Abstract
Background
In cystic fibrosis (CF), recurrent infections suggest impaired mucosal immunity but whether production of secretory immunoglobulin A (S-IgA) is impaired remains elusive. S-IgA is generated following polymeric immunoglobulin receptor (pIgR)-mediated transepithelial transport of dimeric (d-)IgA and represents a major defence through neutralisation of inhaled pathogens like Pseudomonas aeruginosa (Pa).
Methods
Human lung tissue (n = 74), human sputum (n = 118), primary human bronchial epithelial cells (HBEC) (cultured in air-liquid interface) (n = 19) and mouse lung tissue and bronchoalveolar lavage were studied for pIgR expression, IgA secretion and regulation.
Findings
Increased epithelial pIgR immunostaining was observed in CF lung explants, associated with more IgA-producing plasma cells, sputum and serum IgA, especially Pa-specific IgA. In contrast, pIgR and IgA transport were downregulated in F508del mice, CFTR-inhibited HBEC, and CF HBEC. Moreover, the unfolded protein response (UPR) due to F508del mutation, inhibited IgA transport in Calu-3 cells. Conversely, pIgR expression and IgA secretion were strongly upregulated following Pa lung infection in control and F508del mice, through an inflammatory host response involving interleukin-17.
Interpretation
A complex regulation of IgA secretion occurs in the CF lung, UPR induced by CFTR mutation/dysfunction inhibiting d-IgA transcytosis, and Pa infection unexpectedly unleashing this secretory defence mechanism.
Funding
This work was supported by the Forton's grant of the King Baudouin's Foundation, Belgium, the Fondazione Ricerca Fibrosi Cistica, Italy, and the Fonds National de la Recherche Scientifique, Belgium.
Keywords: Cystic fibrosis, Immunoglobulin A, Lung mucosal immunity, Infection, Endoplasmic reticulum stress
Introduction
Cysticfibrosis (CF) is a lethal genetic autosomal recessive disease, caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene [1], which encodes for an anion channel expressed in epithelial cells. Whereas all epithelia may be affected, lung disease is mainly responsible for the morbidity and mortality of patients with CF [2]. It is characterised by sticky mucus, chronic neutrophilic inflammation, recurrent opportunistic infections, and progressive formation of bronchiectasis. Pseudomonas aeruginosa (Pa) is the most common bacteria found in the CF adult lung and is associated with a worsening of lung function and vital prognosis [3,4]. The susceptibility to opportunistic Pa infection relates to impaired mucosal immunity underlying a permissive environment in CF airways. In the normal lung, the airway epithelium and immune resident cells ensure frontline defence against pathogens through mechanical and immune barrier functions. In addition to mucociliary clearance, the epithelium can sense and respond to pathogens, and is able to secrete several antimicrobial molecules and cytokines/chemokines (tumour necrosis factor-α (TNF-α), interleukin-8 (IL-8/CXCL8) and interleukin-1β (IL-1β)) in order to recruit or activate innate immune cells and, if required, to support activation of adaptative immunity. In CF, multiple aspects of those mechanisms are impaired including mucociliary clearance or production of antimicrobial proteins [5]. In addition, some studies suggest an imbalance between Th1 and Th2 responses, patients with CF with chronic Pa infection displaying a Th2-dominated response, while Th1 response against Pa was related to a better outcome [6,7]. This immune response is accompanied by increased Pa-specific immunoglobulin (Ig) G antibodies in the serum from infected patients with CF [8], as well as by an increased local production of Pa-specific IgA [9,10].
Research in context.
Evidence before this study
Cystic fibrosis (CF) is a lethal genetic autosomal recessive disease, which is caused by mutations on cystic fibrosis transmembrane conductance regulator (CFTR) gene and mainly affects the lungs. Lung disorder is characterised by airway inflammation and Pseudomonas aeruginosa (Pa) infection that affects almost 70% of patients with CF and is associated with poor clinical outcomes. Recurrent infections suggest impaired mucosal immunity in patients and the secretion of immunoglobulin A (IgA) is one of the defence mechanisms. IgA is transported across the epithelium by a transcellular routing mediated by the polymeric immunoglobulin receptor (pIgR). In the bronchial lumen, secretory IgA binds antigens and pathogens, and neutralises them through the so-called immune exclusion. pIgR has been studied in several chronic respiratory diseases, where its expression is downregulated. However, no data is available in CF. Previous studies reported increased IgA concentrations in serum or bronchoalveolar lavage of patients with CF. Conversely, decreased IgA secretion was described in CF saliva and in gastric luminal fluid.
Added value of this study
A multimodal approach was designed to assess epithelial transporter and IgA immunity in the CF lung through lung tissue, sputum, serum and primary epithelial cell cultures, as well as CF mice. Systematic analyses of pIgR expression and IgA production/secretion were performed in the different models (steady state, upon CFTR dysfunction, or upon infection). We observed increased pIgR expression and IgA production in the human CF lung. While CFTR dysfunction per se leads to downregulation of pIgR expression and IgA transcytosis, through activation of endoplasmic reticulum stress, chronic lung Pa infection is associated with an upregulation of pIgR-mediated IgA secretion through a host inflammatory response involving interleukin-17.
Implications of all the available evidence
This study reconciled previous data by showing a complex interplay between epithelial cells, B cells, and bacteria (Pa), which connects CFTR dysfunction, unfolded protein response, and a (Th17) host innate immune response to IgA secretion in the lung. However, we observed in our study that a local specific humoral response to Pa leads to increased Pa-specific IgA in chronically infected patients in both sputum and serum. These measurements could provide a diagnostic tool for Pa chronic infection in the lungs of patients with CF.
Alt-text: Unlabelled box
IgA exerts antimicrobial, antiviral, as well as anti-inflammatory activities. Polymeric – mainly dimeric (d-) – IgA, produced by subepithelial plasma cells, is transported into secretions by polymeric immunoglobulin receptor (pIgR)-mediated transcytosis. After binding of d-IgA, the pIgR/d-IgA complex is sent to the apical pole where the extracellular part of the receptor – called secretory component (SC) – is cleaved, releasing SC bound to IgA to form secretory IgA (S-IgA), as well as free SC [11]. At the epithelial surface, S-IgA may neutralise antigens and pathogens through so-called immune exclusion. In addition, SC itself may contribute to these anti-microbial and anti-inflammatory functions [12,13], particularly by inhibiting the activity of pro-inflammatory molecules such as IL-8/CXCL8, and by protecting S-IgA from proteolytic degradation [14,15]. pIgR expression has been studied in several chronic respiratory diseases [16], [17], [18], where it is downregulated, while no data is available in CF. Previous studies reported increased IgA concentrations in serum [19] or bronchoalveolar lavage (BAL) of patients with CF [20], as well as increased free SC in CF sputum [21]. Conversely, decreased IgA secretion was described in CF saliva and in gastric luminal fluid [22,23].
This study was designed to assess epithelial pIgR and IgA immunity in the CF lung through a multimodal approach including lung tissue, sputum, serum and primary epithelial cell cultures, as well as mice harbouring the F508del CFTR mutation (F508del mice) and CFTR inhibition in primary epithelial cell cultures. We demonstrate that pIgR/SC expression and IgA production are increased in the human CF lung. While CFTR dysfunction per se leads to downregulation of pIgR expression and IgA transcytosis, through activation of endoplasmic reticulum (ER) stress, chronic lung Pa infection is associated with an upregulation of pIgR-mediated IgA secretion through a host inflammatory response involving interleukin-17 (IL-17).
Methods
Patients
Lung tissue were obtained from 74 patients, which included explants obtained from 36 patients with end-stage CF disease compared with lung tissue from 38 control patients undergoing lung surgery for a solitary lung tumour (n = 32) or unused lung donors (n = 6). Sputum and serum were obtained from 118 subjects, with 67 patients with CF at stable state compared with 51 age-matched control subjects with no clinical evidence of lung disease and normal lung function. 25 patients were enrolled for cell culture, which included seven control subjects (undergoing lung resection surgery for a solitary lung tumour) and 18 patients with severe CF (undergoing lung resection surgery for transplantation) (Table 1). Patients with CF were classified according to their state of infection by Pa as follows; 1) chronic infection, when having more than 50% of months, when samples had been taken, positive in a 12-months period, 2) intermittent infection, when 50% or less months, when samples had been taken, positive in a 12-months period, 3) free of infection, when there was no growth of Pa in the last 12 months, and 4) never infected, when Pa was never isolated in culture. Among Pa-never infected patients, eight out of ten were infected by other bacteria (such as Staphylococcus aureus, Achromobacter, or Burkholderia species) and two patients were infected by Aspergillus fumigatus.
Table 1.
Patient characteristics.
| Controls | Patients with CF | All | ||
|---|---|---|---|---|
| Lung tissue series | Subjects, n | 38 | 36 | 74 |
| Sex, F/M | 21/17 | 20/16 | 41/33 | |
| Age, years | 58 ± 14 | 31 ± 9 | 45 ± 18 | |
| Genotype, F508del homozygous/F508del heterozygous/other mutations | NA | 22/10/4 | NA | |
| FEV1, % predicted (n = 68) | 94 ± 18 | 24 ± 8 | 60 ± 38 | |
| Sputum and serum series | Subjects, n | 51 | 67 | 118 |
| Sex, F/M | 35/16 | 36/31 | 71/47 | |
| Age, years | 31 ± 10 | 34 ± 11 | 33 ± 11 | |
| BMI, kg/m² | 23 ± 5 | 22 ± 3 | 22 ± 4 | |
| Genotype, F508del homozygous/F508del heterozygous/other mutations | NA | 36/24/7 | NA | |
| FEV1, % predicted | 100 ± 9 | 67 ± 23 | 81 ± 25 | |
| VC, % predicted | 102 ± 10 | 87 ± 19 | 93 ± 17 | |
| Pa infection status, C/I/F/N [3] | NA | 33/12/12/10 | NA | |
| Human-derived epithelial cells series | Subjects, n | 7 | 18 | 25 |
| Sex, F/M | 1/6 | 9/9 | 10/15 | |
| Age, years | 62 ± 8 | 35 ± 12 | 43 ± 16 | |
| Genotype, F508del homozygous/F508del heterozygous/other mutations | NA | 9/7/2 | NA | |
| FEV1, % predicted (n = 16) | 97 ± 14 | 31 ± 20 | 48 ± 34 | |
| Subjects, n (pIgR expression) | 6 | 8 | 14 | |
| Subjects, n (UPR activation) | 8 | 10 | 18 | |
| Subjects, n (Pa supernatant stimulation) | 0 | 5 | 5 | |
| Subjects, n (IL-17 stimulation) | 0 | 6 | 6 |
Definition of abbreviations: CF, cystic fibrosis; F, female; M, male; FEV1, forced expiratory volume in the first second; NA, not applicable; BMI, body mass index; VC, vital capacity; Pa, Pseudomonas aeruginosa; C, chronically infected by Pa; I, intermittently infected by Pa; F, free of Pa infection; N = never infected by Pa; pIgR, polymeric immunoglobulin receptor; UPR, unfolded protein response. N is specified when data are missing.
Immunohistochemistry for human and mouse pIgR, and human IgA
Lung samples were fixed in 4% formaldehyde and embedded in paraffin wax. Paraffin lung sections (5 μm-thick) were deparaffinised in toluene and rehydrated through a graded series from methanol to water. Except for human pIgR/SC, sections underwent antigen retrieval treatment using cooker pressure treatment during 5 min at a pressure of 15 psi. Endogenous peroxidase, biotin, and streptavidin sites were inactivated by hydrogen peroxide, biotin, and avidin solutions or Bloxall (Vector Laboratories Inc., USA), as appropriate. Nonspecific protein binding sites were blocked with 5% bovine serum albumin or horse serum (Bio-Rad, USA) in tris-buffered saline (TBS) for 1 h, according to the secondary antibody. Rabbit polyclonal anti-human pIgR (1 µg/mL; home-made Lp877), mouse anti-human IgA (4 µg/mL; Thermo Fisher Scientific, Germany), or goat anti-mouse pIgR (R&D Systems, UK) were incubated overnight at 4°C. After washes with TBS 0.1% Tween 20, sections were incubated with goat anti-rabbit IgG (1:3000; Sigma-Aldrich, USA) – followed by streptavidin-horseradish peroxidase (HRP) amplification –, horse anti-mouse Ig (ImmPRESSTM Reagent) or mouse anti-goat IgG (1:100; Sigma-Aldrich), respectively. Revelation was performed by using 3,3′-Diaminobenzidine (Sigma-Aldrich). Sections were counterstained with Hematoxylin (Sigma-Aldrich) and coverslipped. Slides were scanned using Leica SCN400 (Leica, UK) before selecting the ten preserved fields. Quantification was done with Leica software and expressed as percentage of positive area for pIgR expression and for IgA immunostaining as the number of IgA positive cells per mm².
Dual immunohistochemistry for IgA and cluster of differentiation (CD)138, and for CD3 and RAR-related orphan receptor (ROR)γt
As previously, unstained paraffin sections were deparaffinised, rehydrated, and subjected to antigen retrieval. Endogenous peroxidase activity was blocked by incubation in Bloxall (Vector lab, USA) for 15 min and 0.3% hydrogen peroxide in TBS 5% goat serum (Abcam) for 30 min respectively. Each section was subjected to two sequential stainings, each including a blocking with TBS 5% goat serum followed by primary antibody incubation and corresponding secondary HRP-conjugated polymer antibody (Invitrogen). Mouse anti-human IgA (Thermo Fisher Scientific) and rabbit anti-CD138 (Acris, Germany), or rabbit anti-CD3 (Cell Signaling, USA) and mouse anti-RORγt (Sigma) were successively used as primary antibody. Each HRP-conjugated polymer mediated the covalent binding of a different fluorophore using tyramide signal amplification, as previously described [24]. Finally, sections were counterstained with Hoechst (Thermo Fisher Scientific) and mounted with fluorescence mounting medium (Dako, USA). As negative controls, we used corresponding control isotypes diluted at the same concentration as related primary antibody. Images were acquired with Panoramic P250 Flash III slide scanner (3DHistech, Hungary). Quantification of the CD3 and RORγt co-staining was performed with Author software (Visiopharm®) and was expressed as the number of positive cells per µm².
Basal cell isolation
The bronchial section was digested by pronase E from Streptomyces griseus 1 mg/mL (Sigma-Aldrich) in Roswell Park Memorial Institute (RPMI) medium (Lonza, Belgium) supplemented with 200 U/mL penicillin and 200 µg/mL streptomycin (Lonza) overnight at 4°C. Cells were labelled with anti-CD151 conjugated with phycoerythrin (Clone 14A2.H1, BD Biosciences) and anti-CD142 conjugated with allophycocyanin (Clone HTF1, Miltenyi Biotec, Netherlands) antibodies after blocking with FcR Blocking Reagent (Miltenyi Biotec) and dead cells discrimination by Fixable Viability Stain 450 (BD Biosciences, USA). Cell sorting was performed with a FACSAria cell sorter (BD Biosciences). Basal cells (CD151+/CD142+) were selected and seeded in a 75 cm² cell culture flask in bronchial epithelial basal medium (BEBM) (Lonza), supplemented with 200 U/mL penicillin and 200 µg/mL streptomycin (Lonza) (as well as PrimocinTM100 µg/mL (InvitroGen, France) for CF cells), bovine serum albumin 1.5 mg/mL (Sigma-Aldrich) and retinoic acid 100 µM (Sigma-Aldrich). Cells were cultured at 37°C and 5% CO2 to reconstitute a primary bronchial epithelium. When reaching 90% confluency, cells were trypsinised and 100,000 cells were seeded in 0.33 cm² insert with 0.4 µm polyester membrane pore (Corning, USA) in submerged condition for 10–15 days. Then air-liquid interface (ALI) condition was applied for two weeks in BEBM:Dulbecco's Modified Eagle Medium (DMEM) (Lonza) 1:1 supplemented as pure BEBM.
In vitro epithelial assays
Primary human bronchial epithelial cells (HBEC) were treated after culture for two weeks in ALI condition with 25 µL of CFTR inhibitors (GlyH-101 or PPQ-102) (CF Foundation) (1, 5, 10, 20 µM), or 25 µL of Pa supernatant, at the apical pole for 48 h. Basal media, apical washes, and cell lysates (for western blot or reverse transcription and real-time polymerase chain reaction (RT-qPCR)) were then harvested. HBEC cultures were also treated for 48 h with increasing doses of IL-17 (10, 20, 40 ng/mL). Basal media, apical washes, and cell lysates (for western blot or RT-qPCR) were then collected and harvested.
For the transcytosis assay of d-IgA, after two weeks in ALI condition, 1 mg/mL d-IgA was added at basolateral pole for 72 h, and S-IgA was measured by sandwich Enzyme Linked Immunosorbent Assays in the apical wash (300 µL phosphate-buffered saline).
Pa supernatant preparation and stimulation
Pa mucoid clinical strain was processed according to Massion and colleagues to obtain Pa supernatant [25]. Briefly, Pa mucoid clinical strain was seeded in Trypticone Soy Broth medium (BD, USA) and incubated during 72 h, at 37°C. Then, bacteria culture was centrifuged at 10,000 g during 50 min, at 4°C. Supernatant was filtered (0.2 µm) and stored at -80°C. After two weeks in ALI condition, ALI-HBEC were stimulated with 25 µL of Pa supernatant at apical pole for 48 h. Sterile phosphate-buffered saline was used as control condition. Basal media, apical washes, and cell lysates (for western blot or RT-qPCR) were then harvested.
Calu-3 cell culture
Calu-3 cells (passages 10–15) were cultured at 37°C and 5% CO2 in a RPMI medium (Lonza), supplemented with 10% foetal bovine serum, 200 U/mL penicillin and 200 µg/mL streptomycin (Lonza). Cells were maintained in a 75 cm² cell culture flask and split when they reached 90% confluence. For unfolded protein response (UPR) activation, Calu-3 cells were stimulated with thapsigargin (Sigma-Aldrich) (0.2, 0.5, 1, 2 µM) or tunicamycin (Sigma-Aldrich) (2, 5, 10, 20 µg/mL), diluted in dimethyl sulfoxide (DMSO), during 6 h or 24 h. DMSO was used as control condition. Media and cell lysates (for western blot or RT-qPCR) were then harvested. For transcytosis assay of d-IgA, Calu-3 cells (50,000 per insert) were seeded in 0.33 cm² insert with 0.4 µm polyester membrane pore (Corning). After they reached confluency, they were stimulated with 10 µg/mL tunicamycin and 1 mg/mL d-IgA was added at basolateral pole for 48 h. DMSO was used as control condition. Basal media and apical washes or media were then collected. Calu-3 cell cultures were also treated for 48 h with increasing doses of IL-17 (5, 10, 20 ng/mL). Media and cells lysates were then collected and harvested.
Sputum and serum collection, and processing
Sputum from patients with CF was obtained during a physiotherapy session while sputum from healthy subjects was induced by using the method of Pizzichini and colleagues, with minor modifications [26]. Sputum induction was achieved with an aerosol of hypertonic saline generated by the NE-U17 ultrasonic nebulizer (Omron). Healthy subjects equipped with a nose clip, inhaled increasing concentrations of saline (3, 4, and 5%) for 7 min each through a mouthpiece. We then asked subjects to rinse their mouth or swallow to minimise contamination with saliva. Finally, we invited them to cough sputum into a sterile container. Collected sputum was diluted 1:10 (w:v) in phosphate-buffered saline with 10 U/mL DNase (Pulmozyme 2500 U/2.5 mL – Roche, USA) and incubated under agitation, during 30 min, at 37°C. Then, it was filtered with 48 µm pore nylon filter (Prosep, Belgium) and centrifuged at 450 g, during 5 min. Neutrophil elastase activity was measured in supernatant. Remaining supernatant was treated with PMSF Protease Inhibitor 2% v:v in order to inactivate elastase and was stored at -80°C. Cells were processed for cytospin (fixed in methanol and staining by Diff-Quick method (Kwik-Diff™ Stains, Thermo Fisher Scientific) and epithelial contamination was assessed. The blood was centrifuged at 650 g for 20 min, the serum was collected, and stored at -80°C.
F508del mice and model of Pa lung infection with Pa-coated beads
Mice (FVB/129) homozygous for the F508del mutation (F508del mice, kindly received from Dr T. Leal, UCLouvain Brussels, Belgium) and wild-type littermates (WT mice) were housed in a specific pathogen free animal facility, in ventilated cages. 15 F508del mice and 15 WT received no treatment (eight females and seven males in each group). In parallel, nine F508del (eight females and one male) and six WT (four females and two males) mice were instilled with agarose beads, coated with a clinical strain of Pa (isolated from a patient with CF). Sterile beads were used to instil seven F508del (four females and three males) and eight WT (three females and five males), as control. Beads were prepared according to Martin and colleagues [27]. Dissolved agarose (coloured with Indian ink) (Type XII, Sigma-Aldrich®) was mixed with mineral oil (Sigma-Aldrich®) at 50°C, and with around 109 colony-forming units of Pa in culture. This mix was cooled down with ice while mixing for 20 min. After rinsing by centrifugations at 4°C, beads were sieved twice to obtain a homogenous solution of 300 to 400 µm beads, a size considered optimal to penetrate the lower airways, but not the alveoli. In order to control the bacterial load, beads and Pa culture were spread on agar-agar and counted after incubation during 24 h at 37°C. A volume of 40 µL of beads solution at 5% in phosphate-buffered saline (sterile beads or Pa-coated) was instilled in mouse trachea after anaesthesia with Ketamine 15 mg/mL (Nimatek® 100 mg/mL Eurovet, Netherlands) and Xylazine 2 mg/mL (Rompun® 2%, Bayer, GmbH D24106, Germany) at 100 µL/10 g body weight. Eventual respiratory stress could appear after instillation and therefore mice were closely monitored and received oxygen supplementation if needed. After 15 days, mice were sacrificed, BAL was performed, and lungs were collected for immunochemistry, RT-qPCR and western blot, as well as blood to obtain serum.
Western blot for pIgR/SC and (phospho-)eukaryotic initiation factor 2α ((P-)eIF2α)
Cells were lysed with Laemmli's Blue. After boiling for 5 min, lysates were separated by 12% sodium dodecyl sulfate-polyacrylamide gel and proteins were transferred to a nitrocellulose membrane. After blocking with TBS 5% bovine serum albumin, the membrane was incubated with rabbit anti-pIgR (1:6000; homemade Lp877), rabbit anti-GAPDH (1:3000; Sigma-Aldrich), mouse anti-eIF2α (1:2000; Cell Signaling), rabbit anti-P-eIF2α (1:1000; Cell Signaling), and mouse anti-β-actin (1:1000; Sigma-Aldrich) overnight at 4°C, then with goat anti-rabbit IgG (1:2000; Cell Signaling) or anti-mouse IgG (1:5000; Sigma-Aldrich) for 1 h at room temperature. Detection of immunoreactivity was performed with the ECLTM Prime Western Blotting Detection chemiluminescence reagent (AmershamTM ECL, GE Healthcare, UK) blot and quantification was performed by using Quantity One software.
Enzyme Linked Immunosorbent Assays for SC, S-IgA, S-IgM, specific IgA to Pa and IL-17
SC, S-Ig, and total Ig concentrations were measured in HBEC apical washes, sputum supernatants or serums, in plates coated with anti-SC (1:2000; Ch606), anti-IgA (1:1000; ACP17) or anti-IgM (Sigma-Aldrich) as capture antibody. After washes, samples and standard incubated 2 h, at 37°C, and detection was performed with biotinylated anti-SC (1:4000; Ch606) – followed by Streptavidin-HRP amplification – or HRP-linked anti-IgA (1:5000; Sigma-Aldrich) or anti-IgM (1:5000; Sigma-Aldrich).
In sputum supernatants, specific IgA to Pa were determined by coating 2.25 µg of antigens from serotypes 001-015 (Statens Serum Institute, Denmark) and detection was performed with the antibody mentioned above, and IL-17 was measured by Duoset (R&D Systems).
In mouse BAL and serum, concentrations of mouse total IgA, as well as (only in BAL) mouse S-IgA and mouse SC, were measured. Plates were coated with anti-mouse IgA (1:1000; Sigma-Aldrich) or anti-mouse SC (1:200; R&D Systems). After blocking, samples and standard (IgA κ myeloma (Sigma-Aldrich) or pure BAL) were loaded and incubated 2 h, at 37°C. Immunodetection was carried out by using biotinylated anti-mouse IgA (SYnAbs, Belgium) and Streptavidin-HRP amplification. For mouse SC, samples and standard (R&D Systems) were coated in plates and incubated overnight at 4°C. Mouse SC was captured with goat anti-mouse SC (R&D Systems) and detected by biotinylated anti-goat IgG (Sigma-Aldrich) and Streptavidin-HRP amplification.
Reverse transcription and real-time polymerase chain reaction
After extraction, ribonucleic acid (RNA) was reverse-transcribed with RevertAid H minus Reverse transcriptase kit (Thermo Fisher Scientific, UK) with 0.3 µg of random hexamer, 20 U of RNase inhibitor, and 1 mM of each dNTP following the manufacturer's protocol in a thermocycler (Applied Biosystems). For the expression measurement, the reaction mix contained 2.5 µL of complementary desoxyribonucleic acid diluted 10-fold, 200 nM of each primer (Table S1), and 2x iTaq UniverSybr Green® Supermix (Bio-Rad) in a final volume of 20 µL. The cycling conditions were 95°C for 3 min followed by 40 cycles of 95°C for 5 s and 60°C for 30 s. To control the specificity of the amplification products, a melting curve analysis was performed. Copy number was calculated from the standard curve. Expression levels of target genes were normalised to the geometric mean of the values for the housekeeping genes (ribosomal protein S13, S18 and L27 in human lung tissue and primary HBEC, S18 and hypoxanthine-guanine phosphoribosyltransferase 1 in Calu-3, and TATA-box binding protein, hypoxanthine-guanine phosphoribosyltransferase 1 and tyrosine 3-monooxygenase/ tryptophan 5-monooxygenase activation protein zeta in mouse tissue).
Transmission electron microscopy
ALI-HBEC were fixed overnight at 4°C in 2.5% glutaraldehyde BEBM:DMEM 1:1 (Lonza), supplemented as mentioned before, washed in cacodylate buffer (pH 7.4) and postfixed twice in 1% osmium tetroxide (and 1.5% ferrocyanide) for 1 h. Then, cells were stained with 1% uracyl acetate for 1 h at room temperature, dehydrated in ethanol and embedded in epoxy resin (Agar 100 resin; Agar Scientific, UK). Quantification of the ER area was performed with ImageJ software. Results were expressed as the percentage of ER area.
Statistical analysis
GraphPad Prism 8.0.2 software (GraphPad Inc., USA) and SPSS (IBM) were used for statistical analysis. Normality of the data distribution was first assessed. Comparisons between two groups were performed using an unpaired Student's t test, except when data distribution were not normal. In such case, comparisons between two groups were performed using the Mann-Whitney U-test, whereas comparisons between more than two groups were performed using the Kruskal-Wallis test followed by a Dunn's post-hoc test or Friedman test in case of paired values. Comparisons were considered as significant if p-value was under 0.05.
Ethics statement
All patients received information and signed a written consent to the study protocol, which was approved by our local clinical ethical committee (Ref. protocol “CLARA” 2005/22SEP/149 – update 29/11/2016, S51577 S52174 S55877) and CPP Ile de France II #1072 for lung tissue samples, Ref. 2015/09Jan/014 for sputum samples). Murine experiments were approved by our local Faculty Ethical committee (Ref. 2018/UCL/MD/04).
Results
Upregulation of epithelial pIgR and IgA expression in the human CF lung
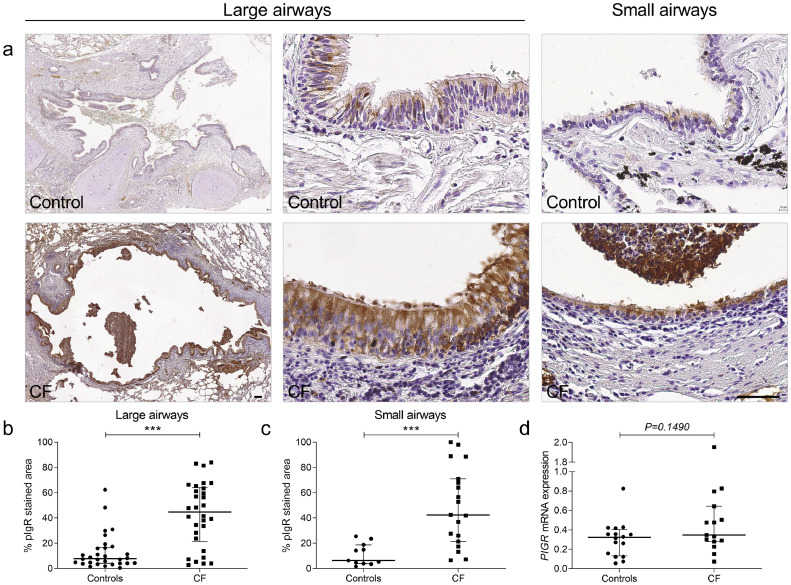
pIgR expression was increased in the bronchial epithelium from patients with CF, compared with controls, in both large and small airways (Fig. 1a–c) while no significant difference was observed at the mRNA level in lung homogenates (Fig. 1d).
Fig. 1.
pIgR expression is increased in airway epithelium from control and patients with CF at the protein level. (a) pIgR immunostaining in a large airway and in a small airway from one representative control and one representative patient with CF. (b) pIgR expression in large airways from 30 patients with CF, as compared with 30 controls (***p < 0.0001, Mann-Whitney test). (c) pIgR expression in small airways from 19 patients with CF, as compared with 11 controls (***p = 0.0003, Mann-Whitney test). (d) PIGR mRNA expression (corrected for housekeeping genes) in lung homogenates from 15 patients with CF, compared with 16 controls (p= 0.1490, Mann-Whitney test). Bars indicate median and interquartile ranges. pIgR, polymeric immunoglobulin receptor; CF, cystic fibrosis. Scale bar, 100 µm.
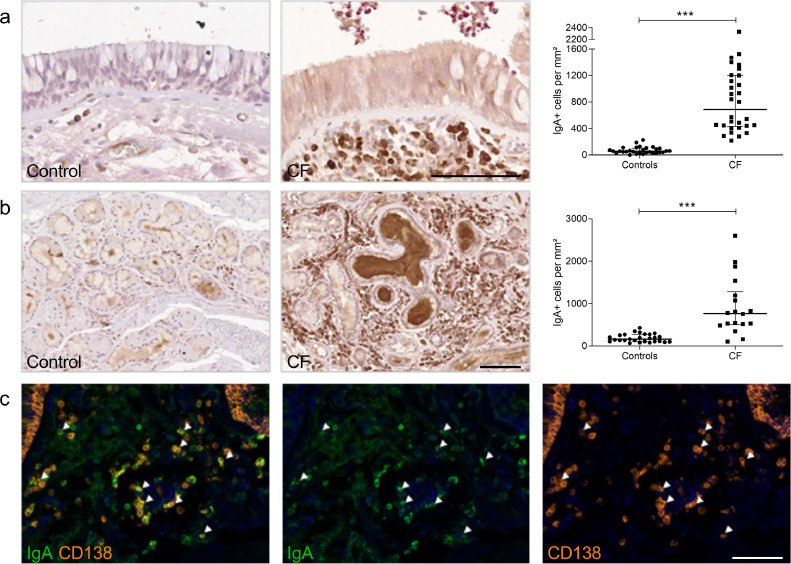
As an increased number of lymphoid aggregates was confirmed in our CF lungs, compared with controls (Fig. S1), IgA immunostaining was carried out on lung tissue, and confirmed a large increase in the number of IgA+ cells in CF lungs in subepithelial areas (Fig. 2a) and in the surrounding submucosal glands (Fig. 2b). A dual immunostaining for IgA and CD138 indicated that >70% of IgA+ cells consisted of IgA+ plasma cells (Fig. 2c).
Fig. 2.
Patients with CF display increased IgA+ cells number. (a) IgA staining in the subepithelial area from one representative control and one representative patient with CF and number of IgA+ cells in the subepithelial area from 30 patients with CF, as compared with 30 controls (***p < 0.0001, Mann-Whitney test). (b) IgA staining in the glandular area from one representative control and from one representative patient with CF and number of IgA+ cells in the glandular area from 18 patients with CF, as compared with 27 controls (***p < 0.0001, Mann-Whitney test). (c) Identification of plasma cells (CD138+ cells (orange)) producing IgA (green; arrows) in the subepithelial area from one patient with CF. Bars indicate median and interquartile ranges. IgA, immunoglobulin A.
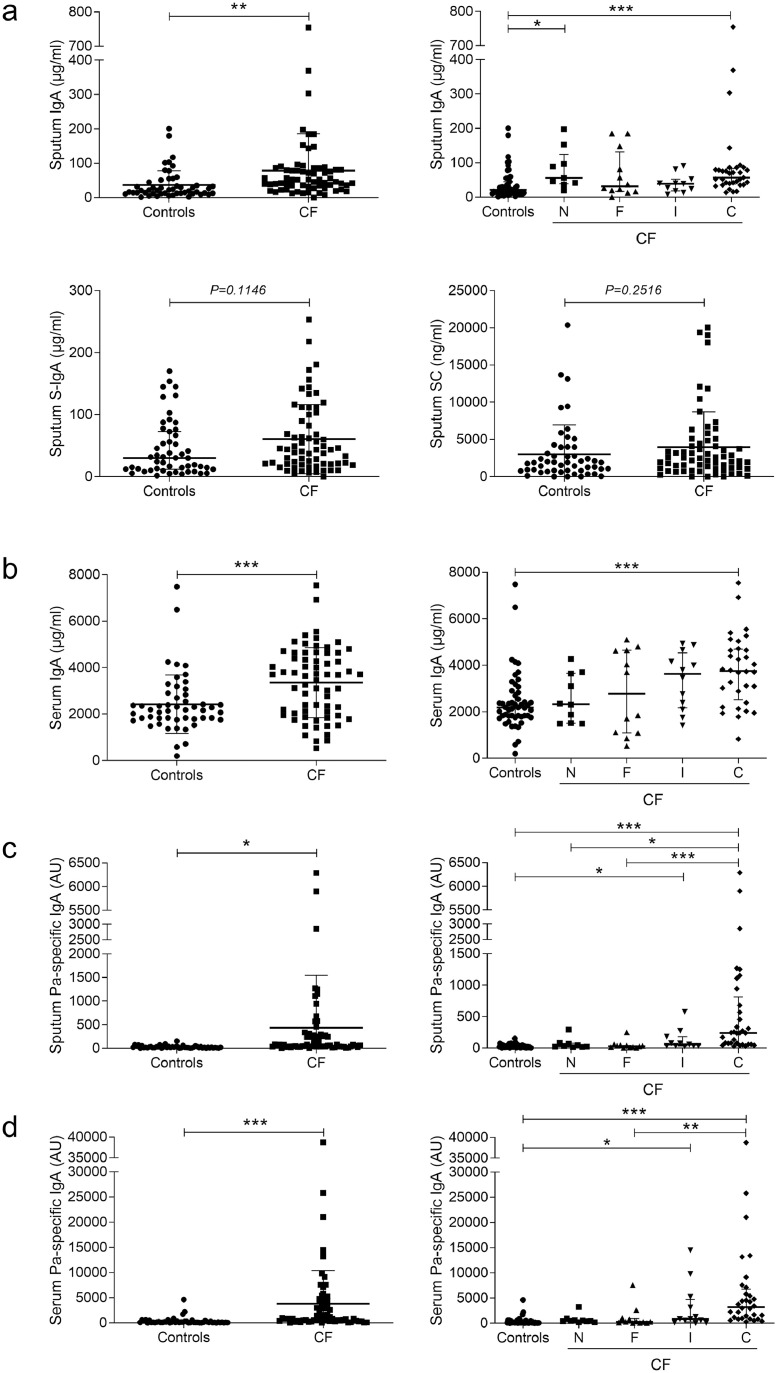
Upregulation of total and Pa-specific IgA in human CF sputum
Despite raised neutrophil elastase activity in sputum from patients with CF (Fig. S2a), which is able to cleave IgA and SC [28], the concentration of IgA was increased in sputum and serum from patients with CF, compared with controls, especially in chronically Pa infected patients (Fig. 3a–b). Never Pa infected patients were nevertheless infected with other bacteria (such as Staphylococcus aureus, Achromobacter, or Burkholderia species) or Aspergillus fumigatus (in eight and two out of ten patients, respectively). A similar increase was observed for IgM (Fig. S2b), also secreted following pIgR-mediated transport. In addition, a trend for increased S-IgA and no change for SC (Fig. 3a) were noticed. Of note, higher concentrations of IgA and SC were measured in sputum from F508del homozygous patients when compared with patients without this mutation (Fig. S2c–f).
Fig. 3.
IgA and Pa-specific IgA concentrations are increased in sputum and serum from patients with CF. (a) IgA concentration in sputum from 51 controls and 65 patients with CF (**p = 0.0092, unpaired Student's t-test), distributed following Leeds criteria (***p < 0.001, **p < 0.01, Kruskal-Wallis test followed by Dunn's test); S-IgA concentration in sputum from 51 controls and 65 patients with CF (p = 0.1146, unpaired Student's t-test); SC concentration in sputum from 51 controls and 65 patients with CF (p = 0.2516, unpaired Student's t-test). (b) IgA concentration in serum from 51 controls and 66 patients with CF (***p = 0.0006, unpaired Student's t-test), distributed following Leeds criteria (***p < 0.001, Kruskal-Wallis test followed by Dunn's test). (c) Pa-specific IgA concentration in sputum from 51 controls and 65 patients with CF (*p = 0.0107, unpaired Student's t-test), distributed following Leeds criteria (***p < 0.001, *p < 0.05, Kruskal-Wallis test followed by Dunn's test). (d) Pa-specific IgA concentration in serum from 51 controls and 66 patients with CF (***p = 0.0003, unpaired Student's t-test), distributed following Leeds criteria (***p < 0.001, **p < 0.01, *p < 0.05, Kruskal-Wallis test followed by Dunn's test). Bars indicate mean and standard deviation or median and interquartile ranges (Leeds distribution). IgA, immunoglobulin A; Pa, Pseudomonas aeruginosa; CF, cystic fibrosis; S-IgA, secretory immunoglobulin A; SC, secretory component; C, chronically infected by Pa; I, intermittently infected by Pa; F, free of Pa infection; N, never infected with Pa.
In addition, a Pa-specific IgA response was observed in sputum and serum from patients with CF, chronically or intermittently infected with Pa (Fig. 3c–d), and in patients carrying at least one F508del mutation (Fig. S2ag–h).
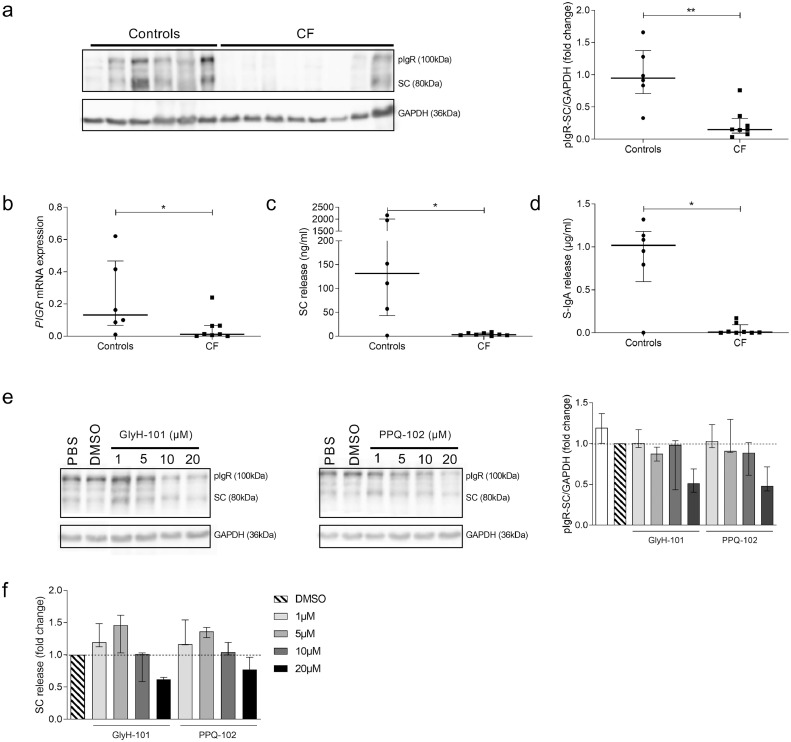
Downregulation of pIgR expression following CFTR dysfunction in the human bronchial epithelium
In order to avoid the major risk of infection of primary CF HBEC, airway basal cells were sorted (by using CD142 and CD151 staining), and cultured in ALI for two weeks to reconstitute in vitro a differentiated CF bronchial epithelium [29]. In contrast to tissue data, a reduced pIgR expression was observed in CF HBEC, compared with the epithelium derived from control lungs both at protein (Fig. 4a) and mRNA level (Fig. 4b). In addition, the CF-derived epithelium displayed strong decreases in SC and S-IgA release in the apical washes (Fig. 4c–d). To confirm this negative link between CFTR dysfunction and pIgR expression, control HBEC were treated at 2 weeks in ALI culture with increasing concentrations of two CFTR inhibitors (GlyH-101 and PPQ-102). After 48 h of treatment, a dose-dependent decrease in pIgR/SC production and SC release in apical washes was observed (Fig. 4e–f).
Fig. 4.
pIgR expression and SC/S-IgA release are downregulated in ALI-HBEC derived from patients with CF and in control ALI-HBEC upon CFTR inhibition. (a) Representative western blot for pIgR in CF ALI-HBEC, compared with control ALI-HBEC and its pIgR-SC/GAPDH expression quantification in eight CF ALI-HBEC, compared with six control ALI-HBEC (**p = 0.0027, Mann-Whitney test). (b) PIGR mRNA expression (corrected for housekeeping genes) in eight CF ALI-HBEC, compared with six control ALI-HBEC (*p = 0.0426, Mann-Whitney test). (c) SC release in apical washes of eight CF ALI-HBEC, compared with six control ALI-HBEC (*p = 0.0293, Mann-Whitney test). (d) After transcytosis assay, S-IgA release in apical washes of eight CF ALI-HBEC, compared with six control ALI-HBEC (*p = 0.0127, Mann-Whitney test). (e) Representative western blot for pIgR in control ALI-HBEC, treated 48 h with increasing concentrations of GlyH-101 and PPQ-102 or DMSO as control and its pIgR-SC/GAPDH expression quantification in three control ALI-HBEC. (f) Concentration of SC released in apical washes of three control ALI-HBEC, treated 48 h with increasing concentrations of GlyH-101 and PPQ-102 or DMSO as control. Bars indicate median and interquartile ranges. pIgR, polymeric immunoglobulin receptor; SC, secretory component; S-IgA, secretory immunoglobulin A; ALI-HBEC, human bronchial epithelial cells in air-liquid interface; CF, cystic fibrosis.
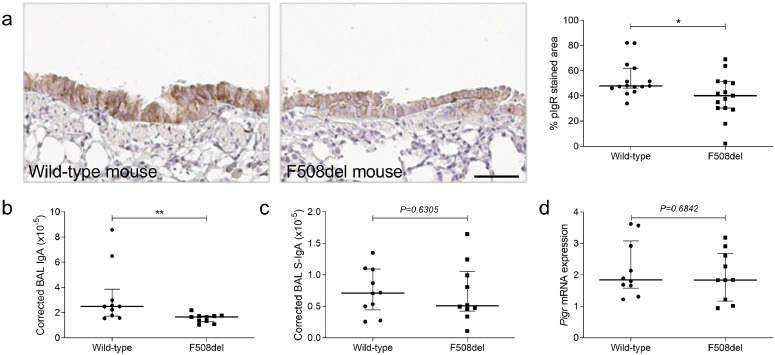
Downregulation of pIgR expression and IgA secretion following CFTR dysfunction in F508del mice
In order to confirm pIgR downregulation in “sterile” environment upon CFTR dysfunction in vivo, we studied pIgR expression in F508del mice. A decreased pIgR expression was observed, as compared with WT mice (Fig. 5a), as well as a lower concentration of total IgA in BAL (Fig. 5b). As in human lung, S-IgA in BAL (Fig. 5c) and Pigr mRNA expression in lung homogenates were not significantly changed (Fig. 5d).
Fig. 5.
pIgR expression and IgA secretion are decreased in airways from F508del mice. (a) pIgR immunostaining in an airway from one representative WT mouse and from one representative F508del mouse and quantification in airways from 15 F508del mice, as compared with 15 WT mice (*p = 0.0344, Mann-Whitney test). (b) IgA concentration, normalised for total protein content, in BAL from ten F508del mice, compared with ten WT mice (**p = 0.0058, Mann-Whitney test). (c) S-IgA concentration, normalised for total protein content in BAL from ten F508del mice, compared with ten WT mice (p = 0.6305, Mann-Whitney test). (d) Pigr mRNA expression (corrected for housekeeping genes) in lung homogenates from ten F508del mice, compared with ten WT mice (p = 0.6842, Mann-Whitney test). Bars indicate median and interquartile ranges. pIgR, polymeric immunoglobulin receptor; IgA, immunoglobulin A; WT, wild-type; S-IgA, secretory immunoglobulin A; BAL, bronchoalveolar lavage. Scale bar, 50 µm.
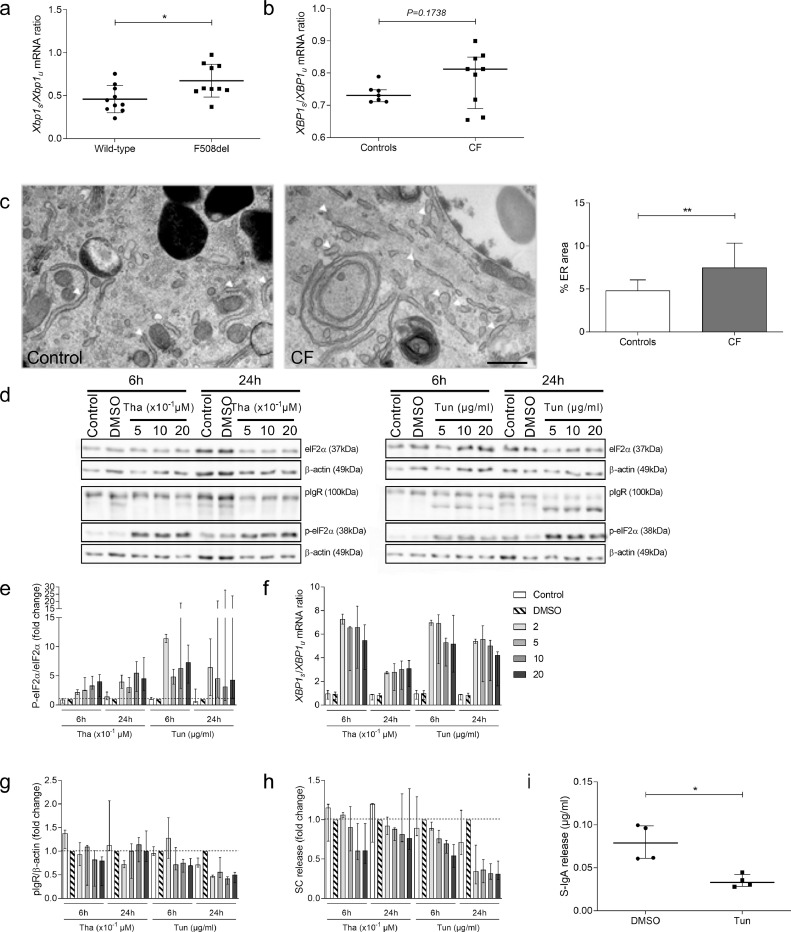
UPR is activated in CF-derived HBEC and F508del mice, and affects SC secretion
To explore the mechanism of pIgR downregulation observed upon CFTR dysfunction, in both human CF HBEC and F508del mice, we hypothesised that the ER stress and UPR could be involved. Indeed, F508del mutation has been associated with an accumulation of misfolded proteins in the ER, resulting in ER stress and UPR activation [30]. UPR activation assessed by the mRNA ratio of active, spliced/unspliced, XBP-1 was confirmed in F508del mouse lungs (Fig. 6a), which received no treatment. A trend was also observed in the reconstituted epithelium from patients with CF (most of them harbouring at least one copy of the F508del mutation) as compared with controls (Fig. 6b). In addition, ER stress was also evidenced by transmission electron microscopy showing dilated ER cavities in CF, while this feature was absent in control HBEC (Fig. 6c). Therefore, we stimulated UPR in Calu-3 cells (by thapsigargin or tunicamycin), an epithelial cell line which stably expresses pIgR at high level, to evaluate whether this regulates pIgR expression. UPR activation leading to phosphorylation of eIF2α (Fig. 6d–e), increased spliced/unspliced XBP1 mRNA ratio (Fig. 6f) and dose-dependently decreased pIgR protein expression (Fig. 6g) and SC release (Fig. 6h). In addition, d-IgA transcytosis was impaired in Calu-3 cells treated with tunicamycin (Fig. 6i). These data indicate that UPR activation per se (or that follows ER stress) could recapitulate the downregulation of pIgR expression and IgA transport observed in CF cell cultures and in CF mouse lungs.
Fig. 6.
UPR is activated in CF-derived human HBEC and in lungs from F508del mice at baseline and it downregulates SC secretion in Calu-3 cells. (a) Xbp1s/Xbp1u mRNA ratio (corrected for housekeeping genes) in lung from ten F508del mice receiving no treatment, compared with ten WT mice (*p = 0.0133, Mann-Whitney test). (b) XBP1s/XBP1u mRNA ratio (corrected for housekeeping genes) in HBEC (cultured in sterile condition) from nine patients with CF, compared with seven controls (p = 0.1738, Mann-Whitney test). (c) Transmission electron microscopy in HBEC (cultured in sterile condition) from one control and from one patient with CF and quantification of ER (arrows) surface in one control and from one patient with CF (**p = 0.0094, unpaired Student's t-test). (d) Representative western blot for eIF2α, P-eIF2α and pIgR in Calu-3 cells treated with increasing concentrations of thapsigargin, tunicamycin or DMSO as control condition. (e) Quantification of P-eIF2α/eIF2α protein expression in Calu-3 cells after treatment with increasing concentrations of thapsigargin and tunicamycin, compared with DMSO as control condition (n = 3). (f) XBP1s/XBP1u mRNA ratio (corrected for housekeeping genes) in Calu-3 cells treated increasing concentrations of thapsigargin and tunicamycin or DMSO as control condition (n = 3). (g) Quantification of pIgR/β-actin expression in Calu-3 cells after treatment with increasing concentrations of thapsigargin and tunicamycin, compared with DMSO as control condition (n = 3). (h) SC secretion upon treatment with increasing concentrations of thapsigargin and tunicamycin, compared with DMSO as control condition (n = 3). (i) After transcytosis assay, S-IgA release upon thapsigargin treatment (*p = 0.0286, Mann-Whitney test) (n = 4). Bars indicate median and interquartile ranges, except for (c) (mean and standard deviation). UPR, unfolded protein response; XBP1s, spliced X-box binding protein 1; XBP1u, unspliced X-box binding protein 1; eIF2α, eukaryotic initiation factor 2 α; P-eIF2α, phospho-eukaryotic initiation factor 2 α; pIgR, polymeric immunoglobulin receptor; SC, secretory component; S-IgA, secretory immunoglobulin A; Tha, thapsigargin; Tun, tunicamycin. Scale bar, 500 nm.
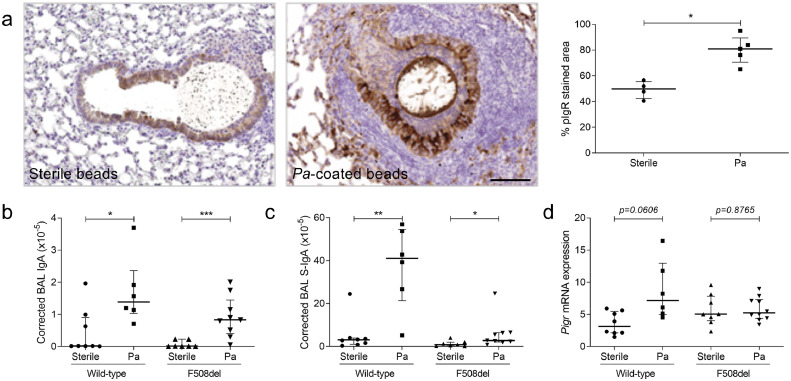
Upregulation of pIgR expression and (S-)IgA production upon Pa infection
In order to reconcile the apparent discrepancy between upregulated pIgR expression in human CF lungs and its downregulation upon CFTR dysfunction in HBEC and in F508del mice, we reasoned that the negative CFTR-pIgR link could be overcome during lung infection with Pa. We observed that WT mice instilled with Pa-coated beads displayed increased pIgR expression in the airway epithelium compared with sterile beads (Fig. 7a). In addition, a strong upregulation of IgA and S-IgA concentrations was observed in BAL upon Pa infection both in WT and F508del mice (Fig. 7b–c), associated with an increase in PIGR mRNA expression (Fig. 7d).
Fig. 7.
pIgR expression and (S-)IgA secretion are upregulated in lungs from mice infected with Pa. (a) pIgR immunostaining in an airway from one representative WT mouse instilled with sterile beads or Pa-coated beads and quantification (in the airway epithelium surrounding the beads) in five WT mice instilled with Pa-coated beads, compared with four WT mice instilled with sterile beads (*p = 0.0159, Mann-Whitney test). (b) IgA concentration, normalised for the total protein content, in BAL from six WT mice instilled with Pa-coated beads, compared with eight WT mice instilled with sterile beads (*p = 0.0200, Mann-Whitney test) and from nine F508del mice instilled with Pa-coated beads, compared with seven F508del mice instilled with sterile beads (***p = 0.0007, Mann-Whitney test). (c) S-IgA concentration, normalised for the total protein content, in BAL from six WT mice instilled with Pa-coated beads, compared with eight WT mice instilled with sterile beads (**p = 0.0013, Mann-Whitney test) and from nine F508del mice instilled with Pa-coated beads, compared with seven F508del mice instilled with sterile beads (*p = 0.0164, Mann-Whitney test). (d) Pigr mRNA expression (corrected for housekeeping genes) in lungs from six WT mice instilled with Pa-coated beads, compared with eight WT mice instilled with sterile beads (*p = 0.0134, Mann-Whitney test). Bars indicate median and interquartile ranges. pIgR, polymeric immunoglobulin receptor; Pa, Pseudomonas aeruginosa; S-IgA, secretory immunoglobulin A; SC, secretory component; BAL, bronchoalveolar lavage. Scale bar, 100 µm.
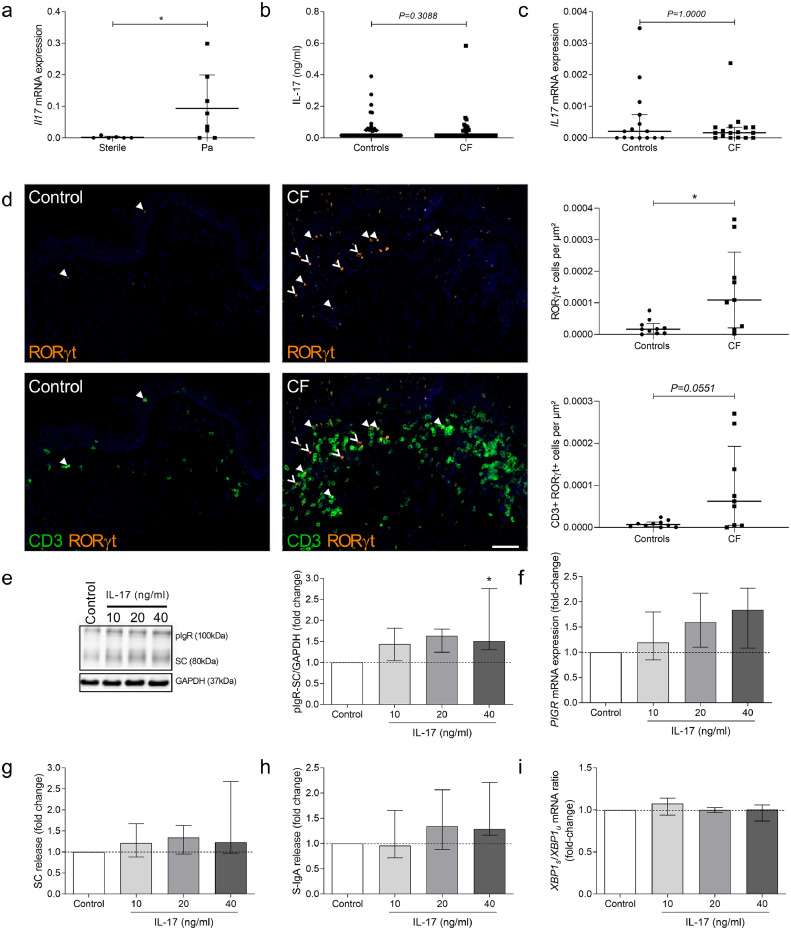
Pa infection upregulates pIgR expression through an indirect mechanism involving IL-17
To address the mechanisms by which Pa could upregulate pIgR expression and IgA secretion, CF HBEC were incubated with the supernatant of a Pa clinical strain culture. Only marginal and not significant changes in PIGR mRNA expression, pIgR protein, SC release and d-IgA transcytosis were observed in CF-derived cells treated for 48 h with Pa supernatant (Fig. S3). An indirect mechanism was then explored by looking at the inflammatory response induced by Pa infection. In particular, we evaluated IL-17 as it is reportedly increased upon Pa infection, and it is able to upregulate PIGR mRNA expression in intestinal cells [31,32]. An induction of Il17 mRNA expression was noticed in the lungs from mice instilled with Pa-coated beads, compared with mice instilled with sterile beads (Fig. 8a). While IL-17A upregulation was not recapitulated in sputum or lung tissue from patients with CF (Fig. 8b–c), immune cells producing IL-17 (RORγt+ cells, including CD3+ RORγt+ Th17 cells) [33,34] were increased in subepithelial areas of patients with CF, compared with controls (Fig. 8d), as well as in total lung tissue (Fig. S4a). When CF-derived cells were stimulated by increasing concentrations of IL-17A, we observed a dose-dependent increase in pIgR expression (Fig. 8e), associated with a trend to increased PIGR mRNA expression, SC release and d-IgA transcytosis (Fig. 8f–h), which did not influence UPR activation through XBP-1 splicing (Fig. 8i). Upregulated PIGR mRNA was also measured Calu-3 cells stimulated by IL-17A (Fig. S4b–d). As shown in CF-derived HBEC, no influence on UPR activation through XBP-1 splicing was observed upon IL-17A treatment (Fig. S4e). These data suggest that Pa infection could stimulate pIgR expression and S-IgA production in the CF lung by activating an inflammatory host response that involves IL-17.
Fig. 8.
IL-17 is increased in lungs from mice infected with Pa and upregulates pIgR expression in ALI-HBEC derived from patients with CF. (a) Il17 mRNA expression (corrected for housekeeping genes) in lungs from six WT mice instilled with Pa-coated beads, compared with eight WT mice instilled with sterile beads (*p = 0.0373, Mann-Whitney test). (b) IL-17 concentration in sputum from 65 patients with CF, compared with 51 controls (p = 0.3088, Mann-Whitney test). (c) IL17 mRNA expression (corrected for housekeeping genes) in lung homogenates from 15 patients with CF, compared with 16 controls (p = 1.0000, Mann-Whitney test). (d) RORγt staining (and CD3) in the subepithelial area from one representative control and one representative patient with CF, and number of RORγt+ cells (filled and no-filled arrows), including CD3+ RORγt+ cells (filled arrows), in the subepithelial area from 9 patients with CF, as compared with 10 controls (*p = 0.0279, p = 0.0551, Mann-Whitney test). (e) Representative western blot for pIgR in CF ALI-HBEC, treated with increasing concentrations of IL-17 and its pIgR-SC/GAPDH expression quantification in six CF ALI-HBEC, treated with increasing concentrations of IL-17 (*p = 0.0437, Friedman test). (f) PIGR mRNA expression (corrected for housekeeping genes) in five CF ALI-HBEC, treated with increasing concentrations of IL-17. (g) SC release by six CF ALI-HBEC, treated with increasing concentrations of IL-17. (h) SC release by six CF ALI-HBEC, treated with increasing concentrations of IL-17. (i) XBP1s/XBP1u mRNA ratio (corrected for housekeeping genes) in five CF ALI-HBEC, treated with increasing concentrations of IL-17. Bars indicate median and interquartile ranges. IL-17, interleukin 17; Pa, Pseudomonas aeruginosa; pIgR, polymeric immunoglobulin receptor; CF, cystic fibrosis; ROR, RAR-related orphan receptor; CD, cluster of differentiation; SC, secretory component; S-IgA, secretory immunoglobulin A; XBP1s/u, spliced/unspliced X-box binding protein 1. Scale bar, 50 µm.
Discussion
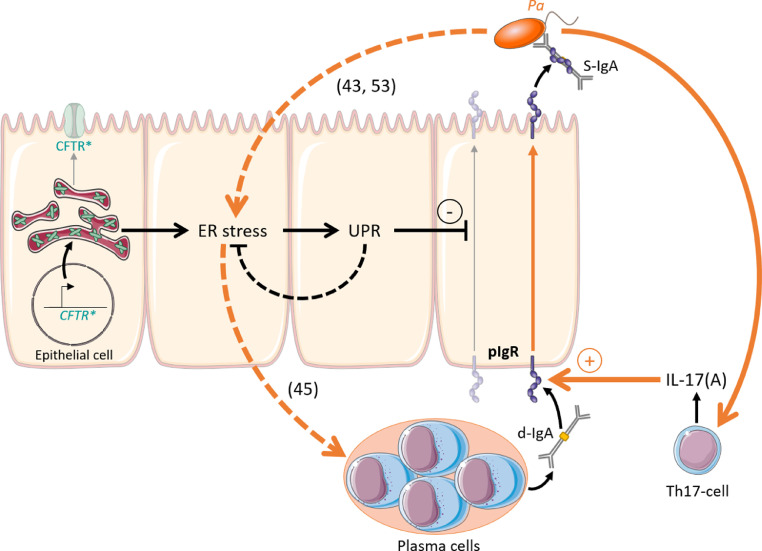
This study shows that epithelial pIgR expression, IgA production, and IgA+ B cells are all upregulated in the CF lung, sputum and serum. This IgA response includes specific antibodies to Pa which represents a major opportunistic bacterium in this disease. Secondly, experiments in airway epithelial basal/stem cell-derived cultures from CF lungs and in F508del mice revealed a constitutive downregulation of pIgR/SC production and d-IgA transcytosis, as recapitulated by adding CFTR inhibitors in control cells. This negative CFTR-pIgR pathway involved UPR activation following the ER stress observed in CF. Finally, an in vivo model of chronic lung infection by Pa-coated microbeads enabled to identify that infection could restore pIgR expression and IgA production in the lungs from F508del mice, through an IL-17 inflammatory host response (Fig. 9).
Fig. 9.
Model of pIgR regulation in CF lung. First, accumulation of misfolded F508del-CFTR (CFTR*) is responsible for ER stress. Consequently, activation of UPR is able to downregulate SC and S-IgA secretion. Second, Pa infection modulates this pathway (orange arrows). Pa drives host IL-17 response which stimulates PIGR expression and further increases ER stress and UPR activation.[43,53] In addition, if ER stress caused by Pa infection becomes insufficiently counterbalanced by UPR activation, it may contribute to IgA upregulation, as previously shown.[45] CFTR, cystic fibrosis transmembrane regulator; UPR, unfolded protein response; SC, secretory component; S-IgA, secretory immunoglobulin A; d-IgA, dimeric immunoglobulin A; Pa, Pseudomonas aeruginosa; IL-17, interleukin 17; pIgR, polymeric immunoglobulin receptor.
In contrast to previous data in COPD or chronic rhino-sinusitis, this study shows that bronchial pIgR expression is upregulated in CF [[16], [17], [18],35]. Numbers of IgA+ B cells are also increased in this disease, as previously observed in COPD [36]. Whereas decreased SC concentration in saliva from patients with CF was previously reported [37], Marshall et al. observed an increased concentration and altered glycosylation of SC in CF sputum [21]. Our study could reconcile the apparent discrepancy between previous studies regarding IgA [20], [21], [22], [23], by suggesting that IgA upregulation may selectively occur in the lungs (but not in the digestive tract) as those are the target of acquired bacterial infection (e.g. Pa) in CF. This increased IgA response is particularly noticed in patients with severe disease, and includes the production of serum/systemic and respiratory/secretory Pa-specific IgA [9,[38], [39], [40], [41], [42]]. Our study robustly confirms increased IgA levels in CF airways, as well as increased serum IgA, and that this response includes a specific reactivity to Pa at both levels.
A major finding of this study is that epithelial pIgR expression and IgA transport are disrupted in primary cultures of HBEC from patients with CF, as well as in the lung from F508del mice. In human lung homogenates, Pigr gene expression was not affected, maybe due to the large amount of non-pIgR expressing cell types into the lung. On the contrary, the use of CF-derived HBEC showed impaired pIgR expression when cultivating in the absence of inflammatory stimulus and allows to distinguish slight difference (notably for PIGR mRNA levels). In vitro experiments using selective CFTR inhibitors could confirm that CFTR dysfunction induces pIgR protein downregulation and defective pIgR protein expression is also demonstrated in non-infected F508del mice. F508del mutation, which was also carried by most of our patients with CF from whom cells were derived, is associated with improper folding and accumulation of the CFTR protein in the ER. Therefore, we hypothesised and confirmed that ER stress and subsequent UPR activation could represent a mechanism involved in pIgR downregulation, in line with previous reports in short-term cultures of CF-derived cells and in a CF cell line [30,43]. We could also demonstrate UPR activation through XBP-1 splicing in the lungs from F508del mice even in the absence of infection. Furthermore, our data show that UPR activation in epithelial cells induces a downregulation of pIgR/SC production as well as of d-IgA transcytosis. In addition, it has been shown that UPR activation in CF not only fails to resolve the ER stress but also sensitises the inflammatory response to Toll-like receptor stimulation [30]. Interestingly, pIgR downregulation (among several other proteins) was also linked to UPR activation in the context of ulcerative colitis [44], whereas another recent study showed that mice with a restricted deletion of XBP-1 in the intestinal epithelium (thus enable to develop UPR to counteract ER stress in these cells) display T cell-independent accumulation of IgA+ plasma cells and increased IgA secretion, which mediates protection against spontaneous enteritis [45]. In line with the latter, our data further show that UPR activation itself disrupts epithelial pIgR expression and that lungs from patients with CF (and presumably increased ER stress) display increased numbers of mucosal IgA+ plasma cells. Taken together, our study and these data globally indicate that ER stress and UPR pathway represent key regulatory checkpoints of IgA production at mucosal surfaces.
Infection with Pa, the most common bacteria in adult patients with CF [3,46], was studied as a plausible mechanism that could underlie the upregulation of IgA in the human (infected) CF lung, and which is absent in cell cultures and CFTR-mutated mice. The model of chronic Pa lung infection favours the retention of the bacteria in the airways and reproduces some features of the CF lung disease such as strong neutrophilic inflammation and peribronchial lymphoid aggregates [47,48]. Only a slight increased inflammatory score in FVB F508del mice was demonstrated, in contrast with C57BL/6J CFTR-KO mice showing some lymphoid aggregates [49,50]. When compared with the same genetic background, CFTR-KO and F508del mice display the same phenotype and, until now, no diseased lung phenotype related to CFTR misfolding has been observed in F508del mice [51]. F508del mice (on FVB/129 background) classically display a reduction in body weight, CF nasal electrophysiological profile and tendency to intestinal mucus obstruction, associated with normal survival [50,52]. Variabilities between murine models are mostly attributed to the strain background [52]. We show that Pa infection overcomes the (mutated) CFTR-driven inhibitory pathway on the pIgR, thereby unleashing IgA production in the CF lung. This phenomenon occurs independently from UPR regulation, as Pa infection further increases ER stress and UPR activation [43,53], and activates the host inflammatory response that includes the release of IL-17. This cytokine is able to stimulate pIgR expression in airway epithelial cells as previously shown in intestinal epithelial cells [32]. Interestingly, Th17-mediated responses (through IL-17A production) were shown to upregulate pIgR expression in the airway epithelium and IgA/M concentrations in BAL from mice [54]. Other inflammatory cytokines such as IL-1β and TNF-α, which are also upregulated upon Pa infection and able to stimulate pIgR transcription in airway cell lines [55,56], could also be implicated. Similarly, the observation of increased levels of IgA in sputum from never infected patients (Fig. 3a) suggests that other opportunistic bacteria (e.g., Staphylococcus aureus, Burkholderia cepacia) could drive similar mechanisms as those evidenced with Pa. However, increased concentration of IL-1β and TNF-α as well as IL-8, and neutrophilic inflammation has been demonstrated in BAL of young infants, without the presence of detectable pathogens, suggesting “sterile inflammation” [57,58]. But these statements remain controversial [59,60]. We could not assess this in our patients with CF cohort (for lung tissues and sputum) since these adult patients (although few were never infected by Pa) were carriers of other pathogens. Recent studies related to CF sterile inflammation (in CF animal models and CF samples) showed the potential responsibility of hypoxic injury, resulting in necrosis, IL-1α release and sterile neutrophilic inflammation [61]. The possible induction of IL-1β and activation of NF-κB by IL-1α might be responsible of pIgR expression early in patients with CF, even before infection detection. As mentioned before, despite showing no CF-like lung phenotype, F508del mice develop a minor but spontaneous inflammation in the lungs. However, we could notice that pIgR expression was still affected in this model although the decrease was less marked than in cellular model. This could be due to isolation of cells from inflammatory microenvironment.
The preserved IgA response to pathogens was already suggested by increased Pa-specific IgA antibodies in saliva and nasal secretions from patients with CF [9], as well as by increased serum specific IgG correlated to the presence of Pa in the sinuses [62]. Such Pa sinusitis may precede intermittent lung infection, suggesting that paranasal sinuses may represent a bacterial reservoir able to re-contaminate the patient after lung eradication [63]. Pa-specific IgA in saliva was also assessed as a predictor of Pa chronic infection [64,65]. Similarly, we observed in our study that a local specific IgA response to Pa is consistently observed in chronically infected patients, both in serum and sputum secretions. Whether those IgA assays could provide the added value to IgG antibodies and bacteriology for the diagnosis of Pa (chronic) infection should be further studied in larger and multicentric clinical cohorts.
One limitation of our study first relates to the end-stage status of patients with CF recruited for tissue samples (lung explants). It is however remarkable that sputum data from a large spectrum of disease severity retrieved consistent findings, indicating that S-IgA upregulation encompasses the full spectrum of adult patients with CF. Second, we encountered difficulties to observe differences in IL-17 levels (and did not observe changes in PIGR expression despite SC alterations) in lung homogenates. While increased number of IL-17-producing cells were shown in CF, we could not confirm increased IL-17 expression or secretion possibly as a result of the dilution of the signal by the nature of the sample (tissue homogenate). As mentioned above, while pIgR mRNA changes were observed in isolated broncho-epithelial cells, no difference was measured in lung homogenates, possibly due to a similar dilution of the signal. In addition, this study did not directly assess whether IgA plays a protective role in the lung from patients. It was reported that overglycosylation of SC in CF reduces its capacity to bind and neutralise IL-8/CXCL8, hence favouring neutrophilic inflammation [21]. It is further possible that accumulated S-IgA in the bronchial lumen (Fig. 2b), following increased production and impaired mucociliary clearance, could form immune complexes that – instead of being eliminated to achieve immune exclusion – activate FcαRI-bearing phagocytes [66]. Thus, the exact role of upregulated lung IgA responses during CF should be further investigated. Finally, pIgR dysregulation in the absence of lung infection was assessed in F508del patients but the state of IgA secretion (through pIgR) remains unclear in non-F508del patients and could be further addressed in a dedicated study of such patients even though these mutations are rarer and most patients suffer from early infection. It would be interesting to also address this point in CFTR-KO mice, compared to F508del mice.
In conclusion, this study in CF reveals a complex interplay between epithelial cells, B cells, and bacteria (Pa), which connects CFTR dysfunction, UPR activation, and a (Th17) host innate immune response to IgA secretion in the lung.
Funding sources
This work was supported by the Forton's grant of the King Baudouin's Foundation, Belgium (grant 2014-J1810150-E004) and the Fondazione Ricerca Fibrosi Cistica, Italy (grant 26/2014), and the Fonds National de la Recherche Scientifique, Belgium, grants 1.M505.15 and 1.R601.17 (S.G.) and 1.R016.16 and 1.R016.18 (C.P.). The funding sources had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report and in the decision to submit the paper for publication.
Declaration of interests
The authors have declared that no conflict of interest exists.
Author contributions
A.M.C. conducted the experiments and analysis and wrote the paper; M.L. performed mice sacrifice, RT-qPCR for human lung tissues and helped with cell cultures and tissue processing for immunochemistry; S.N. and T.L. provided technical knowledge, F508del and WT mice and CFTR inhibitors; B.D. helped with sputum processing and ELISA; F.C. helped with FACS sorting protocol setting and supervised patients database; F.A.N. helped with tyramide multiplex immunochemistry; C.B. helped with quantification of immunohistochemistry; M.V. performed transmission electron microscopy; V.D. provided some sputum samples; L.R., C.M. and P.R.B. provided some lung tissues for immunochemistry, designed and performed Pseudomonas aeruginosa infection in mice; D.H. and S.V. participated in the collection of lung tissue samples for culture, immunochemistry and RNA analysis; A.F. reviewed the paper; C.P. and S.G. designed the study, supervised patient recruitment, data analysis and the writing of the paper.
Acknowledgements
The authors thank C. Fregimilicka and M. De Beukelaer (2IP imaging platform, UCLouvain, Brussels, Belgium) for their help with immunochemistry and tissue processing; D. Brusa (flow cytometry platform, UCLouvain, Brussels, Belgium) for his help with cell sorting; A.-S. Aubriot and N. Bauwens (centre de reference pour la mucoviscidose of the Cliniques UCL St-Luc, Brussels) for sputum collection in patients with CF; V. Lacroix and B. Rondelet (Departments of Thoracic Surgery of the Cliniques UCL St-Luc and CHU Mont-Godinne, Belgium, respectively) for their collaboration with lung surgery and lung tissue sampling; and the IREC Pole of Microbiology (UCL, Brussels, Belgium) for sharing their molecular biology facility.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102974.
Appendix. Supplementary materials
References
- 1.De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med. 2016;4(8):662–674. doi: 10.1016/S2213-2600(16)00023-0. [DOI] [PubMed] [Google Scholar]
- 2.Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primers. 2015;1:15010. doi: 10.1038/nrdp.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2(1):29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 4.Sloane AJ, Lindner RA, Prasad SS. Proteomic analysis of sputum from adults and children with cystic fibrosis and from control subjects. Am J Respir Crit Care Med. 2005;172(11):1416–1426. doi: 10.1164/rccm.200409-1215OC. [DOI] [PubMed] [Google Scholar]
- 5.Yonker LM, Cigana C, Hurley BP, Bragonzi A. Host-pathogen interplay in the respiratory environment of cystic fibrosis. J Cyst Fibros. 2015;14(4):431–439. doi: 10.1016/j.jcf.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartl D, Griese M, Kappler M. Pulmonary T(H)2 response in Pseudomonas aeruginosa-infected patients with cystic fibrosis. J Allergy Clin Immunol. 2006;117(1):204–211. doi: 10.1016/j.jaci.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Moser C, Kjaergaard S, Pressler T, Kharazmi A, Koch C, Hoiby N. The immune response to chronic Pseudomonas aeruginosa lung infection in cystic fibrosis patients is predominantly of the Th2 type. APMIS. 2000;108(5):329–335. doi: 10.1034/j.1600-0463.2000.d01-64.x. [DOI] [PubMed] [Google Scholar]
- 8.Pressler T, Frederiksen B, Skov M, Garred P, Koch C, Hoiby N. Early rise of anti-pseudomonas antibodies and a mucoid phenotype of pseudomonas aeruginosa are risk factors for development of chronic lung infection–a case control study. J Cyst Fibros. 2006;5(1):9–15. doi: 10.1016/j.jcf.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Aanaes K, Johansen HK, Poulsen SS, Pressler T, Buchwald C, Hoiby N. Secretory IgA as a diagnostic tool for Pseudomonas aeruginosa respiratory colonization. J Cyst Fibros. 2013;12(1):81–87. doi: 10.1016/j.jcf.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Gohy ST, Hupin C, Pilette C, Ladjemi MZ. Chronic inflammatory airway diseases: the central role of the epithelium revisited. Clin Exp Allergy. 2016;46(4):529–542. doi: 10.1111/cea.12712. [DOI] [PubMed] [Google Scholar]
- 11.Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 12.Dallas SD, Rolfe RD. Binding of Clostridium difficile toxin A to human milk secretory component. J Med Microbiol. 1998;47(10):879–888. doi: 10.1099/00222615-47-10-879. [DOI] [PubMed] [Google Scholar]
- 13.Perrier C, Sprenger N, Corthesy B. Glycans on secretory component participate in innate protection against mucosal pathogens. J Biol Chem. 2006;281(20):14280–14287. doi: 10.1074/jbc.M512958200. [DOI] [PubMed] [Google Scholar]
- 14.Marshall LJ, Perks B, Ferkol T, Shute JK. IL-8 released constitutively by primary bronchial epithelial cells in culture forms an inactive complex with secretory component. J Immunol. 2001;167(5):2816–2823. doi: 10.4049/jimmunol.167.5.2816. [DOI] [PubMed] [Google Scholar]
- 15.Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17(1):107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- 16.Gohy ST, Detry BR, Lecocq M. Polymeric immunoglobulin receptor down-regulation in chronic obstructive pulmonary disease. Persistence in the cultured epithelium and role of transforming growth factor-beta. Am J Respir Crit Care Med. 2014;190(5):509–521. doi: 10.1164/rccm.201311-1971OC. [DOI] [PubMed] [Google Scholar]
- 17.Pilette C, Godding V, Kiss R. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(1):185–194. doi: 10.1164/ajrccm.163.1.9912137. [DOI] [PubMed] [Google Scholar]
- 18.Hupin C, Rombaux P, Bowen H, Gould H, Lecocq M, Pilette C. Downregulation of polymeric immunoglobulin receptor and secretory IgA antibodies in eosinophilic upper airway diseases. Allergy. 2013;68(12):1589–1597. doi: 10.1111/all.12274. [DOI] [PubMed] [Google Scholar]
- 19.Hodson ME, Morris L, Batten JC. Serum immunoglobulins and immunoglobulin G subclasses in cystic fibrosis related to the clinical state of the patient. Eur Respir J. 1988;1(8):701–705. [PubMed] [Google Scholar]
- 20.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150(2):448–454. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 21.Marshall LJ, Perks B, Bodey K, Suri R, Bush A, Shute JK. Free secretory component from cystic fibrosis sputa displays the cystic fibrosis glycosylation phenotype. Am J Respir Crit Care Med. 2004;169(3):399–406. doi: 10.1164/rccm.200305-619OC. [DOI] [PubMed] [Google Scholar]
- 22.Oh J, McGarry DP, Joseph N, Peppers B, Hostoffer R. Salivary IgA deficiency in a patient with cystic fibrosis (genotype M470V/V520F) Ann Allergy Asthma Immunol. 2018;121(5):619–620. doi: 10.1016/j.anai.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Hallberg K, Mattsson-Rydberg A, Fandriks L, Strandvik B. Gastric IgA in cystic fibrosis in relation to the migrating motor complex. Scand J Gastroenterol. 2001;36(8):843–848. doi: 10.1080/003655201750313379. [DOI] [PubMed] [Google Scholar]
- 24.Aboubakar Nana F, Hoton D, Ambroise J. Increased expression and activation of FAK in small-cell lung cancer compared to non-small-cell lung cancer. Cancers (Basel) 2019;11(10) doi: 10.3390/cancers11101526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massion PP, Inoue H, Richman-Eisenstat J. Novel Pseudomonas product stimulates interleukin-8 production in airway epithelial cells in vitro. J Clin Invest. 1994;93(1):26–32. doi: 10.1172/JCI116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pizzichini E, Pizzichini MM, Efthimiadis A. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154(2 Pt 1):308–317. doi: 10.1164/ajrccm.154.2.8756799. [DOI] [PubMed] [Google Scholar]
- 27.Martin C, Thevenot G, Danel S. Pseudomonas aeruginosa induces vascular endothelial growth factor synthesis in airway epithelium in vitro and in vivo. Eur Respir J. 2011;38(4):939–946. doi: 10.1183/09031936.00134910. [DOI] [PubMed] [Google Scholar]
- 28.Pilette C, Ouadrhiri Y, Dimanche F, Vaerman JP, Sibille Y. Secretory component is cleaved by neutrophil serine proteinases but its epithelial production is increased by neutrophils through NF-kappa B- and p38 mitogen-activated protein kinase-dependent mechanisms. Am J Respir Cell Mol Biol. 2003;28(4):485–498. doi: 10.1165/rcmb.4913. [DOI] [PubMed] [Google Scholar]
- 29.Hajj R, Lesimple P, Nawrocki-Raby B, Birembaut P, Puchelle E, Coraux C. Human airway surface epithelial regeneration is delayed and abnormal in cystic fibrosis. J Pathol. 2007;211(3):340–350. doi: 10.1002/path.2118. [DOI] [PubMed] [Google Scholar]
- 30.Blohmke CJ, Mayer ML, Tang AC. Atypical activation of the unfolded protein response in cystic fibrosis airway cells contributes to p38 MAPK-mediated innate immune responses. J Immunol. 2012;189(11):5467–5475. doi: 10.4049/jimmunol.1103661. [DOI] [PubMed] [Google Scholar]
- 31.Lore NI, Cigana C, Riva C. IL-17A impairs host tolerance during airway chronic infection by Pseudomonas aeruginosa. Sci Rep. 2016;6:25937. doi: 10.1038/srep25937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar P, Monin L, Castillo P. Intestinal Interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity. 2016;44(3):659–671. doi: 10.1016/j.immuni.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brodlie M, McKean MC, Johnson GE. Raised interleukin-17 is immunolocalised to neutrophils in cystic fibrosis lung disease. Eur Respir J. 2011;37(6):1378–1385. doi: 10.1183/09031936.00067110. [DOI] [PubMed] [Google Scholar]
- 34.Eberl G. RORgammat, a multitask nuclear receptor at mucosal surfaces. Mucosal Immunol. 2017;10(1):27–34. doi: 10.1038/mi.2016.86. [DOI] [PubMed] [Google Scholar]
- 35.Polosukhin VV, Cates JM, Lawson WE. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184(3):317–327. doi: 10.1164/rccm.201010-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ladjemi MZ, Lecocq M, Weynand B. Increased IgA production by B-cells in COPD via lung epithelial interleukin-6 and TACI pathways. Eur Respir J. 2015;45(4):980–993. doi: 10.1183/09031936.00063914. [DOI] [PubMed] [Google Scholar]
- 37.Wallwork JC, McFarlane H. The SIgA system and hypersensitivity in patients with cystic fibrosis. Clin Allergy. 1976;6(4):349–358. doi: 10.1111/j.1365-2222.1976.tb01915.x. [DOI] [PubMed] [Google Scholar]
- 38.Brett MM, Ghoneim AT, Littlewood JM. Serum IgA antibodies against Pseudomonas aeruginosa in cystic fibrosis. Arch Dis Child. 1990;65(3):259–263. doi: 10.1136/adc.65.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hassan J, Feighery C, Bresnihan B, Keogan M, Fitzgerald MX, Whelan A. Serum IgA and IgG subclasses during treatment for acute respiratory exacerbation in cystic fibrosis: analysis of patients colonised with mucoid or non-mucoid strains of pseudomonas aeruginosa. Immunol Invest. 1994;23(1):1–13. doi: 10.3109/08820139409063428. [DOI] [PubMed] [Google Scholar]
- 40.Kronborg G, Fomsgaard A, Galanos C, Freudenberg MA, Hoiby N. Antibody responses to lipid A, core, and O sugars of the Pseudomonas aeruginosa lipopolysaccharide in chronically infected cystic fibrosis patients. J Clin Microbiol. 1992;30(7):1848–1855. doi: 10.1128/jcm.30.7.1848-1855.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiotz PO, Hoiby N, Permin H, Wiik A. IgA and IgG antibodies against surface antigens of Pseudomonas aeruginosa in sputum and serum from patients with cystic fibrosis. Acta Pathol Microbiol Scand C. 1979;87C(3):229–233. [PubMed] [Google Scholar]
- 42.Van Bever HP, Gigase PL, De Clerck LS, Bridts CH, Franckx H, Stevens WJ. Immune complexes and Pseudomonas aeruginosa antibodies in cystic fibrosis. Arch Dis Child. 1988;63(10):1222–1228. doi: 10.1136/adc.63.10.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ribeiro CM, Paradiso AM, Schwab U. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280(18):17798–17806. doi: 10.1074/jbc.M410618200. [DOI] [PubMed] [Google Scholar]
- 44.Treton X, Pedruzzi E, Cazals-Hatem D. Altered endoplasmic reticulum stress affects translation in inactive colon tissue from patients with ulcerative colitis. Gastroenterology. 2011;141(3):1024–1035. doi: 10.1053/j.gastro.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 45.Grootjans J, Krupka N, Hosomi S. Epithelial endoplasmic reticulum stress orchestrates a protective IgA response. Science. 2019;363(6430):993–998. doi: 10.1126/science.aat7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhagirath AY, Li Y, Somayajula D, Dadashi M, Badr S, Duan K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm Med. 2016;16(1):174. doi: 10.1186/s12890-016-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest. 1997;100(11):2810–2815. doi: 10.1172/JCI119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frija-Masson J, Martin C, Regard L. Bacteria-driven peribronchial lymphoid neogenesis in bronchiectasis and cystic fibrosis. Eur Respir J. 2017;49(4) doi: 10.1183/13993003.01873-2016. [DOI] [PubMed] [Google Scholar]
- 49.Polverino F, Lu B, Quintero JR. CFTR regulates B cell activation and lymphoid follicle development. Respir Res. 2019;20(1):133. doi: 10.1186/s12931-019-1103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilke M, Buijs-Offerman RM, Aarbiou J. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros. 2011;10(Suppl 2) doi: 10.1016/S1569-1993(11)60020-9. S152–71. [DOI] [PubMed] [Google Scholar]
- 51.van Heeckeren AM, Schluchter MD, Drumm ML, Davis PB. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287(5) doi: 10.1152/ajplung.00387.2003. L944–52. [DOI] [PubMed] [Google Scholar]
- 52.Davidson DJ, Dorin JR. The CF mouse: an important tool for studying cystic fibrosis. Expert Rev Mol Med. 2001;2001:1–27. doi: 10.1017/S1462399401002551. [DOI] [PubMed] [Google Scholar]
- 53.Martino ME, Olsen JC, Fulcher NB, Wolfgang MC, O'Neal WK, Ribeiro CM. Airway epithelial inflammation-induced endoplasmic reticulum Ca2+ store expansion is mediated by X-box binding protein-1. J Biol Chem. 2009;284(22):14904–14913. doi: 10.1074/jbc.M809180200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jaffar Z, Ferrini ME, Herritt LA, Roberts K. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J Immunol. 2009;182(8):4507–4511. doi: 10.4049/jimmunol.0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balloy V, Varet H, Dillies MA. Normal and Cystic Fibrosis Human Bronchial Epithelial Cells Infected with Pseudomonas aeruginosa Exhibit Distinct Gene Activation Patterns. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schjerven H, Brandtzaeg P, Johansen FE. A novel NF-kappa B/Rel site in intron 1 cooperates with proximal promoter elements to mediate TNF-alpha-induced transcription of the human polymeric Ig receptor. J Immunol. 2001;167(11):6412–6420. doi: 10.4049/jimmunol.167.11.6412. [DOI] [PubMed] [Google Scholar]
- 57.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151(4):1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 58.Rosenfeld M, Pepe MS, Longton G, Emerson J, FitzSimmons S, Morgan W. Effect of choice of reference equation on analysis of pulmonary function in cystic fibrosis patients. Pediatr Pulmonol. 2001;31(3):227–237. doi: 10.1002/ppul.1033. [DOI] [PubMed] [Google Scholar]
- 59.Armstrong DS, Grimwood K, Carlin JB. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 1997;156(4 Pt 1):1197–1204. doi: 10.1164/ajrccm.156.4.96-11058. [DOI] [PubMed] [Google Scholar]
- 60.Dakin CJ, Numa AH, Wang H, Morton JR, Vertzyas CC, Henry RL. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165(7):904–910. doi: 10.1164/ajrccm.165.7.2010139. [DOI] [PubMed] [Google Scholar]
- 61.Balazs A, Mall MA. Mucus obstruction and inflammation in early cystic fibrosis lung disease: Emerging role of the IL-1 signaling pathway. Pediatr Pulmonol. 2019;54(Suppl 3):S5–S12. doi: 10.1002/ppul.24462. [DOI] [PubMed] [Google Scholar]
- 62.Johansen HK, Norregaard L, Gotzsche PC, Pressler T, Koch C, Hoiby N. Antibody response to Pseudomonas aeruginosa in cystic fibrosis patients: a marker of therapeutic success?a 30-year cohort study of survival in Danish CF patients after onset of chronic P. aeruginosa lung infection. Pediatr Pulmonol. 2004;37(5):427–432. doi: 10.1002/ppul.10457. [DOI] [PubMed] [Google Scholar]
- 63.Hansen SK, Rau MH, Johansen HK. Evolution and diversification of Pseudomonas aeruginosa in the paranasal sinuses of cystic fibrosis children have implications for chronic lung infection. ISME J. 2012;6(1):31–45. doi: 10.1038/ismej.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alanin MC, Pressler T, Aanaes K. Can secretory immunoglobulin A in saliva predict a change in lung infection status in patients with cystic fibrosis? A prospective pilot study. Health Sci Rep. 2018;1(8):e52. doi: 10.1002/hsr2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mauch RM, Rossi CL, Nolasco da Silva MT. Secretory IgA-mediated immune response in saliva and early detection of Pseudomonas aeruginosa in the lower airways of pediatric cystic fibrosis patients. Med Microbiol Immunol. 2019;208(2):205–213. doi: 10.1007/s00430-019-00578-w. [DOI] [PubMed] [Google Scholar]
- 66.Pasquier B, Launay P, Kanamaru Y. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22(1):31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.