Abstract
Radiation therapy is a frontline treatment option for cancer patients; however, the effects of radiotherapy on non-tumor tissue (e.g. radiation-induced dermatitis) often worsen patient quality of life. Previous studies have implicated the importance of redox balance in preventing dermatitis, specifically in reference to modulation of the nuclear factor (erythroid-derived 2)-like 2 (NRF2) signaling pathway. Due to the cytoprotective functions of transcriptional target genes of NRF2, we investigated how modulation of NRF2 expression could affect DNA damage, oxidative stress, and cell viability in response to radiotherapy. Specifically, it was noted that NRF2 knockdown sensitized human skin keratinocytes to ionizing radiation; likewise, genetic ablation of NRF2 in vivo increased radiosensitivity of murine epidermis. Oppositely, pharmacological induction of NRF2 via the apocarotenoid bixin lowered markers of DNA damage and oxidative stress, while preserving viability in irradiated keratinocytes. Mechanistic studies indicated that topical pretreatment using bixin as an NRF2 activator antagonized initial DNA damage by raising cellular glutathione levels. Additionally, topical application of bixin prevented radiation-induced dermatitis, epidermal thickening, and oxidative stress in the skin of SKH1 mice. Overall, these data indicate that NRF2 is critical for mitigating the harmful skin toxicities associated with ionizing radiation, and that topical upregulation of NRF2 via bixin could prevent radiation-induced dermatitis.
Keywords: NRF2, Bixin, Radiation-induced dermatitis, Radiotherapy, Skin, Cancer
Abbreviations: RT, radiation therapy; IR, ionizing radiation; NRF2, nuclear factor (erythroid-derived 2)-like 2; ROS, reactive oxygen species; GSH, glutathione
Graphical abstract
Topical application of bixin induces epidermal NRF2 signaling, preventing IR-induced DNA damage, oxidative stress, and cell death, all of which contribute to cutaneous radiation damage. Thus, induction of NRF2 via topical bixin application could represent a novel strategy for the prevention of radiation-induced dermatitis.
Highlights
-
•
The apocarotenoid bixin prevents IR-induced damage via the NRF2 signaling pathway.
-
•
Topical application of bixin prevents radiation-induced dermatitis in vivo.
-
•
NRF2 is a critical mediator of bixin protection against IR-induced cutaneous damage.
-
•
Glutathione upregulation contributes to bixin protection against IR-induced ROS and genotoxic stress.
1. Introduction
Radiation therapy (RT) is an indispensable treatment modality, with nearly 50% of cancer patients receiving RT at some point during the course of their illness [1,2] Mechanistically, exposure to ionizing radiation (IR) causes tissue damage due to free radical/reactive oxygen species formation, electrophilic genotoxic stress, and inflammatory signaling, ultimately triggering cancer cell death. However, as many as 95% of patients receiving RT may experience collateral tissue damage as a result of IR exposure [3]. Specifically, IR-induced damage underlies several pathological hallmarks of radiation-induced dermatitis including erythema and desquamation, telangiectasia, keratinocyte DNA damage and apoptosis, sunburn-like inflammatory dysregulation, and fibrotic tissue remodeling [[4], [5], [6], [7], [8]]. Importantly, radiation-induced dermatitis significantly impairs quality of life among cancer patients and survivors, yet treatment options are currently inadequate. Presently, standards of care recommend aqueous creams, saline soaks, and limiting irritant exposure including solar radiation to lessen the burden of radiation-induced dermatitis [1]; however, these methods only reduce pain and do not prevent initial burden. Therefore, development of novel molecular strategies for improved prevention of radiation-induced dermatitis might promise to benefit cancer patients in the near future.
The redox-sensitive transcription factor nuclear factor (erythroid-derived 2)-like 2 (NRF2) orchestrates major cellular defense mechanisms by transcriptional upregulation of Antioxidant Response Element (ARE) bearing genes involved in phase-II detoxification metabolism, glutathione synthesis, redox homeostasis, inflammation, and DNA repair [9,10]; thus, NRF2 has emerged as a promising molecular target for the prevention of tissue damage resulting from exposure to environmental electrophilic stressors (e.g. solar ultraviolet (UV) light and IR) [[11], [12], [13], [14]]. Recent studies strongly suggest a protective role for NRF2-mediated gene expression in the suppression of cutaneous photodamage induced by solar UV radiation, and NRF2 activation has been shown to protect cutaneous keratinocytes and fibroblasts against the cytotoxic effects of UVA and UVB [12,[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]]. Constitutive genetic NRF2 activation protects against acute photodamage and chronic photocarcinogenesis [27]; thus, pharmacological modulation of NRF2 has now attracted considerable attention as a novel approach for skin photoprotection [22,24,28,29]. Our own studies have substantiated the photoprotective effects of pharmacological NRF2 activation in cultured human skin cells, reconstructed epidermal skin, and murine exposure models, which can be attributed to NRF2-dependent upregulation of cellular glutathione level and antioxidant encoding genes (e.g. TXN, TXNRD1, SRXN1, PRDXs, GPXs, GCLC/GCLM (GCS)), upregulation of DNA repair enzyme encoding genes (e.g. OGG1, RAD51, TP53BP1), and increased skin barrier function through induction of structural components (e.g. LCEs, SPRP, KRT) [15,[23], [24], [25], [26],28,30,31]. Strikingly, NRF2 activation also occurs in response to exposure to IR, consistent with the crucial involvement of free radical/ROS formation in driving the oxidative and genotoxic stress that underlies IR-mediated tissue damage [[32], [33], [34], [35], [36], [37]]. Therefore, we tested the feasibility of NRF2 activation for skin radioprotection in a preclinical model, with the ultimate goal of translating said findings to benefit cancer patients receiving RT.
Extensive research has highlighted that induction of NRF2, and thus its cytoprotective target genes, can be utilized in therapeutic intervention to avert and/or repair damage to cells [38]. In prior studies, we have reported that the apocarotenoid bixin is a potent activator of the NRF2 signaling pathway in cultured human skin keratinocytes, that topical administration of bixin activates NRF2 with potent protective effects against solar UV-induced skin damage in SKH1 mice, and that bixin-induced suppression of photodamage is observable in Nrf2+/+ but not in Nrf2−/− mice, confirming the NRF2-dependence of bixin-based anti-oxidant/anti-inflammatory effects [25,26]. As there is a significant overlap between the cellular responses to nonionizing (e.g. UV light) and ionizing (e.g. γ-rays) radiation, as well as their cutaneous phenotypic outcomes (manifestation as sunburn or radiation-induced dermatitis, respectively), we pursued the hypothesis that topical application of bixin could prevent radiation-induced dermatitis.
To test the feasibility of this novel therapeutic approach, we first examined if loss of NRF2 in vitro and in vivo sensitizes skin keratinocytes to IR. Second, we tested whether IR-associated damage could be mitigated by pharmacological induction of the NRF2 signaling pathway via bixin treatment. Indeed, herein we show for the first time that topical application of the apocarotenoid bixin can suppress radiation-induced dermatitis via NRF2 induction in both in vitro and in vivo models.
2. Materials and methods
2.1. Cell culture
Skin keratinocytes (HaCaT) cells were purchased from American Type Culture Collection (ATCC) and were cultured in DMEM with l-Glutamine 4.5 g/L glucose and sodium pyruvate (Corning Cellgro) supplemented with 10% fetal bovine serum (Gibco) and 100 units/mL pen strep. Cells were incubated at 37 °C with 5% CO2. For knockdown, control (Qiagen [1027281]) or NRF2 (NFE2L2) siRNA (Thermo Fisher Scientfic [s9493] and Qiagen [S100657937]) were incubated with serum-free DMEM with HiPerfect (Qiagen) for 20 min prior to addition to HaCaT cells (final concentration 40 nM); cells were irradiated 72 h later.
2.2. Radiation
All radiation exposure was carried out by the Experimental Mouse Shared Resource (EMSR) at the University of Arizona using an isocentrically mounted external beam Co60 γ-source/teletherapy machine (Theratron, Atomic Energy of Canada limited (AECL). With the exception of the comet assay (10–40 Gy) and animal experiments (20–30 Gy), requiring higher radiation doses for the induction of biologically relevant damage endpoints, all cells received 4 Gy radiation.
2.3. Antibodies and reagents
Antibodies were purchased from Santa Cruz Biotechnology (NRF2, GCS, GAPDH), Cell Signaling Technologies (p-p53, γ-H2AX), and Thermo Fisher Scientific (DAPI). Secondary antibodies conjugated with horseradish peroxidase (HRP) were purchased from Sigma. Secondary fluorescent antibodies (Alexa Fluor 594) were purchased from Invitrogen. Bixin was obtained from Spectrum (CAS number: 6983-79-5) as previously described [26]. For animal experiments, bixin was dissolved in polyethelene glycol 400 (PEG400) (EMD Millipore). Thiazolyl Blue Tetrazolium (MTT) was purchased from Sigma and dissolved in phosphate-buffered saline (PBS). N-acetyl-l-cysteine (NAC) and D,L-buthionine-SR-sulfoximine (BSO) were obtained from Sigma, and tris(2-carboxyethly)phosphine hydrochloride (TCEP) was purchased from GoldBio.
2.4. Immunoblotting and immunofluorescence
For immunoblotting experiments, cells were collected in 1x Sample Buffer (50 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate [SDS], 10% glycerol, 100 mM dithiothreitol [DTT], 0.1% bromophenol blue) and boiled for 10 min. Cells were then sonicated using the Bioruptor (Diagenode) for 20 min. Samples were run on a 7.5% SDS-PAGE gel, then transferred to a nitrocellulose membrane (Prometheus). Membranes were blocked in 5% milk for 1 h, prior to incubation with primary antibody overnight at 4 °C. Membranes were washed 4 times for 15 min in 1x PBS then incubated with secondary antibody for 1 h in 5% milk. Following secondary incubation, membranes were washed with PBS (6 times, 10 min each), then developed using an enhanced chemiluminescent (ECL) horseradish peroxidase (HRP) reaction (Thermo Fisher Scientfic) and imaged by the Azure c600 (Azure Biosystems).
At 1 h post radiation (either 10 or 40 Gy), comet assay was started as outlined previously [39]. However, image analysis was done in ImageJ using the OpenComet Plugin; values shown represent average tail moment from individual comets selected across multiple images [40].
For indirect immunofluorescence, HaCaT cells were grown on glass cover slips (Fisher Scientific) to 70–90% confluence in 35-mm plastic cell culture dishes. At 1 h post radiation or times specified in Fig 4A, cells were fixed on cover slips using ice cold methanol for 20 min, washed with PBS 3 times, then incubated with (γ-H2AX) antibody diluted in 10% FBS in PBS for 1 h. Next, cover slips were washed 3 times in PBS, then incubated with a fluorescent secondary antibody (Alexa Fluor 594 [rabbit]) diluted in 10% FBS in PBS for 1 h. Cells were then mounted to glass slides using antifade mounting medium and imaged. All images were taken using the Zeiss Observer.Z1 microscope using Slidebook 4.2.0.11 computer software (Intelligent Imaging Innovations, Inc.).
Fig. 4.
Induction of NRF2 suppresses an early IR-induced keratinocyte genotoxic stress response. (a) Images of indirect immunofluorescence analysis of γ-H2AX at 0, 30, 60, 120, or 240 min post radiation in HaCaT cells treated with bixin (24 h) prior to IR exposure (4 Gy) (scale bar represents 10 μm) (inset: magnified nuclei). (b) Quantification of γ-H2AX foci per cell from (a) (n = 3 images). (c) Immunoblot analysis of p-p53 in HaCaT cells at 0, 30, 60, 120, and 240 min post radiation with and without bixin pretreatment. (d) Densitometry analysis of (d) (n = 3); (*p < 0.05). (e) Total glutathione levels in HaCaT cells at 72 h post siRNA (control or NRF2) transfection with bixin or NAC treatment (24 h) prior to measurement (n = 3). (f) Reactive oxygen species were measured 1 h post radiation in HaCaT cells that were transfected with siRNA 72 h prior to radiation and treated with bixin (24 h) prior to radiation (n = 3).
2.5. Cell viability
Cell viability was detected using an MTT assay. In a 96 well, 20 μL of a 2 mg/mL MTT in PBS solution was added directly to the cell culture media and allowed to incubate for 2 h. Media was then removed and isopropanol/HCl was added to cells and absorbance was measured at 570 nm via a SpectraMax iD5 Multi-Mode Microplate Reader (Molecular Devices). Confluence was determined via images taken by the IncuCyte (Essen Biosciences) and analyzed using ZOOM software (blue lines in images outline empty space); timepoints outlined in results/figure legends. Cells were counted using a hemocytometer.
2.6. Histology
Briefly, after harvesting, skin tissues were fixed in 10% formalin and embedded in paraffin. Staining for both hematoxylin and eosin (H&E) was done at 21–22 days post radiation, whereas GCS and γ-H2AX staining were performed at 1 h post radiation as described previously [26]. For both staining types, images were taken via a Nikon Eclipse 50i microscope using Nikon NIS Elements F 4.0 software.
2.7. Electron paramagnetic resonance (EPR)
EPR was performed as outlined previously [41]. Briefly, relative production of reactive oxygen species is represented as the nanomolar concentration of oxidized spin trap divided by the time of trap incubation, then normalized to the total milligrams of protein per well. At 30 min post radiation, cells were incubated with spin trap in the presence of metal chelators (200 μM CMH, 25 μM DF, and 5 μM DETC in filtered KREBS-HEPES buffer) (Noxygen) for 30 min prior to collection and measurement. Cells were harvested in 1x RIPA buffer (10 mM sodium phosphate [pH 7.2], 150 mM NaCl, 1% sodium deoxycholate, 2 mM EDTA, 0.1% SDS, 1% NP-40), and protein concentration was determined via BCA kit. Mouse skin was collected 1 h post radiation and relative levels of reactive oxygen species were determined as described above.
2.8. Glutathione-Glo assay
Glutathione levels were detected 24 h post pharmacological modulation [bixin (40 μM), NAC (500 μM), BSO (1 mM), or a cotreatment of bixin and BSO] and/or genetic modulation [NRF2 siRNA] using a GSH-Glo assay kit (Promega) as per the manufacturer’s protocol including the use of TCEP (1 mM).
2.9. Image analysis, quantifications, and statistics
Results are normalized to their respective controls. All image analysis was carried out using ImageJ software (NIH). Densitometry and erythema of mouse backs were measured via pixel density across an equal area of measurement. Epidermal thickness was measured via comparison to scale bars spanning the stained epidermis. A significant value was determined using t-test and is indicated by an ‘*’ and represents a p value of <0.05; no significance is indicated by use of ‘n.s.’. All values are represented as the mean ± Standard Error of the Mean (SEM).
2.10. Animal work
All animal studies in the manuscript were carried out in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and protocols were approved by the University of Arizona Institutional Animal Care and Use Committee. For knockout experiments, SKH1 Nrf2+/+ and SKH1 Nrf2−/−, between 8 and 12 weeks old, were irradiated with 20 Gy of radiation. Mice were placed in a manner where only the back skin was exposed to radiation, while the remainder of the body was protected via lead blocks. Post radiation mice were monitored and imaged for 22 d prior to sacrifice and collection of skin tissue for staining. For bixin experiments, SKH1 Nrf2+/+ mice between 8 and 12 weeks old were randomly allocated into either control (PEG400) or treatment [1% bixin in PEG400 (w/w)] groups. Mice received topical treatment 48 and 24 h prior to 30 Gy radiation exposure to the back skin exclusively. Mice were monitored and imaged for 21 d post radiation and then sacrificed for skin collection.
3. Results
3.1. Genetic ablation of NRF2 sensitizes skin to IR-induced dermatitis in vivo
First, to evaluate the role of NRF2 in the response to radiotherapy in vivo, we generated an SKH1 Nrf2−/− hairless mouse and monitored radiation-induced dermatitis, a phenotypic indicator of post-irradiation skin damage, in irradiated Nrf2+/+ and Nrf2−/− mice. Nrf2+/+ and Nrf2−/− mice received 20 Gy of γ-radiation specifically to the back and were monitored for the next 22 days (Fig. 1a). Images were taken starting at d 10 coinciding with the appearance of dermatitis. Our results show that Nrf2−/− mice developed a severity of radiation-induced dermatitis that surpassed that of Nrf2+/+ SKH1 mice, with knockout mice exhibiting a 2-fold increase in erythema over the 22 day observation period as compared to their wildtype counterparts (Fig. 1b–c). At d 22, mice were sacrificed and skin was collected and subjected to hematoxylin and eosin (H&E) staining to assess epidermal thickening, an indicator of actinic skin damage; Nrf2−/− mice had an approximately 10 μm (~4.5-fold) thicker epidermis than Nrf2+/+ mice (Fig. 1d–e). These data support a protective role for NRF2 in mitigating radiation-induced dermatitis, as loss of NRF2 enhances the inflammation and epidermal thickening caused by IR in vivo.
Fig. 1.
Genetic ablation of NRF2 sensitizes skin to radiation-induced dermatitis in vivo. (a) Scheme indicating experimental timeline of radiation, images taken, and sacrifice of animals. (b) Dorsal images of SKH1 Nrf2+/+ and Nrf2−/− mice at 0, 10, 13, 16, 19 d post radiation (20 Gy). (c) Quantification of dorsal erythema from (b) (n = 3). (d) At 22 d post radiation, mice were sacrificed, and back tissue was harvested and subjected to staining (scale bar represents 10 μm). (e) Using images from (d) epidermal thickness was quantified (n = 6); (*p < 0.05).
3.2. Loss of NRF2 sensitizes skin keratinocytes to IR-induced damage
Next, the effects of NRF2 status on radiation-induced dermatitis in vitro were assessed using an siRNA-based knockdown approach and subsequent measurement of sensitization of skin keratinocytes (HaCaT cells) to IR-induced cell death. Numerous prior studies have used HaCaT skin keratinocytes as a valid cellular model to study cutaneous effects of ionizing radiation [[42], [43], [44], [45], [46], [47]]. Specifically, at 24 h post radiation, cell confluence decreased approximately 20% in NRF2 knockdown HaCaT cells (Fig. 2a–b). The loss of viable cells was further confirmed using an MTT assay, which indicated that at 24 h post radiation, viability decreased by approximately 30% (Fig. 2c). Next, the modulatory role of NRF2 on the DNA damage response following IR was assessed; to this end, DNA double strand breaks were detected via examining γ-H2AX foci formation in irradiated HaCaT cells post siRNA knockdown of NRF2 (Fig. S1). As expected, 1 h post exposure, foci formation increased ~5.5-fold in irradiated control cells; however, knockdown of NRF2 increased foci formation nearly 8-fold compared to the non-irradiated control (Fig. 2d–e). Furthermore, knockdown of NRF2 enhanced activation of the IR-induced cellular DNA repair response as evidenced by an approximately 10-fold increase in p-p53 levels compared to an only ~6-fold increase in wildtype cells (Fig. 2f–g). To measure genotoxic stress, genomic integrity was assessed by alkaline gel electrophoresis (comet assay). At 1 h post radiation exposure, there was an approximately 2-fold increase in tail moment in NRF2 knockdown irradiated HaCaT cells as compared to control (Fig. 2h–i). Based on the established role of ROS in causing DNA damage in response to IR, and the known involvement of NRF2 in antagonizing ROS formation, the effect of NRF2 deficiency on sensitizing irradiated cells to formation and turnover of free radicals was assessed via electron paramagnetic resonance (EPR) spin trapping. As expected, loss of NRF2 itself in nonirradiated cells increased free radical levels slightly; however, when irradiated, these cells displayed nearly a 2-fold increase in ROS levels at 1 h post radiation (Fig. 2j). Overall, these data indicate that loss of NRF2 expression sensitizes skin keratinocytes to IR by increasing lethal DNA damage and ROS production.
Fig. 2.
Loss of NRF2 sensitizes skin keratinocytes to IR-induced damage. HaCaT cells were transiently transfected with either control or NRF2 siRNA for 72 h prior to radiation (4 Gy). (a) Representative images of cell confluence at 24 h post radiation, which were quantified in (b) (n = 8). (c) At 24 h post radiation viability was tested via MTT assay (n = 8). (d) To assess DNA damage, indirect immunofluorescence analysis was done on HaCaT cells 1 h post radiation for γ-H2AX (scale bar represents 10 μm) (inset shows magnified nuclei). (e) Number of γ-H2AX foci was quantified from the images in (d) (n = 3 images). (f) Immunoblot analysis showing DNA damage marker (p-p53) protein levels 1 h post radiation that was quantified in (g) (n = 3). (h) DNA damage as assessed via comet assay 1 h post radiation (10 Gy) that was quantified in (i) (n = 3–6 images). (j) Reactive oxygen species levels as measured 1 h post radiation via electron paramagnetic resonance spectroscopy (n = 3); (*p < 0.05).
3.3. Pharmacological induction of NRF2 protects skin keratinocytes from IR-induced damage
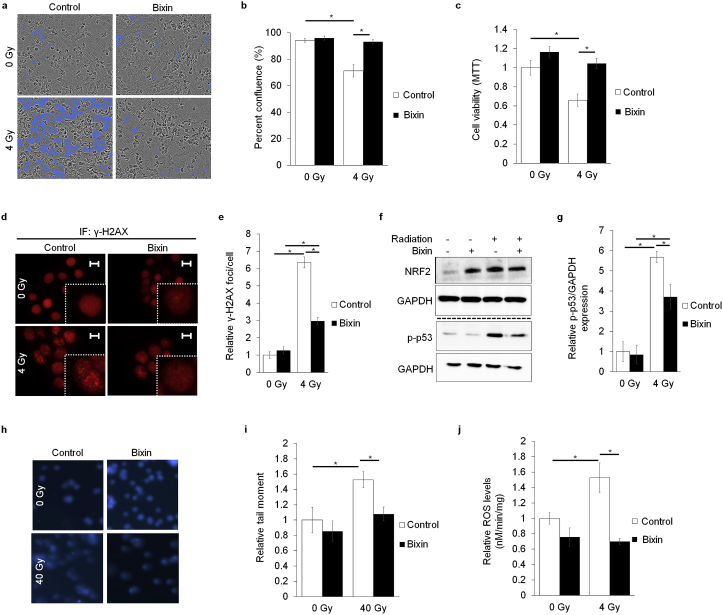
Next, after observing that loss of NRF2 sensitized skin to IR, the efficacy of pharmacological activation of NRF2 in protecting non-tumor tissue from the effects of radiotherapy was tested in HaCaT cells. Following our previous prototype studies, bixin, a potent topical NRF2 inducer with pronounced solar photoprotective activity when applied to mouse skin, was selected to assess IR-directed radioprotective efficacy of NRF2 modulation. Cell viability, DNA damage response, and ROS levels were measured as a function of bixin-dependent radioprotection. Our results demonstrate that bixin pretreatment significantly attenuated cell death 6 d post radiation (Fig. 3a–b). Additionally, bixin pretreatment prevented cell death over an extended period of time (up to 10 d post radiation exposure), as bixin treatment prevented the approximately 40% decrease in cell viability observed in untreated irradiated cells (Fig. 3c). While IR increased γ-H2AX foci formation approximately 6-fold in untreated HaCaT cells, pretreatment with bixin attenuated foci formation by more than 50% (Fig. 3d–e). Additionally, immunoblot analysis indicated that bixin-mediated induction of NRF2 attenuated IR-induced upregulation of p-p53 levels as compared to controls (Fig. 3f–g). Furthermore, bixin pretreatment suppressed IR-induced impairment of genomic integrity as assessed by comet analysis (Fig. 3h–i); likewise, IR-induced ROS formation was attenuated by bixin pretreatment (Fig. 3j). Overall, these data indicate that bixin-induced NRF2 activation attenuates IR-damage in cultured human keratinocytes.
Fig. 3.
Pharmacological induction of NRF2 protects skin keratinocytes from IR-induced damage. HaCaT cells were treated with 40 μM bixin (24 h) prior to radiation (4 Gy). (a) Representative images of cell confluence 6 d post radiation that were quantified in (b) (n = 3). (c) Cell viability measured via MTT at 10 d post radiation (n = 5–6). (d) Images of γ-H2AX foci measured via indirect immunofluorescence analysis 1 h post radiation (scale bar represents 10 μm) (inset shows magnified nuclei). (e) Quantification of foci per cell from (d) (n = 3 images). (f) Immunoblot analysis of DNA damage marker (p-p53) at 1 h post radiation; quantified in (g) (n = 3). (h) Comet images 1 h post radiation (40 Gy). (i) Quantification of tail moment from (h) (n = 3–4 images). (j) Reactive oxygen species as detected 1 h post radiation using electron paramagnetic resonance spectroscopy (n = 3); (*p < 0.05).
3.4. Induction of NRF2 suppresses an early IR-induced keratinocyte genotoxic stress response
As NRF2 regulates a wide array of target genes that are involved in DNA repair, but also key redox defense systems, our further examination focused on mechanisms that might contribute to NRF2-control of cutaneous radiation damage [9]. First, occurrence of IR-induced γ-H2AX foci was examined, demonstrating that number of foci per cell was significantly suppressed in cells pretreated with bixin compared to untreated cells, an observation applicable to the entire time course of the experiment (Fig. 4a–b). Additionally, bixin pretreatment affected the DNA damage response in irradiated HaCaT cells, as p-p53 levels were suppressed throughout each timepoint post radiation in bixin pretreated cells compared to control cells receiving IR (Fig. 4c). Furthermore, quantitative immunoblot analysis indicated a pronounced attenuation of IR-induced p-p53 levels as a result of bixin treatment up to 240 min post radiation (Fig. 4d). To examine if an NRF2-dependent increase in cellular glutathione (GSH) levels could contribute to radioprotection, GSH levels following bixin or NAC preincubation were assessed in HaCaT cells transiently transfected with either control or NRF2 siRNA. After 24 h exposure, GSH levels increased approximately 1.5-fold in both bixin and NAC treated control siRNA skin keratinocytes; however, when NRF2 was knocked down, bixin was unable to increase GSH levels relative to control whereas NAC maintained its ability to elevate GSH (Fig. 4e). To further explore if the protective effects of bixin occur through the NRF2 signaling pathway impacting cellular oxidative stress, IR-induced ROS levels were measured following NRF2 knockdown plus bixin pretreatment. While bixin was able to reduce ROS levels following radiation in control siRNA transfected HaCaT cells, the ability of bixin to attenuate ROS levels was lost when NRF2 was knocked down (Fig. 4f). Further confirmation of NRF2-dependent protective effects of bixin was obtained by assessment of viability and DNA damage (Fig. S2). Furthermore, the protective effects of bixin were predominately attributed to increased GSH levels as BSO, a pharmacological inhibitor of GSH synthesis, prevented bixin protection of skin keratinocytes against radiation-induced DNA damage (Fig. S3). Thus, bixin-dependent upregulation of NRF2 leading to increased cellular GSH levels might be involved in diminishing initial DNA damage inflicted by IR-induced ROS.
3.5. Topical application of bixin prevents radiation-induced dermatitis
Next, to test if pharmacological induction of NRF2 could prevent radiation-induced dermatitis in vivo, topical application of bixin, an established NRF2 inducer known to activate the pathway in murine skin, was employed. To this end, wildtype SKH1 (Nrf2+/+) mice received topical application of either PEG400 (control) or bixin to skin 48 and 24 h prior to 30 Gy radiation exposure to the back (Fig. 5a). Images taken at 7 d were used as a baseline control because no dermatitis had yet developed; however, at 10, 13, and 16 d post radiation severity of erythema was increased up to 3-fold in control mice compared to bixin-pretreated mice (Fig. 5b–c). When mouse skin was examined at d 21, histochemical analysis indicated a ~4-fold epidermal thickening observable in carrier-treated versus bixin-treated mice (Fig. 5d–e). Additionally, in an independent acute exposure model (30 Gy), at 1 h post IR, an increased γ-H2AX nuclear staining was detectable in control epidermis, consistent with radiation damage-induced DNA strand breaks, that was attenuated by bixin-pretreatment (Fig. 5f). Furthermore, in this experiment, immunohistochemical staining analysis indicated that at 1 h post radiation, both irradiated and nonirradiated bixin pretreated skin had increased GCS levels, compared to control treated mice (Fig. 5g). This indicates that pretreatment with bixin promotes upregulation of NRF2 target gene expression to confer protection from radiation. It should be noted that in the context of this analysis skin tissues were harvested 1 h after IR-exposure, and therefore, at the time point, while γ-H2AX reaches a peak, NRF2 and its target genes are not yet expected to be notably induced by radiation.
Fig. 5.
Topical application of bixin prevents radiation-induced dermatitis. (a) Scheme of timeline indicating that SKH1 mice received topical dorsal application of either PEG400 (control) or 1% bixin (w/w) 48 and 24 h prior to radiation exposure (30 Gy). Images were taken of animals 7, 10, 13, 16 d post radiation, prior to sacrifice and tissue collection (d 21). (b) Dorsal images of SKH1 mice (7, 10, 13, 16 d post radiation). (c) Quantification of erythema of images in (b) (n = 5). (d) Representative images (d 21 post radiation) of back tissue subjected to H&E staining (scale bar: 10 μm). (e) Images from (d) were analyzed using ImageJ determining epidermal thickness (n = 3). At 1 h post radiation, irradiated and nonirradiated skin was harvested for IHC staining of (f) γ-H2AX and (g) GCS (scale bars represents 20 μm). (h) At 1 h post radiation, skin was harvested from the backs of mice and reactive oxygen species were measured via electron paramagnetic resonance spectroscopy (n = 3); (*p < 0.05).
As an independent measure of cutaneous free radical and ROS burden, EPR spin trapping was performed using skin tissue harvested 1 h post radiation (Fig. 5h). Indeed, quantitative comparison of spin trap EPR-signal intensity indicated that skin from bixin-pretreated mice displayed reduced ROS levels (~2-fold attenuation of signal intensity) as compared to irradiated control skin. Overall, these murine experiments suggest that topical application of bixin could serve as a protectant against IR-induced cutaneous damage.
4. Discussion
The concept of NRF2-directed topical radioprotection and prevention of radiation-induced dermatitis has remained largely unexplored [1,[33], [34], [35], [36], [37],[48], [49], [50]]. Herein, we have elucidated the critical role that NRF2 plays in mitigating IR-induced damage and tested the efficacy of topical application of bixin to negate the effects of RT in non-tumor tissue, specifically radiation-induced dermatitis. Building on its excellent safety record as an FDA-approved food additive, bixin has demonstrated antigenotoxic and antioxidant cytoprotective activities, and topical use of bixin has been shown previously to display anti-inflammatory activity and enhance skin wound healing [25]. Bixin as an experimental NRF2 inducer is of particular interest because of its water solubility, lack of provitamin A activity, and impressive safety record as documented extensively in mice and humans [28,[51], [52], [53], [54], [55]]. Importantly, bixin ADI (acceptable daily intake) over a lifetime without an appreciable health risk surpasses that of any other carotenoid approved as a food additive [56]. Interestingly, other prior studies have presented experimental evidence that NRF2 activators may protect against radiation-induced dermatitis; however, these studies utilize synthetic triterpenoid NRF2 activators, whereas in our study we repurposed an FDA approved food and cosmetic additive [37,49]. In our own studies, bixin was identified as the result of a screen for diet-derived small molecule NRF2 activators targeting oxidative stress and redox dysregulation in epithelial cells [15,24,26,28,57,58]. This current research examines for the first time the efficacy of bixin-based topical activation of cutaneous NRF2 for skin radioprotection and suppression of radiation-induced dermatitis. Additionally, the use of our established genetic mouse model unequivocally demonstrates mechanistic involvement of topical NRF2 activation in bixin-based skin radioprotection, paving the way toward translational development of this FDA-approved phytochemical.
Overall, this study demonstrates that modulation of NRF2 is a critical determinant of the cutaneous IR response. Specifically, genetic ablation of NRF2 worsens radiation-induced dermatitis in vivo, suggesting that NRF2 is necessary to combat the toxicities of radiotherapy (Fig. 1). Moreover, genetic downregulation of NRF2 renders skin keratinocytes sensitive to DNA damage, oxidative damage, and IR-induced cell death caused by radiotherapy (Fig. 2). Oppositely, pretreatment of skin keratinocytes with bixin lowered initial DNA damage, ROS, and cell death caused by radiation, thus suggesting the protective effects of NRF2 induction in skin (Fig. 3). Specifically, the results demonstrated that bixin pretreatment increased GSH levels and lowered overall DNA damage response following IR exposure (Fig. 4, S2, S3); thus, the NRF2 dependent radioprotective effects observed in our experiments may be attributed to antioxidant modulation antagonizing oxidative damage associated with IR. Remarkably, topical application of bixin was effective at preventing collateral skin damage that occurs as a consequence of IR exposure, mitigating IR-induced ROS levels and epidermal thickening in vivo (Fig. 5). By reducing dose limiting cutaneous toxicity, topical bixin application might not only increase patient quality of life but may also allow the utilization of a higher dose regimen improving therapeutic outcome of RT. In addition to substantiating radioprotection, these data are of further clinical relevance as increased ROS levels are associated with several other complications, including secondary cancer development, thus, negation of ROS via NRF2 induction supports the therapeutic potential of bixin [59]. While our data indicate that loss of GSH is critical in mediating the IR-induced DNA damage response (Fig. S3), IR can also have a non-ROS dependent genotoxic effect; NRF2 control of DNA repair factors is well substantiated, and future considerations should therefore explore the specific role of NRF2 upregulation in facilitating DNA repair following IR exposure [9].
Interestingly, studies have shown that systemic upregulation of NRF2 can be radioprotective [60], while our own previous work has indicated that bixin can upregulate NRF2 systemically causing skin photoprotection [28,61]. However, in the context of this study, topical bixin administration seems preferable as it could limit NRF2 induction to the skin, thus minimizing NRF2 modulation throughout the body, and ultimately maintaining the desired sensitivity of specific tissues to radiotherapy [[62], [63], [64]]. While IR effects on cultured and epidermal keratinocytes are the primary focus of these experiments, the role of inflammatory factors including immune cell infiltration and response, all of which might be subject to modulation by NRF2, deserves further studies as it was not addressed in this prototype investigation. Future work should focus on the systemic upregulation of NRF2 as a whole-body protectant against IR, with particular observance of the resistance of cancer cells with and without pharmacological NRF2 activation. As tumors with constitutively active NRF2 should be unresponsive to NRF2-inducing pharmacological intervention, systemic administration of bixin could serve as a whole-body protectant against IR without further desensitizing the tumor to RT. Thus currently, modulation of NRF2 levels continues to have a context- and temporal-dependent relationship in cancer treatment [65].
Taken together, our data make a link between the cytoprotective effects of bixin and mitigation of radiation-induced dermatitis. As NRF2 is a crucial factor in redox homeostasis and cell survival, we suggest that by upregulating NRF2 via bixin prior to IR exposure, we can lower initial DNA damage and ROS levels in the cell, ultimately preventing cell death. Our in vivo data provides stark evidence that modulation of the NRF2 signaling pathway correlates with radiation-induced dermatitis. We are the first to establish that bixin, an FDA-approved food additive, acts as a radioprotectant against IR in skin. Due to its limited off-target effects and high ADI, topical bixin may represent a promising translational approach to mitigate radiation-induced dermatitis that might benefit cancer patients receiving RT.
Declaration of competing interest
All authors declare that there are no conflicts of interest to disclose.
Acknowledgement
We would like to acknowledge the Experimental Mouse Shared Resource at the University of Arizona, specifically Gillian Paine-Murrieta. Additionally, the authors are funded by the following grants from the National Institutes Health: ES007091[C.J.S.], CA009213 [J.P.], ES031575 [D.D.Z.], ES004940 [D.D.Z.], ES029579 [G.T.W.], ES006694 [center grant], DK109555 [D.D.Z.], CA229112 [G.T.W.], CA229418 [G.T.W.], CA230949 [G.T.W.] and CA023074 [center grant].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101714.
Contributor Information
Donna D. Zhang, Email: dzhang@pharmacy.arizona.edu.
Georg T. Wondrak, Email: wondrak@pharmacy.arizona.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article
figs1.
Figure S1: γ-H2AX staining is confined to nuclei as substantiated by DAPI co-staining. (a) Exemplary DAPI co-staining was performed together with γ-H2AX visualization. Overlay analysis demonstrates γ-H2AX is confined to the nucleus (scale bar represents 10 μm).
figs2.
Figure S2: Bixin cannot prevent IR-induced damage when NRF2 is knocked down. HaCaT cells were treated with bixin (24 h) prior to radiation after being transiently transfected with either control or NRF2 siRNA for 72 h prior to radiation (4 Gy). (a) Representative images at 24 h post radiation of cell confluence, that were quantified in (b) (n = 3). (c) At 1 h post radiation, indirect immunofluorescence analysis was done on HaCaT cells for γ-H2AX (scale bar represents 10 μm) (inset shows magnified nuclei). (d) Number of γ-H2AX foci per cells was quantified from (c) (n = 3 images). (e) Immunoblot analysis of NRF2 24 h post bixin treatment in HaCaT cells transfected with control or NRF2 siRNA for 72 h.
figs3.
Figure S3: Bixin cannot prevent IR-induced damage when GSH synthesis is inhibited. HaCaT cells were treated with bixin, BSO, or a cotreatment of bixin and BSO (24 h) prior to radiation (4 Gy). (a) At 1 h post radiation, indirect immunofluorescence analysis assessed γ-H2AX foci (scale bar represents 10 μm) (inset shows magnified nuclei). (b) Number of γ-H2AX foci were quantified per cell from (a) images (n = 3 images). (c) Total GSH levels were detected 24 h post treatment (n = 3)
References
- 1.Leventhal J., Young M.R. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park) 2017;31(12):94–99. 885-7. [PubMed] [Google Scholar]
- 2.Jaffray D.A., Gospodarowicz M.K. Radiation therapy for cancer. In: Gelband H., Jha P., Sankaranarayanan R., Horton S., editors. third ed. vol. 3. 2015. (Cancer: Disease Control Priorities). Washington (DC) [Google Scholar]
- 3.Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin. Cosmet. Invest. Dermatol. 2016;9:473–482. doi: 10.2147/CCID.S94320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ame J.C., Fouquerel E., Gauthier L.R., Biard D., Boussin F.D., Dantzer F. Radiation-induced mitotic catastrophe in PARG-deficient cells. J. Cell Sci. 2009;122(Pt 12):1990–2002. doi: 10.1242/jcs.039115. [DOI] [PubMed] [Google Scholar]
- 5.Bucci B., Misiti S., Cannizzaro A., Marchese R., Raza G.H., Miceli R. Fractionated ionizing radiation exposure induces apoptosis through caspase-3 activation and reactive oxygen species generation. Anticancer Res. 2006;26(6B):4549–4557. [PubMed] [Google Scholar]
- 6.Daly M.J. Death by protein damage in irradiated cells. DNA Repair. 2012;11(1):12–21. doi: 10.1016/j.dnarep.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Iliakis G. The role of DNA double strand breaks in ionizing radiation-induced killing of eukaryotic cells. Bioessays. 1991;13(12):641–648. doi: 10.1002/bies.950131204. [DOI] [PubMed] [Google Scholar]
- 8.Terakado N., Shintani S., Nakahara Y., Mihara M., Tomizawa K., Suzuki K. Expression of Cu,Zn-SOD, Mn-SOD and GST-pi in oral cancer treated with preoperative radiation therapy. Oncol. Rep. 2000;7(5):1113–1117. doi: 10.3892/or.7.5.1113. [DOI] [PubMed] [Google Scholar]
- 9.Dodson M., de la Vega M.R., Cholanians A.B., Schmidlin C.J., Chapman E., Zhang D.D. Modulating NRF2 in disease: timing is everything. Annu. Rev. Pharmacol. Toxicol. 2019;59:555–575. doi: 10.1146/annurev-pharmtox-010818-021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., Katoh Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T., Motohashi H., Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Saw C.L., Huang M.T., Liu Y., Khor T.O., Conney A.H., Kong A.N. Impact of Nrf2 on UVB induced skin inflammation/photoprotection and photoprotective effect of sulforaphane. Mol. Carcinog. 2011;50:479–486. doi: 10.1002/mc.20725. [DOI] [PubMed] [Google Scholar]
- 13.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schafer M., Werner S. Nrf2-A regulator of keratinocyte redox signaling. Free Radic. Biol. Med. 2015;88:243–252. doi: 10.1016/j.freeradbiomed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Wondrak G.T., Cabello C.M., Villeneuve N.F., Zhang S., Ley S., Li Y., Sun Z., Zhang D.D. Cinnamoyl-based Nrf2-activators targeting human skin cell photo-oxidative stress. Free Radic. Biol. Med. 2008;45:385–395. doi: 10.1016/j.freeradbiomed.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirota A., Kawachi Y., Itoh K., Nakamura Y., Xu X., Banno T., Takahashi T., Yamamoto M., Otsuka F. Ultraviolet A irradiation induces NF-E2-related factor 2 activation in dermal fibroblasts: protective role in UVA-induced apoptosis. J. Invest. Dermatol. 2005;124:825–832. doi: 10.1111/j.0022-202X.2005.23670.x. [DOI] [PubMed] [Google Scholar]
- 17.Dinkova-Kostova A.T., Jenkins S.N., Fahey J.W., Ye L., Wehage S.L., Liby K.T., Stephenson K.K., Wade K.L., Talalay P. Protection against UV-light-induced skin carcinogenesis in SKH-1 highrisk mice by sulforaphane-containing broccoli sprout extracts. Canc. Lett. 2006;240:243–252. doi: 10.1016/j.canlet.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Benedict A.L., Knatko E.V., Dinkova-Kostova A.T. The indirect antioxidant sulforaphane protects against thiopurine-mediated photooxidative stress. Carcinogenesis. 2012;33:2457–2466. doi: 10.1093/carcin/bgs293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber F., Mayer H., Lengauer B., Mlitz V., Sanders J.M., Kadl A., Bilban M., de Martin R., Wagner O., Kensler T.W., Yamamoto M., Leitinger N., Tschachler E. NF-E2- related factor 2 regulates the stress response to UVA-1-oxidized phospholipids in skin cells. Faseb. J. 2010;24:39–48. doi: 10.1096/fj.09-133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian F.F., Zhang F.F., Lai X.D., Wang L.J., Yang L., Wang X., Singh G., Zhong J.L. Nrf2-mediated protection against UVA radiation in human skin keratinocytes. Biosci Trends. 2011;5:23–29. doi: 10.5582/bst.2011.v5.1.23. [DOI] [PubMed] [Google Scholar]
- 21.Hirota A., Kawachi Y., Yamamoto M., Koga T., Hamada K., Otsuka F. Acceleration of UVBinduced photoageing in nrf2 gene-deficient mice. Exp. Dermatol. 2011;20:664–668. doi: 10.1111/j.1600-0625.2011.01292.x. [DOI] [PubMed] [Google Scholar]
- 22.Kalra S., Knatko E.V., Zhang Y., Honda T., Yamamoto M., Dinkova-Kostova A.T. Highly potent activation of Nrf2 by topical tricyclic bis(cyano enone): implications for protection against UV radiation during thiopurine therapy. Cancer Prev Res (Phila) 2012;5:973–981. doi: 10.1158/1940-6207.CAPR-12-0041. [DOI] [PubMed] [Google Scholar]
- 23.Schafer M., Dutsch S., auf dem Keller U., Navid F., Schwarz A., Johnson D.A., Johnson J.A., Werner S. Nrf2 establishes a glutathione-mediated gradient of UVB cytoprotection in the epidermis. Genes Dev. 2010;24:1045–1058. doi: 10.1101/gad.568810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao S., Justiniano R., Zhang D.D., Wondrak G.T. The Nrf2-inducers tanshinone I and dihydrotanshinone protect human skin cells and reconstructed human skin against solar simulated UV. Redox Biol. 2013;1:532–541. doi: 10.1016/j.redox.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojo de la Vega M., Krajisnik A., Zhang D.D., Wondrak G.T. Targeting NRF2 for improved skin barrier function and photoprotection: focus on the achiote-derived apocarotenoid bixin. Nutrients. 2017;9:1371. doi: 10.3390/nu9121371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rojo de la Vega M., Zhang D.D., Wondrak G.T. Topical bixin confers NRF2-dependent protection against photodamage and hair graying in mouse skin. Front. Pharmacol. 2018;9:287. doi: 10.3389/fphar.2018.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knatko E.V., Ibbotson S.H., Zhang Y., Higgins M., Fahey J.W., Talalay P., Dawa R., Ferguson J., Huang J.T., Clarke R., Zheng S., Saito A., Kalra S., Benedict A.L., Honda T., Proby C.M., Dinkova-Kostova A.T. Nrf2 activation protects against solar- simulated ultraviolet radiation in mice and humans. Cancer Prev Res (Phila) 2015;8(6):475–486. doi: 10.1158/1940-6207.CAPR-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao S., Park S.L., Rojo de la Vega M., Zhang D.D., Wondrak G.T. Systemic administration of the apocarotenoid bixin protects skin against solar UV-induced damage through activation of NRF2. Free Radic. Biol. Med. 2015;89:690–700. doi: 10.1016/j.freeradbiomed.2015.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chun K.S., Kundu J., Kundu J.K., Surh Y.J. Targeting Nrf2-Keap1 signaling for chemoprevention of skin carcinogenesis with bioactive phytochemicals. Toxicol. Lett. 2014;229:73–84. doi: 10.1016/j.toxlet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Lieder F., Reisen F., Geppert T., Sollberger G., Beer H.D., auf dem Keller U., Schafer M., Detmar M., Schneider G., Werner S. Identification of UV-protective activators of nuclear factor erythroid-derived 2-related factor 2 (Nrf2) by combining a chemical library screen with computer-based virtual screening. J. Biol. Chem. 2012;287:33001–33013. doi: 10.1074/jbc.M112.383430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schafer M., Farwanah H., Willrodt A.H., Huebner A.J., Sandhoff K., Roop D., Hohl D., Bloch W., Werner S. Nrf2 links epidermal barrier function with antioxidant defense. EMBO Mol. Med. 2012;4:364–379. doi: 10.1002/emmm.201200219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischer N., Seo E.J., Efferth T. Prevention from radiation damage by natural products. Phytomedicine. 2018;47:192–200. doi: 10.1016/j.phymed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 33.McDonald J.T., Kim K., Norris A.J., Vlashi E., Phillips T.M., Lagadec C., Della Donna L., Ratikan J., Szelag H., Hlatky L., McBride W.H. Ionizing radiation activates the Nrf2 antioxidant response. Canc. Res. 2010;70:8886–8895. doi: 10.1158/0008-5472.CAN-10-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S.B., Pandita R.K., Eskiocak U., Ly P., Kaisani A., Kumar R., Cornelius C., Wright W.E., Pandita T.K., Shay J.W. Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2949–E2955. doi: 10.1073/pnas.1207718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sekhar K.R., Freeman M.L. Nrf2 promotes survival following exposure to ionizing radiation. Free Radic. Biol. Med. 2015;88:268–274. doi: 10.1016/j.freeradbiomed.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cameron B.D., Sekhar K.R., Ofori M., Freeman M.L. The role of Nrf2 in the response to normal tissue radiation injury. Radiat. Res. 2018;190:99–106. doi: 10.1667/RR15059.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagami Y., Masuda K. A novel Nrf2 activator from microbial transformation inhibits radiation induced dermatitis in mice. J. Radiat. Res. 2016;57:567–571. doi: 10.1093/jrr/rrw039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu X., Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat. Res. 2005;591(1–2):93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Roberts M.J., Wondrak G.T., Laurean D.C., Jacobson M.K., Jacobson E.L. DNA damage by carbonyl stress in human skin cells. Mutat. Res. 2003;522(1–2):45–56. doi: 10.1016/s0027-5107(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 40.Gyori B.M., Venkatachalam G., Thiagarajan P.S., Hsu D., Clement M.V. OpenComet: an automated tool for comet assay image analysis. Redox Biol. 2014;2:457–465. doi: 10.1016/j.redox.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dodson M., de la Vega M.R., Harder B., Castro-Portuguez R., Rodrigues S.D., Wong P.K. Low-level arsenic causes proteotoxic stress and not oxidative stress. Toxicol. Appl. Pharmacol. 2018;341:106–113. doi: 10.1016/j.taap.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behrends U., Peter R.U., Hintermeier-Knabe R., Eissner G., Holler E., Bornkamm G.W., Caughman S.W., Degitz K. Ionizing radiation induces human intercellular adhesion moleule-1 in vitro. J. Invest. Dermatol. 1994 Nov;103(5):726–730. doi: 10.1111/1523-1747.ep12398607. [DOI] [PubMed] [Google Scholar]
- 43.Petit-Frère C., Capulas E., Lyon D.A., Norbury C.J., Lowe J.E., Clingen P.H., Riballo E., Green M.H., Arlett C.F. Poptosis and cytokine release induced by ionizing or ultraviolet B radiation in primary and immortalized human keratinocytes. Carcinogenesis. 2000;21(6):1087–1095. [PubMed] [Google Scholar]
- 44.Baghdoyan S., Lamartine J., Castel D., Pitaval A., Roupioz Y., Franco N., Duarte M., Martin M.T., Gidrol X. Id2 reverses cell cycle arrest indiced b {gamma}-irradiation in human HaCaT keratinocytes. J. Biol. Chem. 2005 Apr 22;280(16):15836–15841. doi: 10.1074/jbc.M414216200. [DOI] [PubMed] [Google Scholar]
- 45.Trémezaygues L., Seifert M., Vogt T., Tilgen W., Reichrath J. 1,25-dihydroxyvitamin D3 modulate effects of ionizing radiation (IR) on human keratnocytes: in vitro analysis of cell viability/proliferation, DNA-damage and -repair. J. Steroid Biochem. Mol. Biol. 2010 Jul;121(1–2):324–327. doi: 10.1016/j.jsbmb.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Curtis J.J., Vo N.T.K., Seymour C.B., Mothersill C.E. Serotinin and 5-HT3 receptors sensitize human skin cells to direct irradiation cell death but not to soluble radiation-induced bystander signals. Environ. Res. 2020 Jan;180:108807. doi: 10.1016/j.envres.2019.108807. [DOI] [PubMed] [Google Scholar]
- 47.Qian X., Wang Z., Ning J., Qin C., Gao L., He N., Lin D., Zhou Z., Li G. Protecting HaCaT cells from ionizing radiaton using persimmon tannin-Aloe gel composite. Pharm. Biol. 2020 Dec;58(1):510–517. doi: 10.1080/13880209.2020.1767158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan R.J., Webster J., Chung B., Marquart L., Ahmed M., Garantziotis S. Prevention and treatment of acute radiation-induced skin reactions: a systematic review and meta- analysis of randomized controlled trials. BMC Canc. 2014;14(53) doi: 10.1186/1471-2407-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reisman S.A., Lee C.Y., Meyer C.J., Proksch J.W., Sonis S.T., Ward K.W. Topical application of the synthetic triterpenoid RTA 408 protects mice from radiation-induced dermatitis. Radiat. Res. 2014;181:512–520. doi: 10.1667/RR13578.1. [DOI] [PubMed] [Google Scholar]
- 50.Xue J., Yu C., Sheng W., Zhu W., Luo J., Zhang Q., Yang H., Cao H., Wang W., Zhou J., Wu J., Cao P., Chen M., Ding W.Q., Cao J., Zhang S. The nrf2/GCH1/BH4 Axis Ameliorates RadiationInduced skin injury by modulating the ROS cascade. J. Invest. Dermatol. 2017;137:2059–2068. doi: 10.1016/j.jid.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 51.Ulbricht C., Windsor R.C., Brigham A., Bryan J.K., Conquer J., Costa D., Giese N., Guilford J., Higdon E.R., Holmes K., Isaac R., Jingst S., Kats J., Peery L., Rusie E., Savinainen A., Schoen T., Stock T., Tanguay-Colucci S., Weissner W. An evidence- based systematic review of annatto (Bixa orellana L.) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2012;9:57–77. doi: 10.3109/19390211.2012.653530. [DOI] [PubMed] [Google Scholar]
- 52.Stohs S.J. Safety and efficacy of Bixa orellana (achiote, annatto) leaf extracts. Phytother Res. 2014;28:956–960. doi: 10.1002/ptr.5088. [DOI] [PubMed] [Google Scholar]
- 53.Levy L.W., Regalado E., Navarrete S., Watkins R.H. Bixin and norbixin in human plasma: determination and study of the absorption of a single dose of Annatto food color. Analyst. 1997;122:977–980. doi: 10.1039/a701304c. [DOI] [PubMed] [Google Scholar]
- 54.Junior A.C., Asad L.M., Oliveira E.B., Kovary K., Asad N.R., Felzenszwalb I. Antigenotoxic and antimutagenic potential of an annatto pigment (norbixin) against oxidative stress. Genet. Mol. Res. 2005;4:94–99. [PubMed] [Google Scholar]
- 55.WHO Evaluation of certain food additives and contaminants. World Health Organ Tech Rep Ser. 2013;983:1–75. [PubMed] [Google Scholar]
- 56.WHO Evaluation of certain food additives and contaminants. Thirty-fifth report of the joint FAO/WHO expert committee on food additives. Tech Rep Ser. 1990;789:1–48. [PubMed] [Google Scholar]
- 57.Long M., Tao S., Rojo de la Vega M., Jiang T., Wen Q., Park S.L., Zhang D.D., Wondrak G.T. Nrf2-dependent suppression of azoxymethane/dextran sulfate sodium-induced colon carcinogenesis by the cinnamon-derived dietary factor cinnamaldehyde. Cancer Prev Res (Phila) 2015;8:444–454. doi: 10.1158/1940-6207.CAPR-14-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Long M., Rojo de la Vega M., Wen Q., Bharara M., Jiang T., Zhang R., Zhou S., Wong P.K., Wondrak G.T., Zheng H., Zhang D.D. An essential role of NRF2 in diabetic wound healing. Diabetes. 2015;65(3):780–793. doi: 10.2337/db15-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liao Z., Chua D., Tan N.S. Reactive oxygen species: a volatile driver of field cancerization and metastasis. Mol. Canc. 2019;18(1):65. doi: 10.1186/s12943-019-0961-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das U., Manna K., Khan A., Sinha M., Biswas S., Sengupta A. Ferulic acid (FA) abrogates gamma-radiation induced oxidative stress and DNA damage by up-regulating nuclear translocation of Nrf2 and activation of NHEJ pathway. Free Radic. Res. 2017;51(1):47–63. doi: 10.1080/10715762.2016.1267345. [DOI] [PubMed] [Google Scholar]
- 61.Tao S., Rojo de la Vega M., Quijada H., Wondrak G.T., Wang T., Garcia J.G. Bixin protects mice against ventilation-induced lung injury in an NRF2-dependent manner. Sci. Rep. 2016;6:18760. doi: 10.1038/srep18760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zimta A.A., Cenariu D., Irimie A., Magdo L., Nabavi S.M., Atanasov A.G., Berindan-Neagoe I. The role of Nrf2 activity in cancer development and progression. Cancers. 2019;8(11):1755. doi: 10.3390/cancers11111755. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klinge S.A., Sawyer G.A. Effective and safety of topical versus oral nonsteroidal anti- inflammatory drugs: a comprehensive review. Phys Sportsmed. 2013;41(2):64–74. doi: 10.3810/psm.2013.05.2016. [DOI] [PubMed] [Google Scholar]
- 64.Wang W., Lang J. Strategies to optimize radiotherapy based on biological responses of tumor and normal tissue. Exp Ther Med. 2012;4(2):175–180. doi: 10.3892/etm.2012.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sporn M.B., Liby K.T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Canc. 2012;12(8):564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]