Abstract
Environmental and occupational exposures to copper (Cu) play a pivotal role in the etiology of some neurological diseases and reduced cognitive functions. However, the precise mechanisms of its effects on cognitive function have not been yet thoroughly established. In our study, we aimed to investigate the behavior and neurochemical alterations in hippocampus of male and female rats, chronically exposed to copper chloride (CuCl2) and the possible involvement of oxidative stress. Twenty-four rats, for each gender, were divided into control and three test groups (n = 6), and were injected intraperitoneally with saline (0.9% NaCl) or CuCl2 (0.25 mg/kg, 0.5 mg/kg and 1 mg/kg) for 8 weeks. After the treatment period, Y-maze test was used for the evaluation of spatial working memory and the Morris Water Maze (MWM) to test the spatial learning and memory. Biochemical determination of oxidative stress levels in hippocampus was performed. The main results of the present work are working memory impairment in spatial Y-maze which induced by higher Cu intake (1 mg/kg) in male and female rats. Also, In the MWM test, the spatial learning and memory were significantly impaired in rats treated with Cu at dose of 1 mg/kg. Additionally, markers of oxidative stress such as catalase, superoxide dismutase, lipid peroxidation products and nitric oxide levels were significantly altered following Cu treatments. These data propose that compromised behavior following Cu exposure is associated with increase in oxidative stress.
Keywords: Copper, Memory, Oxidative stress, Sex differences, Chronic toxicity
Introduction
In our environment, heavy metals are prevalent in air, soil and water. Virtually, they exist everywhere [1]. Among the heavy metals, copper (Cu) is an essential trace mineral which becomes harmful when the safe dose is surpassed [2]. Generally, Cu can ensure the proper functioning of various biological systems such as the central nervous system (CNS) in different animal species [3–5]. It can regulate several biochemical processes [6, 7] and participates in neurotransmitter synthesis, energy metabolism and antioxidative defense [8].
The brain is highly rich in Cu [9]. This metal can cross the blood–brain barrier (BBB) and is easily distributed in the brain [10]. Cu is found in larger quantities in the hippocampus, striatum and frontal cortex [9, 11]. Abnormal levels of Cu exposure, in the form of deficiency or excess, can seriously influence brain functions [6, 12]. Epidemiological studies have shown that, Cu at high concentrations is associated with some neurological disorders such as Alzheimer’s disease [13], Menkes disease, Parkinson´s and Huntington diseases [14–16]. A significant association between Cu and neurobehavioral performance was revealed [17–19]. Our previous data have demonstrated that chronic Cu exposure modulates affective behaviors [20].
The role of Cu in the memory dysfunction is controversial [7]. Normal level of Cu is necessary for memory and learning [21], this metal facilitates the long-term potentiation (LTP) and it influences reuptake of glutamate, glutamate release and free radicals production [21, 22]. But conversely, excess of Cu affects the memory. Several studies in humans and animals, have reported that elevated level of Cu in the blood can have negative effects on memory function, suggesting a probable role for the metal in this pathologie [23] and its direct or indirect impact on the neurotransmission involved in cognitive abilities including cholinergic, glutamatergic and GABAergic systems [24–26].
Additionally, excessive exposure to Cu provokes oxidative stress (OS) in the CNS which is considered to be among the most affected systems [27], especially the hippocampus, a structure of the brain which plays a very important role in memory functioning [26]. In this sense, an association between the OS and the etiology of cognitive dysfunction was shown in divers studies [28–30].
In view of the forgoing, this study aims to evaluate the spatial learning and memory performance and neurochemical alterations in hippocampus of male and female rats chronically exposed to Cu, and to establish the possible involvement of OS pathways.
Materials and methods
Animals and experimental conditions
Adult Wistar rats of both genders (approximately 60 days; 120 ± 20 g) which born and raised in Animal House of the Faculty of Sciences in Ibn Tofaïl University, were used in this study. The adult rodents were maintained under a 12:12 h light/dark cycle at a controlled room temperature (22 ± 1 °C) with free access to standard laboratory rat water and food. The experimental procedures were approved by the Animal Ethics Committee (Local Institutional Research Committee).
Forty-eight rats were separated (for each gender) into four different groups of six adult rats: Group I: control animals, received NaCl (0.9%) intraperitoneally once daily. Group II: animals were administered Copper at dose of 0.25 mg/kg once daily. Group III: animals were administered Copper (0.5 mg/kg) once daily. Group IV: animals were administered Copper (1 mg/kg) once daily. The concentrations of Cu used in this study were chosen based on our previous experiment evaluating neurobehavioral alterations in rat [20].
Copper Chloride (CuCl2) was obtained from SIGMA-ALDRICH. Saline solution and all other chemicals used in our study were purchased from standard commercial suppliers. By Intraperitoneal route, CuCl2 and NaCl (0.9%) were administered once daily (between the hours of 16:00 and 16:30) during two months.
Neurobehavioral tests
After the treatment period (60 days), all rats were subjected to behavioral analysis by monitoring memory function in Y-maze and MWM tests. The Behavioral studies were effectuated between the hours of 8 am and 12 am.
Y-maze test
The working memory state in rodents was tested by monitoring spontaneous alternation behavior in the Y maze test as previously described [31]. The apparatus was made of painted wood in the form of Y with three arms (45 × 35 × 12 cm). It involved placing a rat in the area of intersection of the three arms of the apparatus and allowing the animal for 5 min (from 3 to 8 min) to make arm decisions afterwards. The % of alternation was determined following this equation: (spontaneous alternation/(total number of arm entries − 2)) × 100.
Morris Water Maze Test
To test the spatial learning and memory performances, the MWM test is employed. The test was performed according to the method previously described by Morris [32, 33]. A stainless circular pool (50 cm in height and 110 cm in diameter) which is full of opaque water (24 ± 1 °C) was used. The apparatus was divided into four equally quadrants. An invisible platform was placed in one of the four quadrants. The maze was located in a large quiet test room (5.2 m × 2.4 m), surrounded by numerous extra maze cues, which could be used by the animals for spatial orientation. The time taken to reach the invisible platform was recorded throughout the acquisition trials (the first 4 days). This time served as a measure of spatial learning performance. In order to assess the spatial memory, the platform was tacked off in the fifth day (probe test). We recorded the time expended in the quadrant which formerly contained the platform (the correct quadrant).
Biochemical examination
After the behavioral tests period, all animals were sacrificed by decapitation; the brains were removed and the cold Hippocampi were isolated and homogenized in 50 mM of phosphate buffer (PH: 7.4, W/V) and then centrifuged at 4 °C for 10 min at 1500 rpm. The resulting supernatants were used for analysis [34].
Determination of Lipid peroxidation levels
Hippocampal LPO was estimated according to the procedure of Draper and Hadley [35]. The formation of oxidized lipids was determined by measuring the TBARS (thiobarbituric-acid-reacting substances). The reaction contained tissue homogenates, 10% TCA (trichloroacetic acid) and TBA (thiobarbituric acid) at 0.67%. For 15 min, the mixture was carried out in a boiling water bath. Just after, butanol (2:1 V/V) was added. The mixture was centrifuged 800g for 5 min. Then, the reaction product was determined at 532 nm [36].
Determination of Nitric oxide levels
Hippocampal NO was determined using Griess reagent [37]. Briefly, 150 µL of sample were incubated at room temperature with 20 mL of Griess reagent and 130 µL of distilled water for 30 min. NO levels were determined at 540 nm. Tissue NO levels were calculated as µmol/g of tissue.
Determination of antioxidant enzymes activities
The SOD and CAT activities were analyzed using the protocol of Beauchamp and Fridovich [38] and the protocol of Aebi [39], respectively. The two antioxidant enzymes activities were expressed in U/g of tissue. For SOD, one unit being the amount inhibiting the photoreduction of the nitroblue tetrazolium (NBT) by 50%. The reduction of NBT was followed at 560 nm, while for CAT, one unit being the amount of H2O2 destroyed/min under temperature of 25 °C at 240 nm.
Data analysis
The statistical result analyses were analyzed using SPSS (version 22). Differences among the animal groups were assessed by two-way, whereas, the learning phase data of the MWM were analyzed by ANOVA repeat measures. Behavioral data are represented as the mean ± SEM Statistical significance was set at either p < 0.05.
Results
Copper effect on memory
Y Maze Test (Fig. 1)
Fig. 1.
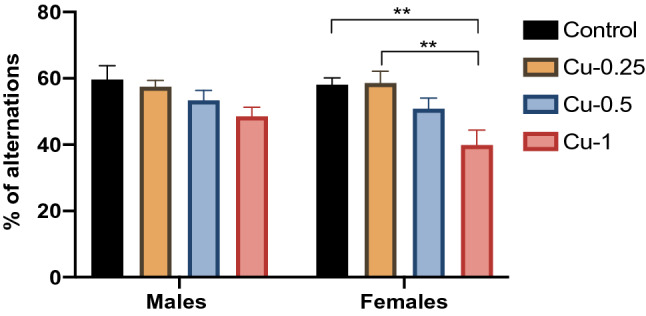
Effect of Cu administrations (0.25, 0.5 and 1 mg/kg i.p.) on Spontaneous alternation percentage measured in Y-maze. Results are represented as mean ± SEM. **p < 0.01
In male rats, Cu did not provoke any significant alteration in Percentage of spontaneous alternation (F(1.32) = 1.61, p > 0.05). In contrast, in female rats, the % of alternation was significantly decreased following Cu treatment at 1 mg/kg compared to control (F(3.32) = 8.54, p < 0.01), while no significant change was observed for the other doses (p > 0.05).
Morris water maze
Spatial learning (Fig. 2)
Fig. 2.
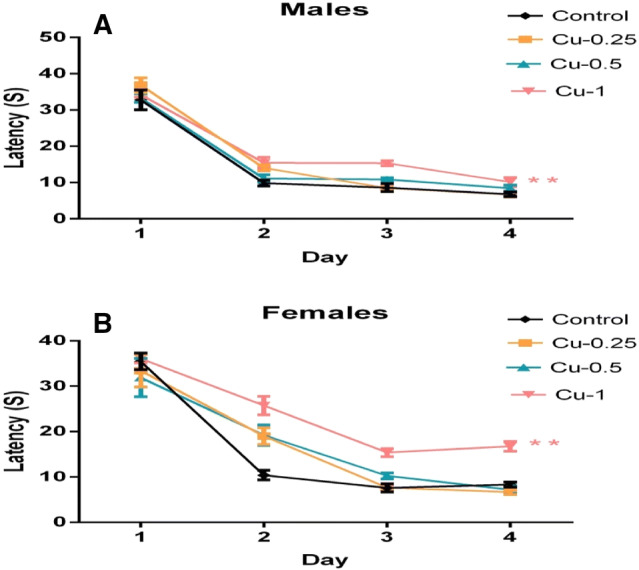
Effect of Cu injections (0.25, 0.5 and 1 mg/kg i.p.) on Latency to reach the hidden platform on each of the 4 days of learning phase in the MWM, in male (a) and female rats (b). Results are represented as mean ± SEM. **p < 0.01
The statistical analysis showed that; a significantly higher latency value was recorded in male and female rats exposed to Cu (1 mg/kg) when compared with the control group (p < 0.01). Cu had a longer effect on the latency of female than male in the 1 mg/kg treated group. In addition, no significant difference is observed for the other doses in rats of both genders (p > 0.05).
Percentage time spent in the correct quadrant (Fig. 3)
Fig. 3.
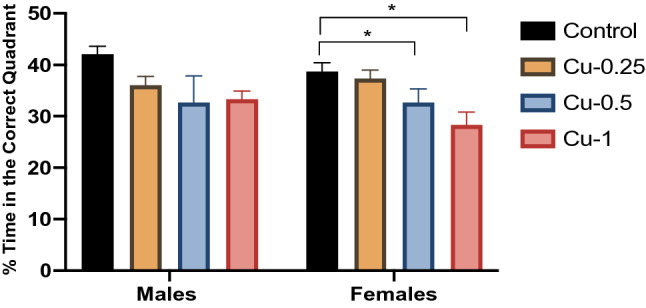
Effect of Cu administrations (0.25, 0.5 and 1 mg/kg i.p.) on Percentage of time spent in the correct quadrant in the probe trial of the MWM. Results are represented as mean ± SEM. *p < 0.05
During the probe test, the effects of Cu on the % of time spent in the correct quadrant in male rats were similar in all groups throughout this study (p > 0.05). In contrast, the % of time spent in the correct quadrant was significantly reduced by 27% in female Cu-treated rats (1 mg/kg) compared to control female rats (F(3,32) = 5.33; p < 0.05).
Copper effect on oxidative stress
LPO levels in hippocampus (Fig. 4)
Fig. 4.
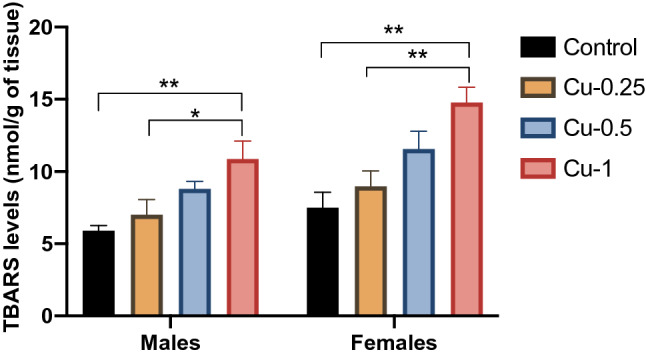
Effect of Cu administrations (0.25, 0.5 and 1 mg/kg i.p.) on TBARS levels in hippocampus. Results are represented as mean ± SEM. *p < 0.05 and **p < 0.01
Alterations in the levels of LPO were recorded in the hippocampus of adult rats exposed to Cu. As shown in Fig. 4, there was a significant increase in LPO levels (TBARS) in the hippocampus of the male and female Cu-treated animals, in a dose-dependent fashion as compared to the controls (F(3.32) = 14.43, p < 0.01).
NO levels in hippocampus (Fig. 5)
Fig. 5.
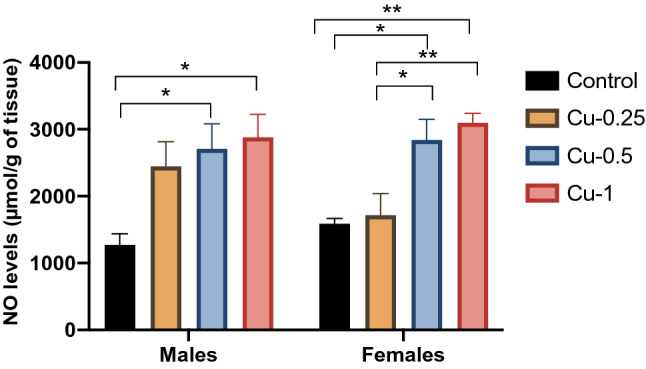
Effect of Cu administrations (0.25, 0.5 and 1 mg/kg i.p.) on Nitric Oxide (NO) levels in hippocampus. Results are represented as mean ± SEM. *p < 0.05 and **p < 0.01
Statistical analysis has shown that Cu-0.5 and Cu-1 significantly increase levels of Hippocampal NO in both sexes by 112% and 126% respectively (F(3.32) = 12.30, p < 0.05), while Cu-0.25 non-significantly increased NO levels (+ 91%) (p < 0.05). Also, in female rats, Cu treatments at 0.5 and 1 mg/kg significantly increased the level of NO in a dose-dependent fashion compared to adult control rats by 78% (p < 0.05) and 94% (p < 0.01) respectively.
Antioxidant enzymes variation in hippocampus (Figs. 6, 7)
Fig. 6.
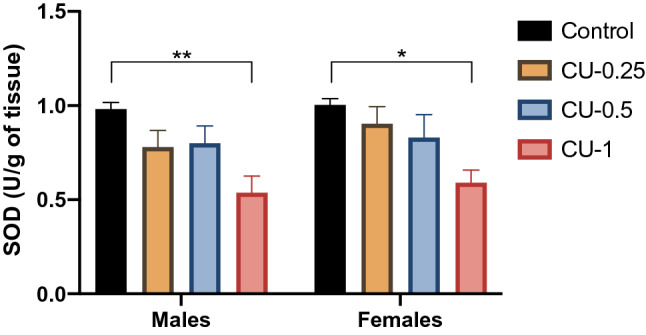
Effect of Cu administrations (0.25, 0.5 and 1 mg/kg i.p.) on Superoxide Dismutase activity (SOD) in hippocampus. Results are represented as mean ± SEM. *p < 0.05 and **p < 0.01
Fig. 7.
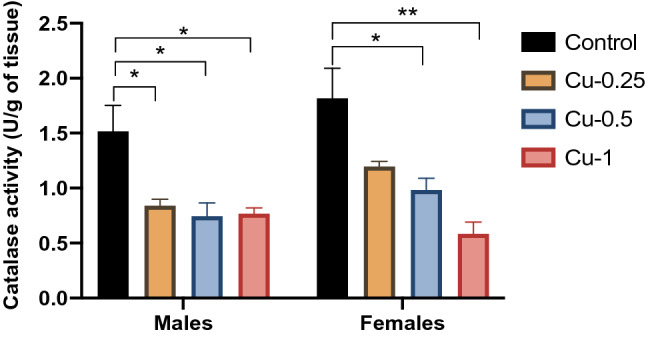
Effect of Cu injections (0.25, 0.5 and 1 mg/kg i.p.) on Catalase activity (CAT) in hippocampus. Results are represented as mean ± SEM. *p < 0.05 and **p < 0.01
Alterations in the activities of antioxidant enzymes were recorded in the hippocampus of adult rats exposed to Cu. As shown in Fig. 6, SOD activity was significantly reduced in a rats of both sexes following Cu treatment (1 mg/kg) when compared with the control group (F(3.32) = 9.5, p < 0.01), while hippocampal SOD activities were non-significantly lower (p > 0.05) in rats exposed to Cu at 0.25 and 0.5 mg/kg compared to the control group.
On the other hand, exposure to all three Cu concentrations led to a significant decrease in hippocampal CAT activities of male adult rats (F(3.32) = 16.72, p < 0.05) (Fig. 7). In females, Cu injections at 0.5, 1 mg/kg significantly decreased activity of CAT compared to control rats by 54% (p < 0.05) and 68% (p < 0.01) respectively.
Discussion
This study was undertaken with the objective to evaluate the harmful effects of intraperitoneally administered Cu on rats’ memory functions and the possible involvement of OS in the toxic mechanisms of Cu exposure.
We demonstrate here that; in Y maze, the rats treated with Cu at a dose of 1 mg/kg have an impaired working memory compared to control rats. This finding is consistent with the study reported by Kumar et al. which demonstrated that % of alterations was significantly decreased in rats that received chronically (30 days to 90 days) oral Cu in dose of 200 mg/kg [24]. Another finding of this study was the impaired spatial learning and memory in rats of both genders in MWM test; Chronic Cu-intoxicated animals showed a significant prolongation of escape latency during the fourth acquisition days in comparison with the control rats. Also, a deficit of memorization was also shown in Cu-1 group during the Probe test in MWM. These effects appeared for the lower and the higher dose of the metal, especially in the group receiving the higher dose of Cu (1 mg/kg). Such results seems to confirm other recent findings, which have revealed that Cu administration can provoke impairments in spatial learning and memory of rats in the MWM [25, 40–42]. Conflicting finding have been reported in the study of Leiva et al. which demonstrated that Cu administered intraperitoneally at dose of 1 mg/kg for 30 days does not interfere with learning or memory process in the MWM [43]. A possible explanation for this discrepancy might be the duration of exposure to Cu or/and the variable response of different animal species to this metal. It has been shown that exposure to Cu for less than 30 days has no undesirable effects on memory function [44]. In addition, research studies on rats have reported an adverse effects on memory induced by Cu, while this is not the case for the mice [45]. Excessive Cu levels may result in cognitive problem in humans [46–48].
After crossing the BBB, Cu is distributed in different regions of the brain, in particular in the hippocampus which contains high levels of this metal [10, 49]. Some studies demonstrated that Cu is disponible in hippocampal neurons [50, 51]. In this sense, the impaired memory may be the result of the perturbation of the hippocampal circuits following Cu administration [52]. Several works have evaluated the effect of Cu on the neurotransmission systems, which perturbations can lead to cognitive dysfunction, such as cholinergic, glutamatergic and GABAergic systems [24–26]. As known, the cholinergic system is necessary in the memory’s process [53]. Pal et al. reported that Cu causes memory impairment by interfering with acetylcholine (ACh) function at the synapse and partly by decreasing acetylcholine esterase (AChE) activity in the hippocampus of rats [54]. The AChE Cu inhibition may be due to the impact of Cu on the catalytic site of the enzyme by an electrostatic interaction implicating specific aminoacidic residues in the active site [55]. Concerning the effect of Cu on the glutamatergic system, it was demonstrated by Zhang et al. that; Cu can induce an increase of Glu content in the hippocampus [25], an excitatory neurotransmitter which participates in the signaling process through different types of glutamatergic receptors and contributes to memory formation [56]. Then, accumulation of excess extracellular Glu and subsequent overstimulation of ionotropic Glu receptors, leads subsequently to neuronal death.
In addition to monoamines impairments, OS induced by Cu was considered as one of the main mechanisms of Cu neurotoxicity [57, 58]. In fact, several studies reported that Cu-induced memory impairment is linked with increased OS within the hippocampus [21, 59]. As known, OS come as a result of an imbalance between the capacity of antioxidant defenses and formations of free radicals including NO [60, 61], which subsequently attack membrane lipids, thus generating LPO [62]. Then, LPO leads to the gradual increase in membrane permeability and changes in receptor functions [63]. In our experiment, we have shown that LPO levels are significantly elevated in rat’s hippocampus following chronic administration of Cu during 2 months. Other authors also showed altered LPO levels following Cu exposure [21, 42]. Zhang et al. observed that higher doses of Cu (2–20 mg/kg) caused a significant increase in LPO [25].
The increased LPO suggested alteration of antioxidants functions or/and over production of free radicals. This is confirmed by the findings of our study. We have revealed that the increased LPO levels induced by Cu in hippocampus tissue are accompanied by a remarkable decrease in SOD and CAT activities, indicating the prooxidant action of Cu. These results are in accordance with the works of Behzadfar et al. and Kalita et al. whom demonstrated that Cu administration enhanced the LPO damage with concomitant alterations in the enzymatic antioxidant defense status [21, 42]. The reduced synthesis of enzyme proteins provoked by high intracellular levels of Cu might be the cause of the decrease in both enzyme activities. As shown in the study of Yu et al.; Hippocampal mitochondrial proteomic revealed that Cu treatment caused an abnormal expression of proteins associated to OS. Among these abnormally expressed proteins, SOD and Protein disulfideisomerase A3 (PDIA3), a protein that defend against OS-induced apoptosis [41], were significantly decreased in mouse hippocampus [64].
In addition, the decrease in antioxidant enzymes in the rat hippocampus observed in the present study, may be the result of the incapacity of SOD and CAT to cope with the influx of NO, produced following Cu administration. It was reported that, excessive Cu encourage free radical generation [9]. In our study, Cu-provoked OS was linked with an elevated production of hippocampal NO, a free radical which can induce several neurotoxic effects [65]. Other research declared that Cu can increase the NO levels in the brain of rats [66, 67]. Moreover, Manto et al. reported that Cu can increase the generation of NO by stimulating the transcription of NO synthase (NOS) [68], an enzyme catalyzing the production of NO [69]. Once in excess, NO can form the ion peroxynitrite (ONOO−) after its reaction with the superoxide ion (O2−), a toxic molecule which can oxidize several molecules including lipids, ending by LPO [70]. Taken together, Cu contributes to increasing NO levels and severely weakens the antioxidant systems; such effects increase oxidative damage which subsequently disrupts neuronal function [71], thus causing a progressive deterioration of the memory function [72, 73].
As observed in our work; the effects of Cu on memory functions and OS were sex dependent, they being slightly marked in female compared to male rats. A possible explanation of these differences suggests the implication of gonadal hormones in the modulation of cognitive impairments. The estrogens were found to favorite the Cu retention [74], which makes the females more vulnerable to the neurotoxic effects of Cu. The sex-related effect is shown also for other metals such Cd, Ni and Al [75–77]. Further experiences are needed to explicate the gender difference in the neurocognitive effect of this metal.
In conclusion, our study showed that chronic intraperitoneal injection of different concentrations of Cu alters rat learning ability and memory in a manner that correlates with the OS in the hippocampus, which may be the possible mechanism.
Acknowledgements
Thanks to Dr Y. CHAHIROU for interest in this work and helpful discussion.
Compliance with ethical standards
Conflict of interest
No potential conflict of interest was declared by the authors.
Footnotes
Work supervisor: Ali Ouichou.
Contributor Information
Mouloud Lamtai, Email: mouloud-lamtai@hotmail.fr.
Ali Ouichou, Email: ouichou@hotmail.com.
References
- 1.Kamal Emam Mahmoud Combined effect of vanadium and nickel on lipid peroxidation and selected parameters of antioxidant system in liver and kidney of male rat. Afr J Biotechnol. 2011;10:18319–18325. doi: 10.5897/AJB11.2949. [DOI] [Google Scholar]
- 2.Kicinski M, Vrijens J, Vermier G, Den Hond E, Schoeters G, Nelen V, Bruckers L, Sioen I, Baeyens W, Van Larebeke N, Viaene MK, Nawrot TS. Neurobehavioral function and low-level metal exposure in adolescents. Int J Hyg Environ Health. 2015;218:139–146. doi: 10.1016/j.ijheh.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Abdellatif A, Omar ELH, Halima G. The neuronal basis of copper induced modulation of anxiety state in rat. Acta Histochem. 2017;119:10–17. doi: 10.1016/j.acthis.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Carotenuto R, Capriello T, Cofone R, Galdiero G, Fogliano C, Ferrandino I. Impact of copper in Xenopus laevis liver: histological damages and atp7b downregulation. Ecotoxicol Environ Saf. 2019;188:109940. doi: 10.1016/j.ecoenv.2019.109940. [DOI] [PubMed] [Google Scholar]
- 5.Pilehvar A, Town RM, Blust R. The effect of copper on behaviour, memory, and associative learning ability of zebrafish (Danio rerio) Ecotoxicol Environ Saf. 2020;188:109900. doi: 10.1016/j.ecoenv.2019.109900. [DOI] [PubMed] [Google Scholar]
- 6.Scheiber IF, Dringen R. Astrocyte functions in the copper homeostasis of the brain. Neurochem Int. 2013;62:556–565. doi: 10.1016/j.neuint.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Jiang X, Li Y, Yu H, Li S, Zhang Z, Xu H, Yang Y, Liu G, Zhu F, Ren X, Zou L, Xu B, Liu J, Spencer PS, Yang X. Low-dose oral copper treatment changes the hippocampal phosphoproteomic profile and perturbs mitochondrial function in a mouse model of Alzheimer’s disease. Free Radic Biol Med. 2019;135:144–156. doi: 10.1016/j.freeradbiomed.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Młyniec K, Gaweł M, Doboszewska U, Starowicz G, Pytka K, Davies CL, Budziszewska B. Essential elements in depression and anxiety. Part II. Pharmacol Rep. 2015;67:187–194. doi: 10.1016/j.pharep.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Desai V, Kaler SG. Role of copper in human neurological disorders. Am J Clin Nutr. 2008;88:855–858. doi: 10.1093/ajcn/88.3.855S. [DOI] [PubMed] [Google Scholar]
- 10.Magura IS, Rozhmanova OM. Oxidative stress and neurodegenerative disorders. Biopolym Cell. 1997;13:513–515. doi: 10.7124/bc.0004B0. [DOI] [Google Scholar]
- 11.Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- 12.Bulcke F, Dringen R. Handling of copper and copper oxide nanoparticles by astrocytes. Neurochem Res. 2016;41:33–43. doi: 10.1007/s11064-015-1688-9. [DOI] [PubMed] [Google Scholar]
- 13.Brewer GJ. The risks of copper toxicity contributing to cognitive decline in the aging population and to alzheimer’s disease. J Am Coll Nutr. 2009;28:238–242. doi: 10.1080/07315724.2009.10719777. [DOI] [PubMed] [Google Scholar]
- 14.Gerd M, Andrea S, Lars H, Dirk B, Thomas R, Colin LM, Konrad B. The amyloid precursor protein of Alzheimer’s disease in the reduction of copper (II) to copper (I) Science. 1996;271:2–4. doi: 10.1126/science.271.5254.1406. [DOI] [PubMed] [Google Scholar]
- 15.Lutsenko S, Bhattacharjee A, Hubbard AL. Copper handling machinery of the brain. Metallomics. 2010;2:596–608. doi: 10.1039/c0mt00006j. [DOI] [PubMed] [Google Scholar]
- 16.Scheiber IF, Mercer JFB, Dringen R. Metabolism and functions of copper in brain. Prog Neurobiol. 2014;116:33–57. doi: 10.1016/j.pneurobio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Crayton JW, Walsh WJ. Elevated serum copper levels in women with a history of post-partum depression. J Trace Elem Med Biol. 2007;21:17–21. doi: 10.1016/j.jtemb.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Nowak G, Zieba A, Dudek D, Krosniak M, Szymaczek M, Schlegel-Zawadzka M. Serum trace elements in animal models and human depression. Part I. Zinc. Hum Psychopharmacol. 1999;14:83–86. doi: 10.1002/(SICI)1099-1077(199903)14:2<83::AID-HUP74>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Russo AJ. Analysis of plasma zinc and copper concentration, and perceived symptoms, in individuals with depression, post zinc and anti-oxidant therapy. Nutr Metab Insights. 2011;4:S6760. doi: 10.4137/NMI.S6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamtai M, Ouakki S, Zghari O, Mesfioui A, El Hessni A, Ouichou A. Affective behavior dysregulation was induced by chronic administration of copper in wistar rats. Neurosci Med. 2019;10:134–149. doi: 10.4236/nm.2019.102009. [DOI] [Google Scholar]
- 21.Kalita J, Kumar V, Misra UK, Bora HK. Memory and learning dysfunction following copper toxicity: biochemical and immunohistochemical basis. Mol Neurobiol. 2017;55:3800–3811. doi: 10.1007/s12035-017-0619-y. [DOI] [PubMed] [Google Scholar]
- 22.Vlachová V. Copper modulation of NMDA responses in mouse and rat cultured hippocampal neurons. Eur J Neurosci. 1996;8:2257–2264. doi: 10.1111/j.1460-9568.1996.tb01189.x. [DOI] [PubMed] [Google Scholar]
- 23.Salustri C, Barbati G, Ghidoni R, Quintiliani L, Ciappina S, Binetti G, Squitti R. Clinical Neurophysiology Is cognitive function linked to serum free copper levels? A cohort study in a normal population. Clin Neurophysiol. 2010;121:502–507. doi: 10.1016/j.clinph.2009.11.090. [DOI] [PubMed] [Google Scholar]
- 24.Kumar V, Kalita J, Misra UK, Bora HK. A study of dose response and organ susceptibility of copper toxicity in a rat model. J Trace Elem Med Biol. 2015;29:269–274. doi: 10.1016/j.jtemb.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Lu W, Han M, Li H, Luo H, Li W, Luo W, Lin Z. Biphasic effects of copper on rat learning and memory in the morris water maze. Ann Clin Lab Sci. 2016;46:346–352. [PubMed] [Google Scholar]
- 26.Neely CLC, Lippi SLP, Lanzirotti A, Flinn JM. Localization of free and bound metal species through X-Ray synchrotron fluorescence microscopy in the rodent brain and their relation to behavior. Brain Sci. 2019;9:74. doi: 10.3390/brainsci9040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishihara K, Kawashita E, Shimizu R, Nagasawa K, Yasui H, Sago H, Yamakawa K, Akiba S. Copper accumulation in the brain causes the elevation of oxidative stress and less anxious behavior in Ts1Cje mice, a model of Down syndrome. Free Radic Biol Med. 2019;134:248–259. doi: 10.1016/j.freeradbiomed.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Lima FD, Souza MA, Furian AF, Rambo LM, Ribeiro LR, Martignoni FV, Hoffmann MS, Fighera MR, Royes LFF, Oliveira MS, de Mello CF. Na + , K + -ATPase activity impairment after experimental traumatic brain injury: relationship to spatial learning deficits and oxidative stress Behav. Brain Res. 2008;193:306–310. doi: 10.1016/j.bbr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 29.Rahman MF, Wang J, Patterson TA, Saini UT, Robinson BL, Newport GD, Murdock RC, Schlager JJ, Hussain SM, Ali SF. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol Lett. 2009;187:15–21. doi: 10.1016/j.toxlet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Kucukatay V, Aǧar A, Gumuslu S, Yargiçoǧlu P. Effect of sulfur dioxide on active and passive avoidance in experimental diabetes mellitus: relation to oxidant stress and antioxidant enzymes. Int J Neurosci. 2007;117:1091–1107. doi: 10.1080/00207450600934531. [DOI] [PubMed] [Google Scholar]
- 31.Sierksma ASR, Van Den Hove DLA, Pfau F, Philippens M, Bruno O, Fedele E, Ricciarelli R, Steinbusch HWM, Vanmierlo T, Prickaerts J. Improvement of spatial memory function in APPswe/PS1dE9 mice after chronic inhibition of phosphodiesterase type 4D. Neuropharmacology. 2014;77:120–130. doi: 10.1016/j.neuropharm.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Morris R. Spatial localization does not require local cues the presence of. Learn Motiv. 1981;12:239–260. doi: 10.1016/0023-9690(81)90020-5. [DOI] [Google Scholar]
- 33.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Method. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 34.Kaoud HA, Kamel MM, Abdel-Razek AH, Kamel GM, Ahmed KA. Neurobehavioural, neurochemical and neuromorphological effects of cadmium in male rats. J Am Sci. 2010;202:54–63. [Google Scholar]
- 35.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-I. [DOI] [PubMed] [Google Scholar]
- 36.Freitas RM, Sousa FCF, Vasconcelos SMM, Viana GSB, Fonteles MMF. Pilocarpine-induced status epilepticus in rats: lipid peroxidation level, nitrite formation, GABAergic and glutamatergic receptor alterations in the hippocampus, striatum and frontal cortex. Pharmacol Biochem Behav. 2004;78:327–332. doi: 10.1016/j.pbb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Injury via a nitric oxide mechanism Activated microglia mediate oxide neuronal cell injury via a nitric mechanism’. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 38.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 39.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 40.Abbaoui A, Gamrani H. Obvious anxiogenic-like effects of subchronic copper intoxication in rats, outcomes on spatial learning and memory and neuromodulatory potential of curcumin. J Chem Neuroanat. 2019;96:86–93. doi: 10.1016/j.jchemneu.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Yu H, Jiang X, Lin X, Zhang Z, Wu D, Zhou L, Liu J, Yang X. Hippocampal subcellular organelle proteomic alteration of copper-treated mice. Toxicol Sci. 2018;164:250–263. doi: 10.1093/toxsci/kfy082. [DOI] [PubMed] [Google Scholar]
- 42.Behzadfar L, Abdollahi M, Sabzevari O, Hosseini R, Salimi A, Naserzadeh P, Sharifzadeh M, Pourahmad J. Potentiating role of copper on spatial memory deficit induced by beta amyloid and evaluation of mitochondrial function markers in the hippocampus of rats. Metallomics. 2017;9:969–980. doi: 10.1039/C7MT00075H. [DOI] [PubMed] [Google Scholar]
- 43.Leiva J, Palestini M, Infante C, Goldschmidt A, Motles E. Copper suppresses hippocampus LTP in the rat, but does not alter learning or memory in the morris water maze. Brain Res. 2009;1256:69–75. doi: 10.1016/j.brainres.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 44.Palizvan MR, Jand A, Jand Y, Taherinejad MR. A study on the effects of orally administered copper sulfate on learning and spatial memory of wistar rats. J Babol Univ Med Sci. 2016;18:31–36. [Google Scholar]
- 45.Lu J, Zheng Y, Wu D, Sun D, Shan Q, Fan S. Trace amounts of copper induce neurotoxicity in the cholesterol-fed mice through apoptosis. FEBS Lett. 2006;580:6730–6740. doi: 10.1016/j.febslet.2006.10.072. [DOI] [PubMed] [Google Scholar]
- 46.Brewer GJ, Kanzer SH, Zimmerman EA, Celmins DF, Heckman SM, Dick R. Copper and ceruloplasmin abnormalities in Alzheimers disease. Am J Alzheimers Dis Other Demen. 2010;25:490–497. doi: 10.1177/1533317510375083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam PK, Kritz-Silverstein D, Barrett-Connor E, Milne D, Nielsen F, Gamst A, Morton D, Wingard D. Plasma trace elements and cognitive function in older men and women: the Rancho Bernardo study. J Nutr Heal Aging. 2008;12:22–27. doi: 10.1007/BF02982160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Squitti R, Siotto M, Polimanti R. Low-copper diet as a preventive strategy for Alzheimer’s disease. Neurobiol Aging. 2014;35:S40–S50. doi: 10.1016/j.neurobiolaging.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 49.D’Ambrosi N, Rossi L. Copper at synapse: release, binding and modulation of neurotransmission. Neurochem Int. 2015;90:36–45. doi: 10.1016/j.neuint.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Schlief ML. NMDA receptor activation mediates copper homeostasis in hippocampal neurons. J Neurosci. 2005;25:239–246. doi: 10.1523/JNEUROSCI.3699-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlief ML, Gitlin JD. Copper homeostasis in the CNS: a novel link between the NMDA receptor and copper homeostasis in the hippocampus. Mol Neurobiol. 2006;33:81–90. doi: 10.1385/MN:33:2:81. [DOI] [PubMed] [Google Scholar]
- 52.Decker MW, McGaugh JL. The role of interactions between the cholinergic system and other neuromodulatory systems in learing and memory. Synapse. 1991;7:151–168. doi: 10.1002/syn.890070209. [DOI] [PubMed] [Google Scholar]
- 53.Flicker C, Dean RL, Watkins DL, Fisher SK, Bartus RT. Behavioral and neurochemical effects following neurotoxic lesions of a major cholinergic input to the cerebral cortex in the rat. Pharmacol Biochem Behav. 1983;18:973–981. doi: 10.1016/S0091-3057(83)80023-9. [DOI] [PubMed] [Google Scholar]
- 54.Pal A, Badyal RK, Vasishta RK, Attri SV, Thapa BR, Prasad R. Biochemical, histological, and memory impairment effects of chronic copper toxicity: a model for non-wilsonian brain copper toxicosis in Wistar rat. Biol Trace Elem Res. 2013;153:257–268. doi: 10.1007/s12011-013-9665-0. [DOI] [PubMed] [Google Scholar]
- 55.Franciscato C, Bueno TM, Moraes-Silva L, Duarte FA, Flores ÉMM, Dressler VL, Pereira ME. High doses of zinc and copper alter neither cerebral metal levels nor acetylcholinesterase activity of suckling rats. EXCLI J. 2009;8:138–147. [Google Scholar]
- 56.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:406–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stern BR, Solioz M, Krewski D, Aggett P, Aw TC, Baker S, Crump K, Dourson M, Haber L, Hertzberg R, Keen C, Meek B, Rudenko L, Schoeny R, Slob W, Starr T. Copper and human health: biochemistry, genetics, and strategies for modeling dose-response relationships. J Toxicol Environ Health B Crit Rev. 2007;10:157–222. doi: 10.1080/10937400600755911. [DOI] [PubMed] [Google Scholar]
- 58.Donnelly PS, Caragounis A, Du T, Laughton KM, Volitakis I, Cherny RA, Sharples RA, Hill AF, Li QX, Masters CL, Barnham KJ, White AR. Selective intracellular release of copper and zinc ions from bis(thiosemicarbazonato) complexes reduces levels of Alzheimer disease amyloid-β peptide. J Biol Chem. 2008;283:4568–4577. doi: 10.1074/jbc.M705957200. [DOI] [PubMed] [Google Scholar]
- 59.Ma Q, Ying M, Sui X, Zhang H, Huang H, Yang L, Huang X, Zhuang Z, Liu J, Yang X. Chronic copper exposure causes spatial memory impairment, selective loss of hippocampal synaptic proteins, and activation of PKR/eIF2α pathway in mice. J Alzheimer’s Dis. 2014;43:1413–1427. doi: 10.3233/JAD-140216. [DOI] [PubMed] [Google Scholar]
- 60.Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complem Altern Med. 2008;8:1–10. doi: 10.1186/1472-6882-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Piñol-Ripoll G, Fuentes-Broto L, Millán-Plano S, Reyes-Gonzáles M, Mauri JA, Martínez-Ballarín E, Reiter RJ, García JJ. Protective effect of melatonin and pinoline on nitric oxide-induced lipid and protein peroxidation in rat brain homogenates. Neurosci Lett. 2006;405:89–93. doi: 10.1016/j.neulet.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 63.Albendea CD, Gómez-Trullén EM, Fuentes-Broto L, Miana-Mena FJ, Millán-Plano S, Reyes-Gonzales MC, Martínez-Ballarín E, García JJ. Melatonin reduces lipid and protein oxidative damage in synaptosomes due to aluminium. J Trace Elem Med Biol. 2007;21:261–268. doi: 10.1016/j.jtemb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Emerit J, Klein JM, Coutellier A, Congy F. Free radicals and lipid peroxidation in cell biology: physiopathologic prospects. Pathol Biol. 1991;39:316–327. [PubMed] [Google Scholar]
- 65.Garry PS, Ezra M, Rowland MJ, Westbrook J, Pattinson KTS. The role of the nitric oxide pathway in brain injury and its treatment—from bench to bedside. Exp Neurol. 2015;263:235–243. doi: 10.1016/j.expneurol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 66.Cuzzocrea S, Persichini T, Dugo L, Colasanti M, Musci G. Copper induces type II nitric oxide synthase in vivo. Free Radic Biol Med. 2003;34:1253–1262. doi: 10.1016/S0891-5849(03)00110-2. [DOI] [PubMed] [Google Scholar]
- 67.Hu HL, Ni XS, Duff-Canning S, Wang XP. Oxidative damage of copper chloride overload to the cultured rat astrocytes. Am J Transl Res. 2016;8:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 68.Manto M. Abnormal copper homeostasis: mechanisms and roles in neurodegeneration. Toxics. 2014;2:327–345. doi: 10.3390/toxics2020327. [DOI] [Google Scholar]
- 69.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moncada S, Bolaños JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676–1689. doi: 10.1111/j.1471-4159.2006.03988.x. [DOI] [PubMed] [Google Scholar]
- 71.Bouayed J, Rammal H, Soulimani R. Oxidative stress and anxiety : relationship and cellular pathways. Oxid Med Cell Longev. 2009;2:63–67. doi: 10.4161/oxim.2.2.7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krumschnabel G, Manzl C, Berger C, Hofer B. Oxidative stress, mitochondrial permeability transition, and cell death in Cu-exposed trout hepatocytes. Toxicol Appl Pharmacol. 2005;209:62–73. doi: 10.1016/j.taap.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amtage F, Birnbaum D, Reinhard T, Niesen WD, Weiller C, Mader I, Meyer PT, Rijntjes M. Estrogen intake and copper depositions: implications for alzheimer’s disease? Case Rep Neurol. 2014;6:181–187. doi: 10.1159/000363688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zghari O, Rezqaoui A, Ouakki S, Lamtai M, Chaibat J, Mesfioui A, El Hessni A, Rifi E-H, Essamri A, Ouichou A. Effect of chronic aluminum administration on affective and cognitive behavior in male and female rats. J Behav Brain Sci. 2018;8:179–196. doi: 10.4236/jbbs.2018.84012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lamtai M, Chaibat J, Ouakki S, Berkiks I, Rifi E, El Hessni A, Mesfioui A, Hbibi AT, Ahyayauch H, Essamri A, Ouichou A. Effect of chronic administration of cadmium on anxiety-like, depression-like and memory deficits in male and female rats: possible involvement of oxidative stress mechanism. J Behav Brain Sci. 2018;8:240–268. doi: 10.4236/jbbs.2018.85016. [DOI] [Google Scholar]
- 77.Lamtai M, Chaibat J, Ouakki S, Zghari O, Mesfioui A, El Hessni A, Rifi E-H, Marmouzi I, Essamri A, Ouichou A. Effect of chronic administration of nickel on affective and cognitive behavior in male and female rats: possible implication of oxidative stress pathway. Brain Sci. 2018;8:141. doi: 10.3390/brainsci8080141. [DOI] [PMC free article] [PubMed] [Google Scholar]


