Abstract
Introduction
Respiratory complications (RC) are a leading cause of death after spinal cord injury (SCI) due to compromised immune function and respiratory muscle weakness. Thus, individuals with SCI are at high risk of developing COVID-19 related RC. Results of a SCI clinical trial showed a supervised respiratory muscle training (RMT) program decreased risk of developing RC. The feasibility of conducting unsupervised RMT is not well documented. Four publications (n = 117) were identified in which unsupervised RMT was performed. Significant improvements in respiratory outcomes were reported in two studies: Maximal Inspiratory and Expiratory Pressure (MIP40% and MEP25%, respectively), Peak Expiratory Flow (PEF9%), seated and supine Forced Vital Capacity (FVC23% and 26%, respectively), and Peak Cough Flow (28%). This review and case report will attempt to show that an inspiratory muscle training (IMT) home exercise program (HEP) is feasible and may prepare the respiratory system for RC associated with COVID-19 in patients with SCI.
Case presentation
A 23-year-old with tetraplegia (P1), history of mechanical ventilation, and hospitalization for RC, completed 27 IMT HEP sessions in one month. MIP and sustained MIP (SMIP) increased from baseline by 28% and 26.5%, respectively. Expiratory volumes and rates also improved (FVC, FEV1, and PEF: 11.7%, 8.3%, and 14.2%, respectively).
Discussion
The effects of COVID-19 on patients with SCI remains inconclusive, but recent literature and the results of this case suggest that unsupervised IMT is feasible and may limit the severity of RC in patients with SCI who contract COVID-19.
Subject terms: Rehabilitation, Risk factors
Introduction
Respiratory complications are a leading cause of death for people with spinal cord injury (SCI), and this rate is rising [1–6]. A change in neurological input to the respiratory system and a physical change in breathing mechanics after SCI are the likely cause of respiratory muscle complications including respiratory failure, atelectasis, aspiration, sputum retention, and the need for mechanical ventilation (MV) [2, 7]. Pneumonia, a prevalent cause of death for individuals with SCI, appears to be a common, and serious manifestation of COVID-19 [8–11]. As such, individuals with SCI are at a high risk of developing complications secondary to COVID-19 due to compromised immune function and underlying respiratory weakness [12–14]. There have been case reports of 16 individuals with SCI and concomitant COVID-19 reported thus far. While one study reported an asymptomatic case of an individual with acute SCI due to the increased screening efforts found at many hospitals [15], nine of these individuals had some form of imaging evidence indicating pneumonia [14, 16–19]. Even though pneumonia was a prevalent clinical feature of their illness, clinical symptoms such as dyspnea and cough were not as predominant as fever [14, 16–19]. Fever appears to be the most prevalent, and often the first symptom of COVID-19 for individuals with SCI, followed by lymphocytopenia [14, 16–19]. In addition, all individuals who were tracheostomy-reliant prior to COVID-19 required aspiration and hyperinflation techniques for secretion removal, when only half of them required these maneuvers prior to COVID-19 diagnosis [14]. Interestingly, six of the 16 patients required some form of non-invasive oxygen therapy during the course of the virus [14, 16–18], and only one patient required intubation with successful extubation [19]. Three deaths have been reported in individuals with SCI and a comorbid COVID-19 diagnosis [15, 18]. In one of these cases, COVID-19 did not appear to be the cause of death for an individual with acute SCI, but factors characteristic of COVID-19 such as increased coagulopathy or cardiac issues may have played a role in their death [18]. The other two patients who did not survive had chronic SCI and were over 80 years old [15]. The relatively low death rate of individuals with SCI due to COVID-19 may be related to a muted immunoresponse limiting symptoms and severity of this novel coronavirus in the SCI population [14, 16, 17]. It is still recommended that these individuals receive close monitoring and preventative therapies where available [14, 16–18].
In 14 of the 16 reported cases patients were already admitted to a care facility when they contracted the virus and were thus able to receive immediate care [16, 19]. This forewarns that favorable outcomes associated with early intervention may not continue to be the norm in this population as one of these individuals required intubation [19]. Additionally, fifty-eight percent of participants in a global survey of SCI clinicians stated that patients with SCI and disease (SCI/D) in their care were given emergency room or hospital level care due to COVID-19 [20]. About 60% of practitioners also felt that their patients with SCI/D were not given enough information about COVID-19. Over one third (34.1%) of responding clinicians had been contacted by their patients with COVID-19 related concerns [20]. The most common anxiety reported by patients with SCI/D was vulnerability to infection (76.9%) [20]. Additional concerns included the inability to obtain testing (28.5%) or transportation to medical appointments (21.3%) [20].
In light of the recent increases in COVID-19 infections in both the SCI and non-SCI populations, and still limited information on risk mitigation and infection outcomes, it is important to provide as much education and preventative therapies as possible. A home-based inspiratory muscle training (IMT) program has been proposed as an option for COVID-19 and pandemic preparation [21]. Respiratory muscle training (RMT) has shown benefits in pulmonary function in people with SCI but its feasibility to be conducted in the home setting is not well documented [22, 23]. IMT will not prevent COVID-19 contraction but may limit the need for supplemental oxygen therapy or decrease the impact of pneumonia on this vulnerable population if completed before or early after diagnosis. A home-based RMT program would also limit the transportation needed to attend multiple appointments and limit unnecessary exposure for patients.
This article reviews home-based RMT programs completed by individuals with SCI that have been published since January 1, 2019 and incorporates data from a single case study to show the respiratory improvements that are possible for this population. The purpose of this brief review and case report is to show that a home-based IMT program is both feasible and may be an effective therapy to prepare the respiratory system of patients with SCI for COVID-19 and its secondary impacts, first postulated by Sanchez-Raya and Sampol [20].
Review of recent literature
The term “Spinal Cord Injury” was paired with “Respiratory Muscle Training,” “Inspiratory Muscle Training,” and “Expiratory Muscle Training” as search criteria in PubMed and Google Scholar. Results were limited to articles published no earlier than January 1, 2019. These search criteria identified 28 articles. An additional case study known to the author was added. Articles were included if they (a) specifically stated that the respiratory interventions were not supervised or (b) provided evidence of a logbook or training diary. Four publications met all inclusion criteria (Table 1) [24–27].
Table 1.
Review table of respiratory muscle training interventions completed at home or mostly without supervision.
| Shin 2019 | Repecki 2019 | Gee 2019 | Leatham 2019 | Current Case | |
|---|---|---|---|---|---|
| Location | Tertiary University Hospital | Community Program/HEP | HEP (most likely) | Community Program/HEP | HEP |
| (n) and sample description | (104) inpatients with acute to chronic injury | (1) 29YOM, chronic SCI, C5-6 AIS B | (6) wheelchair rugby athletes | (10 but 4 dropped out: n = 6) community volunteers | (1) 23 YOM, chronic SCI, C3 AIS B |
| Intervention | Glossophrangeal breathing, IMT with incentive spirometer: 2 sets of 20 reps, ≥ 5 days/week (Coach 2 Device), Air Stacking with resuscitation bag | 6 weeks: SMX classes 1x/week 8 Weeks: SMX classes 2x/week All Weeks: IMT (device not reported) at home and Aerobic/Strength work 3x/wk | IMT and EMT 5 days /week with Powerlung device- 30 breaths per session | SMX classes 1x/week with IMT at home with goal of 30 breaths over 2 sessions/day, 5 days/week with Threshold device | Daily IMT with PrO2FIT device |
| Intensity | Not reported | Not reported | Started at ~60% of MIP/MEP and increased to ~80% | Initial training intensity: “[participants] can complete ten breaths without symptoms of hyperventilation.” | 80% of Max breath |
| Supervision | 1x/week by Physiotherapist | At home, unsupervised | No | Not reported | 1x/week by Physical Therapist |
| Duration | 4–8 weeks | 14 weeks | 6 weeks | 8 weeks | 4 weeks |
| Compliance | Not Reported | Participant did not turn in IMT training diary | Required at least 80% for participation. | 4 participants dropped out (2 in hospital, 2 did not complete IMT) | 96% |
| Actual: 98% | Not Reported | ||||
| Key Respiratory findings (as Pre/Post percent change) | No Respiratory Outcomes Reported | MIP: +40% | No Respiratory Outcomes Reported | MIP: +28% | |
| FVC in supine: +26% | MEP: +25% | SMIP: +26.5% | |||
| FVC in sitting: +23% | FVC: +1.6% ns | FVC: +11.7% | |||
| PCF: +28% | FEV1: +1.5% ns | FEV1: +8.3% | |||
| PEF: +9% | PEF: +14.8% | ||||
| MEP: +/− 0% |
SMX Spinal Mobility X, IMT inspiratory muscle training, EMT expiratory muscle training, MIP maximal inspiratory pressure, FVC forced vital capacity, FEV1 forced expiratory volume in one second, PEF peak expiratory flow, PCF peak cough flow, MEP maximal expiratory pressure.
One hundred-seventeen individuals in these four publications completed a variant of RMT as a home exercise program (HEP). The largest study, enrolling 104 participants, was conducted in a tertiary hospital but patients were instructed to complete the IMT-based HEP on their own with a weekly check-in [27]. All other studies appeared to have participants training in their homes and reporting compliance with a training log or diary [24–26]. Only one study reported treatment compliance (98%) [24]. Repecki et al. reported on a case of a 29 year-old-male who was completing a community-based exercise program in addition to undergoing an IMT HEP, but did not return his training diary [26].
Table 1 describes the diverse interventions reported by these four studies and provides examples of the multiple options available for individuals with SCI to improve pulmonary function in the home setting. The Repecki case study and the Leatham et al. publication described similar study interventions, with participants completing both an IMT HEP and a weekly or bi-weekly community fitness class [25, 26]. The patients included in the article by Shin and colleagues were instructed on a multistep program including glossopharyngeal breathing, IMT with an incentive spirometer, and air stacking [27]. While the athletes in the study conducted by Gee et al. completed RMT, by jointly performing both IMT and expiratory muscle training (EMT) [24]. The authors reported goal frequencies of about 5 days per week with treatment durations ranging from four to fourteen weeks.
Similar to the interventions detailed in this collection of RMT studies, the outcomes reported in these four studies were varied. Two of the four studies provided respiratory-based results [24, 27], while the community-based program studies reported positive findings in functional outcomes including the T-shirt Test, Timed Transfer Test, hand held dynamometry, and Modified Functional Reach [25, 26]. The wheelchair athletes saw significant improvement in maximal inspiratory pressure (MIP) (40%), maximal expiratory pressure (MEP) (25%) and peak expiratory flow rate (PEF) (9%), while forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) did not change significantly (1.6 and 1.5%, respectively) [24]. The authors determined that these highly trained athletes already possessed high pulmonary function and thus did not experience further improvement [24]. The less fit patients studied by Shin et al. experienced significant improvements in FVC in the seated (23%) and supine (26%) positions, as well as in peak cough flow (PCF) (28%) [27]. The significant improvements in pulmonary function measured during expiratory maneuvers are especially interesting because the HEP intervention used by these inpatients focused heavily on IMT [27].
Overall, these studies show that individuals with SCI have the ability to successfully perform RMT exercise in a non-supervised setting. This review also highlights that varied types of respiratory training can be completed and both IMT- and RMT-based programs can improve pulmonary function. These data provide evidence that education and initiation of these types of training paradigms are valuable in the setting of a current global pandemic to improve upon underlying respiratory muscle weakness and bolster respiratory function prior to and after COVID-19 infection.
Case presentation
P1 is a 23-year-old male who sustained a SCI after a motor vehicle accident (MVA) in August 2013. His current International Standards Neurological Classification Spinal Cord Injury (ISNCSCI) level of injury is C3 and the American Spinal Injury Association Impairment Scale (AIS) grade is B with a motor score of 4 at C5 bilaterally. His past medical history includes multiple pulmonary complications post-SCI. Initially, the MVA caused large bilateral pneumothoraces requiring at least three chest tubes. He was intubated and required mechanical ventilation for about two months and then weaned to a tracheostomy. He was decannulated after 2 years post-tracheostomy. P1 has a more recent history of being hospitalized for sepsis and bronchitis (April 2019).
Respiratory function testing, including inspiratory and expiratory assessment, was completed prior to and upon completion of four weeks of IMT. Inspiratory performance, including MIP measured in cm H2O, sustained MIP (SMIP) measured in pressure time units (PTU), and inspiratory duration (ID) measured in seconds, were assessed using the PrO2FIT device (Smithfield, RI). Pulmonary function tests (PFT) were administered with the Micro I Spirometer (Lewiston, ME) and maximal expiratory pressure (MEP) was assessed with the MicroMPM (Lewiston, ME). All initial PFT scores for P1 were at or below 33% of the predicted values based on the NHANES III criteria (Table 2).
Table 2.
PO1 respiratory performance throughout the study.
| MIP | SMIP | ID | FVC | FVC % predicted | FEV1 | FEV1 % predicted | PEF | PEF % predicted | MEP | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 50 | 196 | 9.9 | 1.71 | 28% | 1.68 | 33% | 3.51 | 31% | 50 |
| Week 2 | 59 | 208 | 9.1 | 1.9 | 32% | 1.87 | 37% | 4.22 | 38% | 46 |
| Week 3 | 61 | 244 | 8.9 | 1.8 | 31% | 1.73 | 35% | 3.97 | 36% | 45 |
| Week 4 | 60 | 235 | 10.7 | 1.85 | 31% | 1.82 | 36% | 3.35 | 30% | 48 |
| Follow-up | 64 | 248 | 10 | 1.91 | 32% | 1.82 | 36% | 4.01 | 36% | 50 |
| Actual change | 14 | 52 | 0.1 | 0.2 | 4% | 0.14 | 3% | 0.5 | 5% | 0 |
| Percent change | 28% | 26.5% | 1% | 11.7% | 8.3% | 14.2% | 0% |
MIP maximal inspiratory pressure measured in cmH2O, SMIP sustained MIP measured in pressure time units, ID inspiratory duration measured in seconds, FVC forced vital capacity measured in liters, FVC % Predicted percent of predicted FVC based on NHANES criteria, FEV1 forced expiratory volume in one second measured in liters, FEV1 % Predicted percent of predicted FEV1 based on NHANES criteria, PEF peak expiratory flow measured in liters/second, PEF % Predicted percent of predicted PEF based on NHANES criteria, MEP maximal expiratory pressure.
P1 completed 27 sessions of IMT administered over 28 days using the PrO2FIT device, including four supervised in-person sessions occurring during a scheduled weekly check-in. The PrO2FIT device is a resisted inspiratory trainer with a 2 mm leak that transmits inspiratory information to the device application on a Bluetooth-paired tablet to create a live graph of the user’s inspiratory function (Fig. 1). Each training session with the PrO2FIT device began with a baseline test created by a maximal effort breath where P1 was instructed to exhale to residual volume and inhale as fast and strongly as possible and then continue to inspire until total lung capacity was reached. P1 would then train at 80% of this maximum effort for 42 breaths as guided by the application, with visual and auditory feedback provided to reinforce MIP and SMIP training goals. The 42 breaths were performed in 7 sets of 6 breaths, with a decreasing amount of rest time between each active breath per set (i.e., Set 1 rest time: 40 s; Set 2 rest time: 30 s; Set 3 rest time: 25 s;…; Set 7 rest time: 5 s). Training sessions occupied 26 min on average. Rate of Perceived Exertion scores recorded in a logbook after each training session ranged from 3 to 7 on a 0–10 Borg Categorical Scale (mode: 6).
Fig. 1. User interface during Inspiratory Muscle Training with the PrO2FIT device and accompanying tablet application.
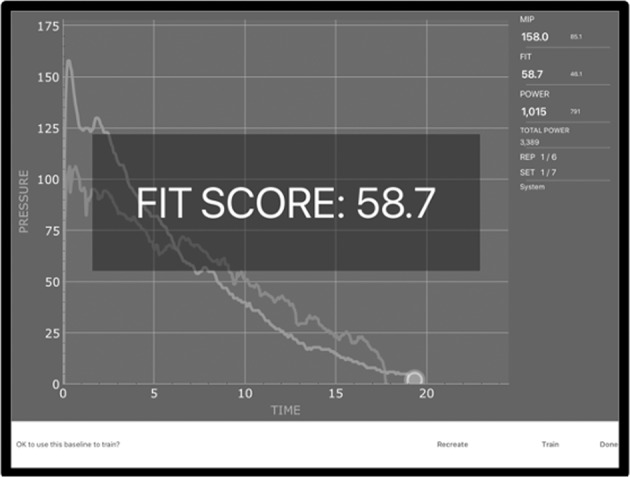
The FIT score is a measure created by the PrO2 manufacturer and was not investigated in this case study.
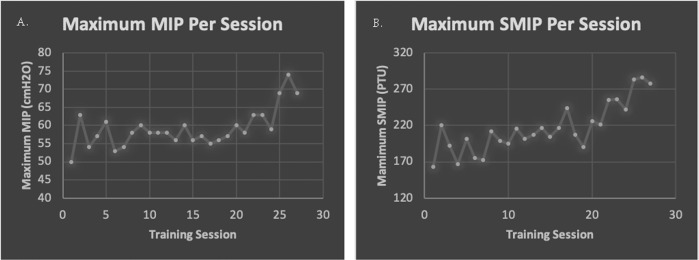
All respiratory measures except MEP improved after 27 treatment sessions. MIP and SMIP increased from baseline by 28% and 26.5%, respectively. Even though training emphasized inspiratory performance, improvements in expiratory volumes and rates were observed (FVC: +11.7%, FEV1: +8.3%, PEF: +14.2%). It is likely that the functional increase in FVC is clinically significant as the change in percent predicted (4%) falls within the MCID reported for individuals with scleroderma [28]. ID improved by 1% and MEP did not change from baseline to follow-up testing. The largest improvement came from Total Power (TP), or the summation of SMIPs created during a training period (Fig. 2). The TP of the first training session was 5,775 PTU, and the TP of the 27th session increased by 86% to 10,724 PTU. Improvements in MIP and SMIP are observed in the highest pressures created per training session, as sometimes P1 was able to improve upon the baseline score within the session (Fig. 3a, b). Functionally, P1 stated that he and his mother noticed an improved ability to sneeze which he attributed to the training.
Fig. 2. Total Power plotted throughout the training period.
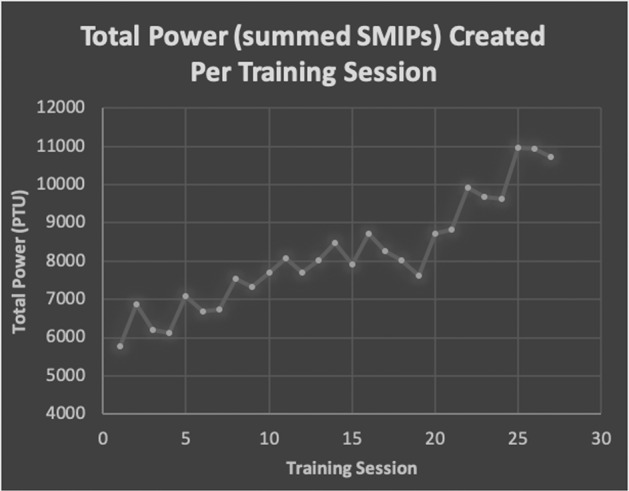
The summed sustained maximal inspiratory pressures (SMIPs) created during a training session with 42 breaths with a target of 80% of a maximum effort baseline breath.
Fig. 3. The change in MIP and SMIP throughout the training period.
Plots depicting the highest maximal inspiratory pressure (MIP) (a) and sustained MIP (SMIP) (b) reached by P1 throughout each training session. These data may or may not include baseline measures.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Discussion
This case presentation and review of recent publications demonstrate both feasibility and respiratory system benefits of a home-based IMT program for use by persons with SCI. Table 1 shows recent home-based RMT programs that report similar home training paradigms (Table 1) [24–27]. Due to varied treatment intervals, interventions, and study endpoints, it is difficult to compare the RMT HEPs to one other. This case study, however, shows that a non-athlete with cervical SCI can perform IMT and improve upon their inspiratory and expiratory function.
In addition to providing evidence for feasibility, the observed improvements in pulmonary function may provide a preventive health benefit. IMT has been used to prepare individuals for surgery and reduce instances of post-surgical pulmonary compromise, including pneumonia [29–32]. Additionally, Boswell-Ruys et al. recently reported the results of a 6-week supervised RMT randomized clinical trial, which showed that individuals with SCI in the intervention group experienced significantly fewer respiratory complications in the following year than participants with SCI undergoing sham treatment [33]. This study reported a significantly higher MIP but not MEP in the intervention group post-RMT, and found significant correlations between MIP and inspiratory capacity, vital capacity, and PEF while coughing. No significant relationships between MEP and these measures were observed [33]. These findings could explain why P1 experienced increases in pulmonary function but not MEP. This inspiratory driven relationship more strongly associating improved cough, vital capacity, and peak cough flow, to MIP than MEP has been reported in multiple SCI-based studies, and is the major reason IMT was not paired with EMT in this case study [34–36].
While MIP has been extensively studied in SCI and other populations, SMIP is a newer measure of respiratory health. Fromiga et al. reported a stronger relationship among SMIP, lung function, and symptom severity than with MIP in a sample of veterans with COPD [37]. In addition, SMIP was a more sensitive and specific predictor for successful extubation outcome than MIP, further linking lung function and SMIP [38]. Thus, patients with greater SMIP appear to wean more easily from mechanical ventilation which would be beneficial for patients with COVID-19 and other infectious diseases. Furthermore, it is possible that greater levels of SMIP may even prevent the need for mechanical ventilation in some patients with SCI, but investigation of this is needed. While SMIP has not been previously reported in patients with SCI, we report a 26.5% increase in SMIP, an 86% increase in TP, and a 28% increase in MIP in this case subject. Furthermore, these inspiratory performance improvements occurred following just four weeks of IMT in the home setting.
The current case study, paired with recent SCI-based RMT publications, provides support for the implementation of preventative IMT HEPs to lessen the severity of infection-related respiratory compromise in some patients with SCI [20]. Additionally, P1 had a urinary tract infection on the post-IMT testing day and it is unknown how this may have impacted his respiratory performance. Lastly, the 4-week time period of this study may not have allowed us to fully capture improvements in respiratory function based on the upward trends of the data found in the figures provided.
In conclusion, evidence to understand how COVID-19 will impact the SCI community remains incomplete. This population may be at a great risk of developing significant morbidity due to COVID-19, and anxieties related to COVID-19 have already been reported to clinicians globally by those living with SCI. This case study, along with the recent literature of RMT HEPs provides evidence that a home-based IMT program is feasible and may be an effective prophylaxis to respiratory dysfunction in patients with SCI who contract the COVID-19 virus.
Compliance with ethical standards
Conflict of interest
This research was supported through funding by the Craig H. Neilsen Foundation in conjunction with the American Spinal Injury Association and the Foundation for Physical Therapy Research. The Miami Project to Cure Paralysis offered space and access to a database of possible participants for recruiting purposes.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Savic G, DeVivo MJ, Frankel HL, Jamous MA, Soni BM, Charlifue S. Causes of death after traumatic spinal cord injury-a 70-year British study. Spinal Cord. 2017;55:891–97. doi: 10.1038/sc.2017.64. [DOI] [PubMed] [Google Scholar]
- 2.McDonald T, Stiller K. Inspiratory muscle training is feasible and safe for patients with acute spinal cord injury. J Spinal Cord Med. 2018:1–8. 10.1080/10790268.2018.1432307. [DOI] [PMC free article] [PubMed]
- 3.Krause JS, Cao Y, DeVivo MJ, DiPiro ND. Risk and protective factors for cause-specific mortality after spinal cord injury. Arch Phys Med Rehabil. 2016;97:1669–78. doi: 10.1016/j.apmr.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Berlly M, Shem K. Respiratory management during the first five days after spinal cord injury. J Spinal Cord Med. 2007;30:309–18. doi: 10.1080/10790268.2007.11753946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopp MA, Watzlawick R, Martus P, Failli V, Finkenstaedt FW, Chen Y, et al. Long-term functional outcome in patients with acquired infections after acute spinal cord injury. Neurology. 2017;88:892–900. doi: 10.1212/WNL.0000000000003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leite VF, Souza DR, Imamura M, Battistella LR. Post-discharge mortality in patients with traumatic spinal cord injury in a Brazilian hospital: a retrospective cohort. Spinal Cord. 2019;57:134–40. doi: 10.1038/s41393-018-0183-y. [DOI] [PubMed] [Google Scholar]
- 7.Claxton AR, Wong DT, Chung F, Fehlings MG. Predictors of hospital mortality and mechanical ventilation in patients with cervical spinal cord injury. Can J Anaesth. 1998;45:144–9. doi: 10.1007/BF03013253. [DOI] [PubMed] [Google Scholar]
- 8.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed]
- 12.López-Dolado E, Gil-Agudo A. Lessons learned from the coronavirus disease 2019 (Covid-19) outbreak in a monographic center for spinal cord injury. Spinal Cord. 2020 doi: 10.1038/s41393-020-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malfatti CG, Cortés PB, Fernández A, Ureña PJC, Baquero EV, Henares FG, et al. Respiratory rehabilitation protocol for patients with spinal cord injury and COVID-19. Secondary Respiratory rehoabilitation protocol for patients with spinal cord injury and COVID-19 2020. https://www.iscos.org.uk/uploads/CV-19/updated%20files%204%2024%2020/ENG_SCI_and_COVID_19_Respiratory.pdf. Accessed Date: 30 May 2020.
- 14.Gil-Agudo A, Jimenez-Velasco I, Gutierrez-Henares F, Lopez-Dolado E, Gambarrutta-Malfatti C, Vargas-Baquero E. Clinical features of coronavirus disease 2019 (COVID-19) in a cohort of patients with disability due to spinal cord injury. MedRXiv. 2020. 10.1101/2020.04.20.20072918. [DOI] [PMC free article] [PubMed]
- 15.Sánchez-Raya J, Sampol J. Spinal cord injury and COVID-19: some thoughts after the first wave. Spinal Cord. 2020. 10.1038/s41393-020-0524-5. [DOI] [PMC free article] [PubMed]
- 16.Righi G, Del Popolo G. COVID-19 tsunami: the first case of a spinal cord injury patient in Italy. Spinal Cord Ser Cases. 2020;6:22. doi: 10.1038/s41394-020-0274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korupolu R, Stampas A, Gibbons C, Hernandez Jimenez I, Skelton F, Verduzco-Gutierrez M. COVID-19: Screening and triage challenges in people with disability due to Spinal Cord Injury. Spinal Cord Ser Cases. 2020;6:35. doi: 10.1038/s41394-020-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pattanakuhar S, Tangvinit C, Kovindha A. A patient with acute cervical cord injury and COVID-19: a first case report. Am J Phys Med Rehabil. 2020. 10.1097/PHM.0000000000001485. [DOI] [PMC free article] [PubMed]
- 19.Ayyildiz A, Kuran B, Altoparlak B, Dogu B, Yilmaz F. Complications of spinal cord injury can hide fever and cough associated to COVID-19. Asian J Case Rep. Med Health. 2020;3:1–5. [Google Scholar]
- 20.Stillman MD, Capron M, Alexander M, Di Giusto ML, Scivoletto G. COVID-19 and spinal cord injury and disease: results of an international survey. Spinal Cord Ser Cases. 2020;6:21. doi: 10.1038/s41394-020-0275-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Severin R, Arena R, Lavie CJ, Bond S, Phillips SA. Respiratory muscle performance screening for infectious disease management following COVID-19: a highly pressurized situation. Am J Med. 2020. 10.1016/j.amjmed.2020.04.003. [DOI] [PMC free article] [PubMed]
- 22.Tamplin J, Berlowitz DJ. A systematic review and meta-analysis of the effects of respiratory muscle training on pulmonary function in tetraplegia. Spinal Cord. 2014;52:175–80. doi: 10.1038/sc.2013.162. [DOI] [PubMed] [Google Scholar]
- 23.Lemos JR, da Cunha FA, Lopes AJ, Guimarães FS, do Amaral Vasconcellos FV, dos Santos Vigário P. Respiratory muscle training in non-athletes and athletes with spinal cord injury: A systematic review of the effects on pulmonary function, respiratory muscle strength and endurance, and cardiorespiratory fitness based on the FITT principle of exercise prescription. J Back Musculoskelet Rehabilitation. 2019;Preprint:1–13. doi: 10.3233/BMR-181452. [DOI] [PubMed] [Google Scholar]
- 24.Gee CM, Williams AM, Sheel AW, Eves ND, West CR. Respiratory muscle training in athletes with cervical spinal cord injury: effects on cardiopulmonary function and exercise capacity. J Physiol. 2019;597:3673–85. doi: 10.1113/JP277943. [DOI] [PubMed] [Google Scholar]
- 25.Leathem JM, Macht-Sliwinski M, Boak S, Courville A, Dearwater M, Gazi S, et al. Community exercise for individuals with spinal cord injury with inspiratory muscle training: a pilot study. J Spinal Cord Med. 2019:1–9. 10.1080/10790268.2019.1655200. [DOI] [PMC free article] [PubMed]
- 26.Repecki C, Sliwinski M, Harding L. Supporting the need for community exercise programs: a case study. Spinal Cord Ser Cases. 2019;5:95. doi: 10.1038/s41394-019-0236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin JC, Han EY, Cho KH, Im SH. Improvement in Pulmonary Function with Short-term Rehabilitation Treatment in Spinal Cord Injury Patients. Sci Rep. 2019;9:17091. doi: 10.1038/s41598-019-52526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kafaja S, Clements PJ, Wilhalme H, Tseng C, Furst DE, Kim G, et al. Reliability and minimal clinically important differences of FVC. Results from the Scleroderma Lung Studies (SLS-I and SLS-II) Am J Resp Crit Care. 2018;197:644–52. doi: 10.1164/rccm.201709-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dronkers J, Veldman A, Hoberg E, van der Waal C, van Meeteren N. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: a randomized controlled pilot study. Clin Rehabil. 2008;22:134–42. doi: 10.1177/0269215507081574. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths SV, Conway DH, Investigators P-C, Sander M, Jammer I, Grocott MPW, et al. What are the optimum components in a care bundle aimed at reducing post-operative pulmonary complications in high-risk patients? Perioper Med (Lond) 2018;7:7. doi: 10.1186/s13741-018-0084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulzebos EH, Helders PJ, Favie NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA. 2006;296:1851–7. doi: 10.1001/jama.296.15.1851. [DOI] [PubMed] [Google Scholar]
- 32.Thybo Karanfil EO, Moller AM. Preoperative inspiratory muscle training prevents pulmonary complications after cardiac surgery - a systematic review. Dan Med J. 2018;65:A5450. [PubMed] [Google Scholar]
- 33.Boswell-Ruys CL, CRH Lewis, Wijeysuriya NS, McBain RA, Lee BB, McKenzie DK, et al. Impact of respiratory muscle training on respiratory muscle strength, respiratory function and quality of life in individuals with tetraplegia: a randomised clinical trial. Thorax. 2020;75:279–88. doi: 10.1136/thoraxjnl-2019-213917. [DOI] [PubMed] [Google Scholar]
- 34.Postma K, Vlemmix LY, Haisma JA, de Groot S, Sluis TA, Stam HJ, et al. Longitudinal association between respiratory muscle strength and cough capacity in persons with spinal cord injury: an explorative analysis of data from a randomized controlled trial. J Rehabil Med. 2015;47:722–6. doi: 10.2340/16501977-1986. [DOI] [PubMed] [Google Scholar]
- 35.Park JH, Kang SW, Lee SC, Choi WA, Kim DH. How respiratory muscle strength correlates with cough capacity in patients with respiratory muscle weakness. Yonsei Med J. 2010;51:392–7. doi: 10.3349/ymj.2010.51.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang SW, Shin JC, Park CI, Moon JH, Rha DW, Cho DH. Relationship between inspiratory muscle strength and cough capacity in cervical spinal cord injured patients. Spinal Cord. 2006;44:242–8. doi: 10.1038/sj.sc.3101835. [DOI] [PubMed] [Google Scholar]
- 37.Formiga MF, Vital I, Urdaneta G, Campos MA, Cahalin LP. Beyond inspiratory muscle strength: Clinical utility of single-breath work capacity assessment in veterans with COPD. Respir Med. 2019;147:13–18. doi: 10.1016/j.rmed.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Bruton A. A pilot study to investigate any relationship between sustained maximal inspiratory pressure and extubation outcome. Heart Lung. 2002;31:141–9. doi: 10.1067/mhl.2002.122840. [DOI] [PubMed] [Google Scholar]