Abstract
A modified SDS–Trizol method was optimized for isolation of total RNA from the stored maize seeds at regular interval of one month for 4 months. Use of SDS extraction buffer before the use of Trizol reduced the co-precipitation problem associated with high carbohydrate content in the seed. Recorded mean RNA yield from seeds across the storage intervals was 978.6 ± 65.46 ng/µl. Average spectrophotometric values (A260/280) of isolated RNA varied from 1.974 ± 0.033 to 1.998 ± 0.022. Attempts to isolate RNA from green leaves using Trizol method also ensured comparable quality and quantity of the isolated RNA. RNA yield from fresh leaves was recorded 1008.2 ± 77.088 ng/µl which is slightly higher than the mean RNA yield from seeds across months. Observed mean A260/280 values of isolated RNA were 1.984 ± 0.030. DNase treatment further improved the A260/280 ratio in both seeds (2.003 ± 0.006) and leaves (2.012 ± 0.037). High quality and quantity along with integrity of the isolated RNA was ensured through downstream analysis after RNA extraction such as first-strand cDNA synthesis and normal PCR. Extraction of RNA from the stored seeds using modified SDS-based Trizol method and from fresh leaves using Trizol method opened new possibility of understanding role of key genes involving developmental steps especially in the stored seeds.
Keywords: RNA, Seed, Leaf, Dry condition, Starch, Storage
Introduction
Maize serves as a staple food, feed and industrial by-products worldwide (Prasanna et al. 2020). Understanding the genetic makeup is key to the success of development of cultivars as per the need (Wimalanathan et al. 2018). In the post-genomic era, considerable successes have been achieved to structurally dissect the whole genome of crops and functional annotation of target genes (Bouchez and Hofte 1998). However, spatial and temporal expressions of the genes in various parts of the tissue made it more difficult to understand the whole genetic make-up of crop species (Koltunow et al. 1990; Dutta et al. 2019a). In this context, isolation of RNA becomes an initial step to understand the functionality of genome (Tan and Yiap 2009).
RNA is one of the key elements involving in entire machinery of the living system (Chomczynski and Sacchi 1987). Therefore, isolation of high-quality RNA from different part of the plant tissues becomes an important step to understand the fundamental biological processes. However, quality of the isolated RNA varies among various tissues due to varying chemical composition (Pereira et al. 2017; Liu et al. 2018). In the crop like cereals, both seed and green leaf are the most important tissues for studying the gene expression as they take part in several morphogenesis and developmental stages of the plant (Shewry and Halford 2002). Therefore, isolation of the high-quality RNA from both seeds as well as green leaves is a significant step for studying expression pattern of the genes involved in morphogenesis and developmental pathways.
In the present study, both stored seeds and green leaves of maize plants were taken as starting materials for optimization of RNA extraction protocol. As maize seeds possess high levels of starch, polysaccharides and other secondary metabolites, it is difficult to isolate high-quality RNA from the seed (Wang et al. 2012). Several methods have been developed for isolating the RNA from the materials rich in polysaccharides, and secondary metabolites (Li and Trick 2005; Wang et al. 2012). However, few of these methods yielded the satisfactory result for isolation of high quality of RNA from the maize seeds. The major problem for RNA isolation from eukaryotic tissues is presence of endogenous RNase enzyme and its introduction to external sources hinder quality of RNA from any tissues (Rosenberg 2008). Trizol or guanidinium isothiocyanate-based method was widely used for extraction of RNA from various organisms including plants, animals and bacteria (Chomczynski and Sacchi 1987). However, maize seed with high amount of starch and polysaccharides create a solidification problem of samples in RNA extraction buffers (Li and Trick 2005). Isolated RNA further gets contaminated with starch, polysaccharides and other secondary metabolites due to co-precipitation, which results in reduced quality of the RNA, further hindering the downstream analysis (Liu et al. 2018). Along with reduced quality, starch tends to co-precipitate with the RNA pellets, making it very difficult to re-dissolve the RNA in double distilled water (Li and Trick 2005). Attempt was undertaken to isolate the RNA from maize seeds using Trizol, CTAB (cetyl trimethyl ammonium bromide) and boric acid associated with SDS methods. However, all of these methods possess limitation to isolate high-quality RNA from maize seeds due to co-precipitation problem (Wang et al. 2012). Isolation of RNA through Trizol method was mainly affected due to formation of sticky, gel-like homogenate mixtures in extraction buffer which made it difficult to separate two phases after high-speed centrifugation (Li and Trick 2005). Though CTAB-based method helped in isolating RNA, it yielded very low quantity of RNA (180 μg/g) contaminated with genomic DNA from maize seeds; besides being a time consuming and labour intensive procedure (Gambino et al. 2008). Commercially available RNA isolation kit also failed to isolate high quality RNA from maize seeds.
Several attempts were taken to isolate RNA from seed materials in various plant species including Arabidopsis (Onate-Sanchez and Vicente-Carbajosa 2008; Kanai et al. 2017; Footitt et al. 2018), Jerusalem artichoke (Mornkham et al. 2013), Dendrobium huoshanens (Liu et al. 2018), Brassica oleracea (Footitt et al. 2018), Sinapis arvensis (Footitt et al. 2018), Phaseolus vulgaris (Pereira et al. 2017), Helianthus annuus (Ma and Yang 2011) and Elaeis guinensis (Qadri et al. 2019). However, there are limited reports on isolation of RNA from the dried seeds of maize stored over a period (Wang et al. 2012). Therefore, efforts were made to optimize the RNA extraction protocol from stored seeds to address the above-mentioned problem and the protocol was equally effective to isolate RNA from leaf sample too. This method will help for expression study of the tissue-specific genes by real-time quantitative PCR and further allow us to clone those gene(s) after appropriate external treatment.
Materials and methods
Plant materials
An inbred (PMI-PV3) was grown at IARI Experimental Farm, New Delhi (28°08′N, 77°12′E, 229 MSL) during rainy season (2017) under recommended agronomic conditions. Young leaf samples were collected at 30 days after sowing and immediately fixed with liquid N, followed by storing at − 80 °C until RNA extraction. Mature seeds of inbred were collected after self-pollination and a part of it was stored in − 80 °C refrigerator as ‘fresh seeds’. The remaining seeds were then stored in muslin cloth bag under ambient room temperature. Seeds from the muslin cloth bag were then stored for 4 months at ambient temperature (25 ± 2 °C). At monthly intervals (30 DAS [days after storage], 60 DAS, 90 DAS and 120 DAS) seeds were kept under deep freeze condition at − 80 °C until RNA extraction. All the experiments were carried out with three replicates.
Chemicals and reagents
Sodium dodecyl sulphate (SDS) was purchased from Amresco® Biochemicals; Tris (pH 8.0), NaCl, β-mercaptoethanol, chloroform, isopropanol and ethylenediaminetetraacetic acid (EDTA) from HiMedia; Trizol (Ambion); ethanol (Analytical CSS); formaldehyde (Biomatik); Turbo DNase (Invitrogen, USA); diethyl pyrocarbonate (DEPC); RNaseOUT (G-Biosciences, USA); Verso cDNA Kit (Thermo Scientific, USA) and One PCR™ mix (Gene Direx) were used in this study. All the reagents were stored according to the manufacturer’s guideline and solutions were prepared immediately before use. SDS extraction buffer was prepared with a concentration of 100 mM Tris (pH 8.0), 150 mM NaCl, 50 mM EDTA, 1.5% SDS, 2% of β-mercaptoethanol. The composition of the extraction buffer remained same as described by Li et al. (2005) with some minor modifications.
Steps for isolation of RNA from seeds
Extraction of RNA from the seeds stored at different storage intervals was pursued following Trizol method (Chomczynski and Sacchi 1987), which was optimized (Modified SDS based-Trizol method) in Maize Genetics Unit, IARI, New Delhi.
Three to four seeds stored in muslin cloth at each month intervals (fresh seed, 30 DAS, 60 DAS, 90 DAS and 120 DAS) were ground using liquid N to make fine powder with mortar and pestle pre-chilled with liquid N.
The 80–100 mg of ground samples were transferred to pre-chilled 1.5 ml DEPC treated micro-centrifuge tube and 500 µl of sodium dodecyl sulphate (SDS) extraction buffer was added immediately to the ground powder, followed by vortexing and then kept sample in ice bath for 15 min.
The samples were centrifuged at 12,000 rpm for 12 min at 4 °C. Supernatant was transferred to a fresh 1.5 ml centrifuge tube.
Equal volume of Trizol was added to the reaction mixture and mixed properly by inverting the tubes gently. The samples were then kept for incubation for 10 min at room temperature.
Half volume of chloroform was added followed by gentle mixing and the samples were kept in ice for another 10 min.
Samples were then centrifuged at 12,000 rpm for 12 min at 4 °C and aqueous phase (around 600–700 µl) was carefully transferred without disturbing the interface. An equal volume of pre-chilled isopropanol was added and kept in − 20 °C for 45 min to 1 h to allow the sample for precipitation.
The samples were centrifuged again at 12,000 rpm for 10 min at 4 °C. After discarding the supernatant, the pellet was washed with 70% ethanol and centrifuged at 8000 rpm for 5 min, supernatant was discarded and the centrifugation was repeated again without adding ethanol.
Residual ethanol was taken out with the help of pipette without disturbing the pellet and the sample was dried in ice keeping the tube opened (~ 30–45 min).
Pellet was dissolved in 50–60 µl of DEPC treated water and the tubes were kept at 4 °C for 2 h. Afterwards, the samples were stored at − 20 °C for further use.
To check the integrity and quality of the RNA, the samples were run in 1% agarose gel supplemented with 1% formaldehyde.
RNA was quantified by Nanodrop UV-spectrophotometer (Thermo Fisher, USA). The 260/280 nm reading was considered as an indicator for purity checking and a value around 2.00 was considered as good quality RNA without DNA contamination.
DNase treatment was given to the sample using Turbo DNase kit (Invitrogen, USA), following manufacturer’s instruction @ 1 μL TURBO DNase (2 U) for up to 10 μg of RNA in a 50 μL reaction for an incubation period of 20–30 min at 37 °C. These reaction conditions will remove up to 2 μg of genomic DNA from total RNA in a 50 μL reaction mixture.
DNase-treated samples were again run on 1% agarose gel supplemented with 1% formaldehyde to confirm the further quality assurance of RNA sample, followed by visualization under gel documentation system (BioRad, USA). Later on, RNA was quantified using Nanodrop UV spectrophotometer (Thermo Scientific, USA).
Steps for isolation of RNA from tender leaves
For isolation of RNA from leaf sample, Trizol method was pursued with few modifications at the initial stages.
Fresh leaf sample was collected and immediately fixed with liquid N, followed by storing the leaf at − 80 °C for further procedure.
Around 80–100 mg of leaf sample was ground using liquid N to make fine powder with mortar and pestle pre-chilled with liquid N.
Ground leaf sample was centred in mortar with help of spatula chilled with liquid N and 1 ml of Trizol was poured gently on the centred sample and waited until thawing of the sample. Care was taken during pouring of Trizol on the sample to ensure that entire powdered leaf sample was properly covered with Trizol.
The tip of the micro tips was cut to pipette out the sample from mortar after thawing and the sample was dispensed in 1.5 ml DEPC-treated eppendorf tube and kept it for 10 min ambient at room temperature.
200 µl of chloroform was added to the sample and after mixing properly, the tubes were kept immediately in ice bath for 10 min.
Rest of the procedure followed was same as for isolation of RNA from the seeds viz. step No. 6–13. [Note: all the glasswares and plasticwares used in the above methodology were washed with autoclaved DEPC water followed by autoclaving at 120 °C at 15 psi for 1 h and working bench was washed with RNase OUT (G-Biosciences; USA) before starting of the experiment].
First-strand cDNA synthesis for PCR amplification
First-strand complementary DNA (cDNA) was synthesized using Verso cDNA Kit (Thermo Scientific, USA) according to manufacturer’s guideline @ 4 µl of 5X cDNA synthesis buffer, 2 µl of dNTP Mix, 1 µl of a blend of random hexamers and anchored oligo-dT 3:1 (v/v), 1 µl of Verso Enzyme Mix, 1 ng of template (RNA) and nuclease-free water to make a final volume of 20 µl. cDNA samples were quantified using Nanodrop UV spectrophotometer (Thermo Scientific, USA) to make a final concentration of 20 ng/µl. Primers designed by Delobel et al. (2008) were used for specific PCR amplification of part of Alcohol dehydrogenase1 (Adh1) gene. The forward and reverse primers used for Adh1 gene amplification were F: 5′-CGTCGTTTCCCATCTCTTCCT-3′ and R: 5′-CCACTCCGAGACCCTCAGTC-3′, respectively, and thus yields an amplicon size of 135 bp (Delobel et al. 2008). The purified and lyophilized form of oligonucleotide primers was custom-synthesized (M/s. Macrogen, Taiwan) followed by dilution with Milli-Q water to a concentration of 10 μM. The PCR reactions was performed using Veriti96-Thermal Cycler (Applied Biosystems, USA) with a final volume 20 μl containing 1X One PCR™ mix (Ready-to-use PCR mix, Gene Direx), 50 ng of cDNA, 0.5 μM of forward and reverse primers each. The PCR conditions followed for amplification were: an initial denaturation at 94 °C for 5 min; followed by 35 cycles of denaturation at 94 °C for 45 s, annealing at 60ºC for 45 s and primer extension at 72 °C for 45 s; and a final extension step at 72 °C for 10 min. The resulted PCR product of Adh1 gene was separated through 4% agarose gel electrophoresis stained with ethidium bromide (10 mg/ml) using 1X-TBE buffer following the standard procedure and visualised under gel documentation system (BioRad, USA).
Results
In the present study, a little amount of sample (80–100 mg) was used for extraction of the high quality of RNA following our optimized RNA extraction protocol and thus eventually resulted in high quality as well as quantity of RNA. Integrity of the RNA was verified by sharp intact band of 28S, 18S, and 5S rRNA; and lack of smear in the 1% agarose gel (Figs. 1, 2). Modified SDS-based Trizol method followed in this study yielded sufficient quantity of RNA (ng/µl) from seeds at different storage intervals (Table 1).
Fig. 1.
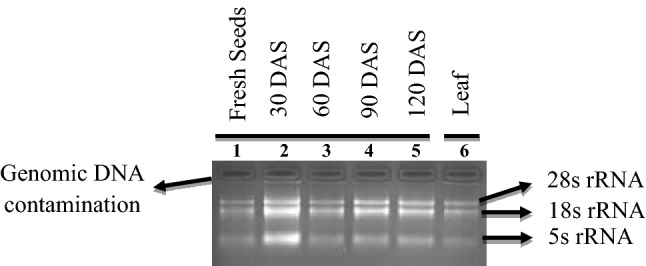
Isolation of RNA from seed and leaf tissues. Lane no. 1 to 5: seed sample at monthly intervals (up to 4 months); Lane no. 6: fresh leaf sample
Fig. 2.
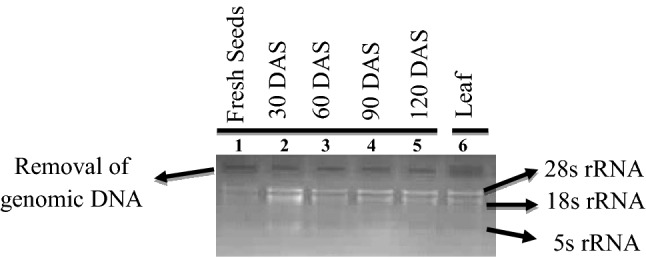
Removal of DNA contamination in the samples after DNase treatment. Lane no. 1–5: seed sample at monthly intervals (up to 4 months); Lane no. 6: fresh leaf sample
Table 1.
Concentration and quality measures of the RNA isolated using the standardized protocol
| S. no. | Tissue type | RNA yield (ng/µl) | A260/280a | A260/280b |
|---|---|---|---|---|
| 1. | Fresh seed (0 DAS) | 922.4 ± 85.958 | 1.974 ± 0.033 | 2.006 ± 0.040 |
| 2. | Seed at 30 DAS | 983.8 ± 134.622 | 1.998 ± 0.022 | 2.010 ± 0.027 |
| 3. | Seed at 60DAS | 914.4 ± 95.806 | 1.986 ± 0.019 | 2.004 ± 0.037 |
| 4. | Seed at 90 DAS | 1076.2 ± 178.781 | 1.968 ± 0.040 | 2.006 ± 0.020 |
| 5. | Seed at 120 DAS | 996.2 ± 95.667 | 1.984 ± 0.024 | 1.992 ± 0.038 |
| 6. | Leaf sample | 1008.2 ± 77.088 | 1.984 ± 0.030 | 2.012 ± 0.037 |
Data are shown as mean ± standard deviation with three replicates
aBefore DNase treatment
bAfter DNase treatment
The recorded yield of RNA from the seed sample across four months of storage was 978.6 ± 65.46 ng/µl. In freshly harvested seeds (0 DAS), the recorded mean RNA yield was 922.4 ± 85.958 ng/µl. Average RNA yield was 983.8 ± 134.622 ng/µl at 30 DAS, followed by 914.4 ± 95.806 ng/µl, 1076.2 ± 178.781 ng/µl and 996.2 ± 95.667 ng/µl at 60, 90 and 120 DAS, respectively (Fig. 3). At every months of storage, the average RNA yield was satisfactory for the downstream procedure. However, mean RNA yield at different storage intervals was not affected by their duration to isolate RNA from seeds. This result suggested no effect of storage intervals on RNA yield (Fig. 3). The recorded RNA yield from fresh leaves using Trizol method was 1008.2 ± 77.088 ng/µl which is marginally higher than the average RNA yield from seeds across months (978.6 ± 65.46 ng/µl) using modified SDS-based Trizol method.
Fig. 3.
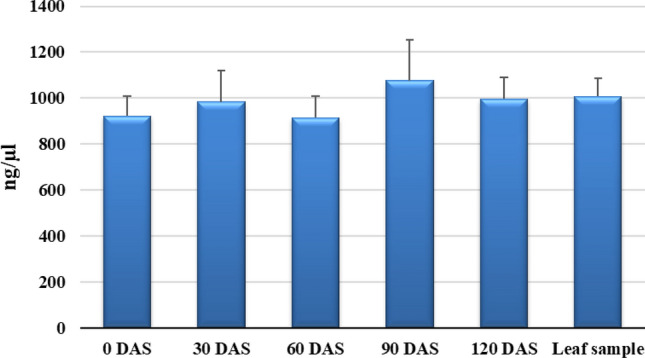
Average RNA yield (ng/µl) form seeds and leaf sample. DAS days after storage for seeds; error bar represents standard deviation around mean
The quality of the isolated RNA was assessed though spectrophotometric absorbance at 260 nm and 280 nm. Before DNase treatment, mean spectrophotometric ratio (A260/280) from freshly harvested seeds (0 DAS) was 1.974 ± 0.033. In the subsequent months, A260/280 ratio was 1.998 ± 0.022, 1.986 ± 0.019, 1.968 ± 0.040 and 1.984 ± 0.024 at 30, 60, 90 and 120 DAS; respectively (Table1). The A260/280 ratio for leaves was 1.984 ± 0.030 which was comparable with the seeds across storage intervals (1.982 ± 0.011). Mean A260/280 ratio for seeds at different storage intervals and leaves centered around 2.000 which indicated less contamination of the isolated RNA with genomic DNA.
Across all the samples, purity of the RNA was further improved following DNase treatment (Table 1). Lack of band due to genomic DNA contamination at the opening of well on 1% gel electrophoresis confirmed the removal of the DNA (Fig. 2). The mean A260/280 ratio was improved from the initial value of 1.982 ± 0.011 to 2.003 ± 0.006 in seeds across storage intervals. The recorded mean for A260/280 after DNase treatment was 2.006 ± 0.040, 2.010 ± 0.027, 2.004 ± 0.037, 2.006 ± 0.020 and 1.992 ± 0.038 at 0, 30, 60, 90 and 120 DAS; respectively. The observed ratio of A260/280 in RNA preparation isolated from leaves using Trizol method was 2.012 ± 0.037 compared to the initial value of 1.984 ± 0.030 before DNase treatment. Improvement in A260/280 ratio indicates removal of contaminated genomic DNA from RNA preparations. Use of DNase treatment further intensified the sharpness of 28S and 18S rRNA band and lack of smear in the 1% agarose gel (Fig. 2).
The next step following the RNA extraction was cDNA preparation through reverse transcription using isolated RNA as template. To confirm the efficient cDNA preparation, PCR reaction was performed using cDNA as template to amplify the desired fragment of Adh1 gene. A sharp and intact band of 135 bp of fragment of Adh1 gene was separated on 2% gel electrophoresis (Fig. 4). This ensures the accurate synthesis of cDNA from all the RNA preparation isolated from seed samples under storage using modified SDS-based Trizol method. Sharpness and thickness of the band amplified from cDNA prepared from seeds was similar that of band amplified using cDNA prepared from leaves using Trizol method. This result suggested the equal effectiveness of both modified SDS-based Trizol method to isolate RNA from seeds and Trizol method to isolate RNA from leaves.
Fig. 4.
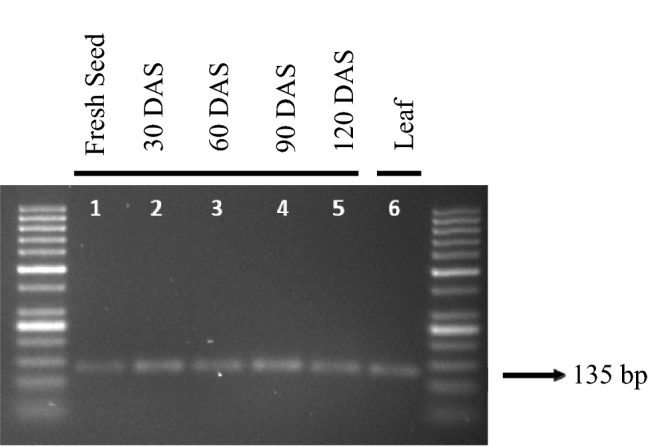
Amplification of Adh1 gene in the samples. Lane no. 1–5: seed sample at monthly intervals (up to 4 months); Lane no. 6: fresh leaf sample
Discussion
Higher concentration of starch and polysaccharides limits the isolation of high quality and quantity of RNA from maize; and here we standardised protocol to isolate high quality and quantity of RNA both from the stored seed and green leaves. We observed intact band of 28S, 18S, and 5S rRNA with no smear after agarose gel run. Integrity of the RNA is hampered mainly due to introduction of endogenous RNase enzyme to external sources and interaction of RNase enzyme with cellular RNA from eukaryotic tissues (Rio et al. 2010). Although RNase enzyme has evolved as an adaptive advantage of an organism (Harder and Schroder 2002; Dyer and Rosenberg 2006; Luhtala and Parker 2010), its ubiquitous nature makes it difficult to isolate RNA from different biological tissues (Chomczynski and Sacchi 1987; Wang et al. 2012). Trizol-based method has been widely used for extraction of RNA from different tissues due to its role in inactivation of RNase enzyme (Chomczynski and Sacchi 1987; Rio et al. 2010). Several methods have been developed for isolating the RNA from various organisms including plants, animals and bacteria using Trizol reagent. Various researchers have optimized methods to isolate RNA from plant tissues including leaf, root, flower, and various other part of the tender seedlings in different plant species (Onate-Sanchez and Vicente-Carbajosa 2008; Pereira et al. 2017; Qadri et al. 2019).
However, cereals especially maize seeds contain large proportion of starch and polysaccharides (Nelson and Pan 1995; James et al. 2003) which causes the solidification of samples in RNA extraction buffers (Gao et al. 2001). In our study, modified SDS-based Trizol method yielded good quality and quantity of RNA from fresh seeds (0 DAS) and seeds under storage. Initially, the use of SDS separates the proteins from nucleic acids. The presence of sodium chloride in the SDS buffer at high concentration creates an environment where nucleic acids can precipitate but polysaccharides remain soluble (Fang et al. 1992). The same principle was used for separation of polysaccharides from the sample while using Dellaporta DNA extraction method (Dellaporta et al. 1983). In coffee, difficulty of RNA extraction due to high polysaccharides and polyphenol content was also resolved through SDS-based protocol (Huded et al. 2018). In the very first step, application of the SDS extraction buffer made all the downstream analysis easier through removal of starch and polysaccharides. Therefore, the co-precipitation problem was resolved at the very initial stage of the protocol. In case of maize leaves; however, no such kind of problem exists. Therefore, Trizol reagent without any SDS extraction buffer was sufficient enough to yield high amount of RNA with good quality while preparing from leaves. In case of Cuminum cyminum, the phenol chloroform extraction method was found very promising as compared to other methods in term of high yield and good quality RNA from root and shoot samples (Kanai et al. 2017). CTAB-based method also was proven to isolate RNA from different tissues of grapevine and other woody plants; however, it yielded very low amount (180 μg/g) of RNA and purity was affected due to contamination with genomic DNA (Gambino et al. 2008).
In our study, RNA yield (ng/µl) was also promising from both seeds and leaves using modified SDS-based Trizol method and Trizol method, respectively. Mean RNA yield for seed at monthly intervals varied from 914.40 ng/µl to 1076.2 ng/µl, whereas it was 1008.2 ng/µl from maize leaves. In case of cumin, maximum RNA yield was observed using Trizol method from both root (8199.56 ng/µl) and shoot (2975.40 ng/µl) (Kanai et al. 2017). The quantity of RNA in the samples may also differ due to handling error at the time of RNA preparation (Kuang et al. 2018). Yield of RNA might also vary because of diverse response of different species and different tissues towards extraction protocol (Mornkham et al. 2013; Kanai et al. 2017; Huded et al. 2018). In the present study, seeds from a single maize inbred (PMI-PV3) at different storage interval was subjected to RNA isolation. It was found that amount of RNA did not change with the varying duration of storage suggesting no effect of the duration of storage on the extraction protocol. However, the composition of the RNA within the pool may vary as the level of expression of genes at different storage intervals may differ. Expression study on many key genes involved in the carotenoid biosynthesis pathway in potato tubers also supported varying composition of RNA in the pools at different storage intervals (Li et al. 2012).
For quality assessment of the isolated RNA, spectrophotometric absorbance at three wave lengths (230, 260 and 280 nm) was considered (Kuang et al. 2018). Absorbance at 280 nm and 230 nm wave lengths provides specific measurement of protein and background contamination, respectively, whereas absorbance at 260 nm wave length measures nucleic acid (DNA and RNA) concentration. Reading at 260 nm absorbance equal to 1.000 at pH 7.5 indicates 40 µg/ml of RNA and 50 µg/ml double-stranded DNA (Kuang et al. 2018). The ratios of A260/230 and A260/280 are considered as indicator of for assessing the quality of nucleic acid preparation. Pure RNA generally should give value of 2.00 or slightly above for A260/230 ratio. Low A260/230 ratio could be because of salts, carbohydrates, peptides and aromatic compounds present in RNA preparation (Ahlfen and Schlumpberger 2010). However, it is not clear which contaminants underwrites to a low A260/230 ratio and, therefore, there is no acceptable limit for it (Ahlfen and Schlumpberger 2010; Kuang et al. 2018). The most widely accepted parameter for assessing RNA quality was A260/280 ratio (Kuang et al. 2018). In the present study, therefore, A260/280 ratio was considered for the quality assessment. Mean A260/280 ratios of isolated RNA varied from 1.974 ± 0.033 to 1.998 ± 0.022 from seeds using modified SDS-based Trizol method. The average A260/280 ratio was 1.984 ± 0.030 from leaves using Trizol method. A260/280 ratio in both seeds (2.003 ± 0.006) and leaves (2.012 ± 0.037) further improved following DNase treatment. Mornkham et al. (2013) also observed varying range of A260/280 ratio using different RNA extraction protocols from seed samples of two Jerusalem artichoke accessions (Mornkham et al. 2013). Generally, A260/280 ratio of around 2.00 indicate less contamination of RNA samples with the genomic DNA (Manchester 1996); and this confirms the high quality of RNA isolated using the protocol optimised in this study.
The protocol optimized in this study would also be useful for downstream procedure as confirmed through normal PCR using cDNA. It is generally advisable to design primers for PCR from the exon–exon boundary to detect the unwanted amplification from the genomic DNA. Presence of a large intron in between forward and reverse primer designed from two exons can resolve this problem through optimizing the extension time at time of PCR. However, these steps are essential if no DNA elimination steps were performed at the time of RNA preparation. In our experiment, TURBO DNase treatment was given to remove contaminating DNA from RNA preparations. Using TURBO DNase buffer, contaminating DNA is digested to levels below the limit of detection by routine PCR. The protocol subsequently removed accessory DNase enzyme and divalent cations such as magnesium and calcium from the samples. Genomic DNA contamination can also be detected through melting curve analysis at time of real-time PCR (Kuang et al. 2018). For cDNA preparation, reverse transcriptase enzyme was used which needs RNA as template and short oligonucleotide as primers. Several reverse transcriptase enzymes and priming strategies have been described to prepare first strand cDNA with their advantages and disadvantages (Nolan et al. 2006). In our experiment, Verso cDNA Kit (Thermo Scientific, USA) was used for cDNA preparation. Verso enzyme mix contains RNA-dependent DNA polymerase with a significantly attenuated RNase H activity that can synthesize up to 11 kb long cDNA strands. It also contains RNase inhibitor that protects the RNA template from degradation at the time of cDNA preparation. For priming, random hexamers or anchored oligo-dT or blend of random hexamers and anchored oligo-dT can be used for first strand cDNA synthesis. To achieve unbiased representation of the 5′ and 3′ region of the genes, it is generally advisable to use mixture of random hexamers and anchored oligo-dT (Kuang et al. 2018). In our experiment, we used blend of random hexamers and anchored oligo-dT to ensure the unbiased representation of the 5′ and 3′ region of the genes. Use of a mixture of random hexamers and anchored oligo-dT could, therefore, be used to generate entire length first strand cDNA.
Optimization of the protocol to isolate high quality and quantity of RNA from both seed and leaf can, therefore, extends its applicability which otherwise was difficult following conventional RNA isolation protocols. Expression analysis of several gene families can be undertaken in maize in different part of tissues (Liu et al. 2019; Dutta et al. 2019a, b). Isolated RNA can further be used for functional genomics study.
Conclusion
In this study, an effective, robust, and efficient method for RNA extraction from seeds was developed. The optimized method was equally effective for leaf sample to isolate high-quality RNA. This method can be used for studying regulatory genes under dormant condition of seed during storage and also deciphering the role of the key genes in adult stage.
Acknowledgements
The first author is thankful to Indian Council of Agricultural Research for the Junior Research Fellowship to pursue his master’s degree programme.
Author contributions
Conduct of the experiment: SD; RNA isolation from seeds: MV; RNA isolation from leaves: RC; field experiment and sample preparation: RUZ; drafting of the manuscript: SD, MV and FH; design of experiment: MV and FH.
Funding
Financial support from the ICAR funded Consortia Research Platform on ‘Biofortification of Selected Crops for Nutritional Security-Maize Component’ [IARI Project Code No.: 12-131] is thankfully acknowledged.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. The authors declare that they have no conflict of interests.
Ethical approval
Not applicable.
Availability of data and material
All supporting data are included within the article.
Code availability
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All the authors have read the content and consented to submit the manuscript.
References
- Ahlfen SV, Schlumpberger M (2010) Effects of low A260/A230 ratios in RNA preparations on downstream applications. Qiagen Gene Exp Newslett 15
- Bouchez D, Hofte H. Functional genomics in plants. Plant Physiol. 1998;118(3):725–732. doi: 10.1104/pp.118.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. doi: 10.1007/BF02712670. [DOI] [Google Scholar]
- Delobel C, Lacher S, Mazzara M, Van Den Eede G. Event specific method for quantification of maize line Bt11 using real time PCR. Eur Comm Jt Res Centre. 2008 doi: 10.2788/4370. [DOI] [Google Scholar]
- Dutta S, Muthusamy V, Zunjare RU, Bhowmick R, Hossain F. Genome wide study of fatty acid hydroxylase (FAH) superfamily containing β-carotene hydroxylase (crtRB1) in maize (Zea mays L.) Pharma Innov. 2019;J8:422–428. [Google Scholar]
- Dutta S, Muthusamy V, Zunjare RU, Hossain F. Analysis of paralogous genes of Carotenoid dioxygenase affecting carotenoid biosynthesis pathway in maize (Zea mays L.) Journal of Pharmacognosy and Phytochemistry. 2019;8:524–530. [Google Scholar]
- Dyer KD, Rosenberg HF. The RNase a superfamily: generation of diversity and innate host defense. Mol Divers. 2006;10:585–597. doi: 10.1007/s11030-006-9028-2. [DOI] [PubMed] [Google Scholar]
- Fang G, Hammar S, Grumet R. A quick and inexpensive method for removing polysaccharides from plant genomic DNA. Biotechniques. 1992;13:52–54. [PubMed] [Google Scholar]
- Footitt S, Awan S, Finch-Savage WE. An improved method for the rapid isolation of RNA from Arabidopsis and seeds of other species high in polyphenols and polysaccharides. Seed Sci Res. 2018;28:360–364. doi: 10.1017/S0960258518000296. [DOI] [Google Scholar]
- Gambino G, Perrone I, Gribaudo I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem Anal. 2008;19:520–525. doi: 10.1002/pca.1078. [DOI] [PubMed] [Google Scholar]
- Gao J, Liu J, Li B, Li Z. Isolation and purification of functional total RNA from blue-grained wheat endosperm tissues containing high levels of starches and flavonoids. Plant Mol Biol Rep. 2001;19:185–186. doi: 10.1007/BF02772163. [DOI] [Google Scholar]
- Harder J, Schroder JM. RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J Biol Chem. 2002;277:46779–46784. doi: 10.1074/jbc.M207587200. [DOI] [PubMed] [Google Scholar]
- Huded AKC, Jingade P, Mishra MK. A rapid and efficient SDS-based RNA isolation protocol from different tissues of coffee. 3Biotech. 2018;8:183. doi: 10.1007/s13205-018-1209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MG, Denyer K, Myers AM. Starch synthesis in the cereal endosperm. Curr Opin Plant Biol. 2003;6:215–222. doi: 10.1016/S1369-5266(03)00042-6. [DOI] [PubMed] [Google Scholar]
- Kanai M, Mano S, Nishimura M. An efficient method for the isolation of highly purified RNA from seeds for use in quantitative transcriptome analysis. JoVE J Vis Exp. 2017;119:e55008. doi: 10.3791/55008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow AM, Truettner J, Cox KH, Wallroth M, Goldberg RB. Different temporal and spatial gene expression patterns occur during anther development. Plant Cell. 1990;2:1201–1224. doi: 10.2307/3869340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang J, Yan X, Genders AJ, Granata C, Bishop DJ. An overview of technical considerations when using quantitative real-time PCR analysis of gene expression in human exercise research. PLoS ONE. 2018;13:e0196438. doi: 10.1371/journal.pone.0196438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Trick HN. Rapid method for high-quality RNA isolation from seed endosperm containing high levels of starch. Biotechniques. 2005;38:872–876. doi: 10.2144/05386BM05. [DOI] [PubMed] [Google Scholar]
- Li L, Yang Y, Xu Q, Owsiany K, Welsch R, Chitchumroonchokchai C, Thannhauser TW. The Or gene enhances carotenoid accumulation and stability during post-harvest storage of potato tubers. Mol Plant. 2012;5:339–352. doi: 10.1093/mp/ssr099. [DOI] [PubMed] [Google Scholar]
- Liu L, Han R, Yu N, Zhang W, Xing L, Xie D, Peng D. A method for extracting high-quality total RNA from plant rich in polysaccharides and polyphenols using Dendrobium huoshanense. PLoS ONE. 2018;13:e0196592. doi: 10.1371/journal.pone.0196592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Huang L, Ma Z, Sun W, Wu Q, Tang Z, Bu T, Li C, Chen H. Genome-wide identification, expression analysis and functional study of the GRAS gene family in Tartary buckwheat (Fagopyrum tataricum) BMC Plant Biol. 2019;19:1–17. doi: 10.1186/s12870-018-1600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhtala N, Parker R. T2 family ribonucleases: ancient enzymes with diverse roles. Trends Biochem Sci. 2010;35:253–259. doi: 10.1016/j.tibs.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XB, Yang J. An optimized preparation method to obtain high-quality RNA from dry sunflower seeds. Genet Mol Res. 2011;10:160–168. doi: 10.4238/vol10-1gmr979. [DOI] [PubMed] [Google Scholar]
- Manchester KL. Use of UV methods for measurement of protein and nucleic acid concentrations. Biotechniques. 1996;20:968–970. doi: 10.2144/96206bm05. [DOI] [PubMed] [Google Scholar]
- Mornkham T, Wangsomnuk PP, Fu YB, Wangsomnuk P, Jogloy S, Patanothai A. Extractions of high quality RNA from the seeds of Jerusalem artichoke and other plant species with high levels of starch and lipid. Plants. 2013;2:302–316. doi: 10.3390/plants2020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson O, Pan D. Starch synthesis in maize endosperms. Annu Rev Plant Biol. 1995;46:475–496. doi: 10.1146/annurev.pp.46.060195.002355. [DOI] [Google Scholar]
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1:1559. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- Onate-Sanchez L, Vicente-Carbajosa J. DNA-free RNA isolation protocols for Arabidopsis thaliana, including seeds and siliques. BMC Res Notes. 2008;1:93. doi: 10.1186/1756-0500-1-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira WJ, Bassinello PZ, Brondani C, Vianello RP. An improved method for RNA extraction from common bean seeds and validation of reference genes for qPCR. Crop Breed Appl Biotechnol. 2017;17:150–158. doi: 10.1590/1984-70332017v17n2a22. [DOI] [Google Scholar]
- Prasanna BM, Palacios-Rojas N, Hossain F, Muthusamy V, Menkir A, Dhliwayo T, Ndhlela T, San Vicente F, Nair SK, Vivek BS, Zhang X. Molecular breeding for nutritionally enriched maize: status and prospects. Front Genet. 2020;10:1392. doi: 10.3389/fgene.2019.01392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri R, Iqbal A, Wu Y, Li J, Nisar N, Azam M, Yang Y. A modified protocol for total RNA isolation from different oil palm (Elaeis guineensis) tissues using cetyltrimethyl ammonium bromide. Curr Sci. 2019;116:479–482. doi: 10.18520/cs/v116/i3/479-482. [DOI] [Google Scholar]
- Rio DC, Ares M, Hannon GJ, Nilsen TW. Purification of RNA using TRIzol (TRI reagent) Cold Spring Harbor Protocols. 2010;2010:5439. doi: 10.1101/pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- Rosenberg HF. RNase A ribonucleases and host defense: an evolving story. J Leukoc Biol. 2008;83:1079–1087. doi: 10.1189/jlb.1107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewry PR, Halford NG. Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot. 2002;53:947–958. doi: 10.1093/jexbot/53.370.947. [DOI] [PubMed] [Google Scholar]
- Tan SC, Yiap BC. DNA, RNA, and protein extraction: the past and the present. Biomed Res Int. 2009;2009:574398. doi: 10.1155/2009/574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Wang G, Zhang X, Wang F, Song R. Isolation of high quality RNA from cereal seeds containing high levels of starch. Phytochem Anal. 2012;23:159–163. doi: 10.1002/pca.1337. [DOI] [PubMed] [Google Scholar]
- Wimalanathan K, Friedberg I, Andorf CM, Lawrence-Dill CJ. Maize GO annotation—methods, evaluation, and review (maize-GAMER) Plant Direct. 2018;2:e00052. doi: 10.1002/pld3.52. [DOI] [PMC free article] [PubMed] [Google Scholar]


