Abstract
Background
Patients with signs and symptoms of myocardial ischemia and non-obstructive coronary artery disease (CAD) frequently have coronary functional abnormalities, including coronary microvascular dysfunction. Those with the latter are grouped under the term “microvascular angina” (MVA). Although diagnostic criteria exist for MVA, as recently proposed by our COVADIS (COronary VAsomotor Disorders International Study) group and the condition has been increasingly recognized in clinical practice, the clinical characteristics and long-term prognosis of MVA patients in the current era remain to be fully elucidated.
Aims
In the present study, we aimed to prospectively assess the clinical characteristics and long-term prognosis of MVA subjects in the current era in an international, multicenter, observational, and prospective registry study.
Methods
A total of 15 medical centers across 7 countries (USA, UK, Germany, Spain, Italy, Australia, and Japan) enrolled subjects fulfilling the COVADIS diagnostic criteria for MVA as follows; (1) signs and/or symptoms of myocardial ischemia, (2) absence of obstructive CAD, and (3) objective evidence of myocardial ischemia and/or coronary microvascular dysfunction. The primary endpoint was the composite of major cardiovascular events (MACE), including cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, hospitalization due to heart failure or unstable angina. Between July 2015 and December 2018, a total of 706 subjects with MVA (M/F 256/450, 61.1 ± 11.8 [SD] yrs.) were registered. Subjects will be followed for at least 1 year.
Summary
The present study will provide important information regarding the clinical characteristics, management, and long-term prognosis of MVA patients in the current era.
Keywords: Coronary microvascular dysfunction, Microvascular angina
1. Background
Angina pectoris has been considered for a long time to be mainly caused by obstructive atherosclerotic epicardial coronary artery disease (CAD) [1]. At present, however, it is well known that up to 50% of patients undergoing diagnostic coronary angiography for typical chest pain are found to have “normal” or “near-normal” coronary arteries [2]. Indeed, recent studies suggest that at least 3–4 million women and men with signs/symptoms suggestive myocardial ischemia have no obstructive CAD in the US, and they are associated with poor quality of life, psychological distress, and health-care costs that approximate those with obstructive CAD [3]. In such cases, coronary functional abnormalities are likely to be involved, including increased vasoconstrictive reactivity and/or reduced vasodilator function [4]. Coronary microvessels are known to contribute to >50% of total coronary vascular resistance and regulate coronary blood flow [5]. Coronary microvascular dysfunction (CMD) is typically defined as increased coronary vascular resistance and/or impaired dilatation of those microvessels, leading to inadequate increase in blood flow in response to stress with resultant myocardial ischemia [5], [6], [7]. Thus, CMD may be considered to be the underlying mechanism in a large proportion of angina patients. Indeed, Camici and Crea proposed the original clinical and pathogenetic classifications of CMD, as follows; (1) CMD in the absence of myocardial disease or obstructive CAD, (2) CMD in myocardial diseases, (3) CMD in obstructive CAD, and (4) iatrogenic CMD [6], [7].
The ISCHEMIA trial has recently demonstrated that as compared with optimal medical therapy alone, additional coronary revascularization therapy, such as percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), has no prognostic benefits in patients with stable CAD and moderate to severe ischemia [8], which could be explained by coronary functional abnormalities (including CMD) in this population with obstructive CAD.
The term microvascular angina (MVA), which was originally proposed by Cannon and Epstein in 1988 [9], is applicable to angina/myocardial ischemia attributable to type 1 CMD of the above classification. Several studies demonstrated that patients with MVA have significantly higher rates of cardiovascular events compared to patients with non-obstructed coronary arteries, but no anginal symptoms, highlighting the importance of identifying such patients [10], [11]. Previously, progress in the understanding of MVA was limited because studies were usually underpowered, used either restrictive inclusion criteria, or included rather heterogenous groups of patients [7]. Moreover, tools for assessment of coronary microcirculation were not available until recently in clinical practice. Recently, however, several studies using both invasive and non-invasive techniques for assessment of coronary physiology have produced a large wealth of data, leading to a better understanding of CMD and microvascular ischemia [12], [13]. Based on the recent discussions on the clinical implication of MVA due to CMD, the COronary VAsomotor Disorders International Study (COVADIS) group, which was established in 2012 by clinician scientists with expertise in coronary vasomotor abnormalities, proposed diagnostic criteria to define MVA [14]. As shown in Table 1, the diagnosis of MVA can be made if patients present with symptoms of myocardial ischemia in the absence of relevant epicardial CAD and have objective evidence of myocardial ischemia as well as impaired coronary microvascular function defined by any of the following, reduced coronary flow reserve (CFR), microvascular spasm, increased index of microcirculatory resistance (IMR), or the coronary “slow flow phenomenon” [14]. Although the diagnostic criteria for MVA exist and the condition has been increasingly recognized in clinical practice, the clinical characteristics and long-term prognosis of patients with a diagnosis of MVA in the current era remain to be fully elucidated. Thus, in the present study, we aimed to assess the clinical characteristics and long-term prognosis of MVA patients in the current era, utilizing an international, multicenter, observational, and prospective cohort study.
Table 1.
Clinical Criteria for Suspecting Microvascular Angina (MVA) by COVADIS.
| 1. Symptoms of myocardial ischemia |
| a. Effort and/or rest angina |
| b. Angina equivalents (i.e. shortness of breath) |
| 2. Absence of obstructive coronary artery disease (<50% diameter reduction or FFR > 0.80) by |
| a. Coronary CTA |
| b. Invasive coronary angiography |
| 3. Objective evidence of myocardial ischemia |
| a. Ischemic ECG changes during an episode of chest pain |
| b. Stress-induced chest pain and/or ischemic ECG changes in the presence or absence of transient/reversible |
| abnormal myocardial perfusion and/or wall motion abnormality |
| 4. Evidence of impaired coronary microvascular function |
| a. Impaired coronary flow reserve (cut-off values depending on methodology use between <2.0 and <2.5) |
| b. Coronary microvascular spasm, defined as reproduction of symptoms, ischemic ECG changes but no epicardial |
| spasm during acetylcholine provocation test |
| c. Abnormal coronary microvascular resistance indices (e.g. IMR > 25) |
| d. Coronary slow flow phenomenon, defined as TIMI frame count > 25 |
| Definitive MVA: all four criteria are present for a diagnosis of microvascular angina. |
| Suspected MVA: symptoms of ischemia are present with no obstructive coronary artery disease but only objective |
| evidence of myocardial ischemia, or evidence of impaired coronary microvascular function alone. |
CTA, computed tomographic angiography; ECG, electrocardiogram; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; TIMI, thrombolysis in myocardial infarction.
2. Methods
2.1. Overall study design
This is a multinational, multicenter, observational, prospective, and longitudinal cohort study. Fifteen medical centers in 7 countries from 4 continents participated in the study (Fig. 1). Data collection was performed via an electronic case report system established by the Japanese Coronary Spasm Association [15]. Subjects underwent clinical assessments and received standard medical care as determined by attending physicians. Subjects did not receive experimental intervention or directed treatment because of their participation in the study. The ethics committee of Tohoku University Graduate School of Medicine approved the study protocol (No. 2016-1-643), which was performed in compliance with the Declaration of Helsinki (UMIN000035177). All subjects provided a written informed consent before study entry.
Fig. 1.
Participating institutions of the present study.
2.2. Study population
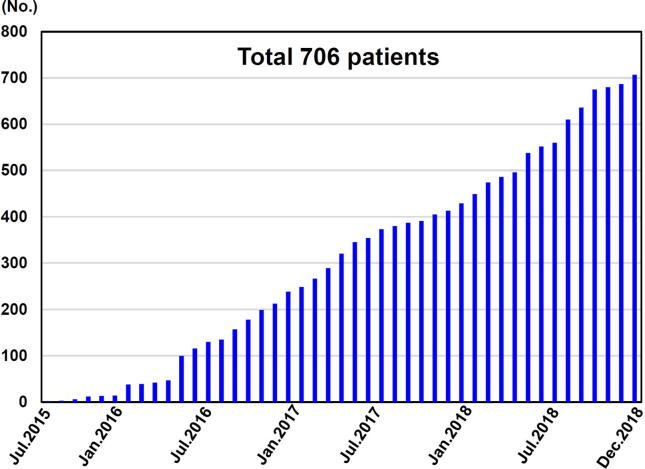
We enrolled subjects fulfilling the COVADIS diagnostic criteria for MVA as follows; (1) signs and/or symptoms of myocardial ischemia, (2) absence of obstructive CAD, and (3) objective evidence of myocardial ischemia and/or coronary microvascular dysfunction (Table 1), as determined by the clinical site [14]. More specifically, symptoms of myocardial ischemia were as determined by the treating clinician. Obstructive CAD was defined as any coronary stenosis of >50% diameter reduction by conventional angiography or computed tomography angiography, whereby patients were excluded. Objective evidence of myocardial ischemia was obtained with rest/stress ECG and/or non-invasive imaging by assessing either myocardial perfusion with single photon emission computed tomography (SPECT), positron emission tomography (PET), cardiac magnetic resonance (CMR), left ventricular wall motion abnormality with stress echocardiography, or positive troponin levels due chronic myocardial damage. Evidence of impaired microvascular function was assessed by using coronary functional testing, including measurements of CFR and/or microvascular resistance or visual constriction, and acetylcholine provocation testing for coronary microvascular spasm. During the period from July 1, 2015 to December 31, 2018, we prospectively enrolled 706 subjects (Fig. 2).
Fig. 2.
Enrollment of subjects in the present study.
2.3. Study endpoints
The primary endpoint is the composite of major cardiovascular events (MACE), including cardiovascular death, non-fatal myocardial infarction (MI), non-fatal stroke, hospitalization due to heart failure or unstable angina (UA). We only counted the number of patients with the first occurrence of an event in the MACE during the follow-up period. Definition of MI is based on the third universal definition [16]. Definition of UA is based on the presence of ischemic chest pain and hospitalization within 24 h of most recent symptoms, without elevation in cardiac biomarkers but with evidence of myocardial ischemia. Stroke was defined as neurological deficit due to an ischemic or hemorrhagic central nervous system event with residual systems > 24 h after onset or leading to death. Other objectives are to elucidate patient characteristics, diagnostic approaches, and the trend of medical therapies for contemporary MVA patients, particularly in terms of the racial and sex differences.
2.4. Data collection
All subjects who met the eligibility criteria determined at the site were registered following site ethical review board approval. Data collection was performed through the use of the electronic case report form established by the Japanese Coronary Spasm Association [15]. The investigators at each study site registered information on demographics, relevant medical history, cardiovascular risk factors, quality of life (e.g. Seattle Angina Questionnaire, SAQ), diagnostic approaches for myocardial ischemia, anatomical and/or functional status of epicardial coronary arteries and coronary microcirculation, and medications. The follow-up of each patient was conducted from study entry to the end of December 2019 either by a telephone call or personal visit, depending on the approach considered most practical and effective. The minimum follow-up period for included subjects was 1 year.
2.5. Study variables
Variables obtained at enrolment/baseline included subject demographics (sex, age, height, weight), cardiovascular risk factors (hypertension, hyperlipidemia, diabetes mellitus, smoking, menopause), past history and family history of cardiovascular disease, type of angina episodes (effort, rest, mixed), circadian distribution of angina attacks, ECG leads of ST-segment elevation or depression at rest, arrhythmias during spontaneous attack, use of non-invasive diagnostic modalities for myocardial ischemia (SPECT, PET, CMR, stress echocardiography or electrocardiography), laboratory tests (renal function tests, lipid profile, hemoglobin, white blood cell count, glycated hemoglobin, brain natriuretic peptide, high sensitive C-reactive protein), information regarding interventional diagnostic procedures for assessment of coronary vasodilatation (e.g. coronary flow reserve, index of microcirculatory resistance, hyperemic microvascular resistance) or assessment for propensity to coronary vasoconstriction (e.g. spasm provocation test), medications (calcium channel blocker, nitrate, statin, ACE-I, ARB, antiplatelet/anticoagulant agent, beta-blocker, hormone replacement therapy, and antioxidant agent), patient-reported angina status assessed by the SAQ, and outcomes during acute hospitalization (death or discharge alive).
During the follow-up period, clinical outcomes (cardiovascular death, non-fatal MI, non-fatal stroke, hospitalization due to heart failure, and UA), presence or absence of anginal symptoms, laboratory tests, self-reported anginal status by the SAQ, and medications were collected.
2.6. Statistical methods
Statistical methods appropriate for epidemiological studies will be used for the analysis of the collected data. Baseline continuous variables will be presented as means ± SD or medians and interquartile range, depending on the distribution of the data that will be tested by Shapiro-Wilk normality test. Categorical variables will be presented as counts and percentages. Events will be analyzed as time from enrolment until the first occurrence of any component from the primary composite of MACE. The incidence rate of the primary endpoint will be stratified for the inclusion risk factors, such as age, sex, hypertension, diabetes, smoking habits, obesity, heart failure, region, and race. In addition, to reduce confounding effects related to differences in baseline characteristics, the association of fore-mentioned covariates, especially region or race, and the occurrence of MACEs is planned to be explored with a multivariate Cox proportional-hazards regression and/or a propensity-score matching method.
2.7. Ethics approval
This study was performed in accordance with ethical principles that are consistent with the Declaration of Helsinki, International Conference on Harmonization of Good Clinical Practice guidelines, and the applicable legislation on non-interventional studies. The final protocol was approved by the site ethics committee. The investigator at each site ensured that the subject was given full and adequate oral and written information in the local language about the nature, purpose, possible risk, and benefit of this study.
2.8. Study organization
The Coronary Vasomotor Disorder International Study (COVADIS) group was established in 2012 to define the nomenclature and stimulate interest into coronary vasomotor disorders. The COVADIS Steering Committee served as the principal investigators for the COVADIS Microvascular Angina Registry, including the Steering Committee co-chairs and the data coordinating center (DCC). The Steering Committee members are as follows; John Beltrame (COVADIS co-chair, Australia), Colin Berry (PI, United Kingdom), Paolo Camici (PI, Italy), Filippo Crea (PI, Italy), Juan Carlos Kaski (PI, United Kingdom), C. Noel Bairey Merz (COVADIS co-chair, USA), Peter Ong (PI, Germany), Carl J Pepine (PI, USA), Udo Sechtem (PI, Germany), and Hiroaki Shimokawa (Study Chair, DCC, Japan).
2.9. Baseline clinical characteristics of subjects
From July 2015 to December 2018, 706 subjects with MVA (M/F 256/450, 61.1 ± 11.8 [SD] yrs.) were registered (Fig. 2). Subjects’ clinical characteristics are summarized in Table 2. Almost two thirds (64%) of the cohort were female and the main ethnic groups were Caucasian (61%), Asian (28%), and Hispanic (6%). More than half of the subjects had hypertension (52%) or dyslipidemia (53%), whereas relatively fewer subjects had diabetes mellitus (17%) or were current smokers (16%). The predominant symptom was chest pain or chest discomfort (68%), especially at rest (35%), and shortness of breath on exertion (19%).
Table 2.
Baseline Clinical Characteristics of Subjects.
| Overall (n = 706) | |
|---|---|
| Ethnicity | |
| Caucasian, n(%) | 434 (61) |
| Asian, n(%) | 201 (28) |
| Hispanic, n(%) | 40 (6) |
| Black, n(%) | 16 (2) |
| Age (mean ± SD), yrs. | 61.1 ± 11.8 |
| Female, n(%) | 450 (64) |
| Hypertension, n(%) | 370 (52) |
| Dyslipidemia, n(%) | 371 (53) |
| Diabetes mellitus, n(%) | 119 (17) |
| Current smoking, n(%) | 111 (16) |
| Previous history of CAD, n(%) | 241 (34) |
| Previous PCI, n(%) | 66 (9) |
| Symptoms | |
| Angina, n(%) | 480 (68) |
| Rest angina, n(%) | 247 (35) |
| Effort angina, n(%) | 106 (15) |
| Rest and effort angina, n(%) | 127 (18) |
| Shortness of breath, n(%) | 133 (19) |
| Asymptomatic, n(%) | 58 (9) |
| Others, n(%) | 76 (11) |
Results are expressed as mean ± standard deviation or n (%).
CAD, coronary artery disease; PCI, percutaneous coronary intervention.
Symptoms can be overlapped.
3. Discussion
Although the importance of coronary functional abnormalities in patients with chest pain and non-obstructive CAD, including coronary microvascular dysfunction, has been established, their pathogenesis and prognostic implications remain to be fully elucidated. To the best of our knowledge, this is the first international study that focused on the risk factors, clinical features, diagnostic methods, treatments and long-term prognosis of contemporary patients with MVA diagnosed using standardized criteria.
Long-term prognosis of MVA patients has consistently been reported to be good [17], a considerable number of patients with potential markers of worse outcome are included [12], [13]. Of those, invasive physiological indices including CFR and IMR are considered to be predictive markers of adverse cardiac events in MVA patients [10], [13]. In the present international study, we aim to elucidate the clinical characteristics and long-term prognosis of contemporary MVA patients in a large population in a prospective manner. Furthermore, in previous studies of patients with chest pain and no obstructive CAD, women were more likely than men to have angina without significant coronary artery stenosis, and coronary microvascular dysfunction has been proposed as one of the explanations of this phenomenon [18], [19]. Further investigation by the WISE Study revealed that almost one-half of women with angina and non-obstructive CAD had abnormal coronary microvascular function, suggesting that coronary microvascular dysfunction is a cause of angina [10]. However, there has been no large cohort study that compared the actual prevalence of coronary microvascular dysfunction between sexes. In addition, it has been reported that CMD appears to be involved in functional and structural alterations associated with aging, hypertension, diabetes mellitus, dyslipidemia and insulin resistance [7], although the prevalence of these arteriosclerotic risk factors in MVA patients remains unknown. One of the purposes of the present study is to elucidate associated clinical characteristics, including the prevalence of risk factors of MVA patients in a large population.
CMD can develop in variable clinical settings and can be triggered by several pathogenetic mechanisms [6]. From a pathophysiological point of view, and independent of the underlying mechanisms, the development of MVA is attributable to varying degrees of disruption of the normal coronary physiology, which may subsequently impair the capacity of myocardial blood flow to adapt to changes in myocardial oxygen demand. However, a precise diagnostic algorithm of MVA patients, especially in terms of invasive techniques to evaluate coronary microvascular function, still remains to be fully established. In the present study, all subjects were registered based on objective evidence of myocardial ischemia and/or impaired microvascular function according to the international standardized diagnostic criteria for MVA proposed by the COVADIS [14] (Table 1). The standard criteria for investigation of MVA due to CMD make it possible to examine different diagnostic strategies including non-invasive and invasive assessments for their feasibility, safety, and accuracy in the present study.
The management of MVA represents a major unmet need because the lack of large, randomized studies involving homogeneous patient groups makes it difficult to generate evidence-based recommendations [20]. Furthermore, the treatment for CMD has so far been empirical because its pathophysiology appears multifactorial, with overlapping phenotypes that often coexist. Recent papers have discussed the management of MVA patients and suggested some potential therapies for the disorder [3], [20]. Anti-atherothrombosis treatments with statins, angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers (ARB), and low-dose aspirin may improve symptoms and outcomes in MVA patients [21], [22]. Although conventional anti-anginal therapies, including beta-blockers, calcium channel blockers, and nitrates, are reasonable as first-line regimens for MVA patients given the underlying pathophysiology, there has been limited data on their beneficial effects on recurrent anginal attacks [23], [24]. On the other hand, some novel therapies (e.g. ranolazine, ivabradine and fasudil) may reduce symptoms and improve coronary microvascular function in MVA patients [25], [26], [27]. In the present study, we aimed to describe the current treatments for MVA patients in contemporary clinical practice and provide insights to the optimal treatment for patients at risk of future cardiac events.
4. Summary
This study will provide important information regarding the clinical characteristics, management, and long-term prognosis of MVA patients in the current era.
CRediT authorship contribution statement
Akira Suda: Software, Data curation, Writing - original draft. Jun Takahashi: Software, Data curation, Writing - original draft. John F. Beltrame: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Colin Berry: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Paolo G. Camici: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Filippo Crea: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Javier Escaned: Data curation. Tom Ford: Data curation. Juan Carlos Kaski: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Takahiko Kiyooka: Data curation. Puja K. Metha: Data curation. Peter Ong: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Yukio Ozaki: Data curation. Carl Pepine: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Ornella Rimoldi: Data curation. Basmah Safdar: Data curation. Udo Sechtem: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Kenichi Tsujita: Data curation. Eric Yii: Data curation. C. Noel Bairey Merz: Conceptualization, Methodology, Investigation, Writing - review & editing, Supervision. Hiroaki Shimokawa: Conceptualization, Methodology, Investigation, Software, Writing - original draft, Writing - review & editing, Supervision.
Declaration of Competing Interest
F.C. reports speaker fees from AstraZeneca, Amgen and Servier and institutional agreements between his employ-er, the Catholic University, and Biotronik, Boheringer Ingelheim. C.N.B.M. reports lecturer fees from Abbott Diagnostics and BoardDirector fees from iRhythm. C.B. declares institutional agreementsbetween his employer, the University of Glasgow, and AbbottVascular, AstraZeneca, Boehringer Ingelheim, Coroventis, DalCor, GSK, HeartFlow, Novartis, and Philips. P.G.C. reports personal consultant fees from Servier. P.O. reports personal fees from Bayer Healthcare, Pfizer and Philips/Volcano. T.F. has acted as a speaker for Abbott Vascular, Boehringer Ingelheim and Novartis. None of the declared interests regard the submitted work. All other authors have nothing to disclose.
Acknowledgments
Acknowledgments
We thank all the staff who will support this study as follows; Chassidi Garrett, Sonoka Goto, Koichi Kaikita, Hideki Kawai, Sarah Long, Sabine Nägele, Valeria Martínez Pereyra, and Andreas Seitz. We also thank the subjects who participated in the registry.
Funding
The Japanese Coronary Spasm Association (funded by the Japan Heart Foundation). CB received funding from the British Heart Foundation (PG/17/2532884; RE/18/6134217). CNBM and CJP received funding from contracts from the National Heart, Lung and Blood Institutes nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, K23HL105787, T32HL69751, R01 HL090957, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources, the National Center for Advancing Translational Sciences Grant UL1TR000124 and UL1TR000064. JB received funding from The Hospital Research Foundation. PO and US received funding from the Berthold-Leibinger-Foundation, Germany.
References
- 1.Ohman E.M. Chronic stable angina. N. Engl. J. Med. 2016;374:1167–1176. doi: 10.1056/NEJMcp1502240. [DOI] [PubMed] [Google Scholar]
- 2.Patel M.R., Peterson E.D., Dai D. Low diagnostic yield of elective coronary angiography. N. Engl. J. Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merz C.N.B., Pepine C.J., Walsh M.N. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation. 2017;135:1075–1092. doi: 10.1161/CIRCULATIONAHA.116.024534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimokawa H. 2014 Williams Harvey lecture: Importance of coronary vasomotion abnormalities - From bench to bedside. Eur. Heart J. 2014;35:3180–3193. doi: 10.1093/eurheartj/ehu427. [DOI] [PubMed] [Google Scholar]
- 5.Camici P.G., D’Amati G., Rimoldi O. Coronary microvascular dysfunction: Mechanisms and functional assessment. Nat. Rev. Cardiol. 2015;12:48–62. doi: 10.1038/nrcardio.2014.160. [DOI] [PubMed] [Google Scholar]
- 6.Camici P.G., Crea F. Coronary microvascular dysfunction. N. Engl. J. Med. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 7.Crea F., Camici P.G., Merz C.N.B. Coronary microvascular dysfunction: An update. Eur. Heart J. 2014;35:1101–1111. doi: 10.1093/eurheartj/eht513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maron D.J., Hochman J.S., Reynolds H.R. Initial invasive or conservative strategy for stable coronary disease. N. Engl. J. Med. 2020;382:1395–1407. doi: 10.1056/NEJMoa1915922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannon R.O., Epstein S.E. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am. J. Cardiol. 1988;61:1338–1343. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 10.Pepine C.J., Anderson R.D., Sharaf B.L. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia. Results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) Study. J. Am. Coll. Cardiol. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.M., Choi K.H., Hwang D. Prognostic implication of thermodilution coronary flow reserve in patients undergoing fractional flow reserve measurement. JACC Cardiovasc Interv. 2018;11:1423–1433. doi: 10.1016/j.jcin.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Ford T.J., Stanley B., Good R. Stratified medical therapy using invasive coronary function testing in angina: The CorMicA Trial. J. Am. Coll. Cardiol. 2018;72:2841–2855. doi: 10.1016/j.jacc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Suda A., Takahashi J., Hao K. Coronary functional abnormalities in patients with angina and non-obstructive coronary artery disease. J. Am. Coll. Cardiol. 2019;74:2350–2360. doi: 10.1016/j.jacc.2019.08.1056. [DOI] [PubMed] [Google Scholar]
- 14.Ong P., Camici P.G., Beltrame J.F. International standardization of diagnostic criteria for microvascular angina. Int. J. Cardiol. 2018;250:16–20. doi: 10.1016/j.ijcard.2017.08.068. [DOI] [PubMed] [Google Scholar]
- 15.Sato K., Takahashi J., Odaka Y. Clinical characteristics and long-term prognosis of contemporary patients with vasospastic angina: Ethnic differences detected in an international comparative study. Int. J. Cardiol. 2019;291:13–18. doi: 10.1016/j.ijcard.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K., Alpert J.S., Jaffe A.S. Third universal definition of myocardial infarction. Glob Heart. 2012;7:275–295. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Kaski J.C., Rosano G.M.C., Collins P. Cardiac syndrome X: Clinical characteristics and left ventricular function. Long-term follow-up study. J. Am. Coll. Cardiol. 1995;25:807–814. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 18.Bugiardini R., Merz C.N.B. Angina with “Normal” coronary arteries. JAMA. 2005;293:477–484. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi Y., Fearon W.F., Honda Y. Effect of sex differences on invasive measures of coronary microvascular dysfunction in patients with angina in the absence of obstructive coronary artery disease. JACC Cardiovasc. Interv. 2015;8:1433–1441. doi: 10.1016/j.jcin.2015.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merz C.N.B., Pepine C.J., Shimokawa H. Treatment of coronary microvascular dysfunction. Cardiovasc. Res. 2020;116:856–870. doi: 10.1093/cvr/cvaa006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pizzi C., Manfrini O., Fontana F. Angiotensin-converting enzyme inhibitors and 3-hydroxy-3-methylglutaryl coenzyme A reductase in cardiac syndrome X: role of superoxide dismutase activity. Circulation. 2004;109:53–58. doi: 10.1161/01.CIR.0000100722.34034.E4. [DOI] [PubMed] [Google Scholar]
- 22.Kayikcioglu M., Payzin S., Yavuzgil O. Benefits of statin treatment in cardiac syndrome-X. Eur. Heart J. 2003;24:1999–2005. doi: 10.1016/s0195-668x(03)00478-0. [DOI] [PubMed] [Google Scholar]
- 23.Sütsch G., Oechslin E., Mayer I. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int. J. Cardiol. 1995;52:135–143. doi: 10.1016/0167-5273(95)02458-9. [DOI] [PubMed] [Google Scholar]
- 24.Bugiardini R., Borghi A., Biagetti L. Comparison of verapamil versus propranolol therapy in syndrome X. Am. J. Cardiol. 1989;63:286–290. doi: 10.1016/0002-9149(89)90332-9. [DOI] [PubMed] [Google Scholar]
- 25.Mohri M., Shimokawa H., Hirakawa Y. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. J. Am. Coll. Cardiol. 2003;41:15–19. doi: 10.1016/s0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 26.Villano A., Di Franco A., Nerla R. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am. J. Cardiol. 2013;112:8–13. doi: 10.1016/j.amjcard.2013.02.045. [DOI] [PubMed] [Google Scholar]
- 27.Merz C.N.B., Handberg E.M., Shufelt C.L. A randomized, placebo-controlled trial of late Na current inhibition (ranolazine) in coronary microvascular dysfunction (CMD): Impact on angina and myocardial perfusion reserve. Eur. Heart J. 2016;37:1504–1513. doi: 10.1093/eurheartj/ehv647. [DOI] [PMC free article] [PubMed] [Google Scholar]