Abstract
Objective
This prospective study aims to evaluate the efficacy of Continuous upper arm brachial block (modified interscalene block) with an arthroscopic capsular release in the outcome of resistant frozen shoulder cases.
Methods
We studied 123 patients who underwent arthroscopic capsular release and subacromial decompression for resistant frozen shoulder cases between June 2016 and July 2019. Postoperative analgesia was provided with Continuous upper arm brachial block and ambulatory patient-controlled analgesia pump for 2–3 weeks. The patients were started on regular physiotherapy on the first postoperative day. All the patients were followed up at 3rd week, 6th week, 3rdmonth, 6th month, 1st year, and 2nd year with VAS and Constant-Murley scores.
Results
At a mean follow-up period of 18 months, there was a statistically significant improvement in the range of motion, VAS scores, and Constant-Murley scores postoperatively (p < .01). None of the cases required postoperative opioid administration for pain control. Minor neurological complications like recurrent laryngeal nerve palsy and Horner's syndrome were seen in few cases that resolved with titration of the drug dose.
Conclusion
Our study verifies the use of continuous upper arm brachial block (CUABB) with a portable infusion pump for 2–3 weeks in arthroscopic capsular release for resistant frozen shoulder cases. It significantly reduced postoperative pain in the initial two weeks that aided with early recovery of the shoulder movements and functions without an increased incidence of acute or chronic neurologic complications.
Keywords: Capsular release, Frozen shoulder, Modified interscalene block
1. Introduction
Shoulder pathologies are notorious for producing pain. Though newer strategies and methods have evolved, the treatment of frozen shoulder remains a mystery for orthopedic surgeons. Frozen shoulder is a benign, self-limiting condition that resolves spontaneously in 2–3 years in the majority, but some patients remain symptomatic and disabled for many years after the onset of disease.1 Resistant frozen shoulder is refers to the group of cases who fails to show any significant clinical improvement after undergoing at least 3 months of nonsurgical therapy. Only a few patients can afford or will accept months of significant disability affecting their routine life. A treatment that accelerates recovery is indicated in the majority who fail to respond to the initial nonsurgical therapy.There are no definite management guidelines for the frozen shoulder that yield consistent outcomes. A good number of patients respond well to non-surgical measures with analgesics, steroids, physiotherapy, manipulation under anesthesia (MUA) or cervical nerve root block, hydrodistension, and steroid infiltration.2,3 General consensus is to try non-operative measures for at least six months.4 In patients with significant pain and functional disability, early arthroscopic intervention is recommended with the established safety of the procedure and predictable outcomes in the current literature.5 Arthroscopic glenohumeral capsular release has been widely accepted as a proven treatment for refractive frozen shoulder.6 Maintenance of the gained shoulder range of motion (ROM) with early aggressive physical therapy is the key to the success of the arthroscopic capsular release. However, the major hindrance to achieving an early shoulder range of movement in these cases is the postoperative pain. Lack of persistent pain relief following arthroscopic procedures resulted in recurrence or incomplete resolution of symptoms in the majority.
Interscalene brachial plexus block has been used for pain relief in a variety of shoulder surgery procedures such as operative treatment of proximal humeral fractures, arthroplasties, instability, and rotator cuff repairs. Continuous interscalene nerve block (CISB) gives predictable and prolonged analgesia following shoulder surgeries, thus allowing early post-surgical rehabilitation with good functional outcome.7 Despite having increasing evidence, continuous ISB is not universally accepted by the orthopedic surgeons. A 2013 survey showed that only 15% of surgeons elected for continuous ISB whereas 59% elected for single-injection ISB and 26% opted for no peripheral nerve block.8 Concerns regarding the safety of CISB has limited its use for a short duration. In the majority of centers, interscalene block is maintained only for a day or two after the shoulder surgery.
We hypothesized that arthroscopic capsular release and subacromial decompression (SAD) combined with continuous local anesthetic infusion using a modified interscalene block technique, termed continuous upper arm brachial block (CUABB) method, with an indwelling catheter placed for a longer duration (2–3 weeks) in the postoperative period would provide sustained pain relief and improved outcomes in refractory frozen shoulder cases.
2. Material and methods
After getting the institutional research board (IRB) approval and written informed consent, 134 patients were enrolled in this prospective outcome analysis study conducted at a tertiary care hospital, between June 2016 and July 2019. Eleven patients were lost to follow up and 123 patients were included in the study (Table 1). We included all the patients having frozen shoulder with ASA physical status I to II patients, ≥30 years of age, body mass index (BMI) ≤35 kg/m2. We followed the criteria laid by Zuckerman et al. to diagnose frozen shoulder that included (1) insidious onset of true shoulder pain (2) night pain (3) painful restriction of both active and passive elevation to less than 100° and/or external rotation to less than one half of normal and (4) normal radiologic appearance.9 Clinically all the patients had global restriction of movements interfering with their activities of daily living (ADL). MRI showed intact rotator cuff or tendinosis or partial cuff tears less than 50% thickness. All the patients were taken up for surgery after failure of nonoperative measures of at least three months. We also included patients with diabetes mellitus in our study group as they were commonly affected with frozen shoulder. Those patients with sensitivity to local anesthetics, coagulopathy, local site infections, age <30 yrs or >70 yrs, ipsilateral upper limb neurological deficits, renal, pulmonary, hepatic or cardiac contraindications, obstructive sleep apnoea patients, hypothyroid patients, psychiatric or cognitive disorders having difficulty in understanding the instructions for using the infusion pump or pain scales, rotator cuff full thickness or partial thickness tears >50% that needed repair, calcific tendinitis, traumatic bony or labral pathology were excluded from the study.
Table 1.
Patient demographics at baseline.
| Male | 39 |
|---|---|
| Female | 84 |
| Age | 58 (33–74) |
| Right side | 74 |
| Left side | 49 |
| Diabetes | 33 (M-11; F-22) |
| Mean duration of symptoms | 4 months (2–9 months) |
2.1. Continuous upper arm brachial block (CUABB) technique- a modified interscalene block technique
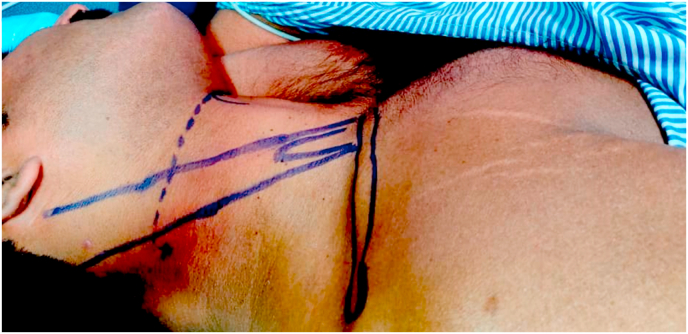
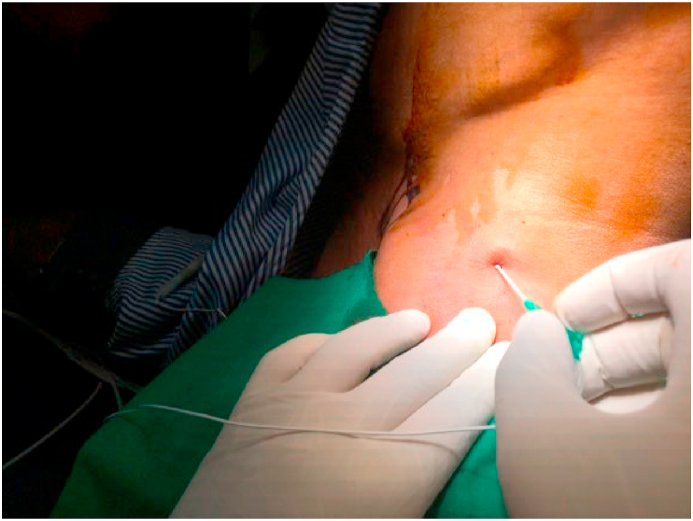
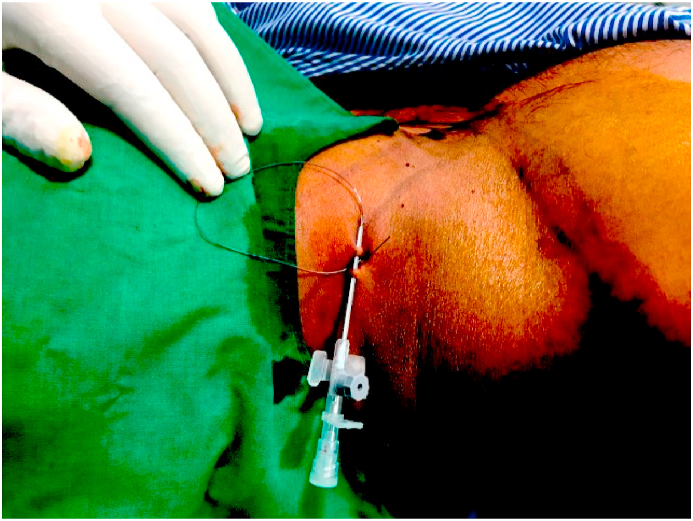
All patients were given intramuscular 0.05%mg/kg of Midazolam 30 min before the induction of anesthesia. Once the vitals are stable, the patient was positioned in the lateral decubitus position with the head in slight lateral flexion to expose the supraclavicular fossa and neck. An assistant gives slight traction in the arm with the elbow flexed to stretch the skin over the lateral side of the neck to make the anatomical landmarks prominent. The posterior edge of the clavicular head of the sternocleidomastoid is palpated by asking the patient to lift the head off the table and marked with a marker pen. Another transverse line is drawn laterally from the thyroid notch intersecting the previous line. The external jugular vein is seen on the way which is an additional landmark. The needle entry point is at the intersection of these two lines posterosuperior to the external jugular vein (Fig. 1). The entry site is infiltrated subcutaneously with a local anesthetic agent (1% Lignocaine). The localized point was punctured using a 55 mm 22G needle (Stimuplex, B.Braun, Germany) directed anteriorly, caudally, and medially (Fig. 2). The needle with the sheath is connected to the nerve stimulator (Stimuplex, B. Braun, Germany) and the circuit is completed by an ECG electrode placed in the ipsilateral arm of the patient. The needle is slowly advanced in the same direction till we noticed the contractions of Supraspinatus muscle followed by contractions of Biceps/Brachialis. The stimulating voltage of nerve stimulator is initially set at 1 mA (2 Hz, 100 μsec) and then gradually the stimulating current is reduced. The block is attempted to get the effect of both suprascapular nerve and axillary nerve block which are the major sensory suppliers to the shoulder joint. The needle tip is advanced beyond the Erb's point down to the posterior cord and positioned in between the two to get the stimulation of C5, C6 area. Contractions of the deltoid muscle are noticed next. The stimulating current is further reduced up to 0.3 mA. At 0.3 mA the muscle contractions should be noticed. The stimulation of the nerve with the minimum current of 0.3 mA indicates that the needle tip is perfectly near to the nerve. The upper trunk block completely involves the suprascapular nerve but axillary nerve escapes. Advancing the needle beyond this point will also block the axillary nerve, so shoulder analgesia could be obtained without separately blocking the suprascapular nerve. With the catheter in place, apart from intraoperative analgesia, postoperative analgesia is also accomplished. Consistent twitching of the supraspinatus, biceps/brachialis, and deltoid with electric stimulation of 1 Hz and 0.3 mA was taken as the correct blocking location. At this point, contractions of the ipsilateral hemidiaphragm are looked for and if noticed, needle direction is adjusted till diaphragmatic contraction disappears. This largely avoids hemidiaphramatic palsy due to the block. Aspirate the needle to rule out intravascular location. Now the stimulating needle is removed by retaining the sheath in situ. The catheter is passed into the catheter sheath and advanced up to 5–10 mm beyond the tip of the sheath. Thereafter, the sheath is removed by paying meticulous attention to retain the catheter in situ. After repeat aspiration to reconfirm the catheter position, we slowly injected 1 ml of 0.2% Ropivacaine. Thereafter, the remaining 10 ml of 0.2% Ropivacaine was injected. Complete loss of pinprick sensation to a blunted needle tip on the skin over the deltoid and loss of active shoulder abduction was defined as a successful upper brachial plexus block.The main deterrent to Continuous nerve block catheters placement at many centers is the frequent dislodgement of the catheter in the postoperative period. To prevent such catastrophe, stable fixation of the catheter is of paramount importance. After the introduction of the catheter, it is first fixed at the entry point with 1-0 black silk. A no. 18 cannula is used to double tunnel the catheter subcutaneously at the entry point (Fig. 3). Both tunnels are fixed with the 1-0 black silk and affixed using an adhesive, clear occlusive dressing at the base of the neck to avoid the surgical field. If the catheter could not be advanced past the end of the needle, the needle hub was moved medially, in small increments, until the catheter could be advanced. Thereafter, the patient was positioned for general anesthesia and arthroscopy procedure.
Fig. 1.
Point of needle insertion at intersection of line drawn along the posterior border of the clavicular head of the sternocleidomastoid and horizontal line along the thyroid notch.
Fig. 2.
Stimuplex needle directed anteriorly, caudally and medially in lateral decubitus.
Fig. 3.
Subcutaneous tunneling of the catheter.
A portable infusion pump Dosi-Fuser, (Leventon SAU, Spain) filled with 250 ml of 0.2% Ropivacaine was attached to the perineural catheter, and infusion started at 4 ml/h in the recovery room. With this pump, drug delivery can be adjusted from 1 ml/h up to 7 ml/h by the patient using the control knob.The option of 0 is there for no drug delivery. If the recurrent laryngeal nerve is blocked, hoarseness of voice may be present which could also be due to endotracheal intubation. But still, all hoarseness of voice in this study was taken as recurrent laryngeal nerve block and the dose and rate of flow of Ropivacaine is adjusted till recurrent laryngeal nerve recovers. Ipsilateral Horner's syndrome suggested blockade of the stellate ganglion. This also warranted drug delivery titration. The continuous upper arm brachial block (CUABB) was given by the experienced senior surgical faculty in all cases.
2.2. Surgical technique arthroscopic capsular release
All the patients were operated in a lateral decubitus position. Gentle manipulation of the shoulder in the flexion-extension axis was done before positioning the patient. No attempt was made to manipulate the shoulder in internal or external rotation. The degree of restricted ROM was noted after the manipulation. This guided the capsular release needed peroperatively. The shoulder joint was entered through the posterior portal. The anterior portal was created under vision. After preliminary evaluation to confirm the diagnosis, the capsular release was started from the anterior portal region using the radiofrequency device (Arthrocare, Sunnyvale, CA). Initially, the rotator interval was released thoroughly along with the release of the coracohumeral (CHL) ligament from the base of the coracoid process. Thereafter, the anteroinferior capsule was released up to 5 o'clock position. All three glenohumeral ligaments were released. The subscapularis tendon was not released. The posterior capsule was released only in cases with gross restriction of the internal rotation after manipulation following the induction of anesthesia. The inferior capsule was released from 5 to 7 o'clock position with utmost care using a hooked RF probe/basket forceps under the vision and maintaining close contact with the glenoid margin to avoid damaging the axillary nerve. Biceps was assessed intraoperatively for any pathology. It was treated with tenotomy/tenodesis based on the degree of damage and age of the patient. The location of tenodesis was decided peroperatively based on the biceps pathology. Following glenohumeral work, subacromial space was inspected and arthroscopic release of the bursal adhesions done. Acromioplasty was not done. The shoulder was again manipulated to assess the improvement in the motion achieved by arthroscopic release after surgery.
2.3. Postoperative treatment
The patients were started on passive mobilization of the shoulder the next day of surgery. They were taught passive assisted mobilization using their non-operated arm. Therapy sessions were conducted twice a day during the inpatient stay by the trained physiotherapist. The arm was supported in a sling throughout the infusion period. Motor and sensory recovery were reviewed in the post-operative period. If the ipsilateral hand or the elbow was completely insensate and paralyzed in the next morning of the surgery, the infusion rate was reduced until sensations and motor recovery were seen. Patients were also enquired regarding any symptoms of local anesthetic toxicity. If there was a motor blockade seen on the next day of the surgery, the rate of the infusion was adjusted. The patients were followed up at 6hr, 12 h, 24 h, 48 h,7thday, 14th, and 21st day. Patients were discharged on the third postoperative day and then seen on 7th, 14th and 21stday. Three doses of intravenous antibiotics were given for all cases. At the time of discharge, patients gained nearly 90deg of passive assisted forward flexion/abduction and around 20–30 deg of external rotation. All the patients were provided with a home exercise regimen to follow. The infusion pump was filled every 4–6 days or earlier if got emptied. Sutures were removed on the 14th postoperative day. Thereafter they were continued on physiotherapy protocol and seen at 3rd week, 6th week, 3rdmonth, 6th month, 1st year, and 2nd year. The patient and caretaker were given standard postoperative instructions. Verbal and written instructions on the use of an infusion pump and catheter were provided. Specific directions were given to recognize the early signs and symptoms of local anesthetic toxicity and catheter site infection. Contact phone numbers of the treating surgeons were provided for any queries. Patients were prescribed a nonsteroidal anti-inflammatory drug (Diclofenac 50 mg, taken 2–3times/d, depending on patient age) be taken in case of pain and four additional occlusive dressings to be applied over the catheter site in case of slackening of the applied patch. In the case of “break-through” pain, patients were advised to first increase the flow rate of the infusion pump. If the pain had not resolved after 30 min, patients were instructed to use oral NSAIDs and to record it in their medication log. The catheter was removed between 10 and 21 days. The pain was assessed on a 10 cm Visual analog scale (VAS) (0 = no pain, 10 maximal pain). Passive movements of both affected and non-affected shoulders were measured using a goniometer. Clinical evaluation was done using Constant-Murley scoreat regular intervals (at three months, six months, one year, and two years/final follow-up).10 Patients were also assessed for oral analgesic consumption, sleep quality, and catheter site discomfort on every follow-up. The average duration of follow-up was 18 months (range: 12–24 months).
3. Results
The mean follow-up period was 18 months (range, 8–24 months). Statistically, there was a significant improvement in the range of motion postoperatively (p < .001).A similar finding was a significant reduction in postoperative pain scores. The average VAS pain score before surgery was 8.2 which reduced to an average of 3.4 by three weeks. (p < .001) (Table 2). At the latest follow up, the average forward elevation improved from a preoperative level of 38.6–172.8 deg and average abduction improved from 44.4 deg to 165.6 deg. External rotation improved from an average of 10.6–55.8 deg. Internal rotation improved from the lateral thigh region to T10 spine levels (p < .05) (Table 3). The average duration of the surgery was 66 mts (54–118 mts). The infusion pump with the catheter was kept for a mean duration of 12 days (10–21days). The constant score increased from an average of 32–84 at the final follow-up (p < .001) (Table 4).The outcome assessment at the final follow up is shown in Table 5.
Table 2.
Postoperative VAS pain scores.
| Preop | 1week | 2week | 3week | 6weeks | 3 mths | 6mths | 1 year | Last Fu |
|---|---|---|---|---|---|---|---|---|
| 8.2 ± 0.96 | 3.6 ± 1.24 | 3.4 ± 1.46 | 3.4 ± 1.36 | 2.6 ± 1.08 | 1.8 ± 1.04 | 1.4 ± 0.46 | 1.4 ± 0.28 | 1.2 ± 0.26 |
Table 3.
Data are presented as mean ± SD.
| Preop | 1wk | 2week | 3week | 6weeks | 3mths | 6mths | 1year | Last fu | |
|---|---|---|---|---|---|---|---|---|---|
| Abductionrowhead | 44.4 ± 16 | 125.2 ± 12a | 148.6 ± 14a | 153.8 ± 16a | 124.8 ± 12a | 152.2 ± 11a | 165.2 ± 08a | 160.4 ± 09a | 165.6 ± 12a |
| Forward Flexionrowhead | 38.6 ± 12 | 153.2 ± 16a | 169.8 ± 08a | 165.2 ± 06a | 125.6 ± 10a | 149.2 ± 06a | 166.4 ± 08a | 168.4 ± 04a | 172.8 ± 04a |
| External Rotationrowhead | 10.6 ± 04 | 34.2 ± 12a | 34.4 ± 10a | 54.2 ± 08a | 33.4 ± 06a | 54.8 ± 06a | 55.4 ± 07a | 55.4 ± 08a | 55.8 ± 06a |
| Internal Rotationrowhead | Lateral thigh | Buttocks | LS junction | L 3 | L3 | L1b | T12b | T10b | T10b |
P < .05 preoperative vs postoperative (student's paired T-test).
P < .05 preoperative vs postoperative (Wilcoxon signed-rank tests).
Table 4.
Summary of Constant scores as a function of time.
| Preop | 3mths | 6mths | 1 year | Final follow-up/2 year |
|---|---|---|---|---|
| 32 | 67 | 74 | 78 | 84 |
Table 5.
Outcome assessment at final follow up.
| Preoperative | Postoperative | P value | |
|---|---|---|---|
| Abduction | 44.4 ± 16 | 165.6 ± 12 | P < 0.001a |
| Forward flexion | 38.6 ± 12 | 172.8 ± 04 | P < 0.001a |
| ER | 10.6 ± 04 | 55.8 ± 06 | P < 0.05a |
| IR | Lateral Thigh | T10 | P < 0.05b |
| CS | 32 | 84 | P < 0.05a |
| VAS | 8.2 ± 0.96 | 1.2 ± 0.26 | P < 0.05a |
P < .05 student's paired T-test.
P < .05 Wilcoxon signed-rank tests.
We found a delay in the achievement of ROM in diabetic cases in the initial months but all of them regained similar ROM as nondiabetic cases by the end of 6 months. 24 patients (19.5%) complained of paresthesia in the C5, 6 distribution areas, which got relieved after the removal of the catheter. Of the 33 diabetic patients, 3 cases (2.4%) developed an infection at the catheter site which required early removal of the catheter in 2 cases. Around 8 cases (6.5%) developed recurrent laryngeal nerve palsy and 19 cases (15.4%) developed Horner's syndrome in the postoperative period which got relieved in the first week with dose titration (Table 6). We found a good number of patients (19.5%) complaining of paresthesia in the C5,6 distribution area. None of the cases required postoperative opioid administration for pain control. There was no significant discomfort at the catheter site apart from those cases with catheter site infection. All the patients reported a significant improvement in the sleep quality post-surgery with an infusion of the local anesthetic. The sleep quality was assessed using sleep questionnaires and sleep diary.
Table 6.
Complications.
| Complications | Number of cases |
|---|---|
| 1. Catheter site infection | 3 |
| 1. Transient recurrent laryngeal palsy | 8 |
| 1. Transient Horner's syndrome | 19 |
| 1. Paresthesia C5,6 distribution | 24 |
3.1. Statistical analysis
The difference in ROM (forward flexion, external rotation, and internal rotation) and VAS pain score were computed for each patient, summarized, and tested using student's paired T-test from before CUABB placement to final follow up. The nonparametric data were analyzed with the use of Wilcoxon signed-rank tests. Results were considered significant if P ≤ .05. Data were analyzed using SPSS software (ver.18, SPSS, Chicago, IL).
4. Discussion
Arthroscopic release and mobilization have become one of the widely accepted treatment modalities for the management of resistant frozen shoulder cases. Early and efficient postoperative rehabilitation therapy is necessary for the successful outcome. Loss of the regained shoulder range of movements in the early postoperative period is at significant risk without aggressive stretching exercises early on in the postoperative period.6,11 Pain, which is severe to very severe in this condition, has become the main factor limiting efficient rehabilitation after the arthroscopic capsular release of the shoulder joint. Adequate control of postoperative shoulder pain following capsular release plays a vital role in patient compliance to an efficient rehabilitation program. We studied the outcome of continuous upper brachial plexus block kept for a prolonged duration of 2–3 weeks in the management of frozen shoulder using a patient-controlled pump and catheter system.
Interscalene blocks (ISB) and catheters have emerged as a safe and effective modality for postoperative pain relief in shoulder surgery.12,13 In addition to postoperative analgesia, ISB has advantages of intra-operative bleeding reduction, good muscle relaxation, and reduced general anesthetic complications.13 We utilized the nerve stimulation technique as the method of controlling the insertion of the needle and catheter as described in previous studies although the use of ultrasound is on the rise.14, 15, 16The main issues sited with the use of the ISB are the complete motor and sensory blockade that interferes with active participation in physiotherapy protocols by the patient. Also, the insertion and maintenance of the ISB catheters are difficult.17 In our technique, the patient was placed in a lateral decubitus position for placement of the catheter which made the insertion technically easier. The catheter was held in position by tunneling through the skin multiple times and retaining the position by water-resistant adhesive plaster. By adjusting the rate of drug flow through the catheter, the motor blockade could be avoided with retention of the sensory blockade for pain relief.
Our approach of upper arm brachial plexus blocks differs from the classical anterior approach by Winnie, posterior approach by Pippa, and modified lateral approach by Borgeat et al. in a few points.18, 19, 20 Firstly, the patient is positioned in the lateral position that gives a good trajectory and a three-dimensional image of the interscalene space for needle insertion and catheter placement. The needle puncture point is at the level of thyroid notch, below the insertion point of Winnie's and Meiers' tech which reduces the chances of piercing the scalene muscles. The entry point of our technique is similar to the point described by Borgeat et al.20,21 None of our patients developed major complications such as total spinal anesthesia, epidural anesthesia, or injection in the spinal cord as reported with Winnie's techniques.22, 23, 24 Similarly, difficulties to insert the catheter were reported by Singelyn et al.in 66% and Tuominenet al. in up to 25% cases where Winnie's approach was used.25,26 These complications were reduced in our approach as it is easier to direct the needle trajectory away from the cervical spine toward the interscalene space in the lateral position.
Minor side effects such as recurrent laryngeal nerve blockade or Horner syndrome were encountered in 4% and 19% of cases in our study. Jochum et al. noticed an incidence of recurrent laryngeal nerve blockade of 3% and an incidence of Horner syndrome of 71% similar to the incidences reported by Vester-Andersen et al. using Winnie's technique.27,28 Borgeat et al. reported an incidence of 0.9% of recurrent laryngeal nerve blockade and 6% incidence of Horner's syndrome which is lower than our findings.29 The more distal administration of the local anesthetic through the catheter placed within the interscalene space makes the preganglionic sympathetic fibers going to the stellate ganglion less exposed to it, thus reducing the incidence of Horner's syndrome and recurrent laryngeal nerve palsy in our group of patients. Maintenance of strict aseptic precautions reduced the incidence of infection around the catheter site to 2.4% in our cases. All these were superficial infections in diabetic patients that resolved with oral antibiotics but necessitated the removal of the catheter in 2 cases.We have used Ropivacaine instead of Bupivacaine as we found better preservation of hand strength and reduced incidence of paresthesias with the former in our routine practice as has been reported by Borgeat et al.20 The use of Ropivacaine as compared to Bupivacaine seems to advantages in terms of better sensorimotor dissociation with the former.30 The concentration of 0.2% Ropivacaine was found to be adequate for most patients.
We performed a limited arthroscopic release as compared to the 360° release for all cases as suggested by some authors.31 The inferior capsule was released with adequate caution from the glenoid side to prevent damage to the axillary nerve. The cadaveric dissection studies by Bowen et al has guided us to determine the regional capsule that needs to be released to regain the lost glenohumeral motion.32 Loss of external rotation mandated a release of the rotator interval, the coracohumeral ligament, the superior and middle glenohumeral ligaments, and/or the intra-articular portion of the subscapularis. Loss of abduction or forward elevation required the release of the anteroinferior capsule, including the anterior band of the inferior glenohumeral ligament. Loss of internal rotation warranted posterosuperior capsular release. We have never released the subscapularis tendon. In our series, the majority of the cases demanded the release of the rotator interval, coracohumeral ligament, all three glenohumeral ligaments including the inferior capsule. The posterior capsule was released only in a few cases of our series (12 cases). As we were able to maintain adequate pain relief for the initial 2–3 weeks with the use of the infusion pump, we could maintain the patients on aggressive physiotherapy without loss of compliance due to pain from their side. We believe that the crux of getting back the movements in frozen shoulder lies in maintaining adequate analgesia in the postoperative period for a sufficient period to tide over the postsurgical inflammatory phase after adequate arthroscopic capsular releases. Retention of analgesia for 2–3 weeks permits patients to follow the aggressive physiotherapy even at their home. This helps to regain the ROM early as compared to other regimens with limited period continuous ISB. We found no significant difference in the final ROM between diabetics and nondiabetics though there was a delay in achieving the comparable ROM between both groups as reported in previous studies.33 This delay was not found to be statistically significant in our study. Though the long term outcomes may be comparable to other studies, our regimen allows early recovery of ROM in resistant frozen shoulder cases.34
Although continuous upper arm brachial plexus block (CUABB) with local anesthetic infusion offers significant improvements in postoperative pain control and shoulder function, there are several potential inherent risks involved in the use of these catheters like catheter site infection, nerve injury, catheter migration, local anesthetic toxicity and even epidural/intrathecal anesthesia.35, 36, 37, 38 Previous studies have demonstrated the safety and efficacy of home administration of perineural blocks in shoulder surgery.39,40 To our knowledge, this is the first study supporting the use of continuous upper brachial plexus block procedure in lateral decubitus position and maintaining it for a prolonged duration of 2–3 weeks to optimize pain control and mobilization of frozen shoulder. Metanalysis has demonstrated good results of continuous ISB in shoulder surgery than single-shot ISB but none of the studies retained the catheter for more than 72 h.7 The data in this study demonstrate that it is possible to achieve adequate pain relief by retaining the catheter and single-use of an infusion pump for a longer duration of 2–3 weeks to aid the rapid rehabilitation in frozen shoulder. In this study, we observed no episodes of iatrogenic instability in the follow-up, although case reports of iatrogenic instability have been recorded.41 Besides, none of our patients required any form of revision surgery. In our study, we found no major complications related to the regional block catheter placement or prolonged local anesthetic infusion. There could be many reasons for the limited use of continuous nerve blocks in shoulder surgery. Lack of a simple, reproducible method of insertion and retention of the perineural catheter, fear of local anesthetic toxicity, catheter site infection and nerve injury-related issues. Singelyn et al. noted the difficulty in the insertion of 84% (21 of 25) of catheters.25 This is in contrast with the ease and success of catheter insertion in this study. Klein et al. reported successful interscalene catheterization for the continuous block for all of their patients similar to our study.42 Our success could be attributed to the increasing exposure and experience of the treating faculties to peripheral nerve blockade in routine arthroscopic surgery as well as the availability of less toxic local anesthetic drugs. However, given the success of long-duration ambulatory perineural blocks in this series of patients, further larger prospective trials to establish the learning curves and safety of the procedure is needed.
A potential limitation is the lack of an analytic determination of the Ropivacaine levels in the plasma. A further limitation is the lack of a control group. One of the strengths of this study lies in its large sample size and follow up. As all the patients are not willing to accept the added responsibility that comes with the catheter and infusion pump system for a long duration, appropriate patient selection is crucial for the success and safety of ambulatory local anesthetic infusion following arthroscopic shoulder surgery.
5. Conclusion
This prospective study validates the use of continuous upper arm brachial plexus (CUABB) block with a portable infusion pump and catheter in resistant frozen shoulder cases. It significantly reduced postoperative pain and opioid use with early recovery of the shoulder movements and functions. Our technique of modified interscalene block in lateral decubitus position and catheter placement in trained hands does not seem to increase the incidence of acute or chronic neurologic complications. The efficacy of continuous upper arm brachial plexus block (CUABB) with ambulatory patient-controlled analgesia pump and patient satisfaction with pain control in the frozen shoulder is high in our study.
CRediT authorship contribution statement
Sibin Surendran: Investigation, Methodology, Writing - original draft. Gopinathan Patinharayil: Conceptualization, Methodology, Resources, Supervision. Raju Karuppal: Investigation, Resources. Anwar Marthya: Supervision, Project administration. Muhammed Fazil: Investigation, Formal analysis. Shibi Mohammed Ali: Conceptualization, Formal analysis, Data curation, Methodology.
Contributor Information
Sibin Surendran, Email: drsibinortho@gmail.com.
Gopinathan Patinharayil, Email: gopinathan.p@gmail.com.
Raju Karuppal, Email: drrajuortho@rediffmail.com.
Anwar Marthya, Email: anwarmh@gmail.com.
Muhammed Fazil, Email: drfazilvv@gmail.com.
Shibi Mohammed Ali, Email: shibimuhammadali@gmail.com.
References
- 1.Hand C., Clipsham K., Rees J.L., Carr A.J. Long-term outcome of frozen shoulder. J Shoulder Elbow Surg. 2008;17(2):231–236. doi: 10.1016/j.jse.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Levine W.N., Kashyap C.P., Bak S.F., Ahmad C.S., Blaine T.A., LU Bigliani. Nonoperative management of idiopathic adhesive capsulitis. J Shoulder Elbow Surg. 2007;16(5):569–573. doi: 10.1016/j.jse.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi R., Kajita Y., Harada Y., Iwahori Y., Deie M. Clinical results of shoulder manipulation under ultrasound-guided cervical nerve root block for frozen shoulder in patients with diabetes. J Orthop. 2020;21:297–301. doi: 10.1016/j.jor.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harryman D.T., 2nd, Sidles J.A., Matsen F.A., 3rd Arthroscopic management of refractory shoulder stiffness. Arthroscopy. 1997;13:133–147. doi: 10.1016/s0749-8063(97)90146-8. [DOI] [PubMed] [Google Scholar]
- 5.Ide J., Takaji K. Early and long term results of the arthroscopic treatment of shoulder stiffness. J Bone Joint Surg. 2004;13:174–179. doi: 10.1016/j.jse.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Warner J.P., Allen A., Marks P., Wong P. Arthroscopic release for chronic, refractory adhesive capsulitis of the shoulder. J Bone Joint Surg Am. 1996;78:1808–1816. doi: 10.2106/00004623-199612000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Vorobeichik L., Brull R., Bowry R., Laffey J.G., Abdallah F.W. Should continuous rather than single-injection interscalene block be routinely offered for major shoulder surgery? A meta-analysis of the analgesic and side-effects profiles. Br J Anaesth. 2018;120:679–692. doi: 10.1016/j.bja.2017.11.104. [DOI] [PubMed] [Google Scholar]
- 8.Moore D.D., Maerz T., Anderson K. Shoulder surgeons' perceptions of interscalene nerve blocks and a review of complications rates in the literature. Physician Sportsmed. 2013;41:77–84. doi: 10.3810/psm.2013.09.2026. [DOI] [PubMed] [Google Scholar]
- 9.Zuckerman J., Cuomo F., Rokito S. Definition and classification of frozen shoulder: a consensus approach. J Shoulder Elbow Surg. 1994;3:S72. doi: 10.1016/j.jse.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Constant C.R., Murley A.H. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 11.Shaffer B., Tibone J., Kerlan R. Frozen shoulder: a long-term follow-up. J Bone Joint Surg Am. 1992;74:738–746. [PubMed] [Google Scholar]
- 12.Brown A., Weiss R., Greenberg C., Flatow E., Bigliani L. Interscalene block for shoulder arthroscopy: comparison with general anesthesia. Arthroscopy. 1993;9:295–300. doi: 10.1016/s0749-8063(05)80425-6. [DOI] [PubMed] [Google Scholar]
- 13.Kinnard P, Truchon R, St-Pierre A, Montreuil J. Interscalene block for pain relief after shoulder surgery. A prospective randomized study. Clin Orthop1994;304:22-24. [PubMed]
- 14.Klein S.M., Nielsen K.C., Martin A. Interscalene brachial plexus block with continuous intraarticular infusion of ropivacaine. Anesth Analg. 2001;93:601–605. doi: 10.1097/00000539-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Singelyn F.J., Seguy S., Gouverneur J.M. Interscalene brachial plexus analgesia after open shoulder surgery: continuous versus patient-controlled infusion. Anesth Analg. 1999;89:1216–1220. [PubMed] [Google Scholar]
- 16.Goebel S., Stehle J., Schwemmer U., Reppenhagen S., Rath B., Gohlke F. Interscalene brachial plexus block for open-shoulder surgery: a randomized, double-blind, placebo-controlled trial between single-shot anesthesia and patient-controlled catheter system. Arch Orthop Trauma Surg. 2010;130(4):533‐540. doi: 10.1007/s00402-009-0985-7. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi K., Sethi N., Bauer G.S. Postoperative pain control following arthroscopic release of adhesive capsulitis: a short-term retrospective review study of the use of an intra-articular pain catheter. Arthroscopy. 2002;18(4):359‐365. doi: 10.1053/jars.2002.32311. [DOI] [PubMed] [Google Scholar]
- 18.Winnie A.P. Interscalene brachial plexus block. Anesth Analg. 1970;49(3):455‐466. [PubMed] [Google Scholar]
- 19.Pippa P., Cominelli E., Marinelli C., Aito S. Brachial plexus block using the posterior approach. Eur J Anaesthesiol. 1990;7:411–420. [PubMed] [Google Scholar]
- 20.Borgeat A., Ekatodramis G. Anaesthesia for shoulder surgery. Best Pract Res Clin Anaesthesiol. 2002;16(2):211‐225. doi: 10.1053/bean.2002.0234. [DOI] [PubMed] [Google Scholar]
- 21.Meier G., Bauereis C., Heinrich C. Interscalene brachial plexus catheter for anesthesia and postoperative pain therapy: experience with a modified technique. Anaesthesist. 1997;46:715–719. doi: 10.1007/s001010050458. [DOI] [PubMed] [Google Scholar]
- 22.Dutton R., Eckhart W., 3rd, Sunder N. Total spinal after interscalene blockade of the brachial plexus. Anesthesiology. 1994;80:939–941. doi: 10.1097/00000542-199404000-00028. [DOI] [PubMed] [Google Scholar]
- 23.Scammell S.J. Inadvertent epidural anaesthesia as a complication of interscalene brachial plexus block. Anaesth Intensive Care. 1979;7:56–57. doi: 10.1177/0310057X7900700109. [DOI] [PubMed] [Google Scholar]
- 24.Benumof J.L. Permanent loss of cervical spinal cord function associated with interscalene block performed under general anesthesia. Anesthesiology. 2000;93:1541–1544. doi: 10.1097/00000542-200012000-00033. [DOI] [PubMed] [Google Scholar]
- 25.Singelyn F.J., Seguy S., Gouverneur J.M. Interscalene brachial plexus analgesia after open shoulder surgery: continuous versus patient-controlled infusion. Anesth Analg. 1999;89:1216–1220. [PubMed] [Google Scholar]
- 26.Tuominen M., Haasio J., Hekali R., Rosenberg P.H. Continuous interscalene brachial plexus block: clinical efficacy, technical problems and bupivacaine plasma concentration. Acta Anaesthesiol Scand. 1989;33:84–88. doi: 10.1111/j.1399-6576.1989.tb02866.x. [DOI] [PubMed] [Google Scholar]
- 27.Jochum D., Roedel R., Gleyze P., Balliet J.M. Bloc interscalénique et chirurgie de l’épaule: etude prospective d’unesérie continue de 167 patients. Ann Fr Anesth Reanim. 1997;16:114–119. doi: 10.1016/s0750-7658(97)87191-2. [DOI] [PubMed] [Google Scholar]
- 28.Vester-Andersen T., Christiansen C., Hansen A., Sorensen M., Meisler C. Interscalene brachial plexus block: area of analgesia, complications and blood concentrations of local anesthetics. Acta Anaesthesiol Scand. 1981;25:81–84. doi: 10.1111/j.1399-6576.1981.tb01612.x. [DOI] [PubMed] [Google Scholar]
- 29.Borgeat A., Dullenkopf A., Ekatodramis G., Nagy L. Evaluation of the lateral modified approach for continuous interscalene block after shoulder surgery. Anesthesiology. 2003;99(2):436–442. doi: 10.1097/00000542-200308000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg P.H., Heinonen E. Differential sensitivity of A and C nerve fibres to long-acting amide local anaesthetics. Br J Anaesth. 1983 Feb;55(2):163–167. doi: 10.1093/bja/55.2.163. [DOI] [PubMed] [Google Scholar]
- 31.Cvetanovich G.L., Leroux T.S., Bernardoni E.D. Clinical outcomes of arthroscopic 360° capsular release for idiopathic adhesive capsulitis in the lateral decubitus position. Arthroscopy. 2018;34(3):764–770. doi: 10.1016/j.arthro.2017.08.249. [DOI] [PubMed] [Google Scholar]
- 32.Bowen M.K., Warren R.F. Ligamentous control of shoulder stability based on selective cutting and static translation experiments. Clin Sports Med. 1991;10:757–782. [PubMed] [Google Scholar]
- 33.Yanlei G.L., Keong M.W., TijauwTjoen D.L. Do diabetic patients have different outcomes after arthroscopic capsular release for frozen shoulder? J Orthop. 2019;16(3):211–215. doi: 10.1016/j.jor.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Lievre H.M., Murrell G.A. Long-term outcomes after arthroscopic capsular release for idiopathic adhesive capsulitis. J Bone Joint Surg Am. 2012;94(13):1208–1216. doi: 10.2106/JBJS.J.00952. [DOI] [PubMed] [Google Scholar]
- 35.Borgeat A., Ekatodramis G., Kalberer F., Benz C. Acute and nonacute complications associated with interscalene block and shoulder surgery: a prospective study. Anesthesiology. 2001;95:875–880. doi: 10.1097/00000542-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 36.Hogan Q., Dotson R., Erickson S. Local anesthetic myotoxicity: a case and review. Anesthesiology. 1994;80:942–947. doi: 10.1097/00000542-199404000-00029. [DOI] [PubMed] [Google Scholar]
- 37.Tuominen M.K., Pere P., Rosenberg P.H. Unintentional arterial catheterization and bupivacaine toxicity associated with continuous interscalene brachial plexus block. Anesthesiology. 1991;75:356–358. doi: 10.1097/00000542-199108000-00026. [DOI] [PubMed] [Google Scholar]
- 38.Cook L.B. Unsuspected extradural catheterization in an interscalene block. Br J Anaesth. 1991;67:473–475. doi: 10.1093/bja/67.4.473. [DOI] [PubMed] [Google Scholar]
- 39.Rawal N., Axelsson K., Hylander J. Postoperative patient-controlled local anesthetic administration at home. Anesth Analg. 1998;86(1):86‐89. doi: 10.1097/00000539-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Ilfeld B.M., Morey T.E., Wright T.W., Chidgey L.K., Enneking F.K. Continuous interscalene brachial plexus block for postoperative pain control at home: a randomized, double-blinded, placebo-controlled study. Anesth Analg. 2003;96(4) doi: 10.1213/01.ANE.0000049824.51036.EF. [DOI] [PubMed] [Google Scholar]
- 41.Drakos M.C., Green D.M., Dodson C.C., Allen A.A., Warren R.F. Shoulder dislocation after mobilization procedures for adhesive capsulitis. Orthopedics. 2008;31(12) doi: 10.3928/01477447-20081201-06. [DOI] [PubMed] [Google Scholar]
- 42.Klein S.M., Grant S.A., Greengrass R.A. Interscalene brachial plexus block with a continuous catheter insertion system and a disposable infusion pump. Anesth Analg. 2000 Dec;91(6):1473–1478. doi: 10.1097/00000539-200012000-00033. [DOI] [PubMed] [Google Scholar]