Abstract
Aflatoxin B1 (AFB1), a mycotoxin produced by Aspergillus spp., was proved as one of the major causes of human hepatocellular carcinoma (HCC) when chronically consumed. An efflux of AFB1 was reported to be associated with breast cancer resistance protein (BCRP) whose activity could also be modulated by green tea catechins. The purpose of this study was, therefore, to examine the impacts of green tea catechins on BCRP activity in Caco-2 cells by H33342 (bis-benzamide, BCRP substrate) accumulation and AFB1 efflux. Results showed a significant decrease (p < 0.05) of AFB1 in the efflux ratio following the incubation with Ko143, a specific BCRP inhibitor, and sodium fluoride, confirming the association of BCRP in AFB1 efflux transport across the cells. Pre-incubation with green tea and gallate catechins (ECG and EGCG) significantly reduced the efflux ratio of AFB1 (p < 0.05) and significantly increased the intracellular H33342 substrate (p < 0.05) in Caco-2 cells, clearly indicating the inhibitory effects of green tea and gallate catechins on BCRP function. Further study on H33342 accumulation revealed a dose-dependent increment of intracellular H33342 when co-administered with increasing concentrations of AFB1. This result implied a possible role of AFB1 as a BCRP competitive inhibitor. The findings from this study concluded the roles of BCRP as an efflux transporter for AFB1 and could be modulated by the exposure of green tea catechins. Owing to a reduction of its efflux, an inhibitory effect of BCRP when pre-exposed with green tea catechins could be crucial for AFB1 cellular accumulation.
Keywords: Mycotoxins, Transporter, Absorption, Polyphenols
Introduction
Aflatoxin B1 (AFB1) is the secondary metabolite produced by fungus Aspergillus spp. including A. flavus, A. parasiticus and A. nomius [1]. AFB1 was detected in many foodstuffs especially corn, in which was found the highest level of 970.32 µg/kg in China [2]. It can be converted by cytochrome P-450 enzyme systems into AFB1 8,9-exo-epoxide which is highly reactive and unstable. Chronic exposure of AFB1 is found to be associated with gene mutation, hepatotoxicity and hepatocellular carcinoma (HCC) in animals and humans [1, 3]. Following oral ingestion, AFB1 is readily absorbed in the gastrointestinal tract by passive diffusion and membrane transport proteins play a fundamental role in the cellular accumulation and toxicity of the toxin. A previous study reported that systemic exposure of AFB1 was restricted through the function of breast cancer resistance protein (BCRP/ABCG2) efflux pump by decreasing its cellular accumulation in MDCK-II cells transduced with BCRP, rendering the protection of the body against the dietary carcinogens [4].
Breast cancer resistance protein is the membrane transporter protein in the ATP-binding cassette (ABC) family which expresses in the apical side of the epithelium in the small intestine and colon and plays a critical role in the efflux of both endogenous compounds and exogenous compounds. So that, BCRP takes part in the absorption and elimination of some water insoluble or low water-soluble drugs as well as toxins [5].
Seeing that there is an increasing trend for natural product consumption, natural substances such as flavonoids have been studied for their interactions with drugs and toxins. Some of them can inhibit or induce phase I and/or phase II metabolism enzymes that may increase drug toxicity or decrease drug efficacy [6] while some of them can interact with transport proteins and interfere with drug accumulation [7]. The structure–activity relationship study also confirmed an inhibitory effect of some flavonoids on BCRP activity that may alter the absorption of BCRP substrates as well [8].
One of the major flavonoid sources which is consumed worldwide is green tea. Most flavonoids in green tea are characterized as catechins or flavan-3-ols that can be divided into four major types including (−)-epicatechin (EC), (−)-epicatechin gallate (ECG), (−)-epigallocatechin (EGC) and (−)-epigallocatechin gallate (EGCG). Apart from catechins, quercetin is also a major constituent in green tea [9]. Not only does it have beneficial effects on many diseases such as cardiovascular disease, diabetes, neurodegenerative diseases, liver disease, anti-inflammation, skin irritation and cancer [10, 11], but catechins have also been proved as a BCRP inhibitor that may lead to an improvement in the bioavailability of some medicines especially chemotherapy [12].
However, there is no evidence regarding the influence of green tea catechins on BCRP activity that may lead to the accumulation of some toxins, especially AFB1, inside the cells and potentiate the toxicity from AFB1 to human health. Therefore, the aim of this study was to investigate the efflux of AFB1 via BCRP transporting protein in Caco-2 cells and the effect of green tea catechins on BCRP function.
Materials and methods
Materials
All the chemicals, unless otherwise specified, were purchased from Sigma-Aldrich (St. Louis, MO, USA). Caco-2 cells (HTB-37™) were obtained from American Type Culture Collection (Manassas, VA, USA). Dulbecco’s Modification of Eagle’s Medium (DMEM), Fetal bovine serum (FBS), Penicillin–Streptomycin, Trypsin–EDTA and Phosphate-Buffered Saline (PBS, 1X) were purchased from Mediatech Inc (Manassas, VA, USA).
Caco-2 cell culture
Caco-2 cells were grown in DMEM supplemented with 10% FBS, 1% Penicillin/Streptomycin in 75-cm2 tissue culture T-flasks at 37 °C in a humidified 5% CO2 atmosphere. The culture medium was changed every 2–3 days. Caco-2 cells (passage 45–60) were seeded onto 24-Transwell plates for AFB1 efflux test or 24-well plates for H33342 accumulation assay for 14 days.
Caco-2 cell permeability and cell viability assays
For the permeability test, phenol red was used as a paracellular transport marker to test the integrity of Caco-2 cells monolayers [13]. Phenol red solution was added into the apical side of the cell culture insert and incubated for 4 h. Cell culture medium from the basolateral side was collected and determined at 560 nm using a microplate reader and %Permeability of Caco-2 cells was calculated based on the amount of phenol red capable of passing through the insert. The procedure was repeated every 2–3 days (day 0–day 10).
For cell viability assays, sulforhodamine B colorimetric assay (SRB) assay was used to measure cellular protein content by binding to the protein in viable cells and reflex the cell viability [14]. Caco-2 cells (10,000 cells/well) were cultured for 2 days and then treated along with test compounds for 2 days with 0.5% DMSO as a control. After that, the cells were fixed with 100 µl iced-cold 10% Trichloroacetic acid and incubated at 2–8 °C for an hour. The plate was washed 4 times with deionized water and placed at room temperature. Next, 50 µl of 0.4% SRB in 1% acetic acid was added and left at room temperature for 30 min. The plate was quickly rinsed 4 times with 1%acetic acid and left overnight. The cells were then solubilized with 200 µl of 10 mM Tris base (pH 10.5). The plate was later shaken for 15 min and the absorbance was measured at 540 nm.
Hoechst33342 (H33342) accumulation assay
H33342, a fluorescent dye, was used as a BCRP substrate to assess the activity of BCRP in Caco-2 cells [15, 16]. Prior to the accumulation assay, 3 × 105 cells/mL of Caco-2 cells were subcultured into a 24 well-plate for 14 days. In case of the addition of green tea and catechins, they were pre-incubated for 2 days. After that, cell culture medium was removed and replaced with or without Ko143 in the presence or absence of sodium fluoride (NaF) for an hour. Then 20 µM of H33342 was diluted by cell culture medium and treated with or without AFB1 for an hour. Caco-2 cells were washed twice with 1 mL of ice-cold PBS and lysed with 500 µl of cell lysis buffer (0.1% Triton-X solution in 0.3% NaOH). The amount of intracellular dye was measured at 370 nm (excitation) and 450 nm (emission) by a fluorescence spectrophotometer.
AFB1 efflux test and analysis by HPLC
Caco-2 cell suspension (1 × 105 cells) were seeded on the apical side of the cell insert with the blank media in the basolateral side. Cell culture medium in both sides was replaced every 2–3 days. In case of the addition of green tea and catechins, they were incubated for 2 days. To perform the efflux test, the cell culture medium was removed and replaced with new medium with or without Ko143 in the presence or absence of NaF for an hour prior to the addition of AFB1 (400 ng/mL final concentration) onto the apical side of the insert. Sample mediums in both the apical and basolateral sides were collected at 4 h after adding AFB1. The AFB1 sample was extracted by Solid Phase Extraction (SPE) column, evaporated by N2 gas and derivatized with trifluoroacetic acid. The supernatant was centrifuged and detected by HPLC with fluorescence detector (λex = 360 nm, λem = 440 nm). The efflux ratio of AFB1 was calculated by the apparent permeability coefficients (Papp) values in the basolateral to apical (B to A) direction versus Papp in the apical to basolateral (A to B) direction [17].
Statistical analysis
Data was statistically analyzed using the IBM SPSS Statistics program version 19. All results were expressed as mean ± standard deviation (SD). The data was analyzed by one-way ANOVA followed by Dunnett’s post hoc test for parametric results and the Kruskal–Wallis Test for non-parametric results.
Results
Caco-2 cell permeability and cell viability assays
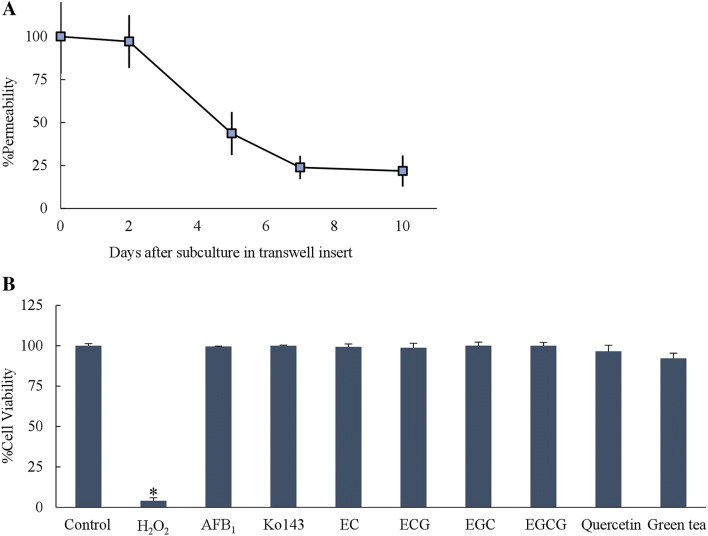
Caco-2 cells were initially tested for cellular integrity of tight junction prior to the evaluation of AFB1 transport across the cells. Following the incubation with phenol red (day 0, 2, 5, 7 and 10), the ratio of phenol red permeation considerably decreased from 97.1 to 21.8% from day 2 to day 7 and constantly stayed at a low level until day 10 (Fig. 1a). This could be presumed the readiness of the Caco-2 cell model for the transport of AFB1 in this study when cultured for up to 10 days.
Fig. 1.
a Percentage of permeability of phenol red across Caco-2 cells cultured on transwell inserts from day 0–day 10 (n = 4). b Caco-2 cell viability test by SRB assay after adding 1000 ng/mL AFB1, 100 µM Ko143, 50 µM of each catechins, 50 µM Quercetin and 1 mg/mL green tea infusion for 2 days (n = 6–8). 0.5% H2O2 was used as a positive control. Data is presented as mean ± SD (*p < 0.001)
AFB1 toxicity against Caco-2 cells were also tested. At the concentration up to 1000 ng/mL, AFB1 did not cause the decrease of Caco-2 cell viability compared to a negative control. Likewise, 100 µM Ko143, 1 mg/mL of green tea infusion, 50 µM quercetin and each catechins (EC, ECG, EGC and EGCG) also did not decrease Caco-2 cell viability (Fig. 1). In this study, each catechins in 1 mg/ml of green tea infusion detected by HPLC were about 10–600 µM (Data not shown). Therefore, theses concentrations of tested compounds were then selected for further experiments to avoid cytotoxic effects on Caco-2 cells.
H33342 accumulation assay
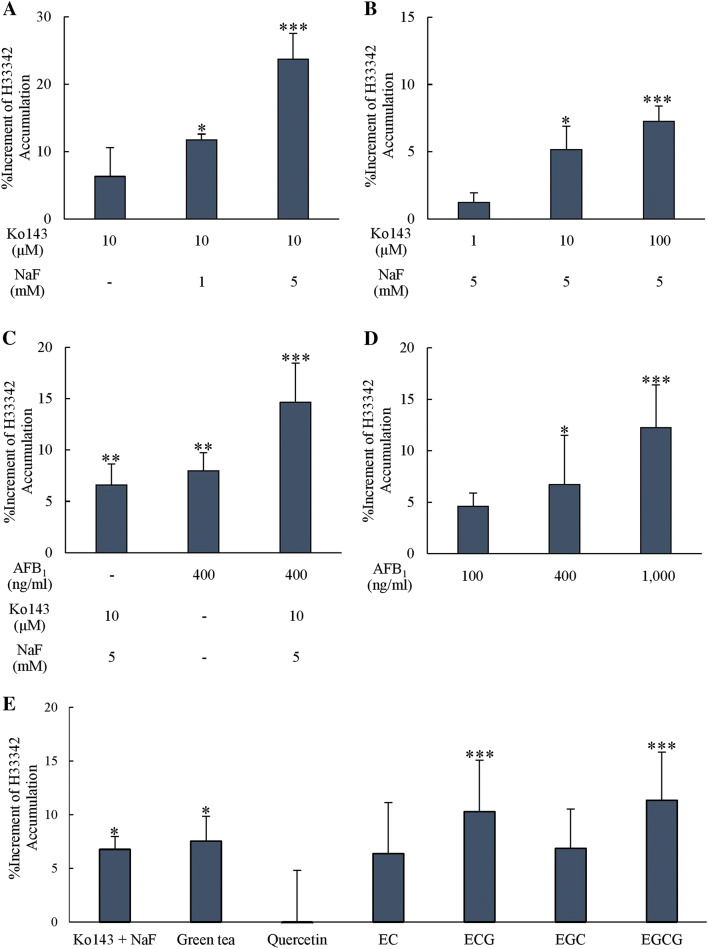
The detection of a fluorescence signal following the incubation of H33342 with Caco-2 cells could represent as passing through and detaining of the dye to be accumulated inside the cells. A low fluorescence signal was detected when H33342 alone was incubated with Caco-2 under normal culture conditions. The addition of Ko143, a selective BCRP inhibitor, led to a slight increase of the fluorescence signal. However, when Ko143 was co-incubated with sodium fluoride, an esterase inhibitor, H33342 accumulation was significantly enhanced regarding the increased concentrations of NaF (1 mM and 5 mM) (Fig. 2a), implying the presence of BRCP on Caco-2 cells and the involvement of BCRP in H33342 accumulation. The increased concentration of Ko143 (1–100 µM) in the presence of NaF showed a dose-dependently inhibition of BCRP function as determined by the increased accumulation of H33342 and reached a significant level at 10 and 100 mM (Fig. 2b). To verify the role of BCRP on AFB1 efflux, 400 ng/mL AFB1 was added into Caco-2 cells in the presence of H33342. An elevated fluorescence signal of H33342 accumulated inside Caco-2 cells was detected and the signal was even higher with the addition of NaF (Fig. 2c). Further results on varied concentrations of AFB1 confirmed the competition between H33342 and AFB1 to be transported out of the cells when the concentration of AFB1 was increased (100–1000 ng/mL) (Fig. 2d). These results indicated a competitive inhibition of AFB1 with BCRP substrate H33342.
Fig. 2.
Increment of H33342 (20 µM) accumulated in Caco-2 cells after adding a 1 or 5 mM NaF with 10 µM Ko143, b 1–100 µM Ko143 with 5 mM NaF, c 400 ng/mL AFB1 with or without 10 µM Ko143 and 5 mM NaF, d 100, 400 and 1000 ng/mL AFB1, e 10 µM Ko143 with 5 mM NaF, 1 mg/mL green tea infusion, 50 µM quercetin and each catechins (n = 4–6). Data is presented as mean ± SD compared to control at zero percent increment (*p < 0.05, **p < 0.01, ***p < 0.001)
Furthermore, the accumulations of H33342 in Caco-2 cells were significantly elevated when pre-incubating with 1 mg/mL green tea infusion, 50 µM ECG and 50 µM EGCG for 2 days compared with the control group (p < 0.05 for green tea infusion and p < 0.001 for ECG and EGCG) (Fig. 2e). However, 50 µM EC and EGC slightly increased the accumulation but did not reach the significant level (p > 0.05).
AFB1 efflux test and analysis by HPLC
For the determination of AFB1, culture medium was collected, extracted via the SPE column and analyzed by HPLC. The actual concentration of AFB1 was calculated based on the comparison with a standard curve of standard AFB1 (2–400 ng/mL) constructed by the same sample preparation procedure.
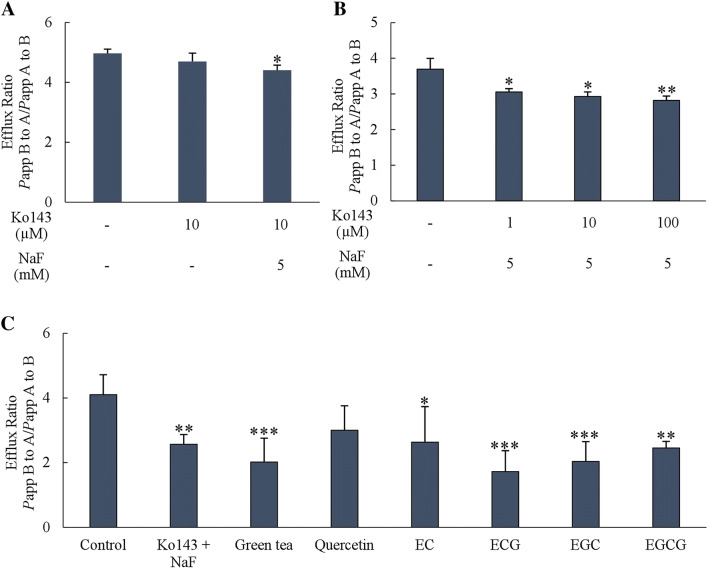
The efflux ratio of AFB1 (Papp B to A per Papp A to B) when 10 µM Ko143 plus 5 mM NaF (BCRP inhibitor) were added declined significantly (p < 0.05) compared to the corresponding control (Fig. 3a, b). For green tea catechins exposure, pre-incubating with 1 mg/mL green tea infusion, 50 µM ECG, EGC and EGCG for 2 days significantly reduced the efflux ratio (p < 0.001). Similarly, pre-incubating with 50 µM EC significantly reduced A/B ratio (p < 0.05) (Fig. 3c). The result substantiated the effect of green tea catechins on the inhibition of BCRP activity.
Fig. 3.
Efflux ratio of AFB1 (400 ng/ml) calculated from Papp (B to A) per Papp (A to B) after adding a 10 µM Ko143 with or without 5 mM NaF, b 1 to 100 µM Ko 143 with 5 mM NaF, c 1 mg/mL green tea infusion, 50 µM quercetin and each catechins (n = 4–6). Data is presented as mean ± SD compared to control (*p < 0.05, **p < 0.01, ***p < 0.001)
Discussion
Caco-2 cells are an epithelial like cell that developed from human colorectal adenocarcinoma. Caco-2 cells also express many transporting proteins including BCRP that can transport both endogenous and exogenous substances out of the cells so they were used for testing drugs as a substrate of many efflux proteins [18, 19]. In this study, Ko143 was used as a BCRP inhibitor due to its specificity to BCRP and low toxicity [20]. However, Caco-2 cells have a high expression of carboxylesterase-1 (hCE-1) [21] which is a major enzyme to metabolize Ko143 (an ester form) to an inactive form (an acid form) [22]. Accordingly, NaF (an esterase inhibitor) was added with Ko143 to prevent the metabolism and maintain the level and activity of Ko143 throughout the test period [20]. The current study showed that NaF can increase an efficacy of Ko143 as a BCRP inhibitor in both H33342 accumulation assay and the AFB1 efflux test. This is the first study reporting on the effectiveness of NaF as an esterase inhibitor combined with Ko143 in cell culture.
The main goal of the current study was to determine the permeability and efflux of AFB1 via BCRP transporting protein in Caco-2 cells. From the AFB1 efflux test, a decrease of AFB1 in apical side when Ko143 plus NaF were added convinced that AFB1 is a substrate of BCRP. This finding confirms the association between AFB1 and BCRP that was reported in previous research about a reduction of systemic exposure of 2 µM AFB1 by BCRP [4]. Apart from a BCRP substrate that was proved, it can be inferred about a competitive inhibition of AFB1 when co-administered with H33342, BCRP substrate. A dose-dependent inhibition of AFB1 assure its role as a substrate for BCRP as well.
As mentioned in the literature review, numerous flavonoids were investigated as bifunctional modulators of drug efflux in the MDR cell. They can partly bind to both ATP and the steroid binding site of ABC transporting proteins [23]. Regarding BCRP, quercetin which is the major flavonol in green tea [9] is a notable example of a BCRP inhibitor [24, 25]. Nonetheless, quercetin has been reported as having an inductive effect on BCRP. It can increase both mRNA and protein expression via interaction with the antioxidant response element (AREs) in the promotor of target genes [26]. Astonishingly, quercetin could neither induce nor inhibit BCRP activity of both H33342 and AFB1 in this study. Owing to the inconclusive results, it may be caused by the difference of material used; dose, time of administration and type of cell or tissue [27].
In this study, green tea and gallate catechins (ECG and EGCG) could significantly inhibit BCRP activity in both H33342 and AFB1 substrates. This finding corresponded with the previous evidence that EGCG was able to downregulate BCRP activity in a tamoxifen (BCRP substrate) resistant MCF-7 cell line [28].Gallate catechins in green tea also had an ability to inhibit the BCRP function when co-administered with mitoxantrone. The hydrophobic property was considered to be crucial for the BCRP inhibitory activity of catechins [12]. In contrast, an activity of non-gallate catechins (EC and EGC) seems to be indecisive. They could inhibit an efflux of AFB1 but did not significantly effect H33342 accumulation. Nevertheless, another previous research disputed that four green tea components at 50 µM did not show an inhibitory effect on the uptake of Dasatinib in BCRP-overexpression cells [24]. The structure–activity relationship (SAR) study explained an inhibitory effect of flavonoids to BCRP protein, likewise [8]. It is believed that strong a BCRP inhibitory structure should have a double bond between position 2 and 3, a methoxy or hydroxy group at position 5 and a methoxy group in position 3. All catechins structures conversely, do not have these three components but just gallate catechins containing gallate moisty at position 3 which might play a key role in inhibiting the BCRP function more than the hydroxyl group of non-gallate catechins [8].
Ultimately, these findings make several contributions to the current literature. First, it confirms an intestinal efflux of AFB1 via BCRP and an implication of these findings is a competitive inhibition of AFB1 when co-administered with BCRP substrate. Second, this research extends our knowledge of green tea catechins especially gallate catechins as a BCRP inhibitor. Exposure of green tea catechins could potentially affect cellular accumulation of AFB1 due to a reduction of its efflux. Even though, the findings of this study have several important implications for future practice, in vivo study is still necessary to verify this effect within the organism. Not to mention, chronic exposure of green tea might interfere with an excretion of other mycotoxins or toxic substances. This is an important issue for further research.
Acknowledgements
This study was financially supported by a research grant from the National Research Council of Thailand (NRCT) and the Faculty of Pharmaceutical Science, Khon Kaen University, Thailand.
Abbreviations
- ABC
ATP-binding cassette
- AFB1
Aflatoxin B1
- BCRP
Breast cancer resistance protein
- DMEM
Dulbecco’s modification of eagle’s medium
- DMSO
Dimethyl sulfoxide
- EC
(−)-Epicatechin
- ECG
(−)-Epicatechin gallate
- EGC
(−)-Epigallocatechin
- EGCG
(−)-Epigallocatechin gallate
- H33342
Hoechst 33342 dye substrate
- HPLC
High performance liquid chromatography
- NaF
Sodium fluoride
- P-gp
P-glycoprotein
- Papp
Apparent permeability coefficients
- SPE
Solid phase extraction
- SRB
Sulforhodamine B
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Omotayo OP, Omotayo AO, Mwanza M, Babalola OO. Prevalence of mycotoxins and their consequences on human health. Toxicol Res. 2019;35:1–7. doi: 10.5487/TR.2019.35.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J, Liu X. Contamination of aflatoxins in different kinds of foods in China. Biomed Environ Sci. 2007;20:483–487. [PubMed] [Google Scholar]
- 3.Bbosa GS, Kitya D, Odda J, Ogwal-Okeng J. Aflatoxins metabolism, effects on epigenetic mechanisms and their role in carcinogenesis. Health. 2013;5:14–34. doi: 10.4236/health.2013.510A1003. [DOI] [Google Scholar]
- 4.Van Herwaarden AE, Wagenaar E, Karnekamp B, Merino G, Jonker JW, Schinkel AH. Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis. 2006;27:123–130. doi: 10.1093/carcin/bgi176. [DOI] [PubMed] [Google Scholar]
- 5.Misaka S, Müller F, Fromm MF. Clinical relevance of drug efflux pumps in the gut. Curr Opin Pharmacol. 2013;13:847–852. doi: 10.1016/j.coph.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Kyselova Z. Toxicological aspects of the use of phenolic compounds in disease prevention. Interdiscip Toxicol. 2011;4:173–183. doi: 10.2478/v10102-011-0027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang E, Barecki-Roach M, Johnson WW. Elevation of P-glycoprotein function by a catechin in green tea. Biochem Biophys Res Commun. 2002;297:412–418. doi: 10.1016/S0006-291X(02)02219-2. [DOI] [PubMed] [Google Scholar]
- 8.Pick A, Müller H, Mayer R, Haenisch B, Pajeva IK, Weigt M, Bönisch H, Müller CE, Wiese M. Structure-activity relationships of flavonoids as inhibitors of breast cancer resistance protein (BCRP) Bioorg Med Chem. 2011;19:2090–2102. doi: 10.1016/j.bmc.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 9.Peterson J, Dwyer J, Bhagwat S, Haytowitz D, Holden J, Eldridge AL, Beecher G, Aladesanmi J. Major flavonoids in dry tea. J Food Compos Anal. 2005;18:487–501. doi: 10.1016/j.jfca.2004.05.006. [DOI] [Google Scholar]
- 10.Min KJ, Kwon TK. Anti-cancer effects and molecular mechanisms of epigallocatechin-3-gallate. Integr Med Res. 2014;3:16–24. doi: 10.1016/j.imr.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HK, Choi SY, Chang HK, Baek SY, Chung JO, Rha CS, Kim BJ, Kim MN. Human skin safety test of green tea cell extracts in condition of allergic contact dermatitis. Toxicol Res. 2012;28:113–116. doi: 10.5487/TR.2012.28.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugihara N, Kuroda N, Watanabe F, Choshi T, Kamishikiryo J, Seo M. Effects of catechins and their related compounds on cellular accumulation and efflux transport of mitoxantrone in caco-2 cell monolayers. J Food Sci. 2017;82:1224–1230. doi: 10.1111/1750-3841.13680. [DOI] [PubMed] [Google Scholar]
- 13.Martel F, Monteiro R, Lemos C. Uptake of serotonin at the apical and basolateral membranes of human intestinal epithelial (caco-2) cells occurs through the neuronal serotonin transporter (SERT) J Pharmacol Exp Ther. 2003;306:355–362. doi: 10.1124/jpet.103.049668. [DOI] [PubMed] [Google Scholar]
- 14.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116. doi: 10.1038/nprot.2006.179. [DOI] [PubMed] [Google Scholar]
- 15.Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M, Dean M, Sharp JG, Cowan K. The multidrug resistance transporter ABCG2 (Breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin Cancer Res. 2002;8:22–28. [PubMed] [Google Scholar]
- 16.Paturi DK, Kwatra D, Ananthula HK, Pal D, Mitra AK. Identification and functional characterization of breast cancer resistance protein in human bronchial epithelial cells (Calu-3) Int J Pharm. 2010;384:32–38. doi: 10.1016/j.ijpharm.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang F, Ouyang H, Yang JZ, Borchardt RT. Bidirectional transport of rhodamine 123 and Hoechst 33342, fluorescence probes of the binding sites on P-glycoprotein, across MDCK-MDR1 cell monolayers. J Pharm Sci. 2004;93:1185–1194. doi: 10.1002/jps.20046. [DOI] [PubMed] [Google Scholar]
- 18.Sevin E, Dehouck L, Fabulas-da Costa A, Cecchelli R, Dehouck MP, Lundquist S, Culot M. Accelerated caco-2 cell permeability model for drug discovery. J Pharmacol Toxicol Methods. 2013;68:334–339. doi: 10.1016/j.vascn.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Xia CQ, Liu N, Yang D, Miwa G, Gan LS. Expression, localization, and functional characteristics of breast cancer resistance protein in caco-2 cells. Drug Metab Dispos. 2005;33:637–643. doi: 10.1124/dmd.104.003442. [DOI] [PubMed] [Google Scholar]
- 20.Weidner LD, Zoghbi SS, Lu S, Shukla S, Ambudkar SV, Pike VW, Mulder J, Gottesman MM, Innis RB, Hall MD. The inhibitor Ko143 is not specific for ABCG2. J Pharmacol Exp Ther. 2015;354:384–393. doi: 10.1124/jpet.115.225482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imai T, Imoto M, Sakamoto H, Hashimoto M. Identification of esterase expressed in caco-2 cells and effects of their hydrolyzing activity in predicting human intestinal absorption. Drug Metabol Dispos. 2005;33:1185–1190. doi: 10.1124/dmd.105.004226. [DOI] [PubMed] [Google Scholar]
- 22.Liu K, Zhu J, Huang Y, Li C, Lu J, Sachar M, Li S, Ma X. Metabolism of KO143, an ABCG2 Inhibitor. Drug Metab Pharmacok. 2017;32:193–200. doi: 10.1016/j.dmpk.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Conseil G, Baubichon-Cortay H, Dayan G, Jault JM, Barron D, Di Pietro A. Flavonoids: a class of modulators with bifunctional interactions at vicinal ATP- and steroid-binding sites on mouse p-glycoprotein. Proc Natl Acad Sci. 1998;95:9831–9836. doi: 10.1073/pnas.95.17.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleisher B, Unum J, Shao J, An G. Ingredients in fruit juices interact with dasatinib through inhibition of BCRP: a new mechanism of beverage-drug interaction. J Pharm Sci. 2015;104:266–275. doi: 10.1002/jps.24289. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004;65:1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- 26.Ebert B, Seidel A, Lampen A. Phytochemicals induce breast cancer resistance protein in caco-2 cells and enhance the transport of benzo[a]pyrene-3-sulfate. Toxicol Sci. 2007;96:227–236. doi: 10.1093/toxsci/kfl147. [DOI] [PubMed] [Google Scholar]
- 27.Tras B, Cetin G, Uney K, Dik B, Corum O, Atalay S. Effects of BCRP and P-gp modulators on the penetration of aflatoxin B1 into the mouse brain. Kafkas Univ Vet Fak Derg. 2017;23:95–100. [Google Scholar]
- 28.Farabegoli F, Papi A, Bartolini G, Ostan R, Orlandi M. (−)-Epigallocatechin-3-gallate downregulates Pg-P and BCRP in a tamoxifen resistant MCF-7 cell line. Phytomedicine. 2010;17:356–362. doi: 10.1016/j.phymed.2010.01.001. [DOI] [PubMed] [Google Scholar]