Abstract
The aim of the present study was to determine the effect of microRNA (miR)-132 on cardiac fibrosis in myocardial infarction (MI)-induced heart failure and angiotensin (Ang) II-treated cardiac fibroblasts (CFs). Experiments were carried out in Sprague-Dawley rat treatment with ligation of left coronary artery to induce heart failure, and in CFs administration of Ang II to induce fibrosis. The level of miR-132 was increased in the heart of rats with MI-induced heart failure and the Ang II-treated CFs. In MI rats, left ventricle (LV) ejection fraction, fractional shortening, the maximum of the first differentiation of LV pressure (LV +dp/dtmax) and decline (LV -dp/dtmax) and LV systolic pressure (LVSP) were reduced, and LV end-systolic diameter (LVESD), LV end-diastolic diameter (LVEDD), LV volumes in systole (LVVS) and LV volumes in diastole (LVVD) were increased, which were reversed by miR-132 agomiR but deteriorated by miR-132 antagomiR. The expression levels of collagen I, collagen III, transforming growth factor-β (TGF-β), and α-smooth muscle actin (α-SMA) were increased in the heart of rat with MI-induced heart failure and CFs administration of Ang II. These increases were inhibited by miR-132 agomiR but enhanced by miR-132 antagomiR treatment. MiR-132 inhibited PTEN expression, and attenuated PI3K/Akt signal pathway in CFs. These results indicated that the up-regulation of miR-132 improved the cardiac dysfunction, attenuated cardiac fibrosis in heart failure via inhibiting PTEN expression, and attenuating PI3K/Akt signal pathway. Up-regulation of miR-132 may be a strategy for the treatment of heart failure and cardiac fibrosis.
Keywords: cardiac dysfunction, cardiac fibroblasts, fibrosis, heart failure, microRNA -132
Introduction
Heart failure, a complex syndrome resulting from structural or functional cardiac disorders, can disable the ventricle from ejecting or filling blood [1–3]. Despite the progress in diagnosis, heart failure is still a leading cause of morbidity and mortality worldwide [4–6]. Heart failure is preceded by left ventricular (LV) remodeling, which is characterized by the formation of cardiac interstitial fibrosis, including the increases of collagen I, collagen III, α-smooth muscle actin (α-SMA), and transforming growth factor-β (TGF-β) [7–9].
MicroRNAs (miRs), a group of small, naturally occurring and non-coding RNAs, can negatively regulate gene expressions through promoting mRNA degradation or inhibiting mRNA translation [10–13]. Many miRNAs have been recognized as biomarkers and possible therapeutic targets for the diagnosis and treatment of diseases [14]. Human studies and animal experiments have found multiple miRs, including miR-24, -199b, -100, -195, -208, and -133, are dysregulated in heart failure [15–19]. However, the relevant mechanisms are far from being understood.
Circulating miR-132 level was associated with heart failure, and it rose with the severity of heart failure [20]. However, another study found that miR-132 expression was down-regulated in the blood of heart failure patients [21]. Moreover, overexpression of miR-132 dramatically enhanced the antioxidant stress and antiapoptotic ability of H9C2 cells [21]. MiR-132 activated the phosphateidylinositol 3-kinase/protein kinase (PI3K/Akt) signal transduction pathway via inhibiting phosphatase and tensin homolog (PTEN) expression, thus facilitating cardiocyte proliferation and attenuating apoptosis and cardiac fibrosis in rats with doxorubicin-induced dilated cardiomyopathy (DCM) [22]. However, whether miR-132 attenuates heart failure and reduces cardiac fibrosis in MI-induced heart failure is still not well known. The purpose of the present study was to explore the curative effects of miR-132 on cardiac dysfunction and heart fibrosis in rats with MI-induced heart failure.
Materials and methods
Animals
The experiments were performed at Animal Core Facility of Nanjing Medical University using male Sprague-Dawley (SD) rats (180-200g, Vital River Biological Co., Ltd, Beijing, China). The rats were kept in a temperature-controlled room on a 12 h light–dark cycle with free access to standard chow and tap water. All procedures were approved by the Experimental Animal Care and Use Committee of Nanjing Medical University (Nanjing, China) in 2017 (No.14051386), and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1996).
Myocardial infarction model
The myocardial infarction (MI) in the rat model was induced by coronary artery ligation with sterile techniques as previously reported [23]. Briefly, the rats (180–200 g), anesthetized with sodium pentobarbital (50 mg/kg, i.p.), were randomly subjected to the ligation of the left anterior descending coronary artery or the sham-operated (Sham) groups. The heart was exposed through a left intercostal thoracotomy with the left coronary artery looped by a single nylon suture. Then, the heart was quickly repositioned into the chest. The Sham rats were treated in the same way as the coronary ligation rats except that their left anterior descending coronary arteries were not ligated.
Angiotensin II rat model
Male SD rats weighing 180–200 g were infused with Ang II (500 ng/kg/min, Sigma, MO, U.S.A.) or saline (solvent control) via osmotic minipumps (model 2004; ALZET, CA, U.S.A.) with an infusion rate of 0.25 μl/h that were surgically placed below the neck for 4 weeks.
MiRNA-132 agomiR and antagomiR treatment in rats
To determine the effects of miR-132 on MI-induced HF, the rats were injected with miR-132 agomiR (a 2′OME + 5′chol modified miR-132 agonist, 40 mg/kg/day) or antagomiR (a 2′OME+5′chol modified miR-132 inhibitor, 40mg/kg/day) 7 days after MI via tail vein for three consecutive days. The miR-132 agomiR and antagomiR were obtained from RIBOBIO (Guangzhou, China). After 4 weeks of injection, the rats were killed with an overdose of sodium pentobarbital (100 mg/kg, i.p.).
Echocardiography
Transthoracic echocardiography was performed using an ultrasound system (VisualSonics, Toronto, Canada) with a 21-MHz probe under isoflurane (2.0%) anesthesia. Measurements over three consecutive cardiac cycles were averaged. The ejection fraction (EF) and fractional shortening (FS) of left ventricular (LV) in rats were calculated. The LV end-systolic diameter (LVESD), LV end-diastolic diameter (LVEDD), LV volumes in systole (LVVS), and LV volumes in diastole (LVVD) were measured.
Hemodynamic monitoring
The rats were anesthetized with isoflurane (2.0%). A conductance micromanometer catheter (1.4F, Millar Instruments, TX, U.S.A.) was inserted into the LV chamber for hemodynamic monitoring via the left carotid artery. The maximum of the first differentiation of LV pressure (LV +dp/dtmax) and decline (LV -dp/dtmax), LV systolic pressure (LVSP), and LV end-diastolic pressure (LVEDP) were obtained using a PowerLab data acquisition system (AD Instruments, Sydney, Australia).
Quantitative real time-PCR (qRT-PCR)
The rats were killed with an overdose of pentobarbital (100 mg/kg, i.p.), and the hearts were removed. The total RNA in samples was extracted with TRIzol (Ambion, TX, U.S.A.). The cDNA was extracted from the RNA with reverse transcription using random primers in a total volume of 10 μl according to the instructions of the PrimeScript™ RT Master Mix (TaKaRa Biomedical Technology, Beijing, China). All cDNA was stored at − 80°C before use. Collagen I, collagen III, TGF-β, and α-SMA mRNA were determined with SYBR Green I fluorescence. All samples were amplified in triplicates for 45 cycles in a 384-well plate. The relative gene expression was determined by calculating the values of Δcycle threshold (ΔCt) as a relative quantity to the endogenous control. U6 was as a control to miR-132, and GAPDH was as a control to collagen I, collagen III, TGF-β, and α-SMA. The primers (Genscript, Nanjing, China) are shown in Table 1.
Table 1. List of utilized primers for qRT-PCR.
| Gene | Species | Forward primer | Reverse primer |
|---|---|---|---|
| Collagen I | Rat | TCAAGATGGTGGCCGTTAC | CTGCGGATGTTCTCAATCTG |
| Collagen III | Rat | CGAGATTAAAGCAAGAGGAA | GAGGCTTCTTTACATACCAC |
| TGF-β, | Rat | CAGGGAGTAAGGGACACGA | ACAGCAGTTAGGAACCCAGAT |
| α-SMA | Rat | GTCCCAGACATCAGGGAGTAA | TCGGATACTTCAGCGTCAGGA |
| miR-132 | Rat | ACACTCCAGCTGGGTAACA | CTCAACTGGTGTCGTGGA |
| U6 | Rat | GCTTCGGCAGCACATATACTAAAAT | CGCTTCACGAATTTGCGTGTCAT |
| GAPDH | Rat | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
Abbreviations: α-SMA, α-smooth muscle actin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; miR, microRNA; TGF-β, transforming growth factor-β.
Isolation and culture of cardiac fibroblasts (CFs)
Rat CFs were isolated from SD rats (1–3 days). Briefly, CFs were separated from cardiomyocytes by gravity separation and grown to confluence on 10-cm cell culture dishes with DMEM media including 10% FBS, 1% penicillin and 1% streptomycin (Biochannel Biotechnology Co., Ltd, Nanjing, China) at 37°C in humid air with 5% CO2 and 95% O2. The second passage CFs was used in the experiments. CFs were incubated with 10−6 M [24,25] Ang II (Sigma, MO, U.S.A.) for 24 h to induce the fibrotic phenotype, and treated with miR-132 agomiR or antagomiR according to the manufacturers’ instructions.
Bioinformatics analysis and dual-luciferase reporter gene assay
Target gene of miR-132 was determined according to previous study [22]. Briefly, endonuclease sites (SpeI and HindIII) were used to insert PTEN into the pMIR-reporter vector, and the mutation sites of complementary sequences of seed sequences were designed on wild-type PTEN (PTEN-WT). After restriction enzyme cutting, the T4 DNA ligase was used to insert the target fragment into the pMIR-reporter vector. The WT and mutant type (MUT) luciferase reporter plasmids with the correct sequence were cotransfected into HEK-293T with miR-132, respectively. After 48 h of transfection, the cells were collected, disrupted, and centrifuged for 5 min to collect the supernatant. The luciferase kit (Beyotime Biotech Co, Ltd., Shanghai, China) was used to determine the relative light unit (RLU) as its instructions
Statistical analyses
Data are presented as mean ± standard error of the mean (SE). Using GraphPad Prism 6.0 (GraphPad software Inc., CA, U.S.A.), statistical significance among multiple groups was evaluated by one-way analysis of variance (ANOVA) with the Bonferroni post-hoc test. A two-tailed P-value <0.05 was considered statistically significant.
Results
Expression of miR-132 in the heart of heart failure rats and Ang II-treated CFs
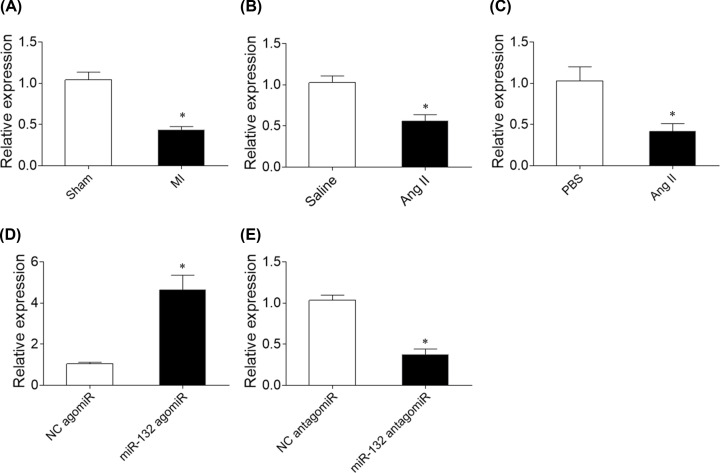
The expression level of miR-132 was reduced in the heart of MI-induced heart failure rats (Figure 1A). MiR-132 expression level was reduced in the heart of rat administration of angiotensin (Ang) II (Figure 1B). MiR-132 expression level was reduced in Ang II-treated CFs (Figure 1C) MiR-132 expression level was increased in the heart of rat treatment with miR-132 agomiR (Figure 1D). E. MiR-132 expression level was reduced in the heart of rat treatment with miR-132 antagomiR (Figure 1E).
Figure 1. Expression of microRNA (miR)-132.
(A) The level of miR-132 was reduced in the heart of myocardial infarction (MI)-induced heart failure rats. (B) MiR-132 expression level was reduced in the heart of rat administration of angiotensin (Ang) II. (C) MiR-132 expression level was reduced in Ang II-treated cardiac fibroblasts (CFs). (D) MiR-132 expression level was increased in the heart of rat treatment with miR-132 agomiR. (E) MiR-132 expression level was reduced in the heart of rat treatment with miR-132 antagomiR. The results are expressed as mean ± SE; N =8; *P<0.05 versus the Sham group (A) or PBS group (B).
Effects of miR-132 agomiR on cardiac function in heart failure rats
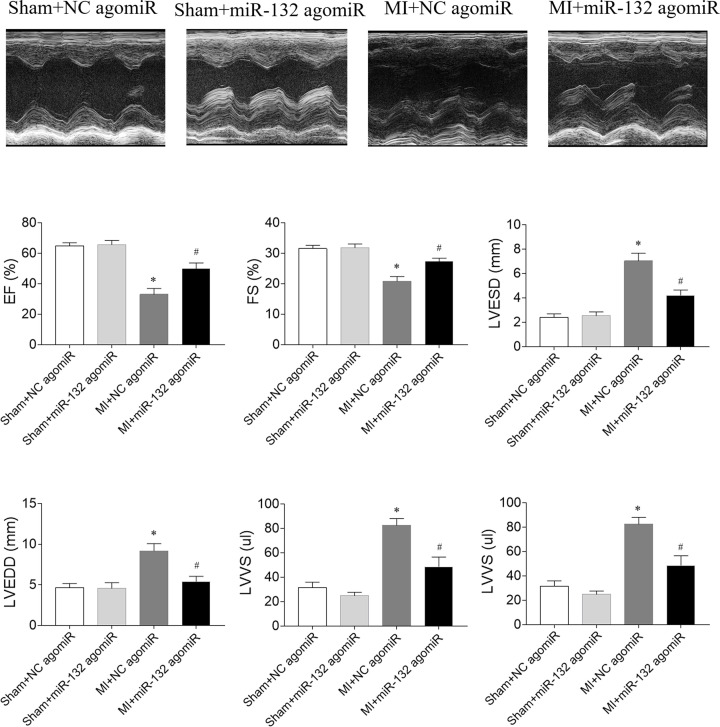
The EF and FS of LV in MI-induced heart failure rats were reduced, which were improved by miR-132 agomiR. LVESD, LVEDD, LVVS, and LVVD were increased in HF rats, and miR-132 agomiR treatment inhibited these increases (Figure 2).
Figure 2. Effects of microRNA (miR)-132 agomiR on cardiac function in myocardial infarction (MI)-induced heart failure rats.
The left ventricular (LV) ejection fraction (EF) and fractional shortening (FS) were reduced, and LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), LV volumes in systole diastole (LVVs) and LV volumes in diastole (LVVd) were increased in MI-induced heart failure rats. These changes were reversed by miR-132 agomiR treatment. The results are expressed as mean ± SE; N=8; *P<0.05 versus the Sham+NC agomiR group; #P<0.05 versus the MI+NC agomiR group.
Effects of miR-132 antagomiR on cardiac function in heart failure rats
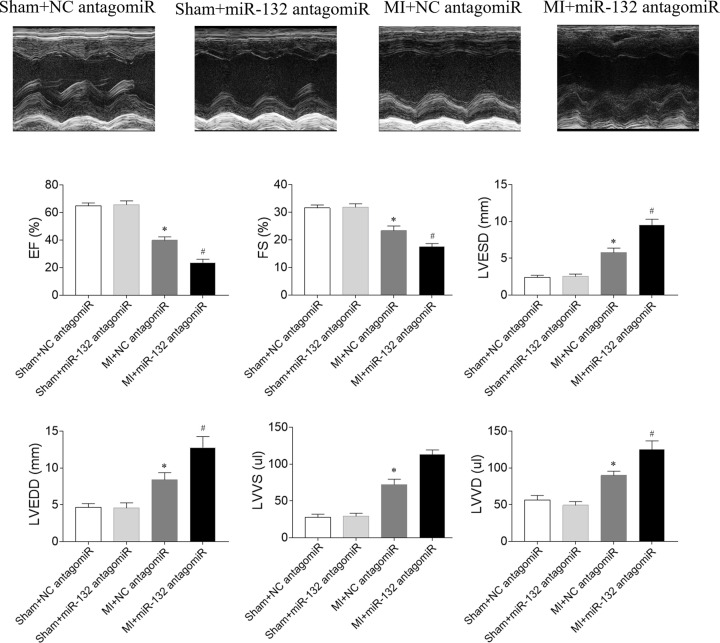
The decreases of EF and FS were aggravated by miR-132 antagomiR in MI-induced heart failure rats. The increases of LVESD, LVEDD, LVVS and LVVD in MI rats were further enhanced by miR-132 agomiR injection (Figure 3).
Figure 3. Effects of microRNA (miR)-132 antagomiR on cardiac function in myocardial infarction (MI)-induced heart failure rats.
The left ventricular (LV) ejection fraction (EF) and fractional shortening (FS) were reduced, and LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD), LV volumes in systole diastole (LVVs) and LV volumes in diastole (LVVd) were increased in MI-induced heart failure rats, and these changes were further aggravated by miR-132 antagomiR treatment. The results are expressed as mean ± SE; N=8; *P<0.05 versus the Sham+NC antagomiR group; #P<0.05 versus the MI+NC antagomiR group.
Effects of miR-132 agomiR on cardiac hemodynamics in heart failure rats
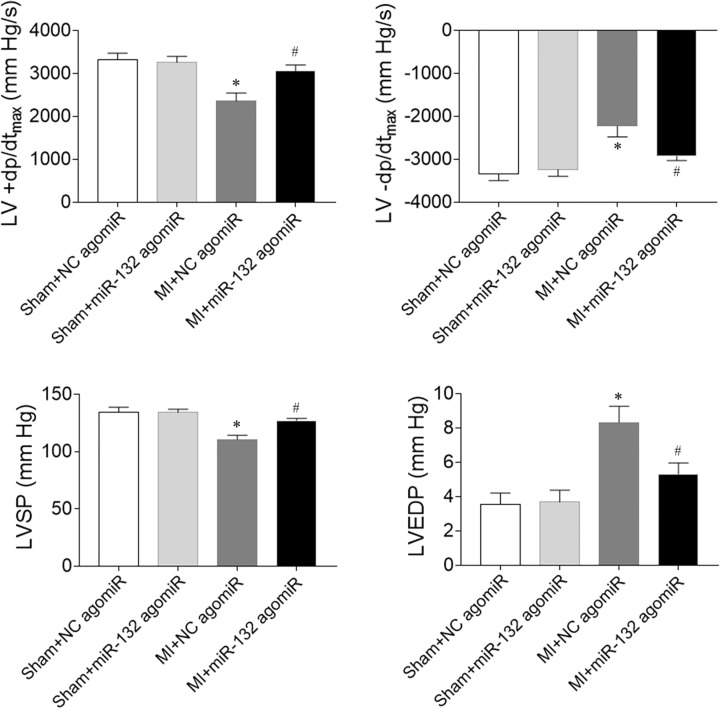
MI-induced heart failure reduced LV +dp/dtmax and LV -dp/dtmax. MiR-132 agomiR treatment increased the reduction of LV +dp/dtmax and LV -dp/dtmax in heart failure rats. LVSP was reduced in MI-induced heart failure rats, which was reversed after miR-132 agomiR injection. LVEDP in heart failure rats was increased, which was inhibited by miR-132 administration (Figure 4).
Figure 4. Effects of microRNA (miR)-132 agomiR on cardiac hemodynamics in myocardial infarction (MI)-induced heart failure rats.
The maximum of the first differentiation of left ventricular pressure (LV +dp/dtmax) and decline (LV -dp/dtmax) and left ventricle systolic pressure (LVSP) were reduced, and LV end-diastolic pressure (LVEDP) was increased in MI-induced heart failure rats, and these changes were reversed by miR-132 agomiR treatment. The results are expressed as mean ± SE; N=8; *P<0.05 versus the Sham+NC agomiR group; #P<0.05 versus the MI+NC agomiR group.
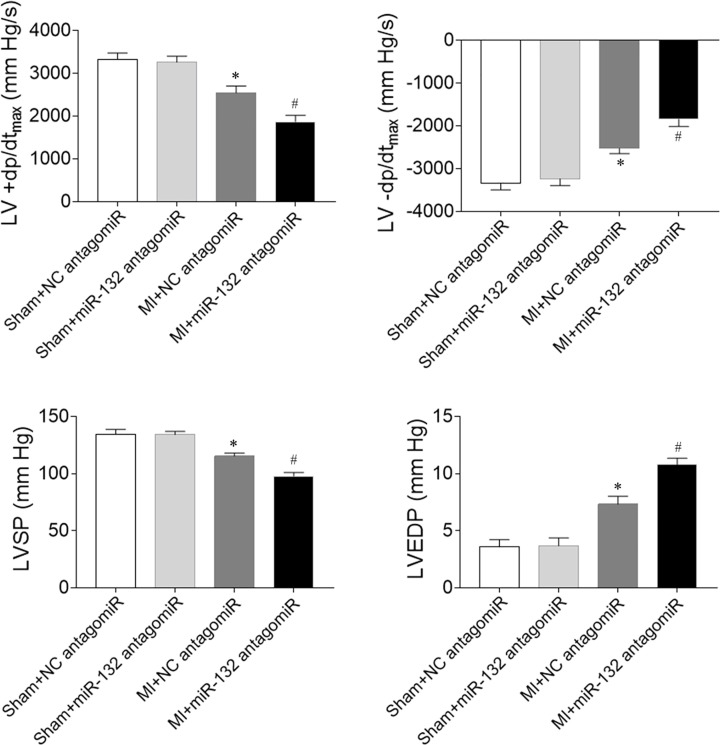
Effects of miR-132 antagomiR on cardiac hemodynamics in heart failure rats
The decreases of LV +dP/dtmax and LV -dP/dtmax were further reduced by miR-132 antagomiR in MI-induced heart failure rats. Treatment with miR-132 antagomiR also further aggravated the decreases of LVSP in MI-induced heart failure rats. The increase of LVEDP in heart failure rats was enhanced after miR-132 antagomiR administration (Figure 5).
Figure 5. Effects of microRNA (miR)-132 antagomiR on cardiac hemodynamics in myocardial infarction (MI)-induced heart failure rats.
The maximum of the first differentiation of left ventricular pressure (LV +dP/dtmax) and decline (LV -dP/dtmax) and left ventricle systolic pressure (LVSP) were reduced, and LV end-diastolic pressure (LVEDP) was increased in MI-induced heart failure rats, and these changes were further aggravated by miR-132 antagomiR treatment. The results are expressed as mean ± SE; N=8; *P<0.05 versus the Sham+NC antagomiR group; #P<0.05 versus the MI+NC antagomiR group.
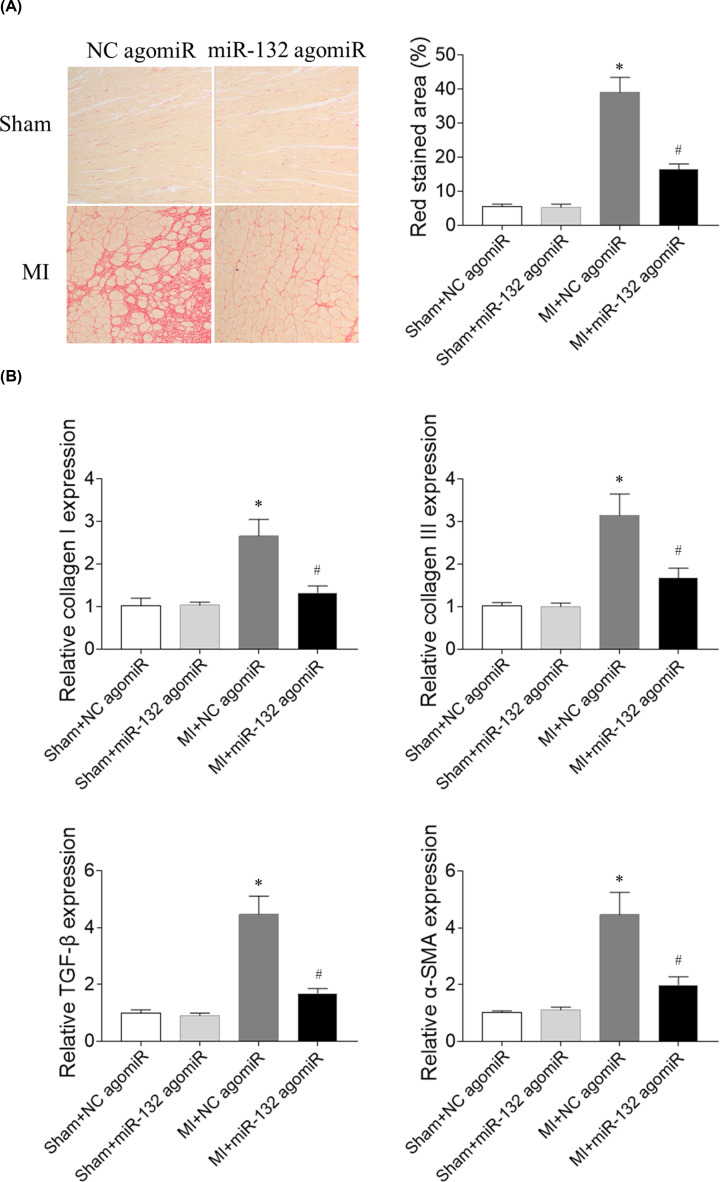
Effects of miR-132 agomiR on cardiac fibrosis in heart failure rats
The expression level of collagen I was increased in the heart of heart failure rats, which was inhibited after miR-132 agomiR administration. The level of collagen III was increased in the heart of MI rats, which was reversed by miR-132 agomiR treatment. TGF-β level was increased in the heart of MI-induced heart failure rats, which was abolished by miR-132 agomiR injection. The α-SMA expression in the heart was increased in the MI-induced heart failure rats, which was blocked by miR-132 agomiR (Figure 6).
Figure 6. Effects of microRNA (miR)-132 agomiR on cardiac fibrosis in myocardial infarction (MI)-induced heart failure rats.
(A) The increase of fibrosis in heart was inhibited by miR-132 agomiR treatment. (B) The expression levels of collagen I, collagen III, transforming growth factor-β (TGF-β), and α-smooth muscle actin (α-SMA) were increased in the heart of MI-induced heart failure rats, and these increases were inhibited by miR-132 agomiR treatment; N=8; *P<0.05 versus the Sham+NC agomiR group; #P<0.05 versus the MI+NC agomiR group.
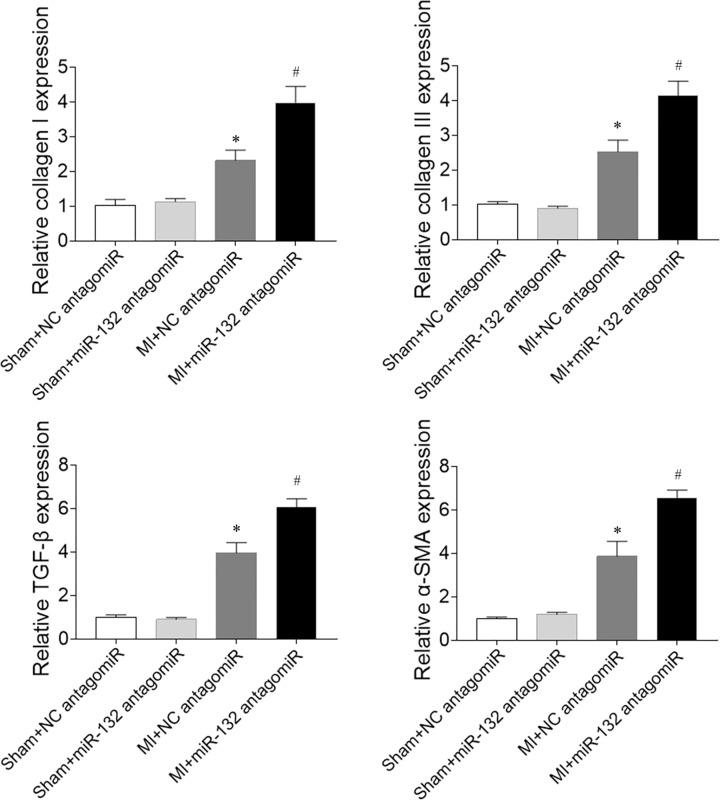
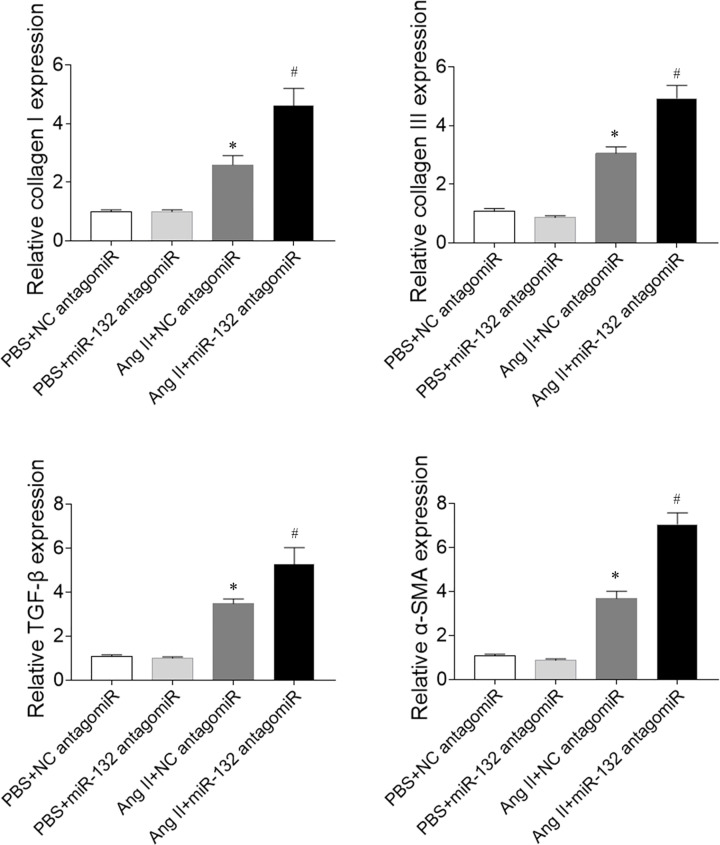
Effects of miR-132 antagomiR on cardiac fibrosis in heart failure rats
The expression levels of collagen I and collagen III in the heart were increased in the MI-induced heart failure rats, and these increases were further enhanced by miR-132 antagomiR administration. MiR-132 antagomiR treatment further elevated the levels of TGF-β and α-SMA compared with NC antagomiR in the heart of MI-induced heart failure rats (Figure 7).
Figure 7. Effects of microRNA (miR)-132 antagomiR on cardiac fibrosis in myocardial infarction (MI)-induced heart failure rats.
The expression levels of collagen I, collagen III, transforming growth factor-β (TGF-β), and α-smooth muscle actin (α-SMA) were increased in the heart of MI-induced heart failure rats, and these increases were further enhanced by miR-132 antagomiR treatment; N=8; *P<0.05 versus the Sham+NC antagomiR group; #P<0.05 versus the MI+NC antagomiR group.
Effects of miR-132 agomiR on fibrosis in CFs induced by Ang II
The collagen I expression was increased in Ang II-treated CFs, which was inhibited by miR-132 agomiR treatment. The collagen III level was increased in Ang II-treated CFs, which was reversed by miR-132 agomiR administration. TGF-β level was increased in the Ang II-treated CFs, and miR-132 agomiR injection abolished this increase. The α-SMA expression increased in Ang II-treated CFs, and miR-132 agomiR blocked the increase (Figure 8).
Figure 8. Effects of microRNA (miR)-132 agomiR on fibrosis in cardiac fibroblasts (CFs) treated with angiotensin (Ang) II.
The expression levels of collagen I, collagen III, transforming growth factor-β (TGF-β), and α-smooth muscle actin (α-SMA) were increased in Ang II-treated CFs, and these increases were inhibited by miR-132 agomiR treatment. *P<0.05 versus the PBS+NC agomiR group; #P<0.05 versus the Ang II+NC agomiR group.
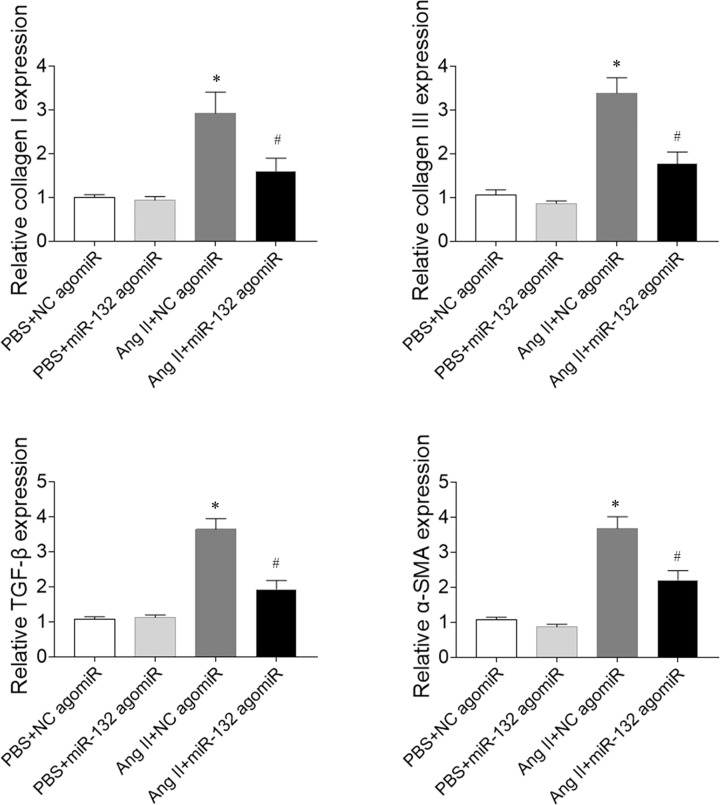
Effects of miR-132 antagomiR on fibrosis in CFs induced by Ang II
The expression levels of collagen I and collagen III in Ang II-treated CFs were increased, which was further enhanced by miR-132 antagomiR administration. The levels of TGF-β and α-SMA in the Ang II-treated CFs were increased, which were also further enhanced by miR-132 antagomiR treatment (Figure 9).
Figure 9. Effects of microRNA (miR)-132 antagomiR on the fibrosis of cardiac fibroblasts (CFs) treated with angiotensin (Ang) II.
The expression levels of collagen I, collagen III, transforming growth factor-β (TGF-β), and α-smooth muscle actin (α-SMA) were increased in Ang II-treated CFs, and these increases were further enhanced by miR-132 antagomiR treatment. *P<0.05 versus the PBS+NC antagomiR group; #P<0.05 versus the Ang II+NC antagomiR group.
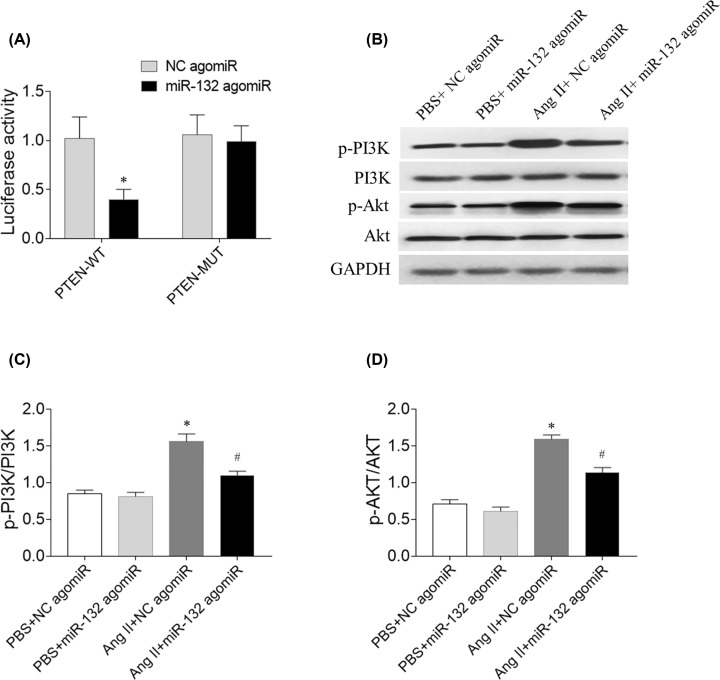
The mechanism of miR-132
Compared with the NC group, the luciferase activity of PTEN-WT 3′-UTR was markedly attenuated by miR-132 agimiR, whereas the PTEN-MUT 3′-UTR luciferase activity was not inhibited (Figure 10A). The levels of p-PI3K and p-Akt were increased in Ang II-treated CFs, and these increases were inhibited by miR-132 agomiR (Figure 10).
Figure 10. The mechanism of miR-132.
(A) The luciferase activity of PTEN-WT 3′-UTR was markedly attenuated by miR-132 agimiR. (B–D) The increases of p-PI3K and p-Akt were inhibited by miR-132 agomiR. *P<0.05 versus the NC agomiR (A) or PBS+NC agomiR (B) group; #P<0.05 versus the Ang II+NC agomiR group.
Discussion
In the present study, we found that attenuated cardiac function in MI-induced heart failure rats, which was improved by miR-132 agomiR but aggravated by miR-132 antagomiR treatment. MI-induced heart failure reduced the cardiac hemodynamics, which was reversed by miR-132 agomiR but deteriorated by miR-132 antagomiR administration. MiR-132 agomiR treatment inhibited the cardiac fibrosis in heart failure rats, while miR-132 antagomiR enhanced the fibrosis in the heart of MI rats. Up-regulation of miR-132 with agomiR attenuated the increase of fibrosis in Ang II-treated CFs, but down-regulation of miR-132 with antagomiR further enhanced the increase of fibrosis in Ang II-induced CFs.
The expression of miR-132 was increased in isoproterenol-induced cardiac hypertrophy [26]. MiR-132 expression was down-regulated in the blood of heart failure patients [21]. However, another study showed that circulating miR-132 levels rose with the severity of heart failure in patients with chronic heart failure [20]. In the present study, we found miR-132 expression in the heart was reduced in MI-induced heart failure rats. Furthermore, miR-132 level was also reduced in Ang II-treated CFs. Our current results demonstrated miR-132 level was down-regulated in the heart of heart failure rats and Ang-II treated CFs. Up-regulation of miR-132 may be a therapeutic strategy to treat heart failure and cardiac fibrosis.
The cardiac function was reduced in MI-induced heart failure rats [27], mice [28,29], rabbits [30], and swines [31]. In the present study, the results showed that the EF and FS of LV in MI-induced heart failure rats were reduced, which were improved by miR-132 agomiR. The LVESD, LVEDD, LVVS, and LVVD were increased in the heart failure rats, and miR-132 agomiR treatment inhibited these increases. The decreases of EF and FS, and the increases of LVESD, LVEDD, LVVS and LVVD in MI rats were further enhanced after the administration of miR-132 antagomiR. These results indicated upregulation of miR-132 improved, but downregulation of miR-132 further deteriorated the cardiac dysfunction in heart failure rats, which is supported by the previous finding that injection of miR-132 mimics into MI mice increased FS and EF [32].
Cardiac hemodynamics were impaired in rats with chronic heart failure, which was manifested by the increase of LVEDP and the reduction of LVSP and +dp/dtmax [23]. In the present study, we found that miR-132 agomiR treatment enhanced the reduction of LV +dP/dtmax, LV -dP/dtmax and LVSP, and attenuated the increase of LVEDP in heart failure rats. The decreases of LV +dP/dtmax, LV -dP/dtmax and LVSP, and the increase of LVEDP was further aggravated by miR-132 antagomiR administration. These results demonstrated that the inhibition of miR-132 further damaged the cardiac hemodynamics in heart failure rats while increasing the miR-132 level improved cardiac hemodynamics.
Cardiac fibrosis is caused by pathological stimulation to the heart. MI-induced heart failure showed markedly fibrosis in the heart [33]. The Ang II-induced activation of cardiac fibroblasts, and hypertrophy and proliferation of cardiomyocytes were significantly inhibited by miR-132 inhibitor anti-miR-132 [34]. Up-regulated miR-132 facilitated cardiocyte proliferation and repressed cardiocyte apoptosis and cardiac fibrosis in dilated cardiomyopathy induced by doxorubicin [22]. In the present study, the results showed that the expression levels of collagen I, collagen III, TGF-β, and α-SMA were increased in the heart of heart failure rats, which was reversed by miR-132 agomiR treatment, but further enhanced by miR-132 antagomiR injection. Moreover, the expression levels of collagen I, collagen III, TGF-β, and α-SMA in Ang II-treated CFs were inhibited by miR-132 agomiR treatment and further increased by miR-132 antagomiR treatment. These results indicated that miR-132 up-regulation attenuated the fibrosis of heart and inhibited the activation of cardiac fibroblasts in MI rats.
Differences genes are targeted by miR-132 in some diseases as previous studies. It showed that miR-132 inhibits cardiomyocyte apoptosis, and ameliorates myocardial remodeling in rats with MI through IL-1β down-regulation [35]. miR-132 exhibited the protective impacts on H9C2 cells against oxygen and glucose deprivation-induced injury via targeting FOXO3A [36]. Our present study found that miR-132 targeted PTEN in CFs, which is supported by previous study [22]. The signal pathway of PI3K/Akt was involved in the fibrosis of the heart [37,38]. The expression of p-Akt was increased in the CFs treated by Ang II [8]. In our present study, the results showed that p-PI3K and p-Akt levels were increased in Ang II-treated CFs, and these results indicated that miR-132 inhibited PTEN expression, and attenuated PI3K/Akt signal pathway in CFs.
In conclusions, up-regulation of miR-132 improved the cardiac dysfunction and the damage to cardiac hemodynamics, and attenuated cardiac fibrosis in heart failure via inhibiting PTEN expression, and attenuating PI3K/Akt signal pathway. MiR-132 may help to improve heart failure and is a potential biomarker for heart failure [39].
Abbreviations
- Ang
angiotensin
- CF
cardiac fibroblast
- CTGF
connective tissue growth factor
- EF
ejection fraction
- FS
fractional shortening
- LV
left ventricle
- LV +dP/dtmax
the maximum of the first differentiation of LV pressure
- LVEDD
LV end-diastolic diameter
- LVESD
LV end-systolic diameter
- LVSP
LV systolic pressure
- LVVD
LV volumes in diastole
- LVVS
LV volumes in systole
- MI
myocardial infarction
- miR
microRNA
- SD
Sprague-Dawley
- SMA
smooth muscle actin
- TGF
transforming growth factor
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This study was supported by the medical innovation team project of Jiangsu Province [grant number CXTDB2017015] and the hospital-level project of Taizhou People’s Hospital [grant number ZL201904].
Author Contribution
G.Y.W.: conceptualization and method; R.Z.W. and Z.B.R.: analysis and investigation; G.Y.W. and L.L.: manuscript written; Y.L. and L.Z.: manuscript revision.
References
- 1.Tsutsui H., Kinugawa S. and Matsushima S. (2011) Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 301, H2181–2190 10.1152/ajpheart.00554.2011 [DOI] [PubMed] [Google Scholar]
- 2.Dickstein K., Cohen-Solal A., Filippatos G. et al. (2008) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur. Heart J. 29, 2388–2442 [DOI] [PubMed] [Google Scholar]
- 3.Hunt S.A., Abraham W.T., Chin M.H. et al. (2009) 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J. Am. Coll. Cardiol. 53, e1–e90 [DOI] [PubMed] [Google Scholar]
- 4.Hamaguchi S., Kinugawa S., Tsuchihashi-Makaya M. et al. (2010) Spironolactone use at discharge was associated with improved survival in hospitalized patients with systolic heart failure. Am. Heart J. 160, 1156–1162 10.1016/j.ahj.2010.08.036 [DOI] [PubMed] [Google Scholar]
- 5.Hamaguchi S., Tsuchihashi-Makaya M., Kinugawa S. et al. (2009) Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ. J. 73, 1442–1447 10.1253/circj.CJ-09-0062 [DOI] [PubMed] [Google Scholar]
- 6.Tsuchihashi-Makaya M., Hamaguchi S., Kinugawa S. et al. (2009) Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ. J. 73, 1893–1900 10.1253/circj.CJ-09-0254 [DOI] [PubMed] [Google Scholar]
- 7.Liu C., Yang C.X., Chen X.R. et al. (2018) Alamandine attenuates hypertension and cardiac hypertrophy in hypertensive rats. Amino Acids 50, 1071–1081 10.1007/s00726-018-2583-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Wang L., Liu C., Chen X. et al. (2019) Alamandine attenuates longterm hypertensioninduced cardiac fibrosis independent of blood pressure. Mol. Med. Rep. 19, 4553–4560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yue Y., Meng K., Pu Y. et al. (2017) Transforming growth factor beta (TGF-beta) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes Res. Clin. Pract. 133, 124–130 10.1016/j.diabres.2017.08.018 [DOI] [PubMed] [Google Scholar]
- 10.Bartel D.P. (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 11.Javadian M., Gharibi T., Shekari N. et al. (2018) The role of microRNAs regulating the expression of matrix metalloproteinases (MMPs) in breast cancer development, progression, and metastasis. J. Cell. Physiol. 234, 5399–5412 [DOI] [PubMed] [Google Scholar]
- 12.van Rooij E. (2011) The art of microRNA research. Circ. Res. 108, 219–234, 108/2/219 [pii] 10.1161/CIRCRESAHA.110.227496 [DOI] [PubMed] [Google Scholar]
- 13.Mohr A.M. and Mott J.L. (2015) Overview of microRNA biology. Semin. Liver Dis. 35, 3–11 10.1055/s-0034-1397344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones Buie J.N., Goodwin A.J., Cook J.A. et al. (2016) The role of miRNAs in cardiovascular disease risk factors. Atherosclerosis 254, 271–281 10.1016/j.atherosclerosis.2016.09.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., Price N.L. and Fernandez-Hernando C. (2019) Non-coding RNAs in lipid metabolism. Vascul. Pharmacol. 114, 93–102 10.1016/j.vph.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan H., Zhang Y., Lu Y. et al. (2009) Downregulation of miR-133 and miR-590 contributes to nicotine-induced atrial remodelling in canines. Cardiovasc. Res. 83, 465–472 10.1093/cvr/cvp130 [DOI] [PubMed] [Google Scholar]
- 17.Ye Y., Perez-Polo J.R., Qian J. et al. (2011) The role of microRNA in modulating myocardial ischemia-reperfusion injury. Physiol. Genomics 43, 534–542 10.1152/physiolgenomics.00130.2010 [DOI] [PubMed] [Google Scholar]
- 18.Schulte C., Karakas M. and Zeller T. (2017) microRNAs in cardiovascular disease - clinical application. Clin. Chem. Lab. Med. 55, 687–704 10.1515/cclm-2016-0576 [DOI] [PubMed] [Google Scholar]
- 19.van Rooij E., Sutherland L.B., Liu N. et al. (2006) A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. U. S. A. 103, 18255–18260 10.1073/pnas.0608791103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Masson S., Batkai S., Beermann J. et al. (2018) Circulating microRNA-132 levels improve risk prediction for heart failure hospitalization in patients with chronic heart failure. Eur. J. Heart Fail. 20, 78–85 10.1002/ejhf.961 [DOI] [PubMed] [Google Scholar]
- 21.Liu X., Tong Z., Chen K. et al. (2018) The Role of miRNA-132 against Apoptosis and Oxidative Stress in Heart Failure. Biomed. Res. Int. 2018, 3452748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C.J., Huang Y., Lu J.D. et al. (2018) Upregulated microRNA-132 rescues cardiac fibrosis and restores cardiocyte proliferation in dilated cardiomyopathy through the phosphatase and tensin homolog-mediated PI3K/Akt signal transduction pathway. J. Cell. Biochem. 120, 1232–1244 [DOI] [PubMed] [Google Scholar]
- 23.Gan X.B., Duan Y.C., Xiong X.Q. et al. (2011) Inhibition of cardiac sympathetic afferent reflex and sympathetic activity by baroreceptor and vagal afferent inputs in chronic heart failure. PLoS One 6, e25784, PONE-D-11-12568 [pii] 10.1371/journal.pone.0025784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z., Zhang X., Guo N. et al. (2016) Substance P Inhibits the Collagen Synthesis of Rat Myocardial Fibroblasts Induced by Ang II. Med. Sci. Monit. 22, 4937–4946 10.12659/MSM.898454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X., Wang Y., Yan S. et al. (2017) Effect of testosterone on the proliferation and collagen synthesis of cardiac fibroblasts induced by angiotensin II in neonatal rat. Bioengineered 8, 14–20 10.1080/21655979.2016.1227141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narasimhan G., Carrillo E.D., Hernandez A. et al. (2018) Protective Action of Diazoxide on Isoproterenol-Induced Hypertrophy Is Mediated by Reduction in MicroRNA-132 Expression. J. Cardiovasc. Pharmacol. 72, 222–230 10.1097/FJC.0000000000000619 [DOI] [PubMed] [Google Scholar]
- 27.Polegato B.F., Minicucci M.F., Azevedo P.S. et al. (2016) Association between Functional Variables and Heart Failure after Myocardial Infarction in Rats. Arq. Bras. Cardiol. 106, 105–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun D. and Yang F. (2017) Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism. Biochem. Biophys. Res. Commun. 486, 329–335 10.1016/j.bbrc.2017.03.036 [DOI] [PubMed] [Google Scholar]
- 29.Adapala R.K., Kanugula A.K., Paruchuri S. et al. (2020) TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation. Basic Res. Cardiol. 115, 14 10.1007/s00395-020-0775-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K., Zhu Z.F., Chi R.F. et al. (2019) The NADPH oxidase inhibitor apocynin improves cardiac sympathetic nerve terminal innervation and function in heart failure. Exp. Physiol. 104, 1638–1649 10.1113/EP087552 [DOI] [PubMed] [Google Scholar]
- 31.Gabisonia K., Prosdocimo G., Aquaro G.D. et al. (2019) MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 569, 418–422 10.1038/s41586-019-1191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L., Wang G.Y., Dong J.H. et al. (2019) MicroRNA-132 improves myocardial remodeling after myocardial infarction. Eur. Rev. Med. Pharmacol. Sci. 23, 6299–6306 [DOI] [PubMed] [Google Scholar]
- 33.Song L.L., Zhang Y., Zhang X.R. et al. (2019) Theacrine attenuates myocardial fibrosis after myocardial infarction via the SIRT3/beta-catenin/PPARgamma pathway in estrogen-deficient mice. Eur. Rev. Med. Pharmacol. Sci. 23, 5477–5486 [DOI] [PubMed] [Google Scholar]
- 34.Ding J., Tang Q., Luo B. et al. (2019) Klotho inhibits angiotensin II-induced cardiac hypertrophy, fibrosis, and dysfunction in mice through suppression of transforming growth factor-beta1 signaling pathway. Eur. J. Pharmacol. 859, 172549 10.1016/j.ejphar.2019.172549 [DOI] [PubMed] [Google Scholar]
- 35.Zhao Z., Du S., Shen S. et al. (2020) microRNA-132 inhibits cardiomyocyte apoptosis and myocardial remodeling in myocardial infarction by targeting IL-1beta. J. Cell. Physiol. 235, 2710–2721 10.1002/jcp.29175 [DOI] [PubMed] [Google Scholar]
- 36.Zhang J., Xu H., Gong L. et al. (2020) MicroRNA-132 protects H9c2 cells against oxygen and glucose deprivation-evoked injury by targeting FOXO3A. J. Cell. Physiol. 235, 176–184 10.1002/jcp.28956 [DOI] [PubMed] [Google Scholar]
- 37.Yang W., Wu Z., Yang K. et al. (2019) BMI1 promotes cardiac fibrosis in ischemia-induced heart failure via the PTEN-PI3K/Akt-mTOR signaling pathway. Am. J. Physiol. Heart Circulatory Physiol. 316, H61–H69 10.1152/ajpheart.00487.2018 [DOI] [PubMed] [Google Scholar]
- 38.Wang X., Lu L., Tan Y. et al. (2019) GPR 30 reduces myocardial infarct area and fibrosis in female ovariectomized mice by activating the PI3K/AKT pathway. Life Sci. 226, 22–32 10.1016/j.lfs.2019.03.049 [DOI] [PubMed] [Google Scholar]
- 39.Panico C. and Condorelli G. (2018) microRNA-132: a new biomarker of heart failure at last? Eur. J. Heart Fail. 20, 86–88 10.1002/ejhf.1044 [DOI] [PubMed] [Google Scholar]