Repositioning of clinically approved drugs that may reduce the severity, hospitalization events, and time of recovery from SARS-CoV2 infection is a global health priority. Clinical reports of drug effects and lung pathology are providing insight into the pathogenesis of Covid-19, and comparison of these findings with our knowledge of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) can be used to develop rational hypotheses to prioritize clinical studies of available therapeutics.
Recently, Cao and colleagues [1] investigated the addition of a lopinavir-ritonavir regimen to standard of care, which previously showed promising activity in an open-label clinical study of patients affected by SARS [2]. However, lopinavir-ritonavir therapy did not confer any clinical benefit for patients with Covid-19, even when patients received glucocorticoids as a supportive drug [1]. Several agents, including remdesivir and chloroquine/hydroxychloroquine alone or in combination with azithromycin, were tested as potential treatment for Covid-19 patients, however available data are still not sufficient to delineate a definitive therapeutic approach [3], [4], [5].
A relevant debate was ongoing concerning the use of non-steroidal anti-inflammatory drugs (NSAIDs) to treat patients with Covid-19 [6,7]. Following a survey by the French National Agency for Medicines and Health Products Safety (ANSM), US Food and Drug Administration (FDA) and European Medicines Agency (EMA) are investigating a potential correlation between NSAIDs and increased severity of Covid-19 symptomatology, supporting possible negative effects of these drugs to exacerbate Covid-19-related viral and bacterial pulmonary infections, leading to increased hospitalization rates and poor health outcomes.
Although no clinical findings were previously reported to suggest any potential risk to use NSAIDs to treat patients with SARS or MERS, NSAID-induced down-regulation of prostaglandins and thromboxanes through cyclooxygenase (COXs) inhibition shifts metabolism of arachidonic acid toward the lipoxygenase (LOX) pathway, resulting in a net increase in leukotrienes production.
Of note, leukotrienes are potent chemotactic and immunomodulatory molecules that exert a key role in the pathological development of multiple lung diseases including acute respiratory distress syndrome (ARDS), a major clinical consequence of Covid-19 in patients admitted to intensive care unit (ICU) [8,9]. By mediating the recruitment of pivotal players of the innate immune response, such as macrophages and neutrophils, leukotrienes enhance the immune system's ability to kill microbes and produce antimicrobial mediators [9].
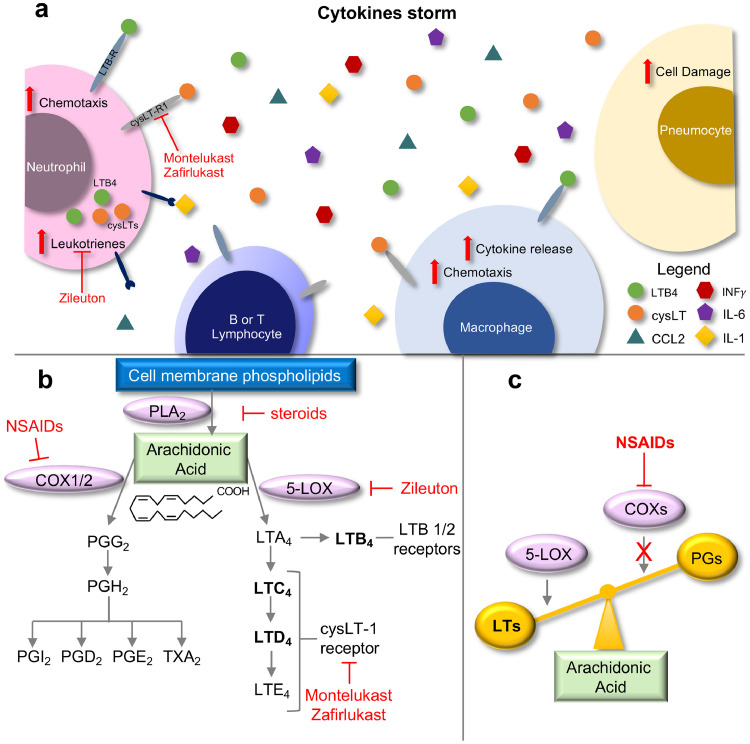
Leukotrienes act in a paracrine and/or autocrine fashion to boost cytokine release in the microenvironment, including TNFα, IL-1, IL-6 and CCL2, which activates leukocytes to amplify the inflammatory response [10], [11]. In the context of respiratory syndromes, this exacerbated inflammatory response can impair organ function [8] (Fig. 1 A). Indeed, leukotrienes have been demonstrated to mediate lung injury in several diseases characterized by inflammation, increased vascular permeability and bronchoconstriction, including ARDS [9].
Fig. 1.
A) Schematic showing how COVID-19 triggers hyper-activation of the immune-response, generating a cytokine storm. LTs acting in an autocrine and/or paracrine manner could positively regulate and amplify the release and action of inflammatory cytokines, which results in a positive feedback loop that further induces LT synthesis and the inflammatory process. B) Arachidonic acid metabolism, drugs and key molecular targets. The schematic highlights how the use of NSAIDs can shift arachidonic acid metabolism towards the 5-LOX and LT biosynthetic pathway. C) Schematic showing the shift of arachidonic acid metabolism towards 5-LOX activity after administration of NSAIDs.
Lung pathological studies from patients with SARS, MERS and Covid-19 have uncovered features that are compatible with a role of leukotrienes in mediating and potentially enhancing a harmful inflammatory response. Pathological analysis of lung tissues among casualties during the SARS outbreak revealed diffuse alveolar damage accompanied by edema, pneumocyte hyperplasia, and hyaline membranes, followed by interstitial fibrosis. Likewise macrophages were the prominent leucocytes infiltrating the alveoli and lung interstitium of patients who succumbed to SARS [12], [13], [14]. Recent pathological findings in Covid-19 patients show signs of microangiopathy with a marked neutrophil-infiltrate in upper and lower respiratory tract, a pathological feature compatible with a robust activation of leukotrienes signaling [15,16]. Of note, an elevated neutrophil-lymphocyte ratio has been proposed as an independent risk-factor of severe SARS-CoV-2 infection, further sustaining the important role of neutrophils in mediating the pathological response to Covid-19 infection [17,18].
At the molecular level, transcriptomic profiling of peripheral leukocytes isolated from Covid-19 patients during early stage recovery showed a dramatic increase in IL-1, IL-6, CCL2 and TNF family cytokine production [19,20]. Because signaling by these mediators can be amplified by leukotrienes, these findings are consistent with leukotriene-activity playing a critical role in Covid-19 pathogenesis and can explain why Covid-19 patients requiring admission to ICU present with exacerbated release of pro-inflammatory mediators compared to patients with less severe illness.
Based on the whole of these findings, we posit that leukotriene-pathway inhibition through leukotriene-receptor inhibitors (montelukast, zafirlukast) or 5-lypoxygenase antagonists (zileuton) could be exploited as an effective and immediate preventive strategy to mitigate the excessive pulmonary inflammation and airway remodeling in symptomatic patients who otherwise will develop a severe Covid-19 disease (Fig. 1B-C) [8,21].
Nevertheless, a possible limitation of this approach may exist in the context of acute tissue damage. The arachidonic acid cascade generates not only pro-inflammatory mediators (including prostaglandins and leukotrienes) but also pro-resolving lipid-mediators (Resolvin E1, Protectin D1). Although data concerning the effects of leukotriene inhibitors in tissue repair are still lacking, in a model of zymosan-initiated peritonitis it has been demonstrated that COX-2-specific (NS-398) and LOX-12/15 (baicalein) inhibition caused a delayed tissue repair [22].
Determining the optimum timing of administration of leukotrienes-inhibitor therapy will be essential to achieve the clinical goal of modulating the immune response in patients with Covid-19 before the onset of severe pneumonia, thus limiting the rate and time of hospitalization, and reducing the mortality rate. These drugs have been in wide use, and there are extensive safety and pharmacology data readily available that can be used to design clinical studies of these agents for treatment of Covid-19 [23,24].
In conclusion, considering the likely endemic nature of Covid-19 infection, the risk of a second surge of new cases and the lack of vaccination strategies for which time is key factor in order to evaluate efficacy and safety, we urge the immediate clinical consideration of a safe, low-cost class of drugs with the double expectation of drastically changing the disease outcome as well as providing an immediate socioeconomical relief.
Declaration of Competing Interest
Authors declare that no conflict of interest exists.
Acknowledgments
This work was supported by the Associazione Italiana per la Ricerca sul Cancro (AIRC-iCare2 fellowship) to F. Citron (#23874).
References
- 1.Cao B., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;(Mar.) doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu C.M., et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59(Mar. (3)):252. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grein J., et al. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020;(Apr.) doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioSci. Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 5.Gautret P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;(Mar.) doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.L. Fang, G. Karakiulakis, and M. Roth, “Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?,” Lancet Respir. Med., doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed]
- 7.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(Feb. (10223)):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(Feb. (10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters-Golden M., Henderson W.R. Leukotrienes. N. Engl. J. Med. 2007;357(Nov. (18)):1841–1854. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 10.Rola-Pleszczynski M., Stankova J. Leukotriene B4 enhances interleukin-6 (IL-6) production and IL-6 messenger RNA accumulation in human monocytes in vitro: transcriptional and posttranscriptional mechanisms. Blood. 1992;80(Aug. (4)):1004–1011. doi: 10.1182/blood.V80.4.1004.1004. [DOI] [PubMed] [Google Scholar]
- 11.Li H., et al. Leukotriene B4 strongly increases monocyte chemoattractant protein-1 in human monocytes. Arterioscler., Thromb., Vasc. Biol. 2004;24(Oct. (10)):1783–1788. doi: 10.1161/01.ATV.0000140063.06341.09. [DOI] [PubMed] [Google Scholar]
- 12.Nicholls J.M., et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361(May (9371)):1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tse G.M.-K., et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS) J. Clin. Pathol. 2004;57(Mar. (3)):260. doi: 10.1136/jcp.2003.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Z., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8(Apr. (4)):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes B.J., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(Apr. (e20200652)) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lämmermann T., et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498(Jun. (7454)):371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y., et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J. Infect. 2020;(Apr.) doi: 10.1016/j.jinf.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B., et al. Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. medRxiv. 2020;(Jan.) doi: 10.1101/2020.03.12.20035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen W., et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. medRxiv. 2020;(Jan.) doi: 10.1101/2020.03.23.20039362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L., et al. Blood single cell immune profiling reveals the interferon-MAPK pathway mediated adaptive immune response for COVID-19. medRxiv. 2020;(Jan.) doi: 10.1101/2020.03.15.20033472. [DOI] [Google Scholar]
- 21.Rubinstein I., Kumar B., Schriever C. Long-term montelukast therapy in moderate to severe COPD—a preliminary observation. Respir. Med. 2004;98(Feb. (2)):134–138. doi: 10.1016/j.rmed.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Schwab J.M., Chiang N., Arita M., Serhan C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447(Jun. (7146)):869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenzel S., et al. The safety and efficacy of zileuton controlled-release tablets as adjunctive therapy to usual care in the treatment of moderate persistent asthma: a 6-month randomized controlled study. J. Asthma. 2007;44(Jan. (4)):305–310. doi: 10.1080/02770900701344199. [DOI] [PubMed] [Google Scholar]
- 24.Thalanayar Muthukrishnan P., Nouraie M., Parikh A., Holguin F. Zileuton use and phenotypic features in asthma. Pulm. Pharmacol. Ther. 2020;60(Feb.) doi: 10.1016/j.pupt.2019.101872. [DOI] [PubMed] [Google Scholar]