Abstract
Background & Aims
Remnant lipoprotein cholesterol (RLP-C) is an atherogenic lipid profile associated with non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease (CVD). With increased rates of CVD seen in adults with NAFLD, RLP-C has the potential to identify individuals with NAFLD who are at increased risk of CVD. This study examined in adolescents sex-different associations among RLP-C, NAFLD, and cardiometabolic risk factors, and whether RLP-C is associated with NAFLD beyond traditional cardiometabolic risk factors.
Methods
Adolescents in the Raine Study had anthropometry, clinical, biochemistry and arterial stiffness measurements recorded at 17 years of age. Fatty liver, subcutaneous and visceral adipose thickness were assessed using abdominal ultrasound. Relationships among RLP-C, NAFLD, liver biochemistry, insulin resistance, adipokines, adiposity and arterial stiffness were assessed.
Results
NAFLD was diagnosed in 15.1% (19.6% females and 10.7% males) of adolescents. Increasing RLP-C levels were associated with increasing severity of hepatic steatosis and metabolic syndrome. Adolescents with NAFLD and serum RLP-C levels in the highest quartile compared with the lowest quartile, had higher serum leptin, homeostatic model assessment of insulin resistance (HOMA-IR), high-sensitivity C-reactive protein, low-density-lipoprotein cholesterol, triglycerides, BMI, subcutaneous and visceral adipose thickness, systolic blood pressure and arterial stiffness, but lower adiponectin and high-density-lipoprotein cholesterol. Using multivariable logistic regression, RLP-C in the lowest quartile compared with the highest quartile was associated with 85% lower odds of NAFLD in males and 55% in females, after adjusting for waist circumference, leptin, ALT, adiponectin and HOMA-IR.
Conclusions
There is an association between RLP-C and NAFLD beyond traditional risk factors of adiposity and insulin resistance in adolescents. Although raised serum RLP-C levels were associated with the severity of hepatic steatosis and markers of cardiometabolic risk, lower serum RLP-C might reflect reduced cardiovascular risk.
Lay summary
Remnant lipoprotein cholesterol (RLP-C) is a part of the blood cholesterol that is linked with heart disease and non-alcoholic fatty liver disease (NAFLD) in adults. In the Raine Study, teenagers with high RLP-C levels had more severe fat accumulation in their liver. Thus, RLP-C might be the hidden link between NAFLD and future risk of heart disease.
Keywords: Metabolic syndrome, NAFLD, Cardiometabolic risk, Lipids, Adiposity, Arterial stiffness, Raine study
Abbreviations: AIx, Aortic Augmentation Index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; C-AGPH-HR75, Central Augmentation Pressure/Pulse Height Ratio at Heart Rate 75; GGT, gamma-glutamyl transpeptidase; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein; IDF, International Diabetes Federation; LDL-C, low-density lipoprotein cholesterol; NAFLD, non-alcoholic fatty liver disease; OR, odds ratio; Q1, lowest (first) quartile; Q2, second quartile; Q3, third quartile; Q4, top (fourth) quartile; RLP-C, remnant lipoprotein cholesterol; T2DM, type 2 diabetes mellitus; TG, triglycerides; VLDL, very-low-density lipoprotein
Graphical abstract
Highlights
-
•
Non-alcoholic fatty liver disease (NAFLD) and heart disease share risk factors.
-
•
Serum remnant lipoprotein cholesterol (RLP-C) is linked with severity of liver fat.
-
•
Males with NAFLD have higher cardiometabolic risk.
-
•
RLP-C may contribute to risk of cardiovascular disease in people with NAFLD.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic liver disorder typified by accumulation of fat in the liver, with variable degrees of inflammation or hepatic fibrosis, in the absence of excessive alcohol.1 It is associated with the metabolic syndrome, primarily abdominal obesity, hypertriglyceridaemia, type 2 diabetes mellitus (T2DM) or fasting glycaemia and low levels of serum high-density cholesterol (HDL-C).2,3 It is the most common cause of chronic liver disease globally, with population estimates ranging from 13.5% to 31.8% depending on the population studied.[2], [3], [4] The prevalence of NAFLD in the general population increases with the number of features of the metabolic syndrome and with age.[2], [3], [4], [5], [6] Previous studies reported population prevalence ranges of 3–18% in adolescents and a global prevalence of up to 25% in adults, increasing to >90% in very obese adults and 38% in obese children.[5], [6], [7], [8], [9], [10], [11] Sex differences in the prevalence and phenotype of NAFLD associated with adiposity distribution have also been reported.5
The global burden of NAFLD has progressively increased over recent decades, likely as a result of an increased prevalence of obesity, T2DM, dyslipidaemia and hypertension, which are concurrent risk factors for cardiovascular disease (CVD). CVD is the leading cause of disease burden in adults globally.4,12,13 Adults with NAFLD have a 2-fold increased risk of coronary artery disease and have increased cardiovascular events.14 Individuals with NAFLD also have a lipid profile referred to as the ‘atherogenic lipid triad’, typified by increased levels of low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG), and low HDL-C.15 A longitudinal community follow-up study in the USA showed an increasing incidence of NAFLD, particularly in adolescents and young adults, and an association between NAFLD and increased cardiovascular events over 10 years.16
Remnant lipoprotein cholesterol (RLP-C), the cholesterol contained in very-low-density lipoprotein (VLDL), was associated with incident coronary heart disease in adults in the Jackson Heart and Framingham Offspring Cohort Studies in the USA.17 More recently, NAFLD was independently associated with higher circulating RLP-C levels that were linked with a higher risk of major adverse cardiovascular and cerebrovascular events in outpatients with NAFLD and features of the metabolic syndrome or atrial fibrillation from Italian hospitals.18 Cardiometabolic risk factors, such as abdominal obesity, dyslipidaemia and hyperinsulinism, have been associated with NAFLD in Raine Study participants aged 17 years.5 Adolescents with NAFLD and increased waist circumference, triglycerides, insulin, systolic blood pressure, but lower HDL-C, also had higher arterial stiffness.19 RLP-C was also recently associated with NAFLD and raised liver transaminases in 17-year-old adolescents from the Avon Longitudinal Study of Parents and Children in the UK.20
As the prevalence of NAFLD continues to rise, knowledge of preclinical cardiometabolic risk factors in young people with NAFLD, using noninvasive assessments, may identify opportunities to ameliorate future cardiovascular risk burden. The primary aim of our study was to examine sex-based associations between RLP-C, NAFLD and a range of cardiometabolic risk factors, including adiposity, homeostatic model assessment of insulin resistance (HOMA-IR), serum alanine transferase (ALT), adipokines and measures of arterial stiffness, in 17-year-old adolescents. The secondary aim was to determine whether RLP-C was associated with NAFLD beyond traditional risk factors of adiposity and insulin resistance in adolescents.
Methods
Study population
The Raine Study was established as a pregnancy cohort examining the impact of repeated antenatal ultrasound examinations on foetal outcomes. The cohort comprised 2,900 predominantly Caucasian women (generation 1 or Gen1) between 16 and 18 weeks' gestation, recruited from the general outpatient antenatal clinic at King Edward Memorial Hospital (Subiaco, WA, Australia) and nearby private practices between 1989 and 1991. The women gave birth to 2,868 live neonates (generation 2 or Gen2), who have since been followed up at ∼1–3-year intervals.21 A 17-year cross-sectional assessment of the cohort was conducted between 2006 and 2009, during which 1,726 Gen2 adolescents were followed up. During this assessment, the Raine Study families were considered representative of the broader Western Australian population.22 Using charts with pictures and descriptions of the Tanner pubertal stages, the adolescents matched their individual pubertal appearance with the appropriate stage, as previously reported.5 Assessments included a detailed health and lifestyle questionnaire, anthropometric and cardiovascular assessments, fasting bloods and an abdominal ultrasound. Institutional ethics committee approval was obtained from the Princess Margaret Hospital for Children Human Research Ethics Committee. Parents or legal guardians provided signed informed consent and the adolescent provided personal assent for participation in the study.
Abdominal ultrasound assessment
Details of the ultrasound protocol for assessing fatty liver (hepatic steatosis) and abdominal fat compartments have been previously reported.5 Trained ultrasonographers conducted the ultrasound examinations and a single specialist radiologist interpreted the resulting images. The ultrasonographers and radiologist were blinded to the clinical and laboratory characteristics of the study participants. The presence or absence of hepatic steatosis was based on scoring of echotexture, deep attenuation and vessel blurring, using scores of 0–3, 0–2 and 0–1, respectively. The diagnosis of fatty liver (hepatic steatosis) required a total score of at least 2, comprising an echotexture score of at least 1. The severity of hepatic steatosis was categorised by the total fatty liver score as 0–1 (no fatty liver), 2–3 (mild fatty liver) and 4–6 (moderate–severe fatty liver). The intraobserver reliability (K statistic) for reporting hepatic steatosis was 0.78 (95% CI 0.73–0.88). Visceral adipose tissue thickness (VAT) was measured as the distance between the anterior wall of the aorta and the internal face of the rectus abdominis muscle perpendicular to the aorta. Subcutaneous adipose tissue thickness (SAT) was measured as the thickness of the fat tissue between the skin–fat interface and the linea alba, avoiding SAT compression. The intraclass correlation coefficient was 0.93 for SAT (95% CI 0.93–0.93) and 0.94 for VAT (95% CI 0.94–0.95).
Definition of NAFLD
NAFLD was diagnosed if hepatic steatosis was detected using liver ultrasound, in the absence of excessive alcohol intake and without identifiable secondary causes. Adolescents with a self-reported alcohol intake threshold of <140 g per week for females and 210 g per week in males, consistent with recent NAFLD diagnosis and management guidelines, were classified as having NAFLD. Testing for viral serology for HBV or HCV was not carried out, given the notification rates for HBV and HCV infections were, on average, <24/100,000 and <23/100,000, respectively, for Western Australian teenagers between the ages of 15 and 19 years over the study period.2,5
Arterial stiffness assessment
Assessment of arterial stiffness was performed using a SphygmoCor Pulse Wave System as previously described.19 Blood pressure was measured by oscillometric sphygmomanometer (DINAMAP vital signs monitor 8100, DINAMAP XL vital signs monitor or DINAMAP ProCare 100). The Pulse Wave Analysis System (SCOR-Px) and SphygmoCor Pulse Wave System (SCOR-Vx) were used. Tonometers were applied to two sites (the carotid artery and distal dorsalis pedis). Pulse wave analysis was collected from the radial artery with the wrist facing upwards. Pulse wave velocity (PWV) was calculated dividing the distance between tonometers by the transit time of the arterial pulse wave. An Aortic Augmentation Index (AIx) was defined as the difference in the second and first systolic pressure peaks as a percentage of pulse pressure. AIx measures the central arterial reflected pressure wave occurring after distension of peripheral vessels with systole. Reduced elasticity of peripheral arterial vessels is reflected by the pressure wave occurring earlier after cardiac ejection, augmenting systolic pressure. Central Augmentation Pressure/Pulse Height Ratio at Heart Rate 75 (C-AGPH-HR75) was calculated to represent an arterial stiffness measure adjusting PWV and AIx for a heart rate of 75.
Anthropometry
Body weight was measured in kilograms (kg) using a Wedderburn Chair Scale (to the nearest 100 g), height in cm with a Holtain Stadiometer (to the nearest 0.1 cm) with participants dressed in light clothes without shoes, and waist circumference (cm) at the umbilicus level with a tape measure (to the nearest 0.1 cm). Skinfold thickness measurements were obtained from the anterior abdominal wall, triceps, subscapular and suprailiac skinfold regions using a skinfold calliper. Metabolic syndrome was defined using International Diabetes Federation (IDF) criteria, which include central obesity with a waist circumference ≥80 cm in females and ≥94 cm in males, plus at least 2 of the following: systemic arterial hypertension, hypertriglyceridaemia, low levels of HDL-C, T2DM or raised fasting glucose.23 MBI was calculated from weight (kg)/height (m)2.
Biochemistry
Venous blood samples taken from an antecubital vein after an overnight fast were assayed for serum glucose, insulin, ALT, aspartate aminotransferase (AST), gamma glutamyl transpeptidase (GGT), triglycerides, total cholesterol (TC), HDL-C, ferritin, transferrin saturation, high-sensitivity C-reactive protein (CRP), adiponectin and leptin. The HOMA-IR score was calculated as [fasting insulin (μU/ml) × fasting glucose (mmol/L)]/22.5. LDL-C was derived using the Friedewald equation.24 RLP-C levels were calculated as total cholesterol – (HDL-C + LDL-C).18
Statistical analysis
Sex-specific analyses were performed because of previously identified sex differences in the prevalence of NAFLD and in associations between NAFLD and cardiometabolic covariates in the Raine Study.5 Continuous descriptive data were summarised as means and standard deviations, with categorical variables as proportions. The main outcome variables were the ultrasound diagnosis of NAFLD and severity of steatosis. Differences in continuous variables between adolescents with or without NAFLD were computed using the independent t test, analysis of variance (ANOVA) or, for nonparametric variables, the Mann-Whitney U test or Kruskal-Wallis test. Differences between categorical variables were determined with the Pearson Chi-square test or Fisher's exact test. Correlation coefficients were sought using Pearson's correlation. We had 95% power to detect a 24% change in odds ratio, at α = 0.05 with a sample size of 1,170 subjects using multivariable logistic regression analysis (G∗Power 3.1). Multivariable logistic regression models were used to calculate the odds of NAFLD from adolescent physical assessment and biochemistry that were statistically significant in univariate analysis, as well as HOMA-IR. All p values are reported as two-sided, and p <0.05 was considered significant. Data were analysed using IBM SPSS statistics for Windows (version 20.0; IBM Corp., Armonk, NY, USA).
Results
NAFLD
Liver ultrasound was performed on 1,170 adolescents (51% male), of whom 1,162 had anthropometry, 1,165 cardiovascular assessment, 992 fasting blood tests and 1,122 arterial stiffness measurements. Mean age was 17.0 (standard deviation 0.3) years. Most adolescents (98% of males and 99% of females) were pubertal or post-pubertal, according to the Tanner scale. The Tanner stage of puberty was not associated with the presence or absence of NAFLD (p>0.05). Among the adolescents, 21.4% had central obesity (33.2% female versus 9.9% male, p <0.05) using IDF waist circumference criteria. By contrast, using BMI criteria, only 7.5% (8.0% female vs. 6.9% male, p = 0.48) were obese, whereas 20.6% (22.1% female and 19.2% male, p = 0.21) were overweight/obese. Median alcohol intake was 10 g per week [interquartile range (IQR) 0–90 g per week] during the preceding 12 months. Three adolescents were excluded from the analysis because of excessive alcohol intake. NAFLD was diagnosed in 177 (15.1%), comprising 19.6% females and 10.7% males (p <0.001). Among the adolescents with NAFLD, 8.7% (12.7% female and 4.9% male) had mild steatosis, whereas 6.1% (6.8% female and 5.4% male) had moderate–severe steatosis. General and subcutaneous adiposity indices, serum triglycerides, LDL-C, RLP-C, ALT, GGT, leptin, insulin, HOMA-IR, hsCRP, C-AGPH-HR75 and Alx were higher, whereas HDL-C and adiponectin were lower in those with NAFLD compared with those without NAFLD (Table 1).
Table 1.
Cohort characteristics.
| Characteristic | Males |
Female |
||||
|---|---|---|---|---|---|---|
| NAFLD (n = 63) | No NAFLD (n = 528) | p value | NAFLD (n = 113) | No NAFLD (n = 463) | p value | |
| Anthropometry | ||||||
| Weight (kg) | 94.5 ± 20.4 | 69.4 ± 11.0 | <0.001 | 72.9 ± 17.2 | 60.9 ± 10.0 | <0.001 |
| BMI (kg/m2) | 29.3 ± 5.9 | 21.9 ± 3.1 | <0.001 | 26.3 ± 5.9 | 22.2 ± 3.4 | <0.001 |
| Waist circumference (cm) | 98.1 ± 15.6 | 78.3 ± 7.7 | <0.001 | 85.3 ± 14.6 | 75.5 ± 9.3 | <0.001 |
| Abdominal obesity (%) | 59.0 | 4.2 | <0.001 | 61.5 | 25.7 | <0.001 |
| Suprailiac SFT (mm) | 26.2 ± 11.0 | 11.4 ± 7.0 | <0.001 | 24.0 ± 9.1 | 17.0 ± 6.7 | <0.001 |
| SAT (mm) | 31.4 ± 14.2 | 12.5 ± 7.7 | <0.001 | 30.0 ± 14.7 | 18.6 ± 6.6 | <0.001 |
| VAT (mm) | 41.6 ± 16.4 | 34.7 ± 9.9 | <0.001 | 31.4 ± 9.8 | 29.5 ± 8.7 | 0.09 |
| Cardiovascular | ||||||
| SBP (mmHg) | 124.3 ± 9.8 | 119.3 ± 10.2 | <0.001 | 110.4 ± 9.3 | 109.3 ± 9.6 | 0.29 |
| DBP (mmHg) | 60.2 ± 7.1 | 59.2 ± 6.6 | 0.29 | 59.8 ± 6.0 | 59.6 ± 6.6 | 0.83 |
| Pulse per minute | 66.7 ± 12.1 | 62.5 ± 10.2 | 0.003 | 67.5 ± 9.6 | 66.8 ± 9.9 | 0.46 |
| Biochemistry | ||||||
| ALT (U/L) | 39.1 ± 23.6 | 22.1 ± 9.9 | <0.001 | 19.7 ± 13.3 | 18.1 ± 10.2 | 0.20 |
| AST (U/L) | 31.4. ± 14.5 | 27.0 ± 8.3 | 0.001 | 21.3 ± 5.4 | 22.0 ± 5.1 | 0.19 |
| GGT (U/L) | 23.7 ± 14.1 | 15.4 ± 7.3 | <0.001 | 13.9 ± 6.8 | 13.0 ± 6.8 | 0.24 |
| Glucose (mmol/L) | 5.0 ± 0.5 | 4.8 ± 0.7 | 0.08 | 4.6 ± 0.4 | 4.7 ± 0.4 | 0.70 |
| Total cholesterol (mmol/L) | 4.0 ± 0.9 | 3.9 ± 0.7 | 0.34 | 4.4 ± 0.9 | 4.3 ± 0.7 | 0.41 |
| HDL-C (mmol/L) | 1.1 ± 0.2 | 1.2 ± 0.2 | <0.001 | 1.3 ± 0.3 | 1.4 ± 0.3 | 0.001 |
| LDL-C (mmol/L) | 2.4 ± 0.8 | 2.2 ± 0.6 | 0.16 | 2.5 ± 0.7 | 2.4 ± 0.6 | 0.13 |
| Triglycerides (mmol/L) | 1.3 ± 0.6 | 1.0 ± 0.6 | <0.001 | 1.1 ± 0.6 | 1.0 ± 0.5 | 0.01 |
| RLP-C (mmol/L) | 0.6 ± 0.3 | 0.5 ± 0.3 | 0.007 | 0.5 ± 0.3 | 0.5 ± 0.1 | 0.006 |
| Leptin (μg/L) | 12.8 (6.2–28.4) | 2.3 (1.4–5.2) | <0.001 | 43.3 (27.1–66.4) | 22.4 (13.9–35.8) | <0.001 |
| Adiponectin (mg/L) | 6.6 ± 2.8 | 8.4 ± 5.2 | 0.01 | 9.3 ± 4.6 | 11.9 ± 6.4 | <0.001 |
| hsCRP (mg/L) | 1.2 (0.6–2.5) | 0.4 (0.2–0.8) | <0.001 | 1.3 (0.4–4.6) | 0.7 (0.3–1.8) | 0.001 |
| Fasting insulin (mU/L) | 10.5 (6.6–19.4) | 6.8 (4.4–9.8) | <0.001 | 9.7 (6.8–15.6) | 7.5 (5.1–10.7) | <0.001 |
| HOMA-IR | 2.3 (1.4–4.1) | 1.4 (0.9–2.1) | <0.001 | 2.1 (1.3–3.1) | 1.5 (1.0–2.2) | <0.001 |
| Metabolic syndrome (%) | 21.8% | 1.6% | <0.001 | 9.9 | 1.7% | 0.001 |
| Arterial stiffness measurements | ||||||
| C-AGPH-HR75 | −3.3 (−14.8 to 3.5) | −10.5 (−18.3 to −3.0) | <0.001 | −6.3 (−12.3 to 0.0) | −6.5 (−13.5 to 1.0) | 0.66 |
| Arterial augmentation index | 105.0 (93.5–110.4) | 97.0 (89.50–105.5) | 0.002 | 98.5 (91.5–106.1) | 99.0 (92.4–107.5) | 0.93 |
| Arterial pulse wave velocity | 6.6 (6.2–7.2) | 6.6 (6.2–7.1) | 0.48 | 6.3 (5.9–6.7) | 6.3 (5.8–6.7) | 0.59 |
Data are presented as mean ± standard deviation, median (interquartile range) or as proportions. Differences in continuous variables between adolescents with or without NAFLD were computed using the independent t test or the Mann-Whitney U test. Differences between categorical variables were determined with the Pearson Chi-square test or Fisher's exact test. p values are for associations between RLP-C and other variables. p values <0.05 are considered statistically significant.
Metabolic syndrome is defined using International Diabetes Federation (IDF) criteria. Abdominal obesity was defined as waist circumference ≥80 cm in females and ≥94 cm in males.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; C-AGPH-HR75, Central Augmentation Pressure/Pulse Height Ratio at Heart Rate 75; DBP, diastolic blood pressure; GGT, gamma-glutamyl transpeptidase; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment for insulin resistance; hsCRP, high sensitivity CRP; LDL-C, low density lipoprotein cholesterol; RLP-C, remnant lipoprotein cholesterol; SAT, subcutaneous adipose thickness; SBP, systolic blood pressure; SST, suprailiac skinfold thickness; VAT, visceral adipose thickness.
Remnant lipoprotein cholesterol
Fasting serum RLP-C levels were positively correlated with general and subcutaneous adiposity, systolic blood pressure, serum GGT, insulin, HOMA-IR, total cholesterol, LDL-C, triglycerides and leptin, but negatively correlated with HDL-C and adiponectin. RLP-C was not significantly associated with visceral adiposity, serum ALT or AST (Table 2).
Table 2.
Correlations between RLP-C, anthropometric, cardiovascular and biochemical variables in the whole cohort.
| Variable | Males |
Females |
||
|---|---|---|---|---|
| r | pvalue | r | pvalue | |
| Weight (kg) | 0.24 | <0.001 | 0.16 | <0.001 |
| Body mass index (kg/m2) | 0.28 | <0.001 | 0.20 | <0.001 |
| Waist (cm) | 0.03 | 0.58 | 0.18 | <0.001 |
| SAT (mm) | 0.25 | <0.001 | 0.12 | 0.008 |
| SST (mm) | 0.26 | <0.001 | 0.19 | <0.001 |
| VAT (mm) | 0.11 | 0.03 | 0.11 | 0.03 |
| SBP (mmHg) | 0.17 | <0.001 | 0.12 | 0.01 |
| DBP (mmHg) | 0.12 | 0.008 | 0.04 | 0.45 |
| Pulse (bpm) | 0.04 | 0.38 | 0.12 | 0.007 |
| ALT (U/L) | 0.16 | <0.001 | 0.04 | 0.35 |
| AST (U/L) | 0.03 | 0.50 | 0.07 | 0.14 |
| GGT (U/L) | 0.22 | <0.001 | 0.20 | <0.001 |
| TG (mmol/L) | 0.99 | <0.001 | 0.99 | <0.001 |
| Total cholesterol (mmol/L) | 0.27 | <0.001 | 0.32 | <0.001 |
| HDL-C (mmol/L) | −0.27 | <0.001 | −0.20 | <0.001 |
| LDL-C (mmol/L) | 0.003 | 0.94 | 0.11 | 0.02 |
| Insulin (mU/L) | 0.33 | <0.001 | 0.30 | <0.001 |
| HOMA-IR | 0.33 | <0.001 | 0.29 | <0.001 |
| Glucose (mmol/L) | 0.08 | 0.07 | 0.02 | 0.70 |
| hsCRP (mg/L) | −0.002 | 0.97 | 0.07 | 0.16 |
| Leptin (μg/L) | 0.26 | <0.001 | 0.26 | <0.001 |
| Adiponectin (mg/L) | −0.12 | 0.008 | −0.18 | <0.001 |
Data are presented as Pearson's correlation coefficients (r). p values are for associations between RLP-C and other variables. p values <0.05 are considered statistically significant.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; GGT, gamma-glutamyl transpeptidase; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment for insulin resistance; hsCRP, high sensitivity CRP; LDL-C, low density lipoprotein cholesterol; r, correlation coefficient; RLP-C, remnant lipoprotein cholesterol; SAT, subcutaneous adipose thickness; SBP, systolic blood pressure; SST, suprailiac skinfold thickness; TG, triglycerides; VAT, visceral adipose thickness.
Relationship between NAFLD, obesity and serum RLP-C
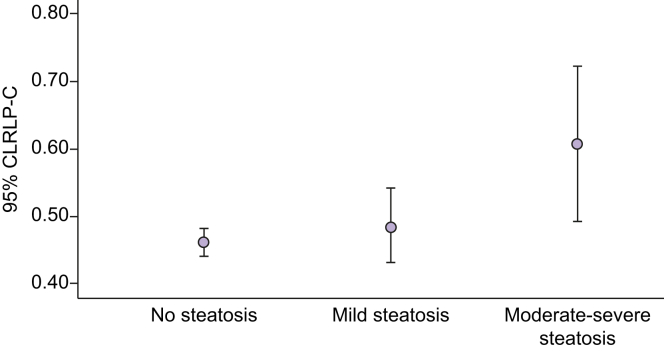
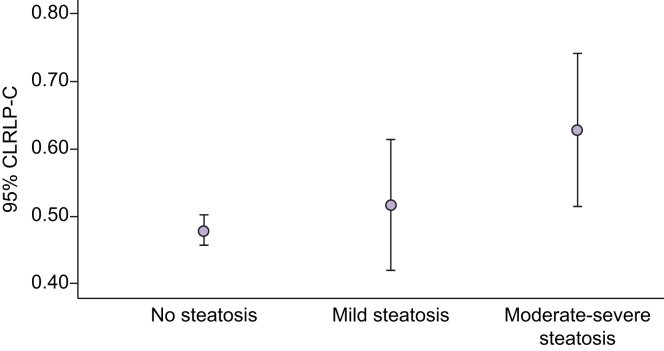
NAFLD was more prevalent in obese adolescents than in nonobese adolescents. Serum RLP-C levels were higher in adolescents with NAFLD compared with those without NAFLD (Table 1). Increasing serum RLP-C levels were associated with increasing severity of hepatic steatosis [mean (SD) RLP-C 0.47 (0.24) mmol/L, 0.50 (0.23) mmol/L and 0.62 (0.31) mmol/L] for absent steatosis, mild steatosis and moderate-severe steatosis, respectively (p <0.001) (Figs 1 and 2).
Fig. 1.
Association between serum RLP-C levels and steatosis severity in female subjects.
RLP-C, remnant lipoprotein cholesterol.
Fig. 2.
Association between serum RLP-C levels and steatosis severity in male subjects.
RLP-C, remnant lipoprotein cholesterol.
Relationship between serum RLP-C and other serum biochemical characteristics
Adolescents with RLP-C in the highest quartile had higher serum GGT, leptin, total cholesterol, LDL-C, triglycerides, fasting insulin, HOMA-IR and hsCRP compared with those in the lowest quartile of RLP-C. Males, but not females, in the highest quartile of RLP-C also had raised serum ALT (Table 3).
Table 3.
Comparison of lowest and highest RLP-C quartiles in adolescents with non-alcoholic fatty liver disease.
| Variable | Non-alcoholic fatty liver disease |
|||||
|---|---|---|---|---|---|---|
| Males |
Females |
|||||
| RLP-C Q1 (n = 33) | RLP-C Q4 (n = 62) | p value | RLP-C Q1 (n = 204) | RLP-C Q4 (n = 187) | p value | |
| Weight (kg) | 81.5 (19.5) | 104.6 (19.0) | 0.002 | 67.7 (13.5) | 76.0 (15.5) | 0.046 |
| BMI (kg/m2) | 24.60 (4.75) | 32.9 (5.1) | <0.001 | 24.1 (4.5) | 27.7 (5.8) | 0.02 |
| Waist (cm) | 94.1 (17.9) | 96.7 (15.8) | 0.69 | 81.7 (11.4) | 88.2 (14.2) | 0.09 |
| SAT (mm) | 21.3 (12.4) | 36.9 (11.1) | 0.001 | 26.2 (12.7) | 34.3 (14.8) | 0.04 |
| SST (mm) | 20.4 (9.9) | 32.8 (6.7) | 0.001 | 24.2 (9.0) | 25.9 (8.6) | 0.51 |
| VAT (mm) | 35.1 (12.8) | 47.9 (20.1) | 0.10 | 31.8 (11.5) | 36.3 (10.2) | 0.22 |
| SBP (mmHg) | 110.85 (9.18) | 129.3 (10.0) | 0.001 | 107.0 (6.2) | 112.0 (12.7) | 0.10 |
| DBP (mmHg) | 57.7 (6.8) | 62.3 (8.1) | 0.11 | 58.9 (5.7) | 59.4 (6.6) | 0.75 |
| Pulse (bpm) | 64.0 (10.2) | 70.1 (12.2) | 0.11 | 63.7 (9.7) | 70.3 (10.9) | 0.02 |
| ALT (U/L) | 29.0 (12.1) | 49.1 (27.7) | 0.03 | 20.4 (20.7) | 21.9 (11.6) | 0.72 |
| AST (U/L) | 27.8 (5.9) | 35.1 (19.9) | 0.27 | 21.0 (7.2) | 21.7 (5.2) | 0.71 |
| GGT (U/L) | 15.2 (6.1) | 29.6 (17.3) | 0.01 | 11.1 (4.8) | 16.9 (8.6) | 0.003 |
| TG (mmol/L) | 0.6 (0.1) | 1.8 (0.6) | <0.001 | 0.6 (0.1) | 1.7 (0.5) | <0.001 |
| Total cholesterol (mmol/L) | 3.1 (0.7) | 4.5 (0.7) | <0.001 | 3.8 (0.8) | 4.8 (0.9) | <0.001 |
| HDL-C (mmol/L) | 1.2 (0.2) | 1.0 (0.2) | 0.02 | 1.4 (0.3) | 1.2 (0.3) | 0.01 |
| LDL-C (mmol/L) | 1.7 (0.6) | 2.6 (0.8) | 0.001 | 2.2 (0.6) | 2.8 (0.8) | 0.004 |
| HOMA-IR | 1.3 (0.8–2.6) | 3.3 (2.2–5.4) | 0.002 | 1.6 (1.2–2.4) | 2.6 (1.6–3.7) | 0.04 |
| Glucose (mmol/L) | 4.9 (0.3) | 5.1 (0.5) | 0.20 | 4.7 (0.4) | 4.6 (0.4) | 0.82 |
| Insulin (mU/L) | 6.1 (3.5–10.9) | 14.0 (10.1–21.6) | 0.003 | 7.7 (6.1–12.0) | 12.5 (8.1–17.7) | 0.02 |
| hsCRP (mg/L) | 0.8 (0.2–1.4) | 1.6 (0.9–4.1) | 0.007 | 0.4 (0.2–2.1) | 1.6 (0.7–5.6) | 0.02 |
| Leptin (μg/L) | 6.1 (3.2–18.0) | 23.2 (11.7–39.3) | 0.001 | 31.4 (24.4–45.7) | 49.2 (35.3–67.3) | 0.01 |
| Adiponectin (mg/L) | 8.2 (2.8) | 6.1 (2.4) | 0.03 | 12.5 (5.8) | 7.8 (3.4) | <0.001 |
| Abdominal obesity (%) | 40.0 | 52.0 | 0.78 | 42.9 | 73.5 | 0.04 |
| Metabolic syndrome (%) | 0.0 | 50.0 | <0.001 | 0.0 | 26.5 | <0.001 |
| C-AGPH-HR75 | −9.4 (10.5) | −1.2 (10.8) | 0.045 | −8.0 (5.9) | −6.1 (9.4) | 0.42 |
| Arterial augmentation index | 97.5 (9.2) | 104.6 (11.2) | 0.08 | 99.8 (7.0) | 98.4 (9.7) | 0.59 |
| Arterial pulse wave velocity | 6.4 (0.6) | 6.8 (0.9) | 0.18 | 6.1 (0.7) | 6.3 (0.6) | 0.36 |
Data are presented as mean (standard deviation), median (interquartile range) or as proportions. Differences in continuous variables between adolescents in the lowest quartile and top quartile of RLP-C were computed using the independent t test or the Mann-Whitney U test. Differences between categorical variables were determined with the Pearson Chi-square test or Fisher's exact test. p values are for comparisons between Q1 and Q4 of RLP-C. p values <0.05 are considered statistically significant.
Metabolic syndrome is defined using International Diabetes Federation (IDF) criteria. Abdominal obesity was defined as waist circumference ≥80 cm in females and ≥94 cm in males.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; C-AGPH-HR75, Central Augmentation Pressure/Pulse Height Ratio at Heart Rate 75; DBP, diastolic blood pressure; GGT, gamma-glutamyl transpeptidase; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment for insulin resistance; hsCRP, high sensitivity CRP; LDL-C, low density lipoprotein cholesterol; RLP-C, remnant lipoprotein cholesterol; SAT, subcutaneous adipose thickness; SBP, systolic blood pressure; SST, suprailiac skinfold thickness; VAT, visceral adipose thickness.
Relationship between serum RLP-C, anthropometric characteristics and cardiovascular measures in adolescents with NAFLD
Among adolescents with NAFLD, those in the highest quartile of RLP-C had higher rates of central obesity, greater prevalence of metabolic syndrome, as well as higher general and subcutaneous adiposity. Males in the highest quartile of RLP-C also had higher systolic blood pressure and C-AGPH-HR75 (Table 3).
Prediction of NAFLD
Using multivariable logistic regression analysis, serum RLP-C levels in the lowest quartile were associated with reduced risk of NAFLD (Table 4). In males, serum RLP-C levels in the lowest quartile were associated with 85% reduced odds of being found to have NAFLD, whereas higher waist circumference, serum ALT and leptin were associated with a NAFLD diagnosis after adjusting for serum adiponectin, HOMA-IR and hsCRP. By contrast, in females, serum RLP-C levels in the lowest quartile were associated with 55% reduced odds of being found to have NAFLD, whereas higher waist circumference and serum leptin and lower adiponectin were associated with NAFLD after adjusting for HOMA-IR and serum hsCRP (Table 4). These associations remained significant after adjusting for serum triglycerides, HDL-C and LDL-C.
Table 4.
Prediction models for non-alcoholic fatty liver disease.
| Characteristics | Multivariable analysis in males (OR, 95% CI) | p value | Multivariable analysis in females (OR, 95% CI) | p value |
|---|---|---|---|---|
| Waist circumference (cm) | 1.19 (1.13–1.25) | <0.001 | 1.04 (1.01–1.07) | <0.001 |
| RLP-C (mmol/L) | ||||
| Q1 | 0.15 (0.03–0.72) | 0.12 | 0.44 (0.20–0.99) | 0.18 |
| Q2 | 0.49 (0.14–1.77) | 0.02 | 0.61 (0.29–1.31) | 0.048 |
| Q3 | 0.36 (0.10–1.37) | 0.28 | 0.86 (0.42–1.76) | 0.21 |
| Q4 | Reference | 0.14 | Reference | 0.69 |
| ALT (U/L) | 1.05 (1.01–1.08) | 0.005 | ||
| Leptin (μg/L) | 1.14 (1.08–1.19) | <0.001 | 1.02 (1.01–1.03) | 0.007 |
| Adiponectin (mg/L) | 0.94 (0.888–0.996) | 0.04 |
Data are presented as odds ratios and 95% confidence intervals (CIs). Multivariable logistic regression models were used to calculate the odds of NAFLD from adolescent physical assessment and biochemistry that were statistically significant in univariate analysis, adjusted for homeostasis model assessment for insulin resistance (HOMA-IR), adiponectin and high sensitivity CRP (hsCRP) in each sex. p values <0.05 are considered statistically significant.
ALT, alanine aminotransferase; OR, Odds ratio; Q1, lowest (first) quartile; Q2, second quartile; Q3, third quartile; Q4, top (fourth) quartile; RLP-C, remnant lipoprotein cholesterol.
Discussion
In this cohort study of well-characterised population-based adolescents, there was a 15% prevalence of NAFLD. NAFLD was associated with fasting serum RLP-C levels after adjusting for traditional risk factors of adiposity and insulin resistance. Furthermore, the severity of hepatic steatosis increased with increasing serum RLP-C, providing a potential further link between adolescent NAFLD and atherogenic dyslipidaemia, metabolic syndrome, general and subcutaneous adiposity, hyperleptinaemia and insulin resistance.
In adolescents with NAFLD, serum RLP-C levels were positively associated with serum ALT, GGT, hsCRP, and cardiometabolic risk factors, including fasting insulin, HOMA-IR, subcutaneous and visceral adiposity, systolic blood pressure, pulse rate and measures of arterial stiffness. There was a sex-related association between NAFLD and cardiometabolic risk factors. Male adolescents with NAFLD or with the highest quartile of RLP-C also had increased visceral adiposity, systolic blood pressure and features of arterial stiffness. This might imply a higher future risk of CVD in males compared with females with NAFLD, as previously described using the Framingham risk score.25 Although waist circumference, serum RLP-C and leptin levels were associated with NAFLD in both sexes, serum ALT was also associated with NAFLD only in male adolescents, whereas adiponectin was associated with NAFLD only in female adolescents.
A recent study showed an association between higher circulating RLP-C, severity of hepatic steatosis and prevalence of CVD and cerebrovascular disease in adults with NAFLD.18 Furthermore, other studies reported an increasing prevalence and burden of NAFLD in global populations, mirroring the increasing rates of the metabolic syndrome.6,11 Several population studies, systematic reviews and meta-analyses have identified CVD as the leading cause of morbidity and mortality in adults with NAFLD.[26], [27], [28], [29] Notably, NAFLD is associated with endothelial dysfunction, increased carotid intima thickness, arterial stiffness and left ventricular mass, and left ventricular diastolic dysfunction.30 By contrast, a population-based study using primary care databases from three European countries recently reported no significant increase in CVD related to fatty liver.31 However, the case definition of NAFLD was not clearly stated, NAFLD prevalence was extraordinarily low, the duration of follow-up was relatively short and the study population included adolescents and young adults who would normally be expected to be at low risk for cardiovascular outcomes. A possible role for RLP-C in predicting risk of cardiovascular events in adults treated with statins or with T2DM and chronic kidney disease has been described in Japanese studies.32,33 Consequently, our findings could contribute to the identification of adolescents at future risk of CVD who could benefit from cardiometabolic risk reduction.
The strengths of our study include the large population size, from a well-characterised cohort of adolescents with ultrasound assessment of NAFLD and with detailed metabolic risk factor assessment.5 A limitation of the study is the use of ultrasound, rather than histology or MRI to diagnose NAFLD. However, ultrasound is the preferred initial non-invasive test for NAFLD supported by the American Association for the Study of Liver Disease and the European Association for the Study of the Liver.2,34 In addition, liver biopsy in healthy, population-based adolescents could not be justified.
Conclusion
In conclusion, RLP-C was associated with NAFLD after adjusting for adiposity and insulin resistance. Raised serum RLP-C levels were associated with increased risk of the metabolic syndrome, severity of hepatic steatosis and increased adiposity in adolescents. However, those with NAFLD and lower RLP-C levels appeared to have reduced severity of cardiometabolic risk factors. Consequently, the demonstration of an association between RLP-C and cardiovascular risk in individuals with NAFLD could aid in the identification of those who might benefit from targeted risk factor assessment and management before the development of adverse cardiovascular outcomes.
Financial support
The 17-year follow-up of the Raine Study was funded by a National Health and Medical Research Council of Australia (NHMRC) Program grant 353514 and Project grant 403981. O.T.A. is supported by a Western Australian Department of Health/Raine Medical Research Foundation Clinician Research Fellowship. T.A.M. is supported by a NHMRC Fellowship (grant number 1136046). R-C.H. is supported by a NHMRC Fellowship (grant number 1053384).
Authors' contributions
J.C., study design, manuscript preparation; T.A.M., L.A.A., L.J.B. and J.K.O., data acquisition and manuscript review; R-C.H., manuscript review; O.T.A., study design, data acquisition, data analysis and manuscript preparation.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We thank the Raine Study participants and their families. We also thank the Raine Study team for cohort coordination and data collection. The National Health and Medical Research Council (NHMRC) is thanked for its long-term contribution to funding the Raine Study since its inception. The Raine Medical Research Foundation; The University of Western Australia (UWA); Curtin University; Telethon Kids Institute; UWA Faculty of Medicine, Dentistry and Health Sciences; Womens and Infants Research Foundation; Edith Cowan University; Murdoch University and The University of Notre Dame Australia are acknowledged for their support and funding of the core management of the Raine Study. We also thank Derrick Van Rooyen for illustrating the graphical abstract.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100150.
Supplementary data
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 3.Farrell G.C., Larter C.Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology. 2006;43(2 Suppl 1):S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 5.Ayonrinde O.T., Olynyk J.K., Beilin L.J., Mori T.A., Pennell C.E., de Klerk N. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology. 2011;53:800–809. doi: 10.1002/hep.24097. [DOI] [PubMed] [Google Scholar]
- 6.Younossi Z.M. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 7.Schwimmer J.B., Deutsch R., Kahen T., Lavine J.E., Stanley C., Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 8.Lawlor D.A., Callaway M., Macdonald-Wallis C., Anderson E., Fraser A., Howe L.D. Nonalcoholic fatty liver disease, liver fibrosis, and cardiometabolic risk factors in adolescence: a cross-sectional study of 1874 general population adolescents. J Clin Endocrinol Metab. 2014;99:E410–E417. doi: 10.1210/jc.2013-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song P., Yu J., Wang M., Chang X., Wang J., An L. Prevalence and correlates of suspected nonalcoholic fatty liver disease in Chinese children. Int J Environ Res Public Health. 2017;14:465. doi: 10.3390/ijerph14050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doycheva I., Watt K.D., Alkhouri N. Nonalcoholic fatty liver disease in adolescents and young adults: the next frontier in the epidemic. Hepatology. 2017;65:2100–2109. doi: 10.1002/hep.29068. [DOI] [PubMed] [Google Scholar]
- 11.Welsh J.A., Karpen S., Vos M.B. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr. 2013;162:496–500. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Australian Institute of Health and Welfare . AIHW; Canberra: 2018. Australia's Health 2018. [Google Scholar]
- 13.WHO . WHO; Geneva: 2018. Global Health Estimates 2016: Disease Burden by Cause, Age, Sex, by Country and by Region, 2000-2016. [Google Scholar]
- 14.Wong C.R., Lim J.K. The association between nonalcoholic fatty liver disease and cardiovascular disease outcomes. Clin Liver Dis (Hoboken) 2018;12:39–44. doi: 10.1002/cld.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alkhouri N., Carter-Kent C., Elias M., Feldstein A.E. Atherogenic dyslipidemia and cardiovascular risk in children with nonalcoholic fatty liver disease. Clin Lipidol. 2011;6:305–314. doi: 10.2217/clp.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen A.M., Therneau T.M., Larson J.J., Coward A., Somers V.K., Kamath P.S. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67:1726–1736. doi: 10.1002/hep.29546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joshi P.H., Khokhar A.A., Massaro J.M., Lirette S.T., Griswold M.E., Martin S.S. Remnant lipoprotein cholesterol and incident coronary heart disease: the Jackson heart and Framingham offspring cohort studies. J Am Heart Assoc. 2016;5:e002765. doi: 10.1161/JAHA.115.002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pastori D., Baratta F., Novo M., Cocomello N., Violi F., Angelico F. Remnant lipoprotein cholesterol and cardiovascular and cerebrovascular events in patients with non-alcoholic fatty liver disease. J Clin Med. 2018;7:378. doi: 10.3390/jcm7110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang R.C., Beilin L.J., Ayonrinde O., Mori T.A., Olynyk J.K., Burrows S. Importance of cardiometabolic risk factors in the association between nonalcoholic fatty liver disease and arterial stiffness in adolescents. Hepatology. 2013;58:1306–1314. doi: 10.1002/hep.26495. [DOI] [PubMed] [Google Scholar]
- 20.Hartley A., Santos Ferreira D.L., Anderson E.L., Lawlor D.A. Metabolic profiling of adolescent non-alcoholic fatty liver disease. Wellcome Open Res. 2018;3:166. doi: 10.12688/wellcomeopenres.14974.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newnham J.P., Evans S.F., Michael C.A., Stanley F.J., Landau L.I. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet. 1993;342:887–891. doi: 10.1016/0140-6736(93)91944-h. [DOI] [PubMed] [Google Scholar]
- 22.Straker L., Mountain J., Jacques A., White S., Smith A., Landau L. Cohort profile: the Western Australian pregnancy cohort (raine) study-generation 2. Int J Epidemiol. 2017;46:1384–1385j. doi: 10.1093/ije/dyw308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome--a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 24.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 25.Motamed N., Rabiee B., Poustchi H., Dehestani B., Hemasi G.R., Khonsari M.R. Non-alcoholic fatty liver disease (NAFLD) and 10-year risk of cardiovascular diseases. Clin Res Hepatol Gastroenterol. 2017;41:31–38. doi: 10.1016/j.clinre.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Targher G., Day C.P., Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341–1350. doi: 10.1056/NEJMra0912063. [DOI] [PubMed] [Google Scholar]
- 27.Assy N., Djibre A., Farah R., Grosovski M., Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology. 2010;254:393–400. doi: 10.1148/radiol.09090769. [DOI] [PubMed] [Google Scholar]
- 28.Wu S., Wu F., Ding Y., Hou J., Bi J., Zhang Z. Association of non-alcoholic fatty liver disease with major adverse cardiovascular events: a systematic review and meta-analysis. Sci Rep. 2016;6:33386. doi: 10.1038/srep33386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams L.A., Anstee Q.M., Tilg H., Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 30.Francque S.M., van der Graaff D., Kwanten W.J. Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J Hepatol. 2016;65:425–443. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Alexander M., Loomis A.K., van der Lei J., Duarte-Salles T., Prieto-Alhambra D., Ansell D. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. BMJ. 2019;367:l5367. doi: 10.1136/bmj.l5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura T., Obata J.E., Hirano M., Kitta Y., Fujioka D., Saito Y. Predictive value of remnant lipoprotein for cardiovascular events in patients with coronary artery disease after achievement of LDL-cholesterol goals. Atherosclerosis. 2011;218:163–167. doi: 10.1016/j.atherosclerosis.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen S.V., Nakamura T., Uematsu M., Fujioka D., Watanabe K., Watanabe Y. Remnant lipoproteinemia predicts cardiovascular events in patients with type 2 diabetes and chronic kidney disease. J Cardiol. 2017;69:529–535. doi: 10.1016/j.jjcc.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 34.European association for the study of the liver (EASL); European association for the study of diabetes (EASD); European association for the study of obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.