Summary
Increased consumption of fats and added sugars has been associated with an increase in metabolic syndromes. Here we show that mice chronically fed an energy-rich diet (ERD) with high fat and moderate sucrose have enhanced the absorption of a gastrointestinal fructose load, and this required expression of the arrestin domain protein Txnip in the intestinal epithelial cells. ERD feeding induced gene and protein expression of Glut5, and this required the expression of Txnip. Furthermore, Txnip interacted with Rab11a, a small GTPase that facilitates the apical localization of Glut5. We also demonstrate that ERD promoted Txnip/Glut5 complexes in the apical intestinal epithelial cell. Our findings demonstrate that ERD facilitates fructose absorption through a Txnip-dependent mechanism in the intestinal epithelial cell, suggesting that increased fructose absorption could potentially provide a mechanism for worsening of metabolic syndromes in the setting of a chronic ERD.
Subject Areas: Human Metabolism, Molecular Biology
Graphical Abstract
Highlights
-
•
Consumption of energy-rich diets (ERDs) promotes intestinal fructose absorption
-
•
Intestinal Txnip is required for increased fructose absorption by ERD
-
•
Txnip increases fructose transporter Glut5 protein and gene expression
-
•
Txnip forms a complex with Rab11a to facilitate the apical localization of Glut5
Human Metabolism; Molecular Biology
Introduction
The consumption of energy-dense diets or the “western diet” has coincided with an increase in metabolic diseases including obesity and type 2 diabetes (Chatterjee et al., 2017). An increase in “added sugars,” particularly in liquid forms such as in sugar-sweetened beverages, has also been associated with an increase in metabolic diseases (Bray et al., 2004; Khan and Sievenpiper, 2016; Schwarz et al., 2017). Sucrose, or table sugar, is composed of one molecule of fructose and one molecule of glucose. Although they are isomers, fructose metabolism differs from glucose metabolism as fructose can readily be diverted to the liver into a de novo lipogenesis pathway (Lyssiotis and Cantley, 2013). Recent evidence suggests that fructose consumption at low doses may simply be providing more glucose, as intestinal cells can metabolize low doses of fructose into glucose (Jang et al., 2018). However, at higher doses, fructose may be absorbed rapidly into the liver, bypassing key regulatory steps in glycolysis, and thereby stimulating hepatic fat synthesis (Jang et al., 2018).
Txnip, a member of arrestin domain-containing protein family (Patwari and Lee, 2012), is a multifunctional intracellular protein that coordinates signaling pathways, including oxidative stress, endoplasmic reticulum stress, apoptosis, DNA damage, and inflammation (Spindel et al., 2011). The function of Txnip has been defined through in vivo studies as a regulator of carbohydrate metabolism (Chutkow et al., 2008; Parikh et al., 2007; Waldhart et al., 2017). Txnip regulates glucose metabolism (Parikh et al., 2007) by binding to glucose transporters, Glut1 and Glut4 (Waldhart et al., 2017; Wu et al., 2013). Txnip can also regulate fructose metabolism in the setting of severe streptozotocin-induced diabetes (Dotimas et al., 2016), possibly through binding to fructose transporters, Glut5 and Glut2, in intestinal epithelial cells. Previous epidemiological studies have demonstrated the association of fructose consumption with type 2 diabetes (Malik et al., 2010; Tappy and Le, 2010). Thus, to explore the relationship of an energy-rich diet (ERD) and fructose metabolism, we studied acute fructose absorption in mice fed with an ERD.
Results and Discussion
Energy-Rich Diet Promotes Fructose Absorption and Elevates Txnip Expression
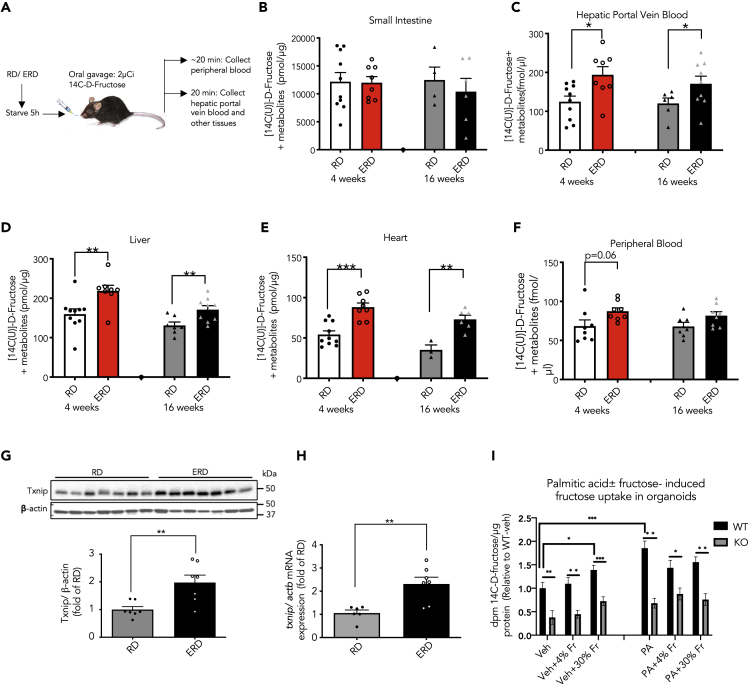
As hyperglycemia increases Txnip expression significantly (Dotimas et al., 2016), we studied normoglycemic mice fed with an ERD, generally called a high-fat diet (HFD); this diet includes 7% calories as sucrose or 3.5% calories as fructose. C57Bl/6J male mice at ages 7–9 weeks were placed on RD (regular diet) or ERD for 4 or 16 weeks and their metabolic profiles (Table S1) were obtained. As seen in Tables S1 and S2, the fasting blood glucose levels did not change with ERD feeding, suggesting that fasting blood glucose levels did not influence the differences in fructose absorption in our study. As outlined in the experimental procedures (Figure 1A), the mice were then administered 2 μCi of radiolabeled [14C-(U)]-D fructose dissolved in 1:1 fructose mannitol solution using intragastric gavage to examine intestinal fructose absorption. Although fructose is generally consumed together with glucose, our data indicate that ERD increases acute fructose absorption in the presence of either glucose or mannitol in the gavage solution (Figure S1A). We assayed tissues at 20 min after gavage as this is the peak time for fructose absorption in normal and diabetic mice (Dotimas et al., 2016). There was no difference in the isotope retained in the small intestine, which includes both lumenal and absorbed fructose (Figure 1B); but interestingly, in both the short- (4 weeks) and long-term (16 weeks) ERD-fed mice, the amount of absorbed isotope from gastrointestinal fructose was significantly increased in the portal vein, liver, and heart, as depicted in Figures 1C–1E. Furthermore, a trend for increased 14C-fructose + metabolites (hereafter termed only as 14C-fructose) level in the peripheral blood of ERD mice versus RD mice was also observed, consistent with the known first-pass effect of fructose by the liver (Lyssiotis and Cantley, 2013) (Figure 1F). Our acute bolus fructose dose of approximately 1.0 g/kg was on the same order as most of the experiments performed by Jang et al. (Jang et al., 2018) in their study. We did not measure the amount of fructose transmitted to the colon in our study due to the early times of sacrifice (20 min versus 60–120 min in the experiments by Jang et al., 2018) required to access the portal vein, but it is possible that it would differ in the setting of ERD or added fructose to drinking water. Collectively, these data indicate that ERD feeding increased fructose absorption from small intestine via the hepatic portal vein.
Figure 1.
Energy-Rich Diet Promotes Fructose Absorption and Elevates Txnip Expression
(A) Schematic representation of the experimental procedure.
(B–F) Fructose absorption (i.e., 14C-fructose + metabolites) by various tissues from 4 weeks (n = 7–8) and 16 weeks (n = 3–8) RD/ERD diet-fed mice after the intragastric oral gavage of 14C-fructose. Values are shown as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, or ∗∗∗p < 0.001 as calculated by unpaired t test.
(G) A representative western blot and quantitative analysis of Txnip (molecular weight: 50kDa) and β-actin (loading control, molecular weight: 42kDa) in the jejunal lysates of RD/ERD-fed mice (n = 7 mice/diet).Values are shown as mean ± SEM. ∗∗p < 0.01as calculated by unpaired t test.
(H) Gene expression of Txnip normalized to actb, house-keeping gene, in the jejunal samples from RD/ERD-fed mice (n = 6–7 mice/diet). Values are shown as mean ± SEM. ∗∗p < 0.01 as calculated by unpaired t test.
(I) Intestinal uptake of fructose was performed in the intestinal organoids extracted from Txnip wild-type (WT) and knockout (KO) mice. Both palmitic acid (PA) and 30% fructose (veh+30% Fr) significantly increased fructose uptake in WT organoids when compared with WT-veh. However, the deletion of Txnip significantly reduced both fructose-induced and PA-induced fructose uptake. There was no significant increase in the PA-induced fructose uptake by Txnip WT organoids after the addition of either 4% Fr or 30% Fr (n = 7–8 wells).
Values are shown as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, or ∗∗∗p < 0.001 as calculated by unpaired t test. WT, wild-type; KO, knockout; veh, vehicle/BSA; PA, palmitic acid; and Fr, fructose.
See also Tables S1 and S2 and Figures S1 and S2.
Consistent with increased hepatic Txnip protein expression previously reported in HFD/ERD-fed mice (Shao et al., 2012), the jejunum from the ERD-fed mice had a significant increase in Txnip protein (Figure 1G and 2.0 ± 0.3-fold, p < 0.01) and Txnip gene (Figure 1H and 2.3 ± 0.3-fold, p < 0.01) expressions when compared with the RD-fed mice. These data show that ERD feeding promotes fructose absorption and Txnip expression in the jejunum. To determine if the absorbed gastrointestinal fructose load remained in the form of fructose in the intestinal epithelial cell, a mass spectrometry-based analysis was performed using [U-13C]-fructose using an identical protocol as that described in the previous paragraph for experiments with [14C-(U)]-D fructose. The intestine contained primarily 13C-labeled fructose and a small amount of isotope-labeled glucose metabolite (labeled fructose 391.89 ± 37.60 nmol/mg tissue versus 0.48 ± 0.33 nmol/mg tissue, n = 10 mice, ∗∗∗p < 0.001 versus labeled glucose), consistent with recently published results with high fructose influx (Jang et al., 2018). Of note, when compared with RD-fed mice, ERD-fed villin cre mice showed a trend of increased 13C-fructose-1-phosphate in the jejunum (Figure S1B).
We also determined the impact of chronic fructose consumption on fructose absorption. For this, we conducted a 4-week-long study wherein we placed C57BL/6J mice on an RD or ERD with and without 30% fructose solution in drinking water, as described previously (Softic et al., 2017). Mice on RD and ad libitum access to water were used as the control group. As illustrated in Figure S2C, there was an increase in acute fructose uptake with chronic consumption of fructose in drinking water, irrespective of the diet consumed. In addition, fructose in drinking water accelerated the time to peak fructose absorption from 20 min to 10 min, although the differences between groups were qualitatively similar. We speculate that there could be an evolutionary advantage as to why ERD feeding promotes fructose absorption. ERD may lead to an inflammatory state, and in this setting, adaptation to harness more calories could be beneficial. This concept is similar to a theory of insulin resistance suggested for HFD and type 2 diabetes (Soeters and Soeters, 2012).
To further explore our in vivo finding, we extracted intestinal organoids from Txnip wild-type (WT) and knockout (KO) mice to assess fructose absorption according to a protocol described previously (Zietek et al., 2015). As illustrated in Figure 1I, we subjected the intestinal organoids to an “energy-rich environment” using 50 μM palmitic acid (PA) or BSA (vehicle) as previously reported for the intestinal organoids (Beyaz et al., 2016), as well as added either 4% (approximating the percentage of fructose in the ResearchDiets Inc. High Fat Diet, D12492) or 30% fructose to the solution for 3 h. Interestingly, as shown in Figure 1I, there was a significant increase in fructose uptake by the PA-treated WT organoids (p < 0.001 versus WT-vehicle) and 30% fructose-treated WT organoids (p < 0.05 versus WT-vehicle). However, there was no significant difference between PA-treated groups after the addition of 4% fructose (p = 0.08 versus PA alone) or 30% fructose (p = 0.1 versus PA alone). We also found that exogenous fructose alone significantly increased fructose uptake in the WT versus Txnip KO organoids (p < 0.001), indicating that Txnip is crucial for fructose-induced acute fructose absorption by organoids. Overall, when compared with ERD, the data from Figure S2 suggest that fructose in drinking water has a larger impact on fructose absorption than ERD. We have previously demonstrated that fructose in drinking water promoted fructose absorption in intestinal epithelial cells, and for this, Txnip was necessary (Dotimas et al., 2016). Thus, in the current study, we sought to explore the effect of ERD on fructose absorption.
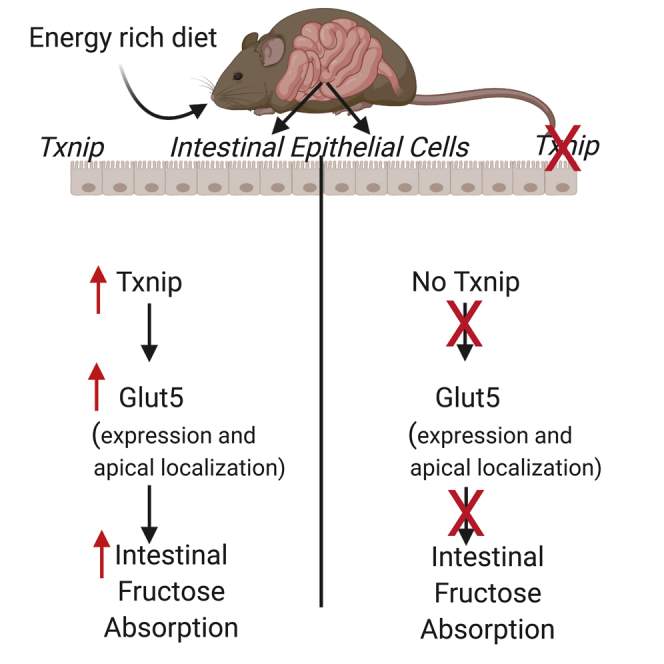
Deletion of Txnip in the Intestinal Epithelial Cells Mitigates ERD-Induced Fructose Absorption
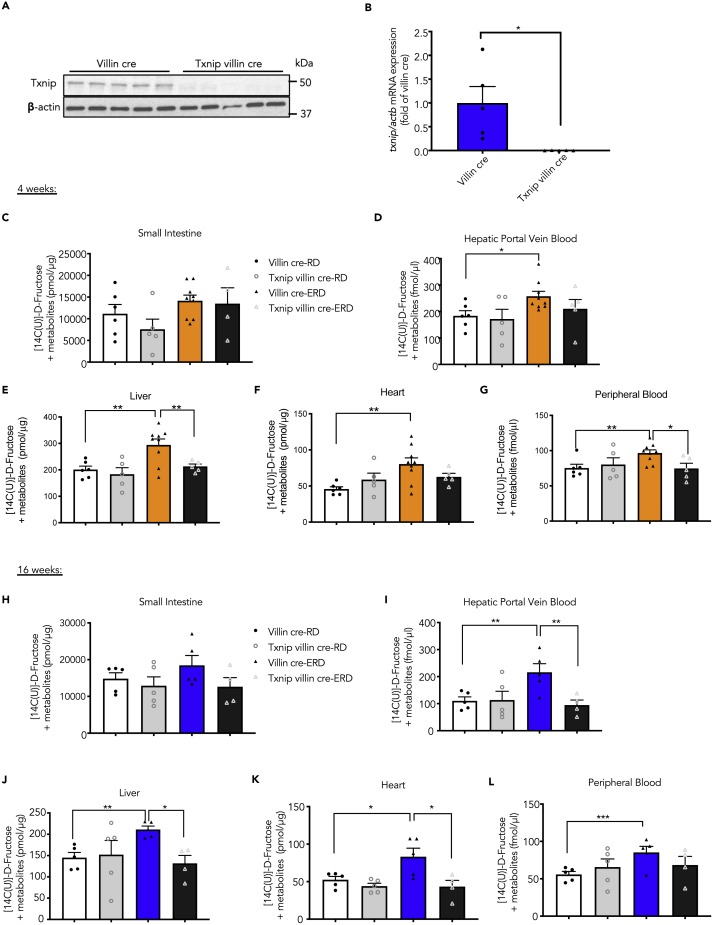
Next, we explored in vivo if Txnip is an essential component of ERD-induced fructose absorption. As a majority of the fructose is absorbed by the proximal part of the small intestine via intestinal epithelial cells (Bray et al., 2004), we studied mice with genetic deletion of Txnip in intestinal epithelial cells by crossing Txnipflox/flox (Txnipfl/fl) mice with villin cre mice. We confirmed Txnip deletion in the villi of Txnipfl/fl villin cre+/- (hereafter called Txnip villin cre) mice when compared with the villi of villin cre-controls (Txnip+/+ villin cre+/-, hereafter called villin cre) by both western analysis and qRT-PCR (Figures 2A and 2B). Moreover, unlike systemic Txnip-KO mice (Chutkow et al., 2008), the metabolic profiles displayed by the Txnip villin cre mice were similar to those of the villin cre mice (Tables S1 and S2), implying that the experimental outcomes were due to the intestinal epithelium deletion of Txnip and not due to the global metabolic effects of Txnip deletion. Also, note that insulin levels were increased by the ERD as anticipated (Table 1), and this could influence transport of isotope from fructose into the liver and heart. Interestingly, from the metabolic studies performed, the only differences noted between the ERD-fed Txnip villin cre mice and villin-cre controls on the same diet were in their total food consumption (1.6 ± 0.2-fold versus ERD-fed villin cre, n = 4, p < 0.05) and energy expenditure (ERD-fed Txnip villin cre versus ERD-fed villin cre (kcal/h/kg): 22.5 ± 0.3 versus 21.3 ± 0.3, n = 4, p < 0.05), which were higher in the ERD-fed Txnip villin cre mice (Table 1).
Figure 2.
Deletion of Txnip in the Intestinal Epithelial Cells Mitigates ERD-Induced Fructose Absorption
(A) A representative western blot demonstrating successful abolition of Txnip in the villi of Txnip villin cre mice (n = 5).
(B) Gene expression of Txnip normalized to actb in villi from villin cre and Txnip villin cre mice (n = 5). Values are shown as mean ± SEM. ∗p < 0.05 as calculated by unpaired t test.
(C–L) (C–G) Fructose absorption (i.e., 14C-fructose + metabolites) by various tissues from 4 weeks (n = 4–8) and (H–L) 16 weeks (n = 4–5) in RD/ERD-fed mice after the oral gavage of 14C-fructose.
Values are shown as mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, or ∗∗∗p < 0.001 as calculated by unpaired t test.
See also Table S2.
Table 1.
Metabolic Outcomes from Villin Cre and Txnip Villin Cre Mice
| Metabolic Parameters | Villin Cre |
Txnip Villin Cre |
||
|---|---|---|---|---|
| RD (n = 4) | ERD (n = 4) | RD (n = 4) | ERD (n = 4) | |
| Fat mass (g) | 2.9 ± 0.5 | 19.5 ± 0.5 | 2.4 ± 0.4 | 17.3 ± 1.9 |
| Lean mass (g) | 26.0 ± 1.3 | 27.2 ± 0.9 | 24.9 ± 1.4 | 25.8 ± 1.4 |
| Body weight (g) | 30.4 ± 1.4 | 47.7 ± 0.5 | 28.7 ± 1.3 | 43.8 ± 3.2 |
| AUC (ipGTT) | 17,132 ± 918 | 24,707 ± 1,858 | 17,070 ± 2,193 | 26,256 ± 2,919 |
| 24-h food intake(kcal) | 12.5 ± 0.6 | 5.2 ± 0.5 | 11.8 ± 0.6 | 8.3 ± 1.0∗ |
| 24-h water intake (mL) | 3.4 ± 0.2 | 0.8 ± 0.2 | 3.2 ± 0.3 | 1.5 ± 0.2∗ |
| VO2 consumption rate (mL/h/kg) | 4,110 ± 74 | 4,466 ± 69 | 4,050 ± 70 | 4,700 ± 71∗ |
| VCO2 production rate (mL/h/kg) | 3,673 ± 62 | 3,347 ± 56 | 3,661 ± 68 | 3,599 ± 58∗∗ |
| Energy expenditure rate (kcal/h/kg) | 20.3 ± 0.4 | 21.3 ± 0.3 | 20.0 ± 0.3 | 22.5 ± 0.3∗ |
| Respiratory exchange ratio | 0.9 ± 0.0 | 0.7 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.0∗∗ |
| Total physical activity (counts/h) | 6,958 ± 475 | 2,722 ± 360 | 4,653 ± 337∗∗∗ | 3,245 ± 188 |
| Liver triglycerides (μmol/g) | 12.8 ± 1.7 | 18.3 ± 4.6 | 11.8 ± 2.4 | 22.1 ± 6.0 |
| Plasma cholesterol (mg/dL) | 81 ± 2.0 | 210 ± 15.0 | 78 ± 3.0 | 198 ± 14.0 |
| HDL (mg/dL) | 71 ± 2.0 | 172 ± 12.0 | 68 ± 3.0 | 155 ± 4.0 |
| LDL (mg/dL) | 74 ± 3.0 | 172 ± 6.0 | 68 ± 4.0 | 170 ± 17.0 |
| Insulin (ng/mL) | 0.56 ± 0.2 (n = 5) | 2.2 ± 0.5# (n = 4) | 0.56 ± 0.1 (n = 5) | 2.7 ± 0.5## (n = 4) |
AUC, area under the curve; HDL, high-density lipoprotein; ipGTT, intraperitoneal glucose tolerance test; LDL, low-density lipoprotein;
Results are expressed as mean ± SEM (n=4 mice/ group).
p values were calculated using an unpaired Student’s t test. ∗p<0.05, ∗∗p<0.01, and ∗∗∗p<0.001 versus villin cre with the same diet; # p<0.05, ##p<0.01 versus RD-fed mice from the same strain.
We then randomized a second cohort of 7- to 9-week-old Txnip villin cre and villin cre mice to RD versus ERD for short-term (4 weeks, Figures 2C–2G) or long-term (16 weeks, Figures 2H–2L) experiments and assessed fructose absorption. By 4 weeks of ERD feeding, consistent with aforementioned data on RD/ERD-fed C57Bl/6J mice, we found a significant increase in fructose absorption into the hepatic portal vein, liver, heart, and peripheral blood in the villin cre mice (Figures 2D–2G). In contrast, we did not observe increased uptake of the isotope in the livers of the ERD-fed Txnip villin cre mice, where Txnip was absent in the intestinal epithelium. The impact of deletion of Txnip in intestinal epithelial cells was even more pronounced in the Txnip villin cre mice that were placed on ERD for 16 weeks when compared with villin cre mice placed on the same diet, with significant reductions in isotope uptake in the hepatic portal vein, liver, and heart (Figures 2I–2L). These findings were supported by the fructose uptake experiments performed on intestinal organoids (Figure 1I), wherein we showed that deletion of Txnip significantly mitigated fructose absorption (p < 0.01 for WT-vehicle versus KO-vehicle). Moreover, despite the presence of PA, Txnip KO organoids failed to show enhanced fructose uptake (p < 0.001 versus Txnip WT-PA). The data are consistent with the hypothesis that Txnip is required for a chronic ERD to promote intestinal fructose absorption. Collectively, although basal level of Txnip is adequate to drive physiological/normal fructose absorption, an increased Txnip expression stimulated by ERD is necessary for ERD-induced fructose absorption.
Txnip Is Required for the Energy-Rich Diet-Induced Glut5 Expression
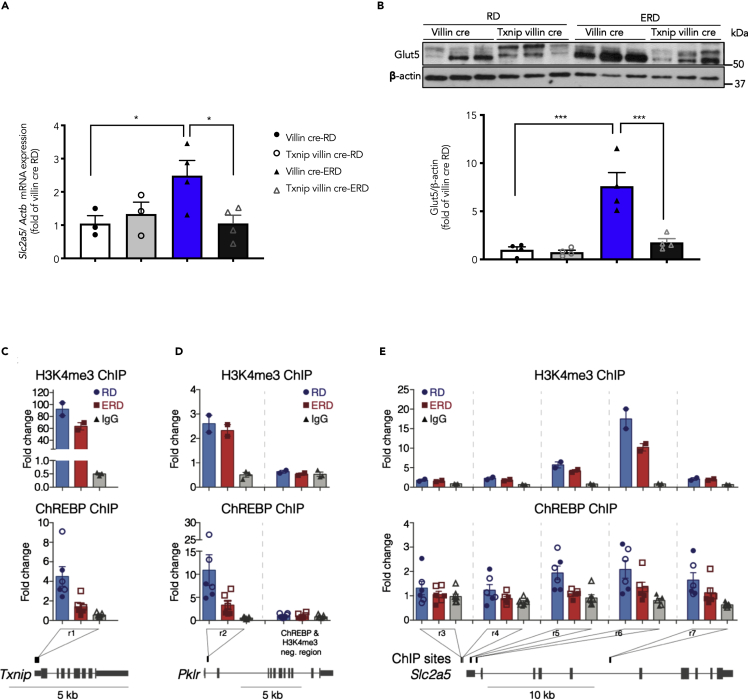
We next explored the mechanism for increased fructose absorption by Txnip under an ERD. Although a reciprocal association between Txnip and tissue responsiveness to nitric oxide has been reported before (Sverdlov et al., 2013), we examined the changes in NO levels in intestinal lysates obtained from villin cre and Txnip villin cre mice on RD/ERD to investigate whether NO may be responsible for the difference observed in fructose absorption and thus serve as a potential mechanism. As seen in Figure S3, the presence or absence of Txnip in the intestinal mucosal lysates did not appear to have a substantial role in regulating parameters of nitric oxide effect. Next, we assessed the expression of Glut2 and Glut5, the fructose transporters in the intestine, in the intestinal mucosa from these mice. We performed gene expression of Glut2 (Slc2a2) in the jejunal tissues, and there were no significant differences in the gene expression of Glut2 with diets and/or deletion of Txnip in epithelial cells (n = 4 animals per group). Interestingly, we observed a significant increase in the Slc2a5 gene (2.5 ± 0.5-fold versus villin cre RD, n = 3–4, p < 0.05) and Glut5 protein (7.6 ± 1.4-fold versus villin cre RD, n = 4, p < 0.001, double bands) (Figures 3A and 3B) with ERD feeding. Deletion of Txnip in the intestinal epithelium dramatically reduced Glut5 gene and protein expression under ERD feeding (Figures 3A and 3B). These findings reveal that Txnip is essential for ERD-induced Glut5 expression in the intestine.
Figure 3.
Txnip Is Required for the Energy-Rich Diet-Induced Glut5 Expression
Villin cre and Txnip villin cre mice were placed on RD/ERD for 16 weeks.
(A) Gene expression of Slc2a5 normalized to actb in mucosal lysates extracted from jejuna (n = 3–4/group).
(B) A representative western blot (top) with quantitation (bottom) from the mucosal lysates obtained from jejuna (n = 4/group) showing the Glut5 protein levels. Values are shown as mean ± SEM. ∗p < 0.05 or ∗∗∗p < 0.001 as calculated by unpaired t test.
(C and D) ChIP for ChREBP and H3K4me3 on intestinal tissues from mice on an RD or an ERD, followed by quantitative PCR to assay enrichment at Txnip, Pklr, and Slc2a5 genomic loci. H3K4me3 enrichment shown in the top panel (one of two independent biological replicates shown). Error bars represent the mean ± SEM of n = 2 ChIPs for mice on RD and ERD and n = 3 IgG ChIPs. Positive control regions for ChREBP at the (C) Txnip promoter (r1) (Poungvarin et al., 2015) and (D) pyruvate kinase L/R (Pklr) promoter (r2) (Kim et al., 2017) along with a previously reported ChREBP negative control region (Kim et al., 2017).
(E) Slc2a5 promoter and gene body showing five different ChIP regions assayed (assay was conducted on 2 biological replicates and 3 technical replicates). Regions (r3, r4, and r7) contain previously identified ChREBP-binding sites (Kim et al., 2017; Oh et al., 2018). Regions (r5 and r6) overlap with the Slc2a5 promoter and are in proximity to previously reported ChREBP-binding sites (Oh et al., 2018). Error bars indicate the mean ± SEM of two independent ChREBP ChIP experiments for chromatin from n = 2 mice on RD (n = 3 ChIPs per biological replicate indicated by open or solid circles), n = 2 mice on ERD (n = 3 ChIPs per biological replicate indicated by open or solid squares), and control IgG (n = 3 to 4 ChIPs per biological replicate indicated by open or solid gray triangles) with either RD or ERD chromatin. Two of seven IgG samples for r4 have undetermined Ct values and therefore are not shown. Genomic loci shown are from University of Santa Cruz Genome Browser (UCSC), mm10.
See also Figures S3 and S4.
Recently, it was reported that carbohydrate responsive element-binding protein (ChREBP) bound directly to Slc2a5 (Kim et al., 2017) in the intestine. As Txnip promotes the de-phosphorylation and nuclear translocation of ChREBP (Chen et al., 2014), we hypothesized that ERD-induced Txnip would enhance the binding of ChREBP to the promoter region of Slc2a5. To test for this possible mechanism, we performed ChREBP chromatin immunoprecipitation (ChIP)-qPCR on intestinal samples from mice that were fed either an RD or ERD and also confirmed that the ChREBP ChIP assay responded to classical stimuli (Figure S4). We assayed previously reported ChREBP-binding sites in the thioredoxin-interacting protein (Txnip) (Poungvarin et al., 2015) and pyruvate kinase L/R (Pklr) promoter regions (Kim et al., 2017), including observing changes in their gene expressions after challenging with ERD (Figure S4C). At the Txnip and Pklr promoter regions, we found an enrichment of H3K4me3, therefore indicating an active promoter (Figures 3C and 3D, top panel). We also found enrichment for ChREBP at the Txnip (r1) and Pklr (r2) promoters in both RD and ERD samples when compared with control IgG samples as well as compared with a previously reported ChREBP-negative control region (Kim et al., 2017) (Figures 3C and 3D). We noted that a previous ChREBP-binding region in the Slc2a5 locus (Kim et al., 2017) suggested to be the promoter contains enrichment of chromatin marks such as H3K4me1 and H3K27ac, which are consistent with a potential role as an enhancer element (Creyghton et al., 2010). We then assayed ChREBP enrichment at the Slc2a5 promoter and gene body at previously reported ChREBP-binding regions (r3, r4, r7) (Kim et al., 2017; Oh et al., 2018). We noticed ChREBP enrichment at three of five regions in mice fed an RD (r5, r6, and r7) and in two of five regions in mice fed an ERD (r6 and r7) (Figure 3E, bottom panel). We then determined if there was a difference in ChREBP enrichment in mice fed an RD compared with an ERD. In one of five regions examined (r5), we observed a reduced trend in ChREBP-bound chromatin in mice fed an ERD compared with an RD (Figure 3E, bottom panel). It is also notable that in our ChREBP control regions (Txnip and Pklr promoters) we also observed a decreased trend in ChREBP-bound chromatin in mice fed an ERD compared with an RD (Figures 3C and 3D, bottom panel). ChREBP has been described to have a role as a transcriptional activator or repressor on gene expression (Adamson et al., 2006; Bricambert et al., 2010; Caron et al., 2013; Jeong et al., 2011; Noordeen et al., 2010; Sae-Lee et al., 2016). Thus, the decrease in ChREBP binding at the Txnip and Slc2a5 promoters in mice fed an ERD suggests that the ERD-induced increase of Txnip (Figure 1H) and Slc2a5 (Figure 3A) expression likely functions by an alternative transcriptional mechanism that has yet to be identified. Furthermore, we did not study if loss or gain in Txnip regulates methylation of the Slc2a5 gene. Future comprehensive studies of promoter and enhancer regulation could support the role of changes in Txnip level in gene regulation.
Txnip Interacts Endogenously with Rab11a and Is Needed for Apical Localization of Glut5
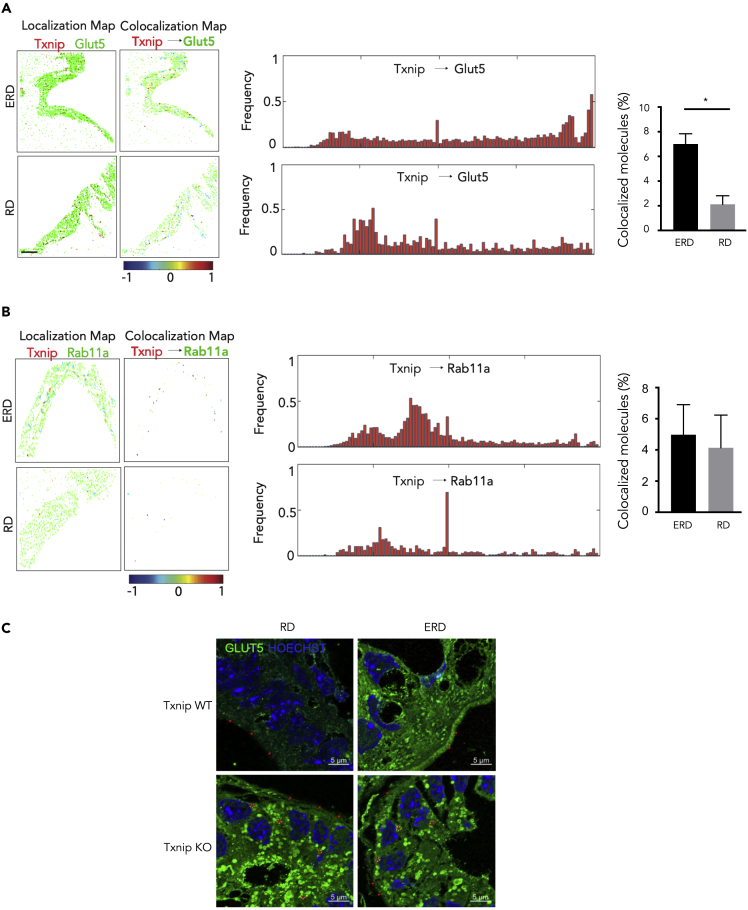
In addition to augmenting Glut5 gene and protein expressions, we also studied another potential mechanism for how Txnip might promote fructose uptake under ERD. Rab11a, a GTPase Rab-family member that plays a role in membrane trafficking and vesicle formation, is crucial for the apical localization of Glut5 in the intestinal epithelial cells (Patel et al., 2015). An interaction between Txnip and Rab11a was suggested in mass spectrometry data from an unbiased proteomics experiment in our previous studies (Lee et al., 2014). As Rab11a-mediated Glut5 trafficking to the apical membrane is necessary for fructose uptake (Patel et al., 2015), we speculated that Txnip could be facilitating the apical localization of Glut5 by forming a super-complex with Rab11a. To assess the difference in the co-localization of these proteins in the jejuna of RD and ERD mice, we performed an imaging analysis with dSTORM, a super-resolution digital microscope. A major advantage of using STORM is that it combines high-accuracy localization of the individual fluorophores in three dimensions, allowing for the most precise visualization of molecular interactions at the nanoscopic level (Schmider et al., 2019). We compared the effects of an RD and ERD on the colocalization of Txnip with Glut5 (Figure 4A) or Rab11a at the apical brush border (Figure 4B). The apical brush border was selected using the dSTORM acquisitions where we selected the region of interest (ROI) from the apical side of the tissue to the nuclei. Clus-DoC was then used to determine the colocalization of Txnip with either Glut5 (Figure 4A) or Rab11a (Figure 4B). Localization maps and colocalization maps of Txnip relative to Glut5 or Rab11a from ERD- or RD-fed mice are shown (left panels). Frequency histograms from all tissue sections analyzed are shown (middle graphs). The colocalization of Txnip with Glut5 was increased over 3-fold in ERD-fed mice compared with RD-fed mice (right panels, Figures 4A and 4B, p < 0.05). Although there was colocalization, no significant difference in percentage of colocalization was observed with Txnip and Raba11 (Figure 4B). Cluster maps of Txnip with Glut5 or Rab11a for the whole ROIs analyzed are shown in Figure S5, along with the analysis of various cluster properties of Glut5 and Txnip clusters that did not change. These results suggest that ERD may increase fructose transport by increasing the number of Txnip/Glut5 interactions in the apical brush border.
Figure 4.
Txnip Interacts with Rab11a and Is Needed for Apical Localization of Glut5
Semi-thin sections (1 μM) from the small intestine of mice were imaged following ERD or RD using two-color dSTORM. Localization data was analyzed using the Clus-DoC algorithm. Representative images of Txnip and Glut5 and Txnip and Rab11a are shown.
(A) Localization maps for Txnip (red) and Glut5 (green) and colocalization maps for Txnip relative to Glut5 (right panels). Txnip molecules are color-coded according to their degree of colocalization (DoC) scores (color scale bar at bottom). Frequency histograms of DoC scores of all molecules for Txnip from all cells analyzed (middle panels). The percent colocalization of Txnip molecules with Glut5 is shown in the bar graph.
(B) Localization maps, histograms, and bar graphs for the colocalization of Txnip (red) and Rab11a (green). Statistical significance was assessed by two-way ANOVA with multiple comparisons and a Tukey post-test with significance indicated by ∗p < 0.05. Bars show mean ± SEM from 5–13 ROIs over 3 separate mice (scale bar, 10 μm). See Figure S5 for more details.
(C). Representative images showing the distribution of Glut5 in enterocytes of Txnip WT and KO mice. One-micron sections from jejuna of RD- or ERD-fed Txnip WT and KO mice (n = 3 mice/group) were stained for Glut5. Expression of Glut5 (green) is remarkably elevated at the apical brush border (red arrows) of WT-ERD versus WT-RD. Notably, Glut5 is trapped in vesicles (as shown by ∗) in the Txnip KO enterocytes. Scale bars, 5 μm, magnification = 63× with Airyscan.
We also performed confocal microscopy with Airyscan mode (for super-resolution images) on the cryostat sections obtained from Txnip WT and KO mice that were fed RD/ERD for 1 month to assess the localization of Glut5 in enterocytes. As shown in Figure 4C, we saw that the intensity of Glut5 staining was elevated at the apical brush border of WT-ERD versus WT-RD (for quantified cellular Glut5 expression, please refer to Figures 3B and 4A). Notably, Glut5 appeared to be located in vesicles in the Txnip KO enterocytes, suggesting that Txnip is required for Glut5 apical localization.
Conclusion
These data collectively demonstrate that a chronic high-energy diet promotes acute fructose absorption from the intestine and the arrestin domain protein Txnip in intestinal epithelial cells plays a crucial role in this process. As seen in Figure 5, we show two molecular mechanisms for this, which we speculate may be working in concert. First, a high-energy diet increases Glut5 protein and gene expression, and Txnip is required for this process. Second, by forming a complex with Rab11a as well as Glut5, Txnip may be promoting Glut5 trafficking to the apical membrane for enhanced fructose uptake. Thus, our findings suggest that an ERD increases absorption of fructose. This could then have synergistic effects in the liver by promoting more hepatic de novo lipogenesis, a key factor in the pathogenesis of metabolic diseases (Schwarz et al., 2017). It is important to note that although the basal level of Txnip is sufficient to conduct fructose absorption and that the higher expression of Txnip leads to increased fructose absorption, this does not prove that the increase in Txnip was solely responsible for the increased fructose absorption. Our study cannot exclude other possibilities that could have been driven by ERD.
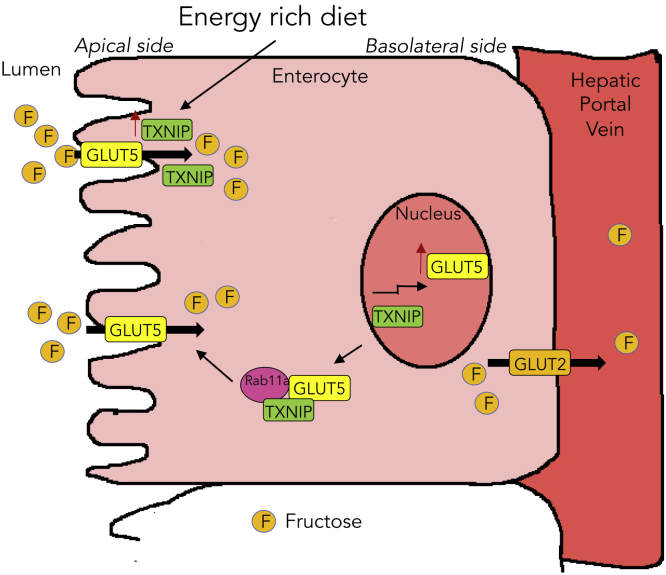
Figure 5.
Schematic Representation of Intestinal Fructose Absorption
Energy-rich diet increases intestinal Txnip expression, which in turn increases fructose absorption by elevating both Glut5 protein and gene expressions. Second, Txnip binds with Rab11a, a small GTPase protein essential for Glut5 apical localization, to potentially promote Glut5 trafficking to the apical surface for more fructose uptake.
Limitations of the Study
The experiments in this study did not isolate high fat content from the moderate sucrose content of the ERD. Chronic fructose consumption can increase acute fructose absorption (Dotimas et al., 2016), and thus the modest amount of fructose in the ERD may be partially responsible for inducing the acute fructose absorption effect. However, our experiments show that, in this model of metabolic disease, Txnip is a molecular mediator of an increase in acute fructose absorption. Future experiments will attempt to isolate an HFD away from all dietary fructose, to determine if high fat alone can promote fructose absorption.
Resource Availability
Lead Contact
Correspondence and requests for materials should be addressed to the Lead Contact, Richard T. Lee (Richard_lee@harvard.edu).
Materials Availability
No new unique reagents were generated in this study. The mice for conditional deletion of Txnip were previously generated by our laboratory and deposited for the public at jax.org.
Data and Code Availability
No dataset or codes were used in this study. The primer sequences used for the ChIP assays were obtained from various literature, and they are listed as well as cited in the Transparent Methods.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Supported by NIH/NIDDK to R.T.L. (1R01DK107396) and J.K.K. (5U2C-DK093000), the Glenn Foundation for Medical Research to A.J.W., and Diabetes Canada Post-Doctoral Fellowship to A.S. (PF-3-16-5176-AS). R.J.S. was supported by National Institutes of Health—1R01AR065538, 1R01CA193520, R01DK062472, and S10RR027931 and the MGH Molecular Imaging Core. A.B.S. was supported by National Institutes of Health—K01DK089145 and R01DK062472. We would also like to thank the Harvard Center for Biological Imaging (CBI) core for their technical advice and guidance with image processing.
Author Contributions
Conceptualization, R.T.L. and A.S.; Methodology, A.S., S.D., J.K.K., A.J.W., R.J.S., and R.T.L.; Supervision: A.S., S.D., J.K.K., A.J.W., R.J.S., and R.T.L.; Investigation, A.S., S.D., E.M.R., J.P.L., A.B.S., N.N., H.H., C.V., R.H.F., and H.N.; Formal Analysis, A.S., S.D., A.B.S., J.P.L., N.N., C.V. R.H.F., and H.N.; Writing – Original Draft, A.S. and R.T.L.; Writing – Review and Editing, A.S., S.D., A.B.S., J.P.L., N.N., E.M.R., H.H., C.V., J.K.K., A.J.W., R.J.S., and R.T.L.
Declaration of Interests
The authors declare no competing interests.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101521.
Supplemental Information
References
- Adamson A.W., Suchankova G., Rufo C., Nakamura M.T., Teran-Garcia M., Clarke S.D., Gettys T.W. Hepatocyte nuclear factor-4alpha contributes to carbohydrate-induced transcriptional activation of hepatic fatty acid synthase. Biochem. J. 2006;399:285–295. doi: 10.1042/BJ20060659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaz S., Mana M.D., Roper J., Kedrin D., Saadatpour A., Hong S.J., Bauer-Rowe K.E., Xifaras M.E., Akkad A., Arias E. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531:53–58. doi: 10.1038/nature17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G.A., Nielsen S.J., Popkin B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004;79:537–543. doi: 10.1093/ajcn/79.4.537. [DOI] [PubMed] [Google Scholar]
- Bricambert J., Miranda J., Benhamed F., Girard J., Postic C., Dentin R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J. Clin. Invest. 2010;120:4316–4331. doi: 10.1172/JCI41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron S., Huaman Samanez C., Dehondt H., Ploton M., Briand O., Lien F., Dorchies E., Dumont J., Postic C., Cariou B. Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes. Mol. Cell Biol. 2013;33:2202–2211. doi: 10.1128/MCB.01004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Khunti K., Davies M.J. Type 2 diabetes. Lancet. 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- Chen J., Jing G., Xu G., Shalev A. Thioredoxin-interacting protein stimulates its own expression via a positive feedback loop. Mol. Endocrinol. 2014;28:674–680. doi: 10.1210/me.2014-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chutkow W.A., Patwari P., Yoshioka J., Lee R.T. Thioredoxin-interacting protein (Txnip) is a critical regulator of hepatic glucose production. J. Biol. Chem. 2008;283:2397–2406. doi: 10.1074/jbc.M708169200. [DOI] [PubMed] [Google Scholar]
- Creyghton M.P., Cheng A.W., Welstead G.G., Kooistra T., Carey B.W., Steine E.J., Hanna J., Lodato M.A., Frampton G.M., Sharp P.A. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. U S A. 2010;107:21931–21936. doi: 10.1073/pnas.1016071107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotimas J.R., Lee A.W., Schmider A.B., Carroll S.H., Shah A., Bilen J., Elliott K.R., Myers R.B., Soberman R.J., Yoshioka J. Diabetes regulates fructose absorption through thioredoxin-interacting protein. Elife. 2016;5:e18313. doi: 10.7554/eLife.18313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang C., Hui S., Lu W., Cowan A.J., Morscher R.J., Lee G., Liu W., Tesz G.J., Birnbaum M.J., Rabinowitz J.D. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 2018;27:351–361 e353. doi: 10.1016/j.cmet.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y.S., Kim D., Lee Y.S., Kim H.J., Han J.Y., Im S.S., Chong H.K., Kwon J.K., Cho Y.H., Kim W.K. Integrated expression profiling and genome-wide analysis of ChREBP targets reveals the dual role for ChREBP in glucose-regulated gene expression. PLoS One. 2011;6:e22544. doi: 10.1371/journal.pone.0022544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T.A., Sievenpiper J.L. Controversies about sugars: results from systematic reviews and meta-analyses on obesity, cardiometabolic disease and diabetes. Eur. J. Nutr. 2016;55:25–43. doi: 10.1007/s00394-016-1345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Astapova I.I., Flier S.N., Hannou S.A., Doridot L., Sargsyan A., Kou H.H., Fowler A.J., Liang G., Herman M.A. Intestinal, but not hepatic, ChREBP is required for fructose tolerance. JCI Insight. 2017;2:e96703. doi: 10.1172/jci.insight.96703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Min Kim S., Dotimas J., Li L., Feener E.P., Baldus S., Myers R.B., Chutkow W.A., Patwari P., Yoshioka J. Thioredoxin-interacting protein regulates protein disulfide isomerases and endoplasmic reticulum stress. EMBO Mol. Med. 2014;6:732–743. doi: 10.15252/emmm.201302561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyssiotis C.A., Cantley L.C. Metabolic syndrome: F stands for fructose and fat. Nature. 2013;502:181–182. doi: 10.1038/502181a. [DOI] [PubMed] [Google Scholar]
- Malik V.S., Popkin B.M., Bray G.A., Despres J.P., Willett W.C., Hu F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen N.A., Khera T.K., Sun G., Longbottom E.R., Pullen T.J., da Silva Xavier G., Rutter G.A., Leclerc I. Carbohydrate-responsive element-binding protein (ChREBP) is a negative regulator of ARNT/HIF-1beta gene expression in pancreatic islet beta-cells. Diabetes. 2010;59:153–160. doi: 10.2337/db08-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh A.R., Sohn S., Lee J., Park J.M., Nam K.T., Hahm K.B., Kim Y.B., Lee H.J., Cha J.Y. ChREBP deficiency leads to diarrhea-predominant irritable bowel syndrome. Metabolism. 2018;85:286–297. doi: 10.1016/j.metabol.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh H., Carlsson E., Chutkow W.A., Johansson L.E., Storgaard H., Poulsen P., Saxena R., Ladd C., Schulze P.C., Mazzini M.J. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007;4:e158. doi: 10.1371/journal.pmed.0040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel C., Douard V., Yu S., Gao N., Ferraris R.P. Transport, metabolism, and endosomal trafficking-dependent regulation of intestinal fructose absorption. FASEB J. 2015;29:4046–4058. doi: 10.1096/fj.15-272195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwari P., Lee R.T. An expanded family of arrestins regulate metabolism. Trends Endocrinol. Metab. 2012;23:216–222. doi: 10.1016/j.tem.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poungvarin N., Chang B., Imamura M., Chen J., Moolsuwan K., Sae-Lee C., Li W., Chan L. Genome-Wide analysis of ChREBP binding sites on male mouse liver and white adipose chromatin. Endocrinology. 2015;156:1982–1994. doi: 10.1210/en.2014-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sae-Lee C., Moolsuwan K., Chan L., Poungvarin N. ChREBP regulates itself and metabolic genes implicated in lipid accumulation in beta-cell line. PLoS One. 2016;11:e0147411. doi: 10.1371/journal.pone.0147411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmider A.B., Vaught M., Bauer N.C., Elliott H.L., Godin M.D., Ellis G.E., Nigrovic P.A., Soberman R.J. The organization of leukotriene biosynthesis on the nuclear envelope revealed by single molecule localization microscopy and computational analyses. PLoS One. 2019;14:e0211943. doi: 10.1371/journal.pone.0211943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz J.M., Clearfield M., Mulligan K. Conversion of sugar to fat: is hepatic de Novo lipogenesis leading to metabolic syndrome and associated chronic diseases? J. Am. Osteopath. Assoc. 2017;117:520–527. doi: 10.7556/jaoa.2017.102. [DOI] [PubMed] [Google Scholar]
- Shao W., Yu Z., Chiang Y., Yang Y., Chai T., Foltz W., Lu H., Fantus I.G., Jin T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS One. 2012;7:e28784. doi: 10.1371/journal.pone.0028784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeters M.R., Soeters P.B. The evolutionary benefit of insulin resistance. Clin. Nutr. 2012;31:1002–1007. doi: 10.1016/j.clnu.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Softic S., Gupta M.K., Wang G.X., Fujisaka S., O'Neill B.T., Rao T.N., Willoughby J., Harbison C., Fitzgerald K., Ilkayeva O. Divergent effects of glucose and fructose on hepatic lipogenesis and insulin signaling. J. Clin. Invest. 2017;127:4059–4074. doi: 10.1172/JCI94585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindel O.N., World C., Berk B. Thioredoxin Interacting Protein (TXNIP): redox dependent and independent regulatory mechanisms. Antioxid. Redox Signal. 2011;16:587–596. doi: 10.1089/ars.2011.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverdlov A.L., Chan W.P., Procter N.E., Chirkov Y.Y., Ngo D.T., Horowitz J.D. Reciprocal regulation of NO signaling and TXNIP expression in humans: impact of aging and ramipril therapy. Int. J. Cardiol. 2013;168:4624–4630. doi: 10.1016/j.ijcard.2013.07.159. [DOI] [PubMed] [Google Scholar]
- Tappy L., Le K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- Waldhart A.N., Dykstra H., Peck A.S., Boguslawski E.A., Madaj Z.B., Wen J., Veldkamp K., Hollowell M., Zheng B., Cantley L.C. Phosphorylation of TXNIP by AKT mediates acute influx of glucose in response to insulin. Cell Rep. 2017;19:2005–2013. doi: 10.1016/j.celrep.2017.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu N., Zheng B., Shaywitz A., Dagon Y., Tower C., Bellinger G., Shen C.H., Wen J., Asara J., McGraw T.E. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol. Cell. 2013;49:1167–1175. doi: 10.1016/j.molcel.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zietek T., Rath E., Haller D., Daniel H. Intestinal organoids for assessing nutrient transport, sensing and incretin secretion. Sci. Rep. 2015;5:16831. doi: 10.1038/srep16831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No dataset or codes were used in this study. The primer sequences used for the ChIP assays were obtained from various literature, and they are listed as well as cited in the Transparent Methods.