Abstract
The Framingham Heart Study (FHS) is one of the largest and established longitudinal populational cohorts. CT cohorts of the FHS since 2002 provided a unique opportunity to assess non-cardiac thoracic imaging findings. This review deals with image-based phenotyping studies from recent major publications regarding interstitial lung abnormalities (ILAs), pulmonary cysts, emphysema, pulmonary nodules, pleural plaques, normal spectrum of the thymus, and anterior mediastinal masses, concluding with the discussion of future directions of FHS CT cohorts studies in the era of radiomics and artificial intelligence.
Keywords: Framingham Heart Study, CT, Lung, Thymus, Interstitial lung abnormalities, Pleural plaques
1. Introduction
The Framingham Heart Study (FHS) was initiated in 1948 to investigate epidemiologic risk factors of cardiovascular diseases [1]. CT scans obtained among this cohort since 2002 provided a unique window to assess the lungs and mediastinum on cross-sectional imaging in thousands of presumably healthy participants for the first time in history. The results of the analyses in cardiovascular disease have been extensively published. The purpose of this review is to summarize the non-cardiovascular results of analyses (lung and non-cardiac mediastinal structure).
We review the following results from the FHS chest CT cohorts that deal with interstitial lung abnormalities (ILAs), pulmonary cysts, emphysema, pulmonary nodules, pleural plaques, normal spectrum of the thymus, and anterior mediastinal masses (Table 1). With respect to the unique and less biased nature of the FHS cohorts, these phenotyping studies of preclinical or incidental lesions, in addition to age-related conditions, have been well studied. We will also discuss the future direction of the FHS chest CT cohorts in this era of artificial intelligence and radiomics features.
Table 1.
Summary of CT-based phenotyping in the Framingham Heart Study cohorts.
| CT findings | Prevalence | Comments | References |
|---|---|---|---|
| Interstitial lung abnormalities (ILAs) | 7% (177/2633) | -Associated with older age and MUC5B gene polymorphism | Hunninghake et al [4] |
| -Progression of ILA is associated with lung function decline and increased mortality | Araki et al [5] | ||
| Putman et al [6] | |||
| Pulmonary cysts | 7.6 % (200/2633) | -A few pulmonary cysts are likely part of aging changes of the lungs | Araki et al [9] |
| -Not seen in individuals under 40y | |||
| Emphysema | Pure paraseptal: 3% (86/2633) | -Associated with presence of ILAs | Araki et al [11] |
| Mixed: 8% (214/2633) | -Participants living closer to a major road had higher lung volume measure on CT | Rice et al [12] | |
| Pulmonary nodules | Non-calcified solid: 35 % (924/2633) | -Prevalence of solid nodules increases with aging | Non-published preliminary data |
| Subsolid: 2% (64/2633) | |||
| Pleural plaques/thickening | 1.5 % (40/2633) | -Prevalence is decreased from prior reports in the US | Araki et al [17] |
| -In addition to posterior distribution (88−93%), anterior involvement is also common (78%) | |||
| Normal spectrum of the thymus | Predominantly soft-tissue thymus: 1% (36/2540) | -Women have more solid soft tissue thymus than men | Araki et al [22] |
| Half soft tissue: 7% (172/2540) | -Smoking and higher BMI are associated with advanced fatty degeneration of the thymus | ||
| Anterior mediastinal masses | 0.9 % (23/2571) | -Most often appear as an oval mass at the midline location | Araki et al [2] |
| -May grow in observation for 5−7 years despite small size (6−8 mm) at baseline. |
2. Framingham Heart Study
Initiated in 1948, the Framingham Heart Study (FHS) is the largest and most established longitudinal population-based cohort study designed to identify epidemiologic risk factors of cardiovascular disease. The Original Cohort of the FHS consisted of over 5000 participants randomly selected from adult residents in the town of Framingham, Massachusetts [1]. From 2002–2005, the Third Generation and Offspring cohorts of the FHS underwent cardiac CT (MDCT1). Subsequently, from 2009 to 2011, follow-up chest CT (MDCT2), which covers entire lungs and mediastinum, enabled epidemiological approach of CT findings in the lungs and mediastinum in correlation with demographic and clinical features. These FHS cohorts with CT are suitable for the investigation of the prevalence of specific abnormalities in the chest, such as lung diseases, pleural plaques, and mediastinal masses. Furthermore, preclinical abnormalities and the normal range of age-related changes have also been assessed in these FHS cohorts.
CT techniques are as follows: For MDCT2, participants underwent non-contrast chest CT in supine at full inspiration using 64-detector-row CT scanner (Discovery, GE Healthcare, Waukesha, WI) with 120 kV and 300−400 mA (optimized with body weight), a gantry rotation time of 0.35 s and slice thickness of 0.63 mm [2]. For MDCT1, participants underwent ECG gated non-contrast cardiac CT in supine using 8-detector-row CT scanner (Lightspeed, GE Healthcare, Waukesha, WI) with 120 kV, 320−400 mA, a gantry rotation time of 0.5 s and slice thickness of 2.5 mm. The cardiac scans cover from 2 cm below the carina to the apex of the heart, which do not cover entire lungs, however, still useful for comparison noted on MDCT2 [2].
3. Disease phenotyping in the lungs and pleura
3.1. Interstitial lung abnormalities
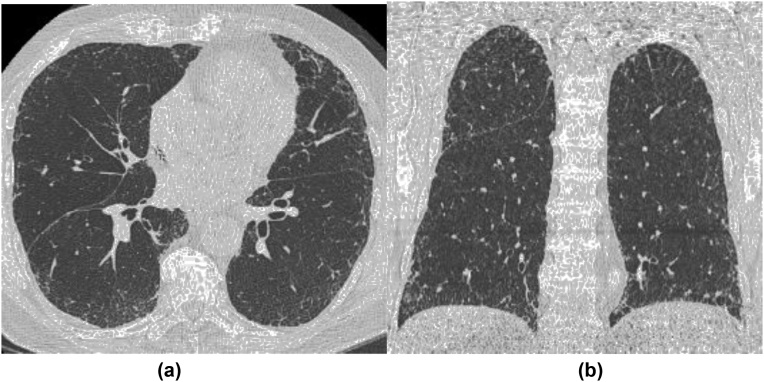
Interstitial lung abnormalities (ILAs) are CT findings suggestive of various patterns of lung parenchymal damages due to inflammation and fibrosis (Fig. 1). These are now known to be a subclinical form of idiopathic pulmonary fibrosis (IPF) [3]. ILAs are defined as nondependent changes affecting more than 5% of any lung zone, including ground-glass or reticular abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, or traction bronchiectasis [4]. It is of great importance to know the prevalence of ILAs in the general population and to assess the consequence of this preclinical condition. These were seen in 7% in the FHS MDCT2 and associated with older age and MUC5B gene polymorphism [4]. Comparison with prior cardiac CT (MDCT1, mean difference of 6.4 years) showed that progression of ILAs was associated with an increased rate of pulmonary function impairment and increased risk of death in these presumably healthy individuals [5]. Further assessment of ILAs with multiple large cohorts of FHS, AGES-Reykjavik Study, COPDGene Study, and ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) confirmed association of ILAs with increased mortality [6].
Fig. 1.
A representative case of interstitial lung abnormalities (ILAs). Axial (a) and coronal (b) CT images demonstrate subpleural reticulation and ground-glass opacities associated with mild traction bronchiectasis involving nondependent lateral and anterior aspects of both lungs.
3.2. Pulmonary cysts
A pulmonary cyst is defined as a round, usually thin-walled, parenchymal lucency or low-attenuating area with a well-defined interface with normal lung on chest CT [7]. A pneumatocele is a similar condition that is secondary to parenchymal necrosis and check-valve airway obstruction often due to infection or trauma [7]. Pulmonary cysts are often seen incidentally on chest CT, which may not necessarily be mentioned in a radiology report. However, when appeared in a multiple form, they need to be investigated for possible cystic lung diseases such as pulmonary Langerhans cell histiocytosis (PLCH), lymphangioleiomyomatosis (LAM), Birt-Hogg-Dube syndrome and lymphoid interstitial pneumonia (LIP) [8]. The prevalence of pulmonary cysts in the FHS was 7.6 % and more frequently seen in older participants, but not seen in those younger than 40 years [9]. Pulmonary cysts are not associated with cigarette smoking or impairment in measures of spirometry [9]. The FHS contributed to prove that incidental pulmonary cysts are not clinically significant and likely part of aging changes [10].
3.3. Emphysema
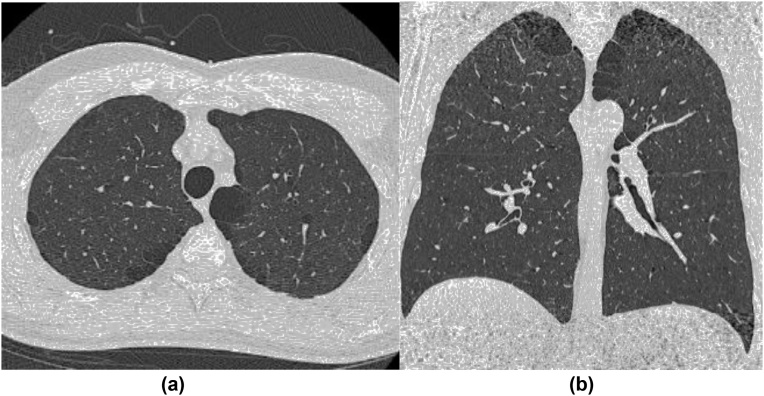
Emphysema is characterized by irreversible airspace enlargement distal to the terminal bronchiole with destruction of alveolar walls. On CT, emphysema appears as focal areas or regions of low attenuation, usually without visible walls [7]. The presence of a thin wall is a key finding to differentiate between pulmonary cysts of emphysema [9]. There are three major subtypes according to the distribution in the secondary pulmonary lobules: centrilobular, paraseptal, and panlobular emphysema. Paraseptal emphysema appears bounded by pleural surface and interlobular septa (Fig. 2). If paraseptal emphysema occurs in pure or predominant form, it tends to be asymptomatic, and therefore, could be underrecognized clinically [11]. In the FHS CT cohort, pure paraseptal emphysema was seen in 3%, and mixed type of paraseptal and centrilobular emphysema was in 8% of participants. Paraseptal emphysema was associated with decreased pulmonary function [11]. Rice et al. reported that higher lung volume measure on CT in the FHS CT cohort, which suggests subclinical emphysema or small airway disease, was associated with participants living close to a major road who are at risk for exposure of ambient air pollution [12]. The COPDGene is one of the largest cohort studies with high-risk participants with smoking history [13]. However, such high-risk smoker cohorts are likely to have relatively severe emphysema cases, and subclinical milder cases may be buried and difficult to analyze. The FHS is a suitable cohort to investigate preclinical paraseptal emphysema, in the same manner of investigation of preclinical ILAs.
Fig. 2.
A representative case of paraseptal emphysema. Axial (a) and coronal (b) CT images demonstrate upper lobe predominant focal lucencies bounded by pleural surface aligned mostly in a single layer.
3.4. Pulmonary nodules
Incidental pulmonary nodules are commonly found because of the increasing use of CT for daily clinical practice and lung cancer screening. Nodules with a size larger size than 6 mm showing growth on follow up imaging are concerning for malignancy. However, the management of small nodules is important in regard to control both health economic costs. In 2017, the Fleischner Society published a guideline for the management and follow-up recommendations of incidental pulmonary nodules, which suggests no routine follow-up for solid or subsolid nodules smaller than 6 mm in low-risk adult individuals [14]. European and American screening cohorts reported the prevalence of non-calcified pulmonary nodules of 13–51 % at baseline participants [15]. However, the results may vary depending on detection size criteria or imaging protocol and could be biased and deviated from the general population because screening cohorts recruited only high-risk participants. For example, The National Lung Screening Trial (NLST) adopted eligibility criteria of age between 55 and 74 years and smoking history of at least 30 pack-years [16]. On the other hand, the FHS CT cohorts were recruited regardless of risk factors and included participants with a wider age range (34–92 years) [9]. Preliminary results from the FHS CT cohort showed non-calcified nodules in 37.5 %, including solid nodules in 35.1 % and subsolid nodules in 2.4 % of all 2633 participants. The prevalence of solid nodules was higher in older age groups (19–29 % in the age of 30−50 s, and 43–53 % in 60−80 s). These findings from the FHS allow for a better perspective of pulmonary nodules in the US population that does not have enough smoking history or age range to be qualified for the lung cancer screening exam.
3.5. Asbestos-related pleural disease
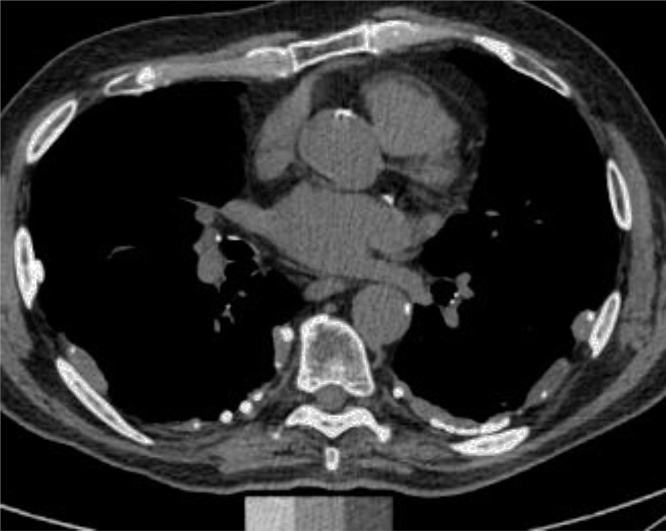
Pleural plaques and diffuse pleural thickening are non-neoplastic pleural abnormalities typically associated with asbestos exposure (Fig. 3). The presence of pleural plaques is an important index of asbestos exposure and a risk factor for associated malignancies such as malignant mesothelioma and lung cancer. In the US, the use of asbestos peaked in 1973 and then declined rapidly with the awareness of health issues related to asbestos. However, regulation of asbestos use varies in regions or countries, and the latent period of the asbestos-related disease could be as long as several decades. Therefore, the encounter of incidental findings of pleural abnormalities is still not rare. The prevalence of pleural plaques and thickening on CT was 1.5 % in the FHS cohort (2009–2011) [17], which is less than half of the prevalence (3.9 %) reported previously based on X-ray findings in NHANES II cohort (1976–1980) [18]. Pleural abnormalities are associated with older age, male sex, and cigarette smoking [17]. Direct assessment of asbestos or any other harmful materials is difficult and may require dedicated equipment. Therefore, longitudinal observation of the general population with CT image findings is a useful measure to assume the occupational or general exposure of the associated materials [12].
Fig. 3.
A representative case of Asbestosis related pleural disease (pleural plaques). Axial CT image demonstrates multiple well-demarcated soft-tissue thickening of pleura with partial calcifications, mostly involving the posterior aspect of the pleura.
4. Mediastinum
4.1. Normal spectrum of thymus and thymic hyperplasia
The thymus demonstrates morphological change according to age. The thymic gland reaches a peak in size and density at puberty and eventually undergoes involution, which is a gradual decrease in size and density with fatty degeneration [19]. Male sex and aging are reported to be associated with facilitated involution [20]. Thymic hyperplasia consists of two types, true hyperplasia and lymphoid hyperplasia. True hyperplasia is an enlargement of the thymic gland with normally organized thymic tissue, which occurs after stress, such as chemoradiation for cancer treatment, steroid therapy, trauma, or thermal burns. On the other hand, lymphoid hyperplasia refers to an increased number of lymphoid follicles in the thymic gland, which is associated with myasthenia gravis and other autoimmune diseases [19,21]. Thymectomy often improves symptoms of myasthenia gravis. However, true thymic hyperplasia does not usually require any invasive intervention or surgical resection, although it needs to be differentiated from thymic neoplasms. The finding obtained from the meticulous investigation of the FHS CT cohort contributed to defining the normal appearance of the thymus. In addition to age and sex, the FHS study showed that smoking status and body mass index (BMI) are associated with increased fatty degeneration of the thymus [22]. Knowledge of the CT appearance of the normal spectrum of the thymus is essential because prominently solid-appearing normal thymus or thymic hyperplasia could be misdiagnosed as an anterior mediastinal mass and may result in unnecessary invasive or surgical intervention.
4.2. Anterior mediastinal mass
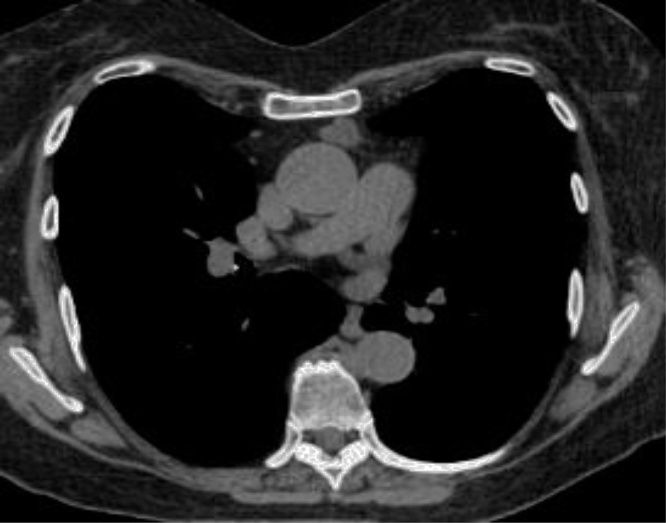
Anterior mediastinal mass can represent a broad-spectrum disease such as thymic epithelial tumors, thymic cyst, thymic hyperplasia, teratoma, or lymphoma. Incidental encounter of anterior mediastinal masses on CT is common (Fig. 4). The prevalence of anterior mediastinal mass was 0.9 % in the FHS cohort [2]. Observation for 5−7 years showed growth in size in six of eight masses even though the baseline size is as small as 6−8 mm, which suggests that long term follow-up may be necessary [2]. The prevalence was 0.4 % in the Early Lung Cancer Action Project (ELCAP), which included smokers with high risk for lung cancer [23]. These findings contributed to ACR recommendations for the management of incidental mediastinal mass [24]. Further work-up with MRI is recommended for non-cystic anterior mediastinal mass [24]. A large cohort study in Korea with low dose screening CT showed that the prevalence of anterior mediastinal mass was 0.7 % [25]. Most of the masses were benign even those showed some growth on follow up imaging study, which supports conservative management. While incidental findings can be observed in large screening cohorts, the results could be biased in the hospital-based or screening cohort, which has higher risk or possession of active diseases. Therefore, the FHS CT cohorts still provide epidemiologically significant data and a unique opportunity to assess demographic features and risk factors with less bias.
Fig. 4.
A representative case of an anterior mediastinal mass. Axial CT image shows a well-demarcated nodular lesion in the anterior mediastinum which is somewhat triangular-shaped and partially lobulated.
5. Future directions in image-based phenotyping, quantification, machine learning, and artificial intelligence
The FHS CT cohorts have provided important results of chest image findings in epidemiology, which are distinct from those obtained in the screening or hospital-based cohorts. A comparison of the findings between the FHS and other cohorts is useful to better understand the prevalence or nature of each finding and disease in the general population. Of note, the FHS cohorts are suitable for the investigation of incidental findings and preclinical conditions of asymptomatic patients in addition to defining normal age-related changes. Most of the previous studies in the FHS CT cohorts are based on visual assessment using dichotomous or semi-quantitative classification. Only a few FHS studies utilized quantitative evaluation of the lungs, such as lung densitometry or measurement of airway wall thickness [26,27]. Therefore, further investigation of the FHS CT cohorts with quantitative measures may provide a robust radiomics database [12], which is suitable for and could contribute to establishing machine learning models and artificial intelligence radiology. Previously provided visual evaluation results of the FHS CT cohorts could be used as training data to build and adjust the ML models. Furthermore, combined data could be analyzed to answer clinically or epidemiologically essential questions. For example, which one of the lung diseases such as ILAs, lung cancer, COPD, or pulmonary hypertension may determine participants’ long-term mortality? The FHS CT cohorts are promising to further accumulate more useful data from different perspectives of various phenotypes of lungs and mediastinum.
6. Conclusion
The FHS CT cohorts provide a unique opportunity to investigate various lung and mediastinal diseases, which is distinct from other screening cohorts. The FHS CT cohorts are suitable for investigation of preclinical conditions of asymptomatic patients, age-related changes and incidental findings. Further investigation of the FHS CT cohort with qualitative measures and combining them with previous results of visual assessment could contribute to building machine learning models and establishing artificial intelligence radiology in the future.
Disclosure and funding
Araki: Nothing to disclose.
Washko:
Schiebler: Shareholder of Stemina Biomarker Discovery, Inc., X-Vax, Inc., and Healthmyne, Inc.
O’Connor: the National Institutes of Health grants (N01-HC-25195 and R01 HL111024).
Hatabu: grants from Canon Medical System Inc, grants from Konica Minolta Inc, other from Mitsubishi Chemical Inc, other from Canon Medical System Inc, outside the submitted work.
Conflicts of interest and source of funding
Tetsuro Araki has no conflicts of interest on this manuscript and nothing to disclose.
Contributor Information
Tetsuro Araki, Email: arakitex@gmail.com.
George R. Washko, Email: gwashko@bwh.harvard.edu.
Mark L. Schiebler, Email: MSchiebler@uwhealth.org.
George T. O’Connor, Email: goconnor@bu.edu.
Hiroto Hatabu, Email: hhatabu@bwh.harvard.edu.
References
- 1.Mendis S. The contribution of the Framingham Heart Study to the prevention of cardiovascular disease: a global perspective. Prog. Cardiovasc. Dis. 2010;53(1):10–14. doi: 10.1016/j.pcad.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Araki T., Nishino M., Gao W., Dupuis J., Washko G.R., Hunninghake G.M., Murakami T., O’Connor G.T., Hatabu H. Anterior mediastinal masses in the Framingham Heart Study: prevalence and CT image characteristics. Eur. J. Radiol. Open. 2015;2:26–31. doi: 10.1016/j.ejro.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araki T., Dahlberg S.E., Hida T., Lydon C.A., Rabin M.S., Hatabu H., Johnson B.E., Nishino M. Interstitial lung abnormality in stage IV non-small cell lung cancer: a validation study for the association with poor clinical outcome. Eur. J. Radiol. Open. 2019;6:128–131. doi: 10.1016/j.ejro.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunninghake G.M., Hatabu H., Okajima Y., Gao W., Dupuis J., Latourelle J.C., Nishino M., Araki T., Zazueta O.E., Kurugol S., Ross J.C., Est́epar R.S.J., Murphy E., Steele M.P., Loyd J.E., Schwarz M.I., Fingerlin T.E., Rosas I.O., Washko G.R., O’Connor G.T., Schwartz D.A. MUC5B promoter polymorphism and interstitial lung abnormalities. N. Engl. J. Med. 2013;368(23):2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Araki T., Putman R.K., Hatabu H., Gao W., Dupuis J., Latourelle J.C., Nishino M., Zazueta O.E., Kurugol S., Ross J.C., Estépar R.S.J., Schwartz D.A., Rosas I.O., Washko G.R., O’Connor G.T., Hunninghake G.M. Development and progression of interstitial lung abnormalities in the Framingham Heart Study. Am. J. Respir. Crit. Care Med. 2016;194(12):1517–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Putman R.K., Hatabu H., Araki T., Gudmundsson G., Gao W., Nishino M., Okajima Y., Dupuis J., Latourelle J.C., Cho M.H., El-Chemaly S., Coxson H.O., Celli B.R., Fernandez I.E., Zazueta O.E., Ross J.C., Harmouche R., Estépar R.S.J., Diaz A.A., Sigurdsson S., Gudmundsson E.F., Eiríksdottír G., Aspelund T., Budoff M.J., Kinney G.L., Hokanson J.E., Williams M.C., Murchison J.T., MacNee W., Hoffmann U., O’Donnell C.J., Launer L.J., Harrris T.B., Gudnason V., Silverman E.K., O’Connor G.T., R.Washko G., Rosas I.O., Hunninghake G.M. Association between interstitial lung abnormalities and all-cause mortality. JAMA - Journal of the American Medical Association. 2016;315(7):672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansell D.M., Bankier A.A., MacMahon H., McLoud T.C., Müller N.L., Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 8.Seaman D.M., Meyer C.A., Gilman M.D., McCormack F.X. Diffuse cystic lung disease at high-resolution CT. Am. J. Roentgenol. 2011;196(6):1305–1311. doi: 10.2214/AJR.10.4420. [DOI] [PubMed] [Google Scholar]
- 9.Araki T., Nishino M., Gao W., Dupuis J., Putman R.K., Washko G.R., Hunninghake G.M., O’connor G.T., Hatabu H. Pulmonary cysts identified on chest CT: are they part of aging change or of clinical significance? Thorax. 2015;70:1156–1162. doi: 10.1136/thoraxjnl-2015-207653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Copley S.J. Morphology of the aging lung on computed tomography. J. Thorac. Imaging. 2016;31(3):140–150. doi: 10.1097/RTI.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 11.Araki T., Nishino M., Zazueta O.E., Gao W., Dupuis J., Okajima Y., Latourelle J.C., Rosas I.O., Murakami T., O’Connor G.T., Washko G.R., Hunninghake G.M., Hatabu H. Paraseptal emphysema: prevalence and distribution on CT and association with interstitial lung abnormalities. Eur. J. Radiol. 2015;84(7):1413–1418. doi: 10.1016/j.ejrad.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rice M.B., Li W., Dorans K.S., Wilker E.H., Ljungman P., Gold D.R., Schwartz J., Koutrakis P., Kloog I., Araki T., Hatabu H., San Jose Estepar R., O’Connor G.T., Mittleman M.A., Washko G.R. Exposure to traffic emissions and fine particulate matter and computed tomography measures of the lung and airways. Epidemiology. 2018;29(3):333–341. doi: 10.1097/EDE.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Washko G.R., Hunninghake G.M., Fernandez I.E., Nishino M., Okajima Y., Yamashiro T., Ross J.C., San R., Estépar J., Lynch D.A., Brehm J.M., Andriole K.P., Diaz A.A., Khorasani R., Aco K.D., Sciurba F.C., Silverman E.K., Hatabu H., Rosas I.O. Lung volumes and emphysema in smokers with interstitial lung abnormalities ter for pulmonary functional imaging. N. Engl. J. Med. 2011;364(10):897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacMahon H., Naidich D.P., Goo J.M., Lee K.S., Leung A.N., Mayo J.R., Mehta A.C., Ohno Y., Powell C.A., Prokop M., Rubin G.D., Schaefer-Prokop C.M., Travis W.D., Van Schil P.E., Bankier A.A. Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Society 2017. Radiology. 2017;284(1):228–243. doi: 10.1148/radiol.2017161659. [DOI] [PubMed] [Google Scholar]
- 15.Walter J.E., Heuvelmans M.A., Oudkerk M. Small pulmonary nodules in baseline and incidence screening rounds of low-dose CT lung cancer screening. Transl. Lung Cancer Res. 2017;6(1):42–51. doi: 10.21037/tlcr.2016.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aberle D.R., Adams A.M., Berg C.D., Black W.C., Clapp J.D., Fagerstrom R.M., Gareen I.F., Gatsonis C., Marcus P.M., Sicks J.R.D. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011;365(5):395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Araki T., Yanagawa M., Sun F.J., Dupuis J., Nishino M., Yamada Y., Washko G.R., Christiani D.C., Tomiyama N., O’Connor G.T., Hunninghake G.M., Hatabu H. Pleural abnormalities in the Framingham Heart Study: prevalence and CT image features. Occup. Environ. Med. 2017;74(10):756–761. doi: 10.1136/oemed-2016-104178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogan W.J., Ragan N.B., Dinse G.E. X-ray evidence of increased asbestos exposure in the US population from NHANES I and NHANES II, 1973-1978. National Health Examination Survey. Cancer Causes Control. 2000;11:441–449. doi: 10.1023/a:1008952426060. [DOI] [PubMed] [Google Scholar]
- 19.Nishino M., Ashiku S.K., Kocher O.N., Thurer R.L., Boiselle P.M., Hatabu H. The thymus: a comprehensive review. Radiographics. 2006;26(2):335–348. doi: 10.1148/rg.262045213. [DOI] [PubMed] [Google Scholar]
- 20.Ackman J.B., Kovacina B., Carter B.W., Wu C.C., Sharma A., Shepard J.-A.O., Halpern E.F. Sex difference in normal thymic appearance in adults 20-30 years of age. Radiology. 2013;268(1):245–253. doi: 10.1148/radiol.13121104. [DOI] [PubMed] [Google Scholar]
- 21.Araki T., Sholl L.M., Gerbaudo V.H., Hatabu H., Nishino M. Imaging characteristics of pathologically proven thymic hyperplasia: Identifying features that can differentiate true from lymphoid hyperplasia. Am. J. Roentgenol. 2014;202(3):471–478. doi: 10.2214/AJR.13.11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araki T., Nishino M., Gao W., Dupuis J., Hunninghake G., Murakami T., Washko G., O’Connor G., Hatabu H. Normal thymus in adults: appearance on CT and associations with age, sex, BMI and smoking. Eur. Radiol. 2016;26(1):16–24. doi: 10.1007/s00330-015-3796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henschke C.I., Lee I.J., Wu N., Farooqi A., Khan A., Yankelevitz D., Altorki N.K. CT screening for lung cancer: prevalence and incidence of mediastinal masses. Radiology. 2006;239(2):586–590. doi: 10.1148/radiol.2392050261. [DOI] [PubMed] [Google Scholar]
- 24.Munden R.F., Carter B.W., Chiles C., MacMahon H., Black W.C., Ko J.P., McAdams H.P., Rossi S.E., Leung A.N., Boiselle P.M., Kent M.S., Brown K., Dyer D.S., Hartman T.E., Goodman E.M., Naidich D.P., Kazerooni E.A., Berland L.L., Pandharipande P.V. Managing incidental findings on thoracic CT: mediastinal and cardiovascular findings. A white paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2018;15(8):1087–1096. doi: 10.1016/j.jacr.2018.04.029. [DOI] [PubMed] [Google Scholar]
- 25.Yoon S.H., Choi S.H., Kang C.H., Goo J.M. Incidental anterior mediastinal nodular lesions on chest CT in asymptomatic subjects. J. Thorac. Oncol. 2018;13(3):359–366. doi: 10.1016/j.jtho.2017.11.124. [DOI] [PubMed] [Google Scholar]
- 26.Kliment C.R., Araki T., Doyle T.J., Gao W., Dupuis J., Latourelle J.C., Zazueta O.E., Fernandez I.E., Nishino M., Okajima Y., Ross J.C., Estépar R.S.J., Diaz A.A., Lederer D.J., Schwartz D.A., Silverman E.K., Rosas I.O., Washko G.R., O’Connor G.T., Hatabu H., Hunninghake G.M. A comparison of visual and quantitative methods to identify interstitial lung abnormalities. BMC Pulm. Med. 2015;15(1) doi: 10.1186/s12890-015-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller E.R., Putman R.K., Diaz A.A., Xu H., Estepar R.S.J., Araki T., Nishino M., De Frias S.P., Hida T., Ross J., Coxson H., Dupuis J., O’Connor G.T., Silverman E.K., Rosas I.O., Hatabu H., Washko G., Hunninghake G.M. Increased airway wall thickness in interstitial lung abnormalities and idiopathic pulmonary fibrosis. Ann. Am. Thorac. Soc. 2019;16(4):447–454. doi: 10.1513/AnnalsATS.201806-424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]