Abstract
Fluorides are thought to be a major cause of osteocarcinogenesis, due to their widespread industrial use, ability to accumulate in bone tissue, and genotoxic and probable carcinogenic properties. In vitro experiments investigating the genotoxic potential of fluorides in bone tissue models can provide valuable indirect information on their involvement in osteocarcinogenesis. Here, we investigated whether sodium fluoride (NaF) has the ability to induce DNA damage and chromosomal abnormalities in human osteosarcoma cells after 48 and 72 h of exposure. The cell cultures were treated with NaF in concentrations of 0, 20, 100 and 200 μg/ml. The level of DNA damage was assessed by the comet assay, and the frequency of chromosomal abnormalities by a micronucleus test. A significant increase in DNA damage indicators was noted in the samples treated with fluoride concentrations of 100 and 200 µg/ml, after 48 and 72 h of exposure. The micronucleus test revealed a dose-dependent increase in cells with micronuclei, nucleoplasmic bridges and nuclear protrusions. Increasing the concentration of NaF led to an increase in the prevalence of cytogenetic indicators after both treatment durations. This demonstrated ability of fluorine to exert genotoxic effects on bone cells indirectly indicates the possible importance of fluoride in the aetiology of osteosarcoma.
Keywords: Genotoxicity, NaF, In vitro, DNA comets, Micronucleus test
Introduction
Despite their relative rarity, primary oncological diseases of bone tissue pose a significant problem for modern biomedicine. Along with these cancers being therapeutically challenging, the causes of pathology also remain uncertain. Determining the aetiology of this type of disease is thus an important issue, as knowing its cause(s) will allow us to achieve significant progress in prevention and treatment. The main factor in the development of osteocarcinogenesis is believed to be environmental exposure. Fluorides are thought to be the environmental factors, due to their widespread industrial use, ability to accumulate in bone tissue [1], and genotoxic [2] and probable carcinogenic properties [3, 4]. This assumption is based on previous ecological-epidemiological studies [5] and studies of the correlation of fluoride in sera with the frequency of oncogenic mutations of the p53 gene in human tumour tissues [6]. However, some publications have presented evidence against the involvement of fluoride in osteocarcinogenesis [7, 8]. To test the fluoride hypothesis, an experimental assessment of the genotoxic effects of fluoride-ion exposure on human osteoblast cells is necessary. To date, a limited number of experiments on non-human bone cell cultures have been conducted. When cultured with sodium fluoride (NaF), an increase in the frequency of chromosomal aberrations in the primary culture of rat vertebral cells was observed [9], as was an increase in DNA damage in the UMR 106 rat osteosarcoma [10]. Surprisingly, though, no studies on human bone models have been conducted.
In vitro experiments investigating the genotoxic potential of fluorides in bone tissue models can provide valuable indirect information on the involvement of these species in osteocarcinogenesis. Hence, the purpose of this work was to determine the genotoxicity of NaF to human bone cells.
Materials and methods
Cell culture
The human osteosarcoma cell line (HOS) was used as a model of bone tissue, and was obtained from the collection of the State Research Center of Virology and Biotechnology VECTOR (Novosibirsk, Russia). Removal of culture from cryopreservation was performed by rapid warming in a water bath (37 °C), with further washing of the cryoprotectant by centrifugation (1000 × g), followed by replacing the environment and seeding the cells in a culture flask. The seeding concentration was 75,000–100,000 cells per ml. The culture environment consisted of 90% Dulbecco’s modified Eagle’s medium (DMEM) as nutrient (containing l-glutamine and an antibiotic Penicillin–Streptomycin (Biolot, St. Petersburg, Russia)) and 10% blood serum of cow embryo (HyClone, Logan, United States). The cells were incubated at 37 °C and in a humidified atmosphere of 5% CO2. Passaging was carried out every 3–5 days. The cells were removed from the monolayer using trypsin–Versen solution (1:1) (Biolot, St. Petersburg, Russia). After the accumulation of cell mass, they were seeded in culture plates. The maximum exposure concentration was determined by a preliminary assessment of substance cytotoxicity in different concentrations. An experiment was conducted with final concentrations of NaF of 50, 100, 200, 300 and 400 μg/ml. The cytokinesis-block proliferation index (CBPI) was evaluated and the permissible concentrations of the test substance were determined. Subsequently, an experiment was conducted with NaF concentrations causing cytotoxicity of less than 55% (CBPI > 1.55).
The process of cell growth and plating in culture plates was repeated. Next, 24 h after passage, the samples were treated with PBS (Eco-service, St. Petersburg. Russia) (the control group) or NaF (Vecton, St. Petersburg, Russia) to a final concentration of 0, 20, 100 and 200 μg/ml (the same concentrations that were used in similar studies on other types of cell culture. Additionally, part of the samples was cultured as a positive control with mitomycin C (Sigma-Aldrich) at a final concentration of 12.5 ng/ml for the micronucleus test and with hydrogen peroxide for the comet assay (100 μM for 2 min, with further cultivation for 48 or 72 h). Subsequently, the cells were cultured for 48 or 72 h. Six samples were tested for each combination of a concentration of NaF and duration of exposure. The samples intended for the micronucleus test with cytochalasin blocking were treated 24 h before the end of cultivation of with cytochalasin B (Pan Eco, Moscow, Russia), to a final concentration of 6 μg/ml. After the end of cultivation, the cells were transferred to suspension and subjected to sample preparation for the micronucleus test and the comet assay. The experiment was repeated 3 times.
Micronucleus test
The micronucleus test was performed in accordance with the recommendations of Fenech [11]. After cultivation, the preparations were treated with a hypotonic solution of KCl (Vecton, St. Petersburg, Russia) and fixed using Carnoy’s fixative. Then, staining was performed using a 2% solution of Giemsa stain (Pan Eco, Moscow, Russia). The resulting preparations were analysed using a Nikon Eclipse 80i microscope at 1000 × magnification. The selection criteria for the cells to be included in the analysis, and the criteria for the recording of cytogenetic damage, were consistent with generally accepted recommendations [12]. In each preparation, the nuclei of 1000 binuclear cells were analysed, in which micronuclei (Fig. 1), nucleoplasmic bridges (Fig. 2) and nuclear protrusions (Fig. 3) were observed.
Fig. 1.
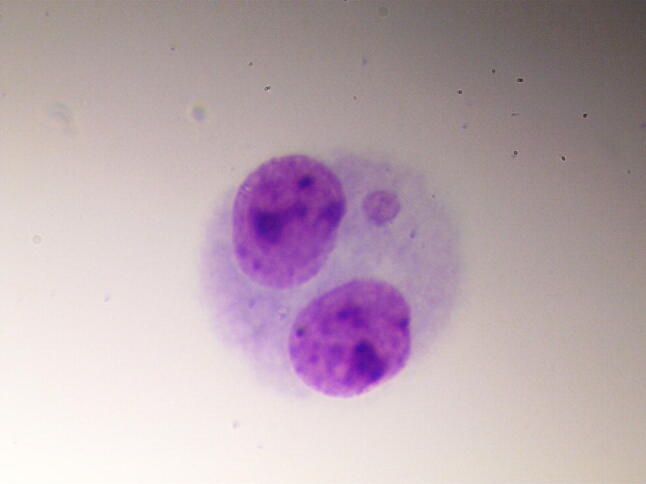
MNBN in HOS
Fig. 2.
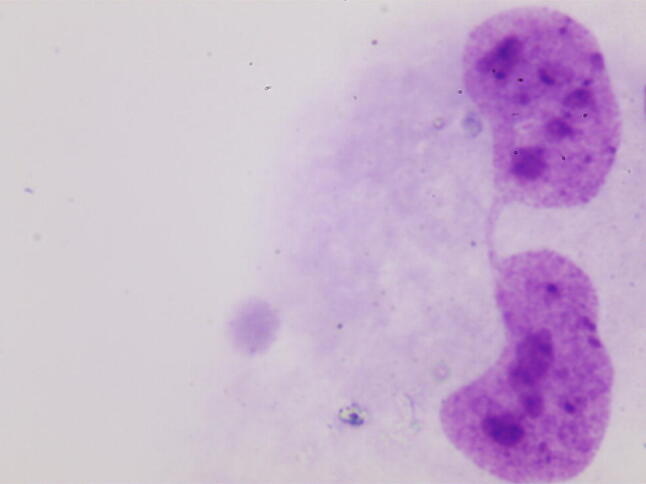
Binucleated cell with nucleoplasmic bridge
Fig. 3.
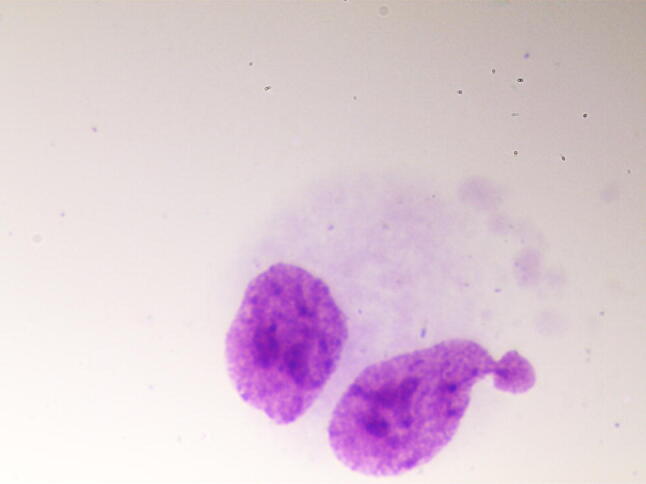
Binucleated cell with nuclear protrusion
Comet assay
The comet assay was performed in an alkaline modification developed by Singh and colleagues [13]. The fragmentation parameters were estimated by photomicrographing preparations stained with SYBR Green using a Zeiss Axio Imager 2 fluorescence microscope. A total of 200 randomly selected comets from each sample were photographed with a magnification of 200 × (Fig. 4). The subsequent processing of the photographs was carried out using the CASP software package (Krzysztof Konca, http://casplab.com). Two parameters, namely the percentage of DNA in the comet’s tail and the DNA-comet index, were calculated as follows: index of DNA comet (IDC) = (0n0 + 1n1 + 2n2 + 3n3 + 4n4)/Σ, where n0–n4 are the numbers of comets of each type, Σ is the sum of the calculated DNA-comets.
Fig. 4.
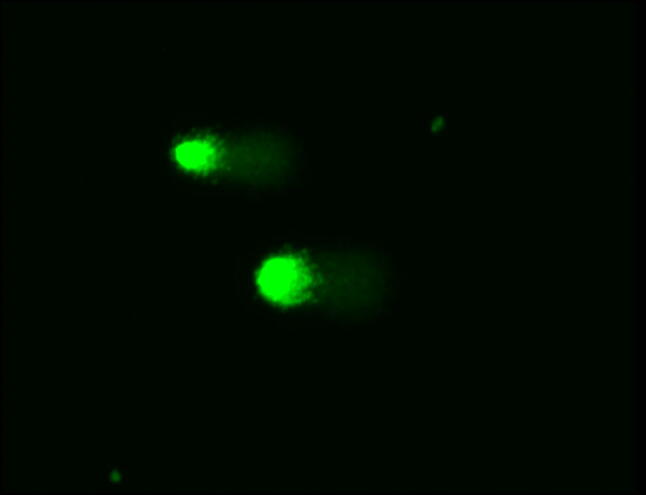
DNA-Comets in HOS
Statistical analysis
Statistical analysis of the data was carried out using the Statistica 10.0 package (StatSoft, TIBCO Software). The average values and the limit of the 95% confidence interval (CI 95) were calculated for the two quantitative indicators. Group comparisons were performed using the Mann–Whitney U-test.
Results
Comet assay
The highest average value of percentage of DNA in the comet’s tail was observed in the samples cultured for 48 h in 200 µg/ml NaF (Table 1).
Table 1.
The percentage of DNA in the comet’s tail, in the studied samples, means [95%, CI], %
| Cultivation time | The percentage of DNA in the comet’s tail, in the studied samples (%) | ||||
|---|---|---|---|---|---|
| 0 µg/ml NaF | 20 µg/ml NaF | 100 µg/ml NaF | 200 µg/ml NaF | H2O2 100 μM for 2 min |
|
| 48 h | 1.38 [1.24–1.52] | 3.79 [3.46–4.13]** | 4.09 [3.86–4.33]** | 4.47 [4.20–4.76]**,## | 4.89 [4.65–5.13] |
| 72 h | 1.18 [1.01–1.36] | 1.15 [1.01–1.30] | 2.16 [1.89–2.43]**,## | 2.76 [2.30–3.21]**,## | 3.42 [3.05–3.80] |
**p < 0.01, Significant differences against samples treated with 0 µg/ml of sodium fluoride; ##p < 0.01, Significant differences against samples treated with 20 µg/ml of sodium fluoride
A significant increase in the percentage of DNA in the comet’s tail was observed in samples with a NaF concentration of 20, 100 and 200 µg/ml with 48 h exposure (p < 0.01), and in those with concentrations of 100 and 200 µg/ml with 72 h exposure (p < 0.01), compared with the control samples (0 μg/ml). In addition, a significant increase in the percentage of DNA in the comet’s tail was observed with an increase in the NaF concentration from 20 to 200 µg/ml and after 48 h of exposure.
Similar to the trend observed with the percentage of DNA in the comet’s tail, the highest value of the DNA-comet index was observed in samples exposed for 48 h to a NaF concentration of 200 µg/ml (Table 2). Compared with the control, significant increases in the DNA-comet index were observed in samples with a NaF concentration of 20, 100 and 200 µg/ml with 48 h of exposure (p < 0.01), and in the samples with 100 and 200 µg/ml NaF with 72 h of exposure (p < 0.01). In addition, a significant increase in this index was observed with an increase in the NaF concentration from 20 µg/ml to 200 µg/ml with 48 h of exposure (p < 0.05). Additionally, samples exposed to 100 and 200 µg/ml NaF for 72 h showed a significant increase in the DNA-comet index compared with those exposed to only 20 μg/ml NaF for the same duration (p < 0.05). There was also a significant decrease in the DNA-comet index in the positive control samples with an exposure time of 72 h compared with those exposed for 48 h (p < 0.01).
Table 2.
Index of the DNA-Comets, means [95%, CI]
| Cultivation time | Index of the DNA-Comets | ||||
|---|---|---|---|---|---|
| 0 µg/ml NaF | 20 µg/ml NaF | 100 µg/ml NaF | 200 µg/ml NaF | H2O2 100 μM for 2 min |
|
| 48 h | 0.31 [0.28–0.42] | 0.88 [0.80–0.95]** | 0.97 [0.92–1.03]** | 1.07 [1.00–1.14]**,# | 1.17 [1.11–1.23] |
| 72 h | 0.24 [0.21–0.27] | 0.26 [0.23–0.29] | 0.54 [0.47–0.61]**,# | 0.72 [0.60–0.84]**,# | 0.90 [0.79–1.01] |
**p < 0.01, Significant differences against samples treated with 0 µg/ml of sodium fluoride; #p < 0.05, Significant differences against samples treated with 20 µg/ml of sodium fluoride
Micronucleus test
The results of the micronucleus test are presented in Table 3. Compared with the control, the concentration of NaF of 20 µg/ml in the culture medium did not generate a statistically significant increase in the frequency of binucleated cells with a micronucleus (MNBN), nucleoplasmic bridges or nuclear protrusion at either exposure time. In the case of a NaF concentration of 100 µg/ml, compared with the control, a statistically significant increase was observed in the numbers of MNBNs (p < 0.05 with 48 h exposure and p < 0.01 with 72 h exposure), binucleated cells with nucleoplasmic bridges (p < 0.01 at 48 exposures and p < 0.05 at 72 h exposure) and binucleated cells with nuclear protrusions at 72 h exposure (p < 0.05). In the case of a NaF concentration of 200 μg/ml in the culture medium, compared with the control there was a statistically significant increase in the number of MNBNs (p < 0.01 at 48 h exposure and p < 0.01 at 72 h exposure), binucleated cells with nucleoplasmic bridges (p < 0.05 at 48 h exposure and p < 0.01 at 72 h exposure) and binucleated cells with nuclear protrusions (p < 0.05 at 48 h exposure and p < 0.05 at 72 h exposure). In addition, a statistically significant increase in the number of MNBNs was observed with an increase in exposure time from 48 to 72 h in samples with a NaF concentration of 200 μg/ml (p < 0.01).
Table 3.
The results of the micronucleus test, means [95%, CI] (‰)
| Cultivation time | The frequency of micronucleus test’s indicators (‰) | ||||
|---|---|---|---|---|---|
| 0 µg/ml NaF | 20 µg/ml NaF | 100 µg/ml NaF | 200 µg/ml NaF | 12.5 ng/ml Mitomycin C | |
| MNBN | |||||
| 48 h | 32.33 [25.16–9.50] | 42.00 [24.90–9.09] | 60.67 [51.26–70.07]* | 69.11 [62.08–76.13]** | 88.78 [82.57–94.98] |
| 72 h | 35.00 [17.09–2.91] | 32.00 [25.42–8.57] | 73,91 [61.34–86.48]** | 90.21 [88.82–91.60]** | 100.07 [76.42–123.71] |
| Binucleated cells with nucleoplasmic bridges | |||||
| 48 h | 7.67 [0–15.41] | 9.33 [1.74–16.92] | 23.66 [19.87–27.46]** | 21.82 [19.22–24.42]* | 33.98 [28.05–39.90] |
| 72 h | 7.66 [1.93–13.40] | 12.67 [6.63–18.71] | 17.32 [9.52–25.13]* | 24.54 [20.44–28.64]** | 38.09 [29.29–46.89] |
| Binucleated cells with nuclear protrusion | |||||
| 48 h | 11.67 [7.87–15.46] | 6.67 [0–13.84] | 18.67 [7.46–29.87] | 33.43 [25.03–41.83]* | 27.02 [19.5–34.54] |
| 72 h | 7.33 [5.90–8.77] | 7.67 [4.80–10.53] | 22.73 [18.61–26.85]* | 23.41 [21.16–25.67]* | 29.19 [22.36–36.01] |
*p < 0.05, Significant differences against samples treated with 0 µg/ml of sodium fluoride; **p < 0.01, Significant differences against samples treated with 0 µg/ml of sodium fluoride
Discussion
The results of this study showed that NaF was able to induce damage to the DNA of osteoblast cells. In the comet assay, both the percentage of DNA in the comet’s tail and the DNA-comet index increased with the concentration of fluorine in the growth medium. In the case of 48 h of exposure, both parameters differed significantly in samples with NaF concentrations of 20 or 100 µg/ml, compared with the control sample (0 µg/ml). In samples with a concentration of 200 µg/ml, a further significant increase was noted relative to the cell cultures exposed to only 20 µg/ml. In the case of 72 h of exposure the level of DNA damage was lower, possibly because of the activation of DNA repair or apoptotic programs in cells with most DNA damage.
Nonetheless, the average extent of genotoxic effects increased with the concentration of NaF in the growth environment, as in the case of 48 h exposure. In the positive control, the DNA-comet index decreased in the case of 72-h cultivation compared with 48-h cultivation, in agreement with the abovementioned conclusions. The extent of DNA damage in samples with an NaF concentration of 100 µg/ml and 200 µg/ml was far greater than that in samples with concentrations of 0 and 20 µg/ml. The obtained data are consistent with the results from similar studies conducted on mammalian cells. Specifically, several authors have studied the ability of NaF in concentrations from 0 to 100 mM to cause DNA damage, using methods for assessing the extent of DNA fragmentation. It has been determined that exposure to fluoride ions can lead to dose-dependent DNA damage in rat osteosarcoma cell lines [10], primary mouse hepatocytes [14, 15] and primary rat kidney cells [16].
When assessing the extent of chromosomal damage with a micronucleus test, a dose-dependent increase in cells with micronuclei, nucleoplasmic bridges and nuclear protrusions was noted. An increase in the concentration of NaF for both exposure durations led to an increase in the numbers of these cytogenetic indicators. Notably, an increase in the exposure time did not lead to a decrease in the number of cytogenetic markers, in contrast to the increased extent of DNA damage. On the contrary, a significant increase in the number of MNBN cells was observed at 72 h of exposure, as opposed to 48 h.
The obtained results are consistent with data from other authors. Cultivation of cells in the presence of fluoride ions led to an increase in chromosome aberration and sister chromatid exchanges in cell cultures of the Syrian hamster embryo [17, 18], Indian muntzhak [19] and the red bone marrow, hippocampal neurons and tracheal epithelium [20–22] of rats. In vitro experiments on human cell lines showed that fluoride was significantly genotoxicity at low concentrations. The ability of fluoride ions to induce DNA damage and increase the frequency of clastogenic effects in human leukocytes has been noted [23–25] for buccal epithelial cells [26], fibroblasts [27], JHU-1 foreskin fibroblasts [28], HL-60 human leukaemia cell lines [29] and human primary hepatocytes [30]. The results of these studies, as well as the present work, are in conflict with a number of other works in which the genotoxic potential of fluoride ions was not confirmed in experiments on primary cells of mice, sheep, cows [31, 32] and human leukocytes [33].
Taken together, the published data strongly suggest that genotoxicity is connected to the ability of fluoride ions to induce mitochondrial damage and oxidative stress. Such events usually culminate in either apoptosis of cells, subsequent to activation of proapoptotic caspases (caspase 3, 9 and others), or necrosis. Some authors have demonstrated that fluoride in low concentrations induces oxidative stress, leading to apoptosis of human lymphocytes in vitro [34]. Similar observations have been made in rat foetal hepatocytes [30]. According to other research, the influence of fluoride contributes to the synthesis of active forms of oxygen with the induction of SIRT1/autophagy, via signalling of the c-Jun N-terminal kinase in ameloblasts. Other observed effects of fluoride are its triggering of the release of cytochrome C, weakening of ATP synthesis and phosphorylation of γH2AX [35]. Simultaneous treatment of a cell line with fluoride ions and catalase greatly reduced the apoptosis of cells, suggesting that the reactive oxygen species (ROS)-mediated genotoxic mechanism of fluoride is a primary mechanism underpinning its activity [36]. There is also evidence of fluoride ions’ ability to inhibit the active state of enzymes involved in the biotransformation of phase I and II xenobiotics, as well as enzyme markers of oxidative stress [37]. Moreover, it has been noted that low concentrations of fluoride ions affected the progression of the cell cycle [34] and, that they lead to DNA fragmentation through activation of caspase 3 [38].
In this study it has been shown that NaF influenced the genomic integrity of human bone cells. It was noted that short-term cultivation of tumour osteoblasts in the presence of NaF increased the extent of DNA damage and chromosomal aberrations. The results indirectly testify to the possible roles of fluorides in the aetiology of osteosarcoma.
Acknowledgements
The study was conducted with financial support from the Russian Foundation for Basic Research and the administration of the region of Kemerovo (No 18-44-420012 p_a, agreement with AKO No 8, 28 June 2018), and from a stipend for young scientists from the President of the Russian Federation.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Lav KH, Baylink DJ. Molecular mechanism of action of fluoride on bone cells. J Bone Miner Res. 1998;13:1660–1667. doi: 10.1359/jbmr.1998.13.11.1660. [DOI] [PubMed] [Google Scholar]
- 2.Perumal E, Paul V, Govindarajan V, Panneerselvam L. A brief review on experimental fluorosis. Toxicol Lett. 2013;223:236–251. doi: 10.1016/j.toxlet.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Purohit SD, Gupta RC, Mathur AK, Gupta N, Jeswani ID, Choudhary VK. Experimental pulmonary fluorosis. Indian J Chest Dis Allied Sci. 1999;41:27–34. [PubMed] [Google Scholar]
- 4.Grandjean P, Olsen JH, Jensen OM, Juel K. Cancer incidence and mortality in workers exposed to fluoride. J Natl Cancer Inst. 1992;84:1903–1909. doi: 10.1093/jnci/84.24.1903. [DOI] [PubMed] [Google Scholar]
- 5.Bassin EB, Wypij D, Davis RB, Mittleman MA. Age-specific fluoride exposure in drinking water and osteosarcoma. Cancer Causes Control. 2006;17:421–428. doi: 10.1007/s10552-005-0500-6. [DOI] [PubMed] [Google Scholar]
- 6.Ramesh N, Vuayaraghavan AS, Desai BS, Natarajan M, Murthy PB, Pillai KS. Low levels of p53 mutations in Indian patients with osteosarcoma and the correlation with fluoride levels in bone. J Environ Pathol Toxicol Oncol. 2001;20:237–243. [PubMed] [Google Scholar]
- 7.Archer NP, Napier TS, Villanacci JF. Fluoride exposure in public drinking water and childhood and adolescent osteosarcoma in Texas. Cancer Causes Control. 2016;27:863–868. doi: 10.1007/s10552-016-0759-9. [DOI] [PubMed] [Google Scholar]
- 8.Gelberg KH, Fitzgerald EF, Synian H, Robert D. Fluoride exposure and childhood osteosarcoma: a case-control study. Am J Public Health. 1995;85:1678–1683. doi: 10.2105/AJPH.85.12.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mihashi M, Tsutsui T. Clastogenic activity of sodium fluoride to rat vertebral body-derived cells in culture. Mutat Res. 1996;368:7–13. doi: 10.1016/S0165-1218(96)90034-8. [DOI] [PubMed] [Google Scholar]
- 10.Hirano S, Ando M. Fluoride mediates apoptosis in osteosarcoma UMR 106 and its cytotoxicity depends on the pH. Arch Toxicol. 1997;72:52–58. doi: 10.1007/s002040050468. [DOI] [PubMed] [Google Scholar]
- 11.Fenech M. The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutat Res. 1993;285:35–44. doi: 10.1016/0027-5107(93)90049-L. [DOI] [PubMed] [Google Scholar]
- 12.Fenech M. The in vitromicronucleus technique. Mutat Res. 2000;455:81–95. doi: 10.1016/S0027-5107(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 13.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 14.Das J, Ghosh J, Manna P, Sil PC. Taurine provides antioxidant defense against NaF-induced cytotoxicity in murine hepatocytes. Pathophysiology. 2008;15:181–190. doi: 10.1016/j.pathophys.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh J, Das J, Manna P, Sil PC. Cytoprotective effect of arjunolic acid in response to sodium fluoride mediated oxidative stress and cell death via necrotic pathway. Toxicol In Vitro. 2008;22:1918–1926. doi: 10.1016/j.tiv.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Jia L, Zhang Z, Zhai L, Zhang Y, Sun G. DNA damage induced by fluoride in rat kidney cells. Fluoride. 2008;41:297–300. [Google Scholar]
- 17.Aardema MJ, Gibson DP, LeBoeuf RA. Sodium fluoride-induced chromosome aberrations in different stages of the cell cycle: a proposed mechanism. Mutat Res. 1989;223:191–203. doi: 10.1016/0165-1218(89)90047-5. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsui T, Suzuki N, Ohmori M. Exchanges, and unscheduled DNA synthesis in cultured Syrian transformation, chromosome aberrations, sister chromatid sodium fluoride-induced morphological and neoplastic hamster embryo cells. Cancer Res. 1984;44:938–941. [PubMed] [Google Scholar]
- 19.He W, Liu A, Bao H, Wang Y, Cao W. Effect of sodium fluoride and fluoroacetamide on sister chromatid exchanges and chromosomal aberrations in cultured Red Muntjac (Muntjacus muntjak) cells. Acta Scient Circumst. 1983;3:94–100. [Google Scholar]
- 20.Zhang M, Wang A, Xia T, He P. Effects of fluoride on DNA damage, S-phase cell-cycle arrest and the expression of NF-kappaB in primary cultured rat hippocampal neurons. Toxicol Lett. 2008;179:1–5. doi: 10.1016/j.toxlet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Khalil AM. Chromosome aberrations in cultured in bone marrow cells treated with inorganic fluorides. Mutat Res. 1995;343:67–74. doi: 10.1016/0165-1218(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 22.Lee TC, Jan KY, Wang TC. Induction of sister chromatid exchanges by arsenite in primary rat tracheal epithelial cells. Bull Inst Zool Acad Sin. 1988;27:105–110. [Google Scholar]
- 23.Tiwari H, Rao MV. Curcumin supplementation protects from genotoxic effects of arsenic and fluoride. Food Chem Toxicol. 2010;48:1234–1238. doi: 10.1016/j.fct.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Pant HH, Rao MV. Evaluation of in vitro anti-genotoxic potential of melatonin against arsenic and fluoride in human blood cultures. Ecotoxicol Environ Saf. 2010;73:1333–1337. doi: 10.1016/j.ecoenv.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Jachimczak D, Skotarczak B. The effect of fluoride and lead ions on the chromosomes of human leukocytes in vitro. Genet Polon. 1978;19:353–357. [Google Scholar]
- 26.Kleinsasser NH, Weissacher H, Wallner BC, et al. Cytotoxicity and genotoxicity of fluorides in human mucosa and lymphocytes. Laryngorhinootologie. 2001;80:187–190. doi: 10.1055/s-2001-13760. [DOI] [PubMed] [Google Scholar]
- 27.Scott D (1985) Cytogenetic effects of sodium fluoride in cultured human fibroblasts. In: 4th international conference on environmental mutagens (Conference proceedings). Stockholm
- 28.Hayashi N, Tsutsui T. Cell cycle dependence of cytotoxicity and clastogenicity induced by treatment of synchronized human diploid fibroblasts with sodium fluoride. Mutat Res. 1993;290:293–302. doi: 10.1016/0027-5107(93)90170-K. [DOI] [PubMed] [Google Scholar]
- 29.Anuradha CD, Kanno S, Hirano S. Fluoride induces apoptosis by caspase-3 activation in human leukemia HL-60 cells. Arch Toxicol. 2000;74:226–230. doi: 10.1007/s002040000132. [DOI] [PubMed] [Google Scholar]
- 30.Wang A, Xia T, Chu Q, et al. Effects of fluoride on lipid peroxidation, DNA damage and apoptosis in human embryo hepatocytes. Biomed Environ Sci. 2004;17:217–222. [PubMed] [Google Scholar]
- 31.Kishi K, Ishidab T. Clastogenic activity of sodium fluoride in great ape cells. Mutat Res. 1993;301:183–188. doi: 10.1016/0165-7992(93)90076-8. [DOI] [PubMed] [Google Scholar]
- 32.Jagiello G, Lin JS. Sodium fluoride as potential mutagen in mammalian eggs. Arch Environ Health. 1974;29:230–235. doi: 10.1080/00039896.1974.10666574. [DOI] [PubMed] [Google Scholar]
- 33.Thomson EJ, Kilanowski, Perry PE. The effect of fluoride on chromosome aberration and sister chromatid exchange frequencies in cultured human lymphocytes. Mutat Res. 1985;144:89–92. doi: 10.1016/0165-7992(85)90008-9. [DOI] [PubMed] [Google Scholar]
- 34.Jothiramajayam M, Sinha S, Ghosh M, et al. Sodium fluoride promotes apoptosis by generation of reactive oxygen species in human lymphocytes. J Toxicol Environ Health A. 2014;77:1269–1280. doi: 10.1080/15287394.2014.928658. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Bandoski C, Bartlett JD. Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling. Free Radic Biol Med. 2015;89:369–378. doi: 10.1016/j.freeradbiomed.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen Ngoc TD, Son YO, Lim SS, Shi X, Kim JG, Heo JS, Choe Y, Jeon YM, Lee JC. Sodium fluoride induces apoptosis in mouse embryonic stem cells through ROS-dependent and caspase- and JNK-mediated pathways. Toxicol Appl Pharmacol. 2012;259:329–337. doi: 10.1016/j.taap.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dutta M, Rajak P, Khatun S, Roy S. Toxicity assessment of sodium fluoride in Drosophila melanogaster after chronic sub-lethal exposure. Chemosphere. 2017;166:255–266. doi: 10.1016/j.chemosphere.2016.09.112. [DOI] [PubMed] [Google Scholar]
- 38.Karube H, Nishitai G, Inageda K, Kurosu H, Matsuoka M. NaF activates MAPKs and induces apoptosis in odontoblast-like cells. J Dent Res. 2009;88:461–465. doi: 10.1177/0022034509334771. [DOI] [PubMed] [Google Scholar]


