Highlights
-
•
Cartilage damage is frequently observed on MRI in Olympic Athletes.
-
•
Patellofemoral cartilage damage is most common and associated with certain sports including volleyball and weightlifting.
-
•
Meniscal tears are associated with localized cartilage damage.
-
•
Trend for prevalence of cartilage damage to increase with increasing age of the athlete.
Keywords: Olympics, Knee, MRI, Cartilage, Rio
Abstract
Purpose
To report the MRI patterns of knee cartilage damage and concomitant internal derangement in athletes participating at the Rio de Janeiro 2016 Olympic Games.
Methods
Knee MRIs obtained at the core imaging facility of the International Olympic Committee were blindly, retrospectively reviewed by a board-certified musculoskeletal radiologist for meniscal, ligamentous, and tendon abnormalities. Cartilage assessment was based on the modified Outerbridge criteria.
Results
Of 122 athletes who received a knee MRI, 64 (52.4 %) had cartilage damage. Cartilage damage was more prevalent in the patellofemoral compartment (52 athletes, 42.6 %), followed by lateral (23 athletes, 18.9 %) and medial tibiofemoral compartments (12 athletes, 9.8 %). Patellofemoral cartilage damage was most prevalent in beach-volleyball (100 %), followed by volleyball (8 athletes, 66.7 %) and weightlifting (7 athletes, 70 %). Patellofemoral cartilage damage was most prevalent with quadriceps (8 athletes, 72.7 %) and patellar tendinosis (11 athletes, 61.1 %). Medial and lateral tibiofemoral cartilage damage was significantly associated with medial (8 athletes, 29.6 %) and lateral meniscal tears (16 athletes, 55.2 %), respectively. There was a trend for the percentage of athletes with cartilage damage to increase with age.
Conclusion
The majority of athletes at the 2016 Rio Summer Olympics who had a knee MRI showed cartilage damage. Patellofemoral compartment cartilage damage was most common and frequently observed in certain sports including volleyball, beach volleyball, and weightlifting. Overuse in these sports can contribute to patellofemoral cartilage damage and subsequent development of anterior knee pain. Cartilage damage was also observed with concomitant meniscal tears and older age.
1. Introduction
Elite athletes throughout the world convened in Rio de Janeiro for the 2016 Summer Olympics. These athletes, who have trained intensely for years, are highly susceptible to injuries (acute and overuse) and cartilage damage; MRI often provides a highly sensitive and specific tool for identifying and grading these abnormalities [[1], [2], [3], [4]]. Such information, including type of internal knee derangement and epidemiology [3,[5], [6], [7], [8], [9]], has been reported at prior Olympic games, with knee abnormalities being among the most common [7,10,11]. At the Rio 2016 Olympic Games, 40 % of all injuries led to at least one day of missed participation and 20 % led to at least 7 days missed [9]. A previous study has shown that among the different knee abnormalities in elite athletes, cartilage damage is commonly observed and is associated with a high degree of pain and subsequent functional impairment [12]. Furthermore, cartilage damage, whether caused by repetitive use or acute trauma, can lead to accelerated osteoarthritis if left untreated, potentially limiting mobility [13].
Kompel et al. [14] previously evaluated this same cohort of knee MRIs performed on athletes participating in the 2016 Rio Olympics and showed that cartilage damage was the most frequently observed knee abnormality. However, the location (knee compartment i.e. medial tibiofemoral, lateral tibiofemoral and patellofemoral) of cartilage damage was not specified and the relative prevalence of cartilage damage in each sport was not described. Additionally, there was no correlation performed on athletes with cartilage damage and the presence of additional internal derangement in the knee. Finally, these cartilage abnormalities were not stratified by age and gender.
Therefore, the purpose of this descriptive study is to further detail cartilage damage on MRI in athletes who participated in the 2016 Rio Olympics including the location (stratified by sport), additional internal derangement in athletes with cartilage damage, and to evaluate the prevalence of cartilage damage depending on the athletes’ age and gender.
2. Materials and methods
2.1. Subjects’ MRIs
Knee MRI studies of the Rio 2016 Summer Olympic athletes were systematically reviewed as they were obtained from the Radiological Information System (RIS) and Picture Archiving and Communication System (PACS). These knee MRIs were performed for either injuries sustained during the 2016 Olympic Games or for knee pain during the Games without an inciting event. Medical information was sorted by the athlete’s accreditation number to prevent duplication of results from both NOC data and information from the other medical venues. If duplication was found, the NOC data was retained. The International Olympic Committee (IOC) approved our study and intent to publish this data.
2.2. Confidentiality and ethical approval
For those IOC athletes who obtained a knee MRI, the athlete accreditation number was used to obtain the athlete’s age, gender, nationality, and sport. This data was then de-identified and treated with the strictest confidentiality once the Olympic games concluded. Our study was approved by the medical research ethics committee of the South-Eastern Norway Regional Health Authority (#S-07196C). Informed consent was waived as the epidemiologic data was anonymized and de-identified. The IOC granted approval to use the anonymized imaging and demographic data for publication. A second IRB was obtained from Boston University (#H-36593).
2.3. Data collection
MRI was performed with a 3 T Discovery MR750w or a 1.5 T Optima 450 MRw MR scanner at the official IOC clinic within the Olympic village. The images were then collected retrospectively through RIS. Concurrently, the anonymized epidemiologic data was obtained for the corresponding knee MRI, and then stratified according to gender, age, country, type of sport, and internal derangement classification. Axial, sagittal, and coronal proton density with fat suppression and sagittal T2 and T1-weighted MRI sequences were acquired.
2.4. Imaging interpretation
All knee MRIs were independently reviewed by a board-certified Musculoskeletal Radiologist (AJK) with 7 years of subspecialized musculoskeletal experience, blinded to the initial reports. Reliability was determined by a second reader with 25 years of musculoskeletal experience (AG) through 30 randomly selected cases using prevalence-adjusted and bi-adjusted kappa (PABAK) and overall % agreement (Appendix).
The MRI assessment was similar to that described in Kompel et al. [14]. Cartilage assessment was based on a modified Outerbridge grading system with Grade 1 — areas of intrachondral hyperintensity with normal surface contour; Grade 2 — cartilage loss < 50 % of the thickness; Grade 3 — cartilage loss > 50 % of the thickness; and Grade 4 — full thickness loss [15]. Given the potential low diagnostic accuracy for Grade 1 lesions, only Grades 2, 3, and 4 were considered abnormal [16]. Medial and lateral collateral ligament injury was described as Grade 1 –– intact fibers with high signal on both sides of the ligament; Grade 2 –– high signal around the ligament and partial disruption of the fibers; and Grade 3 –– high signal around the ligament and complete disruption of the fibers [17]. Only grade 2 and 3 ligament sprains were included in the analysis as to only include definite ligament abnormalities [18]. Anterior and posterior cruciate ligament injuries were graded as partial or complete tears [17] however, both injuries types were grouped together for this analysis. Mucoid degeneration of the anterior or posterior cruciate ligaments without tear were not included in the analysis. Meniscal tears were diagnosed based on linear hyperintense signal on two consecutive MRI “slices” extending to an articular surface or abnormal morphology of the meniscus (flap or displaced tear) [19]. Mucoid meniscal degeneration without tear were not included in the analysis (for which there were 2 of the medial meniscus and 0 in the lateral meniscus). Tendon injuries were scored as either partial or complete disruption of the fibers and the presence of tendinosis was recorded [20,21]. The presence of an anterior cruciate ligament (ACL) graft was also noted.
2.5. Statistical analysis
Athletes were categorized based on the absence or presence of structural damage in the knee (other than cartilage damage) including ligament, meniscal, tendon tears, tendinosis, and presence of an ACL graft. Descriptive statistics were used to tabulate the frequency of the presence or absence of these abnormalities with the presence of compartment specific (medial, lateral or patellofemoral) cartilage damage. Fisher exact test was applied to evaluate for significant associations (contingency) between the presence/absence of internal derangement (i.e. torn versus intact medial meniscus) and compartment specific cartilage damage.
3. Results
There were 11,274 athletes at the Rio Olympics 2016; 113 received at least one knee MRI and 9 received bilateral knee MRIs or a second MRI of the same knee. Athletes taking part in Athletics (i.e. Track and Field) received the most MRIs, followed by Handball, Volleyball, and Wrestling.
More athletes had cartilage damage involving the patellofemoral compartment as opposed to the medial and lateral compartments, as demonstrated in Table 1. Out of 122 MRIs, 64 (52.4 %) had some degree of cartilage damage. Out of this group, 52 athletes (42.6 %) had patellofemoral cartilage damage versus medial (12 athletes, 9.8 %) and lateral (23 athletes, 18.9 %) cartilage damage. Patellofemoral cartilage damage was most prevalent among beach volleyball (100 %), volleyball (66 %) and weightlifting athletes (70 %) (Table 1).
Table 1.
Number (%) of athletes in each sport with cartilage loss on MRI and compartment specific location of the cartilage loss.
| Sport | # athletes with MRI | #of athletes with any cartilage loss (%) | Medial cartilage loss (%) | Lateral cartilage loss (%) | Patellofemoral cartilage loss (%) |
|---|---|---|---|---|---|
| Athletics | 27 | 13(48.1 %) | 4(14.8 %) | 4(14.8 %) | 10(37.0 %) |
| Beach volleyball | 7 | 7(100.0 %) | 0(0.0 %) | 0(0.0 %) | 7(100.0 %) |
| Handball | 13 | 7(53.8 %) | 1(7.7 %) | 4(30.8 %) | 4(30.8 %) |
| Hockey | 9 | 2(22.2 %) | 1(11.1 %) | 0(0.0 %) | 1(11.1 %) |
| Judo | 8 | 4(50.0 %) | 1(12.5 %) | 2(25.0 %) | 2(25.0 %) |
| Volleyball | 12 | 9(75.0 %) | 2(16.7 %) | 3(25.0 %) | 8(66.7 %) |
| Weightlifting | 10 | 7(70.0 %) | 0(0.0 %) | 3(30.0 %) | 7(70.0 %) |
| Wrestling | 11 | 4(36.4 %) | 1(9.1 %) | 2(18.2 %) | 3(27.3 %) |
| Combined sportsa | 21 | 11(52.4 %) | 2(10.0 %) | 5(23.8 %) | 10(47.6 %) |
| Aquatics - Water polo, Rowing, Shooting, Tennisb | 4 | 0(0.0 %) | 0(0.0 %) | 0(0.0 %) | 0(0.0 %) |
| Total | 122 | 64(52.5 %) | 12(9.8 %) | 23(18.9 %) | 52(42.6 %) |
Sports with 4 athletes or less, and sports with 1 athlete with cartilage damage were combined.
Sports with 1 athlete each without cartilage damage.
As shown in Table 2, medial tibiofemoral cartilage damage was significantly more prevalent among those with medial meniscal tear (8 athletes, 29.6 %, p < 0.05 compared to an intact medial meniscus. Similarly, lateral cartilage damage was significantly (16 athletes, 55.2 %, p < 0.05) associated with lateral meniscal tears. However, patellofemoral cartilage damage was most prevalent in athletes with quadriceps tendinosis (8 athletes, 72.7 %) and patellar tendinosis (11 athletes, 61.1 %). Ligament tears did not demonstrate any significant association with compartment specific (i.e. medial, lateral and patellofemoral) cartilage damage. However, athletes with an ACL graft demonstrated tricompartmental cartilage damage occurring more often in the lateral (8 athletes, 66.7 %, p < 0.05) and patellofemoral (6 athletes, 50 %) compartments (Table 2). The distribution of cartilage damage in different combinations of meniscal and ligamentous abnormalities is presented in Table 3. Concomitant medial and lateral meniscal tears showed higher prevalence of cartilage damage than any combination of ligamentous and single meniscal tears (Table 3).
Table 2.
Compartment specific cartilage damage and associated internal derangement.
| # athletes | Medial cartilage damage (%) | Lateral cartilage damage (%) | Patellofemoral cartilage damage (%) | |
|---|---|---|---|---|
| ACL | ||||
| Intact | 91 | 8(8.8 %) | 13(14.3 %) | 40(44.0 %) |
| Tear | 17 | 2(11.8 %) | 3(17.6 %) | 4(23.5 %) |
| ACL graft | # | * | ||
| Absent | 96 | 10(10.4 %) | 16(16.7 %) | 44(45.8 %) |
| Present | 12 | 3(25.0 %) | 8(66.7 %) | 6(50.0 %) |
| PCL | ||||
| Intact | 112 | 10(8.9 %) | 21(18.8 %) | 50(44.6 %) |
| Tear | 4 | 1(25.0 %) | 0(0.0 %) | 0(0.0 %) |
| MCL | ||||
| Intact | 90 | 5(5.6 %) | 14(15.6 %) | 40(44.4 %) |
| Sprain | 6 | 1(16.7 %) | 2(33.3 %) | 1(16.7 %) |
| LCL | ||||
| Intact | 114 | 12(10.5 %) | 22(19.3 %) | 50(43.9 %) |
| Sprain | 5 | 0(0.0 %) | 0(0.0 %) | 1(20.0 %) |
| Medial meniscus | * | # | ||
| Normal | 93 | 4(4.3 %) | 14(15.1 %) | 41(44.1 %) |
| Tear | 27 | 8(29.6 %) | 9(33.3 %) | 10(37.0 %) |
| Lateral meniscus | * | |||
| Normal | 93 | 7(7.5 %) | 7(7.5 %) | 39(41.9 %) |
| Tear | 29 | 5(17.2 %) | 16(55.2 %) | 13(44.8 %) |
| Quadriceps tendon | * | # | ||
| Normal | 108 | 11(10.2 %) | 17(15.7 %) | 43(39.8 %) |
| Tendinosis | 11 | 1(9.1 %) | 6(54.5 %) | 8(72.7 %) |
| Tear | 3 | 0(0.0 %) | 0(0.0 %) | 1(33.3 %) |
| Patella tendon | * | |||
| Normal | 96 | 8(8.3 %) | 14(14.6 %) | 38(39.6 %) |
| Tendinosis | 18 | 4(22.2 %) | 7(38.9 %) | 11(61.1 %) |
| Tear | 8 | 0(0.0 %) | 2(25.0 %) | 3(37.5 %) |
*p < 0.05; # p = .0.05−0.1
Table 3.
Compartment specific cartilage damage in athletes with multi-ligamentous and/or meniscal tears.
| Multiple abnormalities in the same knee on MRI | # athletes | Medial cartilage damage | Lateral cartilage damage | Patellofemoral cartilage damage |
|---|---|---|---|---|
| 1 ligament and 1 meniscal tear | 7 | 2 (28.6 %) | 2(28.6 %) | 2(28.6 %) |
| 2 ligament and 1 meniscal tear | 2 | 0(0.0 %) | 0(0.0 %) | 0(0.0 %) |
| 1 ligament and both menisci torn | 5 | 2(40.0 %) | 3(60.0 %) | 2(40.0 %) |
| Both menisci torn, no ligament tear | 4 | 1(25.0 %) | 4(100.0 %) | 3 (75.0 %) |
| Multi-ligamentous, no meniscal tear | 1 | 0(0.0 %) | 0(0.0 %) | 0(0.0 %) |
When stratifying these athletes by age and gender (Table 4), older men had more cartilage damage. A similar trend was seen in the female population but only with lateral and patellofemoral cartilage abnormalities, noting a peak in the 26−30-year old population.
Table 4.
Compartment specific cartilage damage and association with gender and age.
| Gender and Age | # athletes with MRI | Medial cartilage damage | Lateral cartilage damage | Patellofemoral cartilage damage |
|---|---|---|---|---|
| Male <20 | 2 | 0(0.0 %) | 0(0.0 %) | 0(0.0 %) |
| Male 20−25 | 15 | 0(0.0 %) | 1(6.7 %) | 5(33.3 %) |
| Male 26−30 | 27 | 2(7.4 %) | 6(22.2 %) | 11(40.7 %) |
| Male >30 | 16 | 2(12.5 %) | 4(25.0 %) | 11(68.8 %) |
| Female <20 | 2 | 1(50.0 %) | 0(0.0 %) | 0(0.0 %) |
| Female 20−25 | 24 | 2(8.3 %) | 3(12.5 %) | 7(29.2 %) |
| Female 26−30 | 28 | 5(17.9 %) | 9(32.1 %) | 15(53.6 %) |
| Female >30 | 8 | 0(0.0 %) | 0(0.0 %) | 3(37.5 %) |
4. Discussion
Our study found that in those athletes who received an MRI, the patellofemoral joint was the compartment most frequently exhibiting cartilage abnormalities especially in volleyball, beach volleyball, and weightlifting. These sports, such as volleyball, have previously been reported to be associated with patellofemoral cartilage abnormalities [22]. Volleyball players clinically have demonstrated anterior knee pain, especially at the patellar attachments of the quadriceps and patellar tendons, which is likely secondary to repeated jumping and squatting. In a prior study, cartilage damage within volleyball populations (Fig. 1) was also theorized to be due to repetitive jumping [23] as well as distance traveled during a game and the short-term explosive force of the legs [24].
Fig. 1.
MRI of the left knee with axial proton density-weighted fat-suppressed (a) and sagittal T1-weighted non-fat-suppressed (b) sequences. 34-year-old male beach volleyball player with a full thickness cartilage defect over the central sulcus of the trochlea (a, white arrow, Grade 4). The is associated mild subchondral bone marrow edema (a, blue arrow), sclerosis (b, red arrow) and subchondral osteophyte. This athlete had no ligament, meniscal injury or cartilage damage in the medial or lateral compartments. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Knee injuries are common in weightlifting athletes, with a higher likelihood of developing knee osteoarthritis, especially in the patellofemoral compartment [25]. In addition to volleyball sports, our study demonstrated that weightlifters frequently showed patellofemoral cartilage damage which is supported by the literature [25]. Weightlifting has been related to patellofemoral pain syndrome, or anterior knee pain; the proposed mechanism is the sudden extension of the knee when lifting a weight from a squatting position [26] similar to the injury mechanism seen in volleyball players as described by Kujala et al. [22]. The squat, a fundamental technique in weightlifting, has been found to increase compressive forces onto the extensor mechanism (patella, quadriceps and patellar tendons) as the patella moves against the femoral condyles [27] especially when performed in an explosive manner [28]. So, in addition to patellofemoral cartilage damage, these same athletes who participate in volleyball and weightlifting (Fig. 2), are more likely to incur injuries to the patella and quadriceps tendons [22,[29], [30], [31]].
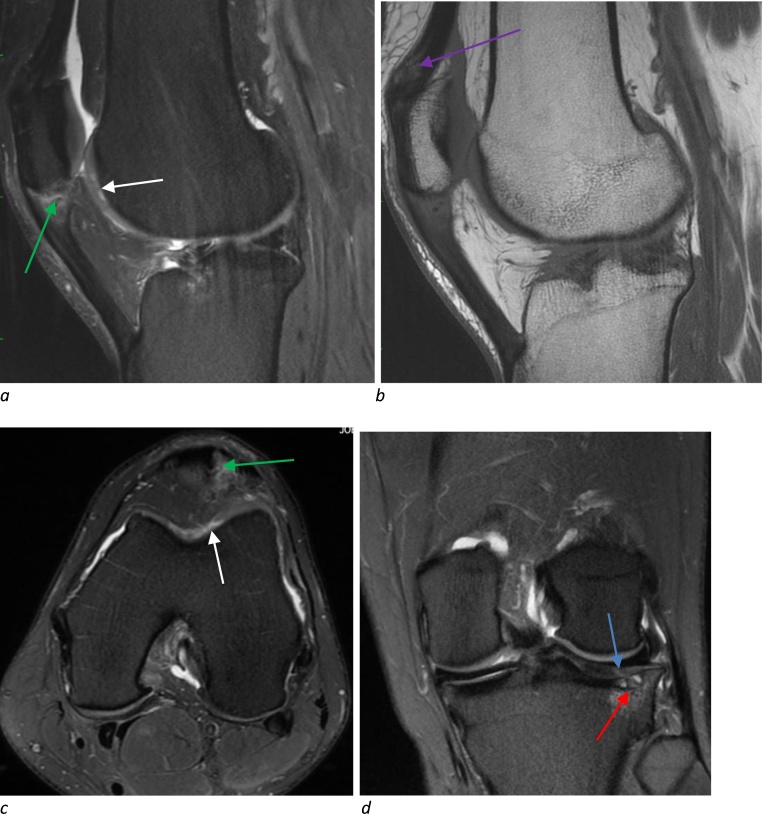
Fig. 2.
MRI of the left knee with sagittal (a), axial (c) and coronal (d) proton density-weighted fat-suppressed and sagittal T1-weighted non-fat-suppressed (b) sequences. 30 year-old female basketball player with multiple abnormalities including full thickness trochlea cartilage defect (Grade 3) with delamination (a,c, white arrow), full thickness cartilage fissure (d, blue arrow, Grade 4) over the lateral tibia with associated bone marrow edema and cystic change (d, red arrow), patella tendon partial tear (a,c, green arrow), and quadriceps tendinosis (b, purple arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The question remains whether patellofemoral chondral abnormalities are secondary to sport specific repetitive motion or pre-existing variant anatomy, such as trochlear geometry and patellofemoral alignment [32]. Given these certain sports had a higher prevalence of patellofemoral cartilage loss when compared to others, the repetitive movements likely contributed or exacerbated any underlying maltracking problem leading to patellofemoral cartilage damage and potentially anterior knee pain [33]. The addition of meniscal pathology can also lead to patellofemoral osteoarthritis as seen by MRI-detected cartilage damage [34]. Furthermore, increased cartilage damage in the patellofemoral compartment has been seen to not only lead to patellofemoral osteoarthritis but to arthritis in all three knee joint compartments, as was seen by Stefanik et al. over a 7-year time period [35].
Kompel et al. previously demonstrated athletics, handball, judo, and wrestling (Fig. 3) had the highest proportion of meniscal tears at the Rio 2016 Summer Olympics [14]. These sports concomitantly had a higher percentage of cartilage damage in the medial and lateral compartments when compared to a majority of the remaining sports. Previous research has shown that meniscal tears lead to cartilage loss [36] and that there can be a localized effect where the cartilage most damaged is adjacent to the region of meniscal tear [37]. Our study supported this localized effect by demonstrating a significant association between medial and lateral meniscal tears and concomitant cartilage damage in the respective compartment.
Fig. 3.
MRI of the right knee with sagittal proton density-weighted fat-suppressed (a) and T1-weighted non-fat-suppressed (b) sequences. 29-year-old male wrestler with a complex tear of the posterior horn of the lateral meniscus (a, white arrow). There is partial thickness cartilage loss in the adjacent femoral condyle (a, blue arrows, Grade 3) and focal full thickness cartilage loss over the tibia (a, red arrow, Grade 4) with associated subchondral sclerosis (b, yellow arrows). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Age also remains a factor in cartilage loss; as age increases, cartilage abnormality increases [38]. In the data presented, females and males generally exhibited a higher percentage of patellofemoral cartilage abnormalities as age increased (noting the small sample size for females older than 30 years could account for the lower numbers of cartilage loss). Studies have found that osteoarthritis, as demonstrated by cartilage loss, usually occurs 10 years after an initial injury such as meniscal or ACL tear [39]. Additionally, athletes with ACL grafts, indicating a more remote ACL injury, had a higher percentage of cartilage loss compared to an intact ACL and those with more recent non-repaired ACL tears [40].
Our study was limited by the lack of clinical information. While MRIs were obtained for either an acute injury during the Games or for pain unrelated to a recent discrete injury while at the Games, this information for specific athletes was not known when retrospectively reviewing the MRIs. For instance, we did not have access to the physical exam findings and mechanisms of injury, nor did we have any clinical follow-up to correlate our findings with subsequent clinical management or surgical treatment. Also, the MRIs evaluated in this study were those obtained at the Olympics polyclinic; any MRIs obtained outside the Olympic village (e.g. if an athlete was transferred to a hospital for a severe or life-threatening injury) would not have been included. Obtaining this data at future Olympic games would help better understand the mechanism of cartilaginous damage and better assess return to play and an athlete’s performance during their trials.
In conclusion, knee MRIs obtained for injury or pain at the Olympic Games can assist in the diagnosis of cartilage damage and concomitant internal derangement of the knee. Patellofemoral cartilage damage is a frequently observed abnormality, especially in such sports as volleyball, beach volleyball and weightlifting. Repetitive overuse, especially in jumping and squatting sports, likely contribute to the development of patellofemoral cartilage loss. Medial and lateral compartment cartilage damage occurred significantly more often in knees that also had concomitant meniscal tears. Age did play a role in the prevalence of cartilage damage, which correlates to the literature about previous injury and subsequent development of osteoarthritis.
Conflict of interest
Ali Guermazi is the president of Boston Imaging Core Lab (BICL), LLC, and a consultant to MerckSerono, AstraZeneca, Pfizer, Galapagos, Roche and TissueGene. Frank Roemer is a shareholder of BICL, LLC. Lars Engebretsen is a consultant to Arthrex and Smith and Nephew. Akira Murakami, Andrew J. Kompel, Zohaib Ahmed, and Mohamed Jarraya have nothing to disclose.
IRB approval
Our study was approved by the medical research ethics committee of the South-Eastern Norway Regional Health Authority (#S-07196C) and Boston University (#H-36593).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Zohaib Ahmad: Writing - original draft, Writing - review & editing. Akira M. Murakami: Writing - review & editing. Lars Engebretsen: Investigation, Resources, Writing - review & editing, Project administration. Mohamed Jarraya: Data curation, Writing - review & editing. Frank W. Roemer: Writing - review & editing, Visualization. Ali Guermazi: Conceptualization, Methodology, Validation, Formal analysis, Writing - review & editing, Visualization. Andrew J. Kompel: Conceptualization, Methodology, Validation, Formal analysis, Writing - review & editing, Visualization.
Contributor Information
Zohaib Ahmad, Email: Zohaib.Ahmad@bmc.org.
Akira M. Murakami, Email: Akira.Murakami@bmc.org.
Lars Engebretsen, Email: lars.engebretsen@medisin.uio.no.
Mohamed Jarraya, Email: mjarraya@mgh.harvard.edu.
Frank W. Roemer, Email: Frank.Roemer@uk-erlangen.de.
Ali Guermazi, Email: Guermazi@bu.edu.
Andrew J. Kompel, Email: Andrew.Kompel@bmc.org.
Appendix A
Table A1.
Reliability measurements (PABAK and overall agreement) of 30 randomly selected cases scored by a second reader.
| PABAK |
Agreement for all scores | Agreement for scores 0 vs >0 | ||
|---|---|---|---|---|
| PABAK | 95 % CI | |||
| Anterior cruciate ligament | 0.91 | 0.79−1.00 | 93.3 | 93.3 |
| Posterior cruciate ligament | 1.00 | 100.0 | 100.0 | |
| Medial collateral ligament | 0.54 | 0.33−0.76 | 63.3 | 70.0 |
| Lateral collateral ligament | 0.90 | 0.77−1.00 | 93.3 | 93.3 |
| Medial meniscus | 0.54 | 0.33−0.76 | 63.3 | 73.3 |
| Lateral meniscus | 0.75 | 0.57−0.94 | 80.0 | 80.0 |
| Medial cartilage | 0.50 | 0.28−0.72 | 60.0 | 63.3 |
| Lateral cartilage | 0.44 | 0.23−0.65 | 53.3 | 66.7 |
| Patellofemoral cartilage | 0.08 | 0−0.28 | 26.7 | 50.0 |
| Quadriceps tendon | 0.46 | 0.24−0.68 | 56.7 | 66.7 |
| Patella tendon | 0.00 | 13.3 | 30.0 | |
| Anterior cruciate ligament graft | 0.00 | 26.7 | 26.7 | |
References
- 1.Guermazi A., Hayashi D., Jarraya M., Crema M.D., Bahr R., Roemer F.W., Grangeiro J., Budgett R.G., Soligard T., Domingues R., Skaf A., Engebretsen L. Sports injuries at the Rio De Janeiro 2016 Summer Olympics: use of diagnostic imaging services. Radiology. 2018;287(3):922–932. doi: 10.1148/radiol.2018171510. [DOI] [PubMed] [Google Scholar]
- 2.Boeve B.F., Davidson R.A., Staab E.V., Jr. Magnetic resonance imaging in the evaluation of knee injuries. South. Med. J. 1991;84(9):1123–1127. doi: 10.1097/00007611-199109000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Bethapudi S., Budgett R., Engebretsen L., O’Connor P. Imaging at London 2012 summer Olympic Games: analysis of demand and distribution of workload. Br. J. Sports Med. 2013;47(13):850–856. doi: 10.1136/bjsports-2013-092345. [DOI] [PubMed] [Google Scholar]
- 4.Crema M.D., Roemer F.W., Marra M.D., Burstein D., Gold G.E., Eckstein F., Baum T., Mosher T.J., Carrino J.A., Guermazi A. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37–61. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engebretsen L., Soligard T., Steffen K., Alonso J.M., Aubry M., Budgett R., Dvorak J., Jegathesan M., Meeuwisse W.H., Mountjoy M., Palmer-Green D., Vanhegan I., Renstrom P.A. Sports injuries and illnesses during the London Summer Olympic Games 2012. Br. J. Sports Med. 2013;47(7):407–414. doi: 10.1136/bjsports-2013-092380. [DOI] [PubMed] [Google Scholar]
- 6.Junge A., Engebretsen L., Mountjoy M.L., Alonso J.M., Renstrom P.A., Aubry M.J., Dvorak J. Sports injuries during the Summer Olympic Games 2008. Am. J. Sports Med. 2009;37(11):2165–2172. doi: 10.1177/0363546509339357. [DOI] [PubMed] [Google Scholar]
- 7.Engebretsen L., Steffen K., Alonso J.M., Aubry M., Dvorak J., Junge A., Meeuwisse W., Mountjoy M., Renstrom P., Wilkinson M. Sports injuries and illnesses during the Winter Olympic Games 2010. Br. J. Sports Med. 2010;44(11):772–780. doi: 10.1136/bjsm.2010.076992. [DOI] [PubMed] [Google Scholar]
- 8.Soligard T., Steffen K., Palmer-Green D., Aubry M., Grant M.E., Meeuwisse W., Mountjoy M., Budgett R., Engebretsen L. Sports injuries and illnesses in the Sochi 2014 Olympic Winter Games. Br. J. Sports Med. 2015;49(7):441–447. doi: 10.1136/bjsports-2014-094538. [DOI] [PubMed] [Google Scholar]
- 9.Soligard T., Steffen K., Palmer D., Alonso J.M., Bahr R., Lopes A.D., Dvorak J., Grant M.E., Meeuwisse W., Mountjoy M., Pena Costa L.O., Salmina N., Budgett R., Engebretsen L. Sports injury and illness incidence in the Rio de Janeiro 2016 Olympic Summer Games: a prospective study of 11274 athletes from 207 countries. Br. J. Sports Med. 2017;51(17):1265–1271. doi: 10.1136/bjsports-2017-097956. [DOI] [PubMed] [Google Scholar]
- 10.Calhoon G., Fry A.C. Injury rates and profiles of elite competitive weightlifters. J. Athl. Train. 1999;34(3):232–238. [PMC free article] [PubMed] [Google Scholar]
- 11.Micheo W.F., Figueroa C. Comparison of the pattern of injuries in children and adult athletes. The first 10 years experience at the Olympic Training Center. Bol. Asoc. Med. P. R. 2006;98(1):7–14. [PubMed] [Google Scholar]
- 12.Murray I.R., Benke M.T., Mandelbaum B.R. Management of knee articular cartilage injuries in athletes: chondroprotection, chondrofacilitation, and resurfacing. Knee Surg. Sports Traumatol. Arthrosc. 2016;24(5):1617–1626. doi: 10.1007/s00167-015-3509-8. [DOI] [PubMed] [Google Scholar]
- 13.Zlotnicki J.P., Naendrup J.H., Ferrer G.A., Debski R.E. Basic biomechanic principles of knee instability. Curr. Rev. Musculoskelet. Med. 2016;9(2):114–122. doi: 10.1007/s12178-016-9329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kompel A.J., Murakami A.M., Engebretsen L., Forster B.B., Lotfi M., Jarraya M., Hayashi D., Roemer F.W., Crema M.D., Guermazi A. MRI-Detected Sports-Related Knee Injuries and Abnormalities at the Rio de Janeiro 2016 Summer Olympic Games. AJR Am. J. Roentgenol. 2018;211(4):880–886. doi: 10.2214/AJR.17.19334. [DOI] [PubMed] [Google Scholar]
- 15.Jungius K.P., Schmid M.R., Zanetti M., Hodler J., Koch P., Pfirrmann C.W. Cartilaginous defects of the femorotibial joint: accuracy of coronal short inversion time inversion-recovery MR sequence. Radiology. 2006;240(2):482–488. doi: 10.1148/radiol.2401050077. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues M.B., Camanho G.L. Mri evaluation of knee cartilage. Rev. Bras. Ortop. 2010;45(4):340–346. doi: 10.1016/S2255-4971(15)30379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naraghi A.M., White L.M. Imaging of athletic injuries of knee ligaments and menisci: sports imaging series. Radiology. 2016;281(1):23–40. doi: 10.1148/radiol.2016152320. [DOI] [PubMed] [Google Scholar]
- 18.Schweitzer M.E., Tran D., Deely D.M., Hume E.L. Medial collateral ligament injuries: evaluation of multiple signs, prevalence and location of associated bone bruises, and assessment with MR imaging. Radiology. 1995;194(3):825–829. doi: 10.1148/radiology.194.3.7862987. [DOI] [PubMed] [Google Scholar]
- 19.Lefevre N., Naouri J.F., Herman S., Gerometta A., Klouche S., Bohu Y. A current review of the meniscus imaging: proposition of a useful tool for its radiologic analysis. Radiol. Res. Pract. 2016;2016:8329296. doi: 10.1155/2016/8329296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sonin A.H., Fitzgerald S.W., Bresler M.E., Kirsch M.D., Hoff F.L., Friedman H. MR imaging appearance of the extensor mechanism of the knee: functional anatomy and injury patterns. Radiographics. 1995;15(2):367–382. doi: 10.1148/radiographics.15.2.7761641. [DOI] [PubMed] [Google Scholar]
- 21.Diederichs G., Issever A.S., Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30(4):961–981. doi: 10.1148/rg.304095755. [DOI] [PubMed] [Google Scholar]
- 22.Kujala U.M., Aalto T., Osterman K., Dahlstrom S. The effect of volleyball playing on the knee extensor mechanism. Am. J. Sports Med. 1989;17(6):766–769. doi: 10.1177/036354658901700607. [DOI] [PubMed] [Google Scholar]
- 23.Boeth H., MacMahon A., Eckstein F., Diederichs G., Schlausch A., Wirth W., Duda G.N. MRI findings of knee abnormalities in adolescent and adult volleyball players. J. Exp. Orthop. 2017;4(1):6. doi: 10.1186/s40634-017-0080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mroczek D., Januszkiewicz A., Kawczynski A.S., Borysiuk Z., Chmura J. Analysis of male volleyball players’ motor activities during a top level match. J. Strength Cond. Res. 2014;28(8):2297–2305. doi: 10.1519/JSC.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 25.Kujala U.M., Kettunen J., Paananen H., Aalto T., Battie M.C., Impivaara O., Videman T., Sarna S. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995;38(4):539–546. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- 26.Waryasz G.R., McDermott A.Y. Patellofemoral pain syndrome (PFPS): a systematic review of anatomy and potential risk factors. Dyn. Med. 2008;7:9. doi: 10.1186/1476-5918-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escamilla R.F. Knee biomechanics of the dynamic squat exercise. Med. Sci. Sports Exerc. 2001;33(1):127–141. doi: 10.1097/00005768-200101000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Kujala U.M., Osterman K., Kvist M., Aalto T., Friberg O. Factors predisposing to patellar chondropathy and patellar apicitis in athletes. Int. Orthop. 1986;10(3):195–200. doi: 10.1007/BF00266208. [DOI] [PubMed] [Google Scholar]
- 29.Biedert R.M., Sanchis-Alfonso V. Sources of anterior knee pain. Clin. Sports Med. 2002;21(3):335–347. doi: 10.1016/s0278-5919(02)00026-1. vii. [DOI] [PubMed] [Google Scholar]
- 30.Fredberg U., Bolvig L. Jumper’s knee. Review of the literature. Scand. J. Med. Sci. Sports. 1999;9(2):66–73. [PubMed] [Google Scholar]
- 31.Pfirrmann C.W., Jost B., Pirkl C., Aitzetmuller G., Lajtai G. Quadriceps tendinosis and patellar tendinosis in professional beach volleyball players: sonographic findings in correlation with clinical symptoms. Eur. Radiol. 2008;18(8):1703–1709. doi: 10.1007/s00330-008-0926-9. [DOI] [PubMed] [Google Scholar]
- 32.Subhawong T.K., Eng J., Carrino J.A., Chhabra A. Superolateral Hoffa’s fat pad edema: association with patellofemoral maltracking and impingement. AJR Am. J. Roentgenol. 2010;195(6):1367–1373. doi: 10.2214/AJR.10.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Utting M.R., Davies G., Newman J.H. Is anterior knee pain a predisposing factor to patellofemoral osteoarthritis? Knee. 2005;12(5):362–365. doi: 10.1016/j.knee.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Hart H.F., Crossley K.M., Felson D., Jarraya M., Guermazi A., Roemer F., Lewis C.E., Torner J., Nevitt M., Stefanik J.J. Relation of meniscus pathology to prevalence and worsening of patellofemoral joint osteoarthritis: the Multicenter Osteoarthritis Study. Osteoarthr. Cartil. 2018;26(7):912–919. doi: 10.1016/j.joca.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stefanik J.J., Guermazi A., Roemer F.W., Peat G., Niu J., Segal N.A., Lewis C.E., Nevitt M., Felson D.T. Changes in patellofemoral and tibiofemoral joint cartilage damage and bone marrow lesions over 7 years: the Multicenter Osteoarthritis Study. Osteoarthr. Cartil. 2016;24(7):1160–1166. doi: 10.1016/j.joca.2016.01.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen S.B., Short C.P., O’Hagan T., Wu H.T., Morrison W.B., Zoga A.C. The effect of meniscal tears on cartilage loss of the knee: findings on serial MRIs. Phys. Sportsmed. 2012;40(3):66–76. doi: 10.3810/psm.2012.09.1983. [DOI] [PubMed] [Google Scholar]
- 37.Chang A., Moisio K., Chmiel J.S., Eckstein F., Guermazi A., Almagor O., Cahue S., Wirth W., Prasad P., Sharma L. Subregional effects of meniscal tears on cartilage loss over 2 years in knee osteoarthritis. Ann. Rheum. Dis. 2011;70(1):74–79. doi: 10.1136/ard.2010.130278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meachim G., Bentley G., Baker R. Effect of age on thickness of adult patellar articular cartilage. Ann. Rheum. Dis. 1977;36(6):563–568. doi: 10.1136/ard.36.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roos H., Adalberth T., Dahlberg L., Lohmander L.S. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthr. Cartil. 1995;3(4):261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 40.Chalmers P.N., Mall N.A., Moric M., Sherman S.L., Paletta G.P., Cole B.J., Bach B.R., Jr. Does ACL reconstruction alter natural history?: a systematic literature review of long-term outcomes. J. Bone Joint Surg. Am. 2014;96(4):292–300. doi: 10.2106/JBJS.L.01713. [DOI] [PubMed] [Google Scholar]