Abstract
The gastrointestinal (GI) tract is innervated by the enteric nervous system (ENS), an extensive neuronal network that traverses along its walls. Due to local reflex circuits, the ENS is capable of functioning with and without input from the central nervous system. The functions of the ENS range from the propulsion of food to nutrient handling, blood flow regulation, and immunological defense. Records of it first being studied emerged in the early 19th century when the submucosal and myenteric plexuses were discovered. This was followed by extensive research and further delineation of its development, anatomy, and function during the next two centuries. The morbidity and mortality associated with the underdevelopment, infection, or inflammation of the ENS highlight its importance and the need for us to completely understand its normal function. This review will provide a general overview of the ENS to date and connect specific GI diseases including short bowel syndrome with neuronal pathophysiology and current therapies. Exciting opportunities in which the ENS could be used as a therapeutic target for common GI diseases will also be highlighted, as the further unlocking of such mechanisms could open the door to more therapy-related advances and ultimately change our treatment approach.
1. Introduction
The gastrointestinal (GI) tract is innervated by an extensive intrinsic network of ganglion-rich nerve connections known as the enteric nervous system (ENS) [1, 2]. The human ENS contains approximately 400-600 million neurons that can be found in two major networks—the myenteric and submucosal plexuses, which are also known as Auerbach's and Meissner's plexus, respectively [3–5]. The ENS is the largest and most complex unit of the peripheral nervous system and is located within the walls of the GI tract, extending from the esophagus to the anal canal [6, 7]. In fact, it has been classified as the third division of the autonomic nervous system in addition to the sympathetic and parasympathetic divisions by Langley during the early 20th century [1, 2, 7, 8]. The submucosal plexus lies just beneath the mucosal layer of the gut and is predominantly found in the small and large intestines, whereas the myenteric plexus is found between the circular and longitudinal layers of smooth muscle and can be found along the entire length of the GI tract [1, 6, 9]. Although it receives central nervous system (CNS) input via the vagus nerve and thoracolumbar and lumbosacral spinal cord, it has been shown very early on to act independently of the CNS [3, 10, 11]. The ENS possesses peristaltic motor, secretory, and immunological function in addition to more complex behaviors such as nonpropulsive mixing or segmentation, slow orthograde propulsion via the migrating myoelectric complex (MMC), retropulsion of noxious substances, and modification of nutrient handling and changing of local blood flow [1, 4]. This system is supported by peripheral glial cells called enteric glia that helps the ENS maintain the integrity of the epithelial barrier and that have been shown to play a role in intestinal inflammation and interaction with the microbiome [4, 12, 13]. In this review [14], we will provide a brief general overview of the history, embryology, anatomy, and function of the ENS to date as it relates to the small intestine in a way that the average reader can understand. We hope to make a novel contribution to the literature by connecting common GI disorders with specific neuronal pathophysiology and therapies and also summarize opportunities for future investigation including the potential role of the ENS and the intestinotrophic effect of glucagon-like peptide 2.
2. History
The study of the ENS dates back to the nineteenth century when German anatomist and neuropathologist, Leopold Auerbach, was credited with the discovery of Plexus myentericus Auerbachi or Auerbach's (myenteric) plexus in the mid-19th century [15, 16]. This was followed by the discovery of Meissner's (submucosal) plexus by German anatomist and physiologist, Georg Meissner, around the same time [16]. In 1899, two English scientists, Bayliss and Starling, published a series of articles detailing their experiments on the function of these plexuses and subsequently described the “Law of the Intestine” [3, 17]. This was the first demonstration of the peristaltic reflex and the ENS' ability to function independently of the CNS. This law was reproduced and further characterized by other early pioneers in the field of neurogastroenterology [1, 10, 18]. More specifically, Trendelenburg was the first to reliably reproduce the peristaltic reflex in a completely isolated intestine of the guinea pig with a stimulus that was easily adjustable [10]. The first attempts of morphological classification were made by Cajal and Dogiel who studied their morphology and microarchitecture identified by silver impregnation methods [15, 19]. In the 20th century, multiple attempts at further classification in an effort to support or refute Dogiel's efforts were made [6, 19, 20].
3. Embryology
The development of the ENS has largely been studied in murine and avian embryo models [21–23]. The majority of progenitor cells have been shown to originate and migrate from the vagal level of the neural crest along defined pathways ahead of the descending vagus nerve fibers, picking up cues from the microenvironment along the way before differentiating within the wall of the GI tract [22–25]. They migrate as chains proximodistally within the outer gut mesenchyme and remain in contact with one another for directional migration [26, 27]. A large subset of the vagal enteric neural crest-derived cells take a shortcut through the dorsal mesentery from the ileum to a loop of postcecal bowel, presumably the ascending colon [26]. Additionally, a small group of sacral crest-derived cells migrate to the bowel through the somatic mesenchyme and enter it with the extrinsic sacral nerves giving rise to approximately 20% of postumbilical neurons [21, 28–30]. However, this has recently become controversial as a recent report has called for the redefining of the sacral innervation [31]. During human gestation, the ENS becomes functional during the last trimester and continues to develop following birth [11]. Given the complexity of its development with the migration of cells from the neural crest, the ENS has many unique organizational features that make it similar to the CNS [32]. It lacks much of the internal collagen that creates connective tissue between neurons, and the supportive cells—enteric glia—resemble the astroglia of the CNS and less so Schwann cells (Figure 1) [12, 33–36]. In this regard, the symptoms of obstruction seen in Hirschsprung's disease (Table 1) occurs when a segment of bowel is deprived of ganglion cells secondary to defective migration of enteric glia. This highlights the importance of the ENS to the gut and its motor function [37].
Figure 1.
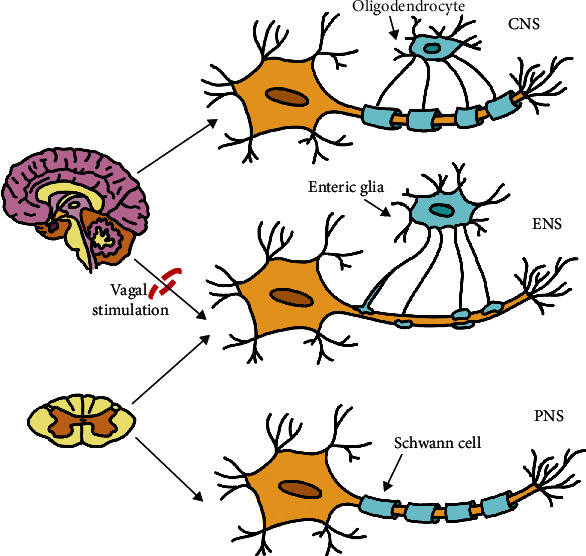
Similarities and differences of the ENS, CNS, and PNS and their supportive cells. CNS: central nervous system; ENS: enteric nervous system; PNS: peripheral nervous system.
Table 1.
Additional examples of ENS involvement in various GI diseases and its role as a therapeutic target.
| Disorder | ENS involvement∗ | Clinical feature(s) | Therapeutic targets† |
|---|---|---|---|
| Gut inflammation | Proinflammatory cytokine-mediated alteration of afferent nerves and enteric glia [49, 50] | Specific to inflammatory disorder (Crohn's, ulcerative colitis, or infectious diarrhea) | IL-1β, TNF-α, mast cell products, 5-HT3 agonists, substance P, and CGRP [49] |
| Hirschsprung's disease | Aganglionosis of myenteric and submucosal plexuses due to defective migration of neural crest cells, disruption of ICC network [52, 53] | Chronic constipation, obstruction, failure to thrive, toxic megacolon [37] | Neuronal stem cell therapy; exploitation of proliferative ICC signaling pathways [53–56] |
| Infectious secretory diarrhea | Prostanoid- and 5HT-mediated stimulation of secretomotor neurons triggered by inflammatory mediators released by mast cells and neutrophils [57–59] | Loose and watery stools, +/- blood, abdominal pain, dehydration, nutrient loss, sepsis [60] | Neural blockade, Loperamide [57–59, 61] |
| Diabetic diarrhea | Diabetic autonomic neuropathy resulting in vagal and sympathetic nerve damage [62] | Nocturnal watery and painless stools, +/- incontinence [63] | Unclear, codeine phosphate [63] Eluxadoline (NCT04313088) |
| Short bowel syndrome | Intestinotrophic effects mediated by presence of GLP-2 receptor on submucosal neurons and endocrine cells [64–66] | Intestinal failure resulting in malabsorption and malnutrition [67] | GLP-2 analogs such as Teduglutide [65, 67–69] |
| Chronic intestinal pseudoobstruction (CIPO) | Hyperactive but disorganized excitatory motor neurons due to dysfunctional or damaged inhibitory motor neurons and loss of ICC [53, 70–72] | Nausea, vomiting, abdominal pain, distention, constipation, diarrhea, malnutrition [71, 72] | Metoclopramide, erythromycin, octreotide, and neostigmine; proliferative ICC pathways [53, 72, 73] |
| Postoperative ileus | Increased sympathetic activity resulting from inhibitory neural reflexes from the spinal cord; release of inhibitory neurotransmitters and ICC loss (NO, VIP, substance P) [74–76] | Nausea, vomiting, abdominal distention, obstipation [77] | Octreotide and CGRP as potential therapies; proliferative ICC pathways [53, 73, 75, 76] |
| Parkinson's disease (PD) and Creutzfeldt-Jakob disease | Deposits of alpha-synuclein and misfolded proteins found in enteric neurons/glia [78, 79] | GI dysfunction, constipation, reservoir of prions [79] | Explore ENS role as a biomarker in these diseases |
∗May not represent a singular pathophysiological process of the disease. †Therapeutic targets may or may not be approved for clinical use.
4. Anatomy and Function
The ENS consists of up to 20 different types of neurons, containing more than all the sympathetic and parasympathetic ganglia combined and a similar amount of neurons to what is in the spinal cord [11, 33]. The major categories as observed by Furness et al. in the Burnstock laboratory include intrinsic primary afferent neurons (IPANs), motor neurons, and interneurons (Figure 2) [1, 9, 11, 15, 38]. These neurons are further classified based on their morphological (Dogiel types I-VII), electrical (types S and AH), chemical (neurotransmitters), and functional properties [15, 20, 39].
Figure 2.
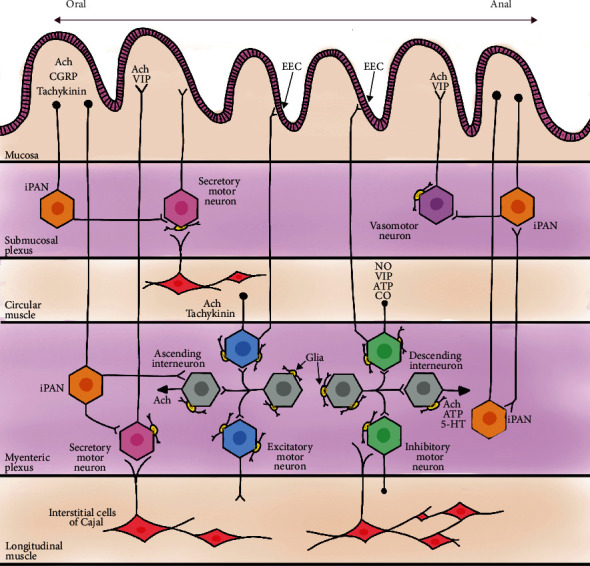
Drawing of the layers of the small intestine showing the complex ENS network, differentiated cells, and neurotransmitters. iPAN: intrinsic primary afferent neuron; EEC: enteroendocrine cell; Ach: acetylcholine; CGRP: calcitonin gene-related peptide; VIP: vasoactive intestinal peptide; ATP: adenosine triphosphate; NO: nitric oxide; CO: carbon monoxide; 5-HT: serotonin.
4.1. Intrinsic Primary Afferent Neurons
The intrinsic primary afferent neurons (IPANs) are some of the first sensory neurons to detect the physical state of the intestine. They are located in the submucosal and myenteric plexuses [40]. The primary neurotransmitters of IPANs are acetylcholine, calcitonin gene-related peptide (CGRP), and tachykinin; the secondary neurotransmitter is undetermined [4, 9, 41]. Morphologically, IPANs are classified as Dogiel type II [40]. They are round or oval in shape and create multiaxonal or pseudounipolar synapses with multiple types of neuronal elements to form intrinsic reflex circuits [40, 42, 43]. In the guinea pig model, myenteric sensory neurons of Dogiel type II morphology make up the majority (~97%) of neurons that project to the mucosa [44]. In the more complicated porcine model, most of the mucosal-projecting neurons live in the submucosal plexus, and a minority (12%) are located in the myenteric plexus. Of the latter group, approximately 23% of the myenteric neurons projecting to the mucosa are Dogiel type II, highlighting a stark difference in the proportion of primary afferent myenteric neurons between both models [20]. Notably, guinea pig and murine models show IPANs to be responsive to mucosal mechanical distortion, to distortion of their processes in the external muscle layers, and to chemicals that interact with the mucosa [40, 45–48]. Within this context, primary afferent nerves such as IPANs have been investigated with respect to their altered excitability and influence on motor activity in inflammatory disorders of the gut (Crohn's, ulcerative colitis, or infectious), and innovative therapeutic targets have been identified (Table 1) [49–51].
4.2. Motor Neurons
Motor neurons of the ENS innervate the circular and longitudinal muscle layers, intrinsic arterioles, and epithelium including enteroendocrine cells [9, 11, 32]. Five broad types have been identified as excitatory, inhibitory, secretomotor, vasomotor, and neurons innervating enteroendocrine cells [9]. The excitatory motor neurons predominantly use acetylcholine as their neurotransmitter with a small component of tachykinins (substance P) [9, 41, 80, 81]. They mainly innervate the circular muscle extending near the boundary of the submucosa and also project more orally compared to the inhibitory neurons [47]. The inhibitory motor neurons primarily use nitric oxide as their neurotransmitter, with vasoactive intestinal peptide (VIP), adenosine triphosphate (ATP), and carbon monoxide (CO) as secondary ones [82–85]. They project to muscle that is close (within 2 mm) to their cell bodies in the anal direction [47]. The excitatory motor neurons stimulate smooth muscle contraction whereas the inhibitory neurons discharge in a continuous fashion, and so inactivity of inhibitory neurons results in propulsive contraction towards the anus [15, 16]. The effects of both excitatory and inhibitory motor neurons have been shown in part to be mediated by the interstitial cells of Cajal (ICC), and this concept is supported by the presence of NO and excitatory tachykinin transmitter receptors on these cells [47, 86–90]. Abnormal excitatory and inhibitory input due to the effect of the autonomic nervous system (sympathetic inhibition of Ach release), neurotransmitters (VIP, NO, substance P, and CGRP), hormones such as corticotropin-releasing factor (CRF), endogenous opioids, and bowel manipulation has been shown to result in various forms of gastrointestinal dysmotility in animal experiments [70, 76, 91]. In addition, the inhibitory effect of anesthetics and morphine on gastrointestinal motility has been demonstrated in humans [76]. These identified mechanisms support the idea that the cause of intestinal pseudoobstruction and postoperative ileus is likely multifactorial and that the targeting of these pathways could lead to preventative and/or curative therapies (Table 1).
Secretomotor neuron cell bodies are located in the submucosal and myenteric plexuses; however, they are a part of secretomotor circuits that involve IPANs with nerve endings in the mucosa [47]. Their activity is initiated through the interaction of luminal contents such as glucose with the mucosa or by toxins such as cholera and enterotoxins [15, 92, 93]. Secretomotor neuron main function is to secrete chloride ions into the intestinal lumen dragging water molecules with them. They consist of a cholinergic and a noncholinergic type [47]. The noncholinergic type uses VIP or a related peptide as its primary neurotransmitter and mediates most of the local reflex response in contrast to the cholinergic neurons that act on muscarinic receptors on the mucosal epithelium [15, 93, 94].
Similar to the secretomotor neurons, the vasomotor neuron cell bodies are located in the submucosal plexus ganglia and their activity is presumed to also be mediated by IPANs, though not significantly [15, 47, 95]. They are the least studied type of motor neuron; however, there is enough evidence to suggest that they are split into cholinergic and noncholinergic neurons, with acetylcholine as the likely primary neurotransmitter and VIP as secondary [47, 95–98]. It is easy to understand how the secretomotor and vasomotor neurons work in tandem to regulate epithelial secretion and blood flow, and it is important to note that these reflexes are under extrinsic modulation via the sympathetics [9].
Enteroendocrine cells are highly specialized cells that reside in the intestinal mucosa interacting with various chemical and mechanical stimuli within the gut's lumen [15]. The major transmitters include, but are not limited to, cholecystokinin (CCK), secretin, somatostatin, serotonin (5-HT), corticotrophin-releasing factor, gastrin, leptin, ghrelin, and glucagon-like peptide 2 (GLP-2) [11, 15, 99]. They are released from these cells and interact with afferent nerve fibers in the lamina propria which in turn communicate with excitatory and inhibitory motor neurons [15]. The production of GLP-2 by enteroendocrine cells is worth highlighting further for the purpose of this review. There is evidence to suggest that these cells detect and participate in the transport of glucose across the mucosa via the activation of glucose transporters by GLP-2 [100]. However, the receptor for GLP-2 is on submucosal neurons as well, which implies that glucose transport could also be mediated by enteric neurons that are excited by GLP-2 [101, 102].
4.3. Interneurons
There are two main types of interneurons—ascending or orally directed interneurons and descending or anally directed interneurons [19]. They are primarily located in the myenteric plexus. Just like the motor neurons, the interneurons' primary neurotransmitter is acetylcholine. Furthermore, ATP has been identified as a secondary neurotransmitter especially in the descending type [47, 103, 104]. However, there is conflicting evidence on whether or not 5-HT is also a secondary neurotransmitter of the descending interneuron [40, 47, 104]. In the guinea pig, one type of ascending and three types of descending interneurons have been identified and have been noted to form chains that extend the length of the GI tract [9, 47]. The majority of the input to the ascending interneurons comes from IPANs, and the remaining input is from other ascending interneurons [47]. In contrast, the descending interneurons receive very little input from IPANs but rather from other descending interneurons. It is therefore thought that the descending interneurons are heavily involved in the MMC of the small intestine [40, 47].
5. Supporting Cells
5.1. Enteric Glia
The enteric glia are the supporting, nonneuronal cells of the myenteric and submucosal plexuses with an approximate ratio of glia to neurons of 2-3 to 1 [36, 79]. They are believed to originate from the neural crest and migrate to the bowel either at the time that the vagal- and sacral crest-derived cells do or later during gut development at the time of extrinsic nerve migration [12, 13]. A unique characteristic is the abundance of glial fibrillary acidic protein (GFAP) that is present in their cytoplasm compared to Schwann cells [12]. This is a result of the large amount of 10 nm intermediate filaments known as “gliofilaments” that they possess [34, 105, 106]. The enteric glia are far more irregular in shape compared to Schwann cells, and they have long processes that radiate out and terminate into small swellings called “end feet” forming an incomplete glial sheath that partially separates the myenteric neurons from the surrounding connective tissue [36]. Several neurotransmitters such as acetylcholine, catecholamines, glutamate, adenosine, and serotonin activate enteric glia [13]. They nourish neurons, maintain homeostasis, and are now being increasingly acknowledged as active regulators of multiple physiological processes [13, 79]. There is some evidence to suggest that enteric glia may have a neurosecretory function, just like astroglia are believed to play a role in controlling ionic flux in the CNS [36]. Enteric glia interact with various other nonneuronal cell types such as enterocytes, enteroendocrine, and immune cells which speak to their emerging role in regulating various intestinal functions and their involvement in pathological disorders such as diarrhea, Parkinson's disease, and Creutzfeldt-Jakob disease (Table 1) [13, 79].
5.2. Interstitial Cells of Cajal
The interstitial cells of Cajal (ICC) have been called the pacemakers of the GI tract due to their ability to produce cyclic spontaneous depolarization and slow waves described as the basic electrical rhythm [107]. They are responsible for initiating slow waves within the GI tract smooth muscle layers due to the lack of unique ion mechanisms within smooth muscle cells necessary to independently produce them [108]. Slow waves are needed to depolarize smooth muscle cells enough to activate calcium influx and trigger excitation-contraction coupling [109]. Furthermore, studies in humans and mice have suggested a mechanosensitive function induced by muscle stretch that then influences slow wave frequency and smooth muscle chronotropy; however, the underlying mechanisms are not fully understood [110, 111]. Experiments in avian and murine models have shown that ICC are derived from mesenchymal cells induced by kit signaling and develop independently from the enteric neuron [53, 112, 113]. Thus, they express c-kit—the marker by which these cells are identified—and a transmembrane receptor that induces receptor tyrosine kinase activity after the binding of its ligand, steel factor (kit ligand or stem cell factor) [107]. As mentioned earlier, they also possess receptors for tachykinins and NO produced by excitatory and inhibitory neurons, respectively, as well as for 5-HT [114, 115]. They are characterized by an elongated, fusiform body with few processes and are located at the junction of motor neurons and smooth muscle cells, forming connections similar to traditional synapses [107–109]. Moreover, ICC are involved in an integrated functional syncytium comprised of smooth muscle cells, ICC, and platelet-derived growth factor-positive cells (i.e., SIP syncytium) [109, 116]. From a pathological perspective, loss of ICC has been observed in a variety of human intestinal motility disorders including chronic intestinal pseudoobstruction (CIPO), Hirschsprung's disease, inflammatory bowel diseases (IBD), mechanical obstruction, and slow transit constipation (Table 1) [53, 109, 117, 118]. Though this remains controversial, ICC have also been suggested as a source of gastrointestinal stromal tumors (GIST) and as one of the reasons for the effectiveness of tyrosine kinase inhibitor, imatinib [53, 119].
6. Current ENS-Targeted Therapies of Common GI Diseases
In addition to the examples summarized in Table 1, several common GI diseases have established ENS-targeted therapies. A number of these therapies will be discussed below in more detail and within the context of the disease.
6.1. Achalasia
Botulinum toxin A is a highly selective neurotoxin that inhibits acetylcholine release from nerve terminals including those of the enteric neurons [120]. It exerts its effects by first gaining entry into the neuron via synaptic vesicle (SV2) receptors [121]. The toxin is produced by the bacterium Clostridium botulinum and was first isolated in the 1940s [121]. Since then, it has been extensively studied and is now used to treat a range of disorders affecting the nervous, urologic, ophthalmologic, dental, and gastrointestinal systems, among others [121, 122]. Achalasia is a rare motility disorder that affects the lower esophageal sphincter—resulting in dysphagia, chest pain, food intolerance, and recurrent aspirations that cause pneumonia [120, 123]. Although the root cause remains elusive, we now know that it is due to the loss of inhibitory neurons of the myenteric plexus resulting in failure of the lower esophageal sphincter (LES) to relax [124, 125]. While nitrates and calcium-channel blockers are often used to pharmacologically treat achalasia by acting on smooth muscle, botulinum toxin A has emerged as a safe and effective treatment that targets the excitatory motor neurons of the ENS [120, 126].
6.2. Slow Transit Constipation
Serotonin type 4 (5-HT4) receptor agonists such as prucalopride, tegaserod, cisapride, velusetrag, and naronapride have been shown to improve colonic motility in patients who suffer from slow transit constipation (STC) [127, 128]. Besides constipation, other symptoms of STC include abdominal pain, nausea, vomiting, and distention [129]. It is characterized by persistent constipation secondary to slow colonic transit that does not respond readily to dietary changes or laxatives [130]. Although the etiology is not entirely clear, this colonic dysmotility has been linked to a disruption of the autonomic and enteric nervous systems, as well as the neuroendocrine system [130–132]. More specifically, a reduced number of myenteric plexus neurons and ICC cells have been demonstrated in patients with slow transit constipation [125, 131–133]. Prokinetic 5-HT4 receptor agonists exert their effect by binding to the 5-HT4 receptor on the enteric neuron leading to the release of acetylcholine and other mediators of excitatory pathways that increase motility [134]. Of note, the nonselective 5-HT4 agonists—tegaserod and cisapride—have fallen out of favor due to their adverse cardiac side effects; however, the more recent and highly selective agonists such as prucalopride, velusetrag, and naronapride have been shown to be safer and much more tolerated [127]. Interestingly, prucalopride has also been shown to have a neuroprotective effect on the enteric nervous system [134].
6.3. Gastroparesis
As is the case in other gastrointestinal dysmotility disorders, 5-HT4 receptor agonists like prucalopride have also proven to be effective in the treatment of gastroparesis [135]. Symptoms of gastroparesis include early satiety, nausea, vomiting, postprandial fullness, and distention which are a result of delayed gastric emptying in the absence of a true mechanical obstruction [136]. The most common etiologies are idiopathy, diabetes, and postsurgery [137]. Both vagal and ENS dysfunctions have been demonstrated in humans with gastroparesis where the loss and injury of ICC and abnormal inhibitory and excitatory motor neurons, as well as a decrease in the number of enteric neurons, all contribute to its pathogenesis [135, 138–140]. Metoclopramide, primarily a D2 receptor antagonist with some 5-HT4 receptor agonism, leads to a gastric prokinetic effect by antagonizing synaptic D2 receptors and stimulating 5-HT4 receptors on the enteric neuron [141, 142]. Due to its ability to cross the blood-brain barrier, it also exerts a central antiemetic effect; however, it can cause extrapyramidal side effects as well which is why its use is limited to 12 weeks [135, 142]. Other pharmacologic treatments of gastroparesis symptoms that act on the enteric nervous system via their respective receptors include domperidone, levosulpiride, erythromycin, motilin, ghrelin, and ondansetron [125, 136, 142–144]. Of note, the approval and use of these medications vary from country to country.
6.4. Irritable Bowel Syndrome
Another class of drugs, not previously discussed, act on the opioid receptors of the ENS (e.g., Eluxadoline and Loperamide) [145–147]. For example, they target the ENS to provide relief of gastrointestinal (primarily diarrheal) symptoms seen in irritable bowel syndrome (IBS) patients [148]. Similarly, 5-HT3 receptor antagonists (e.g., alosetron and ondansetron) have also proven to be of clinical benefit in controlling diarrhea-predominant IBS [149, 150]. IBS is typically diagnosed using the Rome IV criteria—a 3-month history of recurrent abdominal pain for at least 1 day per week in addition to experiencing two or more symptoms such as defecation, change in stool frequency, or change in stool form [148, 151]. It is classified into 4 subtypes (IBS-diarrhea, IBS-constipation, IBS-mixed, and IBS-unsubtyped), but therapies targeting the ENS primarily treat the IBS-diarrhea subtype [152]. Pathogenesis as it pertains to the ENS is due to alterations in sensory and motor function; however, overall, it is not well-understood [148, 153]. Loperamide acts via the mu-opioid receptors on the enteric neuron to slow intestinal transit [152]. The newer therapy of the two, Eluxadoline, acts via the gamma-, kappa-, and mu-opioid receptors to exert its antidiarrheal effects in IBS-diarrhea patients and is currently being investigated in a randomized clinical trial for the treatment of diabetic diarrhea (ClinicalTrials.gov, NCT04313088) [145, 147].
7. GLP-2 and Short Bowel Syndrome
Glucagon-like peptide 2 (GLP-2) is heavily involved in the digestive process and is cosecreted, along with its sister hormone GLP-1, from enteroendocrine L-cells of the small and large intestines [68, 154]. Studies have demonstrated its ability to inhibit gastric emptying and gastric acid secretion stimulated by meals, as well as its role in increasing intestinal barrier function as part of the immune response [68, 155, 156]. Glucagon-like peptide 2 also regulates many intestinal adaptive processes including epithelial proliferation, apoptosis, and inflammation [68, 157]. To exert its effects, GLP-2 interacts with its receptor on the enteric neurons, subepithelial myofibroblasts, and intestinal endocrine cells as demonstrated in the mouse, rat, pig, and human intestines [66, 68, 158–160]. The GLP-2 receptor is highly selective for its cognate ligand, GLP-2, and does not allow effective binding of its structurally related peptide, GLP-1 [68, 159]. When the enteric neuron is exposed to GLP-2, it results in expansion of the mucosal epithelium of the small and large intestines and exerts antiapoptotic actions in the normal and injured intestine by inducing the expression of cell survival genes and proteins [64, 65, 68, 161]. Clinically, this has benefited both adult and pediatric patients who are suffering from short bowel syndrome (SBS) through the development of the GLP-2 analog, Teduglutide (Table 1) [64, 65, 69, 162, 163]. Therefore, the clinical success of Teduglutide makes sense as GLP-2 was previously linked to the regulation of nutrient absorption in several models, as well as in healthy human subjects [164–167]. Furthermore, GLP-2 has been shown to selectively increase visceral blood flow in pigs and healthy humans, as well as in SBS patients [168–170]. In the study of SBS patients, the increase in blood flow correlated with the length of their remaining intestine, implying that GLP-2 exerted metabolic effects on the intestine itself as opposed to the vasculature [170]. In fact, this was demonstrated earlier in a representative porcine model (given its similarity to humans) where GLP-2-induced stimulation of visceral blood flow was mediated by intestinal endocrine cells and the enteric neuron, reenforcing its clinical role in the treatment of SBS patients and making it a potential therapeutic target for low-flow gut diseases such as nonocclusive mesenteric ischemia [66]. Thus, the unlocking of these GLP-2 mechanisms has opened the door to a broad avenue of research looking at the role of GLP-2, the enteric neuron, and the repair, improvement, and maintenance of mucosal integrity and nutrient absorption.
8. Conclusion
The enteric nervous system is the largest and most complex unit of the peripheral nervous system, with ~600 million neurons releasing a multitude of neurotransmitters to facilitate the motor, sensory, absorptive, and secretory functions of the gastrointestinal tract. The enteric nervous system receives regulatory signals from the central nervous system via vagal, thoracolumbar, and lumbosacral input; however, it is also capable of independent function as evidenced by the intestinal peristaltic reflex. The involvement of the enteric nervous system in pathological disorders of the gastrointestinal tract, and the presence of receptors on the enteric neuron for enteric hormones and its transmitters, provides the foundation for current and future targeted therapies that could help patients suffering from a broad range of gastrointestinal disorders.
Acknowledgments
Dr. Daniel Levin's work with the iTHRIV Scholars Program is supported in part by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Numbers UL1TR003015 and KL2TR003016 as well as by the University of Virginia.
Disclosure
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the University of Virginia.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Gershon M. D. The enteric nervous system. Annual Review of Neuroscience. 1981;4(1):227–272. doi: 10.1146/annurev.ne.04.030181.001303. [DOI] [PubMed] [Google Scholar]
- 2.Furness J. B., Costa M. Types of nerves in the enteric nervous system. Neuroscience. 1980;5(1):1–20. doi: 10.1016/0306-4522(80)90067-6. [DOI] [PubMed] [Google Scholar]
- 3.Bayliss W. M., Starling E. H. The movements and innervation of the small intestine. The Journal of Physiology. 1899;24(2):99–143. doi: 10.1113/jphysiol.1899.sp000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furness J. B. The enteric nervous system and neurogastroenterology. Nature Reviews. Gastroenterology & Hepatology. 2012;9(5):286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 5.Schofield G. C. Anatomy of muscular and neural tissues in the alimentary canal. In: Code C. F., editor. Handbook of Physiology. Washington, DC: American Physiological Society; 1968. pp. 1579–1627. [Google Scholar]
- 6.Gabella G. Innervation of the gastrointestinal tract. International Review of Cytology. 1979;59:129–193. doi: 10.1016/S0074-7696(08)61662-9. [DOI] [PubMed] [Google Scholar]
- 7.Rao M., Gershon M. D. Enteric nervous system development: what could possibly go wrong? Nature Reviews. Neuroscience. 2018;19(9):552–565. doi: 10.1038/s41583-018-0041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langley J. N. The Autonomic Nervous System Part I. Cambridge: W. Heffer & Sons Ltd; 1921. [Google Scholar]
- 9.Furness J. B. Types of neurons in the enteric nervous system. Journal of the Autonomic Nervous System. 2000;81(1-3):87–96. doi: 10.1016/S0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 10.Trendelenburg P. Physiologische und pharmakologische Versuche über die Dünndarmperistaltik. Archiv für experimentelle Pathologie und Pharmakologie. 1917;81:55–129. doi: 10.1007/BF01862644. [DOI] [Google Scholar]
- 11.Furness J. B., Callaghan B. P., Rivera L. R., Cho H. J. Advances in Experimental Medicine and Biology. Vol. 817. New York, NY USA: Springer; 2014. The enteric nervous system and gastrointestinal innervation: integrated local and central control; pp. 39–71. [DOI] [PubMed] [Google Scholar]
- 12.Gershon M. D., Rothman T. P. Enteric glia. Glia. 1991;4(2):195–204. doi: 10.1002/glia.440040211. [DOI] [PubMed] [Google Scholar]
- 13.Grubisic V., Gulbransen B. D. Enteric glia: the most alimentary of all glia. The Journal of Physiology. 2017;595(2):557–570. doi: 10.1113/JP271021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming M., II, Ehsan L., Moore S., Levin D. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Preprints; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hansen M. B. The enteric nervous system I: organisation and classification. Pharmacology & Toxicology. 2003;92(3):105–113. doi: 10.1034/j.1600-0773.2003.t01-1-920301.x. [DOI] [PubMed] [Google Scholar]
- 16.Gershon M. D. The Second Brain: A Groundbreaking New Understanding of Nervous Disorders of the Stomach and Intestine. New York, NY: HarperPerennial; 1998. [Google Scholar]
- 17.Bayliss W. M., Starling E. H. The movements and innervation of the small intestine. The Journal of Physiology. 1901;26(3-4):125–138. doi: 10.1113/jphysiol.1901.sp000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez W. C. Bayliss and Starling's law of the INTESTINEorTHE myenteric reflex. The American Journal of Physiology. 1924;69(2):229–248. doi: 10.1152/ajplegacy.1924.69.2.229. [DOI] [Google Scholar]
- 19.Brehmer A., Schrodl F., Neuhuber W. Morphological classifications of enteric neurons — 100 years after Dogiel. Anatomy and Embryology. 1999;200(2):125–135. doi: 10.1007/s004290050267. [DOI] [PubMed] [Google Scholar]
- 20.Hens J., Schrödl F., Brehmer A., et al. Mucosal projections of enteric neurons in the porcine small intestine. The Journal of Comparative Neurology. 2000;421(3):429–436. doi: 10.1002/(SICI)1096-9861(20000605)421:3<429::AID-CNE10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Burns A. J., Douarin N. M. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125(21):4335–4347. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- 22.le Douarin N. Cell line segregation during peripheral nervous system ontogeny. Science. 1986;231(4745):1515–1522. doi: 10.1126/science.3952494. [DOI] [PubMed] [Google Scholar]
- 23.Baetge G., Gershon M. D. Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetal mice: relationship to the development of enteric neurons. Developmental Biology. 1989;132(1):189–211. doi: 10.1016/0012-1606(89)90217-0. [DOI] [PubMed] [Google Scholar]
- 24.Le Douarin N. M., Teillet M. A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. Journal of Embryology and Experimental Morphology. 1973;30(1):31–48. [PubMed] [Google Scholar]
- 25.Yntema C. L., Hammond W. S. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. The Journal of Comparative Neurology. 1954;101(2):515–541. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- 26.Nishiyama C., Uesaka T., Manabe T., et al. Trans-mesenteric neural crest cells are the principal source of the colonic enteric nervous system. Nature Neuroscience. 2012;15(9):1211–1218. doi: 10.1038/nn.3184. [DOI] [PubMed] [Google Scholar]
- 27.Coventry S., Yost C., Palmiter R. D., Kapur R. P. Migration of ganglion cell precursors in the ileoceca of normal and lethal spotted embryos, a murine model for Hirschsprung disease. Laboratory Investigation. 1994;71(1):82–93. [PubMed] [Google Scholar]
- 28.Wang X., Chan A. K. K., Sham M. H., Burns A. J., Chan W. Y. Analysis of the sacral neural crest cell contribution to the hindgut enteric nervous system in the mouse embryo. Gastroenterology. 2011;141(3):992–1002.e6. doi: 10.1053/j.gastro.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Anderson R. B., Stewart A. L., Young H. M. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell and Tissue Research. 2006;323(1):11–25. doi: 10.1007/s00441-005-0047-6. [DOI] [PubMed] [Google Scholar]
- 30.Kapur R. P. Colonization of the murine hindgut by sacral crest-derived neural precursors: experimental support for an evolutionarily conserved model. Developmental Biology. 2000;227(1):146–155. doi: 10.1006/dbio.2000.9886. [DOI] [PubMed] [Google Scholar]
- 31.Espinosa-Medina I., Saha O., Boismoreau F., et al. The sacral autonomic outflow is sympathetic. Science. 2016;354(6314):893–897. doi: 10.1126/science.aah5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gershon M. D. The enteric nervous system: a second brain. Hospital Practice. 1999;34(7):31–52. doi: 10.3810/hp.1999.07.153. [DOI] [PubMed] [Google Scholar]
- 33.Gershon M. D. Nerves, reflexes, and the enteric nervous System. Journal of Clinical Gastroenterology. 2005;39(Supplement 3):S184–S193. doi: 10.1097/01.mcg.0000156403.37240.30. [DOI] [PubMed] [Google Scholar]
- 34.Komuro T., Baluk P., Burnstock G. An ultrastructural study of neurons and non-neuronal cells in the myenteric plexus of the rabbit colon. Neuroscience. 1982;7(7):1797–1806. doi: 10.1016/0306-4522(82)90037-9. [DOI] [PubMed] [Google Scholar]
- 35.Salzer J. L., Zalc B. Myelination. Current Biology. 2016;26(20):R971–r975. doi: 10.1016/j.cub.2016.07.074. [DOI] [PubMed] [Google Scholar]
- 36.Gabella G. Fine structure of the myenteric plexus in the guinea-pig ileum. Journal of Anatomy. 1972;111, Part 1:69–97. [PMC free article] [PubMed] [Google Scholar]
- 37.Bodian M., Stephens F. D., Ward B. C. H. Hirschsprung's Disease And Idiopathic Megacolon. Lancet. 1949;253(6540):6–11. doi: 10.1016/s0140-6736(49)90340-2. [DOI] [PubMed] [Google Scholar]
- 38.Cook R. D., Burnstock G. The altrastructure of Auerbach's plexus in the guinea-pig. I. Neuronal elements. Journal of Neurocytology. 1976;5(2):171–194. doi: 10.1007/BF01181655. [DOI] [PubMed] [Google Scholar]
- 39.Schemann M., Michel K., Peters S., Bischoff S. C., Neunlist M. Cutting-edge technology. III. Imaging and the gastrointestinal tract: mapping the human enteric nervous system. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2002;282(6):G919–G925. doi: 10.1152/ajpgi.00043.2002. [DOI] [PubMed] [Google Scholar]
- 40.Furness J. B., Jones C., Nurgali K., Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Progress in Neurobiology. 2004;72(2):143–164. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Grider J. R. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. The Journal of Pharmacology and Experimental Therapeutics. 2003;307(2):460–467. doi: 10.1124/jpet.103.053512. [DOI] [PubMed] [Google Scholar]
- 42.Pompolo S., Furness J. B. Ultrastructure and synaptic relationships of calbindin-reactive, Dogiel type II neurons, in myenteric ganglia of guinea-pig small intestine. Journal of Neurocytology. 1988;17(6):771–782. doi: 10.1007/BF01216705. [DOI] [PubMed] [Google Scholar]
- 43.Furness J. B. Novel gut afferents: intrinsic afferent neurons and intestinofugal neurons. Autonomic Neuroscience. 2006;125(1-2):81–85. doi: 10.1016/j.autneu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Song Z. M., Brookes S. J., Costa M. Identification of myenteric neurons which project to the mucosa of the guinea-pig small intestine. Neuroscience Letters. 1991;129(2):294–298. doi: 10.1016/0304-3940(91)90484-B. [DOI] [PubMed] [Google Scholar]
- 45.Kirchgessner A. L., Tamir H., Gershon M. D. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. The Journal of Neuroscience. 1992;12(1):235–248. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunze W. A., Furness J. B., Bertrand P. P., Bornstein J. C. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. The Journal of Physiology. 1998;506(3):827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kunze W. A., Furness J. B. The enteric nervous system and regulation of intestinal motility. Annual Review of Physiology. 1999;61(1):117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- 48.Mao Y., Wang B., Kunze W. Characterization of myenteric sensory neurons in the mouse small intestine. Journal of Neurophysiology. 2006;96(3):998–1010. doi: 10.1152/jn.00204.2006. [DOI] [PubMed] [Google Scholar]
- 49.Collins S. M. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology. 1996;111(6):1683–1699. doi: 10.1016/S0016-5085(96)70034-3. [DOI] [PubMed] [Google Scholar]
- 50.Margolis K. G., Gershon M. D. Enteric neuronal regulation of intestinal inflammation. Trends in Neurosciences. 2016;39(9):614–624. doi: 10.1016/j.tins.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linden D. R. Enhanced excitability of guinea pig ileum myenteric AH neurons during and following recovery from chemical colitis. Neuroscience Letters. 2013;545:91–95. doi: 10.1016/j.neulet.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Langer J. C. Hirschsprung disease. Current Opinion in Pediatrics. 2013;25(3):368–374. doi: 10.1097/MOP.0b013e328360c2a0. [DOI] [PubMed] [Google Scholar]
- 53.Huizinga J. D., Zarate N., Farrugia G. Physiology, injury, and recovery of interstitial cells of Cajal: basic and clinical science. Gastroenterology. 2009;137(5):1548–1556. doi: 10.1053/j.gastro.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsai Y. H., Murakami N., Gariepy C. E. Postnatal intestinal engraftment of prospectively selected enteric neural crest stem cells in a rat model of Hirschsprung disease. Neurogastroenterology and Motility. 2011;23(4):362–369. doi: 10.1111/j.1365-2982.2010.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martucciello G., Brizzolara A., Favre A., et al. Neural crest neuroblasts can colonise aganglionic and ganglionic gut in vivo. European Journal of Pediatric Surgery. 2007;17(1):34–40. doi: 10.1055/s-2007-964952. [DOI] [PubMed] [Google Scholar]
- 56.Mosher J. T., Yeager K. J., Kruger G. M., et al. Intrinsic differences among spatially distinct neural crest stem cells in terms of migratory properties, fate determination, and ability to colonize the enteric nervous system. Developmental Biology. 2007;303(1):1–15. doi: 10.1016/j.ydbio.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones S. L., Blikslager A. T. Role of the enteric nervous system in the pathophysiology of secretory diarrhea. Journal of Veterinary Internal Medicine. 2002;16(3):222–228. doi: 10.1111/j.1939-1676.2002.tb02361.x. [DOI] [PubMed] [Google Scholar]
- 58.Bern M. J., Sturbaum C. W., Karayalcin S. S., Berschneider H. M., Wachsman J. T., Powell D. W. Immune system control of rat and rabbit colonic electrolyte transport. Role of prostaglandins and enteric nervous system. The Journal of Clinical Investigation. 1989;83(6):1810–1820. doi: 10.1172/JCI114086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood J. D. Enteric nervous system: sensory physiology, diarrhea and constipation. Current Opinion in Gastroenterology. 2010;26(2):102–108. doi: 10.1097/MOG.0b013e328334df4f. [DOI] [PubMed] [Google Scholar]
- 60.Anand S., Mandal S., Patil P., Tomar S. K. Pathogen-induced secretory diarrhea and its prevention. European Journal of Clinical Microbiology & Infectious Diseases. 2016;35(11):1721–1739. doi: 10.1007/s10096-016-2726-5. [DOI] [PubMed] [Google Scholar]
- 61.Regnard C., Twycross R., Mihalyo M., Wilcock A. Loperamide. Journal of Pain and Symptom Management. 2011;42(2):319–323. doi: 10.1016/j.jpainsymman.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Duchen L. W., Anjorin A., Watkins P. J., Mackay J. D. Pathology of autonomic neuropathy in diabetes mellitus. Annals of Internal Medicine. 1980;92(2_Part_2):301–303. doi: 10.7326/0003-4819-92-2-301. [DOI] [PubMed] [Google Scholar]
- 63.Hosking D. J., Bennett T., Hampton J. R. Diabetic autonomic neuropathy. Diabetes. 1978;27(10):1043–1054. doi: 10.2337/diab.27.10.1043. [DOI] [PubMed] [Google Scholar]
- 64.Jeppesen P. B., Gilroy R., Pertkiewicz M., Allard J. P., Messing B., O'Keefe S. J. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60(7):902–914. doi: 10.1136/gut.2010.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeppesen P. B., Sanguinetti E. L., Buchman A., et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54(9):1224–1231. doi: 10.1136/gut.2004.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guan X., Karpen H. E., Stephens J., et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130(1):150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Carroll R. E., Benedetti E., Schowalter J. P., Buchman A. L. Management and complications of short bowel syndrome: an updated review. Current Gastroenterology Reports. 2016;18(7) doi: 10.1007/s11894-016-0511-3. [DOI] [PubMed] [Google Scholar]
- 68.Drucker D. J., Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annual Review of Physiology. 2014;76(1):561–583. doi: 10.1146/annurev-physiol-021113-170317. [DOI] [PubMed] [Google Scholar]
- 69.Kim E. S., Keam S. J. Teduglutide: a review in short bowel syndrome. Drugs. 2017;77(3):345–352. doi: 10.1007/s40265-017-0703-7. [DOI] [PubMed] [Google Scholar]
- 70.Wood J. D., Alpers D. H., Andrews P. L. Fundamentals of neurogastroenterology. Gut. 1999;45(Supplement 2):ii6–ii16. doi: 10.1136/gut.45.2008.ii6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stanghellini V., Camilleri M., Malagelada J. R. Chronic idiopathic intestinal pseudo-obstruction: clinical and intestinal manometric findings. Gut. 1987;28(1):5–12. doi: 10.1136/gut.28.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Downes T. J., Cheruvu M. S., Karunaratne T. B., de Giorgio R., Farmer A. D. Pathophysiology, diagnosis, and management of chronic intestinal pseudo-obstruction. Journal of Clinical Gastroenterology. 2018;52(6):477–489. doi: 10.1097/MCG.0000000000001047. [DOI] [PubMed] [Google Scholar]
- 73.Cullen J. J., Eagon J. C., Kelly K. A. Gastrointestinal peptide hormones during postoperative ileus. Digestive Diseases and Sciences. 1994;39(6):1179–1184. doi: 10.1007/BF02093781. [DOI] [PubMed] [Google Scholar]
- 74.Barquist E., Bonaz B., Martinez V., Rivier J., Zinner M. J., Taché Y. Neuronal pathways involved in abdominal surgery-induced gastric ileus in rats. The American Journal of Physiology. 1996;270, 4 Part 2:R888–R894. doi: 10.1152/ajpregu.1996.270.4.R888. [DOI] [PubMed] [Google Scholar]
- 75.Zittel T. T., Lloyd K. C. K., Rothenhöfer I., Wong H., Walsh J. H., Raybould H. E. Calcitonin gene-related peptide and spinal afferents partly mediate postoperative colonic ileus in the rat. Surgery. 1998;123(5):518–527. doi: 10.1067/msy.1998.88090. [DOI] [PubMed] [Google Scholar]
- 76.Luckey A., Livingston E., Tache Y. Mechanisms and treatment of postoperative ileus. Archives of Surgery. 2003;138(2):206–214. doi: 10.1001/archsurg.138.2.206. [DOI] [PubMed] [Google Scholar]
- 77.Vather R., Bissett I. Management of prolonged post-operative ileus: evidence-based recommendations. ANZ Journal of Surgery. 2013;83(5):319–324. doi: 10.1111/ans.12102. [DOI] [PubMed] [Google Scholar]
- 78.Scheperjans F., Derkinderen P., Borghammer P. The gut and Parkinson's disease: hype or hope? Journal of Parkinson's Disease. 2018;8(s1):S31–s39. doi: 10.3233/JPD-181477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sharkey K. A. Emerging roles for enteric glia in gastrointestinal disorders. The Journal of Clinical Investigation. 2015;125(3):918–925. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brookes S. J., Steele P. A., Costa M. Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience. 1991;42(3):863–878. doi: 10.1016/0306-4522(91)90050-X. [DOI] [PubMed] [Google Scholar]
- 81.Holzer P., Holzer-Petsche U. Tachykinins in the gut. Part I. expression, release and motor function. Pharmacology & Therapeutics. 1997;73(3):173–217. doi: 10.1016/S0163-7258(96)00195-7. [DOI] [PubMed] [Google Scholar]
- 82.Fahrenkrug J., Haglund U., Jodal M., Lundgren O., Olbe L., de Muckadell O. B. Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiological implications. The Journal of Physiology. 1978;284(1):291–305. doi: 10.1113/jphysiol.1978.sp012541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Costa M., Furness J. B., Pompolo S., et al. Projections and chemical coding of neurons with immunoreactivity for nitric oxide synthase in the guinea-pig small intestine. Neuroscience Letters. 1992;148(1-2):121–125. doi: 10.1016/0304-3940(92)90819-S. [DOI] [PubMed] [Google Scholar]
- 84.Sanders K. M., Ward S. M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. The American Journal of Physiology. 1992;262, 3 Part 1:G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- 85.Xue L., Farrugia G., Miller S. M., Ferris C. D., Snyder S. H., Szurszewski J. H. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(4):1851–1855. doi: 10.1073/pnas.97.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Young H. M., McConalogue K., Furness J. B., de Vente J. Nitric oxide targets in the guinea-pig intestine identified by induction of cyclic GMP immunoreactivity. Neuroscience. 1993;55(2):583–596. doi: 10.1016/0306-4522(93)90526-L. [DOI] [PubMed] [Google Scholar]
- 87.Shuttleworth C. W., Xue C., Ward S. M., de Vente J., Sanders K. M. Immunohistochemical localization of 3',5'-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience. 1993;56(2):513–522. doi: 10.1016/0306-4522(93)90350-O. [DOI] [PubMed] [Google Scholar]
- 88.Vigna S. R., Bowden J. J., McDonald D., et al. Characterization of antibodies to the rat substance P (NK-1) receptor and to a chimeric substance P receptor expressed in mammalian cells. The Journal of Neuroscience. 1994;14(2):834–845. doi: 10.1523/JNEUROSCI.14-02-00834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sternini C., Su D., Gamp P. D., Bunnett N. W. Cellular sites of expression of the neurokinin-1 receptor in the rat gastrointestinal tract. The Journal of Comparative Neurology. 1995;358(4):531–540. doi: 10.1002/cne.903580406. [DOI] [PubMed] [Google Scholar]
- 90.Portbury A. L., Furness J. B., Young H. M., Southwell B. R., Vigna S. R. Localisation of NK1 receptor immunoreactivity to neurons and interstitial cells of the guinea-pig gastrointestinal tract. The Journal of Comparative Neurology. 1996;367(3):342–351. doi: 10.1002/(SICI)1096-9861(19960408)367:3<342::AID-CNE2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 91.Hansen M. B. Small intestinal manometry. Physiological Research. 2002;51(6):541–556. [PubMed] [Google Scholar]
- 92.Frieling T., Wood J. D., Cooke H. J. Submucosal reflexes: distension-evoked ion transport in the guinea pig distal colon. The American Journal of Physiology. 1992;263, 1 Part 1:G91–G96. doi: 10.1152/ajpgi.1992.263.1.G91. [DOI] [PubMed] [Google Scholar]
- 93.Cooke H. J. Neuroimmune signaling in regulation of intestinal ion transport. The American Journal of Physiology. 1994;266, 2 Part 1:G167–G178. doi: 10.1152/ajpgi.1994.266.2.G167. [DOI] [PubMed] [Google Scholar]
- 94.Reddix R., Kuhawara A., Wallace L., Cooke H. J. Vasoactive intestinal polypeptide: a transmitter in submucous neurons mediating secretion in guinea pig distal colon. The Journal of Pharmacology and Experimental Therapeutics. 1994;269(3):1124–1129. [PubMed] [Google Scholar]
- 95.Vanner S., Surprenant A. Cholinergic and noncholinergic submucosal neurons dilate arterioles in guinea pig colon. The American Journal of Physiology. 1991;261, 1 Part 1:G136–G144. doi: 10.1152/ajpgi.1991.261.1.G136. [DOI] [PubMed] [Google Scholar]
- 96.Vanner S., Jiang M. M., Surprenant A. Mucosal stimulation evokes vasodilation in submucosal arterioles by neuronal and nonneuronal mechanisms. The American Journal of Physiology. 1993;264, 2 Part 1:G202–G212. doi: 10.1152/ajpgi.1993.264.2.G202. [DOI] [PubMed] [Google Scholar]
- 97.Vanner S., Surprenant A. Neural reflexes controlling intestinal microcirculation. The American Journal of Physiology. 1996;271, 2 Part 1:G223–G230. doi: 10.1152/ajpgi.1996.271.2.G223. [DOI] [PubMed] [Google Scholar]
- 98.Neild T. O., Shen K. Z., Surprenant A. Vasodilatation of arterioles by acetylcholine released from single neurones in the guinea-pig submucosal plexus. The Journal of Physiology. 1990;420(1):247–265. doi: 10.1113/jphysiol.1990.sp017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sjölund K., Sandén G., Håkanson R., Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85(5):1120–1130. doi: 10.1016/S0016-5085(83)80080-8. [DOI] [PubMed] [Google Scholar]
- 100.Margolskee R. F., Dyer J., Kokrashvili Z., et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(38):15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shirazi-Beechey S. P., Moran A. W., Batchelor D. J., Daly K., al-Rammahi M. Glucose sensing and signalling; regulation of intestinal glucose transport. The Proceedings of the Nutrition Society. 2011;70(2):185–193. doi: 10.1017/S0029665111000103. [DOI] [PubMed] [Google Scholar]
- 102.Sigalet D. L., Wallace L., de Heuval E., Sharkey K. A. The effects of glucagon-like peptide 2 on enteric neurons in intestinal inflammation. Neurogastroenterology and Motility. 2010;22(12):1318–1350. doi: 10.1111/j.1365-2982.2010.01585.x. [DOI] [PubMed] [Google Scholar]
- 103.Surprenant A. Slow excitatory synaptic potentials recorded from neurones of guinea-pig submucous plexus. The Journal of Physiology. 1984;351(1):343–361. doi: 10.1113/jphysiol.1984.sp015249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Monro R. L., Bertrand P. P., Bornstein J. C. ATP participates in three excitatory postsynaptic potentials in the submucous plexus of the guinea pig ileum. The Journal of Physiology. 2004;556(2):571–584. doi: 10.1113/jphysiol.2004.060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jessen K. R., Thorpe R., Mirsky R. Molecular identity, distribution and heterogeneity of glial fibrillary acidic protein: an immunoblotting and immunohistochemical study of Schwann cells, satellite cells, enteric glia and astrocytes. Journal of Neurocytology. 1984;13(2):187–200. doi: 10.1007/BF01148114. [DOI] [PubMed] [Google Scholar]
- 106.Cook R. D., Burnstock G. The ultrastructure of Auerbach's plexus in the guinea-pig. II. Non-neuronal elements. Journal of Neurocytology. 1976;5(2):195–206. doi: 10.1007/BF01181656. [DOI] [PubMed] [Google Scholar]
- 107.Pasternak A., Szura M., Gil K., Matyja A. Interstitial cells of Cajal - systematic review. Folia Morphologica. 2016;75(3):281–286. doi: 10.5603/FM.a2016.0002. [DOI] [PubMed] [Google Scholar]
- 108.Horowitz B., Ward S. M., Sanders K. M. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annual Review of Physiology. 1999;61(1):19–43. doi: 10.1146/annurev.physiol.61.1.19. [DOI] [PubMed] [Google Scholar]
- 109.Sanders K. M., Ward S. M., Koh S. D. Interstitial cells: regulators of smooth muscle function. Physiological Reviews. 2014;94(3):859–907. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Strege P. R., Ou Y., Sha L., et al. Sodium current in human intestinal interstitial cells of Cajal. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2003;285(6):G1111–G1121. doi: 10.1152/ajpgi.00152.2003. [DOI] [PubMed] [Google Scholar]
- 111.Won K. J., Sanders K. M., Ward S. M. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(41):14913–14918. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lecoin L., Gabella G., Le Douarin N. Origin of the c-kit-positive interstitial cells in the avian bowel. Development. 1996;122(3):725–733. doi: 10.1242/dev.122.3.725. [DOI] [PubMed] [Google Scholar]
- 113.Young H. M., Ciampoli D., Southwell B. R., Newgreen D. F. Origin of interstitial cells of Cajal in the mouse intestine. Developmental Biology. 1996;180(1):97–107. doi: 10.1006/dbio.1996.0287. [DOI] [PubMed] [Google Scholar]
- 114.Wouters M. M., Farrugia G., Schemann M. 5-HT receptors on interstitial cells of Cajal, smooth muscle and enteric nerves. Neurogastroenterology and Motility. 2007;19(Supplement 2):5–12. doi: 10.1111/j.1365-2982.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 115.Vannucchi M. G. Receptors in interstitial cells of Cajal: identification and possible physiological roles. Microscopy Research and Technique. 1999;47(5):325–335. doi: 10.1002/(SICI)1097-0029(19991201)47:5<325::AID-JEMT4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 116.Song N. N., Xu W. X. Physiological and pathophysiological meanings of gastrointestinal smooth muscle motor unit SIP syncytium. Sheng li xue bao:[Acta physiologica Sinica] 2016;68(5):621–627. [PubMed] [Google Scholar]
- 117.Antonucci A., Fronzoni L., Cogliandro L., et al. Chronic intestinal pseudo-obstruction. World Journal of Gastroenterology. 2008;14(19):2953–2961. doi: 10.3748/wjg.14.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burns A. J. Disorders of interstitial cells of Cajal. Journal of Pediatric Gastroenterology and Nutrition. 2007;45(Supplement 2):S103–S106. doi: 10.1097/MPG.0b013e31812e65e0. [DOI] [PubMed] [Google Scholar]
- 119.Kwon J. G., Hwang S. J., Hennig G. W., et al. Changes in the structure and function of ICC networks in ICC hyperplasia and gastrointestinal stromal tumors. Gastroenterology. 2009;136(2):630–639. doi: 10.1053/j.gastro.2008.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boeckxstaens G. E., Zaninotto G., Richter J. E. Achalasia. Lancet. 2014;383(9911):83–93. doi: 10.1016/S0140-6736(13)60651-0. [DOI] [PubMed] [Google Scholar]
- 121.Jankovic J. Botulinum toxin: state of the art. Movement Disorders. 2017;32(8):1131–1138. doi: 10.1002/mds.27072. [DOI] [PubMed] [Google Scholar]
- 122.Brashear A. Botulinum toxin type A: exploring new indications. Drugs of Today. 2010;46(9):671–682. doi: 10.1358/dot.2010.46.9.1524356. [DOI] [PubMed] [Google Scholar]
- 123.Schlottmann F., Neto R. M. L., Herbella F. A. M., Patti M. G. Esophageal Achalasia: Pathophysiology, Clinical Presentation, and Diagnostic Evaluation. The American Surgeon. 2018;84(4):467–472. doi: 10.1177/000313481808400415. [DOI] [PubMed] [Google Scholar]
- 124.Ates F., F Vaezi M. The pathogenesis and management of achalasia: current status and future directions. Gut and Liver. 2015;9(4):449–463. doi: 10.5009/gnl14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schemann M., Neunlist M. The human enteric nervous system. Neurogastroenterology and Motility. 2004;16(Supplement 1):55–59. doi: 10.1111/j.1743-3150.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 126.Pasricha P. J., Ravich W. J., Hendrix T. R., Sostre S., Jones B., Kalloo A. N. Intrasphincteric botulinum toxin for the treatment of achalasia. The New England Journal of Medicine. 1995;332(12):774–778. doi: 10.1056/NEJM199503233321203. [DOI] [PubMed] [Google Scholar]
- 127.Tack J., Camilleri M., Chang L., et al. Systematic review: cardiovascular safety profile of 5‐HT4 agonists developed for gastrointestinal disorders. Alimentary Pharmacology & Therapeutics. 2012;35(7):745–767. doi: 10.1111/j.1365-2036.2012.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tillou J., Poylin V. Functional disorders: slow-transit constipation. Clinics in Colon and Rectal Surgery. 2017;30(1):76–86. doi: 10.1055/s-0036-1593436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mollen R. M., Hopman W. P. M., Kuijpers H. H. C., Jansen J. B. M. J. Abnormalities of upper gut motility in patients with slow-transit constipation. European Journal of Gastroenterology & Hepatology. 1999;11(7):701–708. doi: 10.1097/00042737-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 130.El-Salhy M. Chronic idiopathic slow transit constipation: pathophysiology and management. Colorectal Disease. 2003;5(4):288–296. doi: 10.1046/j.1463-1318.2003.00498.x. [DOI] [PubMed] [Google Scholar]
- 131.Wedel T., Roblick U. J., Ott V., et al. Oligoneuronal hypoganglionosis in patients with idiopathic slow-transit constipation. Diseases of the Colon and Rectum. 2002;45(1):54–62. doi: 10.1007/s10350-004-6114-3. [DOI] [PubMed] [Google Scholar]
- 132.Schouten W. R., ten Kate F. J. W., de Graaf E. J. R., Gilberts E. C. A. M., Simons J. L., Klück P. Visceral neuropathy in slow transit constipation: an immunohistochemical investigation with monoclonal antibodies against neurofilament. Diseases of the Colon and Rectum. 1993;36(12):1112–1117. doi: 10.1007/BF02052258. [DOI] [PubMed] [Google Scholar]
- 133.Frattini J. C., Nogueras J. J. Slow transit constipation: a review of a colonic functional disorder. Clinics in Colon and Rectal Surgery. 2008;21(2):146–152. doi: 10.1055/s-2008-1075864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bianco F., Bonora E., Natarajan D., et al. Prucalopride exerts neuroprotection in human enteric neurons. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2016;310(10):G768–G775. doi: 10.1152/ajpgi.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grover M., Farrugia G., Stanghellini V. Gastroparesis: a turning point in understanding and treatment. Gut. 2019;68(12):2238–2250. doi: 10.1136/gutjnl-2019-318712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Camilleri M., Chedid V., Ford A. C., et al. Gastroparesis. Nature Reviews. Disease Primers. 2018;4(1) doi: 10.1038/s41572-018-0038-z. [DOI] [PubMed] [Google Scholar]
- 137.von Arnim U. Gastroparese. Der Internist. 2015;56(6):625–630. doi: 10.1007/s00108-014-3604-9. [DOI] [PubMed] [Google Scholar]
- 138.Grover M., Farrugia G., Lurken M. S., et al. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology. 2011;140(5):1575–1585.e8. doi: 10.1053/j.gastro.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Faussone-Pellegrini M. S., Grover M., Pasricha P. J., et al. Ultrastructural differences between diabetic and idiopathic gastroparesis. Journal of Cellular and Molecular Medicine. 2012;16(7):1573–1581. doi: 10.1111/j.1582-4934.2011.01451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Buysschaert M., Donckier J., Dive A., Ketelslegers J. M., Lambert A. E. Gastric acid and pancreatic polypeptide responses to sham feeding are impaired in diabetic subjects with autonomic neuropathy. Diabetes. 1985;34(11):1181–1185. doi: 10.2337/diab.34.11.1181. [DOI] [PubMed] [Google Scholar]
- 141.Shakhatreh M., Jehangir A., Malik Z., Parkman H. P. Metoclopramide for the treatment of diabetic gastroparesis. Expert Review of Gastroenterology & Hepatology. 2019;13(8):711–721. doi: 10.1080/17474124.2019.1645594. [DOI] [PubMed] [Google Scholar]
- 142.Tonini M., Cipollina L., Poluzzi E., Crema F., Corazza G. R., de Ponti F. clinical implications of enteric and central D2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Alimentary Pharmacology & Therapeutics. 2004;19(4):379–390. doi: 10.1111/j.1365-2036.2004.01867.x. [DOI] [PubMed] [Google Scholar]
- 143.Xu L., Depoortere I., Tomasetto C., et al. Evidence for the presence of motilin, ghrelin, and the motilin and ghrelin receptor in neurons of the myenteric plexus. Regulatory Peptides. 2005;124(1-3):119–125. doi: 10.1016/j.regpep.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 144.McCallum R. W., George S. J. Gastric dysmotility and gastroparesis. Current Treatment Options in Gastroenterology. 2001;4(2):179–191. doi: 10.1007/s11938-001-0030-6. [DOI] [PubMed] [Google Scholar]
- 145.Lembo A. J., Lacy B. E., Zuckerman M. J., et al. Eluxadoline for irritable bowel syndrome with diarrhea. The New England Journal of Medicine. 2016;374(3):242–253. doi: 10.1056/NEJMoa1505180. [DOI] [PubMed] [Google Scholar]
- 146.Ford A. C., Moayyedi P., Lacy B. E., et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. American Journal of Gastroenterology. 2014;109(Supplement 1):S2–26. doi: 10.1038/ajg.2014.187. [DOI] [PubMed] [Google Scholar]
- 147.Fragkos K. C. Spotlight on eluxadoline for the treatment of patients with irritable bowel syndrome with diarrhea. Clinical and Experimental Gastroenterology. 2017;10:229–240. doi: 10.2147/CEG.S123621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sultan S., Malhotra A. Irritable bowel syndrome. Annals of Internal Medicine. 2017;166(11):Itc81–itc96. doi: 10.7326/AITC201706060. [DOI] [PubMed] [Google Scholar]
- 149.Andresen V., Montori V. M., Keller J., West C. P., Layer P., Camilleri M. Effects of 5-hydroxytryptamine (serotonin) type 3 antagonists on symptom relief and constipation in nonconstipated irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials. Clinical Gastroenterology and Hepatology. 2008;6(5):545–555. doi: 10.1016/j.cgh.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Garsed K., Chernova J., Hastings M., et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63(10):1617–1625. doi: 10.1136/gutjnl-2013-305989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schmulson M. J., Drossman D. A. What is new in Rome IV. Journal of Neurogastroenterology and Motility. 2017;23(2):151–163. doi: 10.5056/jnm16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ford A. C., Lacy B. E., Talley N. J. Irritable bowel syndrome. The New England Journal of Medicine. 2017;376(26):2566–2578. doi: 10.1056/NEJMra1607547. [DOI] [PubMed] [Google Scholar]
- 153.Shim L., Talley N. J., Boyce P., Tennant C., Jones M., Kellow J. E. Stool characteristics and colonic transit in irritable bowel syndrome: evaluation at two time points. Scandinavian Journal of Gastroenterology. 2013;48(3):295–301. doi: 10.3109/00365521.2012.758767. [DOI] [PubMed] [Google Scholar]
- 154.Baggio L. L., Drucker D. J. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 155.Wøjdemann M., Wettergren A., Hartmann B., Holst J. J. Glucagon-like peptide-2 inhibits centrally induced antral motility in pigs. Scandinavian Journal of Gastroenterology. 1998;33(8):828–832. doi: 10.1080/00365529850171486. [DOI] [PubMed] [Google Scholar]
- 156.Wøjdemann M., Wettergren A., Hartmann B., Hilsted L., Holst J. J. Inhibition of sham feeding-stimulated human gastric acid secretion by glucagon-like peptide-2. The Journal of Clinical Endocrinology and Metabolism. 1999;84(7):2513–2517. doi: 10.1210/jcem.84.7.5840. [DOI] [PubMed] [Google Scholar]
- 157.Pedersen J., Pedersen N. B., Brix S. W., et al. The glucagon-like peptide 2 receptor is expressed in enteric neurons and not in the epithelium of the intestine. Peptides. 2015;67:20–28. doi: 10.1016/j.peptides.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 158.Bjerknes M., Cheng H. Modulation of specific intestinal epithelial progenitors by enteric neurons. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12497–12502. doi: 10.1073/pnas.211278098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yusta B., Huang L., Munroe D., et al. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology. 2000;119(3):744–755. doi: 10.1053/gast.2000.16489. [DOI] [PubMed] [Google Scholar]
- 160.Ørskov C., Hartmann B., Poulsen S. S., Thulesen J., Hare K. J., Holst J. J. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regulatory Peptides. 2005;124(1-3):105–112. doi: 10.1016/j.regpep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 161.Drucker D. J., Erlich P., Asa S. L., Brubaker P. L. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(15):7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Carter B. A., Cohran V. C., Cole C. R., et al. Outcomes from a 12-week, open-label, multicenter clinical trial of teduglutide in pediatric short bowel syndrome. The Journal of Pediatrics. 2017;181:102–111.e5. doi: 10.1016/j.jpeds.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 163.Jeppesen P. B., Pertkiewicz M., Messing B., et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143(6):1473–1481.e3. doi: 10.1053/j.gastro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 164.Chance W. T., Foley-Nelson T., Thomas I., Balasubramaniam A. Prevention of parenteral nutrition-induced gut hypoplasia by coinfusion of glucagon-like peptide-2. The American Journal of Physiology. 1997;273, 2 Part 1:G559–G563. doi: 10.1152/ajpgi.1997.273.2.G559. [DOI] [PubMed] [Google Scholar]
- 165.Scott R. B., Kirk D., MacNaughton W., Meddings J. B. GLP-2 augments the adaptive response to massive intestinal resection in rat. The American Journal of Physiology. 1998;275(5):G911–G921. doi: 10.1152/ajpgi.1998.275.5.G911. [DOI] [PubMed] [Google Scholar]
- 166.Hsieh J., Longuet C., Maida A., et al. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137(3):997–1005.e4. doi: 10.1053/j.gastro.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 167.Meier J. J., Nauck M. A., Pott A., et al. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology. 2006;130(1):44–54. doi: 10.1053/j.gastro.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 168.Guan X., Stoll B., Lu X., et al. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology. 2003;125(1):136–147. doi: 10.1016/S0016-5085(03)00667-X. [DOI] [PubMed] [Google Scholar]
- 169.Bremholm L., Hornum M., Henriksen B. M., Larsen S., Holst J. J. Glucagon-like peptide-2 increases mesenteric blood flow in humans. Scandinavian Journal of Gastroenterology. 2009;44(3):314–319. doi: 10.1080/00365520802538195. [DOI] [PubMed] [Google Scholar]
- 170.Bremholm L., Hornum M., Andersen U. B., Hartmann B., Holst J. J., Jeppesen P. B. The effect of glucagon-like peptide-2 on mesenteric blood flow and cardiac parameters in end-jejunostomy short bowel patients. Regulatory Peptides. 2011;168(1-3):32–38. doi: 10.1016/j.regpep.2011.03.003. [DOI] [PubMed] [Google Scholar]


