Abstract
The soybean cyst nematode (SCN) (Heterodera glycines Ichinohe) is a devastating pest of soybean (Glycine max (L.) Merr.) in the world. Three soybean QTLs for resistance to SCN race 1 were detected through QTL analyses using recombinant inbred lines (RILs) derived from a cross between ‘Tokei 758’ (susceptible) and ‘To-8E’ (resistant to races 1 and 3, derived from ‘PI 84751’ and ‘Gedenshirazu’). Two of the three QTLs appear to be rhg1 and Rhg4 from their locations on the linkage map. The third QTL, detected around Satt359 on chromosome 11, was tentatively identified as rhg2. All RILs resistant to race 1 had all three QTLs. We developed lines carrying the three loci in various combinations, including all and none, from descendants of a cross between ‘NIL-SCN’ (with resistance derived from ‘PI 84751’ in the ‘Natto-shoryu’ background) and ‘Natto-shoryu’. Evaluating these lines in a race 1-infected field in Mito, Ibaraki, showed that resistance to race 1 required all three loci. Through field evaluation of 10 recombinant fixed pairs that we developed, we located the rhg2 locus to an 821 kb-region between SSR markers Sat_123 (=WGSP11_0140) and BARCSOYSSR11_1420 on chromosome 11.
Keywords: soybean cyst nematode resistance, rhg2, SCN, QTL, marker-assisted selection, soybean, Glycine max (L.) Merr.
Introduction
The soybean cyst nematode (SCN), Heterodera glycines Ichinohe, is a devastating pest of soybean (Glycine max (L.) Merr.) worldwide (Matsuo et al. 2017). In the USA, it is considered the most economically damaging pest of soybean (Allen et al. 2017). By infecting soybean roots, SCN suppresses plant growth, causing stunting and chlorosis (Noel 1999). The female’s body develops into a tough, lemon-shaped brown “cyst”, which protects the eggs for several years in the soil (Jackson 2014). SCN can enter the roots of both susceptible and resistant soybeans, but resistant soybean disrupts syncytium formation (Mahalingam and Skorupska 1996).
In Japan, SCN races 1, 3, and 5 were identified (Inagaki 1979) in a four-cultivar race test (Golden et al. 1970). Races 1 and 3 were dominant (Inagaki 1979, Shimizu and Momota 1992). Their HG (=“H. glycines”) types as determined by a seven-cultivar test (Niblack et al. 2002) are unknown. As donors of resistance to SCN, ‘Gedenshirazu’ (resistant to race 3) and ‘PI 84751’ (resistant to races 1 and 3) have been used in breeding programs. ‘Gedenshirazu’, a local cultivar from Akita, has been used since the 1950s, and its descendants include cultivars of the Toyomasari brand in Hokkaido (‘Yukihomare’, ‘Toyomusume’, ‘Toyokomachi’, ‘Toyoharuka’, and ‘Toyomizuki’) and ‘Ryuhou’, a main cultivar in the Tohoku region. ‘PI 84751’, from Korea, has been used as a donor of resistance to SCN races 1 and 3 since the 1960s. In 1980, ‘Suzuhime’, which own resistance derived from ‘PI 84751’, was released for natto production. ‘PI 84751’-type resistance was also introduced into ‘Yukihomare’, and ‘Yukihomare R’ was released in 2010.
Genes involved in resistance are still not clear, but some major genes have been identified. In the USA, where race 3 is predominant (Jackson 2014), resistance loci rhg1, rhg2, rhg3 (Caldwell et al. 1960), rhg4 (Matson and Williams 1965), and Rhg5 (Rao-Arelli et al. 1992) were reported. Those on chromosomes (Chrs.) 18 (rhg1) and 8 (Rhg4) have been consistently mapped as major quantitative trait loci (QTLs) (Concibido et al. 2004). The rhg1 type was classified as rhg1-a in Peking-type soybeans and rhg1-b in PI 88788-type soybeans (Brucker et al. 2005). rhg1-b can confer resistance to race 3 by itself (Concibido et al. 2004). In cultivars such as ‘Forrest’, with rhg1-a resistance, resistance to race 3 requires both rhg1 and dominant Rhg4 (Meksem et al. 2001). In rhg1-b cultivars, a high copy number of three genes (Glyma18g02580, Glyma18g02590, and Glyma18g02610 in Wm82.a1; Glyma.18g022400, Glyma.18g022500, and Glyma.18g022700 in Wm82.a2) in a 31-kb segment from PI 88788 contributes to PI 88788-type resistance (Cook et al. 2012). In rhg1-a cultivars, only one of the three genes is needed: Peking-type GmSNAP18 (Glyma18g02590 in Glyma1.0; Glyma.18g022500 in Glyma2.0) was enough to confer resistance in combination with Rhg4, which encodes a serine hydroxymethyltransferase (Liu et al. 2017).
In Japan, resistance derived from ‘PI 84751’ was due to three or four loci including rhg1, rhg2, (rhg3), and Rhg4 (Suzuki et al. 2012). Resistance to race 1 was due to rhg1 and Rhg4 derived from ‘PI 84751’ in progeny derived from crosses of lines resistant to race 3 (donor: ‘Gedenshirazu’) and lines resistant to races 1 and 3 (donor: ‘PI 84751’). Resistance to race 3 was stronger in ‘PI 84751’ than in ‘Gedenshirazu’ (Suzuki et al. 2012).
To identify more resistance genes, we performed QTL analysis using a hybrid population derived from a cross between a susceptible line and a resistant line developed from ‘PI 84751’. We detected three QTLs, two near rhg1 and Rhg4. Resistance to race 1 required all three QTLs. Using recombinant fixed pairs, we located the new QTL, tentatively identified as rhg2, in an 821-kb region on Chr. 11.
Materials and Methods
Plant materials
For QTL analyses, we used 199 RILs (‘Tokei 758’/’To-8E’) derived from a cross between susceptible ‘Tokei 758’ and resistant ‘To-8E’ developed in Tokachi, Hokkaido, by the single-seed-descent method. Both parents were distributed by the Hokkaido Research Organization.
To develop recombinant fixed pairs rapidly, we used ‘NIL-SCN’ and ‘Natto-shoryu’, distributed by the Ibaraki Agricultural Center (Ibaraki Prefecture, Japan). ‘NIL-SCN’ is a near-isogenic line of ‘Natto-shoryu’ with SCN resistance from ‘PI 84751’ and Soybean mosaic virus (SMV) resistance from ‘Seiken 2’. ‘NIL-SCN’ was surveyed its background using 349 SSR markers distributed in whole genome, and 41 out of 349 (11.7%) showed genotype which was different from ‘Natto-shoryu’. Because of its small seed size, ‘Natto-shoryu’ produces more seeds than cultivars of the usual seed size. So even in winter, it is possible to harvest enough number of seeds in greenhouse. In field and a greenhouse, we grew three generations per year. In August 2017, ‘NIL-SCN’ was crossed with ‘Natto-shoryu’, and eight resultant F1 seeds were planted in the greenhouse in November and selfed. In January 2018, we harvested F2 seeds and selected 44 out of 478 seeds carrying rhg1 and a recombination in the rhg2 region using DNA extracted from cotyledon of seed. We also selected F2 seeds carrying rhg1, rhg2, and Rhg4 in various combinations. In March, the selected F2 seeds were planted in the greenhouse (13 h dark/11 h light for 6 weeks) and the flowers were selfed. F3 seeds were harvested in May. From 40 F3 seeds, we selected fixed genotypes to make two recombinant fixed pairs, which we grew in the 2018 field test. From 368 F3 seeds, we selected eight pairs and planted them in a field in Tsukuba. In November 2018, F4 seeds were harvested, and eight pairs were obtained as lines.
QTL analysis and SSR markers
A linkage map of the 199 F9 (‘Tokei 758’/‘To-8E’) RILs carrying 162 SSR markers is described in Sayama et al. (2010). QTL analyses were performed by composite interval mapping as implemented in QTL Cartographer 2.5 software (Wang et al. 2005). Genome-wide threshold values (α = 0.05) were used to detect putative QTLs (1000 permutations) (Churchill and Doerge 1994), and markers with LOD > 2.5 were considered significant.
On the linkage map, and to develop recombinant fixed pairs, we used candidate polymorphic SSR markers (Song et al. 2004), GMES markers (Hisano et al. 2007), BARCSOYSSR markers (BARCSOYSSR_1.0; Song et al. 2010), WGSP markers (Fujii et al. 2018), and T markers (Watanabe et al. 2018). In developing F2 (‘NIL-SCN’/‘Natto-shoryu’) lines, we used ‘SCN_Res Bridge’ (Cook et al. 2012), which detects rhg1-b, to select seeds with rhg1, and ‘T000808358s’, which detects a 2-bp indel in intron 3 of Rhg4, to select seeds with Rhg4.
The locations of the markers were based on the genomic sequence of G. max ‘Williams 82’ assembly 2 pseudomolecules (Wm82.a2; Department of Energy Joint Genome Institute Community Sequencing Program 2013).
Evaluation of resistance to SCN race 1 in phytotron
The seeds of the 199 F11 (‘Tokei 758’/‘To-8E’) RILs, their parents, and the susceptible control cultivar ‘Suzumaru’ were sown in plastic trays (5 × 10 cells; 28 cm × 45 cm) filled with soil infested with a race 1 population (Golden et al. 1970). After 34 days, we counted the cysts on the roots of three plants. Numbers of cysts were indexed to that of ‘Suzumaru’ = 100.
Field trials and evaluation of resistance to SCN race 1
We planted the 10 recombinant fixed pairs and their parents, ‘NIL-SCN’ and ‘Natto-shoryu’, in a race 1-infested field at the Ibaraki Agricultural Center, Mito, in late June 2018 and 2019. We evaluated SCN resistance in 5 to 7 plants of each line, with two replicates. The numbers of cysts on roots were evaluated in late August to early September. We scored replicates in which two control lines with or without resistance to race 1 was significantly different at least at 1% level.
Cysts on soybean roots were scored from 0 (resistant) to 5 (susceptible) thus: 0, no cysts; 1, 0–10 cysts; 2, 11–30 cysts; 3, 31–50 cysts; 4, 51–100 cysts; and 5, >100 cysts. Scores of five plants were averaged for each line or cultivar.
We compared the two lines in each recombinant fixed pair and compared ‘Natto-shoryu’ and the F2 lines by F-test and t-test in JMP 13.2.1 software (SAS Institute, Cary, NC, USA). We also compared the numbers of cysts of two groups of RILs which genotype was ‘To-8E’-type or ‘Tokei 758’-type in each QTL region in the same way. We compared the numbers of cysts of each genotype (defined by the three resistance genes) with the ‘Natto-shoryu’ control by Tukey’s multiple comparison test in JMP 13.2.1.
Results
QTL analyses for resistance to SCN race 1
In the 199 RILs, we detected three QTLs on Chrs. 4 and 18 (Table 1, top). That with the largest effect was detected around Sat_210 (=WGSP18_0020, 1.6 Mb on the physical map) on Chr. 18. It accounted for 29% of the total variation, and its ‘To-8E’ allele conferred resistance to SCN race 1. We considered this QTL to be rhg1. The other two QTLs accounted for only 4% and 7% of the total variation. RILs were grouped according to genotype of the nearest marker in each QTL and compared by t-test (Supplemental Table 1). The number of cysts was significantly different between ‘To-8E’-type and ‘Tokei 758’-type only in rhg1.
Table 1.
QTLs for resistance to soybean cyst nematode race 1 detected in RILs (‘Tokei 758’/‘To-8E’) (LOD > 2.5)
| Chromosome | Marker intervala | cM | LODb | R2 (%)c | Additive effectd | Resistance allele | Reported resistance gene/QTL at the location |
|---|---|---|---|---|---|---|---|
| Using all 199 RILs | |||||||
| 4 | Satt607–AW277661 | 33.4–49.0 | 4.9 | 4 | 10 | To-8E | – |
| 18 | Sat_210–Satt235 | 0.0–36.6 | 18.1 | 29 | 27 | To-8E | rhg1 |
| 18 | Satt199–Satt288 | 77.4–103.4 | 4.2 | 7 | –12 | Tokei 758 | – |
| Using 58 RILs with rhg1 | |||||||
| 8 | Sat_162–Sat_215 | 10.5–15.9 | 5.6 | 26 | 20 | To-8E | Rhg4 |
| 11 | Satt359–Satt453 | 130.7–149.7 | 5.5 | 30 | 23 | To-8E | QTL on linkage group B1 |
a Nearest marker is underlined.
b LOD score of the nearest marker.
c Percentage of phenotypic variation explained by the nearest marker.
d Additive effect = ((‘P1’ allele effect) – (‘P2’ effect))/2.
To detect QTLs which confer resistance in combination with rhg1, we selected RILs with rhg1 and performed QTL analysis again. In 58 such RILs, we detected two QTLs on Chrs. 8 and 11, which accounted for 26% and 30% of the total variation (Table 1, bottom). We considered the QTL near Sat_162 (=WGSP08_0060, 8.3 Mb) on Chr. 8 to be Rhg4. We tentatively designated that near Satt_359 (32.4 Mb) on Chr. 11, with a larger effect than Rhg4, as rhg2.
Alleles of rhg2 locus
The genotype of the three loci in the parents of the RILs and the donors of resistance showed that the resistant parent, ‘To-8E’, had PI 84751-type rhg1 and Rhg4 and Gedenshirazu-type rhg2 (Table 2). So ‘To-8E’, which is resistant to race 1, was found to have Gedenshirazu-type rhg2 and PI 84751-type rhg1 and Rhg4. The susceptible parent, ‘Tokei 758’, had non-PI 84751-type, non-Gedenshirazu-type rhg1, Rhg4, and rhg2.
Table 2.
Genotypes of three resistance loci in parents of RILs and donors of resistance
| Cultivar/line | SCN resistance | rhg1 | Rhg4 | rhg2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| race 1 | race 3 | Sat_210 | Sat_162 | Sat_123 | Satt453a | ||||
| PI 84751 | R | R | P | P | P | P | |||
| Gedenshirazu | S | R | G | G | G | G | |||
| P1: Tokei 758 | S | S | other | other | other | other | |||
| P2: To-8E | R | R | P | P | G | G | |||
P, PI 84751-type; G, Gedenshirazu-type. Note that PI 84751 (resistant to SCN race 1) own rhg1, Rhg4 and rhg2, and that Gedenshirazu (resistant to race 3) own only rhg1 and rhg2.
a Satt453 (34.2 Mb on Chromosome 11) is 348 kb downstream of Satt484.
Segregation distortion in RILs
QTL analyses detected three QTLs, but the segregation ratios of the resistant RILs did not fit the expected ratio of 1:7: only eight RILs were resistant among the 199 RILs (Table 3), fitting the segregation ratio of 1:15 (resistant:susceptible), at which four loci are expected (Supplemental Table 2). This result suggests segregation distortion.
Table 3.
Genotypes of three loci for resistance to SCN and phenotype of RILs (‘Tokei 758’/‘To-8E’)
| Resistance genes | QTL | ||||||
|---|---|---|---|---|---|---|---|
| Markera | rhg1 | Rhg4 | rhg2 | Number of RILsb | Number of cystsc | ||
| Sat_210 | Sat_162 | Satt359 | Average SDd | ||||
| + | + | + | 8 | 4.9 ± 7.0 | a | ||
| + | + | – | 25 | 41.2 ± 9.6 | b | ||
| + | – | + | 8 | 40.9 ± 13.3 | bc | ||
| – | + | + | 26 | 47.7 ± 5.0 | c | ||
| + | – | – | 17 | 43.1 ± 12.7 | bc | ||
| – | + | – | 41 | 49.2 ± 5.1 | c | ||
| – | – | + | 25 | 47.4 ± 4.9 | bc | ||
| – | – | – | 47 | 48.8 ± 5.3 | c | ||
a + ‘To-8E’-type (resistant to race 1); – ‘Tokei 758’-type (susceptible).
b RILs heterozygous or not detected at any marker are not shown.
c Number of cysts was indexed that of ‘Suzumaru’ = 100.
d Tukey’s multiple comparison test.
Resistance in genotypes with three resistance loci in various combinations
All RILs were grouped into eight genotypes by three markers, Sat_210 (rhg1), Sat_162 (Rhg4), and Satt_359 (rhg2). The eight RILs with the ‘To-8E’ allele at all three markers (Table 3, top row) had significantly fewer cysts than all other RILs. Thus, soybean resistance to SCN race 1 requires all three loci.
We evaluated F2 lines derived from ‘NIL-SCN’ × ‘Natto-shoryu’ in the race 1 field. Of the two replicates, we scored only one replicate since two control lines (‘Natto-shoryu’ and ‘NIL-SCN’) was not significantly different at 1% level. Only one F2 line, F2-1, having all three loci, had a lower cyst number score than ‘Natto-shoryu’ (Table 4).
Table 4.
Genotypes of three loci for resistance to SCN and phenotype of F2 (‘NIL-SCN’/‘Natto-shoryu’) lines
| Cultivar or linea | Marker | Resistance genes | QTL | Number of plants | Score of cyst number | ||||
|---|---|---|---|---|---|---|---|---|---|
| rhg1 | Rhg4 | rhg2 | Average SD | Pb | |||||
| SCN_Res Bridge | T000808358s | Satt359 (WGSP11_0150) | |||||||
| P1 | Natto-shoryu | –c | – | – | 5 | 4.2 ± 0.4 | |||
| P2 | NIL-SCNd | + | + | + | 5 | 0.8 ± 0.4 | <0.0001*** | ||
| F2 | F2-1 | + | + | + | 5 | 1.2 ± 0.4 | 0.0018** | ||
| F2-2 | + | + | – | 5 | 3.0 ± 1.0 | ns | |||
| F2-3 | + | + | – | 4 | 3.5 ± 0.9 | ns | |||
| F2-4 | + | + | – | 5 | 3.9 ± 0.2 | ns | |||
| F2-5 | + | + | – | 5 | 4.1 ± 0.5 | ns | |||
| F2-6 | + | – | + | 5 | 3.5 ± 0.3 | ns | |||
| F2-7 | + | – | – | 5 | 3.2 ± 1.1 | ns | |||
| P1 | Natto-shoryu | – | – | – | 5 | 3.6 ± 0.8 | |||
| P2 | NIL-SCNd | + | + | + | 5 | 1.2 ± 0.4 | 0.0018** | ||
| F2 | F2-8 | – | + | – | 5 | 3.7 ± 0.2 | ns | ||
| F2-9 | – | – | + | 5 | 3.2 ± 1.1 | ns | |||
| F2-10 | – | – | – | 5 | 3.7 ± 0.4 | ns | |||
a F2 lines from a cross between ‘NIL-SCN’ and ‘Natto-shoryu’. ‘NIL-SCN’ is a near-isogenic line with resistance from ‘PI 84751’ in the ‘Natto-shoryu’ background.
b ‘NIL-SCN’ and all F2 lines were compared with ‘Natto-shoryu’, their background.
c + ‘NIL-SCN’-type (resistant to race 1); – ‘Natto-shoryu’-type (susceptible).
d ‘NIL-SCN’ (resistance from ‘PI 84751’) was compared with ‘Natto-shoryu’ to verify the range of scores of cyst number.
Mapping rhg2 using recombinant fixed pairs in race 1 field
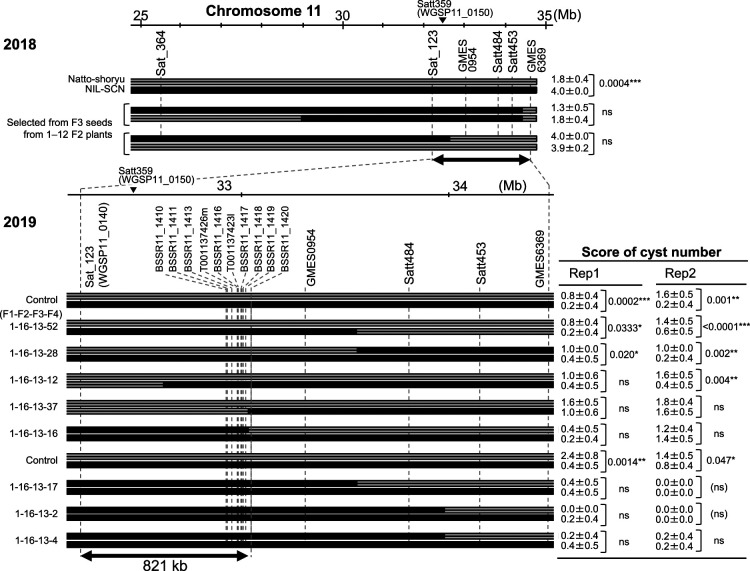
To locate the QTL on Chr. 11, we evaluated the recombinant fixed pairs in the race 1 field in Mito (Fig. 1). We evaluated two recombinant fixed pairs (selected from F3 (‘NIL-SCN’/‘Natto-shoryu’)) in 2018 (Fig. 1, top) and eight pairs (F4 lines) in 2019 (Fig. 1, bottom). An 821-kb region between Sat_123 (WGSP11_0140) and BARCSOYSSR11_1420 on Chr. 11 contained the resistance QTL.
Fig. 1.
Graphical genotypes of recombinant fixed pairs carrying ‘NIL-SCN’ fragments around the QTL regions on chromosomes 11. Except for ‘Natto-shoryu’, all lines and ‘NIL-SCN’ have rhg1 and Rhg4 in common. Top: F3 seeds selected in 2018. Bottom: F4 lines in 2019. Controls in 2019 were F4 lines with or without an ‘NIL-SCN’ fragment that covers this QTL region.  Positions of SSR markers. ‘BARCSOYSSR’ (part of the name of some markers) is abbreviated ‘BSSR’.
Positions of SSR markers. ‘BARCSOYSSR’ (part of the name of some markers) is abbreviated ‘BSSR’.  ‘Natto-shoryu’ fragments;
‘Natto-shoryu’ fragments;  ‘NIL-SCN’ fragments.
‘NIL-SCN’ fragments.  Nearest marker in QTL analysis. Scales (in Mb) are based on the ‘Williams 82’ genome (Wm82.a2). The significance of differences in scores of cyst number of recombinant fixed lines is shown on the right; N = 5.
Nearest marker in QTL analysis. Scales (in Mb) are based on the ‘Williams 82’ genome (Wm82.a2). The significance of differences in scores of cyst number of recombinant fixed lines is shown on the right; N = 5.
Discussion
We identified three QTLs for race 1 resistance in RILs derived from a cross between a susceptible parent and the resistant line ‘To-8E’, with ‘PI 84751’ and ‘Gedenshirazu’ ancestors (Table 1, Supplemental Fig. 1). We identified two of the three QTLs as rhg1 and Rhg4 from their locations. All three QTLs were needed for resistance: if any one of them was lacking, the plant was susceptible (Tables 3, 4). The third QTL, which we tentatively identified as rhg2, was detected in the marker interval Satt359–Satt453 and the nearest marker was Satt359 (WGSP11_0150) (Table 1). Since ‘To-8E’ had Gedenshirazu-type rhg2 (Table 2), this rhg2 was derived from ‘Gedenshirazu’.
In the QTL analysis using 199 RILs, rhg2 and Rhg4 were not detected (Table 1, above). The resolution power would be insufficient since only eight out of 199 RILs had all three loci (Table 2). We solved this problem by another QTL analysis using 58 RILs which had rhg1 (Table 1, bottom).
Suzuki et al. (2012) reported that rhg2 derived from either ‘PI 84751’ or ‘Gedenshirazu’ would suffice to make soybean plants resistant to race 1, as both rhg1 and Rhg4 were derived from ‘PI 84751’. This statement is consistent with the fact that ‘To-8E’, which is resistant to race 1, has Gedenshirazu-type rhg2 and PI 84751-type rhg1 and Rhg4 (Table 2). ‘To-8E’ has both ‘PI 84751’ and ‘Gedenshirazu’ as ancestors (Supplemental Fig. 1) (Tanaka and Yumoto 2002, Tanaka et al. 2015). In its pedigree, race 1 resistance was continuously selected, so plants with PI 84751-type rhg1 and Rhg4 and PI 84751- or Gedenshirazu-type rhg2 would have been selected.
We also located rhg2 derived from ‘PI 84751’ in an 821-kb region between SSR markers Sat_123 (=WGSP11_0140) and BARCSOYSSR11_1420, on Chr. 11, as shown through evaluation of recombinant fixed pairs with the ‘Natto-shoryu’ background (Fig. 1). This 821-kb region overlapped the region which QTL was detected although rhg2 derived from ‘Gedenshirazu’ and that derived from ‘PI 84751’ have not confirmed to be the same gene. The location of rhg2 lay within the region in which ‘QTL on linkage group B1’ associated with resistance to races 2 and 5 was detected (Table 1, right, Guo et al. 2005). Further research is needed to clarify whether they are the same QTL or not.
Race 1 resistance in PI 84751 is independently controlled by rhg1, Rhg4, and rhg2 (and possibly rhg3) (Suzuki et al. 2012). We demonstrated that race 1 resistance is controlled by three genes, two of which are apparently rhg1 and Rhg4 (Table 1). Segregation distortion of the ratio of resistant plants in hybrid populations might explain why more than three genes were reported in the 1960s (Caldwell et al. 1960, Matson and Williams 1965). Here, we observed segregation distortion in the hybrid population used in the QTL analyses (Supplemental Table 2): the observed segregation ratio was lower than expected, and fitted the hypothesis in which not three but four loci were involved.
In the USA, PI 88788-type rhg1 confers resistance to SCN race 3. The combination of Peking-type rhg1 and Rhg4 confers resistance to races 1 and 3. Our results show that the combination of PI 84751-type rhg1 and Rhg4 and Gedenshirazu-type rhg2 is needed to confer resistance to race 1. Since PI 84751 is resistant to race 1, the combination of PI 84751-type rhg1 and Rhg4 and PI 84751 or Gedenshirazu-type rhg2 can confer resistance to race 1. This combination would confer resistance to race 3 also since all cultivars which are resistant to race 1 are resistant to race 3 also (Suzuki et al. 2012). The reason why rhg2 is also needed is unknown: The rhg1 genes in PI 88788, Peking, and PI 84571 might be different from each other. The SCN race 1 population in Japan might not be the same as that in the USA, but races in Japan have not been classified by HG type (Niblack et al. 2002). Further research is needed to identify why resistance derived from ‘PI 84751’ needs rhg2 in addition to rhg1 and Rhg4.
In conclusion, our detection of a third SCN resistance QTL, tentatively designated rhg2, in addition to rhg1 and Rhg4, through QTL analyses using RILs, one parent of which had race 1 resistance derived from ‘PI 84751’, shows that all three QTLs are needed to confer resistance to race 1. By evaluating recombinant inbred pairs, we located rhg2 to an 821-kb region on Chr. 11. For the selection of plants with rhg2 for breeding, further mapping is needed to obtain closer markers to rhg2 than ours at 821 kb.
Author Contribution Statement
CS, YY and RO performed the QTL analyses. FT-S designed the experiments and developed recombinant inbred pairs, which CI, MI and TM evaluated in the field. FT-S wrote the paper.
Supplementary Material
Acknowledgments
This work was supported by grants from the Ministry of Agriculture, Forestry and Fisheries of Japan (‘Development of mitigation and adaptation techniques to global warming in the sectors of agriculture forestry and fisheries’ Soybean 2002 and ‘Project for Climate Change’ Soybean BGW3301).
Literature Cited
- Allen T.W., Bradley C.A., Sisson A.J., Byamukama E., Chilvers M.I., Coker C.M., Collins A.A., Damicone J.P., Dorrance A.E., Dufault N.S. et al. (2017) Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Prog. 18: 19–27. [Google Scholar]
- Brucker E., Carlson S., Wright E., Niblack T. and Diers B. (2005) Rhg1 alleles from soybean PI437654 and PI88788 respond differentially to isolates of Heterodera glycines in the greenhouse. Theor. Appl. Genet. 111: 44–49. [DOI] [PubMed] [Google Scholar]
- Caldwell B.E., Brim C.A. and Ross J.P. (1960) Inheritance of resistance of soybeans to the cyst nematode, Heterodera glycines. Agron. J. 52: 635–636. [Google Scholar]
- Churchill G.A. and Doerge R.W. (1994) Empirical threshold values for quantitative trait mapping. Genetics 138: 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concibido V.C., Diers B.W. and Arelli P.R. (2004) A decade of QTL mapping for cyst nematode resistance in soybean. Crop Sci. 44: 1121–1131. [Google Scholar]
- Cook D.E., Lee T.G., Guo X., Melito S., Wang K., Bayless A.M., Wang J., Hughes T.J., Willis D.K., Clemente T.E. et al. (2012) Copy number variation of multiple genes at rhg1 mediates nematode resistance in soybean. Science 338: 1206–1209. [DOI] [PubMed] [Google Scholar]
- Department of Energy Joint Genome Institute Community Sequencing Program (2013) Soybase—Integrating Genetics and Genomics to Advance Soybean Research. https://soybase.org/. Accessed 2 March 2020.
- Fujii K., Sayama T., Takagi K., Kosuge K., Okano K., Kaga A. and Ishimoto M. (2018) Identification and dissection of single seed weight QTLs by analysis of seed yield components in soybean. Breed. Sci. 68: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden A.M., Epps J.M., Riggs R.D., Duclos L.A., Fox J.A. and Bernard R.L. (1970) Terminology and identity of intraspecific forms of the soybean cyst nematode (Heterodera glycines). Plant Dis. Rep. 54: 544–546. [Google Scholar]
- Guo B., Sleper D.A., Arelli P.R., Shannon J.G. and Nguyen H.T. (2005) Identification of QTLs associated with resistance to soybean cyst nematode races 2, 3 and 5 in soybean PI 90763. Theor. Appl. Genet. 111: 965–971. [DOI] [PubMed] [Google Scholar]
- Hisano H., Sato S., Isobe S., Sasamoto S., Wada T., Matsuno A., Fujishiro T., Yamada M., Nakayama S., Nakamura Y. et al. (2007) Characterization of the soybean genome using EST-derived microsatellite markers. DNA Res. 14: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki H. (1979) Race status or five Japanese populations of Heterodera glycines. Jpn. J. Nematol. 9: 1–4. [Google Scholar]
- Jackson, G. (2014) Soybean Cyst Nematode. Starkville, MS: State University Extension Service Publication, 1293. [Google Scholar]
- Liu S., Kandoth P.K., Lakhssassi N., Kang J., Colantonio V., Heinz R., Yeckel G., Zhou Z., Bekal S., Dapprich J. et al. (2017) The soybean GmSNAP18 gene underlies two types of resistance to soybean cyst nematode. Nat. Commun. 8: 14822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam R. and Skorupska H.T. (1996) Cytological expression of early response to infection by Heterodera glycines Ichinohe in resistant PI 437654 soybean. Genome 39: 986–998. [DOI] [PubMed] [Google Scholar]
- Matson A.L. and Williams L.F. (1965) Evidence of a fourth gene for resistance to soybean cyst nematode. Crop Sci. 5: 477. [Google Scholar]
- Matsuo, E., P. Alfonso and T. Sediyama (2017) Resistance to diseases. In: Soybean Breeding. Springer, Switzerland, pp. 329–350. [Google Scholar]
- Meksem K., Pantazopoulos P., Njiti V.N., Hyten L.D., Arelli P.R. and Lightfoot D.A. (2001) ‘Forrest’ resistance to the soybean cyst nematode is bigenic: saturation mapping of the Rhg1 and Rhg4 loci. Theor. Appl. Genet. 103: 710–717. [Google Scholar]
- Niblack T.L., Arelli P.R., Noel G.R., Opperman C.H., Orf J.H., Schmitt D.P., Shannon J.G. and Tylka G.L. (2002) A revised classification scheme for genetically diverse populations of Heterodera glycines. J. Nematol. 34: 279–288. [PMC free article] [PubMed] [Google Scholar]
- Noel, G.R. (1999) Soybean cyst nematode. In: Hartman, G.L., J.B. Sinclair and J.C. Rupe (eds.) Compendium of Soybean Diseases, 4th edn. APS Press, St. Paul, pp. 52–53. [Google Scholar]
- Rao-Arelli A.P., Anand S.C. and Wrather J.A. (1992) Soybean resistance to soybean cyst nematode race 3 is conditioned by an additional dominant gene. Crop Sci. 32: 862–864. [Google Scholar]
- Sayama T., Hwang T.-Y., Yamazaki H., Yamaguchi N., Komatsu K., Takahashi M., Suzuki C., Miyoshi T., Tanaka Y., Xia Z. et al. (2010) Mapping and comparison of quantitative trait loci for soybean branching phenotype in two locations. Breed. Sci. 60: 380–389. [Google Scholar]
- Shimizu, K. and Y. Momota (1992) Classification and races of soybean cyst nematode. The Course of Nematological Research, The Japanese Nematological Society, pp. 24–28. [Google Scholar]
- Song Q.J., Marek L.F., Shoemaker R.C., Lark K.G., Concibido V.C., Delannay X., Specht J.E. and Cregan P.B. (2004) A new integrated genetic linkage map of the soybean. Theor. Appl. Genet. 109: 122–128. [DOI] [PubMed] [Google Scholar]
- Song Q.J., Jia G.F., Zhu Y.L., Grant D., Nelson R.T., Hwang E.Y., Hyten D.L. and Cregan P.B. (2010) Abundance of SSR motifs and development of candidate polymorphic SSR markers (BARCSOYSSR_1.0) in soybean. Crop Sci. 50: 1950–1960. [Google Scholar]
- Suzuki C., Tanaka Y., Takeuchi T., Yumoto S. and Shirai S. (2012) Genetic relationships of soybean cyst nematode resistance originated in Gedenshirazu and PI84751 on Rhg1 and Rhg4 loci. Breed. Sci. 61: 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y. and Yumoto S. (2002) Near isogenic lines To-8E and To-8e for soybean maturity gene E_1. Rep. Hokkaido Branch, Jpn. Soc. Breed., Hokkaido Branch, Crop Sci. Soc. Jpn. 43: 87–88. [Google Scholar]
- Tanaka Y., Shirai S., Yumoto S., Matsukawa I., Hagihara S., Kurosaki H., Yamazaki H., Suzuki C., Ohnishi S. and Tsunoda M. (2015) A new soybean variety ‘Toyoharuka’. Bulletin of Hokkaido Research Organization Agricultural Experiment Stations 99: 47–60. [Google Scholar]
- Wang, S., C.J. Basten and Z.B. Zeng (2005) Windows QTL Cartographer 2.5. Department of Statistics, North Carolina State University, Raleigh, NC. [Google Scholar]
- Watanabe S., Shimizu T., Machita K., Tsubokura Y., Xia Z., Yamada T., Hajika M., Ishimoto M., Katayose Y., Harada K. et al. (2018) Development of a high-density linkage map and chromosome segment substitution lines for Japanese soybean cultivar Enrei. DNA Res. 25: 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.