Abstract
Purpose
The clinical diagnosis of ocular surface squamous neoplasia is challenging, mostly requiring excisional biopsy. Human tumor angiogenesis is characterized by abnormal vessel architecture and transvascular hyperpermeability. This case report describes features of fluorescein and indocyanine green angiography in a case of conjunctival intraepithelial neoplasia.
Observations
Color photography, optical coherence tomography, fluorescein and indocyanine green angiography were performed in a patient with suspected conjunctival intraepithelial neoplasia before excisional biopsy and histologic confirmation of clinical diagnosis. Fluorescein dye showed extensive early extravascular dye leakage within the limits of the lesion. Indocyanine green dye displayed corneal terminal vessel bulbs with early leakage after 70 seconds and showed diffuse intralesional dye leakage after 7 minutes.
Conclusions
Increased fluorescein and early indocyanine green dye leakage can be used to confirm active angiogenesis already in early stages of dysplastic ocular surface squamous neoplasia. Late leakage of indocyanine green dye may be due to chronic transvascular hyperpermeability within intrinsic tumor vessels. The leakage behaviour of intravenous dyes has the potential to serve as a diagnostic indicator of active growth in dysplastic ocular surface neoplastic lesions.
Keywords: Ocular surface neoplasia, Conjunctival intraepithelial neoplasia, Indocyanine green angiography, Leakage
Highlights
-
•
Ocular surface neoplasia allows a uniquely direct view at tumor angiogenesis.
-
•
Vascular hyperpermeability is a hallmark in malignant tumor tissues.
-
•
Angiography dye leakage may aid in the diagnosis of malignant lesions.
1. Introduction
A variety of neoplastic lesions may develop within ocular surface tissues, including melanocytic and squamous lesions such as squamous papillomas, conjunctival intraepithelial neoplasia (CIN, including carcinoma in situ), invasive squamous cell carcinoma (SCC).1 The diagnosis of these lesions based on clinical examination is challenging. Despite the description of characteristics associated with dysplastic or invasive disease such as the presence of feeder vessels or hemorrhage, visible intrinsic tumor vasculature, increased size and thickness, it is often impossible to reliably exclude dysplastic or malignant disease from clinical appearance only.2 Therefore, current diagnosis largely depends on invasive excisional biopsy and subsequent histological analysis.3,4 Vascular alterations, intrinsic tumor vasculature, and inflammation are important clinical findings in both ocular surface squamous and melanocytic neoplasia.2,5 We have recently shown, that the vessel diameter ratio of afferent to efferent vessels is significantly different between benign and malignant melanocytic ocular surface neoplasia (OSN). Using ICGA, we have also shown, that the angiographic filling time is significantly shorter in benign and non-invasive compared to invasive melanocytic and squamous cell OSN.4 It is known, that tumor angiogenesis in neoplastic tissue is associated with chronic transvascular hyperpermeability.6 The angiographic study of intravenous dye leakage may therefore allow the assessment of transvascular hyperpermeability in OSN for the purpose of improved clinical grading of OSN.
1.1. Case report
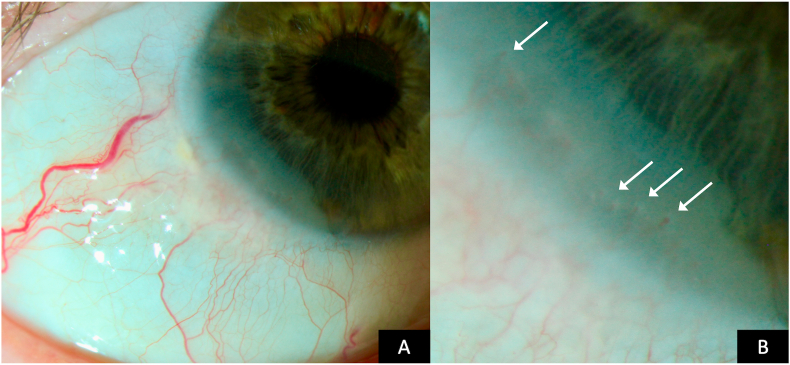
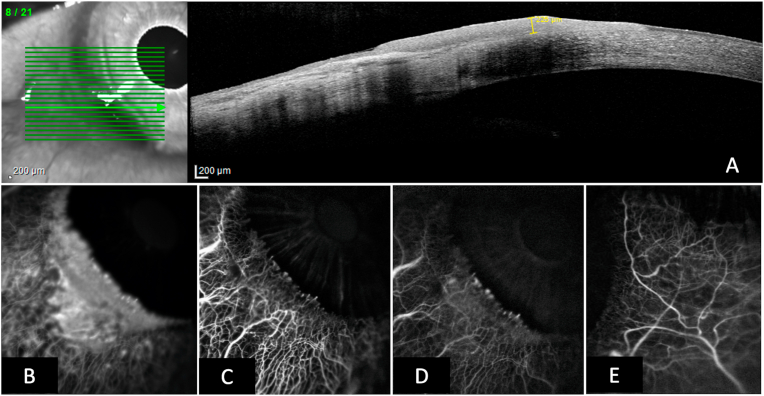
A 73 year-old male caucasian patient was referred to our ocular surface clinic with a suspected diagnosis of conjunctival intraepithelial neoplasia in his right eye, which was incidentally diagnosed during a routine ophthalmologic visite. Visual acuity was 0.9 on a decimal scale. Clinical examination by slit lamp biomicroscopy revealed diffuse greyish inferotemporal conjunctival opacity involving 4 limbal clock hours and extending 3 mm onto the cornea, which was accompanied by intralesional neovascularization forming vascular bulbs along the centripetal corneal lesion border (Fig. 1). A clinical diagnosis of CIN was made and the lesion was imaged using color photography, ICG and fluorescein angiography and high-resolution optical coherence tomography (HR-OCT) using the HRA Spectralis imaging system (Heidelberg Engineering, Heidelberg, Germany). For angiography, single images were taken every 5 seconds for 2 minutes and then every minute for up to 10 minutes. Imaging was performed with approval by the Ethics Committee of the Medical University of Innsbruck under vote number AN2015-0287 356/4.8. HR-OCT confirmed an intraepithelial lesion without destruction of Bowman membrane and with a maximum thickness of 220 μm (Fig. 2A). Fluorescein dye readily leaked from vasculature within the first minute post injection, with leaked fluorescent dye clearly demarcating the clinical extent of the lesion. On ICG angiography, early dye leakage occurred in terminal vessel bulbs along the central corneal border of the lesion. Seven minutes post injection, late ICG leakage occurred diffusely in the lesion, but not from the surrounding conjunctival vasculature (Fig. 2B–D). The lesion was subsequently excised in a no touch double freeze technique. Briefly, the corneal lesion was excised by lamellar keratectomy. Then a line of haemostasis with cautery was made at 2mm from the conjunctival lesion and the lesion was excised entirely along this line with blunt dissection underneath the lesion. 100% alcohol was applied to the whole base for 1 minute. Postoperative corneal re-epithelialization was complete after seven days. Histologic work-up confirmed a diagnosis of a completely excised conjunctival CIN with high-grade dysplasia. Meanwhile, the patient has been followed up for 6 months without signs of clinical recurrence.
Fig. 1.
A. Biomicroscopic color photograph of conjunctival intraepithelial neoplasia. B. High magnification color photograph showing terminal vascular bulbs (arrows) encroaching onto the cornea within the lesion. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
A. High resolution optical coherence tomographic scan of the lesion confirming the intraepithelial location without corneal stromal involvement. Maximum lesional thickness of 220 μm. B. Early fluorescein angiogram (1.5 minutes post injection) depicting diffuse dye leakage within the neoplastic tissue, accentuated in the terminal vascular bulbs on the centripetal border of the lesion. C. Early indocyanine green (ICG, 1.5 minutes post injection) angiogram showing leakage accentuated in the terminal vascular bulbs on the centripetal border of the lesion. D. Late ICG angiogram (9 minutes post injection) showing diffuse dye leakage within the borders of the lesion, but not in surrounding conjunctival tissue. E. Control ICG angiogram of the temporal conjunctiva at 9 minutes post injection. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2. Discussion
Fluorescein dye under physiologic circumstances readily leaks from healthy corneal and conjunctival blood vessels during intravenous angiography. It is particularly useful for measuring the time to leakage of corneal neovascularization providing a reliable indication of vessel maturity.7 ICG is highly (98%) protein bound with no early leakage from corneal or conjunctival vessels and enables imaging and delineation of ocular surface vessels including feeder vessels and intrinsic tumor vasculature in OSN.4,8 We have, however, shown that it leaks from immature vessels during active angiogenesis, most likely due to fenestrations in terminal vascular loops with yet incomplete pericyte coverage and intercellular adhesions.9 We have also observed ICG leakage from conjunctival vessels in states of ocular surface inflammation.10
While vasculature in neoplastic tissue is knowingly characterized by decreased regularity and vessel hierarchy, shunt vessel formation,11 tumor vessels also display a defective endothelial barrier function, incomplete or absent pericyte coverage or basement membrane irregularities, leading to transvascular hyperpermeability in tumors.12
This may be reflected by the observed early and increased fluorescein leakage and late diffuse ICG leakage compared to surrounding conjunctival vessels in the present patient. The focal early leakage of ICG in terminal vascular bulbs can be attributed to immature vessels in active angiogenesis along the centripetal extension of the growing lesion.9 This interpretation is encouraged by results of a recent pilot investigation showing that ICG leakage behaviour from vessels in melanocytic OSN differs between conjunctival naevi and conjunctival melanoma.13 Our interpretation is limited by the fact that we cannot exclude inflammation as a contributing factor known to be associated with conjunctival and corneal early fluorescein and late ICG dye leakage. There were, however, no clinically visible signs of ocular surface inflammation in the affected eye as seen in Fig. 1. Our results suggest the use of ICGA as an additional diagnostic method to evaluate OSN lesions suspecting of malignancy, along with clinical observation and impression cytology.14 The additional information provided by angiography may aid in the clinical decision for primary surgical or medical treatment as with retinoic acid or interferon alpha 2 b.15,16
3. Conclusion
The here presented observation of extravascular dye leakage of ICG from intrinsic tumor vessels in a case of CIN requires a systematic investigation with immunohistopathologic correlation. Angiography may aid in the non-excisional diagnostic evaluation and grading of OSN, potentially reducing the need for excisional biopsy in a proportion of patients.
Patient consent
Consent to publish this case report has been obtained from the patient(s) in writing.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
No author has financial disclosures to make. The authors declare that there is no conflict of interests regarding the publication of this article.
Acknowledgements
This work was funded by Medizinischer Forschungsfonds (MFF), Austria, under project number 294.
Funding
No funding or grant support. No funding was obtained for the conduction of this research. This work has not been submitted elsewhere.
References
- 1.Kenawy N., Garrick A., Heimann H., Coupland S.E., Damato B.E. Conjunctival squamous cell neoplasia: the liverpool ocular oncology centre experience. Graefes Arch Clin Exp Ophthalmol Albrecht Von Graefes Arch Klin Exp Ophthalmol. 2015;253(1):143–150. doi: 10.1007/s00417-014-2860-7. [DOI] [PubMed] [Google Scholar]
- 2.Shields C.L., Alset A.E., Boal N.S. Conjunctival tumors in 5002 cases. Comparative analysis of benign versus malignant counterparts. the 2016 James D. Allen lecture. Am J Ophthalmol. 2017;173:106–133. doi: 10.1016/j.ajo.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 3.Krachmer J.H., Mannis M.J., Holland E.J. third ed. vol. I. Mosby Elsevier; 2011. (Cornea). [Google Scholar]
- 4.Brunner M., Steger B., Romano V. Identification of feeder vessels in ocular surface neoplasia using indocyanine green angiography. Curr Eye Res. 2018;43(2):163–169. doi: 10.1080/02713683.2017.1387273. [DOI] [PubMed] [Google Scholar]
- 5.Shields C.L., Chien J.L., Surakiatchanukul T., Sioufi K., Lally S.E., Shields J.A. Conjunctival tumors: review of clinical features, risks, biomarkers, and outcomes--the 2017 J. Donald M. Gass lecture. Asia-Pac J Ophthalmol Phila Pa. 2017;6(2):109–120. doi: 10.22608/APO.201710. [DOI] [PubMed] [Google Scholar]
- 6.Nagy J.A., Benjamin L., Zeng H., Dvorak A.M., Dvorak H.F. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11(2):109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anijeet D.R., Zheng Y., Tey A., Hodson M., Sueke H., Kaye S.B. Imaging and evaluation of corneal vascularization using fluorescein and indocyanine green angiography. Invest Ophthalmol Vis Sci. 2012;53(2):650–658. doi: 10.1167/iovs.11-8014. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y., Hua R. Ocular surface squamous neoplasia: angiographic characteristics and response to subconjunctival/perilesional 5-fluorouracil injections. Drug Des Dev Ther. 2019;13:1323–1334. doi: 10.2147/DDDT.S191161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palme C., Romano V., Brunner M., Vinciguerra R., Kaye S.B., Steger B. Functional staging of corneal neovascularization using fluorescein and indocyanine green angiography. Transl Vis Sci Technol. 2018;7(5):15. doi: 10.1167/tvst.7.5.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steger B., Romano V., Kaye S.B. Angiographic evaluation of inflammation in atopic keratoconjunctivitis. Ocul Immunol Inflamm. 2018;26(5):685–688. doi: 10.1080/09273948.2016.1247873. [DOI] [PubMed] [Google Scholar]
- 11.Konerding M.A., Fait E., Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Canc. 2001;84(10):1354–1362. doi: 10.1054/bjoc.2001.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald D.M., Choyke P.L. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9(6):713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 13.Wanner A., Palme C., Romano V. Spektrum Augenheilkd; 2019. Indocyanine Green Angiographic Assessment of Melanocytic Ocular Surface Neoplastic Lesions – Pilot Study. Abstracts of the 60th Annual Conference of the Austrian Ophthalmologic Society. [Google Scholar]
- 14.Tananuvat N., Lertprasertsuk N., Mahanupap P., Noppanakeepong P. Role of impression cytology in diagnosis of ocular surface neoplasia. Cornea. 2008;27(3):269–274. doi: 10.1097/ICO.0b013e31815b9402. [DOI] [PubMed] [Google Scholar]
- 15.Krilis M., Tsang H., Coroneo M. Treatment of conjunctival and corneal epithelial neoplasia with retinoic acid and topical interferon alfa-2b: long-term follow-up. Ophthalmology. 2012;119(10):1969–1973. doi: 10.1016/j.ophtha.2012.03.045. [DOI] [PubMed] [Google Scholar]
- 16.Shah S.U., Kaliki S., Kim H.J., Lally S.E., Shields J.A., Shields C.L. Topical interferon alfa-2b for management of ocular surface squamous neoplasia in 23 cases: outcomes based on American joint committee on cancer classification. Arch Ophthalmol. 2012;130(2):159–164. doi: 10.1001/archophthalmol.2011.385. [DOI] [PubMed] [Google Scholar]