Highlights
-
•
Basal ganglia connectivity and its role in gait is not well understood.
-
•
Basal ganglia connectivity grouped into high and low connectivity.
-
•
Groups were not spatially distinct within the basal ganglia.
-
•
Lower connectivity was associated with slower gait speed.
-
•
Association was significant after adjusting for other locomotor risk factors.
Abbreviations: 3MSE, Modified Mini-Mental State Examination; BMI, Body mass index; CES-D, Center for Epidemiologic Studies-Depression; CNS, Central nervous system; CSF, Cerebrospinal fluid; cSVD, cerebral small vessel disease; DSST, Digit symbol substitution task; EPI, Echoplanar imaging; FLAIR, Fluid attenuated inversion recovery; fMRI, Functional magnetic resonance imaging; GMV, Gray matter volume; ICV, Intracranial volume; MNI, Montreal Neurological Institute; MPRAGE, Magnetization prepared rapid acquisition gradient echo; MRI, Magnetic resonance imaging; PD, Parkinson’s Disease; ROI, Region of interest; WMH, White Matter Hyperintensities
Keywords: Connectivity, Resting State, Gait, WMH, Cerebrovascular Burden, Late-Life
Abstract
Background and Aim
The basal ganglia are critical for planned locomotion, but their role in age-related gait slowing is not well known. Spontaneous regional co-activation of brain activity at rest, known as resting state connectivity, is emerging as a biomarker of functional neural specialization of varying human processes, including gait. We hypothesized that greater connectivity amongst regions of the basal ganglia would be associated with faster gait speed in the elderly. We further investigated whether this association was similar in strength to that of other risk factors for gait slowing, specifically white matter hyperintensities (WMH).
Methods
A cohort of 269 adults (79–90 years, 146 females, 164 White) were assessed for gait speed (m/sec) via stopwatch; brain activation during resting state functional magnetic resonance imaging, WMH, and gray matter volume (GMV) normalized by intracranial volume via 3T neuroimaging; and risk factors of poorer locomotion via clinical exams (body mass index (BMI), muscle strength, vision, musculoskeletal pain, cardiometabolic conditions, depressive symptoms, and cognitive function). To understand whether basal ganglia connectivity shows distinct clusters of connectivity, we conducted a k-means clustering analysis of regional co-activation among the substantia nigra, nucleus accumbens, subthalamic nucleus, putamen, pallidum, and caudate. We conducted two multivariable linear regression models: (1) with gait speed as the dependent variable and connectivity, demographics, WMH, GMV, and locomotor risk factors as independent variables and (2) with basal ganglia connectivity as the dependent variable and demographics, WMH, GMV, and locomotor risk factors as independent variables.
Results
We identified two clusters of basal ganglia connectivity: high and low without a distinct spatial distribution allowing us to compute an average connectivity index of the entire basal ganglia regional connectivity (representing a continuous measure). Lower connectivity was associated with slower gait, independent of other locomotor risk factors, including WMH; the coefficient of this association was similar to those of other locomotor risk factors. Lower connectivity was significantly associated with lower BMI and greater WMH.
Conclusions
Lower resting state basal ganglia connectivity is associated with slower gait speed. Its contribution appears comparable to WMH and other locomotor risk factors. Future studies should assess whether promoting higher basal ganglia connectivity in older adults may reduce age-related gait slowing.
1. Introduction
Slow gait speed is common in the elderly and increases the risk of adverse mobility outcomes and traumatic falls, leading to both rapidly rising medical costs and declining capacity for independent living (Studenski, 2019). Although physical therapy may provide short-lasting benefits, the long-term management of mobility disturbances in the elderly has proven difficult.
While age-related changes in peripheral nervous and musculoskeletal systems are well-known contributors of gait slowing (Alexander, 1996), recent evidence suggests an important role for the central nervous system (CNS), in particular cerebral small vessel disease (cSVD) (see reviews (Holtzer et al., 2014, Seidler et al., 2010, Tian et al., 2017, Wennberg et al., 2017, Wilson et al., 2019)). One theory to explain age-related gait slowing is that in older age peripheral and musculoskeletal systems’ impairments make walking more demanding. As these demands increase, they eventually exceed the capacity of the CNS to continue habitual locomotion control. In turn, decreased habitual control may result in a greater need to pay attention while walking (Wu and Hallett, 2005). cSVD-related damage to white matter tracts may also reduce the availability of CNS resources for effective habitual locomotor control, thus magnifying the mismatch between demands and resource availability. Identifying the CNS resources that promote effective habitual control of walking in older age is important as this may inform future therapeutic interventions.
The basal ganglia are a logical candidate for habitual control of locomotion (Nutt et al., 1993). The basal ganglia are comprised of gray matter nuclei located in the brainstem (substantia nigra) and subcortical areas (putamen, pallidum, caudate, nucleus accumbens, subthalamic nucleus), interconnected with each other to form a highly correlated motor control system that works to create synchronous motor movements in a fluid manner. Animal studies and human disease models show that the basal ganglia control planned initiation and execution of coordinated sequences of musculoskeletal activations (Panigrahi et al., 2015) to carry out overlearned tasks such as walking (Wu and Hallett, 2005).
There is an initial indication that basal ganglia would be associated with walking performance in older age. We and others have found that slower gait was associated with smaller gray matter volume (Dumurgier et al., 2012, Rosano et al., 2008a, Rosano et al., 2007, Stijntjes et al., 2016) and lower dopaminergic neurotransmission via Positron Emission Tomography (PET) of the basal ganglia (Bohnen and Cham, 2006, Bohnen et al., 2009, Cham et al., 2007, Cham et al., 2011, Cham et al., 2008). However, the neuroimaging methods used in prior studies do not completely capture the complexity of basal ganglia function or connectivity. While volumetric measures reflect advanced neuronal loss in individual regions, PET or other similar imaging is limited to a specific element of neurotransmission. To understand the contribution of the basal ganglia to locomotion, it is important to examine how individual regions function together.
Patterns of spontaneous regional co-activation at rest, also known as resting state connectivity, are emerging as biomarkers of parenchyma integrity and its functional neural role (Fox and Lancaster, 2002, Smith et al., 2009). For simplicity, we henceforth simply refer to resting state connectivity as connectivity – and other types of connectivity will be explicitly stated, e.g., structural connectivity. Higher co-activation at rest among regions is interpreted as a measure of higher intrinsic connectivity or cohesiveness between those regions. Initial evidence suggests that higher connectivity between basal ganglia and other regions predicts better motor performance in patients with Parkinsonian Syndromes (Filippi et al., 2019) and in healthy adults (Boyne et al., 2018, Zwergal et al., 2012). However, prior studies have not accounted for the contribution of impairment of other age-related factors influencing locomotion including muscle strength (McLean et al., 2014), vision (Chaudhry et al., 2010), joint pain (White et al., 2013), and obesity (Vincent et al., 2010) as well as WMH and brain volume (Rosso et al., 2017). While individual associations are important, quantifying the relative strength of the association in comparison to these locomotor risk factors provides additional information.
While connectivity of the basal ganglia (both within the basal ganglia and with other regions) declines with older age (Bonifazi et al., 2018, Griffanti et al., 2018, Manza et al., 2015, Mathys et al., 2014), its association with slower gait among community dwelling older adults is not known. Most studies relating connectivity with gait speed have examined inter-network connectivity among cortical sensorimotor regions (Crockett et al., 2017, Di Scala et al., 2019, He et al., 2016, Hsu et al., 2018, Hsu et al., 2014, Hugenschmidt et al., 2014, Liem et al., 2017, Lo et al., 2018, Poole et al., 2019, Taniwaki et al., 2007, Yuan et al., 2015) or between cortical motor and default mode networks (see review (Wilson et al., 2019)).
In this study, we characterized the connectivity among the regions of the basal ganglia with the goal to identify clusters of connectivity that would be associated with gait slowing in the elderly. We examined a diverse cohort of community-dwelling older adults without neurodegenerative diseases, and with a rich characterization of locomotor risk factors, including muscle strength (McLean et al., 2014), vision (Chaudhry et al., 2010), joint pain (White et al., 2013), and obesity (Vincent et al., 2010) but also WMH and brain volume (Rosso et al., 2017). We hypothesized that lower connectivity among the regions of the basal ganglia would be associated with slower gait speed. This was based on evidence that: (1) basal ganglia are involved in automatic gait and promoting fluidity of gait; (2) basal ganglia connectivity declines with age and in patient populations appears to be positively correlated with motor performance; and (3) slow gait has been associated with smaller gray matter volume and lower dopaminergic neurotransmission in the basal ganglia. In addition to examining whether this association would be significant after adjusting for WMH, a marker of cSVD, and other locomotor risk factors, we quantified and compared the standardized parameter estimates of connectivity with that of such factors.
2. Methods
2.1. Participants and study design
Health ABC (Aging, Body, Composition) is a longitudinal study to investigate changes in physical and cognitive changes in elderly individuals who were healthy at baseline (1997–1998). Participants (70–79 years old) were recruited to community-based settings from Pittsburgh, PA and Memphis, TN from 1997 to 1998 (Simonsick et al., 2001). Participants were followed annually and completed assessments of physical and cognitive health (Houston et al., 2008). All data is available by request at https://healthabc.nia.nih.gov/.
A set of the participants in Pittsburgh (n = 325) on years 10 and 11 (2006–2008) were recruited to complete MRI scanning if they met inclusion criteria: no assistive devices for walking, eligible for MR scanning (e.g., no metal in body), had a mobility measure on the previous visit, and no history of neurological or psychological illnesses (Nadkarni et al., 2014, Rosso et al., 2014). While all participants had resting state MRI data collected, a set of these participants had poor coverage of the brain or signal dropout identified visually in the basal ganglia (n = 44) while another set did not have gait measures (n = 10) so they were excluded from further analysis. A total of n = 271 participants were included in our final analysis.
2.2. Population characteristics
Gait speed was obtained at time of the brain MRI by asking participants to walk along a 20 m corridor at their usual-paced walking speed from a standing start. This was repeated three times, and the average of the three passes was computed. Participants were instructed to walk “as you normally would.” Walking time was recorded by stopwatch and converted to speed in meters per second. Demographics were obtained at study entry.
Other factors influencing gait slowing were collected close to the time of the MRI visit (<6 months) and included: body mass index (BMI, kg/m2), quadriceps muscle strength (via kin-com), eyesight (self-report, 1-excellent to 6-completely blind), and joint pain (knee, back, other sites, via self-report, present/absent). Isokinetic strength of the right knee extensors (unless injured) was determined at 60 deg/s with a dynamometer (Kin-Com, Chattanooga, TN) with three repetitions (maximal torque was recorded) (Goodpaster et al., 2001). Prevalent cardiometabolic conditions that are often associated with physical function were ascertained from interviews with the participants and confirmed by use of medication: cardiovascular disease (prevalent myocardial infarction, angina, congestive heart failure, stroke and peripheral arterial disease), hypertension (including prevalent treated hypertension and blood pressure level), diabetes, chronic obstructive/restrictive pulmonary disease, and osteoarthritis (hip, knee and unspecified site). Participants were coded as having hypertension if they had systolic blood pressure greater than 140 mm Hg or diastolic blood pressure greater than 90 mm Hg or self-reported hypertension diagnosis/antihypertension medication use at MRI. Participants were defined as having diabetes if their fasting plasma glucose was >126 mg/dL or 2-hour post-challenge >200 Hg/dL or self-reported a diabetes diagnosis or diabetes medication use (Yaffe et al., 2009). Each condition was coded as present/absent; a composite score indicating burden of cardiometabolic conditions was created by summing these conditions, ranging from 0 (no cardiometabolic conditions) to 5 (all five cardiometabolic conditions present). Depressive symptoms were described at the time of MRI based on the 20-item Center for Epidemiologic Studies-Depression (CES-D) scale (Andresen et al., 1994). The Modified Mini-Mental State Examination (3MSE) was used to assess general cognitive function at the time of MRI (Teng and Chui, 1987). The Digit Symbol Substitution Test (DSST) was also administered at the same time to assess processing speed (Wechsler, 1955).
2.3. MRI acquisition
MRI scans were obtained at the MR Research Center of the University of Pittsburgh using a 3T Siemens Tim Trio MR Scanner and a Siemens 12-channel head coil. An axial, whole-brain T1-weighted magnetization prepared rapid gradient echo (MPRAGE) was collected with repetition time (TR) = 2300 ms, echo time (TE) = 3.43 ms, flip angle (FA) = 9 deg, field of view (FOV) = 224x256, 1 mm3 isotropic resolution, no gap, and no acceleration. An axial, whole-brain fluid attenuated inversion recovery (FLAIR) sequence was collected to appropriately identify white/gray matter as well as WMH, a marker of cSVD. This sequence had TR = 9160 ms, TE = 90 ms, FA = 150 deg, FOV = 212x156, 1x1x3mm resolution, 3 mm gap, and no acceleration. We collected an axial, whole brain (excluding the cerebellum) echo-planar imaging (EPI) sequence to measure blood-oxygen dependent responses during resting state. This sequence had a TR = 2000 ms, TE = 34 ms, FA = 90 deg, FOV = 128x128, 2x2x3mm resolution, and no acceleration.
2.4. Structural and functional image processing
Structural Image Processing for Transformation to a Standard Anatomical Space. All processing steps were conducted in SPM12 unless otherwise noted (Penny et al., 2011) (Wellcome Centre for Human Neuroimaging, London, UK). All image space interpolation was performed using 4th degree B-spline method and the similarity metric for registrations was mutual information (for motion correction) or normalized mutual information (coregistration between different image types). FLAIR images were coregistered to the MPRAGE (12 degree of freedom transformations) then a multi-spectral segmentation was conducted of both the MPRAGE and coregistered FLAIR into 6 tissues: gray matter, white matter, cerebrospinal fluid (CSF), skull, soft-tissue, and air. Default values were used for the segmentations in SPM except the number of Gaussians for white matter was set to 2 to include WMH. Segmentation also outputs a deformation field, which can be used to normalize functional images to a standard anatomical space (Montreal Neurological Institute, MNI, space). We created an automated mask by combining threshold maps of gray matter, white matter, and CSF (probability of 0.1) then conducting image filling and image closing (in MATLAB, MathWorks Inc, Natick MA). The total volume of this was extracted and represented the intracranial volume (ICV). We then computed the total gray matter volume (GMV) by counting voxels where the gray matter probability was highest compared to other tissues.
Segmentation of FLAIR Imaging to Compute WMH Volume. We used a semi-automated segmentation procedure for identifying WMH on the FLAIR (Wu et al., 2006). This method identifies seeds above a specified standard deviation of intensities and then uses fuzzy connectedness to grow the seeds. One highly trained analyst (5+ years of experience) viewed the segmentation per individual and ensured that WMH were extracted appropriately. The log of the WMH volume was used as a measure of WMH burden as WMH volume measures are typically skewed and the log WMH is more normally distributed.
Resting State Preprocessing. Resting state data underwent slice-time correction, motion correction, co-registration to the skull-stripped structural image, normalization with the generated deformation field, and smoothing with an 8 mm Gaussian kernel. We then conducted wavelet despiking using the BrainWavelet Toolbox, which uses data-driven and a locally-adaptive denoising method to deal with motion artefacts (Patel et al., 2014). We conducted no scrubbing of scan volumes, instead interpolated across motion artefacts using the wavelet procedure. To account for effects of no interest, we regressed the following features per voxel using SPM: 6 parameters of motion, 5 eigenvariates of white matter and cerebrospinal fluid (i.e., CompCor) (Behzadi et al., 2007), and sinusoids corresponding to unwanted frequencies outside of the band-pass in resting state (i.e., a band-pass filter 0.008–0.15 Hz). This was done using in-house software written in MATLAB. By doing this in one step, we did not reintroduce artifact/noise into our signal (Lindquist et al., 2019). We did not conduct global signal regression due to its controversial nature, where it has been shown to have negative and positive impacts (Murphy and Fox, 2017).
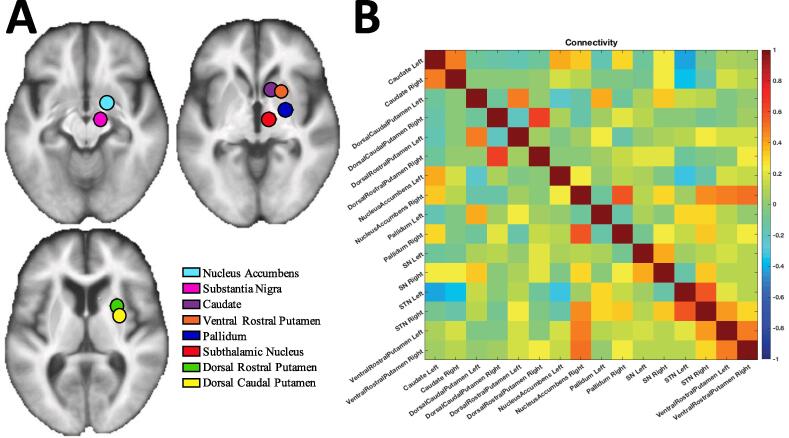
ROI-to-ROI Connectivity. Using a custom script in MATLAB, we conducted region-of-interest (ROI)-based connectivity analysis for each participant using the following ROIs of the basal ganglia (left and right hemispheres separate): caudate, dorsal caudal putamen, dorsal rostral putamen, ventral rostral putamen, nucleus accumbens, pallidum, substantia nigra, and subthalamic nucleus (Di Martino et al., 2008, Tomasi and Volkow, 2014). These locations in MNI space were taken directly from these two manuscripts. We did not separate the internal and external globus pallidus, due to the low resolution of the functional data. These ROIs were created with a 10 mm sphere around the locations determined from several previous studies (Di Martino et al., 2008, Tomasi and Volkow, 2014). ROI-to-ROI based connectivity was computed by computing the eigenvariate of each region and calculating their correlation. This represents the weighted mean across all voxels that explains the maximum amount of variance, which is obtained using principal components analysis. This resulted in 120 unique pairs of connectivity for each participant, which were input into the k-means clustering analysis. The regions and an example of the connectivity matrix are shown in Fig. 1a and Fig. 1b, respectively.
Fig. 1.
(A) Regions-of-interest (ROIs) used of the basal ganglia overlaid on an average structural image of participants in the study. (B) An example of a single participant’s connectivity matrix, which generates 120 unique connectivity pairs (8 regions and 2 hemispheres, 16 × 16 total connections but half used since connectivity is bidirectional and not including diagonal since values equal one). Values represent the Pearson correlation coefficient between any pair of eigenvariate timeseries; negative values often represent low correlation rather than anticorrelation since they may be a product of covariate signal regression rather than a true anticorrelation.
2.4 K-means clustering analysis
Our clustering analysis was conducted with MATLAB 2016b (MathWorks Inc, Natick MA) using the k-means clustering algorithm with squared Euclidean distance as our distance metric. We first computed the number of expected clusters using the Calinski-Harabasz (also known as variance ratio) criterion, which is the ratio between the “between cluster sum-of-squares” and “within cluster sum-of-squares” (Caliński and Harabasz, 1974). The ratio is evaluated for increasing numbers of clusters. The optimal solution should be given by the first local maximum of the ratios. We conducted clustering of our data with 1,000 repetitions and group each individual into a cluster. We re-evaluated our clusters using connectivity data that were standardized to a mean equal to zero and a standard deviation of one, but that did not alter our solution. We used a Silhouette plot (Rousseeuw, 1987), where each value indicates how close each point in one cluster is to points in the neighboring clusters – thus large Silhouette values indicate a good separation. Large numbers of negative Silhouette values indicate a poor fit or that a larger/smaller number of clusters is needed.
We then conducted analysis to test the stability of these solutions using bootstrapping. We randomly resampled our data 5,000 times without replacement only including 75% of the sample. We repeated the k-means clustering analysis each time and then computed the adjusted Rand index (Rand, 1971) to measure the similarity between clustering within the resampled data compared to the entire data. The adjusted Rand index corrects for chance clusters and ranges from zero to one, where a value of 1 indicates perfect agreement between each solution. We then repeated this analysis with 50% of the sampling each time to determine the stability of our solutions. Since we identified two clusters with generally high and generally low basal ganglia connectivity without spatially distributed differences (see results), we calculated a mean basal ganglia connectivity measure across all 120 connectivity pairs.
As there were concerns that two of the regions (dorsal caudal putamen and ventral rostral putamen) were overlapping with other regions, we conducted an additional sensitivity analysis. We excluded the dorsal caudal putamen and ventral rostral putamen then reconducted the clustering without these regions in the connectivity pairs (i.e., 66 connectivity pairs).
2.5. Statistical analysis
We conducted all analyses in SPSS 26 (IBM Corp, Armonk NY). We conducted two multivariable linear regression analyses to understand (1) what factors influenced gait speed and (2) what factors influenced the basal ganglia connectivity measure. In the first analysis we regressed gait velocity (dependent variable) onto the following factors (all collected around the time of MRI): age, race, education, BMI, cardiometabolic conditions index score, depression, total intracranial volume, gray matter volume, WMH volume, Digit-Symbol Substitution score (DSST), eyesight, quadriceps strength, self-reported joint knee pain, and average basal ganglia connectivity. As an exploratory analysis we investigated whether WMH moderated the association between gait and basal ganglia connectivity by testing for the interaction of WMH by basal ganglia connectivity. In the second analysis, we regressed average basal ganglia connectivity (dependent variable) onto all of the factors above except gait speed.
While our k-means clustering analysis was conducted with the full 271 participants, due to missing non-imaging data (e.g., in other clinical and demographic variables) we conducted statistical regression in a reduced set (total sample size of 219 participants). We also verified that the solution of the k-means clustering was not altered when investigating the more limited sample of 219 participants (not shown). Additionally, we tested whether the sample excluded (n = 52) significantly differed on any of the demographic/clinical variables (pairwise deletion of missing data) and found that they did not differ from the complete sample (not shown). We verified that: variance inflation factors were below 2; data were normally distributed (q-q plots); and that the assumption of homoscedasticity was met. Unstandardized as well as standardized estimates are reported (variables are standardized prior to regression by subtracting the mean and dividing by the standard deviation).
In our analyses, we did not adjust for sex, as it was highly collinear (variance inflation factors greater than 5) with quadriceps strength and as we are attempting to understand gait speed, we concluded that we would include quadriceps strength over sex in our models. Instead, we conducted exploratory analyses that included sex instead of quadriceps strength.
3. Results
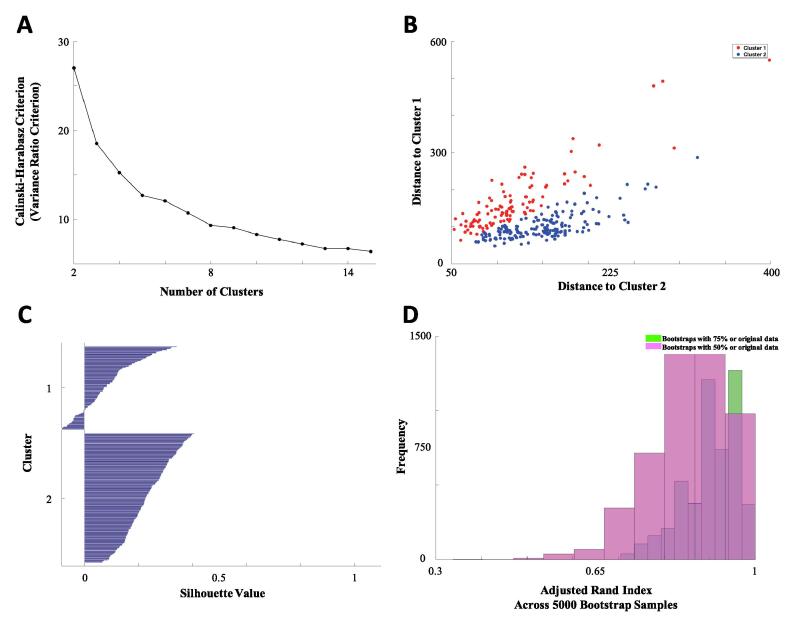
Table 1 shows the demographic and clinical information from our data. We estimated the total number of clusters using the Variance Ratio criterion and found that the optimal number of clusters was equal to two (see Fig. 2a). This resulted in good separation for most individuals in our sample which can be visualized by plotting the distance between each cluster centroid (see Fig. 2b) as well as with a Silhouette plot (see Fig. 2c) (Rousseeuw, 1987). We found that larger numbers of clusters resulted in lower Silhouette values as well as more negative values (not shown). We found that the two major clusters corresponded to low and high connectivity across the entire basal ganglia without spatially distributed differences (Fig. 3a). We conducted an independent t-test for each pairwise connectivity between the two clusters and found that the high connectivity group was significantly higher than the low connectivity group in all pairwise regions (result not shown).
Table 1.
Characteristics of the sample.
| Total Sample (N = 269*) | |
|---|---|
| Gait Speed, meters per second | 0.92 (0.18) |
| Demographics | |
| Age, years | 82.89 (2.7) |
| Female Sex, N (%) | 146 (57%) |
| Education ≤ High school, N (%) | 107 (40%) |
| Black Race, N (%) | 105 (41%) |
| Central Nervous System Factors | |
| Average Basal Ganglia Connectivity | 0.1 (0.49) |
| White Matter Hyperintensity Volume (normalized by total white matter volume) | 0.35 (0.04) |
| Gray Matter Volume (normalized by total intracranial volume) | 0.28 (0.02) |
| Digit Symbol Substitution Test | 37.1 (13.2) |
| Modified Mini-Mental Examination (3MSE) | 93.1 (6.7) |
| Depressive Symptom Severity (CES-D) | 6.7 (6.2) |
| Other Factors related to gait speed | |
| Body Mass Index, kg/m2 | 27.34 (4.49) |
| Peak Quadriceps Muscle Strength, kg | 82 (30.35) |
| Excellent/Good Eyesight, N (%) | 182 (68%) |
| Joint Pain Presence, N (%) | 164 (64) |
| Cardiometabolic index 0/1/2/3/4/5, N | 19/132/53/49/11/4 |
*Means (standard deviation) are reported, unless otherwise noted.
Fig. 2.
(A) Variance Ratio criterion by number of clusters. Optimal clustering should be given by the first local maximum of the ratios, in our case a value of 2 clusters was returned. (B) Each individual has a distance between each cluster centroid (in our case two clusters), and a plot of distance to cluster 1 against distance to cluster 2 helps visualize the separation of the two clusters. (C) Silhouette plot displays the Silhouette value for each individual and is a measure of how close each point in one cluster is to points in the neighboring clusters. Most points have a moderate level of separation in both clusters, however a small number of points in cluster 1 are not well separated. (D) Histogram of adjusted Rand index across the 5,000 random resampling (sampling either 75% or 50% of original data). Adjusted Rand index is a measure of similarity between our original clustering and clustering with only a subset of the sample.
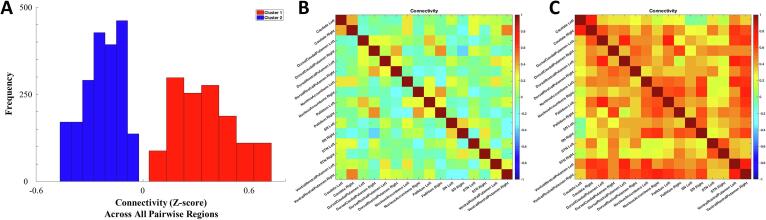
Fig. 3.
(A) Histogram of connectivity (mean zero and standard deviation of 1) across all pairwise connectivity, which indicates that cluster 2 has low basal ganglia connectivity while cluster 1 has high basal ganglia connectivity. Connectivity matrix of a single participant with low basal ganglia connectivity (B) and high basal ganglia connectivity (C) is shown to demonstrate the differences between clusters.
We found that our clusters were highly stable; adjusted Rand indices were greater than 0.8 in 93% of the 5,000 bootstrap samples and adjusted Rand index were greater than 0.9 in 62% of those samples (Fig. 2d). Resampling with 50% of the sample resulted on average with a lower adjusted Rand index, but still showed 80% above an adjusted Rand index of 0.8. We plot the connectivity matrix of a participant with low and one with high connectivity across the entire basal ganglia (Fig. 3b and c, respectively). Since we identified two clusters with generally high and generally low basal ganglia connectivity without spatially distributed differences, we calculated a mean basal ganglia connectivity measure across all 120 connectivity pairs. We found that the mean basal ganglia connectivity was highly correlated to the grouping, as would be expected. The mean allows for a more continuous measure of connectivity such that participants with connectivity that is on the boundary between groups can be more accurately classified. Due to close spatial proximity to other regions, sensitivity analyses excluding dorsal caudal putamen and ventral rostral putamen yield a similar clustering solution: the average connectivity of the basal ganglia with and without these pairs were significantly correlated [r(270) = 0.94, p < 0.001].
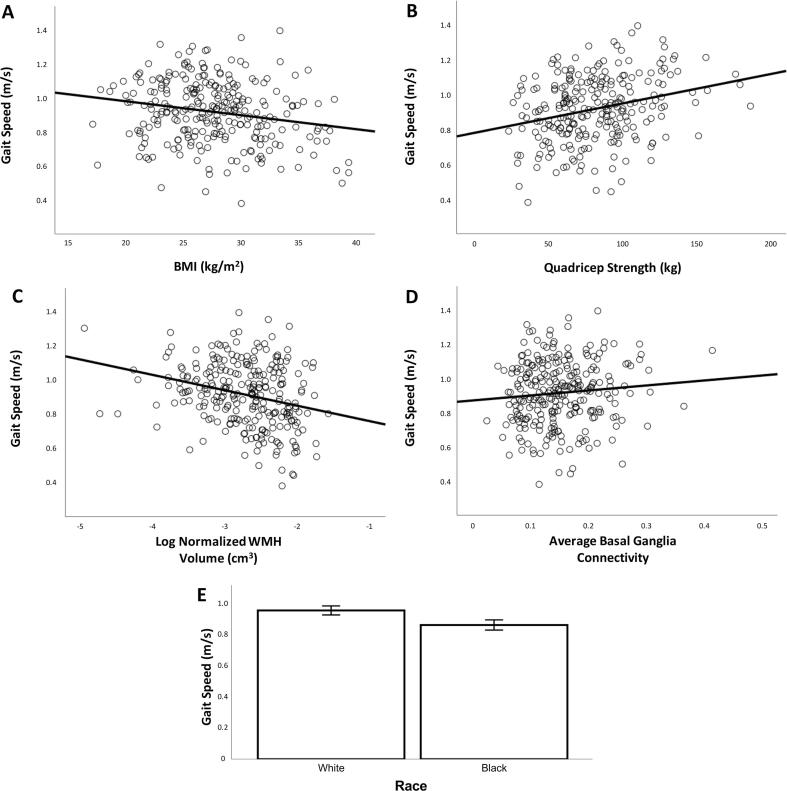
We conducted a regression with gait speed as the outcome and connectivity, demographic factors, central nervous system factors, as well as other factors related to gait speed as predictors. This model explained 32% of the variance in gait speed [F(14,205) = 6.9, p < 0.001, r2 = 0.32]. We found that slower gait speed was associated with being Black race (compared to White race), greater BMI, lower quadricep strength, greater WMH volume, and lower basal ganglia connectivity (see Table 2, Fig. 4). The size of the association with gait was similar but opposite for WMH compared to average basal ganglia connectivity (Table 2, compare standardized regression coefficients). The interaction of basal ganglia connectivity by WMH was not significant (not shown).
Table 2.
Regression explaining variance in Gait Speed (dependent variable).
| Independent Variables | Unstandardized ß | Standard Error | Standardized ß | t-value | p-value |
|---|---|---|---|---|---|
| Intercept | 1.508 | 0.494 | 3.1 | 0.003 | |
| Main independent variable | |||||
| Average Basal Ganglia Connectivity* | 0.373 | 0.186 | 0.123 | 2.0 | 0.046 |
| Demographic Factors | |||||
| Age (years) | −0.007 | 0.004 | −0.092 | −1.5 | 0.134 |
| Race (Ref Cat - White)* | −0.090 | 0.025 | −0.239 | −3.6 | <0.001 |
| Education(Ref Cat – Less than High School) | −0.014 | 0.017 | −0.056 | −0.9 | 0.395 |
| Other Factors Related to Gait Speed | |||||
| BMI (kg/m2)* | −0.011 | 0.003 | −0.255 | −3.9 | <0.001 |
| Cerebrovascular Index | −0.007 | 0.011 | −0.037 | −0.6 | 0.549 |
| Joint Knee Pain (Ref Cat - Yes) | −0.048 | 0.024 | −0.119 | −2.0 | 0.051 |
| Quadricep Strength (kg)* | 0.002 | 0.000 | 0.265 | 4.1 | <0.001 |
| Eyesight (1–6) | 0.005 | 0.015 | 0.021 | 0.3 | 0.736 |
| Central Nervous System Factors | |||||
| Intracranial Volume (cm3) | 0.000 | 0.000 | −0.022 | −0.3 | 0.764 |
| Normalized Gray Matter Volume | 0.138 | 0.556 | 0.017 | 0.2 | 0.805 |
| Normalized WMH Volume* | −0.052 | 0.020 | −0.160 | −2.6 | 0.011 |
| Digit Symbol Substitution Test | 0.001 | 0.001 | 0.068 | 1.0 | 0.322 |
| Depression (CES-D) | −0.002 | 0.002 | −0.066 | −1.1 | 0.278 |
Significant factors are bolded*.
Fig. 4.
Slower gait speed was associated with greater BMI (A), lower quadricep strength (B), greater WMH volume (C), lower average basal ganglia connectivity (D), and being Black race compared to White race (E).
We conducted several exploratory analyses to include the following variables in regression models predicting gait speed: a) 3MSE due to the associations of cognitive function with mobility, and found it was not significantly associated with gait speed; b) sex instead of quadriceps strength, and found sex was associated with gait speed, similar to quadriceps strength; c) interaction of sex with WMH and of sex with basal ganglia connectivity, and found neither was significant (not shown).
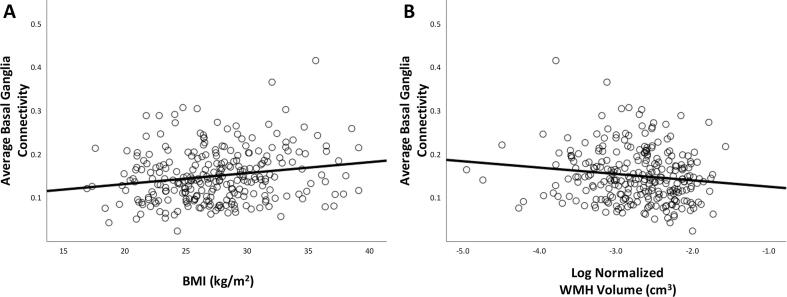
We then conducted a regression with connectivity as the outcome, and demographic factors, central nervous system factors, as well as other factors related to gait speed as predictors. This model explained 13% of the variance in connectivity [F(13,206) = 2.3, p < 0.01, r2 = 0.13]. We found that lower connectivity was associated with lower BMI and greater WMH (see Table 3, Fig. 5).
Table 3.
Regression explaining variance in Basal Ganglia Connectivity (dependent variable).
| Independent Variables | Unstandardized ß | Standard Error | Standardized ß | t-value | p-value |
|---|---|---|---|---|---|
| Intercept | 0.156 | 0.184 | 0.8 | 0.397 | |
| Demographic Factors | |||||
| Age (years) | −0.001 | 0.002 | −0.032 | −0.5 | 0.639 |
| Race (Ref Cat - White) | 0.002 | 0.009 | 0.019 | 0.2 | 0.806 |
| Education(Ref Cat – Less than High School) | −0.005 | 0.006 | −0.055 | −0.7 | 0.461 |
| Other Factors Related to Gait Speed | |||||
| BMI (kg/m2)* | 0.002 | 0.001 | 0.158 | 2.2 | 0.032 |
| Cerebrovascular Index | 0.004 | 0.004 | 0.064 | 0.9 | 0.361 |
| Joint Knee Pain (Ref Cat - Yes) | 0.017 | 0.009 | 0.131 | 1.9 | 0.056 |
| Quadricep Strength (kg) | 0.000 | 0.000 | 0.036 | 0.5 | 0.624 |
| Eyesight (1–6) | 0.000 | 0.006 | 0.005 | 0.1 | 0.946 |
| Central Nervous System Factors | |||||
| Intracranial Volume (cm3) | 0.000 | 0.000 | −0.134 | −1.6 | 0.111 |
| Normalized Gray Matter Volume | 0.073 | 0.208 | 0.028 | 0.4 | 0.725 |
| Normalized WMH Volume* | −0.020 | 0.007 | −0.186 | −2.7 | 0.008 |
| Digit Symbol Substitution Test | 0.000 | 0.000 | −0.028 | −0.4 | 0.712 |
| Depression (CES-D) | −0.001 | 0.001 | −0.089 | −1.3 | 0.193 |
Significant factors are bolded*.
Fig. 5.
Lower average basal ganglia connectivity is significantly associated with lower BMI (A) and greater WMH volume (B).
4. Discussion
In this analysis of community dwelling older adults, we identified a robust cluster of connectivity among the regions of the basal ganglia, which was significantly and positively associated with gait speed. This association was significant after adjusting for other risk factors for gait slowing, including WMH, a marker of cSVD. The association between connectivity and gait speed appeared similar to that of WMH, as indicated by the comparable size of the standardized regression estimates. We also found that the size of the association (i.e., standardized ß) with connectivity was somewhat comparable, albeit less strong, to that of other age-related risk factors for gait slowing. Taken together, our results highlight the basal ganglia connectivity as a potential correlate for gait slowing, in conjunction with WMH and peripheral factors.
4.1. Basal ganglia connectivity and gait speed
It is generally well accepted that walking with age becomes both slower (Studenski et al., 2011) and less ‘automated’, with greater engagement of attention (Atkinson et al., 2007, Hausdorff et al., 2005, Holtzer et al., 2006, Holtzer et al., 2012, Inzitari et al., 2007, Martin et al., 2012, Montero-Odasso et al., 2012, Rosano et al., 2008b, Rosano et al., 2005, Rosano et al., 2012, Rosso et al., 2013, Yogev-Seligmann et al., 2008) and prefrontal resources (Bolandzadeh et al., 2014, Chen et al., 2017, Herold et al., 2017, Holtzer et al., 2015, Mirelman et al., 2017). Greater CNS integrity has been associated with better walking characteristics in older adults (see reviews (Holtzer et al., 2014, Seidler et al., 2010, Tian et al., 2017, Wennberg et al., 2017, Wilson et al., 2019)), although for the most part associations have small effect sizes and lack specificity (e.g., may be more associated with cognitive function in general rather than gait).
Evidence for a link between connectivity and gait speed is recent, and primarily for cortical networks (Boyne et al., 2018, Crockett et al., 2017, Di Scala et al., 2019, He et al., 2016, Hsu et al., 2018, Hsu et al., 2014, Hugenschmidt et al., 2014, Liem et al., 2017, Lo et al., 2018, Poole et al., 2019, Taniwaki et al., 2007, Yuan et al., 2015, Zwergal et al., 2012), with few studies examining subcortical regions and basal ganglia in particular (Boyne et al., 2018, Zwergal et al., 2012). Previous studies have shown both higher and lower connectivity in relation with faster gait. For instance, better walking performance has been associated with lower inter-network connectivity between sensorimotor and default mode network in older adults and in Parkinson patients and also with increases in functional connectivity among subcortical sensorimotor regions (Crockett et al., 2017, Shine et al., 2013, Thibes et al., 2017). This has been interpreted as a mechanism to keep task-related networks focused and preventing “distracting” communications from task-irrelevant networks. While our results add to the literature on the central control of mobility by underscoring the relevance of higher connectivity of the basal ganglia, future studies including both cortical and subcortical connectivity measures are needed to better understand these complex relationships.
4.2. Associations of WMH and BMI with gait speed and connectivity
Basal ganglia resting state connectivity was inversely associated with WMH volume, and both had significant and opposite effects on gait. The association of WMH with gait speed remained similar after adjustment for connectivity and vice versa, and their interaction was not significant, indicating their influence on gait may be via distinct pathways. One potential mechanism through which WMH may affect basal ganglia connectivity is via degeneration of myelin and axons connecting the basal ganglia and the cortex (Scarpelli et al., 1994, Scheltens et al., 1995); this in turn may result in a reconfiguration of the connections within the basal ganglia. Although tract-specific WMH load has been shown to be associated with altered connectivity (Langen et al., 2017), for the most part, prior studies focused on cortical connectivity and not the basal ganglia (Cheng et al., 2017, Zhu et al., 2019), or examined enlarged perivascular space in the basal ganglia gray matter instead of WMH (Acharya et al., 2019); moreover, prior studies examined WMH-connectivity associations in the context of cognitive and not motor function. Future studies should examine tract-specific WMH to better understand how WMH influences basal ganglia connectivity and mobility. It is also possible WMH and connectivity may share risk factors, in other words they may be concurrent manifestations of age-related brain changes that are independent of each other, rather than being causally linked. Of note, the associations of connectivity with cardiometabolic factors commonly associated with WMH were all non-significant (Shaaban et al., 2019). Longitudinal studies need to assess whether basal ganglia connectivity and WMH have non-overlapping risk/protective factors.
The positive association of BMI with basal ganglia connectivity in the presence of its inverse association with gait speed, is seemingly counterintuitive. Prior studies (Coveleskie et al., 2015, Kullmann et al., 2012) showed hyperconnectivity of the reward network within the basal ganglia in individuals who are obese; however, these studies did not examine the influence of BMI on basal ganglia connectivity as a whole, nor its associations with mobility. Perhaps basal ganglia hyperconnectivity in individuals who are obese may help compensate for the negative biomechanical effects of higher BMI on locomotion. Further studies should examine the spatial distribution of BMI-related hyperconnectivity and its influence on gait.
4.3. Negative results and limitations
Our connectivity analyses indicate the lack of spatially distinct clusters of connectivity within the basal ganglia. We would have expected the sensorimotor subregions to be more strongly correlated compared to, for example, the limbic and sensorimotor subregions. We instead found only two distinct clusters of globally high and low connectivity among all regions of the basal ganglia. It is possible that the grouping of basal ganglia connectivity (high vs. low across all pairwise connections) was dependent on spatial resolution. Therefore, it is possible a greater spatial resolution would have resulted in greater number of groups within the basal ganglia. Future studies need to investigate whether a higher resolution fMRI sequence would identify greater number of clusters and spatial patterns among individual regions of the basal ganglia.
One important note about our results is that we did not find an association between cognitive function and gait speed in our results, even though we have previously shown that these are associated in the parent cohort (Rosano et al., 2005) of 3,705 participants. This study examined a subsample of the parent cohort, and this may decrease our ability to detect those effects. Another reasoning is that cognitive function and factors like WMH and volume may include similar variance. Lack of an association with cognitive function does not refute many past results that show there is an association between gait and cognitive function.
Limitations of this study urge caution in interpreting our results. It is important to recognize that usual-paced walking speed is just one measure of gait, which is a complex process with multiple components. Future studies should use a number of different measures of gait repeated several times to fully comprehend the complexity of gait-related impairments. While we focused on the co-activation of the regions of the basal ganglia, which has not been examined in detail, we did not examine the regions that are known to work with the basal ganglia in motor control, notably the thalamus, the sensorimotor cortices and the cerebellum. We also did not examine whether the associations are driven exclusively by the motor networks that are associated with the basal ganglia. It could be that the associative components or reward-related components are contributing to these associations; but we are unable to distinguish these in such a study. Our study is cross-sectional in nature, so it is unclear how these factors change longitudinally, and all associations are bidirectional in nature, thus we could not identify causal pathways. In addition, our sample consists of primarily older adults with a narrow age range of an average age of 83 (SD = 2.8), preventing generalization to the younger elderly.
4.4. Implications of our findings
Our data of higher connectivity in the presence of both age-related impairments (e.g. higher WMH and higher BMI) and faster gait indicate a potential compensatory role for the connectivity among the regions of the basal ganglia. One implication of our results is that there may be potential treatment options to modify resting state connectivity to improve gait speed. There is emerging evidence that connectivity of the basal ganglia to the cortex may increase after exposure to certain neuroprotective factors (Chirles et al., 2017, Gard et al., 2015, McGregor et al., 2018, Yin et al., 2018) or levodopa (Gao et al., 2017). Thus, individuals with impairments in both connectivity of the basal ganglia and gait speed may benefit from treatment with levodopa, while individuals with normal gait speed and low connectivity or abnormal gait speed and high connectivity (where gait impairment may not be related to basal ganglia impairment) may not benefit. One past study in PD showed that gait speed improved after administration of levodopa (Curtze et al., 2015), though not to levels in individuals without PD. Another study showed that in individuals with late-life depression who had slowed processing and gait speed that treatment with levodopa increased both the availability of dopamine in the brain (as measured by positron emission tomography) and improved gait speed, processing speed, and depressive symptoms (Rutherford et al., 2019). Thus, it is possible that if we identify individuals who have gait impairment and lower basal ganglia connectivity, we may be able to more effectively personalize treatments, i.e., only treat with levodopa when impairment is associated with basal ganglia dysfunction as they stand to benefit most from that treatment. Furthermore, this may have even greater benefits in individuals with PD or late-life depression.
5. Conclusion
The field of gerontology is increasingly recognizing the need to identify neural biomarkers of mobility control, especially in older adults without neurological diseases, to design preventions and treatments of age-related mobility impairments (Rosso et al., 2013, Sorond et al., 2015, Varma et al., 2016). Our results provide a potential mechanistic model whereby higher connectivity in the basal ganglia may positively influence gait speed. An implication of this finding is that higher connectivity of the basal ganglia may provide resilience against the detrimental effects of common locomotor risk factors by favoring automated motor control. It may also be interpreted as a compensatory response, however due to the cross-sectional nature of our study that is unclear. Resilience due to the basal ganglia integrity is a potentially promising area of inquiry. Unlike WMH and brain structural impairments, connectivity of the basal ganglia is modifiable, and can serve as a target for interventions. Future studies could investigate if those with low (but not high) basal ganglia connectivity and gait impairment could benefit from interventions targeting this system.
CRediT authorship contribution statement
H.T. Karim: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. A. Rosso: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. H.J. Aizenstein: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing. N.I. Bohnen: Conceptualization, Data curation, Writing - review & editing. S. Studenski: Conceptualization, Data curation, Writing - review & editing. C. Rosano: Conceptualization, Data curation, Formal analysis, Writing - original draft, Writing - review & editing.
Acknowledgements
The Health Aging and Body Composition study was supported by National Institute on Aging (NIA) contracts (N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106). Data acquisition and analysis and interpretation for this manuscript are supported by U01AG061393, 1R01AG037451, R01 AG029232, P30 AG024827, and T32MH019986.
References
- Acharya A., Liang X., Tian W., Jiang C., Han Y., Yi L. White matter hyperintensities relate to basal ganglia functional connectivity and memory performance in aMCI and SVMCI. Front. Neurosci. 2019;13:1204. doi: 10.3389/fnins.2019.01204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander N.B. Gait disorders in older adults. J. Am. Geriatr. Soc. 1996;44:434–451. doi: 10.1111/j.1532-5415.1996.tb06417.x. [DOI] [PubMed] [Google Scholar]
- Andresen E.M., Malmgren J.A., Carter W.B., Patrick D.L. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am. J. Prev. Med. 1994;10:77–84. [PubMed] [Google Scholar]
- Atkinson H.H., Rosano C., Simonsick E.M., Williamson J.D., Davis C., Ambrosius W.T., Rapp S.R., Cesari M., Newman A.B., Harris T.B., Rubin S.M., Yaffe K., Satterfield S., Kritchevsky S.B. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen N.I., Cham R. Postural control, gait, and dopamine functions in parkinsonian movement disorders. Clin. Geriatr. Med. 2006;22(797–812):vi. doi: 10.1016/j.cger.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Bohnen N.I., Muller M.L., Kuwabara H., Cham R., Constantine G.M., Studenski S.A. Age-associated striatal dopaminergic denervation and falls in community-dwelling subjects. J. Rehabil. Res. Dev. 2009;46:1045–1052. doi: 10.1682/jrrd.2009.03.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolandzadeh N., Liu-Ambrose T., Aizenstein H., Harris T., Launer L., Yaffe K., Kritchevsky S.B., Newman A., Rosano C. Pathways linking regional hyperintensities in the brain and slower gait. Neuroimage. 2014;99:7–13. doi: 10.1016/j.neuroimage.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifazi P., Erramuzpe A., Diez I., Gabilondo I., Boisgontier M.P., Pauwels L., Stramaglia S., Swinnen S.P., Cortes J.M. Structure-function multi-scale connectomics reveals a major role of the fronto-striato-thalamic circuit in brain aging. Hum. Brain Mapp. 2018;39:4663–4677. doi: 10.1002/hbm.24312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyne P., Maloney T., DiFrancesco M., Fox M.D., Awosika O., Aggarwal P., Woeste J., Jaroch L., Braswell D., Vannest J. Resting-state functional connectivity of subcortical locomotor centers explains variance in walking capacity. Hum. Brain Mapp. 2018;39:4831–4843. doi: 10.1002/hbm.24326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliński T., Harabasz J. A dendrite method for cluster analysis. Commun. Stat.-Theor. Methods. 1974;3:1–27. [Google Scholar]
- Cham R., Perera S., Studenski S.A., Bohnen N.I. Striatal dopamine denervation and sensory integration for balance in middle-aged and older adults. Gait Posture. 2007;26:516–525. doi: 10.1016/j.gaitpost.2006.11.204. [DOI] [PubMed] [Google Scholar]
- Cham R., Perera S., Studenski S.A., Bohnen N.I. Age-related striatal dopaminergic denervation and severity of a slip perturbation. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:980–985. doi: 10.1093/gerona/glr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham R., Studenski S.A., Perera S., Bohnen N.I. Striatal dopaminergic denervation and gait in healthy adults. Exp. Brain Res. 2008;185:391–398. doi: 10.1007/s00221-007-1161-3. [DOI] [PubMed] [Google Scholar]
- Chaudhry S.I., McAvay G., Ning Y., Allore H.G., Newman A.B., Gill T.M. Geriatric impairments and disability: the cardiovascular health study. J. Am. Geriatr. Soc. 2010;58:1686–1692. doi: 10.1111/j.1532-5415.2010.03022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Pillemer S., England S., Izzetoglu M., Mahoney J.R., Holtzer R. Neural correlates of obstacle negotiation in older adults: an fNIRS study. Gait Posture. 2017;58:130–135. doi: 10.1016/j.gaitpost.2017.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R., Qi H., Liu Y., Zhao S., Li C., Liu C., Zheng J. Abnormal amplitude of low-frequency fluctuations and functional connectivity of resting-state functional magnetic resonance imaging in patients with leukoaraiosis. Brain Behav. 2017;7 doi: 10.1002/brb3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirles T.J., Reiter K., Weiss L.R., Alfini A.J., Nielson K.A., Smith J.C. Exercise training and functional connectivity changes in mild cognitive impairment and healthy elders. J. Alzheimers Dis. 2017;57:845–856. doi: 10.3233/JAD-161151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coveleskie K., Gupta A., Kilpatrick L.A., Mayer E.D., Ashe-McNalley C., Stains J., Labus J.S., Mayer E.A. Altered functional connectivity within the central reward network in overweight and obese women. Nutr. Diab. 2015;5 doi: 10.1038/nutd.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crockett R.A., Hsu C.L., Best J.R., Liu-Ambrose T. Resting state default mode network connectivity, dual task performance, gait speed, and postural sway in older adults with mild cognitive impairment. Front. Aging Neurosci. 2017;9:423. doi: 10.3389/fnagi.2017.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtze C., Nutt J.G., Carlson-Kuhta P., Mancini M., Horak F.B. Levodopa is a double-edged sword for balance and gait in people with Parkinson's disease. Mov. Disord. 2015;30:1361–1370. doi: 10.1002/mds.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D.S., Kelly A.M., Uddin L.Q., Shehzad Z., Biswal B., Walters J.R., Castellanos F.X., Milham M.P. Functional connectivity of human striatum: a resting state FMRI study. Cereb. Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Di Scala G., Dupuy M., Guillaud E., Doat E., Barse E., Dillhareguy B., Jean F.A.M., Audiffren M., Cazalets J.R., Chanraud S. Efficiency of sensorimotor networks: posture and gait in young and older adults. Exp. Aging Res. 2019;45:41–56. doi: 10.1080/0361073X.2018.1560108. [DOI] [PubMed] [Google Scholar]
- Dumurgier J., Crivello F., Mazoyer B., Ahmed I., Tavernier B., Grabli D., Francois C., Tzourio-Mazoyer N., Tzourio C., Elbaz A. MRI atrophy of the caudate nucleus and slower walking speed in the elderly. Neuroimage. 2012;60:871–878. doi: 10.1016/j.neuroimage.2012.01.102. [DOI] [PubMed] [Google Scholar]
- Filippi M., Sarasso E., Agosta F. Resting-state Functional MRI in Parkinsonian Syndromes. Mov Disord Clin Pract. 2019;6:104–117. doi: 10.1002/mdc3.12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox P.T., Lancaster J.L. Mapping context and content: the BrainMap model. Nat. Rev. Neurosci. 2002;3:319. doi: 10.1038/nrn789. [DOI] [PubMed] [Google Scholar]
- Gao L.L., Zhang J.R., Chan P., Wu T. Levodopa effect on basal ganglia motor circuit in Parkinson's disease. CNS Neurosci. Ther. 2017;23:76–86. doi: 10.1111/cns.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard T., Taquet M., Dixit R., Holzel B.K., Dickerson B.C., Lazar S.W. Greater widespread functional connectivity of the caudate in older adults who practice kripalu yoga and vipassana meditation than in controls. Front. Hum. Neurosci. 2015;9:137. doi: 10.3389/fnhum.2015.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster B.H., Carlson C.L., Visser M., Kelley D.E., Scherzinger A., Harris T.B., Stamm E., Newman A.B. Attenuation of skeletal muscle and strength in the elderly: The Health ABC study. J. Appl. Physiol. 2001;1985(90):2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Griffanti L., Stratmann P., Rolinski M., Filippini N., Zsoldos E., Mahmood A., Zamboni G., Douaud G., Klein J.C., Kivimaki M., Singh-Manoux A., Hu M.T., Ebmeier K.P., Mackay C.E. Exploring variability in basal ganglia connectivity with functional MRI in healthy aging. Brain Imaging Behav. 2018;12:1822–1827. doi: 10.1007/s11682-018-9824-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff J.M., Yogev G., Springer S., Simon E.S., Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp. Brain Res. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- He H., Luo C., Chang X., Shan Y., Cao W., Gong J., Klugah-Brown B., Bobes M.A., Biswal B., Yao D. The functional integration in the sensory-motor system predicts aging in healthy older adults. Front. Aging Neurosci. 2016;8:306. doi: 10.3389/fnagi.2016.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold F., Wiegel P., Scholkmann F., Thiers A., Hamacher D., Schega L. Functional near-infrared spectroscopy in movement science: a systematic review on cortical activity in postural and walking tasks. Neurophotonics. 2017;4 doi: 10.1117/1.NPh.4.4.041403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R., Epstein N., Mahoney J.R., Izzetoglu M., Blumen H.M. Neuroimaging of mobility in aging: a targeted review. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:1375–1388. doi: 10.1093/gerona/glu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R., Mahoney J.R., Izzetoglu M., Wang C., England S., Verghese J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage. 2015;112:152–159. doi: 10.1016/j.neuroimage.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R., Verghese J., Xue X., Lipton R.B. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- Holtzer R., Wang C., Verghese J. The relationship between attention and gait in aging: facts and fallacies. Mot. Control. 2012;16:64–80. doi: 10.1123/mcj.16.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D.K., Nicklas B.J., Ding J., Harris T.B., Tylavsky F.A., Newman A.B., Lee J.S., Sahyoun N.R., Visser M., Kritchevsky S.B., Health A.B.C.S. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am. J. Clin. Nutr. 2008;87:150–155. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- Hsu C.L., Best J., Voss M., Handy T., Beauchet O., Lim C., Liu-Ambrose T., 2018. Functional neural correlates of slower gait among older adults with mild cognitive impairment. J. Gerontol.: Med. Sci., in press. [DOI] [PubMed]
- Hsu C.L., Voss M.W., Handy T.C., Davis J.C., Nagamatsu L.S., Chan A., Bolandzadeh N., Liu-Ambrose T. Disruptions in brain networks of older fallers are associated with subsequent cognitive decline: a 12-month prospective exploratory study. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0093673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenschmidt C.E., Burdette J.H., Morgan A.R., Williamson J.D., Kritchevsky S.B., Laurienti P.J. Graph theory analysis of functional brain networks and mobility disability in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:1399–1406. doi: 10.1093/gerona/glu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzitari M., Newman A.B., Yaffe K., Boudreau R., de Rekeneire N., Shorr R., Harris T.B., Rosano C. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S., Heni M., Veit R., Ketterer C., Schick F., Haring H.U., Fritsche A., Preissl H. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum. Brain Mapp. 2012;33:1052–1061. doi: 10.1002/hbm.21268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen C.D., Zonneveld H.I., White T., Huizinga W., Cremers L.G.M., de Groot M., Ikram M.A., Niessen W.J., Vernooij M.W. White matter lesions relate to tract-specific reductions in functional connectivity. Neurobiol. Aging. 2017;51:97–103. doi: 10.1016/j.neurobiolaging.2016.12.004. [DOI] [PubMed] [Google Scholar]
- Liem F., Varoquaux G., Kynast J., Beyer F., Kharabian Masouleh S., Huntenburg J.M., Lampe L., Rahim M., Abraham A., Craddock R.C., Riedel-Heller S., Luck T., Loeffler M., Schroeter M.L., Witte A.V., Villringer A., Margulies D.S. Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage. 2017;148:179–188. doi: 10.1016/j.neuroimage.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Lindquist M.A., Geuter S., Wager T.D., Caffo B.S. Modular preprocessing pipelines can reintroduce artifacts into fMRI data. Hum. Brain Mapp. 2019;40:2358–2376. doi: 10.1002/hbm.24528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo O., Halko M., Zhou J., Cheong W., Harrison R., Wayne P., Lipsitz L., Manor B. Gait variability correlates with resting-state brain network connectivity in aging and disease. Innov. Aging. 2018;2:518. [Google Scholar]
- Manza P., Zhang S., Hu S., Chao H.H., Leung H.C., Li C.R. The effects of age on resting state functional connectivity of the basal ganglia from young to middle adulthood. Neuroimage. 2015;107:311–322. doi: 10.1016/j.neuroimage.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K.L., Blizzard L., Wood A.G., Srikanth V., Thomson R., Sanders L.M., Callisaya M.L. Cognitive function, gait, and gait variability in older people: a population-based study. J. Gerontol. A Biol. Sci. Med. Sci. 2012 doi: 10.1093/gerona/gls224. [DOI] [PubMed] [Google Scholar]
- Mathys C., Hoffstaedter F., Caspers J., Caspers S., Sudmeyer M., Grefkes C., Eickhoff S.B., Langner R. An age-related shift of resting-state functional connectivity of the subthalamic nucleus: a potential mechanism for compensating motor performance decline in older adults. Front. Aging Neurosci. 2014;6:178. doi: 10.3389/fnagi.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor K.M., Crosson B., Krishnamurthy L.C., Krishnamurthy V., Hortman K., Gopinath K., Mammino K.M., Omar J., Nocera J.R. Effects of a 12-week aerobic spin intervention on resting state networks in previously sedentary older adults. Front. Psychol. 2018;9:2376. doi: 10.3389/fpsyg.2018.02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean R.R., Shardell M.D., Alley D.E., Cawthon P.M., Fragala M.S., Harris T.B., Kenny A.M., Peters K.W., Ferrucci L., Guralnik J.M., Kritchevsky S.B., Kiel D.P., Vassileva M.T., Xue Q.L., Perera S., Studenski S.A., Dam T.T. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) sarcopenia project. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:576–583. doi: 10.1093/gerona/glu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman A., Maidan I., Bernad-Elazari H., Shustack S., Giladi N., Hausdorff J.M. Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn. 2017;115:41–46. doi: 10.1016/j.bandc.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M., Verghese J., Beauchet O., Hausdorff J.M. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 2012;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Fox M.D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni N.K., Nunley K.A., Aizenstein H., Harris T.B., Yaffe K., Satterfield S., Newman A.B., Rosano C., Health A.B.C.S. Association between cerebellar gray matter volumes, gait speed, and information-processing ability in older adults enrolled in the Health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:996–1003. doi: 10.1093/gerona/glt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt J.G., Marsden C.D., Thompson P.D. Human walking and higher-level gait disorders, particularly in the elderly. Neurology. 1993;43:268–279. doi: 10.1212/wnl.43.2.268. [DOI] [PubMed] [Google Scholar]
- Panigrahi B., Martin K.A., Li Y., Graves A.R., Vollmer A., Olson L., Mensh B.D., Karpova A.Y., Dudman J.T. Dopamine is required for the neural representation and control of movement vigor. Cell. 2015;162:1418–1430. doi: 10.1016/j.cell.2015.08.014. [DOI] [PubMed] [Google Scholar]
- Patel A.X., Kundu P., Rubinov M., Jones P.S., Vertes P.E., Ersche K.D., Suckling J., Bullmore E.T. A wavelet method for modeling and despiking motion artifacts from resting-state fMRI time series. Neuroimage. 2014;95:287–304. doi: 10.1016/j.neuroimage.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny W.D., Friston K.J., Ashburner J.T., Kiebel S.J., Nichols T.E. Elsevier; 2011. Statistical Parametric Mapping: The Analysis of Functional Brain Images. [Google Scholar]
- Poole V.N., Lo O.Y., Wooten T., Iloputaife I., Lipsitz L.A., Esterman M. Motor-cognitive neural network communication underlies walking speed in community-dwelling older adults. Front. Aging Neurosci. 2019;11:159. doi: 10.3389/fnagi.2019.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand W.M. Objective criteria for the evaluation of clustering methods. J. Am. Stat. Assoc. 1971;66:846–850. [Google Scholar]
- Rosano C., Aizenstein H., Brach J., Longenberger A., Studenski S., Newman A.B. Special article: gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:1380–1388. doi: 10.1093/gerona/63.12.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C., Aizenstein H.J., Studenski S., Newman A.B. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- Rosano C., Newman A.B., Katz R., Hirsch C.H., Kuller L.H. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J. Am. Geriatr. Soc. 2008;56:1618–1625. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C., Simonsick E.M., Harris T.B., Kritchevsky S.B., Brach J., Visser M., Yaffe K., Newman A.B. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology. 2005;24:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- Rosano C., Studenski S.A., Aizenstein H.J., Boudreau R.M., Longstreth W.T., Jr., Newman A.B. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41:58–64. doi: 10.1093/ageing/afr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso A., Studenski S.A., Chen W.G., Aizenstein H.J., Alexander N., Bennett D.A., Black S.E., Camicioli R., Carlson M.C., Ferrucci L., Guralnik J.M., Hausdorff J.M., Kaye J., Launer L.J., Lipsitz L.A., Verghese J., Rosano C. Aging, the central nervous system, and mobility. J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 2013;68:1379–1386. doi: 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso A.L., Olson Hunt M.J., Yang M., Brach J.S., Harris T.B., Newman A.B., Satterfield S., Studenski S.A., Yaffe K., Aizenstein H.J., Rosano C., Health A.B.C.S. Higher step length variability indicates lower gray matter integrity of selected regions in older adults. Gait Posture. 2014;40:225–230. doi: 10.1016/j.gaitpost.2014.03.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso A.L., Studenski S.A., Longstreth W.T., Jr., Brach J.S., Boudreau R.M., Rosano C. Contributors to poor mobility in older adults: integrating white matter hyperintensities and conditions affecting other systems. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72:1246–1251. doi: 10.1093/gerona/glw224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseeuw P.J. Silhouettes: a graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 1987;20:53–65. [Google Scholar]
- Rutherford B.R., Slifstein M., Chen C., Abi-Dargham A., Brown P.J., Wall M.W., Vanegas-Arroyave N., Stern Y., Bailey V., Valente E., Roose S.P. Effects of L-DOPA monotherapy on psychomotor speed and [(11)C]raclopride binding in high-risk older adults with depression. Biol. Psychiatry. 2019;86:221–229. doi: 10.1016/j.biopsych.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Scarpelli M., Salvolini U., Diamanti L., Montironi R., Chiaromoni L., Maricotti M. MRI and pathological examination of post-mortem brains: the problem of white matter high signal areas. Neuroradiology. 1994;36:393–398. doi: 10.1007/BF00612126. [DOI] [PubMed] [Google Scholar]
- Scheltens P., Barkhof F., Leys D., Wolters E.C., Ravid R., Kamphorst W. Histopathologic correlates of white matter changes on MRI in Alzheimer's disease and normal aging. Neurology. 1995;45:883–888. doi: 10.1212/wnl.45.5.883. [DOI] [PubMed] [Google Scholar]
- Seidler R.D., Bernard J.A., Burutolu T.B., Fling B.W., Gordon M.T., Gwin J.T., Kwak Y., Lipps D.B. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban C.E., Jorgensen D.R., Gianaros P.J., Mettenburg J., Rosano C. Cerebrovascular disease: Neuroimaging of cerebral small vessel disease. Prog. Mol. Biol. Transl. Sci. 2019;165:225–255. doi: 10.1016/bs.pmbts.2019.07.008. [DOI] [PubMed] [Google Scholar]
- Shine J.M., Matar E., Ward P.B., Frank M.J., Moustafa A.A., Pearson M., Naismith S.L., Lewis S.J. Freezing of gait in Parkinson's disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain. 2013;136:3671–3681. doi: 10.1093/brain/awt272. [DOI] [PubMed] [Google Scholar]
- Simonsick E.M., Newman A.B., Nevitt M.C., Kritchevsky S.B., Ferrucci L., Guralnik J.M., Harris T., Health A.B.C.S.G. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:M644–649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond F.A., Cruz-Almeida Y., Clark D.J., Viswanathan A., Scherzer C.R., De Jager P., Csiszar A., Laurienti P.J., Hausdorff J.M., Chen W.G., Ferrucci L., Rosano C., Studenski S.A., Black S.E., Lipsitz L.A. Aging, the central nervous system, and mobility in older adults: neural mechanisms of mobility impairment. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:1526–1532. doi: 10.1093/gerona/glv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stijntjes M., de Craen A.J., van der Grond J., Meskers C.G., Slagboom P.E., Maier A.B. Cerebral microbleeds and lacunar infarcts are associated with walking speed independent of cognitive performance in middle-aged to older adults. Gerontology. 2016;62:500–507. doi: 10.1159/000444583. [DOI] [PubMed] [Google Scholar]
- Studenski S. Gait speed reveals clues to lifelong health. JAMA Netw. Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.13112. [DOI] [PubMed] [Google Scholar]
- Studenski S., Perera S., Patel K., Rosano C., Faulkner K., Inzitari M., Brach J., Chandler J., Cawthon P., Connor E.B., Nevitt M., Visser M., Kritchevsky S., Badinelli S., Harris T., Newman A.B., Cauley J., Ferrucci L., Guralnik J. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniwaki T., Okayama A., Yoshiura T., Togao O., Nakamura Y., Yamasaki T., Ogata K., Shigeto H., Ohyagi Y., Kira J., Tobimatsu S. Age-related alterations of the functional interactions within the basal ganglia and cerebellar motor loops in vivo. Neuroimage. 2007;36:1263–1276. doi: 10.1016/j.neuroimage.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Teng E.L., Chui H.C. The modified mini-mental state (3MS) examination. J. Clin. Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Thibes R.B., Novaes N.P., Lucato L.T., Campanholo K.R., Melo L.M., Leite C.C., Amaro E., Jr., Barbosa E.R., Bor-Seng-Shu E., Cardoso E.F., Sato J.R. Altered functional connectivity between precuneus and motor systems in Parkinson's disease patients. Brain Connect. 2017;7:643–647. doi: 10.1089/brain.2017.0534. [DOI] [PubMed] [Google Scholar]
- Tian Q., Chastan N., Bair W.N., Resnick S.M., Ferrucci L., Studenski S.A. The brain map of gait variability in aging, cognitive impairment and dementia-A systematic review. Neurosci. Biobehav. Rev. 2017;74:149–162. doi: 10.1016/j.neubiorev.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Functional connectivity of substantia nigra and ventral tegmental area: maturation during adolescence and effects of ADHD. Cereb. Cortex. 2014;24:935–944. doi: 10.1093/cercor/bhs382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma V.R., Hausdorff J.M., Studenski S.A., Rosano C., Camicioli R., Alexander N.B., Chen W.G., Lipsitz L.A., Carlson M.C. Aging, the central nervous system, and mobility in older adults: interventions. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:1451–1458. doi: 10.1093/gerona/glw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent H.K., Vincent K.R., Lamb K.M. Obesity and mobility disability in the older adult. Obes. Rev. 2010;11:568–579. doi: 10.1111/j.1467-789X.2009.00703.x. [DOI] [PubMed] [Google Scholar]
- Wechsler, D., 1955. Manual for the Wechsler Adult Intelligence Scale.
- Wennberg A.M., Savica R., Mielke M.M. Association between various brain pathologies and gait disturbance. Dement. Geriatr. Cogn. Disord. 2017;43:128–143. doi: 10.1159/000456541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D.K., Niu J., Zhang Y. Is symptomatic knee osteoarthritis a risk factor for a trajectory of fast decline in gait speed? Results from a longitudinal cohort study. Arthritis Care Res. (Hoboken) 2013;65:187–194. doi: 10.1002/acr.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J., Allcock L., Mc Ardle R., Taylor J.P., Rochester L. The neural correlates of discrete gait characteristics in ageing: A structured review. Neurosci. Biobehav. Rev. 2019;100:344–369. doi: 10.1016/j.neubiorev.2018.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Rosano C., Butters M., Whyte E., Nable M., Crooks R., Meltzer C.C., Reynolds C.F., 3rd, Aizenstein H.J. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133–142. doi: 10.1016/j.pscychresns.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Hallett M. The influence of normal human ageing on automatic movements. J. Physiol. 2005;562:605–615. doi: 10.1113/jphysiol.2004.076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K., Fiocco A.J., Lindquist K., Vittinghoff E., Simonsick E.M., Newman A.B., Satterfield S., Rosano C., Rubin S.M., Ayonayon H.N., Harris T.B., Health A.B.C.S. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72:2029–2035. doi: 10.1212/WNL.0b013e3181a92c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z., Chang M., Wei S., Jiang X., Zhou Y., Cui L., Lv J., Wang F., Tang Y. Decreased functional connectivity in insular subregions in depressive episodes of bipolar disorder and major depressive disorder. Front. Neurosci. 2018;12:842. doi: 10.3389/fnins.2018.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev-Seligmann G., Hausdorff J.M., Giladi N. The role of executive function and attention in gait. Mov. Disord. 2008;23:329–342. doi: 10.1002/mds.21720. quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Blumen H.M., Verghese J., Holtzer R. Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: a resting-state fMRI study. Hum. Brain Mapp. 2015;36:1484–1493. doi: 10.1002/hbm.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., Huang H., Yang S., Luo X., Zhu W., Xu S., Meng Q., Zuo C., Zhao K., Liu H., Liu Y., Wang W. Dysfunctional architecture underlies white matter hyperintensities with and without cognitive impairment. J. Alzheimers Dis. 2019;71:461–476. doi: 10.3233/JAD-190174. [DOI] [PubMed] [Google Scholar]
- Zwergal A., Linn J., Xiong G., Brandt T., Strupp M., Jahn K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol. Aging. 2012;33:1073–1084. doi: 10.1016/j.neurobiolaging.2010.09.022. [DOI] [PubMed] [Google Scholar]