Highlights
-
•
We investigated working memory in patients with ECTS and age-matched controls using a verbal working memory task.
-
•
Children with ECTS showed altered behavioral and fMRI responses to verbal working with increasing difficulty level.
-
•
Children with ECTS demonstrated reduced capacity to sustain high WM load.
Keywords: Rolandic epilepsy, BCECTS, Working memory, fMRI, Children with epilepsy, Verbal
Abstract
Background
Previous functional magnetic resonance imaging (fMRI) studies have identified brain systems underlying different components of working memory (WM) in healthy subjects. The aim of this study was to compare the functional integrity of these neural networks in children with self-limited childhood epilepsy with centro-temporal spikes (ECTS) as compared to healthy controls, using a verbal working memory task (WMT).
Methods
Functional MRI of WM in seventeen 6-to-13 year-old children, diagnosed with ECTS, and 17 sex- and age-matched healthy controls were conducted at 3 T. To estimate BOLD responses during the maintenance of low, medium, and high WMT loads, we used a Sternberg verbal WMT. Neuropsychological testing prior to scanning and behavioral data during scanning were also acquired.
Results
Behavioral performances during WMT, in particular accuracy and response time, were poorer in children with ECTS than in controls. Increased WM load was associated with increased BOLD signal in all subjects, with significant clusters detected in frontal and parietal regions, predominantly in the left hemisphere. However, under the high load condition, patients showed reduced activation in the frontal, temporal and parietal regions as compared to controls. In brain regions where WM-triggered BOLD activation differed between groups, this activation correlated with neuropsychological performances in healthy controls but not in patients with ECTS, further suggesting WM network dysfunction in the latter.
Conclusion
Children with ECTS differ from healthy controls in how they control WM processes during tasks with increasing difficulty level, notably for high WM load where patients demonstrate both reduced BOLD activation and behavioral performances.
1. Introduction
Working memory (WM) is a set of cognitive functions and mechanisms that allow us to store, manipulate and use task-relevant information over a short period of time under attentional control (Baddeley and Hitch, 1974). Four modality-specific storage systems exist for holding information in WM: 1) the “phonological loop” which holds speech-based (verbal and acoustic) information (Schweickert and Boruff, 1986), 2) the “visuospatial sketchpad”, which holds the visual and spatial information in a temporary store (Logie, 1995), 3) the “episodic buffer”, i.e., a temporary store of episodes and 4) the “central executive”, which is responsible for control and integration of the information from the first three systems, and manipulating the information within WM (Baddeley, 2000). Executive functions, including working memory (Gur and Gur, 2013), mature throughout adolescence (Best et al., 2011, Gur et al., 2012), with WM showing a tendency for a linear growth trajectory (Conklin et al., 2007) and protracted development into late adolescence (Luna et al., 2010). Several studies have demonstrated that these age-related behavioral improvements were associated with increases in the BOLD signal during tasks requiring working memory (Geier et al., 2009, Satterthwaite et al., 2013).
According to current views, several fronto-parietal regions, such as the inferior and middle frontal gyri, the parietal cortex within and around the intraparietal sulcus, the anterior cingulate and supplementary motor area, as well as the anterior insular cortex, are the neurobiological bases of WM (Casey et al., 1995, Glahn et al., 2002, Miller and Cohen, 2001, Nee et al., 2013, Nee and D'Esposito, 2018, Ranganath and D’Esposito, 2005, Satterthwaite et al., 2012, Sreenivasan et al., 2014, Veltman et al., 2003).In terms of functional specialization within the WM networks, the dorsally located caudal superior frontal sulcus and superior parietal lobule (SPL) are predominantly recruited in the maintenance of spatial information, whereas the mid-lateral prefrontal cortex in the left hemisphere is more sensitive to verbal content (Nagel et al., 2013, Nee et al., 2013). Additionally, Levy & Goldman-Rakic proposed that the ventrolateral prefrontal cortex (VLPFC) is responsible for the processing of verbal non-spatial information of WM while the dorsolateral prefrontal cortex (DLPFC) is responsible for spatial content (Levy and Goldman-Rakic, 2000). This was confirmed by several meta-analyses of fMRI data, where several aforementioned cortical regions within mostly the frontal and parietal lobes (DLPFC, VLPFC, bilateral and medial premotor cortex, dorsal cingulate, frontal pole, bilateral and medial posterior parietal cortex, fusiform gyrus and cerebellum), were activated during verbal WM task (the n-back and Sternberg tasks) (Emch et al., 2019, Owen et al., 2005, Rottschy et al., 2012). Furthermore, in adults, the prefrontal cortex is involved in the active maintenance process of verbal WM and is sensitive to task load (Narayanan et al., 2005). In adults, a linear increase in fMRI activation is observed in parietal and frontal lobe regions as load increases, while this effect is much less pronounced in children (O'Hare et al., 2008, Thomason et al., 2009, Vogan et al., 2016). Generally, increase in load reflects the neural activation linked to the increase in memory demand of information (Cowan et al. 2012) and is primarily associated with activation in the bilateral inferior frontal gyrus (BA 44, BA 45, ventral premotor cortex and caudal lateral prefrontal cortex) (Rottschy et al., 2012).
In many brain disorders expressed early in the childhood, such as attention deficit hyperactivity disorder (ADHD) (Bédard et al., 2014, Martinussen et al., 2005, Massat et al., 2012, van Ewijk et al., 2015) or autistic spectrum disorder (ASD) (Barendse et al., 2013, Barendse et al., 2018, Koshino et al., 2005, Russell, 1997, Vogan et al., 2018), patients experience poor WM skills and show altered WM networks. A recent cross-sectional study of a large cohort of children with epilepsy and matched controls also identified WM difficulties in patients, especially in those with early-onset epilepsy (van Iterson and de Jong, 2018). The WM storage capacity seems to be impacted in children with recently diagnosed idiopathic or cryptogenic epilepsy (Schouten et al., 2002) and remain affected 3–4 years after initial diagnosis (Oostrom et al., 2005). Furthermore, children with new onset childhood absence epilepsy perform worse on verbal WM tasks (Bhise et al., 2010). The performance is also impaired when the demand increases for a WM task (Schouten et al., 2002). Memory and phonological awareness difficulties have been well described in self-limited childhood epilepsy with centro-temporal spikes (ECTS) (Northcott et al., 2007, Northcott et al., 2005).
ECTS, also called Rolandic Epilepsy, and previously known as benign childhood epilepsy with centro-temporal spikes (BCECTS) (Scheffer et al., 2017) is the most common self-limited focal epilepsy of childhood, and considered a developmental disease. It has a characteristic onset between 3 and 13 years of age, a male predominance, a genetic predisposition, and remission during mid-adolescence (ILAE, 1989, Panayiotopoulos, 2005). The EEG pattern is distinctive, with focal uni- or bilateral high-voltage spikes or spike and waves over the centro-temporal region, usually activated by sleep. ECTS is often associated with neuropsychological deficits believed to reflect the interference between the epileptic focus and other brain regions during development. The cognitive dysfunctions observed in children with ECTS, including altered WM, have been linked to an abnormal developmental trajectory of brain structures, particularly those affected by epilepsy (Besseling et al., 2013a, Bourel-Ponchel et al., 2019, Ciumas et al., 2014, Garcia-Ramos et al., 2015, Garcia-Ramos et al., 2016, Hutchinson et al., 2010, Lin et al., 2012, Tosun et al., 2011, Widjaja et al., 2013). Task-based functional neuroimaging studies have shown that ECTS is associated with: 1) reduced activity during verbal generation task within language-related regions, in particular over the anterior language network (Lillywhite et al., 2009), 2) a wider network recruitment during sentence reading comprehension (Malfait et al., 2015), 3) reduced language laterality index, with more bilateral and right-hemispheric activations (Datta et al., 2013) , 4) remodelling of language networks (Vannest et al., 2013) and 5) altered responses to fearful stimuli (Ciumas et al., 2017). Resting state functional MRI have in ECTS have demonstrated altered global brain networks as well as nodal abnormalities in linguistic and ventral and dorsal attention networks (Xiao et al., 2015). Abnormalities in functional connectivity of language‐related circuits (Besseling et al., 2013c, Fang et al., 2017, McGinnity et al., 2017) and between rolandic regions and Broca’s area have also been reported (Besseling et al., 2013b). Regional homogeneity was found to be increased in sensorimotor and left frontal regions while it was decreased in the default mode network (DMN) (Zeng et al., 2015). Finally, a reduced activation was observed in typical areas supporting the DMN which partly normalized during cognitive effort (Oser et al., 2014).
Given that attention, language and WM interact so intently (Baddeley, 2003, Marchetti, 2014) and are all being altered in ECTS, it is important to further investigate the mechanisms of WM dysfunction in ECTS and understand how such dysfunction could be prevented or treated in the affected children. In an attempt to tackle this issue, we have investigated the neural correlates of WM in children with ECTS and age-matched controls using fMRI, an endeavour not previously performed to the best of our knowledge.
2. Materials and methods
2.1. Subjects
Seventeen children (five females and 12 males) from the outpatient clinic of the Department of Epilepsy, Sleep and Pediatric Neurophysiology (HFME, University Hospitals of Lyon, France) diagnosed with epilepsy volunteered for this study. Our selection criteria included: (I) diagnosed with ECTS according to ILAE diagnostic criteria assessed by experienced clinicians (E.P., J.B., A.M., K.O., A.A., P.R.); (II) < 24 months duration of antiepileptic drug (AED) treatment to reduce the potential impact of AEDs on our findings; (III) no other neurological disease; and (IV) normal MRI if available prior to inclusion. Seventeen (five females and 12 males) age- and sex-matched healthy volunteers were recruited as controls (HC). Subjects’ handedness was determined by administering the Edinburgh Handedness Inventory (Oldfield, 1971).
Informed written consent from all children and their parents were obtained. The study was approved by the Ethical Committee of Hospices Civils de Lyon.
2.2. Memory task during imaging
To avoid fatigue, all subjects were tested on an off-school day, in the morning. For training, each child was tested individually in a quiet room, and the experimental task was explained in easy-to-understand terms: first in an animated playful presentation, then in a form similar to the task used in the scanner room. A practice program was run, and each subject practiced the task until they were successful in completing the task. Immediately after the completion of training, subjects were introduced to the MRI scanning room and equipment. Prior to the WM testing, subjects had already completed an fMRI evaluation of other cognitive tasks and were thus already familiar with the environment and the scanner noise.
2.3. Verbal working memory task (WMT) during fMRI
We used the modified Sternberg maintenance task (Sternberg, 1966), similar to the one used by O’Hare et al (O'Hare et al., 2008), according to its capacity to generate working memory induced fMRI activation in the frontal regions (O'Hare et al., 2008, Thomason et al., 2009) typically affected in ECTS. In this procedure, a list of items is first presented and required to be encoded, then, after a few seconds, a probe is presented which the subject shall identify as being part or not of the encoded items.
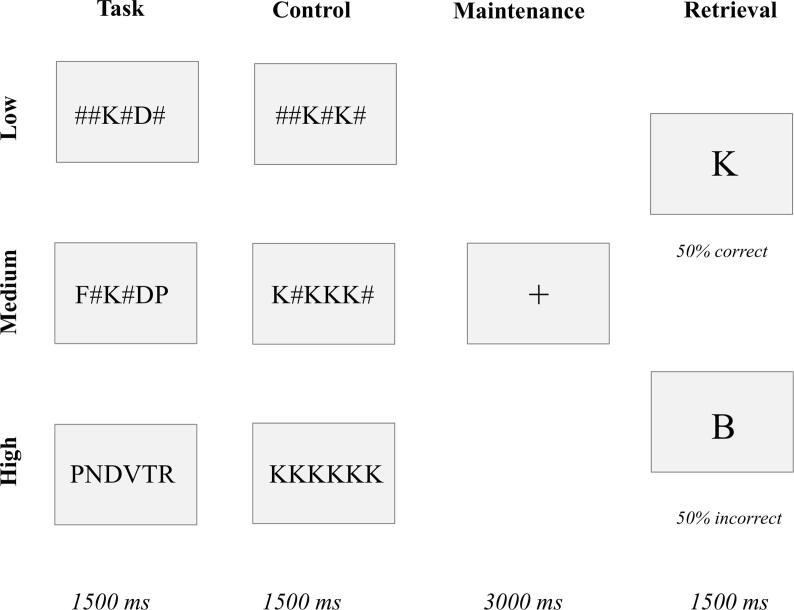
All stimuli were projected on a screen at the rear end of the magnetic bore. Each of the 36 trials started with the presentation of a 400 ms central fixation cross followed by a probe which consisted of a horizontal, central and linear array of two, four or six letters (Fig. 1). This 1500 ms probe-stimulus was replaced by an empty screen during a retention period of 3000 ms. The patient was instructed to remember the letters on the screen until sample presentation. The sample consisted of a letter shortly presented (1500 ms) on the screen. The participant was asked to remember the identity of the letters and respond after the retention period if the presented sample matched the probe. Each trial was followed by a fixation cross with an interstimulus interval between 1000 and 6000 ms. Memory load was manipulated by changing the number of letters to retain — two for low, four for medium, and six for high load. Additionally, 36 trials consisted of a control condition – the same letters appeared – for different loads – two, four or six letters. There was a 50% match in each condition (both control and task trials). All conditions were pseudorandomly assigned across the two runs. Performance data were recorded during scanning, for response time, accuracy (percentage of correctly identified target letters), errors and missed responses.
Fig. 1.
Example of the verbal WMT.
2.4. WM performance assessment
Accuracy (mean percentage of correct responses), response time (milliseconds, ms), missed responses to the probe and errors (incorrect answers, %) were recorded. A mean response time per task was determined for each subject over all correct responses. The response time for incorrect responses was not analysed. Only the first responses were judged as correct or incorrect and were fed into the model. Unwarranted second responses were excluded from the analysis. A repeated measurements ANOVA (SPSS v20, IBM Corp.) with group as the between-subjects factor and memory load as the within-subjects variables was conducted.
2.5. Neuropsychological testing
A neuropsychological battery of tests assessing memory was acquired about 10–14 days before scanning. The current selection of tests refers to the performance on the Wechsler Intelligence Scale for Children (WISC-IV) test (Needelman et al., 2006):
-
1.
Verbal comprehension index (VCI). This is the first of the four indices required for IQ assessment in WISC-IV. It is calculated from three subtests: similarities (evaluates verbal reasoning and concept formation), vocabulary (assesses knowledge of lexicon and the formation of verbal concepts) and comprehension (verbal reasoning, conceptualization, ability to explain practical situations). This index is a relevant measure of crystallized intelligence and a good predictor of academic success and learning.
-
2.
Perceptual reasoning index (PRI). This is one of the second index which is part of the IQ assessment in WISC-IV. It is calculated from three subtests: block design (evaluates the capacity to analyse and to synthesize abstract visual stimuli), pictures concepts (evaluates the aptitude to the categorical reasoning and the abstract reasoning) and matrix reasoning (evaluates fluid reasoning and logical-mathematical reasoning).
-
3.
Working Memory Index (WMI). This is the third index needed for IQ assessment in WISC-IV. It is calculated from two subtests: digits span (evaluates short-term auditory memory and auditory working memory) and letters-numbers sequencing (evaluates auditory WM).
-
4.
Processing speed index (PSI). This is the fourth index needed for IQ assessment in WISC-IV. It is calculated from two subtests: coding (evaluates the speed of grapho-motive processing) and symbol search (working speed of visual exploration).
On the day of scanning, the following two tests were also run:
-
5.
Digit span forward, which primarily evaluates short-term memory. We used an age-calibrated score for this test, whose norm is zero. The lower the score, the lower the performance with significant deficit considered at −2 standard deviations (Wechsler and Psychological, 2004).
-
6.
Digit span backward, a measure of WM where the subject is required to recall a sequence of spoken digits in the reverse order and relies on remembering the sequence exactly. Test begins with two numbers, and gradually increases by one number in each block, until the subject is unable to recall the correct sequences at a particular block. The number of correct trials is scored for each child. Same age-calibration was used as for the digit span forward (Wechsler and Psychological, 2004).
2.6. MRI data acquisition
All 3 T MRI experiments were performed on a human MRI scanner Philips Achieva (Philips Healthcare, Best, Netherlands) with a sensitivity-encoding (SENSE) coil (SENSE Head 32P, Philips Healthcare).
Structural MRI: For the T1-weighted 3D sequence, the parameters were: transversal orientation, 232x217 matrix, 170 slices, TE = 3.3 ms, TR = 7.1 ms, TI = 804 ms, flip angle = 8°, voxel size = 1.1 × 1.1 × 1.2 mm3. Total scan time was 10 min 52 sec.
Functional MRI: We used a standard echo-planar imaging (EPI-FID) sequence (92 × 90 matrix, TE = 30 ms, TR = 2200 ms, FOV (in-plane) = 220 mm, flip angle = 90°, 26 slices, 0.4 mm gap between, slice thickness = 3 mm, voxel size = 2.4 × 2.4 × 3.0 mm3), 2 runs in total.
2.7. fMRI data analysis and statistics
fMRI images were pre-processed using spm12 [Wellcome Department of Imaging Neuroscience, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm12/] on a MATLAB platform (MathWorks Inc., Sherborn, MA). The first four volumes were discarded from the analysis to allow for magnetic saturation effects.
All functional images involved motion correction to the first image of the first run by realigning the remaining volumes using a least-square approach and a six-parameter rigid-body spatial transformation. A mean image was created during the realignment step. Each individual data set was screened for data quality via inspection for image artefacts and excessive head motion (>3 mm head motion or 2° head rotation). Functional images were then co-registered to the T1 structural image. The resulting images were then normalized into standard space using the Montreal Neurological Institute (MNI) template, re-sliced to 2x2x2 mm and smoothed using a Gaussian kernel of full-width half-maximum (FWHM) of 8 mm.
fMRI responses were modelled using the General Linear Model (GLM) across all two runs modelled as separate blocks with a canonical hemodynamic response function (HRF), convolved for the length of each block, normalized to the global signal across the whole brain and the entire session. The data were high-pass filtered at 128 Hz to remove the effects of scanner signal drifts. To account for the intrinsic autocorrelations, autoregressive noise model of order 1 (AR1) was used. Movement parameters and outliers were included as nuisance regressors in the first-level SPM. Individual contrasts were carried out to investigate the BOLD response. Task activity in each load was contrasted with the corresponding control load. The main effect of WMT was tested by contrasting all WMT load (all loads) with all control (no load) conditions [1 1 1–1–1–1]. We were also interested in the areas activated during high and medium WM load vs. low load (six or four items vs two items), since contrasting higher load with lower load is thought to reflect maintenance aspects of WM (Ragland et al., 2002).
These contrast images were taken up to a second level analysis (ANOVA). We looked at the main effect of group, main effect of load and load by group interaction. At the group level and for the between-group analysis we used family wise error (FWE) threshold P = 0.05, k = 5. Age, sex and handedness were included as covariates to control for their effect. Significant BOLD effects were rendered on a normalized MNI template.
At the group level and for the between-group analysis we used family wise error (FWE) threshold P = 0.05, k = 5. Supplementary material also provides results using uncorrected p = 0.001/0.005, k = 300. Age, sex and handedness were included as covariates to control for their effect. Significant BOLD effects were rendered on a normalized MNI template.
In order to better characterize the significant differences between groups, we extracted the contrast parameter estimates for peak voxels from a factorial design (Group as between factor, Load as within factor, significant clusters from Group by Load interaction). Extracted values for each region of interest were submitted to statistical analysis. To identify regions of activation correlated with age, separate regression analyses were performed in each group using age as covariates (multiple regression).
2.8. fMRI activation in relation to clinical parameters and neuropsychological scores
Voxel-based regression analysis: To examine whether the group differences in load-dependent activation varied in relation to subjects’ age and their neuropsychological performance, we applied a multiple regression analysis for all significant contrasts from the within-group analysis. This analysis was performed in both patients and controls. For patients, we also added age of onset, duration of epilepsy, total number of seizures and time after the last seizure as variables.
Post-hoc ROIs-based correlation analysis: To better understand the clinical variables driving the differences in fMRI activation observed in the factorial analysis, we used the clusters identified in the Group by Load interaction contrast to extract the peak of the cluster beta values. Beta values from the BOLD response were extracted from regions of interest centered around clusters’ peak voxel and correlated in each group with neuropsychological scores and performance (correct, missed and errors) measured during scanning using Pearson correlation (SPSS v20, IBM Corp.). Significance values were set after Bonferroni correction for multiple comparisons.
3. Results
3.1. Subjects characteristics
Groups were comparable with regard to age, sex and handedness. The patient group included 17 participants with ECTS and 17 HC (age range between 6.4 and 12.8 years old, (Table 1)). In patients, mean age at the onset of epilepsy was 7.2 years (SD = 1.8, min = 51, max = 10), mean duration of epilepsy from disease onset to the scanning session was 28.3 months (SD = 26.5, min = 2, max = 84) and mean time between the last seizure and scanning session was 6.2 months (SD = 5.2, min = 0.4, max = 20, Median = 4 months, Range = 19.6 months). At the time of inclusion in the study, EEG spike foci were left sided in eight, right sided in seven, and bilateral in two patients. Seven patients took one AED, one took two AEDs, while nine patients were free of any AED. One patient (#4) was additionally treated for co-morbid ADHD with Methylphenidate (Table 2).
Table 1.
Population characteristics.
| Controls (N = 17) | ECTS (N = 17) | p-Value | |||
|---|---|---|---|---|---|
| Age (years), mean, SD | 9.4 | 1.9 | 9.7 | 1.9 | 0.7 |
| Male, Nb | 12 | 70.6% | 12 | 70.6% | 1.000 |
| Female, Nb | 5 | 29.4% | 5 | 29.4% | |
| Range (years) | 6.4 | 12.8 | 6.9 | 12.6 | – |
| Right-handed, Ambidextrous, Left-handed | 12 -- 1 -- 4 | 12 – 2 -- 3 | – | ||
| Full-Scale IQ, mean, SD | 110 | 16 | 104 | 16.8 | 0.353 |
| Verbal comprehension index (VCI) | 111 | 14.6 | 102 | 21.3 | 0.182 |
| Perceptual reasoning index (PRI) | 107 | 10.6 | 107 | 13.6 | 0.909 |
| Working memory index (WMI) | 101 | 15.1 | 100 | 14.4 | 0.939 |
| Processing speed index (PSI) | 110 | 16 | 99 | 15.6 | 0.053 |
| Digit span forward | 5.4 | 1.4 | 5.2 | 0.9 | 0.556 |
| Calibrated score for digit span forward | 0.2 | 1.2 | −0.06 | 0.7 | 0.444 |
| Digit span backward | 4.1 | 1.2 | 3.6 | 0.9 | 0.223 |
| Calibrated score for digit backward | 0.4 | 0.9 | −0.05 | 0.9 | 0.166 |
Table 2.
Patients description.
| # | Age | Sex | Handedness | Diagnosis | EEG focus | Side | Heredity | AED | Age at onset | Total Nb of seizures | GTCS | Last seizure,months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8,6 | F | 100,00% | BCECTS | C-T | Bilateral | No | CLO | 8 | 1 | No | 7 |
| 2 | 12,1 | M | 100,00% | BCECTS | C-T | Bilateral | No | 6 | 5 | No | 11 | |
| 3 | 8,3 | M | 100,00% | BCECTS | C-T | Left | Yes | 8 | 1 | No | 3 | |
| 4* | 8,0 | M | 60,00% | BCECTS | C-T | Left | No | 6 | 1 | No | 4 | |
| 5 | 11,0 | F | −90,00% | BCECTS | C-T | Left | No | VAL | 9 | 6 | No | 2 |
| 6 | 10,9 | M | 80,00% | BCECTS | F-C-T | Left | No | 10 | 1 | No | 4 | |
| 7 | 10,4 | M | 100,00% | BCECTS | C-T | Left | No | CBZ | 9 | 1 | No | 3 |
| 8 | 10,2 | M | −100,00% | BCECTS | C-T | Left | Yes | CBZ | 10 | 3 | No | 0,4 |
| 9 | 12,6 | F | 100,00% | BCECTS | F-C-T | Left | No | VAL | 6 | 2 | No | 13 |
| 10 | 7,2 | M | 100,00% | BCECTS | C | Left | No | 6 | 1 | No | 6 | |
| 11 | 12,0 | M | 20,00% | BCECTS | C | Right | No | VAL | 5 | 4 | Yes | 10 |
| 12 | 9,4 | M | 0,00% | BCECTS | CT | Right | Yes | CLO + VAL | 5 | 1 | No | 1 |
| 13 | 6,9 | M | 100,00% | BCECTS | C-T | Right | No | 6 | 5 | Yes | 10 | |
| 14 | 9,3 | F | 100,00% | BCECTS | C-T | Right | No | 6 | 4 | No | 4 | |
| 15 | 9,6 | M | 80,00% | BCECTS | T | Right | Yes | 7 | 1 | No | 20 | |
| 16 | 11,9 | M | −100,00% | BCECTS | C-T | Right | Yes | 10 | 2 | Yes | 6 | |
| 17 | 6,9 | F | 100,00% | BCECTS | C-T | Right | No | VAL | 6 | 2 | No | 1 |
ECTS –epilepsy with centro-temporal spikes, AED- Anti-epileptic medication, CLO - Clobazam, CBZ- Carbamazepine, VAL- Valproate, Levetiracetam - LEV, Oxcarbazepine - OXZ, *- Methylphenidate (not taken on the day of scanning)
3.2. Neuropsychological and behavioral data
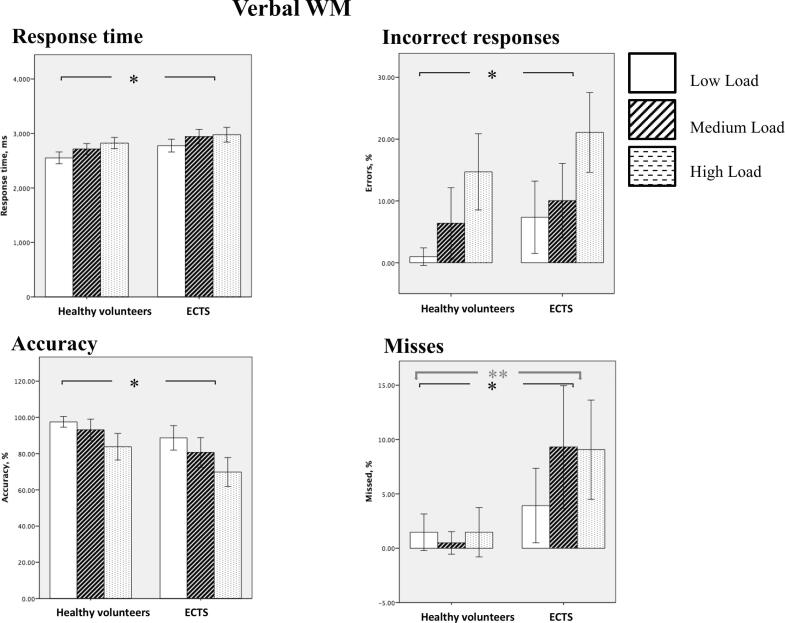
ECTS patients showed comparable neuropsychological scores to that of healthy controls, though full-scale IQ was slightly lower in patients than in HC (Table 1). Regarding performance data collected during scanning, both groups demonstrated a significant association between WM load and concurrent behavioral data, with greater response time (P < 0.001), reduced accuracy (P < 0.001), higher number of errors (P < 0.001) and of missed responses (P = 0.08) for higher WM load. In addition, a significant Group by Load interaction was observed for missed responses. When findings from all WM loads were averaged, ECTS patients showed greater response time (P = 0.006), reduced accuracy (P = 0.001), higher number of errors (P = 0.001) and of missed responses (P = 0.006) than HC (Fig. 2, Table 3).
Fig. 2.
Behavioral analysis. ** - significant Group by Load interaction, * effect of Load.
Table 3.
Behavioral results.
| Load | Healthy volunteers |
ECTS |
Group | WM load | Groups × Load | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Response time, ms | |||||||
| Low | 2552 | 209 | 2777 | 230 | F1.32 = 8.8p = 0.006η2p = 0.213 | F2,64 = 26.1p = 0.000η2p = 0.449 | F2,64 = 0.792p = 0.457η2p = 0.024 |
| Medium | 2716 | 193 | 2943 | 260 | |||
| High | 2824 | 201 | 2977 | 262 | |||
| Accuracy, % | |||||||
| Low | 97.5 | 5.7 | 88.7 | 13.1 | F1.32 = 12.7p = 0.001η2p = 0.285 | F2,64 = 18.9p = 0.000η2p = 0.372 | F2,64 = 0.493p = 0.613η2p = 0.015 |
| Medium | 93.1 | 11.5 | 80.6 | 15.9 | |||
| High | 83.8 | 14.3 | 69.8 | 15.6 | |||
| Errors, % | |||||||
| Low | 1.0 | 2.8 | 7.3 | 11.4 | F1.32 = 4.7p = 0.037η2p = 0.129 | F2,64 = 17.9p = 0.000η2p = 0.359 | F2,64 = 0.219p = 0.804η2p = 0.007 |
| Medium | 6.4 | 11.2 | 10.0 | 11.7 | |||
| High | 14.7 | 12 | 21.1 | 12.5 | |||
| Missed responses, % | |||||||
| Low | 1.5 | 3.3 | 3.9 | 6.7 | F1.32 = 11.4p = 0.002η2p = 0.262 | F2,64 = 2.62p = 0.080η2p = 0.076 | F2,64 = 3.871p = 0.026η2p = 0.108 |
| Medium | 0.5 | 2.0 | 9.3 | 11 | |||
| High | 1.5 | 4.4 | 9.1 | 8.9 | |||
3.3. Impact of clinical variables on neuropsychological and behavioral data
Two neuropsychological scores positively correlated with age across both populations (Digit span forward r = 0.419, p = 0.014, and Digit span backwards r = 0.532, p = 0.001), while behavioral performance did not correlate with age in any group.
ECTS patients demonstrated several positive correlations between the time passed since the last seizure and 1) the WMI score (r = 0.608, P < 0.001), 2) the digit span forward (r = 0.765, P < 0.001), 3) digit span backwards (r = 0.677, P < 0.001), 4) calibrated scores for digit span forward (r = 0.649, P < 0.001) and 5) backwards (r = 0.536, P = 0.003). All these correlations indicated better neuropsychological performances with longer seizure free period before testing and remained significant after Bonferroni correction (correction for bivariate association P = 0.05/9 = P < 0.0055). Age at onset of epilepsy negatively correlated with digit span forward (r = -0.726, P < 0.001) and with calibrated digit span forward (r = -0.743, P < 0.001), indicating better performance with earlier age at onset. In contrast, the total number of seizures, the duration of epilepsy and the presence or absence of AED treatment had no impact on neuropsychological and behavioral findings.
3.4. fMRI data
Table 4 gives an overview of regions significantly activated during WMT. Results of fMRI analysis are displayed in Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6 and the exact dimensions of the peak activation clusters are shown in Table 4. Across all our analyses showing significant WMT-triggered fMRI activation, both patient and control groups mostly showed activation of frontal and parietal regions predominantly in the left hemisphere, consistent with previous investigations of verbal WMT.
Table 4.
fMRI results.
| Healthy Controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| All loads | |||||||||
| Hemi | Lobe | Cluster covering | Brodmann area (BA) | p(FWE-corr) | Cluster size in voxels | T | x | y | z {mm} |
| Left | Frontal | Precentral Gyrus | BA 6, BA 4 | 0.000 | 1373 | 9.63 | −42 | −4 | 42 |
| Sub-lobar | Insula | BA 13 | 0.000 | 396 | 7.49 | −30 | 14 | 14 | |
| Frontal | Precentral Gyrus | BA 44 | 7.22 | −52 | 10 | 10 | |||
| Temporal | Superior Temporal Gyrus | BA 22 | 6.36 | −48 | 10 | 0 | |||
| Temporal | Inferior Temporal Gyrus | BA 19 | 0.016 | 5 | 5.91 | −44 | −58 | −6 | |
| Parietal | Superior Parietal Lobule | BA 7 | 0.000 | 649 | 7.36 | −24 | −68 | 42 | |
| Parietal | Inferior Parietal Lobule | BA 40 | 7.35 | −34 | −40 | 44 | |||
| Frontal | Medial Frontal Gyrus | BA 6 | 0.000 | 1177 | 8.99 | −2 | 18 | 46 | |
| Frontal | Superior Frontal Gyrus | 8.22 | −4 | 6 | 54 | ||||
| Right | 8.66 | 4 | 10 | 52 | |||||
| Frontal | Precentral Gyrus | BA 6 | 0.000 | 172 | 7.39 | 54 | −2 | 46 | |
| Sub-lobar | Insula | BA 13 | 0.000 | 98 | 7.03 | 38 | 16 | 2 | |
| High load | |||||||||
| Left | Frontal | Precentral Gyrus | BA 6 | 0.000 | 1343 | 11.04 | −42 | −4 | 42 |
| Middle Frontal Gyrus | BA 10 | 0.021 | 5 | 5.68 | −38 | 36 | 24 | ||
| Sub-lobar | Insula | BA 13 | 0.000 | 563 | 8.38 | −34 | 22 | 4 | |
| Temporal | Superior Temporal Gyrus | BA 22 | 6.94 | −52 | 10 | −6 | |||
| Frontal | Precentral Gyrus | BA 44 | 6.01 | −56 | 10 | 4 | |||
| Temporal | Inferior Temporal Gyrus | BA 19 | 0.001 | 50 | 7.38 | −44 | −58 | −6 | |
| Parietal | Superior Parietal Lobule | BA 7 | 0.000 | 386 | 7.11 | −24 | −66 | 50 | |
| BA 40 | 0.009 | 14 | 6.18 | −52 | −38 | 54 | |||
| 0.001 | 41 | 6.05 | −40 | −52 | 60 | ||||
| Occipital | Middle Occipital Gyrus | BA 19 | 0.012 | 11 | 5.95 | –32 | −78 | 20 | |
| Sub-lobar | Lentiform Nucleus | Putamen | 0.021 | 5 | 5.85 | −16 | 16 | −6 | |
| Frontal | Medial Frontal Gyrus | BA 6 | 0.000 | 1509 | 10.20 | −2 | 14 | 46 | |
| Right | 8.83 | 8 | 16 | 46 | |||||
| Frontal | Middle Frontal Gyrus | BA 8 | 0.000 | 137 | 8.48 | 38 | 32 | 40 | |
| Sub-lobar | Insula | BA 13 | 0.000 | 233 | 7.76 | 38 | 16 | 0 | |
| Temporal | Superior Temporal Gyrus | BA 38 | 5.69 | 48 | 14 | −6 | |||
| Parietal | Inferior Parietal Lobule | BA 40 | 0.000 | 207 | 6.72 | 48 | −40 | 46 | |
| Frontal | Middle Frontal Gyrus | BA 6 | 0.011 | 12 | 5.99 | 36 | 0 | 60 | |
| BA 9 | 0.007 | 17 | 5.86 | 40 | 44 | 28 | |||
| High load vs low load | |||||||||
| Left | Sub-lobar | Insula | BA 13 | 0.000 | 645 | 8.73 | −38 | 14 | −4 |
| Sub-lobar | Claustrum | * | 7.12 | −28 | 26 | −2 | |||
| Temporal | Middle Temporal Gyrus | BA 22 | 0.000 | 33 | 7.53 | −60 | −38 | 8 | |
| Frontal | Inferior Frontal Gyrus | BA 6 | 0.000 | 337 | 7.43 | −46 | 2 | 30 | |
| Precentral Gyrus | 7.08 | −36 | −4 | 38 | |||||
| Middle Frontal Gyrus | 6.39 | −40 | −2 | 48 | |||||
| Sub-lobar | Lentiform Nucleus | Putamen | 0.001 | 18 | 6.21 | −16 | 4 | 8 | |
| Caudate | Caudate Body | 6.10 | −16 | 2 | 16 | ||||
| Limbic | Cingulate Gyrus | BA 32 | 0.000 | 780 | 7.67 | −2 | 20 | 42 | |
| Right | Frontal | Cingulate Gyrus | BA 32 | 8.46 | 12 | 22 | 34 | ||
| Medial Frontal Gyrus | BA 9 | 7.33 | 8 | 28 | 28 | ||||
| Inferior Frontal Gyrus | BA 47 | 0.000 | 384 | 8.73 | 38 | 16 | −6 | ||
| Sub-lobar | Insula | BA 13 | 6.62 | 30 | 26 | 2 | |||
| Parietal | Inferior Parietal Lobule | BA 40 | 0.001 | 19 | 6.75 | 46 | −42 | 42 | |
| Frontal | Middle Frontal Gyrus | BA 9 | 0.000 | 42 | 6.50 | 32 | 32 | 36 | |
| High load vs medium load | |||||||||
| Right | Frontal | Middle Frontal Gyrus | BA 8 | 0.021 | 8 | 5.94 | 36 | 32 | 40 |
| Medium load | |||||||||
| Left | Parietal | Superior Parietal Lobule | BA 7 | 0.014 | 7 | 5.86 | −24 | −70 | 46 |
| ECTS | |||||||||
| All loads | |||||||||
| Left | Frontal | Inferior Frontal Gyrus, Precentral Gyrus | BA 9, BA 6 | 0.000 | 118 | 7.10 | −54 | 4 | 22 |
| Medial Frontal Gyrus | BA 6 | 0.008 | 10 | 5.92 | 0 | 16 | 46 | ||
| Parietal | Superior Parietal Lobule | BA 7 | 0.000 | 58 | 6.75 | −26 | −62 | 54 | |
| Right | Frontal | Middle Frontal Gyrus | BA 10 | 0.009 | 9 | 5.87 | 40 | 44 | 22 |
| Medium load | |||||||||
| Left | Parietal | Postcentral Gyrus | BA 3 | 0.002 | 23 | 6.47 | −62 | −14 | 26 |
| Precuneus | BA 7, BA 19 | 0.000 | 84 | 6.09 | −28 | −62 | 40 | ||
| Frontal | Inferior Frontal Gyrus, Insula | BA 6 | 0.007 | 14 | 6.05 | −34 | 26 | 2 | |
| Precentral Gyrus | BA 6 | 0.009 | 16 | 5.90 | −40 | 2 | 30 | ||
| Right | Middle Frontal Gyrus | BA 10, BA 46 | 0.006 | 14 | 5.98 | 40 | 44 | 24 | |
| Group comparison | |||||||||
| High load | |||||||||
| Left | Temporal | Superior Temporal Gyrus | BA 22 | 0.001 | 60 | 5.84 | −56 | 10 | −6 |
| Inferior Temporal Gyrus | BA 19 | 0.003 | 33 | 5.83 | −44 | −58 | −6 | ||
| Frontal | Medial Frontal Gyrus | BA 6 | 0.000 | 140 | 6.05 | −4 | 2 | 58 | |
| Middle Frontal Gyrus | BA 6 | 0.003 | 34 | 5.74 | −44 | 2 | 38 | ||
| Right | Inferior Frontal Gyrus | BA 9 | 0.014 | 11 | 5.22 | 54 | 4 | 30 | |
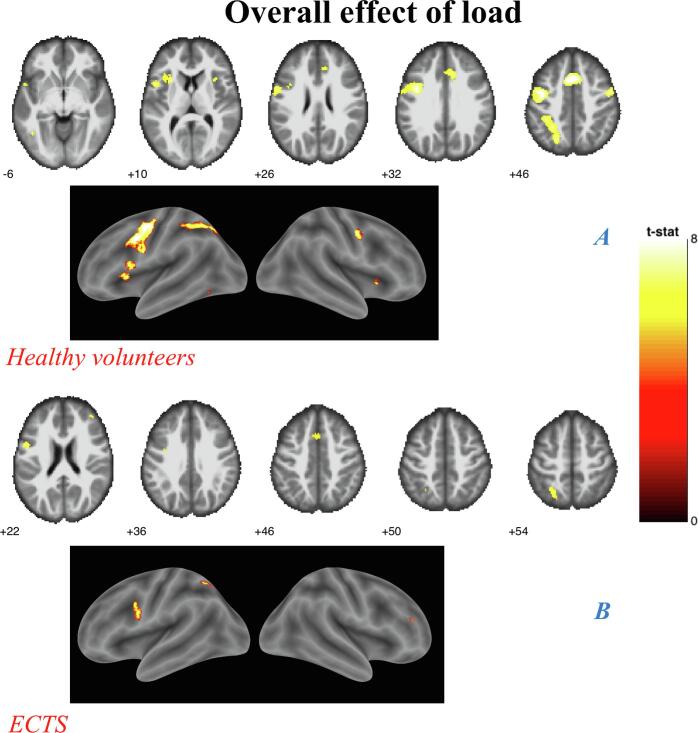
Fig. 3.
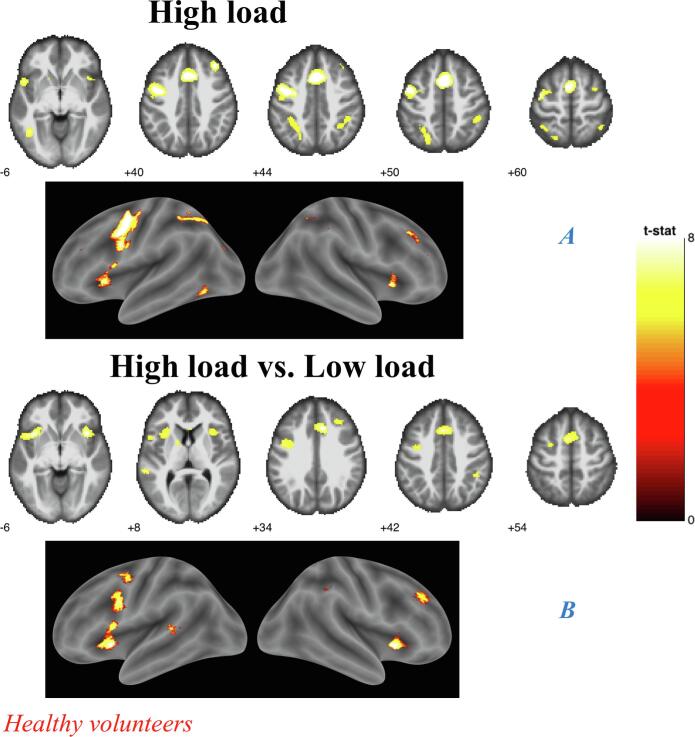
Overall effect of load analyses (A) Controls. (B) ECTS, Results shown on MNI brain at FWE corrected threshold p = 0.05, k = 5, colour bar shows the T-score;
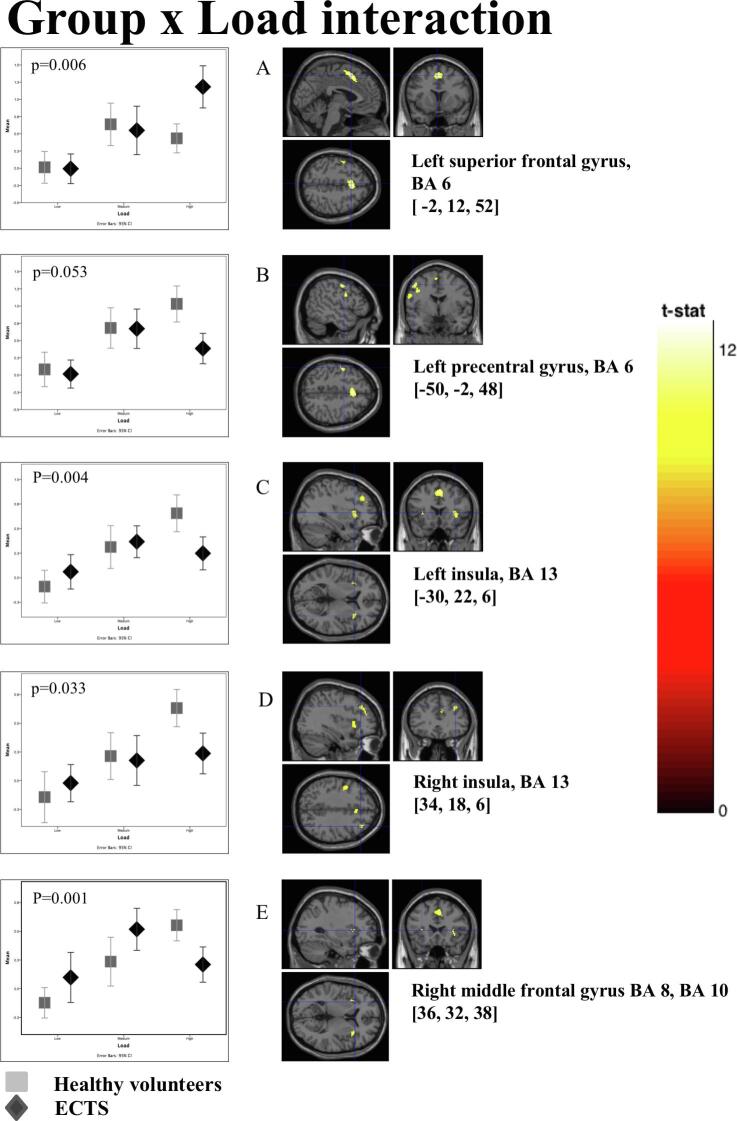
Fig. 4.
Group by Load interaction –Box plots show mean bold signal for each group at different levels of task difficulty.
Fig. 5.
High load analyses. (A) Controls. High load vs. Low load analyses. (B) Controls.
Fig. 6.
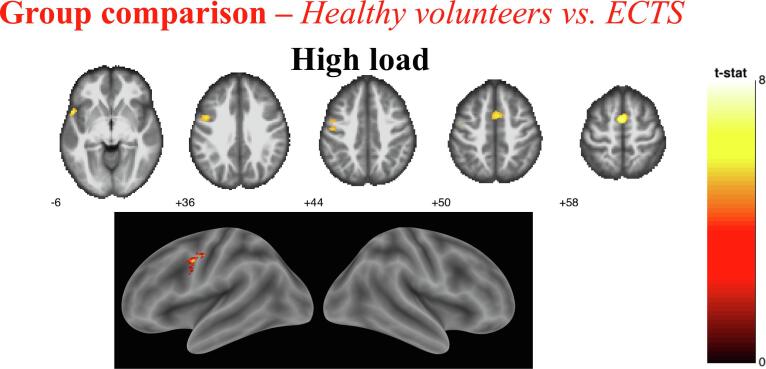
Group comparison analyses, Controls. vs ECTS. High load comparison. Results shown on MNI brain at uncorrected threshold FWE corrected threshold p = 0.05, k = 5, colour bar shows the T-score.
3.5. All loads analyses
In both groups the activation of the left superior parietal lobule (SPL), precentral gyrus and medial frontal gyrus (MFG) was associated with WMT.
In the HC group, additional activations were observed: in the left hemisphere over the superior frontal (SFG) gyri, inferior parietal lobule (IPL), superior and inferior temporal gyrus (STG and ITG, respectively), insula; in the right hemisphere over the precentral gyrus and the insular cortex (Fig. 3A), while in ECTS, we observed further left sided activation of the inferior frontal gyrus (IFG) and middle frontal gyrus (MiFG) (Table 4, Fig. 3B).
The interaction of Group by Load contrast showed significant interaction over five clusters: the left superior frontal gyrus, the left precentral gyrus, the left and the right insula and the right frontal gyrus. The observed differences between groups were greater for high load. This reflected the fact that healthy controls showed a linear increase in activation with increasing WM load (except for the left superior frontal gyrus), while patients showed such an increase only between low and medium load with very little activation for the highest load (Fig. 4).
3.6. High load analyses
High load versus no load showed significant activation only in HC, where it involved the MiFG, MFG, STG and insular cortex bilaterally, as well as the left precentral gyrus, superior parietal lobule (SPL), ITG and middle occipital gyrus, and the right IPL (Fig. 5A). Similarly, high load versus low load showed significant activation only in HC, over the IFG, insular cortex, MiFG and cingulate gyrus bilaterally, the left middle temporal gyrus and dorsal striatum and the right MFG and IPL (Fig. 5B). Finally, high load versus medium load, which primarily reflects WM maintenance, showed an activation in the right MiFG in HC only.
Between-group analyses confirmed that HCs showed significantly greater fMRI activation than ECTS patients for high load versus no load contrast over the left STG, ITG, MFG, MiFG and the right IFG (Fig. 6, Table 4). No region was more activated in patients than in controls.
3.7. Medium load analyses
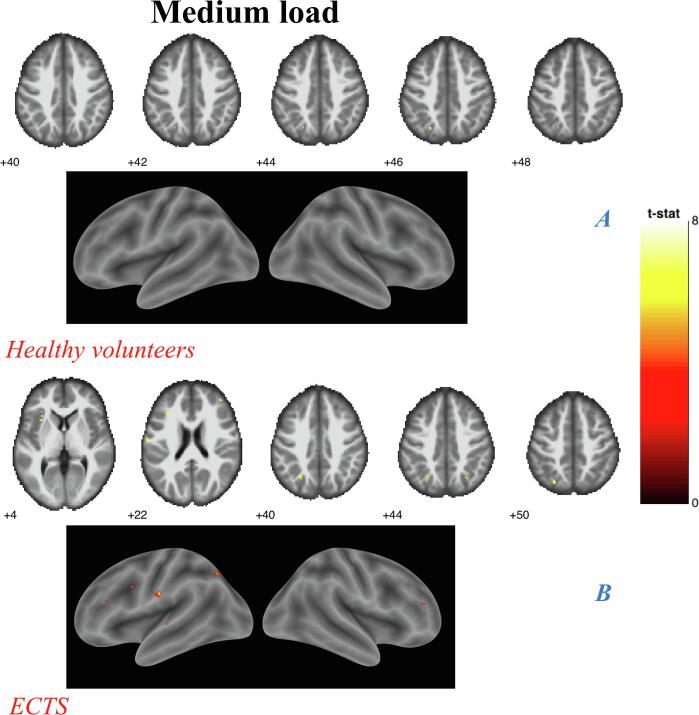
In contrast with high load analyses, medium load versus no load analyses showed larger activated areas in patients than in HC. Indeed, while both HC and patients showed activation in the left SPL, ECTS also showed activation over the left precentral gyrus, postcentral gyrus, insular cortex, IFG and precuneus, and the right MiFG (Fig. 7A and 7B). However, between-group analyses showed no significant difference.
Fig. 7.
Medium load analyses. (A) Controls. (B) ECTS, Results shown on MNI brain at FWE corrected threshold p = 0.05, k = 5, colour bar shows the T-score.
3.8. Low load analyses
There were no significant clusters for the low load versus no load contrast in any of the groups, nor differences between groups.
3.9. fMRI activation in relation to clinical parameters and neuropsychological scores
3.9.1. Voxel-based regression analysis
In healthy controls, several clusters detected for the all loads versus no load contrast showed a positive association with neuropsychological scores: i.e. the left postcentral gyrus cluster correlated with the VCI and WMI score, while the left ITG, MiFG and right SFG clusters correlated with the PRI score. Correlations with PRI score were also observed for the right MiFG and SFG clusters detected in the high load versus no load contrast, as well for the left MiFG cluster detected in the high load vs low load contrast. All these correlations indicated better neuropsychological performances in controls with greater fMRI activation. No such finding was observed in ECTS. There was no correlation with age in any group, nor association with clinical variables such as seizure frequency, age at epilepsy onset, time since the last seizure, duration of epilepsy and total number of seizures.
3.9.2. Post-hoc ROI-based correlation analysis
In ECTS, the extracted values showed a negative correlation with the total number of seizures over the left insula (r = -0.457, P = 0.013) and right MiFG (r = -0.432, P = 0.019) which indicated that the higher the number of seizures, the lower and more abnormal the activation of these regions in ECTS. Patients also showed an association between extracted values in the left SFG and WMI score (r = -0.441, P = 0.012) and calibrated digit span forward span (r = -0.393, P = 0.021) indicating that lower BOLD changes were associated with higher performance scores. In HC a negative correlation was observed between the peak of the cluster beta values of the left precentral gyrus and the WMI score (r = -0.386, P = 0.032). However, none of the above associations survived the Bonferroni correction.
4. Discussion
To the best of our knowledge, this is the first report of WM neural correlates in children with ECTS. Overall, there were greater task-induced activations in age and sex-matched healthy controls than in patients over several brain regions involved in verbal WM. Both groups also demonstrated that the higher the WM load, the greater the fMRI activation in brain areas previously shown to be involved in WM, though this did not apply to the highest WM load in ECTS. Healthy controls responded to increasing WM demands by engaging larger relevant WM-related regions in the dorsal and ventral prefrontal cortex (DLPFC and VLPFC), premotor and motor areas, frontal and supplementary eye field areas (FEF and SEF), insula and parietal cortex. Patients engaged some of these regions to a lesser extent, and maximally at the medium WM load. The between-group comparisons for high WM load showed lower activation in ECTS children over a widespread cortical network including the right DLPFC, the left superior and inferior temporal gyri, FEF and SEF. ECTS also demonstrated lower behavioral WM performance as compared to healthy children and failure to proportionally engage WM network in response to increase in WM load. Finally, ECTS patients failed to show the correlations between WMT-related fMRI activation and neuropsychological performance observed in healthy controls.
None of the groups showed a significant activation during low memory load (two items - a relatively simple condition), probably because the low load did not sufficiently burden the WM system. Indeed, the activation in WM network enhanced when the task become more difficult (Mather and Sutherland, 2011). The mean capacity of WM in our healthy controls group was 4.1 ± 1.2 items, whereas it was 3.6 ± 0.9 in ECTS (see Table 1). Regarding fMRI activation, it is difficult to determine the WM capacity limit as there was no task with five items (intermediate between medium and high load). Furthermore, comparison between high and medium load showed significant clusters in HC, but not in patients. Nevertheless, based on our findings, it appears that ECTS patients reached their limit for the medium WM load, while HC reached it for the high WM load.
Generally, it is considered that the increase in cognitive demand (higher load) results in increased load-dependent activity in areas related to WM (Vogan et al., 2016) and hints towards performance improvements in WM over the course of development (Best et al., 2011, Bunge and Wright, 2007, Best et al., 2011). Executive functions, including WM, develop from childhood till adolescence (Luna et al., 2004), and might even continue to progress until early adulthood (Satterthwaite et al., 2013). However, many WM components are fully functional by the age of 4 (Alloway et al., 2004). Other components of WM maintenance, such as those involved in active verbal rehearsal and attentional refreshing, appears around 7 years of age (Camos and Barrouillet, 2011). Furthermore, while the executive component of WM is appropriately developed by the age of 6, performance on simple and complex WM tasks show a linear increase from age 4 to 14 (Gathercole et al., 2004). This process appears tightly linked to the network level mechanisms of plasticity and its structural and functional coupling, with great intraindividual variability in executive functions, notably of the verbal WM (Baum et al., 2020).
Our patient group was similar to the control group on all demographic variables. Because this experiment was a rare experience for all participants, arousal and motivation factors were expected to be similar in both groups. One might argue that patients might have been more motivated, due to their epilepsy- and health-related concerns, a possibility that would have resulted in greater activation in patients which would then reduce, rather than accounting for, the differences observed between ECTS and HC. Also, the observed differences cannot either be attributed to the female advantage in verbal WM testing (Wang and Carr, 2014), since there were 29.4% female subjects in each group. Furthermore, the sex difference in WM-induced brain activation are observed from late adolescence (Voyer et al., 2017), while the eldest children in our study were 13 years old.
Previous reports on activation during verbal WMTs indicated a frontal and parietal predominance in adolescents and adults (Nagel et al., 2013, Thomason et al., 2009), while in children, activations are primarily observed over the premotor and parietal cortex, anterior insula, dorsal striatum and the cerebellum (Ciesielski et al., 2006). Several areas are responsible for neurodevelopment of verbal WM manipulation such as the lingual gyrus, the occipital poles and the right VLPFC (Brodmann area 9, 10, 46) (Yang et al., 2015). Thus, observing activations in some of these areas in both our groups at the limit of their WM capacity is not surprising.
The prefrontal cortex and parietal cortex play essential roles in active maintenance of WM (Cohen et al., 1997). In particular, dorsolateral prefrontal cortex is associated with manipulating and monitoring information held in WM (Fletcher and Henson, 2001), and is thought to be sensitive to increase in cognitive demand (Thomason et al., 2009). Previous studies have found increased activity in this region at high WM loads (Linden et al., 2003) and with greater task difficulty (Jaeggi et al., 2003). The insular cortex is known to respond in cognitively demanding tasks (Sridharan et al., 2008). The insula has been described as a multimodal region that supports task demands and attention systems and helps disengaging other systems in response to high demands (Eckert et al., 2009). The activation of insular cortex and the left DLPFC during WMT has been associated with increased task difficulty and with monitoring and maintenance of information (Curtis and D'Esposito, 2003). The fact that healthy controls activated the insula for high load and ECTS for medium load task supports the idea that the insula is recruited in the conditions of highest complexity in a given population.
Studies focusing on other forms of epilepsy than ECTS have reported altered WM-triggered fMRI responses. Patients with frontal lobe epilepsy demonstrated lower activation in several brain regions during a verbal WMT (Braakman et al., 2013). In children with temporal lobe epilepsy, WM deficits, notably in accuracy and response time, and reduced WM-triggered BOLD signal, were also reported (Oyegbile et al., 2018). Comparable patterns of decreased fMRI activation and behavioral performance during verbal WMT have been reported in autism (Urbain et al., 2015) and ADHD (Mattfeld et al., 2016). In contrast, patients with juvenile myoclonic epilepsy showed a pattern of activation during a verbal WM task which was similar or greater than those of healthy controls in the motor cortex and supplementary motor area (Vollmar et al., 2011, Wandschneider et al., 2014).
Our fMRI findings should also be interpreted in the context of previously shown structural brain changes in patients with ECTS, including abnormal cortical morphology and white matter development in fronto-parietal regions involved in WM, and reduced structural connectivity in sensorimotor and language areas (Besseling et al., 2013a, Ciumas et al., 2014, Garcia-Ramos et al., 2015, Garcia-Ramos et al., 2016, Hutchinson et al., 2010, Lin et al., 2012, Pardoe et al., 2013, Tosun et al., 2011, Widjaja et al., 2013).
Although the overall intellectual abilities of children with ECTS are preserved, this form of epilepsy is often accompanied by language impairments, particularly for reading, impulsiveness and difficulties in concentration, with a risk of academic underachievement (Croona et al. 1999). It has also been hypothesized that failure to meet WM demands might participate to academic underachievement (Gathercole and Alloway, 2006). In our cohort, children with ECTS demonstrated similar neuropsychological scores than controls, but deficits in WM performance. Memory evaluation in children with ECTS indicates significantly worse performance on tests that include WM testing compared to children without epilepsy (Verrotti et al., 2013, Vintan et al., 2012, Völkl-Kernstock et al., 2006). Consistent with previous studies of verbal WM in this age group, we noticed a significant effect of load on the performance with more errors, less accuracy, and longer response time for higher WM load. While this was observed in both groups, ECTS patients choose more often than controls to not respond to the task if they were not certain of their response.
Several factors might impair WM in patients with epilepsy, including antiepileptic medication, interictal, ictal or post-ictal epileptic activity, and underlying brain lesion and/or epileptogenic process. In our patients, AEDs did not appear to have a significant impact on either the neuropsychological, behavioral and fMRI findings. This might reflect the fact that only half of the patients were treated with AEDs, using a single drug except for one patient, and for a period of less than two years. Ictal and post-ictal activities are also unlikely to play any significant role, since the minimum and median time elapsed since the last seizure was 12 days and 4 months, respectively. Furthermore, the duration of epilepsy and total number of seizures did not have an impact on behavioral and neuropsychological performances. Yet, neuropsychological scores were associated with the time elapsed since the last seizure and age at onset of epilepsy, with better performance for the longest period without a seizure and an earlier age of onset. One hypothesis that could account for the above findings is that late onset of epilepsy and recent seizures are just surrogate markers of a more active ECTS-related neurobiological dysfunction at the time of the study, while a greater proportion of patients with early-onset epilepsy and long delay from last seizure would have already reached the stage of long-term remission. The interictal epileptiform abnormalities which characterize ECTS might well underlie the postulated alteration of WM function (Filippini et al., 2013). Another contributive factor could be that the age-dependent trajectory of WM development would render WM performance more sensitive to late- than early-onset ECTS, accounting for the observed association with age at first seizure but not with disease duration. Data from the literature offer different perspectives. Previous investigations in children with epilepsy (van Iterson and de Jong, 2018) and in those with ECTS (Lopes et al., 2014) found that earlier onset of epilepsy was associated with greater WM memory deficits, while later-onset was associated with a more persistent deficit.
A limitation of this study is its small sample size, precluding the investigation of various variables such as the laterality of the EEG focus, and warranting further studies with a larger number of participants to replicate or extend our findings.
5. Conclusion
Children with ECTS differed from healthy controls in how they modulate WM processes during tasks with increasing difficulty level, with engagement of the fronto-parietal network activity at lower WM load than that responsible for larger activation in HC, and reduced capacity to sustain high WM load from both a behavioral and fMRI point of view.
Data and code availability statement
The data described in this manuscript cannot be made openly available due to ethical and privacy issues of clinical data. Activation maps can be shared upon request from the corresponding author.
Funding
The study was supported by a grant from Picri (Partenariats Institution-Citoyens pour la Recherche et l'Innovation), Ile-de-France, and Epilepsy France Foundation. Dr Ciumas received financial support from Fondation pour la Recherche Médicale.
CRediT authorship contribution statement
Carolina Ciumas: Conceptualization, Methodology, Software, Validation, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Visualization. Alexandra Montavont: Conceptualization, Methodology. Faustine Ilski: Conceptualization, Validation, Formal analysis. Agathe Laurent: Conceptualization, Validation, Formal analysis. Mani Saignavongs: Investigation. Jean-Philippe Lachaux: Conceptualization, Software, Formal analysis. Julitta de Bellescize: Investigation. Eleni Panagiotakaki: Investigation. Karine Ostrowsky-Coste: Investigation. Vania Herbillon: Investigation. Danielle Ibarrola: Methodology, Investigation. Marc Hermier: Methodology, Investigation. Alexis Arzimanoglou: Investigation. Philippe Ryvlin: Conceptualization, Funding acquisition, Validation, Writing - review & editing.
Acknowledgements
We thank all subjects that took part in this research, as well as their parents for the time spent during all evaluations. We are indebted to many healthy volunteers, their families and teaching personnel from the “Sainte Marie Lyon les Maristes” school. We thank Valeriu Savcenco for help with data scripting.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102392.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alloway T.P., Gathercole S.E., Willis C., Adams A.M. A structural analysis of working memory and related cognitive skills in young children. J. Exp. Child. Psychol. 2004;87:85–106. doi: 10.1016/j.jecp.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cognitive Sci. 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory and language: an overview. J. Commun. Disord. 2003;36(3):189–208. doi: 10.1016/s0021-9924(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Baddeley A.D., Hitch G. Working Memory. In: Bower G.H., editor. Psychology of Learning and Motivation. Academic Press; 1974. pp. 47–89. [Google Scholar]
- Barendse E.M., Hendriks M.PH., Jansen J.FA., Backes W.H., Hofman P.AM., Thoonen G., Kessels R.PC., Aldenkamp A.P. Working memory deficits in high-functioning adolescents with autism spectrum disorders: neuropsychological and neuroimaging correlates. J. Neurodevelop. Disord. 2013;5(1) doi: 10.1186/1866-1955-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendse E.M., Schreuder L.J., Thoonen G., Hendriks M.P.H., Kessels R.P.C., Backes W.H., Aldenkamp A.P., Jansen J.F.A. Working memory network alterations in high-functioning adolescents with an autism spectrum disorder: fMRI correlates for memory in ASD. Psychiatry Clin. Neurosci. 2018;72(2):73–83. doi: 10.1111/pcn.12602. [DOI] [PubMed] [Google Scholar]
- Baum G.L., Cui Z., Roalf D.R., Ciric R., Betzel R.F., Larsen B., Cieslak M., Cook P.A., Xia C.H., Moore T.M., Ruparel K., Oathes D.J., Alexander-Bloch A.F., Shinohara R.T., Raznahan A., Gur R.E., Gur R.C., Bassett D.S., Satterthwaite T.D. Development of structure-function coupling in human brain networks during youth. Proc. Natl. Acad. Sci. U S A. 2020;117:771–778. doi: 10.1073/pnas.1912034117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bédard A.-C., Newcorn J.H., Clerkin S.M., Krone B., Fan J., Halperin J.M., Schulz K.P. Reduced Prefrontal Efficiency for Visuospatial Working Memory in Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53(9):1020–1030.e6. doi: 10.1016/j.jaac.2014.05.011. [DOI] [PubMed] [Google Scholar]
- Besseling, R.M., Jansen, J.F., Overvliet, G.M., van der Kruijs, S.J., Ebus, S.C., de Louw, A., Hofman, P.A., Vles, J.S., Aldenkamp, A.P., Backes, W.H., 2013a. Reduced structural connectivity between sensorimotor and language areas in rolandic epilepsy. PLoS One 8, e83568. [DOI] [PMC free article] [PubMed]
- Besseling R.M.H., Jansen J.F.A., Overvliet G.M., van der Kruijs S.J.M., Vles J.S.H., Ebus S.C.M., Hofman P.A.M., Louw A.d., Aldenkamp A.P., Backes W.H. Reduced functional integration of the sensorimotor and language network in rolandic epilepsy. NeuroImage: Clinic. 2013;2:239–246. doi: 10.1016/j.nicl.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseling R.M.H., Overvliet G.M., Jansen J.F.A., van der Kruijs S.J.M., Vles J.S.H., Ebus S.C.M., Hofman P.A.M., de Louw A.J.A., Aldenkamp A.P., Backes W.H. Aberrant functional connectivity between motor and language networks in rolandic epilepsy. Epilepsy Res. 2013;107(3):253–262. doi: 10.1016/j.eplepsyres.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Best J.R., Miller P.H., Naglieri J.A. Relations between executive function and academic achievement from ages 5 to 17 in a large, representative national sample. Learn. Individual Diff. 2011;21(4):327–336. doi: 10.1016/j.lindif.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhise, V.V., Burack, G.D., Mandelbaum, D.E., 2010. Baseline cognition, behavior, and motor skills in children with new-onset, idiopathic epilepsy. Dev Med Child Neurol 52, 22-26. [DOI] [PubMed]
- Bourel-Ponchel E., Mahmoudzadeh M., Adebimpe A., Wallois F. Functional and Structural Network Disorganizations in Typical Epilepsy With Centro-Temporal Spikes and Impact on Cognitive Neurodevelopment. Front Neurol. 2019;10:809. doi: 10.3389/fneur.2019.00809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman H.M.H., Vaessen M.J., Jansen J.F.A., Debeij-van Hall M.H.J.A., de Louw A., Hofman P.A.M., Vles J.S.H., Aldenkamp A.P., Backes W.H. Frontal lobe connectivity and cognitive impairment in pediatric frontal lobe epilepsy: fMRI in Pediatric Frontal Lobe Epilepsy. Epilepsia. 2013;54(3):446–454. doi: 10.1111/epi.12044. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Wright S.B. Neurodevelopmental changes in working memory and cognitive control. Curr. Opin. Neurobiol. 2007;17:243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Camos V., Barrouillet P. Developmental change in working memory strategies: from passive maintenance to active refreshing. Dev. Psychol. 2011;47:898–904. doi: 10.1037/a0023193. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Cohen J.D., Jezzard P., Turner R., Noll D.C., Trainor R.J., Giedd J., Kaysen D., Hertz-Pannier L., Rapoport J.L. Activation of Prefrontal Cortex in Children during a Nonspatial Working Memory Task with Functional MRI. NeuroImage. 1995;2(3):221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Ciesielski K.T., Lesnik P.G., Savoy R.L., Grant E.P., Ahlfors S.P. Developmental neural networks in children performing a Categorical N-Back Task. NeuroImage. 2006;33(3):980–990. doi: 10.1016/j.neuroimage.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Ciumas C., Laurent A., Saignavongs M., Ilski F., de Bellescize J., Panagiotakaki E., Ostrowsky-Coste K., Arzimanoglou A., Herbillon V., Ibarrola D., Ryvlin P. Behavioral and fMRI responses to fearful faces are altered in benign childhood epilepsy with centrotemporal spikes (BCECTS) Epilepsia. 2017;58(10):1716–1727. doi: 10.1111/epi.13858. [DOI] [PubMed] [Google Scholar]
- Ciumas, C., Saignavongs, M., Ilski, F., Herbillon, V., Laurent, A., Lothe, A., Heckemann, R.A., de Bellescize, J., Panagiotakaki, E., Hannoun, S., Marinier, D.S., Montavont, A., Ostrowsky-Coste, K., Bedoin, N., Ryvlin, P., 2014. White matter development in children with benign childhood epilepsy with centro-temporal spikes. Brain 137, 1095-1106. [DOI] [PubMed]
- Cohen J.D., Perlstein W.M., Braver T.S., Nystrom L.E., Noll D.C., Jonides J., Smith E.E. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Conklin H.M., Luciana M., Hooper C.J., Yarger R.S. Working Memory Performance in Typically Developing Children and Adolescents: Behavioral Evidence of Protracted Frontal Lobe Development. Dev. Neuropsychol. 2007;31(1):103–128. doi: 10.1207/s15326942dn3101_6. [DOI] [PubMed] [Google Scholar]
- Cowan, N., Rouder, J.N., Blume, C.L., Saults, J.S., 2012. Models of verbal working memory capacity: what does it take to make them work? Psychol Rev 119, 480-499. [DOI] [PMC free article] [PubMed]
- Croona, C., Kihlgren, M., Lundberg, S., Eeg-Olofsson, O., Eeg-Olofsson, K.E., 1999. Neuropsychological findings in children with benign childhood epilepsy with centrotemporal spikes. Dev Med Child Neurol 41, 813-818. [DOI] [PubMed]
- Curtis C.E., D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn. Sci. 2003;7(9):415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Datta A.N., Oser N., Bauder F., Maier O., Martin F., Ramelli G.P., Steinlin M., Weber P., Penner I.K. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2013;54:487–494. doi: 10.1111/epi.12067. [DOI] [PubMed] [Google Scholar]
- Eckert M.A., Menon V., Walczak A., Ahlstrom J., Denslow S., Horwitz A., Dubno J.R. At the heart of the ventral attention system: The right anterior insula. Hum. Brain Mapp. 2009;30(8):2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emch, M., von Bastian, C.C., Koch, K., 2019. Neural Correlates of Verbal Working Memory: An fMRI Meta-Analysis. Front Hum Neurosci 13, 180. [DOI] [PMC free article] [PubMed]
- Fang J., Chen S., Luo C., Gong Q., An D., Zhou D. Altered language network in benign childhood epilepsy patients with spikes from non-dominant side: A resting-state fMRI study. Epilepsy Res. 2017;136:109–114. doi: 10.1016/j.eplepsyres.2017.07.020. [DOI] [PubMed] [Google Scholar]
- Filippini M., Boni A., Giannotta M., Gobbi G. Neuropsychological development in children belonging to BECTS spectrum: Long-term effect of epileptiform activity. Epilepsy Behav. 2013;28(3):504–511. doi: 10.1016/j.yebeh.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Fletcher P.C., Henson R.N. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramos C., Jackson D.C., Lin J.J., Dabbs K., Jones J.E., Hsu D.A., Stafstrom C.E., Zawadzki L., Seidenberg M., Prabhakaran V., Hermann B.P. Cognition and brain development in children with benign epilepsy with centrotemporal spikes. Epilepsia. 2015;56(10):1615–1622. doi: 10.1111/epi.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Ramos C., Lin J.J., Bonilha L., Jones J.E., Jackson D.C., Prabhakaran V., Hermann B.P. Disruptions in cortico-subcortical covariance networks associated with anxiety in new-onset childhood epilepsy. NeuroImage: Clinic. 2016;12:815–824. doi: 10.1016/j.nicl.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathercole S.E., Alloway T.P. Practitioner Review: Short-term and working memory impairments in neurodevelopmental disorders: diagnosis and remedial support. J. Child. Psychol. Psychiat. 2006;47(1):4–15. doi: 10.1111/j.1469-7610.2005.01446.x. [DOI] [PubMed] [Google Scholar]
- Gathercole S.E., Pickering S.J., Ambridge B., Wearing H. The structure of working memory from 4 to 15 years of age. Dev. Psychol. 2004;40:177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Geier C.F., Garver K., Terwilliger R., Luna B. Development of Working Memory Maintenance. J. Neurophysiol. 2009;101(1):84–99. doi: 10.1152/jn.90562.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn D.C., Kim J., Cohen M.S., Poutanen V.P., Therman S., Bava S., Van Erp T.G., Manninen M., Huttunen M., Lonnqvist J., Standertskjold-Nordenstam C.G., Cannon T.D. Maintenance and manipulation in spatial working memory: dissociations in the prefrontal cortex. NeuroImage. 2002;17:201–213. doi: 10.1006/nimg.2002.1161. [DOI] [PubMed] [Google Scholar]
- Gur, R.C., Gur, R.E., 2013. Memory in health and in schizophrenia. Dialogues in clinical neuroscience 15, 399-410. [DOI] [PMC free article] [PubMed]
- Gur, R.C., Richard, J., Calkins, M.E., Chiavacci, R., Hansen, J.A., Bilker, W.B., Loughead, J., Connolly, J.J., Qiu, H., Mentch, F.D., Abou-Sleiman, P.M., Hakonarson, H., Gur, R.E., 2012. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology 26, 251-265. [DOI] [PMC free article] [PubMed]
- Hutchinson E., Pulsipher D., Dabbs K., y Gutierrez A.M., Sheth R., Jones J., Seidenberg M., Meyerand E., Hermann B. Children with new-onset epilepsy exhibit diffusion abnormalities in cerebral white matter in the absence of volumetric differences. Epilepsy Res. 2010;88(2-3):208–214. doi: 10.1016/j.eplepsyres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILAE Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy 1989. Epilepsia. 1989;30:389–399. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- Jaeggi S.M., Seewer R., Nirkko A.C., Eckstein D., Schroth G., Groner R., Gutbrod K. Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. NeuroImage. 2003;19(2):210–225. doi: 10.1016/s1053-8119(03)00098-3. [DOI] [PubMed] [Google Scholar]
- Koshino H., Carpenter P.A., Minshew N.J., Cherkassky V.L., Keller T.A., Just M.A. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24(3):810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Levy R., Goldman-Rakic P.S. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp. Brain Res. 2000;133(1):23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Lillywhite, L.M., Saling, M.M., Harvey, A.S., Abbott, D.F., Archer, J.S., Vears, D.F., Scheffer, I.E., Jackson, G.D., 2009. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia 50, 2276-2284. [DOI] [PubMed]
- Lin, J.J., Riley, J.D., Hsu, D.A., Stafstrom, C.E., Dabbs, K., Becker, T., Seidenberg, M., Hermann, B.P., 2012. Striatal hypertrophy and its cognitive effects in new-onset benign epilepsy with centrotemporal spikes. Epilepsia 53, 677-685. [DOI] [PMC free article] [PubMed]
- Linden D.E.J., Bittner R.A., Muckli L., Waltz J.A., Kriegeskorte N., Goebel R., Singer W., Munk M.H.J. Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. NeuroImage. 2003;20(3):1518–1530. doi: 10.1016/j.neuroimage.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Logie R.H. L. Erlbaum; Hove: 1995. Visuo-spatial working memory. [Google Scholar]
- Lopes A.F., Monteiro J.P., Fonseca M.J., Robalo C., Simões M.R. Memory Functioning in Children with Epilepsy: Frontal Lobe Epilepsy, Childhood Absence Epilepsy, and Benign Epilepsy with Centrotemporal Spikes. Behav. Neurol. 2014;2014:1–8. doi: 10.1155/2014/218637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., O’Hearn K. What has fMRI told us about the Development of Cognitive Control through Adolescence? Brain Cogn. 2010;72(1):101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait D., Tucholka A., Mendizabal S., Tremblay J., Poulin C., Oskoui M., Srour M., Carmant L., Major P., Lippé S. fMRI brain response during sentence reading comprehension in children with benign epilepsy with centro-temporal spikes. Epilepsy Res. 2015;117:42–51. doi: 10.1016/j.eplepsyres.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Marchetti, G., 2014. Attention and working memory: two basic mechanisms for constructing temporal experiences. Frontiers in Psychology 5. [DOI] [PMC free article] [PubMed]
- Martinussen Rhonda, Hayden Jill, Hogg-Johnson Sheilah, Tannock Rosemary. A Meta-Analysis of Working Memory Impairments in Children With Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(4):377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- Massat, I., Slama, H., Kavec, M., Linotte, S., Mary, A., Baleriaux, D., Metens, T., Mendlewicz, J., Peigneux, P., 2012. Working memory-related functional brain patterns in never medicated children with ADHD. PLoS One 7, e49392. [DOI] [PMC free article] [PubMed]
- Mather M., Sutherland M.R. Arousal-Biased Competition in Perception and Memory. Perspect. Psychol. Sci. 2011;6(2):114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattfeld A.T., Whitfield-Gabrieli S., Biederman J., Spencer T., Brown A., Fried R., Gabrieli J.D.E. Dissociation of working memory impairments and attention-deficit/hyperactivity disorder in the brain. NeuroImage: Clinic. 2016;10:274–282. doi: 10.1016/j.nicl.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnity C.J., Smith A.B., Yaakub S.N., Weidenbach Gerbase S., Gammerman A., Tyson A.L., Bell T.K., Elmasri M., Barker G.J., Richardson M.P., Pal D.K. Decreased functional connectivity within a language subnetwork in benign epilepsy with centrotemporal spikes. Epilepsia Open. 2017;2(2):214–225. doi: 10.1002/epi4.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E.K., Cohen J.D. An Integrative Theory of Prefrontal Cortex Function. Annu. Rev. Neurosci. 2001;24(1):167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Nagel B.J., Herting M.M., Maxwell E.C., Bruno R., Fair D. Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain Cogn. 2013;82(1):58–68. doi: 10.1016/j.bandc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan, N.S., Prabhakaran, V., Bunge, S.A., Christoff, K., Fine, E.M., Gabrieli, J.D., 2005. The role of the prefrontal cortex in the maintenance of verbal working memory: an event-related FMRI analysis. Neuropsychology 19, 223-232. [DOI] [PubMed]
- Nee, D.E., Brown, J.W., Askren, M.K., Berman, M.G., Demiralp, E., Krawitz, A., Jonides, J., 2013. A meta-analysis of executive components of working memory. Cereb Cortex 23, 264-282. [DOI] [PMC free article] [PubMed]
- Nee D.E., D'Esposito M. The Representational Basis of Working Memory. Curr. Top Behav. Neurosci. 2018;37:213–230. doi: 10.1007/7854_2016_456. [DOI] [PubMed] [Google Scholar]
- Needelman H., Schnoes C.J., Ellis C.R. The New WISC-IV: J. Dev. Behav. Pediatr. 2006;27(2):127–128. doi: 10.1097/00004703-200604000-00007. [DOI] [PubMed] [Google Scholar]
- Northcott E., Connolly A.M., Berroya A., McIntyre J., Christie J., Taylor A., Bleasel A.F., Lawson J.A., Bye A.M.E. Memory and phonological awareness in children with Benign Rolandic Epilepsy compared to a matched control group. Epilepsy Res. 2007;75(1):57–62. doi: 10.1016/j.eplepsyres.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Northcott E., Connolly A.M., Berroya A., Sabaz M., McIntyre J., Christie J., Taylor A., Batchelor J., Bleasel A.F., Lawson J.A., Bye A.M.E. The Neuropsychological and Language Profile of Children with Benign Rolandic Epilepsy. Epilepsia. 2005;46(6):924–930. doi: 10.1111/j.1528-1167.2005.62304.x. [DOI] [PubMed] [Google Scholar]
- O'Hare, E.D., Lu, L.H., Houston, S.M., Bookheimer, S.Y., Sowell, E.R., 2008. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. NeuroImage 42, 1678-1685. [DOI] [PMC free article] [PubMed]
- Oldfield R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oostrom, K.J., van Teeseling, H., Smeets-Schouten, A., Peters, A.C., Jennekens-Schinkel, A., Dutch Study of Epilepsy in, C., 2005. Three to four years after diagnosis: cognition and behaviour in children with 'epilepsy only'. A prospective, controlled study. Brain 128, 1546-1555. [DOI] [PubMed]
- Oser N., Hubacher M., Specht K., Datta A.N., Weber P., Penner I.-K. Default mode network alterations during language task performance in children with benign epilepsy with centrotemporal spikes (BECTS) Epilepsy Behav. 2014;33:12–17. doi: 10.1016/j.yebeh.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E.d. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyegbile T.O., VanMeter J.W., Motamedi G., Zecavati N., Santos C., Chun C.L.E., Gaillard W.D., Hermann B. Executive dysfunction is associated with an altered executive control network in pediatric temporal lobe epilepsy. Epilepsy Behav. 2018;86:145–152. doi: 10.1016/j.yebeh.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayiotopoulos, C.P., 2005. Benign Childhood Focal Seizures and Related Epileptic Syndromes. In: International League against Epilepsy. (Ed.), The epilepsies : seizures, syndromes and management : based on the ILAE classifications and practice parameter guidelines. Bladon Medical, Chipping Norton, Oxon., pp. xvi, 541 p.
- Pardoe H.R., Berg A.T., Archer J.S., Fulbright R.K., Jackson G.D. A neurodevelopmental basis for BECTS: Evidence from structural MRI. Epilepsy Res. 2013;105(1-2):133–139. doi: 10.1016/j.eplepsyres.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J.D. Ragland B.I. Turetsky R.C. Gur F. Gunning-Dixon T. Turner L. Schroeder R. Chan R.E. Gur Working memory for complex figures: An fMRI comparison of letter and fractal n-back tasks. Neuropsychology 16 3 370 379 10.1037/0894-4105.16.3.370 http://doi.apa.org/getdoi.cfm?doi=10.1037/0894-4105.16.3.370. [PMC free article] [PubMed]
- Ranganath C., D’Esposito M. Directing the mind's eye: prefrontal, inferior and medial temporal mechanisms for visual working memory. Curr. Opin. Neurobiol. 2005;15(2):175–182. doi: 10.1016/j.conb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Rottschy C., Langner R., Dogan I., Reetz K., Laird A.R., Schulz J.B., Fox P.T., Eickhoff S.B. Modelling neural correlates of working memory: A coordinate-based meta-analysis. NeuroImage. 2012;60(1):830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. Oxford University Press, Oxford; New York: 1997. Autism as an executive disorder. [Google Scholar]
- Satterthwaite T.D., Ruparel K., Loughead J., Elliott M.A., Gerraty R.T., Calkins M.E., Hakonarson H., Gur R.C., Gur R.E., Wolf D.H. Being right is its own reward: Load and performance related ventral striatum activation to correct responses during a working memory task in youth. NeuroImage. 2012;61(3):723–729. doi: 10.1016/j.neuroimage.2012.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T.D., Wolf D.H., Erus G., Ruparel K., Elliott M.A., Gennatas E.D., Hopson R., Jackson C., Prabhakaran K., Bilker W.B., Calkins M.E., Loughead J., Smith A., Roalf D.R., Hakonarson H., Verma R., Davatzikos C., Gur R.C., Gur R.E. Functional Maturation of the Executive System during Adolescence. J. Neurosci. 2013;33(41):16249–16261. doi: 10.1523/JNEUROSCI.2345-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., Hirsch E., Jain S., Mathern G.W., Moshé S.L., Nordli D.R., Perucca E., Tomson T., Wiebe S., Zhang Y.-H., Zuberi S.M. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten, A., Oostrom, K.J., Pestman, W.R., Peters, A.C., Jennekens-Schinkel, A., Dutch Study Group of Epilepsy in, C., 2002. Learning and memory of school children with epilepsy: a prospective controlled longitudinal study. Dev Med Child Neurol 44, 803-811. [DOI] [PubMed]
- R. Schweickert B. Boruff Short-term memory capacity: Magic number or magic spell? Journal of Experimental Psychology: Learning, Memory, and Cognition 12 3 419 425 10.1037/0278-7393.12.3.419 http://doi.apa.org/getdoi.cfm?doi=10.1037/0278-7393.12.3.419. [DOI] [PubMed]
- Sreenivasan K.K., Curtis C.E., D’Esposito M. Revisiting the role of persistent neural activity during working memory. Trends Cogn. Sci. 2014;18(2):82–89. doi: 10.1016/j.tics.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg S. High-Speed Scanning in Human Memory. Science. 1966;153(3736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Race E., Burrows B., Whitfield-Gabrieli S., Glover G.H., Gabrieli J.D.E. Development of Spatial and Verbal Working Memory Capacity in the Human Brain. J. Cognit. Neurosci. 2009;21(2):316–332. doi: 10.1162/jocn.2008.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D., Dabbs K., Caplan R., Siddarth P., Toga A., Seidenberg M., Hermann B. Deformation-based morphometry of prospective neurodevelopmental changes in new onset paediatric epilepsy. Brain. 2011;134(4):1003–1014. doi: 10.1093/brain/awr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C.M. Urbain E.W. Pang M.J. Taylor Atypical spatiotemporal signatures of working memory brain processes in autism Transl Psychiatry 5 8 2015 e617 e617 http://www.nature.com/articles/tp2015107. [DOI] [PMC free article] [PubMed]
- van Ewijk H., Weeda W.D., Heslenfeld D.J., Luman M., Hartman C.A., Hoekstra P.J., Faraone S.V., Franke B., Buitelaar J.K., Oosterlaan J. Neural correlates of visuospatial working memory in attention-deficit/hyperactivity disorder and healthy controls. Psych. Res. Neuroimag. 2015;233(2):233–242. doi: 10.1016/j.pscychresns.2015.07.003. [DOI] [PubMed] [Google Scholar]
- van Iterson L., de Jong P.F. Development of verbal short-term memory and working memory in children with epilepsy: Developmental delay and impact of time-related variables. A cross-sectional study. Epilepsy Behav. 2018;78:166–174. doi: 10.1016/j.yebeh.2017.10.018. [DOI] [PubMed] [Google Scholar]
- Vannest J., Szaflarski J.P., Eaton K.P., Henkel D.M., Morita D., Glauser T.A., Byars A.W., Patel K., Holland S.K. Functional Magnetic Resonance Imaging Reveals Changes in Language Localization in Children With Benign Childhood Epilepsy With Centrotemporal Spikes. J. Child Neurol. 2013;28(4):435–445. doi: 10.1177/0883073812447682. [DOI] [PubMed] [Google Scholar]
- Veltman D.J., Rombouts S.A.R.B., Dolan R.J. Maintenance versus manipulation in verbal working memory revisited: an fMRI study. NeuroImage. 2003;18(2):247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- Verrotti A., Matricardi S., Di Giacomo D.L., Rapino D., Chiarelli F., Coppola G. Neuropsychological impairment in children with Rolandic epilepsy and in their siblings. Epilepsy Behav. 2013;28(1):108–112. doi: 10.1016/j.yebeh.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Vintan M.A., Palade S., Cristea A., Benga I., Muresanu D.F. A neuropsychological assessment, using computerized battery tests (CANTAB), in children with benign rolandic epilepsy before AED therapy. J. Med. Life. 2012;5:114–119. [PMC free article] [PubMed] [Google Scholar]
- Vogan V.M., Francis K.E., Morgan B.R., Smith M.L., Taylor M.J. Load matters: neural correlates of verbal working memory in children with autism spectrum disorder. J. Neurodevelop. Disord. 2018;10(1) doi: 10.1186/s11689-018-9236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogan V.M., Morgan B.R., Powell T.L., Smith M.L., Taylor M.J. The neurodevelopmental differences of increasing verbal working memory demand in children and adults. Dev. Cogn. Neurosci. 2016;17:19–27. doi: 10.1016/j.dcn.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völkl-Kernstock S., Willinger U., Feucht M. Spacial perception and spatial memory in children with benign childhood epilepsy with centro-temporal spikes (BCECTS) Epilepsy Res. 2006;72(1):39–48. doi: 10.1016/j.eplepsyres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Vollmar C., O'Muircheartaigh J., Barker G.J., Symms M.R., Thompson P., Kumari V., Duncan J.S., Janz D., Richardson M.P., Koepp M.J. Motor system hyperconnectivity in juvenile myoclonic epilepsy: a cognitive functional magnetic resonance imaging study. Brain. 2011;134(6):1710–1719. doi: 10.1093/brain/awr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyer D., Voyer S.D., Saint-Aubin J. Sex differences in visual-spatial working memory: A meta-analysis. Psychon. Bull. Rev. 2017;24(2):307–334. doi: 10.3758/s13423-016-1085-7. [DOI] [PubMed] [Google Scholar]
- Wandschneider, B., Centeno, M., Vollmar, C., Symms, M., Thompson, P.J., Duncan, J.S., Koepp, M.J., 2014. Motor co-activation in siblings of patients with juvenile myoclonic epilepsy: an imaging endophenotype? Brain 137, 2469-2479. [DOI] [PMC free article] [PubMed]
- Wang L.u., Carr M. Working Memory and Strategy Use Contribute to Gender Differences in Spatial Ability. Ed. Psych. 2014;49(4):261–282. [Google Scholar]
- Wechsler D., Psychological C. Pearson Education; London: 2004. WISC-IV UK. [Google Scholar]
- Widjaja E., Kis A., Go C., Raybaud C., Snead O.C., Smith M.L. Abnormal white matter on diffusion tensor imaging in children with new-onset seizures. Epilepsy Res. 2013;104(1-2):105–111. doi: 10.1016/j.eplepsyres.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Xiao F., Lei D.u., An D., Li L., Chen S., Chen F., Yang T., Ren J., Huang X., Gong Q., Zhou D. Functional brain connectome and sensorimotor networks in rolandic epilepsy. Epilepsy Res. 2015;113:113–125. doi: 10.1016/j.eplepsyres.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Yang Z., Jutagir D.R., Koyama M.S., Craddock R.C., Yan C.-G., Shehzad Z., Castellanos F.X., Di Martino A., Milham M.P. Intrinsic brain indices of verbal working memory capacity in children and adolescents. Dev. Cogn. Neurosci. 2015;15:67–82. doi: 10.1016/j.dcn.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Ramos C.G., Nair V.A., Hu Y., Liao J., La C., Chen L.i., Gan Y., Wen F., Hermann B., Prabhakaran V. Regional homogeneity (ReHo) changes in new onset versus chronic benign epilepsy of childhood with centrotemporal spikes (BECTS): A resting state fMRI study. Epilepsy Res. 2015;116:79–85. doi: 10.1016/j.eplepsyres.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in this manuscript cannot be made openly available due to ethical and privacy issues of clinical data. Activation maps can be shared upon request from the corresponding author.