Abstract
Background
This study aims to systematically evaluate the effect of moxibustion on the level of inflammatory cytokines in animal models with rheumatoid arthritis (RA) and to provide evidence for the clinical application of moxibustion to the treatment of RA and related basic researches.
Methods
The databases employed in this study include PubMed, EMBASE, Web of Science, China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Periodical Database (VIP), SinoMed, and Wanfang Data Information Site. The retrieval time was from the establishment of these databases to March 2020. The reviewers made use of the CAMARADES 10-item checklist to evaluate the quality of each included study. The inflammatory cytokines were considered as the outcome measure. The Revman 5.3 software was used to conduct meta-analysis on the outcome indicators of the studies included.
Results
A total of 648 articles were retrieved and 18 animal experiments were included in this study. The quality scores of the studies ranged from two to eight with a mean of 5.8. Compared with the effect of the control group, moxibustion reduced the expression of TNF-α (SMD 2.95, 95% CI: 1.99–3.92, P < 0.00001), IL-1β (SMD 4.10, 95% CI: 2.37–5.84, P < 0.00001), IFN-γ (MD 25, 95% CI: 16.17–33.82, P < 0.00001), IL-6 (MD 11.83, 95% CI: 6.22–17.44, P < 0.0001), and IL-17 (MD 99.3, 95% CI: 86.83–111.76, P < 0.00001). At the same time, the level of IL-2 (SMD 8.89, 95% CI: 0.93–16.86, P=0.03), IL-4 (MD 1.79, 95% CI: 0.26–3.32, P=0.02), and IL-10 (MD 5.93, 95% CI: 1.37–10.49, P=0.01) increased after moxibustion treatment. Asymmetric funnel plots indicated that there was publication bias.
Conclusion
The findings of the present review indicate that moxibustion can protect the synovium of joint in animal models with RA by upregulation of the level of anti-inflammatory cytokines and downregulation of the level of proinflammatory cytokines. Moxibustion has the potential to relieve inflammation of RA.
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that starts with the inflammation of synovium. The main symptoms of RA include pain, swelling, and stiffness of the joint. With the progression of the disease, cartilage destruction and bone erosion may occur, eventually leading to severe disability. The occurrence of RA is closely related to autoimmune disorders. Previous studies have revealed that many factors including gene, environment, hormone, and infection are involved in the persistent immunological response [1]. In addition, people with RA have an increased risk of cardiovascular disease, fragility fracture, and venous thromboembolism [2–4]. The incidence of RA varies from country to country and region to region, ranging from 0.5% to 1% in adults [5]. It is estimated that there are over 19 million prevalent cases of RA globally, which increased by 7.4% from 1990 to 2017 [6]. Global Burden of Diseases 2017 found that the crude disability adjusted life years rate increased by 25% during 1990–2017 [7]. The pain and joint dysfunction caused by RA urgently need an effective treatment to alleviate the inflammatory reaction and delay the progression of the disease. Among the RA therapeutic strategies, drug treatment is the most prominent one. However, both anti-inflammatory and biological agents have side effects (such as cardiovascular and gastrointestinal lineages complications) and patients treated with them may develop drug resistance [8, 9]. Therefore, it is of great value to seek a treatment that has significant clinical effect and low recurrence rate without side effects.
Moxibustion is an external therapy based on the theory of traditional Chinese medicine (TCM). As a complementary therapy, its effective mechanism may be associated with the thermal effects, radiation effects, and pharmacological actions produced by the burning of moxa and the influence of meridians and acupoints. The theory of TCM holds that moxibustion can invigorate Qi and stimulate blood circulation, warm meridians, and regulate Yin and Yang [10]. Accumulate studies demonstrate that moxibustion plays an important role in anti-inflammatory and pain relief, especially for the treatment of musculoskeletal diseases, such as primary osteoporosis [11], cervical spondylosis [12], and knee osteoarthritis [13]. Studies found that the therapeutic effects of moxibustion seem to be related to anti-inflammatory and immune regulation. In recent years, a large number of studies, especially animal experiment studies, have illustrated the anti-inflammatory effects of moxibustion for RA.
Reviews based on animal research will be beneficial to the design of animal experiment in the future and provide a basis for clinical studies. Furthermore, animal experiment provides important data and theoretical support for the potential mechanism of moxibustion therapy for RA. Therefore, the current study conducted a systematic review and meta-analysis to analyze the effect of moxibustion on inflammatory cytokines in animal models of RA to provide guidance for future animal experiments and clinical trials.
2. Methods
2.1. Registration
Following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, the manuscript has been registered in PROSPERO (No. CRD 42020171320).
2.2. Search Strategy
Four Chinese databases (CNKI, SinoMed, VIP, and Wanfang) and three English databases (PubMed, EMBASE, and Web of Science) were retrieved. The retrieval time was from the establishment of these databases to March 2020. The languages used in the studies were limited to Chinese and English. Search terms consisted of two aspects: subject (rheumatoid arthritis) and intervention (moxibustion and other related terms).
2.3. Inclusion Criteria
The inclusion criteria are as follows:
subjects: animal models with rheumatoid arthritis,
intervention: the therapy was moxibustion only and the forms of moxibustion were seed-sized moxa cone, suspension moxibustion, mild moxibustion, and so on,
outcome: inflammatory cytokines including tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin-1β (IL-1β), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-10 (IL-10), and interleukin-17 (IL-17) were used to evaluate the anti-inflammatory effects of moxibustion,
language: Chinese or English.
2.4. Exclusion Criteria
Studies conducted in vitro or in humans
Moxibustion therapies combined with other therapies such as Chinese herbal medicine or Western medicine
The control group not treated with purified water injection, saline injection, or with no treatment
Studies without a separate control group
Duplicated publications, reviews, meta-analysis, or case reports; studies with abstract only; studies without extractable data
2.5. Study Selection and Data Extraction
One researcher (YMZ) retrieved the relevant databases according to the search strategy and made a list of all the records. Two evaluators (YNS and WTL) independently assessed the articles based on the inclusion and exclusion criteria. Firstly, the evaluators screened the title and abstract of the articles to exclude the obviously irrelevant literature. Secondly, the reviewers scanned the full text of these selected articles that met the previously mentioned criteria. If there was a disagreement, it would be resolved with negotiation between two researchers (YNS and WTL) or confirmed by the third researcher (LLZ).
Two researchers (YNS and WTL) extracted data from the selected studies. The contents of the data extraction mainly included the following: (1) general information: the first author, the year of publication, and nationality; (2) rat information: the number of rats in the experimental group and control group, gender, species, weight of rats, and modeling method; (3) treatment information: types of moxibustion, acupoint selection, duration, and period of treatment; (4) results: TNF-α, IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-10, and IL-17. When the data in the selected studies was presented as graph, the researchers would try to contact the author to obtain detailed data. If the author had no response after three times of e-mail contact, the study would be excluded since it lacked key information. The third reviewer would make a final decision on any divergence regarding the outcome data.
2.6. Quality Assessment
CAMARADES 10-item checklist was used to assess the methodological quality of the studies included [14]. The checklist included (1) peer-reviewed publication; (2) control of temperature; (3) random allocation to treatment or control; (4) blinded induction of model; (5) blinded assessment of outcome; (6) use of anesthetic without significant intrinsic neuroprotective activity; (7) appropriate animal model; (8) sample size calculation; (9) compliance with animal welfare regulations; (10) statement of potential conflict of interests.
Each study was given a comprehensive quality score out of a possible total of 10 points and the group median was calculated. Two reviewers (YNS and WTL) independently extracted data and assessed the quality of each study. Discrepancies were resolved by the third reviewer.
2.7. Statistical Analysis
Meta-analysis and statistical calculations were performed with the assistance of RevMan 5.3 software. The data of inflammatory cytokines were considered as continuous data. Mean difference (MD) would be employed to estimate the combined effect sizes of the indicators when the outcomes among different studies were tested with the same way. If the measurement unit of the outcome indicator was different, the standardized mean difference (SMD) would be given to estimate the combined effect sizes. The confidence interval (CI) was established at 95%. The I2 statistic was given to assess the heterogeneity among these studies. If I2 < 50%, it indicated that the heterogeneity among the studies included was low and a fixed effect model would be used. Otherwise, a random effect model would be performed. When significant heterogeneity existed, the sensitivity analyses and subgroup analysis would be conducted according to the moxibustion methods, animal species, animal weight, animal gender, and modeling methods. Funnel plot was used to evaluate the publication bias. When P value was less than 0.05, the outcome would be statistically significant.
3. Results
3.1. Study Inclusion
Initially, 648 studies were retrieved from seven databases. After removing duplicates, there were 327 articles left. According to the titles and abstracts of these records, the reviewers excluded 173 articles, because some studies were not about animal experiments, some studies were conducted in vitro, and some were reviews or case reports, while others were related to moxibustion therapies combined with other therapies and so on. After a full-text scanning, 47 articles were excluded. Eventually, 18 studies were selected [15–32]. The flow diagram of the study selection process is shown in Figure 1.
Figure 1.
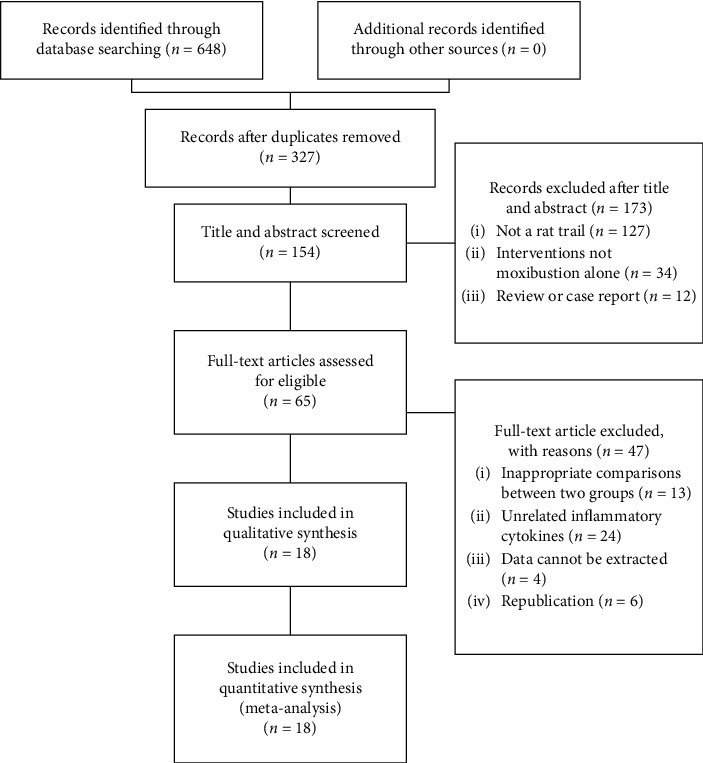
Flow diagram of the study selection process.
3.2. Study Characteristics
The eighteen included studies involved 461 rats, 231 of which were in the moxibustion group and 230 of which were in the control group. Among them, seventeen studies mentioned the weight of the rats, ranging from 110 to 250 g. The age of rats was different and was only stated specifically in four studies [18, 19, 25, 28]. It ranged from seven weeks old to sixteen weeks old. Four studies described rats as adult without offering the specific age of rats [16, 20, 26, 30]. All studies stated the gender of animals. Ten studies selected males [15–18, 21, 22, 24, 25, 27, 28] and eight studies had equal numbers of males and females [19, 20, 23, 26, 29–32]. Different inflammatory cytokines were used as outcome data in these studies: eleven studies reported data as TNF-α [15–25], three studies reported data as IFN-γ [23, 30, 32], eight studies reported data as IL-1β [15–18, 23, 25–27], three studies reported data as IL-2 [25, 28, 29], three studies reported data as IL-4 [23, 27, 30], three studies reported data as IL-6 [21, 26, 31], two studies reported data as IL-10 [30, 32], and two studies reported data as IL-17 [16, 24]. Two species of rats were used in the eighteen studies: thirteen studies used Sprague-Dawley (SD) rats [15–21, 26, 27, 29–32] and five studies used Wistar rats [22–25, 28]. Thirteen out of the eighteen studies were CFA models [16, 19–21, 23, 25–32]. Four studies were CIA models [15, 17, 18, 24]. CFA combined with CIA model was selected in one study [22]. The basic characteristics of the included studies are shown in Table 1.
Table 1.
Characteristics of the 18 included studies.
| Study | Species (Nc/Nm) | Gender | Age (week) | Weight (g) | Model | Moxibustin (acupoints) | Control | Duration (day) | Outcome index | P value |
|---|---|---|---|---|---|---|---|---|---|---|
| Zhang 2018 [15] | SD rat (8/8) | Male | NR | 200–250 | CIA | SM (BL23, ST36) | No treatment | 21 | TNF-α; | P < 0.05 |
| IL-1β | P < 0.05 | |||||||||
| Gao et al. 2017 [16] | SD rat (8/9) | Male | Adult | 200 ± 20 | CFA | SSMC (BL23, ST36) | No treatment | 21 | TNF-α; | P < 0.01 |
| IL-1β; | P < 0.01 | |||||||||
| IL-17 | P < 0.01 | |||||||||
| Wang et al. 2017 [17] | SD rat (8/8) | Male | NR | 200 ± 20 | CIA | SM (BL23, ST36) | No treatment | 21 | TNF-α; | P < 0.01 |
| IL-1β | P < 0.01 | |||||||||
| Wu et al. 2017 [18] | SD rat (8/8) | Male | 7-8 | NR | CIA | SM (BL23, ST36) | No treatment | 21 | TNF-α; | P < 0.05 |
| IL-1β | P < 0.05 | |||||||||
| Su et al. 2015 [19] | SD rat (10/10) | Male/female | 8–10 | 200 ± 20 | CFA | TYM governor meridian | No treatment | 12 | TNF-α | P < 0.05 |
| Wu et al. 2018 [20] | SD rat (24/24) | Male/female | Adult | 180 ± 20 | CFA | SSMC (BL23, ST36) | No treatment | 21 | TNF-α | P < 0.01 |
| Fan et al. 2017 [21] | SD rat (10/10) | Male | NR | 200 ± 20 | CFA | SM (BL23, ST36, GB34) | No treatment | 21 | TNF-α; | P < 0.01 |
| IL-6 | P < 0.01 | |||||||||
| Cui 2019 [22] | Wistar rat (8/8) | Male | NR | 140 ± 15 | CIA + CFA | SSMC (BL23, ST36, SP9, GB34) | No treatment | 20 | TNF-α | P < 0.05 |
| Huo et al. 2020 [23] | Wistar rat (10/10) | Male/female | NR | 180 ± 20 | CFA | SM (BL23, ST36) | No treatment | 21 | TNF-α; | P < 0.05 |
| IL-1β; | P < 0.01 | |||||||||
| IFN-γ; | P < 0.05 | |||||||||
| IL-4 | P < 0.01 | |||||||||
| Yin et al. 2017 [24] | Wistar rat (10/10) | Male | NR | 125 ± 15 | CIA | SM (BL23, ST36) | No treatment | 16 | TNF-α; | P < 0.05 |
| IL-17 | P < 0.05 | |||||||||
| Luo et al. 2011 [25] | Wistar rat (20/20) | Male | 12–16 | 200 ± 20 | CFA | SM (BL23, ST36) | No treatment | 15 | TNF-α; | P < 0.01 |
| IL-1β; | P < 0.01 | |||||||||
| IL-2 | P < 0.01 | |||||||||
| Wu et al. 2018 [26] | SD rat (24/24) | Male/female | Adult | 200 ± 20 | CFA | SSMC (BL23, ST36) | No treatment | 21 | IL-1β; | P < 0.05 |
| IL-6 | P < 0.05 | |||||||||
| Gao et al. 2011 [27] | SD rat (10/10) | Male | NR | 200 ± 20 | CFA | SSMC (BL23, ST36) | No treatment | 21 | IL-1β; | P < 0.01 |
| IL-4 | P < 0.05 | |||||||||
| Zhang et al. 2014 [28] | Wistar rat (8/8) | Male | 12–16 | 160–180 | CFA | SSMC (BL23, behind BL23) | No treatment | 20 | IL-2 | P < 0.01 |
| Bao 2007 [29] | SD rat (10/10) | Male/female | NR | 200 ± 20 | CFA | TM (CV4, GV4) | No treatment | 25 | IL-2 | P < 0.01 |
| Lai 2018 [30] | SD rat (15/15) | Male/female | Adult | 200 ± 20 | CFA | SSMC (BL23, ST36) | No treatment | 21 | IL-4; | P < 0.01 |
| IL-10; | P < 0.05 | |||||||||
| IFN-γ | P < 0.01 | |||||||||
| Ma et al. 2016 [31] | SD rat (24/24) | Male/female | Adult | 200 ± 20 | CFA | SSMC (BL23, ST36) | No treatment | 21 | IL-6 | P < 0.05 |
| Ren et al. 2019 [32] | SD rat (15/15) | Male/female | NR | 200 ± 20 | CFA | SSMC (BL23, ST36) | No treatment | 21 | IFN-γ; | P < 0.05 |
| IL-10 | P < 0.05 |
Nm: number of animals in the moxibustion group, Nc: number of animals in the control group, SD sprague dawley, NR: no record, CIA: collagen-induced arthritis, CFA: complete Freund's adjuvant, SM: suspension moxibustion, SSMC: seed-sized moxa cone, and TYM: tai-yi moxibustion.
3.3. Description of Moxibustion
Various moxibustion techniques were used in terms of the selection of acupoints, manipulation, and stimulation methods. The frequently used acupoints are Zusanli (ST36) and Shenshu (BL23), which have been used in fourteen studies [15–18, 20–27, 31, 32]. One study used governor meridian [19]. One study used Guanyuan (CV4) and Mingmen (GV4) [29]. One study used BL23 and behind Houzusanli (ST36) [28]. The acupoints involved in these studies also included Yanglingquan (GB34), Yinlingquan (SP9), or the combination of the previously mentioned points. The frequency of moxibustion was once a day. Rats received moxibustion treatment from 10 to 30 min per session. The period of moxibustion ranged from 12 to 25 days and most studies were 21 days. Eight studies chose seed-sized moxa cone as the method of stimulation [16, 21, 22, 26–28, 30–32], eight studies used suspension moxibustion [15, 17, 18, 20, 22–25], and the other two studies applied Tian moxibustion [29] and Tai-yi moxibustion, respectively [19]. Details of the moxibustion treatment are listed in Table 1.
3.4. Control Interventions
To better evaluate the anti-inflammatory effects of moxibustion, the current study excluded studies using therapies including western medicine, traditional Chinese medicine, or acupuncture for the control group and chose studies employing therapies like purified water injection, saline injection, or no treatment for the control group. All literature included set no treatment as control interventions.
3.5. Study Quality Assessment
The quality score of the included studies ranged from two to eight. More specifically, one study scored two, two studies scored three, five studies scored four, four studies scored seven, and six studies scored eight. Fourteen studies were published in peer-reviewed journal [16–21, 23–29, 32], but four studies were not because they were master's or doctoral thesis [15, 22, 30, 31]. Eleven studies elaborated the control of temperature [17, 18, 20, 23–26, 28–30, 32]. All studies randomly allocated rats to the control group and the treatment group and adopted appropriate animal models. Blinded induction of model and blinded assessment of outcome were stated, respectively, in four [17, 18, 23, 24] and five studies [20, 26, 28, 31, 32]. Eleven studies reported the use of anesthetic without significant intrinsic neuroprotective activity [16, 17, 20, 22, 23, 25, 26, 28, 29, 31, 32]. No study mentioned sample size calculation. Compliance with animal welfare regulations was stated in eleven studies [17, 18, 20, 22–26, 28,29, 32]. Twelve studies reported the potential conflict of interests [17, 18, 20, 21, 23–29, 32]. The basic characteristics of the study quality are presented in Table 2.
Table 2.
Methodological quality assessment of the studies included.
| Study | ① | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | ⑨ | ⑩ | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang 2018 [15] | √ | √ | 2 | ||||||||
| Gao et al. 2017 [16] | √ | √ | √ | √ | 4 | ||||||
| Wang et al. 2017 [17] | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Wu et al. 2017 [18] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Su et al. 2015 [19] | √ | √ | √ | 3 | |||||||
| Wu et al. 2018 [20] | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Fan et al. 2017 [21] | √ | √ | √ | √ | 4 | ||||||
| Cui 2019 [22] | √ | √ | √ | √ | 4 | ||||||
| Huo et al. 2020 [23] | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Yin et al. 2017 [24] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Luo et al. 2011 [25] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Wu et al. 2018 [26] | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Gao et al. 2011 [27] | √ | √ | √ | √ | 4 | ||||||
| Zhang et al. 2014 [28] | √ | √ | √ | √ | √ | √ | √ | 7 | |||
| Bao 2007 [29] | √ | √ | √ | 3 | |||||||
| Lai 2018 [30] | √ | √ | √ | √ | 4 | ||||||
| Ma et al. 2016 [31] | √ | √ | √ | √ | √ | √ | √ | √ | 8 | ||
| Ren et al. 2019 [32] | √ | √ | √ | √ | √ | √ | √ | √ | 8 |
①, peer-reviewed publication; ②, control of temperature; ③, random allocation to treatment or control; ④, blinded induction of model; ⑤, blinded assessment of outcome; ⑥, use of anesthetic without significant intrinsic neuroprotective activity; ⑦, appropriate animal model; ⑧, sample size calculation; ⑨, compliance with animal welfare regulations; ⑩, statement of potential conflict of interests.
3.6. Outcomes Analysis
3.6.1. Proinflammatory Cytokines Outcomes Analysis
Eleven studies adopted TNF-α as an outcome index [15–25]. The pooled results showed that moxibustion significantly decreased the level of TNF-α compared with the control group (n = 249, SMD 2.95, 95% CI: 1.99–3.92, P < 0.00001; heterogeneity X2 = 63.44, I2 = 84%, Figure 2). IL-1β was reported as outcome in eight studies [15–18, 23, 25–27]. The result showed that moxibustion reduced the level of IL-1β compared with the control group (n = 193, SMD 4.10, 95% CI: 2.37–5.84, P < 0.00001; heterogeneity X2 = 93.04, I2 = 92%, Figure 3). Three studies regarded IFN-γ as an outcome index [23, 30, 32] and the result showed that IFN-γ was significantly different between the moxibustion group and the control group (n = 80, MD 25.0, 95% CI: 16.17–33.82, P < 0.00001; heterogeneity X2 = 5.55, I2 = 64%, Figure 4(a)). IL-6 was used as an outcome indicator in three studies [21, 26, 31]. The pooled results showed that moxibustion significantly decreased IL-6 (n = 116, MD 11.83, 95% CI: 6.22–17.44, P < 0.00001; heterogeneity X2 = 4.05, I2 = 51%, Figure 4(b)). Pooled results of two studies showed that the content of IL-17 was lower in the moxibustion group (n = 37, MD 99.3, 95% CI: 86.83–111.76, P < 0.00001; heterogeneity X2 = 0.13, I2 = 0%, Figure 4(c)).
Figure 2.
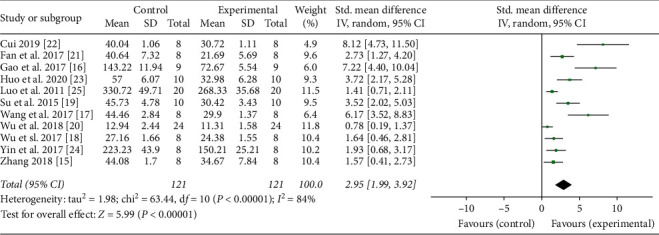
Forest plot showed that the level of TNF-α decreased with moxibustion therapy.
Figure 3.
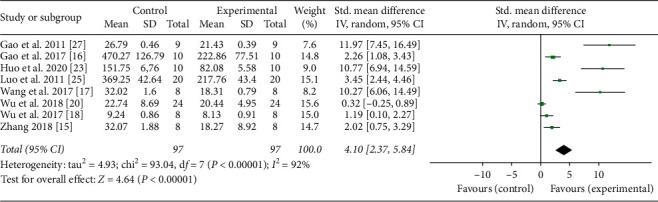
Forest plot showed that the level of IL-1β decreased with moxibustion therapy.
Figure 4.
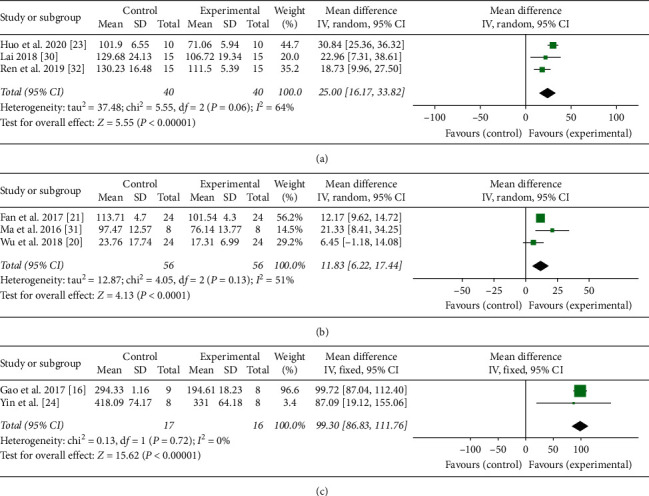
Forest plot showed that the level of proinflammatory cytokines decreased with moxibustion therapy. (a), (b), and (c), respectively, represented the effect of moxibustion on IFN-γ, IL-6, and IL-17.
3.6.2. Anti-Inflammatory Cytokines Outcomes Analysis
Three studies were conducted pooled analysis of IL-2 [25, 28, 29]. The pooled results showed that moxibustion significantly increased the level of IL-2 (n = 76, SMD 8.89, 95% CI: 0.93–16.86, P=0.03; heterogeneity X2 = 60.03, I2 = 97%, Figure 5(a)). The pooled results of other three studies [23, 27, 30] showed that the moxibustion had an effect on IL-4 with a higher expression compared with control groups (n = 70, MD 1.79, 95% CI: 0.26–3.32, P=0.02; heterogeneity X2 = 13.5, I2 = 85%, Figure 5(b)). Two studies [30, 32] reported that the moxibustion increased the level of IL-10 compared with the control group (n = 60, MD 5.93, 95% CI: 1.37–10.49, P=0.01; heterogeneity X2 = 3.36, I2 = 70%, Figure 5(c)).
Figure 5.
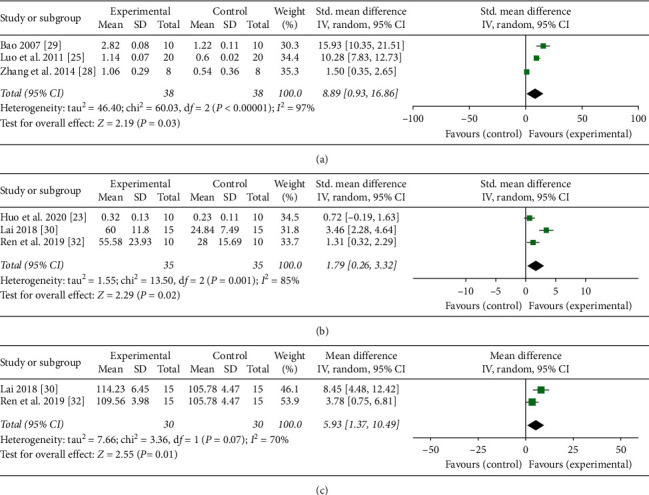
Forest plot showed that the level of anti-inflammatory cytokines increased with moxibustion therapy. (a), (b), and (c), respectively, represented the effect of moxibustion on IL-2, IL-4, and IL-10.
3.7. Subgroup Analyses
3.7.1. TNF-α
The researchers performed a subgroup analysis for the moxibustion methods. The result showed that suspension moxibustion had a significant effect on the reduction of the level of TNF-α (SMD 2.36, 95% CI: 1.51–3.22; P < 0.00001), but no remarkable difference was found in the seed-sized moxa cone (SMD 5.20, 95% CI: −0.25–10.66; P=0.06). Subgroup analysis of the moxibustion methods indicated that suspension moxibustion reduced heterogeneity (I2 = 68%), while seed-sized moxa cone did not (I2 = 94%). Moxibustion was found to have a remarkable effect on the reduction of TNF-α in both Sprague-Dawley rats (SMD 2.96, 95% CI: 1.62–4.30; P < 0.0001) and Wister rats (SMD 3.17, 95% CI: 1.37–4.98; P=0.0006). The present study also conducted a subgroup analysis for the modeling methods and found that CFA model (SMD 2.83, 95% CI: 1.55–4.12; P < 0.0001) and CIA model (SMD 2.37, 95% CI: 1.05–3.69; P=0.0004) performed well in the reduction of the level of TNF-α. In the subgroup analysis, the reviewers compared the effects of different genders on TNT-α. The declining level of TNF-α in male rats (SMD 3.19, 95% CI: 1.97–4.41; P < 0.00001) was higher than that of the male–female experiment (SMD 2.59, 95% CI: 0.38–4.80; P=0.02). The researchers also compared the effects of different body weights on TNF-α in subgroups. The results showed that rats weighing from 180 to 220 g (SMD 3.34, 95% CI: 1.90–4.79; P < 0.00001) had significantly lower levels of TNF-α, while rats weighing from 110 to 150 g (SMD 4.82, 95% CI: −1.24–10.87; P=0.12) and 160 to 200 g (SMD 2.16, 95% CI: −0.72–5.04; P=0.15) had no significant effect on TNF-α levels. Compared with unpublished articles, published articles showed more obvious changes on TNF-α with moxibustion treatment (SMD 2.80, 95% CI: 1.79–3.81; P < 0.00001). A summary of the subgroup analysis is shown in Table 3.
Table 3.
Subgroup analysis for the effect of moxibustion on the reduction of TNF-α.
| Heterogeneity | Effect size | ||||
|---|---|---|---|---|---|
| P | I 2 | 95% CI | P | ||
| Moxibustion | SS | 0.005 | 68 | SMD = 2.36 (1.51, 3.22) | <0.00001 |
| SSMC | 0.00001 | 94 | SMD = 5.20 (−0.25, 10.66) | =0.06 | |
|
| |||||
| Model | CFA | 0.00001 | 87 | SMD = 2.83 (1.55, 4.12) | <0.0001 |
| CIA | 0.02 | 71 | SMD = 2.37 (1.05, 3.69) | =0.0004 | |
|
| |||||
| Species | SD | 0.00001 | 86 | SMD = 2.96 (1.62, 4.30) | <0.0001 |
| Wister | 0.0002 | 85 | SMD = 3.17 (1.37, 4.98) | =0.0006 | |
|
| |||||
| Weight | 110–155 g | 0.0001 | 91 | SMD = 4.82 (−1.24, 10.87) | =0.12 |
| 160–200 g | 0.0005 | 92 | SMD = 2.16 (−0.72, 5.04) | =0.15 | |
| 180–220 g | 0.0001 | 83 | SMD = 3.34 (1.90, 4.79) | <0.00001 | |
|
| |||||
| Gender | Male | 0.00001 | 82 | SMD = 3.19 (1.97, 4.41) | <0.00001 |
| Male/female | 0.0001 | 90 | SMD = 2.59 (0.38, 4.80) | =0.02 | |
|
| |||||
| Publication | Yes | 0.00001 | 84 | SMD = 2.80 (1.79, 3.81) | <0.00001 |
| No | 0.0003 | 92 | SMD = 4.64 (−1.76, 11.05) | =0.16 | |
3.7.2. IL-1β
In the subgroup analysis of IL-1β, the efficacy of suspension moxibustion (SMD 4.58, 95% CI: 2.30–6.86, P < 0.0001) was better than that of the seed-sized moxa cone (SMD 3.60, 95% CI: 0.49–6.71, P=0.02). In regard to the varieties of experimental animals, SD rats (SMD 3.25, 95% CI: 1.47–5.04, P=0.0004) were more sensitive to moxibustion than Wister rats (SMD 6.87, 95% CI: −0.29–14.02, P=0.06). By analyzing different causes of RA models, we found that CFA models (SMD 4.75, 95% CI: 2.19–7.30, P=0.0003) performed better than CIA models (SMD 3.47, 95% CI: 0.67–6.27, P=0.02) in decreasing IL-1β. The study found that IL-1β levels were significantly reduced in experiments using male rats (SMD 3.89, 95% CI: 2.14–5.65, P < 0.0001), but there was no statistically significant difference in experiments using male–female rats (SMD 5.36, 95% CI: −4.87–15.60, P=0.3). Rats with different weights were included in these studies. The reviewers found that rats with weight ranging from 180 to 220 g (SMD 5.71, 95% CI: 2.49–8.94, P < 0.0001) were more sensitive to moxibustion for the improvement of IL-1β than rats with weight ranging from 160 to 200 g (SMD 4.17, 95% CI: 0.72–7.62, P=0.02). Compared with unpublished articles, published articles showed more obvious changes on IL-1β with moxibustion treatment (SMD 4.61, 95% CI 2.58–6.64; P < 0.0001). A summary of the subgroup analysis is shown in Table 4.
Table 4.
Subgroup analysis for the effect of moxibustion on reducing IL-1β.
| Heterogeneity | Effect size | ||||
|---|---|---|---|---|---|
| P | I 2 (%) | 95% CI | P | ||
| Moxibustion | SM | 0.00001 | 90 | SMD = 4.58 (2.30, 6.86) | <0.0001 |
| SSMC | 0.00001 | 94 | SMD = 3.60 (0.49, 6.71) | =0.02 | |
|
| |||||
| Model | CFA | 0.00001 | 95 | SMD = 4.75 (2.19, 7.30) | = 0.0003 |
| CIA | 0.0002 | 88 | SMD = 3.47 (0.67, 6.27) | =0.02 | |
|
| |||||
| Species | SD | 0.00001 | 91 | SMD = 3.25 (1.47, 5.04) | =0.0004 |
| Wister | 0.0003 | 92 | SMD = 6.87 (−0.29, 14.02) | =0.06 | |
|
| |||||
| Weight | 160–200 g | 0.00001 | 96 | SMD = 4.17 (0.72, 7.62) | =0.02 |
| 180–220 g | 0.00001 | 90 | SMD = 5.71 (2.49, 8.94) | <0.00001 | |
|
| |||||
| Gender | Male | 0.00001 | 87 | SMD = 3.89 (2.14, 5.65) | <0.0001 |
| Male/female | 0.00001 | 96 | SMD = 5.36 (−4.87, 15.60) | =0.3 | |
|
| |||||
| Publication | Yes | 0.00001 | 94 | SMD = 4.61 (2.58, 6.64) | <0.00001 |
| No | SMD = 2.02 (0.75, 3.29) | =0.002 | |||
3.8. Biological Mechanism
Several different biological mechanisms were studied to better understand the potential mechanism of the anti-inflammatory effects of moxibustion on the treatment of RA. Among the eighteen studies, fourteen got detailed descriptions about the possible mechanisms. It was found that the main biological mechanisms were categorized into three aspects: regulation of the hypothalamic-pituitary-adrenal axis (HPAA), restoration of the balance between T helper 1 cell (Th1) and T helper 2 cell (Th2), and regulation of the programmed cell death 1 and ligand 1 (PD-1/PD-L1). The summary of the proposed mechanisms is shown in Table 5.
Table 5.
Summary of the proposed mechanisms.
| Study | Findings or proposed mechanisms |
|---|---|
| Zhang 2018 [15] | Decreased TNF-α, IL-1β, PGE2 |
| Adjust abnormal levels of metabolites | |
| Gao et al. 2017 [16] | Reduce TNF-α, IL-1β, IL-17 |
| Wang et al. 2017 [17] | Regulated the immune function |
| Wu et al. 2017 [18] | Decreased TNF-α, IL-1β |
| Su et al. 2015 [19] | Reduced T cell activation |
| Regulated the immune function | |
| Wu et al. 2018 [20] | Regulated the circadian rhythm of TNF-α |
| Fan et al. 2017 [21] | Decreased TNF-α, IL-6, NO, TNF-α mRNA |
| Cui 2019 [22] | Reduced TNF-α, BK |
| Huo et al. 2020 [23] | Decrease TNF-α, IFN-γ, IL-1β, IL-4 |
| Restore the balance between Th1/Th2 | |
| Yin et al. 2017 [24] | Decrease TNF-α, IL-17 |
| Luo et al. 2011 [25] | Increased IL-2 |
| Decreased TNF-α, IL-1β | |
| Wu et al. 2018 [26] | Rhythmic anti-inflammatory |
| Gao et al. 2011 [27] | Increased IL-4 |
| Decreased IL-1β, MT | |
| Restore the balance between Th1/Th2 | |
| Zhang et al. 2014 [28] | Increased IL-2, Bcl-2 |
| Decreased IL-1, caspase-3 | |
| Bao 2007 [29] | Regulated the immune function |
| Lai 2018 [30] | Inhibit the activation of T cell |
| Regulate the PD-1/PD-L1 signaling pathway | |
| Ma et al. 2016 [31] | Regulated the circadian rhythm of IL-6 |
| Regulate the HPAA | |
| Ren et al. 2019 [32] | Regulate the PD-1/PD-L1 signaling pathway |
PGE 2: prostaglandin E2, NO: nitric oxide, BK: bradykinin, MT: melatonin, Th1: T helper 1 cell, Th2: T helper 2 cell, HPAA: the hypothalamic-pituitary-adrenal axis, Bcl-2: B-cell lymphoma-2, PD-1: programmed cell death 1, PD-L1: programmed cell death ligand 1.
3.9. Assessment of Bias
Funnel plot was applied to assess the publication bias. The asymmetry funnel plot indicated that there was publication bias (Figure 6).
Figure 6.
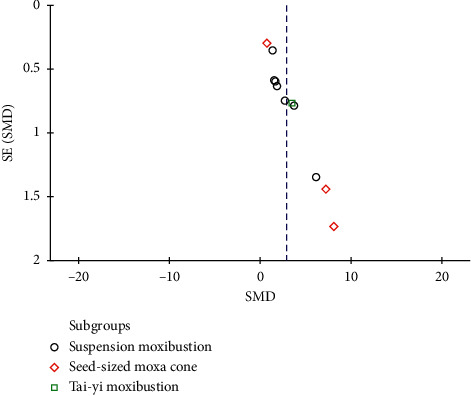
Funnel plot for the effectiveness of moxibustion on TNF-α.
4. Discussion
4.1. Principle Findings
The current study represents the first meta-analysis to evaluate the effect of moxibustion for the treatment of RA in animal experiments with the results of inflammatory cytokines as outcome indicators. This meta-analysis indicated that moxibustion has certain effects on the amelioration of the inflammation in animal models with RA, including the downregulation of the level of proinflammatory cytokines and the upregulation of the level of anti-inflammatory cytokines at the same time. Clinical trials revealed that moxibustion can enhance the anti-inflammatory and analgesic effects of conventional medicine and downregulate HIF-1α/VEGF contents to inhibit angiogenesis [33]. The conclusion is similar to the previous clinical meta-analysis [34].
4.2. Possible Therapeutic Mechanism of the TCM
“The Yellow Emperor's Internal Classic,” an ancient medical canon of TCM in China, holds that the occurrence of RA is closely related to the invasion of three perverse trends of wind, cold, and dampness. The primary pathogenesis is the deficiency of healthy Qi in viscera and the meridian is blocked by accumulated dampness, which has close relationship with spleen, stomach, and kidney. Therefore, warming the meridian and removing dampness through invigorating spleen and kidney are the basic principles of treatment [35]. Almost all the selected acupoints belong to the Stomach Meridian and Kidney Meridian. It is consistent with the basic pathogenesis of the Qi deficiency and pathogenic stagnation, which plays a key role in ensuring effectiveness by warming the meridian and eliminating dampness through invigorating spleen and kidney, dredging channel of Qi and blood, and harmonizing Yin and Yang.
The theory of TCM holds that spleen and stomach provide the material basis of the acquired constitution. ST 36, the he-sea point of Stomach Meridian of Foot-Yangming, can strengthen the function of spleen and stomach and eliminate dampness. The results of previous studies indicated that acupuncture at ST 36 can promote gastrointestinal motility and improve gastrointestinal function [36, 37]. The research found that electroacupuncture at ST 36 might activate AMPK, produce stable ULK1/AMPK compound, and increase the mitochondrial autophagy, which could regulate spleen-stomach and treat spleen deficiency [38]. Meanwhile, acupuncture at ST 36 reduced the lipopolysaccharide- (LPS-) induced serum levels of TNF, IL-6, and INF-γ [39] and could promote eosinophils apoptosis to inhibit the development of inflammatory reaction [40]. BL23 is the back-shu point of kidney. Moxibustion at BL23 for the treatment of RA can not only nourish kidney and benefit essence, but also dispel cold and relieve pain, so as to achieve the effect of treating both symptoms and root causes.
4.3. Possible Modern Biological Mechanism
The combination of the previously mentioned analysis and numerous research results indicates that the biological mechanism of moxibustion in the treatment of RA involves several aspects. Researchers have conducted many studies from the perspective of anti-inflammatory, immune regulation, regulation of circadian rhythm, and bone protection [41–44]. Among them, there are studies concentrating on anti-inflammatory effect as the most important aspect because RA is characterized by synovitis, which may result in cartilage and bone destruction. Previous studies explored the mechanisms of moxibustion in treating RA from several aspects, including restoring the balance between Th1 and Th2 by regulating the levels of inflammatory cytokines [23, 27], modulating the pathological circadian rhythm of TNF-α, IL-1β, and IL-6 [20, 25, 31], regulating the function of HPAA [31], alleviating cartilage degradation through RANKL/OPG signaling pathway [44], and strengthening the negative regulation of PD-1/PD-L1 signaling pathway [30, 32]. This study suggested that moxibustion can relieve synovitis of the joint with RA mainly by inhibiting the secretion of proinflammatory cytokines and enhancing the secretion of anti-inflammatory cytokines to restore the equilibrium between Th1 and Th2. Moxibustion is effective in treating RA, but its mechanism of action involves many aspects, and its anti-inflammatory effect is just one of them. Therefore, it needs to be further explored in future studies.
4.4. The Value of Animal Studies and Systematic Review of the Effectiveness of Moxibustion for RA in Animal Model
As a complementary therapy, moxibustion is safe and effective for people suffering from RA to some extent. However, there is also a controversial view that moxibustion has no therapeutic effect but only a warming effect. Revealing the mechanisms of moxibustion for RA and making sure that the effect is not just warming are significant for the development of moxibustion. To dispel these doubts, it is necessary to objectively investigate the effects of moxibustion on synovium and related tissues, cells, and signal pathways. As is known to all, it is unethical and forbidden to obtain tissues or cells from human body. For this reason, animal researches of moxibustion for RA are of great value and cannot be replaced completely by clinical studies. Meta-analysis is a kind of statistical method that can comprehensively analyze the results of many studies and help researchers promote the methodological quality of preclinical animal researches. Therefore, we conducted this systematic review and meta-analysis to assess the effectiveness of moxibustion in the treatment of RA in animal models and found that moxibustion has a definite effect to relieve inflammation in animal models with RA.
4.5. Subgroup Analysis
The high heterogeneity among the studies based on inflammation cytokines cannot be completely explained. The reviewers carried out subgroup analysis on the included studies based on moxibustion methods, modeling methods, animal species, animal gender, and animal weight, and the researchers also conducted sensitivity analysis, but no evident cause was found. However, in subgroup analysis, it was found that suspended moxibustion was significantly better than seed-sized moxa cone in improving the level of inflammatory cytokines. The reviewers also found that 13 of the 18 studies used ST36 and BL23 points, and the remaining five studies involved acupoints including CV4, GV4, GB34, SP9, and behind ST36 or a combination of the previously mentioned points. The acupoints used were inconsistent. Furthermore, the sample size also covered a wide scope ranging from 16 to 48 and no study mentioned sample size calculation. These findings suggested that, in addition to the differences in moxibustion method, modeling methods and animal species, acupoint combinations, and sample sizes may be another source of heterogeneity among the studies.
4.6. Limitations and Strengths
The present review has several limitations. First, it is the language. The researchers limited the languages of all studies to English and Chinese. Of all the included studies, only one was written in English, which may cause potential publication bias. Second, the scope of sample size still needs to be expanded. Although the researchers conducted a comprehensive literature search and traced the references of the included literature to supplement the relevant literature, no literatures met the inclusion criteria. Third, the quality of the included studies is unsatisfactory and the differences in the selection of acupoints and operation methods may greatly affect the outcome of the meta-analysis. Finally, the blind method was not performed well. Although it is difficult to blind moxibustion for operators, it should be strictly carried out in the process of molding and the assessment of outcome.
According to the above limitations, more databases with different languages should be searched to increase the diversity of the included literature. Meanwhile, in terms of experimental design, especially the random allocation of treatment and control, blind building of model and blind assessment of outcome should be strictly carried out in future animal researches. In addition, a unified standard of moxibustion therapeutic schedule should be seriously performed because the details of moxibustion manipulation are closely related to the efficacy. The choice of moxibustion methods and moxibustion acupoints, duration of each moxibustion, and course of treatment are necessarily to be standardized.
5. Conclusions
The findings of the present review indicate that moxibustion can protect the synovium of joint in animal models with RA by enhancing the level of anti-inflammatory cytokines and decreasing the level of proinflammatory cytokines. Moxibustion has the potential to relieve inflammation in animal models with RA. However, the specific mechanism still needs to be further explored and the quality of studies should also be improved in the future. Therefore, future researches need to be more standardized in terms of experimental design, especially the design of blind method, selection of inflammatory cytokines, and moxibustion methods so as to provide a reliable theoretical basis for the application of basic research achievements to clinical practice.
Acknowledgments
This work was supported by National Key R&D Program of China (2019YFC1709001) and the grants from the National Natural Science Foundation of China (nos. 81674068 and 81973959). The authors would like to extend their gratitude to Qian Chen (Sichuan International Studies University) for her revision of the language.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Alan J. S., Jacqueline E. P. Epidemiology and genetics of rheumatoid arthritis. Arthritis Research & Therapy. 2002;4(3):265–272. doi: 10.1186/ar578. [DOI] [Google Scholar]
- 2.Oyama J.-i., Node K. Rheumatoid arthritis and vascular failure—rheumatoid arthritis is a risk factor for cardiovascular disease- Internal Medicine. 2019;58(10):1373–1374. doi: 10.2169/internalmedicine.2182-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Y.-C., Li Y.-H., Chang C.-H., et al. Rheumatoid arthritis patients with hip fracture: a nationwide study. Osteoporosis International. 2015;26(2):811–817. doi: 10.1007/s00198-014-2968-y. [DOI] [PubMed] [Google Scholar]
- 4.Kim S. C., Schneeweiss S., Liu J., Solomon D. H. Risk of venous thromboembolism in patients with rheumatoid arthritis. Arthritis Care and Research. 2013;65(10):1600–1607. doi: 10.1002/acr.22039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alamanos Y., Voulgari P. V., Drosos A. A. Incidence and prevalence of rheumatoid arthritis, based on the 1987 american college of rheumatology criteria: a systematic review. Seminars in Arthritis and Rheumatism. 2006;36(3):182–188. doi: 10.1016/j.semarthrit.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Moradi-Lakeh M., Forouzanfar M. H., Vollset S. E., et al. Burden of musculoskeletal disorders in the eastern Mediterranean region, 1990–2013: findings from the global burden of disease study 2013. Annals of the Rheumatic Diseases. 2017;76(8):1365–1373. doi: 10.1136/annrheumdis-2016-210146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safiri S., Kolahi A. A., Hoy D., et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: a systematic analysis of the global burden of disease study 2017. Annals of the Rheumatic Diseases. 2019;78(11):1463–1471. doi: 10.1136/annrheumdis-2019-215920. [DOI] [PubMed] [Google Scholar]
- 8.Gaffo A., Saag K. G., Curtis J. R. Treatment of rheumatoid arthritis. American Journal of Health-System Pharmacy. 2006;63(24):2451–2465. doi: 10.2146/ajhp050514. [DOI] [PubMed] [Google Scholar]
- 9.Finckh A., Simard J. F., Gabay C., Guerne P. A. Evidence for differential acquired drug resistance to anti-tumour necrosis factor agents in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2006;65(6):746–752. doi: 10.1136/ard.2005.045062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng H., Shen X. The mechanism of moxibustion: ancient theory and modern research. Evidence-Based Complementary and Alternative Medicine. 2013;2013:7. doi: 10.1155/2013/379291.379291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu D.-M., Xu H., Liu J., et al. Effect of thunder-fire moxibustion on pain, quality of life, and tension of multifidus in patients with primary osteoporosis: a randomized controlled trial. Medical Science Monitor. 2018;24(24):2937–2945. doi: 10.12659/msm.909725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim T. H., Kim K. H., Kang J. W., et al. Moxibustion treatment for knee osteoarthritis: a multi-centre, non-blinded, randomized controlled trial on the effectiveness and safety of the moxibustion treatment versus usual care in knee osteoarthritis patients. PLoS One. 2014;9:p. 8. doi: 10.1371/journal.pone.0101973.e101973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu S. J., Liang Z. H., Fu W. B. Chronic neck pain of cervical spondylosis treated with acupuncture and moxibustion in terms of the heart and kidney theory: a randomized controlled trial. Chinese Acupuncture & Moxibustion. 2012;32(9):769–775. [PubMed] [Google Scholar]
- 14.Macleod M. R., O’Collins T., Howells D. W., Donnan G. A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35(5):1203–1208. doi: 10.1161/01.str.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y. Y. Nanjing, China: Nanjing University of Chinese Medicine; 2018. Study on mechanism of collagen-induced arthritis (CIA) rats treated with moxibustion based on metabonomics. M.S. thesis. [Google Scholar]
- 16.Gao Z. S., Gan X. J., Guo J. Y., et al. Study on the effects of wheat moxibustion on the level of TNF-a and IL-1β in serum in experimental RA rats. Journal of Clinical Acupuncture and Moxibustion. 2017;33(1):37–41+83. [Google Scholar]
- 17.Wang Y., Wu X. Y., Sun Z. L., et al. Therapeutic efficacy of moxibustion at different distances on type II collagen-induced arthritis. Chinese Journal of Tissue Engineering Research. 2017;21(8):1241–1245. doi: 10.3969/j.issn.2095-4344.2017.08.016. [DOI] [Google Scholar]
- 18.Wu X.-y., Wang Y., Sun Z.-l., et al. Experimental study on the effect of different moxibustion durations on rats with rheumatoid arthritis. Journal of Acupuncture and Tuina Science. 2017;15(3):177–183. doi: 10.1007/s11726-017-0997-8. [DOI] [Google Scholar]
- 19.Su R. H., Liu Y., Lv M. Z., et al. Effect of taiyi shenzhen on TNF-α and soluble CD4, CD8 of rheumatoid arthritis rats. Journal of Guizhou Medical University. 2015;40(10):1033–1035. [Google Scholar]
- 20.Wu X., Liu X. G., Jing Z. K., et al. Effects of moxibustion at different time points on foot swelling, serum level of TNF-α and circadian rhythm in rats with rheumatoid arthritis. Chinese Acupuncture & Moxibustion. 2018;38(11):1189–1194. doi: 10.13703/j.0255-2930.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Fan W., Yang J., Xia L. N., et al. Synergistic effect of anti-inflammatory treatment by moxibustion on yanglingquan (GB34) in AA rats based on theory of shaoyang governing bones. Liaoning Journal of Traditional Chinese Medicine. 2017;22(4):852–854. [Google Scholar]
- 22.Cui Y. Y. Chengde, China: Chengde Medical University; 2017. The intervention of moxibustion the “complementary acupoints” Yinlingquan and Yanglingquan to RA rats and the effects about MC release BK, TNF-α. M.S. thesis. [Google Scholar]
- 23.Huo X. H., Li P., Dang H., et al. Effects of Capparis spinosa combined with moxibustion on serum IFN-gamma/IL-4 in rats with rheumatoid arthritis. Liaoning Journal of Traditional Chinese Medicine. 2020;47(4):183–186. [Google Scholar]
- 24.Yin Y., Chen T. W., Zhang R., Ma W. Z. Effect of moxibustion on serum IL-17 and TNF-α levels in collagen-induced arthritis rats. Acupuncture Research. 2017;42(2):149–152+177. [PubMed] [Google Scholar]
- 25.Luo L., Song X. G., Tang Z. L., et al. Effect of moxibustion on the metatarsal joint cytokine in rheumatoid arthritis rats. Global Traditional Chinese Medicine. 2011;4(6):416–419. [Google Scholar]
- 26.Wu X., Jing Z. K., Chen Y., et al. Effects of moxibustion from 7am to 9am and 7pm to 9pm respectively on serum levels of IL-1 and IL-6 in experimental RA rats. Lishizhen Medicine and Materia Medica Research. 2018;29(11):2783–2786. [Google Scholar]
- 27.Gao J., Huang D. J., Zhou H. Y., et al. Impact on IL-1β, IL-4 in the plasma and melatonin in serum of RA rats by the moxibustion treatment. Liaoning Journal of Traditional Chinese Medicine. 2011;38(8):1677–1680. [Google Scholar]
- 28.Zhang C. Y., Cai R. L., Tang Z. L. Influences of moxibustion on inflammatory factors and synoviocytes in rats with rheumatoid arthritis. Journal of Beijing University of Traditional Chinese Medicine. 2014;37(3):190–194+5. [Google Scholar]
- 29.Bao Y. M. Ürümqi, China: Xinjiang Medical University; 2007. Influence of medical vesiculation the cellular immunity in adjuvant arthritis rats. M.S. thesis. [Google Scholar]
- 30.Lai D. L. Chengdu, China: Chengdu University of Traditional Chinese Medicine; 2018. Study on mechanism of PD-1 mediated regulation of T cell over-activation in RA with moxibustion. Ph.D thesis. [Google Scholar]
- 31.Ma W. B., Liu X. G., Qin Y., Zhou H., Yang X. Effects of grain-sized moxibustion from 7am to 9am on circadian rhythm of inflammatory factor IL-6 in rats with rheumatoid arthritis. Chinese Acupuncture & Moxibustion. 2016;36(4):396–401. [PubMed] [Google Scholar]
- 32.Ren J. G., Lei X., Wang D. X., Haiyan Z. Effect of moxibustion on inflammatory factors and expression of synovial tissue type programmed cell death ligand 1 in experimental rheumatoid arthritis rats. Hebei Journal of Traditional Chinese Medicine. 2019;41(4):577–580+586. [Google Scholar]
- 33.Gong Y., Yu Z., Wang Y., et al. Effect of moxibustion on HIF-1α and VEGF levels in patients with rheumatoid arthritis. Pain Research and Management. 2019;2019:9. doi: 10.1155/2019/4705247.4705247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen B. Y., Sun Q., Chen H. Y., et al. Effects of moxibustion on pain behaviors in patients with rheumatoid arthritis: a meta-analysis. Medicine (Baltimore) 2019;98(30):p. 7. doi: 10.1097/MD.0000000000016413.e16413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Z. K., Yang Z. J., Chen F. Effect of electroacupuncture stimulation of “Housanli” (ST 36) and “Zhongwan” (CV 12) on serum leptin and hepatocellular JAK 2-STAT 3 signaling in obese rats. Acupuncture Research. 2015;40(1):1–5. [PubMed] [Google Scholar]
- 36.Lin Y. P., Wan Q. Q., Peng Y., He F. E., Shen J. Effect of electroacupuncture at “Zusanli” (ST 36), etc. on gastrointestinal motility and expression of ghrelin mRNA and growth hormone secretagogue receptor mRNA in diabetic gastroparesis rats. Acupuncture Research. 2015;40(4):290–295. [PubMed] [Google Scholar]
- 37.Chao H.-L., Miao S.-J., Liu P.-F., et al. The beneficial effect of ST-36 (Zusanli) acupressure on postoperative gastrointestinal function in patients with colorectal cancer. Oncology Nursing Forum. 2013;40(2):E61–E68. doi: 10.1188/13.onf.e61-e68. [DOI] [PubMed] [Google Scholar]
- 38.Dong J. Z., Zhang Y., Wei Y. T., et al. Effects of electroacupuncture at “Zusanli” (ST 36) on expression of mitophagy-related proteins in skeletal muscle in rats with spleen deficiency syndrome. Chinese Acupuncture & Moxibustion. 2018;38(7):741–746. doi: 10.13703/j.0255-2930.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Torres-Rosas R., Yehia G., Peña G., et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nature Medicine. 2014;20(3):291–295. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Z. L., Li C. R., Liu Z. L., Zhang Q. R. Effects of acupuncture at “Zusanli” (ST 36) on eosinophil apoptosis and related gene expression in rats with asthma. Chinese Acupuncture & Moxibustion. 2012;32(8):721–725. [PubMed] [Google Scholar]
- 41.Zhang C. Y., Hu L., Cai R. L., Peng C. Y., Yuan J. Toll-like receptor 4/nuclear factor-κb signaling in synovial tissue is involved in the anti-inflammatory effect of moxibustion in rats with rheumatoid arthritis. Acupuncture Research. 2018;43(11):687–691. doi: 10.13702/j.1000-0607.180229. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z., Li X., Zhao C., et al. Effects of moxibustion on Treg/Th17 cell and its signal pathway in mice with rheumatoid arthritis. Zhongguo Zhen Jiu/Chinese Acupuncture and Moxibustion. 2017;37(10):1083–1091. doi: 10.13703/j.0255-2930.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 43.Ma W. B., Liu X. G., Zhou H. Y. Effects of chronological moxibustion on circadian rhythm activities of hypothalamus-pituitary-axis in rheumatoid arthritis rats. Zhen Ci Yan Jiu/Acupuncture Research. 2016;41(2):100–107. [PubMed] [Google Scholar]
- 44.Chen Y., Li H., Luo X., et al. Moxibustion of Zusanli (ST36) and Shenshu (BL23) alleviates cartilage degradation through RANKL/OPG signaling in a rabbit model of rheumatoid arthritis. Evidence-Based Complementary and Alternative Medicine. 2019;2019:8. doi: 10.1155/2019/6436420.6436420 [DOI] [PMC free article] [PubMed] [Google Scholar]


