Abstract
Background
Novel coronavirus disease 2019 (COVID-19) refers to infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen, and has spread to pandemic levels since its inception in December 2019. While several risk factors for severe presentation have been identified, the clinical course for end-stage renal disease (ESRD) patients on maintenance hemodialysis with COVID-19 has been unclear. Previous studies have revealed that some antiviral agents may be effective against COVID-19 in the general population, but the pharmacokinetics and pharmacodynamics of these agents in ESRD patients remain under investigation. Favipiravir, an antiviral agent developed for treatment of influenza, is one candidate treatment for COVID-19, but suitable dosages for patients with renal insufficiency are unknown. Here we provide a first report on the efficacy of favipiravir in a patient with ESRD undergoing hemodialysis.
Case presentation
The case involved a 52-year-old woman with COVID-19 who had been undergoing maintenance hemodialysis three times a week for 3 years due to diabetic nephropathy. She had initially been treated with lopinavir/ritonavir and ciclesonide for 5 days, but developed severe pneumonia requiring invasive positive-pressure ventilation. Those antiviral agents were subsequently switched to favipiravir. She recovered gradually, and after 2 weeks was extubated once the viral load of SARS-CoV-2 fell below the limit of detection. Although concentrations of several biliary enzymes were elevated, no major adverse events were observed.
Conclusion
Favipiravir may be an effective option for the treatment of COVID-19-infected patients with ESRD.
Keywords: SARS-CoV-2, COVID-19, End-stage renal disease, Favipiravir, Hemodialysis
Introduction
The pandemic of novel coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pathogen has spread all over the world since December 2019 [1]. In Japan, the first case of COVID-19 was identified on January 16, 2020 and the number of infected individuals has kept rising. First case of COVID-19 in a hemodialysis patient was reported on March 1 [2]. While several complications have been identified as risk factors for severe presentation, [3] such as hypertension and diabetes, the clinical course of patients with end-stage renal disease (ESRD) on hemodialysis with COVID-19 has not been clarified. Although previous studies revealed that some antiviral agents may be effective for this disease in the general population [4], the pharmacokinetics and pharmacodynamics of these agents in ESRD patients are still under investigation. ESRD patients are estimated to be at higher risk of severe disease and poorer outcomes with COVID-19 due to a suppressed immune system. In addition, these patients usually undergo hemodialysis in relatively close proximity to other patients in hemodialysis facilities, which can lead to the number of COVID-19-infected ESRD patients increasing [5]. Investigation of effective treatments for these patients is thus warranted.
We describe herein the case of ESRD patient undergoing hemodialysis infected with COVID-19 treated with favipiravir, and report on the efficacy of this drug.
Case report
The patient was a 52-year-old woman who has been undergoing maintenance hemodialysis 3 times a week for 3 years due to diabetic nephropathy. She also had a 6-month history of chronic diarrhea and had undergone coronary artery stenting, amputation of the right lower limb, and treatment for latent pulmonary tuberculosis. Medications were aspirin, telmisartan, lovastatin, nalfurafine, ranitidine, carvedilol and potassium aspartate. Six days prior to this admission, she had been seated just behind another COVID-19 patient in a courtesy bus for an outpatient hemodialysis facility. Four days later, when she underwent routine hemodialysis in the morning, she reported feeling feverish, and a nasopharyngeal swab yielded positive results for SARS-CoV-2 by real-time reverse-transcriptase polymerase chain reaction (rRT-PCR) assay the next day.
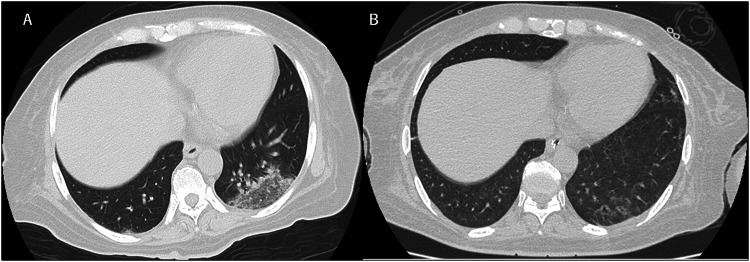
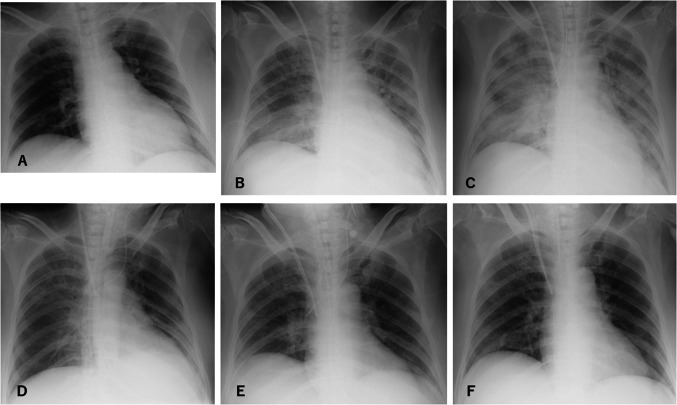
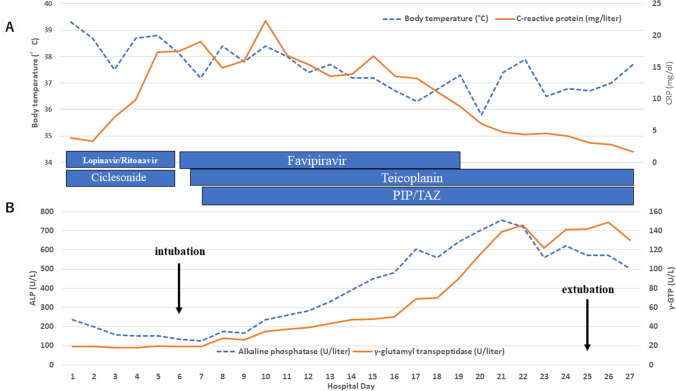
On admission, she reported fatigue, nausea, abdominal pain, and severe diarrhea. Temperature was 39.3 °C, respiratory rate was 18 breaths/min, heart rate was 81 beats/min, blood pressure was 191/97 mmHg, and oxygen saturation was 100% on ambient air. Whereas physical examination showed no abnormalities, chest computed tomography (CT) demonstrated ground-glass opacity in the inferior lobes of both lungs (Fig. 1a). She was admitted to an airborne infection isolation unit. Laboratory tests suggested end-stage renal failure, anemia, inflammatory response, and hypokalemia that could have been caused by chronic diarrhea (Table 1). Blood and stool cultures both yielded negative results. Sequential organ failure assessment (SOFA) score at that time was 5. At this time, only few drugs were approved by ethical committee of our hospital for the treatment with COVID-19, which are ciclesonide, lopinavir/ritonavir, and favipiravir. We avoided to use favipiravir as an initial therapy because of lacking information about dialysis patients. Informed consent was obtained from our patient and treatment with oral lopinavir (800 mg/day) and ritonavir (200 mg/day) was initiated on the evening of admission. In addition, inhaled ciclesonide was administered twice a day. Routine hemodialysis was continued three times a week and body fluid status was tightly controlled properly. We basically followed the same dialysis conditions of her dialysis facility; 4 h, blood flow 150 ml/min, dialysate flow 500 ml/min, dialyzer 1.5 mm2, and heparin as anticoagulant. Abdominal pain and nausea improved immediately, but she was started on supplemental oxygen, delivered by nasal cannula at 2 L/min from the night of hospital day 2. Chest X-rays suggested continued progression of pneumonia (Fig. 2). On day 6, the patient suffered from acute respiratory failure requiring mechanical ventilation with positive end-expiratory pressure as high as 10 cmH2O with fraction of inspired oxygen (FiO2) 1.0. Lopinavir/ritonavir and ciclesonide were then stopped and favipiravir (3600 mg loading dose followed by 1600 mg administered orally daily in two divided doses, comparable to the dose for patients with normal kidney function) was administered for 2 weeks. We monitored laboratory data twice a day to check for adverse events such as pancytopenia and liver dysfunction (Table 1). Transfusions of red blood cells and albumin were conducted as necessary. Mild elevations of alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (γ-GTP) were observed, but these values improved after discontinuation of favipiravir (Fig. 3b). Both parameters improved to normal range, 297U/L and 36U/L respectively, on hospital day 60.
Fig. 1.
Chest CT of the patient. a Ground-glass opacity was observed in the inferior lobes of both lungs on admission. b Density of the lesions decreased when the patient no longer needed mechanical ventilation (Day 27)
Table 1.
Laboratory data by clinical course
| Day 1 | Day 4 | Day 6 | Day 8 | Day 10 | Day 12 | Day 14 | Day 16 | Day 18 | Day 19 | Day 20 | Day 22 | Day 24 | Day 27 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White-cell count (per μl) | 7600 | 6500 | 5400 | 5100 | 7000 | 8000 | 13,900 | 15,400 | 14,200 | 17,000 | 11,900 | 20,000 | 14,800 | 11,300 |
| Absolute lymphocyte count | 900 | 900 | 600 | 600 | 600 | 700 | 1300 | 1100 | 1100 | 1400 | 900 | 1400 | 1700 | 1700 |
| Platelet count (*104 per μl) | 37.0 | 25.7 | 23.9 | 23.6 | 24.7 | 32.9 | 43.1 | 41.8 | 34.2 | 31.1 | 25.1 | 30.9 | 33.3 | 42.0 |
| Hemoglobin (g/dl) | 8.4 | 8.8 | 8.4 | 9.9 | 9.7 | 8.8 | 8.0 | 7.8 | 9.3 | 9.1 | 9.5 | 10.0 | 11.2 | 10.6 |
| Pottasium (mmol/l) | 2.0 | 2.7 | 3.0 | 2.5 | 3.2 | 3.6 | 3.9 | 4.4 | 3.8 | 3.3 | 3.2 | 4.1 | 3.7 | 3.4 |
| Albumin (g/dl) | 2.4 | 2.1 | 2.0 | 2.3 | 2.2 | 2.2 | 2.0 | 1.9 | 2.0 | 2.0 | 2.5 | 2.3 | 3.8 | 3.1 |
| Total bilirubin (mg/dl) | 0.2 | 0.4 | 0.5 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.4 | 0.2 | 0.3 | 0.2 |
| Procalcitonin (ng/ml) | 1.0 | 1.2 | 2.7 | 6.5 | 4.0 | 2.7 | 2.3 | 1.9 | 1.5 | 1.3 | 0.9 | 0.9 | 0.9 | 1.0 |
| Aspartate aminotransferase (U/l) | 13 | 11 | 14 | 17 | 10 | 9 | 13 | 14 | 12 | 12 | 13 | 14 | 17 | 16 |
| Alanin aminotransferase (U/l) | 9 | 6 | 6 | 8 | 4 | 4 | 7 | 10 | 9 | 8 | 9 | 12 | 9 | 13 |
| Lactate dehydrogenase (U/l) | 201 | 229 | 302 | 340 | 261 | 224 | 227 | 235 | 162 | 183 | 179 | 202 | 249 | 162 |
| Alkaline phosphatase (U/l) | 235 | 152 | 133 | 175 | 237 | 283 | 391 | 481 | 560 | 641 | 699 | 720 | 621 | 501 |
| γ-glutamyl transpeptidase (U/l) | 19 | 18 | 19 | 28 | 35 | 39 | 47 | 50 | 70 | 90 | 115 | 146 | 141 | 130 |
| C-reactive protein (mg/dl) | 3.91 | 9.82 | 17.51 | 14.93 | 22.32 | 15.44 | 13.96 | 13.57 | 11.04 | 8.81 | 6.06 | 4.37 | 4.14 | 1.63 |
| Fibrinogen (mg/dl) | 370 | 360 | 385 | 385 | 482 | 551 | 508 | 518 | 509 | 508 | 490 | 529 | 539 | 499 |
| D-dimer (mg/l) | 1.06 | 1.50 | 2.31 | 3.30 | 1.81 | 3.60 | 5.24 | 4.29 | 4.69 | 13.95 | 6.95 | 8.28 | 8.36 | 7.11 |
| Brain natriuretic peptide (pg/ml) | 294.7 | 644.9 | 945.5 | 464.9 | 244.9 | 100.4 | 48.6 | 102.2 | 464.1 | 289.9 | 281.5 | 444.1 | 314.0 | 38.3 |
| P/F ratio | 473 | 268 | 165 | 248 | 214 | 350 | 333 | 327 | 423 | 434 | 475 | 347 | 488 | 461 |
Fig. 2.
Chest X-rays of the patient. Findings worsened until day 6, then gradually improved thereafter. a Day 1; b Day 4; c Day 6; d Day 10; e Day 19; and f Day 24
Fig. 3.
Vital signs and laboratory data. a Body temperature and CRP. Fever gradually improved. CRP peaked on day 10, then decreased. b Concentrations of ALP and γ-GTP. Both ALP and γ-GTP elevated during treatment with favipiravir, but decreased after termination of favipiravir
As CRP levels, P/F ratio and chest X-ray findings gradually improved by day 10 (Fig. 2, 3a Table 1), effect of favipiravir seemed to be appeared approximately in 5 days. Given concerns about concurrent ventilator-associated pneumonia because of the detection of methicillin-resistant Staphylococcus aureus from sputum, teicoplanin (loading dose of 600 mg followed by 200–300 mg administered intravenously every 24 h) and tazobactam/piperacillin (loading dose of 4.5 g followed by 2.25 g administered intravenously every 8 h) was initiated. Body temperature and blood concentration of C-reactive protein (CRP) are shown in Fig. 3a. The worst SOFA score was 9, on hospital day 8, which improved to 5 by hospital day 16. Treatment with favipiravir was terminated on day 19, after 2 weeks of administration. Viral load of SARS-CoV-2 also decreased, falling below the limit of detection on day 24. At this point, the patient was withdrawn from ventilatory support. CT performed on day 27 showed marked improvement of pneumonia (Fig. 1b). No serious thromboembolic events were found during this treatment period.
Discussion
Coronaviruses are positive-sense single-stranded RNA viruses with envelopes of 80–160 nm in diameter. These viruses can be divided into alpha-coronaviruses, causing common cold symptoms, and beta-coronaviruses, which include severe acute respiratory syndrome (SARS) coronaviruses [6] and Middle East respiratory syndrome (MERS) coronaviruses [7]. SARS-CoV-2 is a beta-coronavirus. Many previously reported cases have been middle-aged and the incubation period is 4–7 days [8], as with the patient in this article. The patient seemed to improve slightly at first, respiratory dysfunction rapidly progressed within a week. A previous study noted a similar tendency [9]. A meta-analysis of 46,248 patients from eight studies showed that the most prevalent comorbidities related to severity were hypertension (odds ratio (OR) 2.36, 95% confidence interval (CI) 1.46–3.83), respiratory system disease (OR 2.46, 95%CI 1.76–3.44), and cardiovascular disease (OR 3.42, 95%CI 1.88–6.22) [3]. ESRD patients are also estimated to be at higher risk of severe disease and poorer outcomes from COVID-19 due to suppression of the immune system. However, few experiences with the treatment of COVID-19 in ESRD patients have been reported to date. Considering the structure of the virus, existing agents such as interferon alpha, lopinavir/ritonavir, chloroquine phosphate, ribavirin, and arbidol have been estimated to offer potential efficacy against COVID-19 [4]. The efficacy of lopinavir/ritonavir against COVID-19, which had been expected to be effective against SARS [10], was denied in a previous study [11], and the treatment also seems to have been ineffective in our own case.
Favipiravir is a new type of RNA-dependent RNA polymerase inhibitor that is converted into an active phosphoribosylated form in cells and is recognized as a substrate by viral RNA polymerase, thus inhibiting RNA polymerase activity [12]. Favipiravir is currently undergoing clinical trials for treatment of COVID-19 in Japan. This background led the institutional review board in our hospital to allow treatment of the present patient with favipiravir. According to the report on the deliberating results of favipiravir by ministry of health, labour and welfare published on 2014, favipiravir itself is metabolized mainly in the liver, and plasma concentrations are increased around 1.3-fold in patients with renal insufficiency. However, data are lacking on the extent to which the levels of this drug are impacted by dialysis. Favipiravir hydroxide (M1) is expected to be eliminated by renal excretion and clinical data is lacking regarding clearance by dialysis. Appropriate doses of favipiravir for ESRD patients thus have not been determined and the possibility of adverse events caused by accumulation of M1 should be considered.
The patient in this article showed day-by-day deterioration and required support with mechanical ventilation despite treatment with lopinavir/ritonavir and ciclesonide as first-line treatments. Since the patient had ESRD, heart failure was also suspected, but hemodialysis was performed regularly and body fluid level was tightly controlled by adjusting her dry weight. Respiratory symptoms recovered after starting favipiravir and she was withdrawn from ventilatory support. Although we had been worried about the possibility of pancytopenia or hepatic dysfunction, minimal adverse events were observed along with mild and reversible elevations of ALP and γ-GTP. In addition, we monitored the viral load of SARS-CoV-2 by rRT-PCR, which showed a gradual decrease and eventually fell below the limit of detection. The protocol we used for rRT-PCR conformed to the instructions of LightMix® Modular SARS and Wuhan CoV N-gene, E-gene (Roche, Basel, Switzerland) and LightMix® Modular EAV RNA Extraction Control 660 (Roche, Basel, Switzerland).
The patient had been suffering from chronic diarrhea even before developing COVID-19, so the associations of diarrhea with COVID-19 and favipiravir remain unclear. During the course of treatment, diarrhea and hypokalemia slowly improved with antidiarrheal medicine.
This article is the first report to demonstrate the efficacy of favipiravir in a COVID-19 patient with ESRD undergoing maintenance hemodialysis. In the future, monitoring the concentrations of favipiravir or metabolites and analyzing dialysates when favipiravir is used for ESRD patients on hemodialysis and metabolomics are expected. In conclusion, favipiravir may be one effective option for treating COVID-19-infected ESRD patients.
Author contributions
EK and SS contributed equally to this work. SS, YT, MO, TI, TK and SM participated in deciding the treatment policy of the patient. HH, NJ, HY, MO, YG and AN treated the patient as intensivists. YMi, NT and TY contributed to planning the dosages of antibiotics and antiviral agents administered. IK participated in conducting rRT-PCR assays of SARS-CoV-2. EK and SS participated in writing the manuscript. All authors were involved in drafting, reviewing, and approving the final manuscript.
Funding
The authors have no relevant financial support.
Compliance with ethical standards
Conflict of interest
All the authors have declared no competing interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee at which the studies were conducted and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The use of favipiravir was permitted by the institutional review board in our hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study. Written informed consent for publication of this report was obtained from the patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization [Internet]: Coronavirus disease (COVID-19) Pandemic 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 10 Apr 2020
- 2.Kuroki Y, Hiyama K, Minami J, Takeuchi M, Shojima M, Matsueda S, Nagae H, Nakano T. The first case of COVID-19 pneumonia in a hemodialysis patient in Japan. CEN Case Rep. 2020;18:1–5. doi: 10.1007/s13730-020-00495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020 doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 5.COVID-19 Task Force Committee of the Japanese Association of Dialysis Physicians. Japanese Society for Dialysis Therapy. Japanese Society of Nephrology. Kikuchi K, Nangaku M, Ryuzaki M, Yamakawa T, Hanafusa N, Sakai K, Kanno Y, Ando R, Shinoda T, Nakamoto H, Akizawa T. COVID-19 of dialysis patients in Japan: Current status and guidance on preventive measures. Ther Apher Dial. 2020;24(4):361–365. doi: 10.1111/1744-9987.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 7.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(3):252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(7):449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]