of the candidate in aliquots of 0.2 ml of mixtures (T), from top to bottom. Bar 10 mm
Discussion
National regulatory authorities (NRAs) and NCLs play a central role in ensuring that biological products such as antivenoms available for use in countries have been carefully and thoroughly evaluated against internationally established standards on quality and safety. These institutes of government are pivotal to the process of consolidating systems of regulatory controls for health based on recognized frameworks and technical expertise. WHO guideline for national authorities on quality assurance for biological products stipulate that NRAs should guarantee that available biological products are qualified, safe, and efficacious, and that manufacturers adhere to approved reference standards regarding quality assurance. The responsibilities should also include the implementation of effective national regulations with the enforcement of the setting of appropriate reference standards and control measures [15]. Owing to the large variations in venom composition even within a single species, WHO guidelines for the production control and regulation of snake antivenom immunoglobulins recommend that national reference standard for antivenom be established associated with the snake venoms, which cover the entire interspecies variability [13]. Regional reference standard materials also could be used when countries within a region share a similar distribution of venomous snakes [13]. The candidate for the first NRS for Gloydius snake antivenom assessed for quality in this study was raised against the venom of Gloydius brevicaudus, which was used for establishment of the second NRS for snake venom [12]. This species is widely distributed throughout Korea. Many other species in the same genus of Gloydius also inhabit East Asia, including Japan, China, and Korea. The venoms from these snakes were shown to have very high relationships phylogenetically [16] and similar characteristics on immunoreactivities. The antivenoms raised against the venoms of these snakes were capable of neutralizing those of the variants in the same genus from Korea and Japan [17]. Thus, it is deemed appropriate that the venom of G. brevicaudus has been chosen as an immunogen for raising the candidate for Gloydius snake antivenom as well as the antivenom product which has been used for the treatment of Gloydius snakebites in Korea. It is also considered that the candidate can be used as a reference standard in regions inhabited by Gloydius snakes, such as the regional reference standard for Gloydius snake antivenom established in 2006 [10]. In addition, a reference standard for antivenom is required to have characteristics similar to a commercial antivenom product to improve the accuracy of the quality control tests. Since venom-neutralizing efficacy and specificity can only be compared with antivenom of similar specificity and neutralizing profile [10]. The candidate was obtained from a batch of the final bulk of the antivenom product.
In the quality assessment, the results of the quality control tests, including the preliminary tests on immunodiffusion assay and immune-electrophoresis, fulfilled the requirements regarding the specifications [9], which indicated that the candidate had quality characteristics similar to commercial antivenom products. The real-time stability tests were performed at Korea Vaccine Co., Ltd. where the candidate vials in triplicate were maintained at − 20°C for 0, 3, 6, 9, and 12 months. The control limits for monitoring the stabilities of potencies were set up in the range of 80–120% of the general common potencies, complying with the quality standard of other blood derived products [12]. The lower control limits (LCLs) were 2480 U/ml in the anti-lethal titer and 2400 U/ml in the anti-hemorrhagic titer. The upper control limits (UCLs) were 3720 U and 3600 U per ml in the anti-lethal and anti-hemorrhagic titer, respectively. The results of all were within the range of 80–120% of the control limits, and there were no significant differences among the potencies of the candidates stored at − 20°C for 12 months (data not shown). These results indicated that there was no statistically significant loss of the potencies of the candidates according to storage time. The time interval and design of the continuous real-time stability test were according to the WHO Guidelines [13]. In accelerated stability studies which were also performed at Korea Vaccine Co., Ltd., the candidate vials were exposed to harsh thermal conditions (25 ± 2℃ and 37 ± 2℃ in room humidity of 60 ± 5%) and the stabilities of potencies were assessed over a shorter time span. The results showed that there was no statistically significant degradation in potencies of the candidates stored at these temperatures (data not shown). As a result of the collaborative study, the candidate showed the anti-lethal titer of 3100 U/vial and the anti-hemorrhagic titer of 3000 U/vial. Unfortunately, the collaborative study to establish the assigned potency was conducted at only two Korean facilities and one foreign facilities in Japan. There is one manufacturer of snake antivenom in Korea. Moreover, determination of the anti-hemorrhagic titer is performed routinely for quality control of antivenom products in only Japan and Korea, although the anti-lethal titers of the antivenom products are determined in Japan, Korea, and China as lot-release tests [10]. However, the discriminating capacity of the present study was greatly enhanced by assessing the injected animals over a large number of tests.
This study aimed to establish the first NRS for Gloydius snake antivenom that will be used in routine quality control tests by the NIFDS and manufacturers of the antivenom product in Korea. The NIFDS will secure approximately 2500 vials of the first NRS for Gloydius snake antivenom, which is sufficient for use over the next 10 years as long as the potencies are maintained without observation of a significant decline in their potencies below the control limit. Approximately 50 vials of the NRS for antivenom are used in pairs with the NRS for snake venom for different purposes, which include routine quality control tests of antivenom product by manufacturers and national lot-release tests as well as real-time stability tests in the designed periods by the NIFDS. Furthermore, the candidate preparation showed good quality evaluation on the basis of the results of the estimation for the quality of the candidate and real-time stability tests. In conclusion, the candidate was judged to be suitable to serve as the first Korean NRS for Gloydius snake antivenom.
Acknowledgements
The authors acknowledge Sun Hwa Choi and Young-Jin Lee (Quality control Team, Korea Vaccine Co., Ltd., Korea), Dr. Mayuko Okabe (Department of Immunology, National Institute of Infectious Diseases, Japan) for participating the collaborator study.
Compliance with ethical standards
Conflict of interest
This research was supported by a Grant from the scientific research program (18201MFDS174) and a grant-in-aid (MFDS 4000-4031-301-210-13) at the National Institute of Food Drug Safety Evaluation of the Ministry of Food and Drug Safety, Republic of Korea. None of the authors of this paper has a financial or personal relationship with individuals or organizations that could inappropriately influence and bias the content of the paper.
Footnotes
Kiwon Han and Hojin Song contributed equally to this work.
References
- 1.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, Savioli L, Lalloo DG, de Silva HJ. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim H, Kang HG, Kim KH. Antivenom for snake bite in Korea. J Korean Med Assoc. 2013;56:1091–1103. doi: 10.5124/jkma.2013.56.12.1091. [DOI] [Google Scholar]
- 3.Gumprecht A, Tillack F, Orlov NL, Captain A. Asian Pit vipers. Berlin: Geitje Books; 2004. p. 368. [Google Scholar]
- 4.Eble JA. Matrix biology meets toxinology. Matrix Biol. 2010;29:239–247. doi: 10.1016/j.matbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Kosuge T. Biological toxicity of mamushi-snake venom (Agkistrodon halys) and morphological changes caused by the venom. Kitakanto Med. 1968;18:353–379. doi: 10.2974/kmj1951.18.353. [DOI] [Google Scholar]
- 6.Teteno I, Sawaki Y, Makino M. Current status of mamushi snake (Agkistrodon halys) bite in Japan with special reference to severe and fatal cases. Jpn J Exp Med. 1963;33:331–346. [PubMed] [Google Scholar]
- 7.Omori T, Iwanaga S, Suzuki T. The relationship between the hemorrhagic and lethal of Japanese mamushi (Agkistrodon halys blomhoffii) venom. Toxicon. 1964;2:1–4. doi: 10.1016/0041-0101(64)90027-3. [DOI] [PubMed] [Google Scholar]
- 8.Gutiérrez JM, León G, Lomonte B, Angulo Y. Antivenoms for snakebite envenomings. Inflamm Allergy Drug Targets. 2011;10:369–380. doi: 10.2174/187152811797200669. [DOI] [PubMed] [Google Scholar]
- 9.Korea Food and Drug Administration (2011) Minimum requirement for biological products (in Korean)
- 10.Fukuda T, Iwaki M, Hong SH, Oh HJ, Wei Z, Morokuma K, Ohkuma K, Dianliang L, Arakawa Y, Takahashi M. Standardization of regional reference for mamushi (Gloydius blomhoffii) antivenom in Japan, Korea, and China. Jpn J Infect Dis. 2006;59:20–24. [PubMed] [Google Scholar]
- 11.Yoo SH, Park SJ, Kim SN, Hong CM. Establishment of venom standard for potency test of Agkistrodon antivenom. Biol Prod Saf Monitor Rep. 2004;4:19–26. [Google Scholar]
- 12.Han K, Jung K, Oh H, Song H, Park S, Kim JH, Min G, Lee BH, Nam H, Ato M, Kim YJ, Jeong J, Ahn C. A collaborative study to establish the second Korean national reference standard for snake venom. Toxicol Res. 2018;34:191–197. doi: 10.5487/TR.2018.34.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization (2010) WHO guidelines for the production control and regulation of snake antivenom immunoglobulins. World Health Organization, Geneva. https://www.who.int/bloodproducts/snake_antivenoms/snakeantivenomguide/en. Cited 1 January 2017
- 14.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 15.Guidelines for national authorities on quality assurance for biological products. In: WHO Expert Committee on Biological Standardization. Forty-second Report. World Health Organization, Geneva. 1992, Annex 2 (WHO Technical Report Series, No. 822)
- 16.Xu Y, Myers EA, Wang L, Huang S, He Y, Peng P, Guo P. Molecular phylogeny of the genus Gloydius (Serpentes: Crotalinae) Asian Herpetol Res. 2012;3:127–132. doi: 10.3724/SP.J.1245.2012.00127. [DOI] [Google Scholar]
- 17.Kawashima Y. Study of the immunological relationships between venom of six Asiatic Agkistrodons. Snake. 1974;6:16–26. [Google Scholar]


