Highlights
-
•
Buckwheat protein isolates (BPIs) were treated by varied sonication time and amplitude.
-
•
Sonication increased the in vitro digestibility of BPIs significantly compared to control.
-
•
Sonication changed the molecular conformations (tertiary and secondary) of BPIs.
-
•
Sonication broke BPIs into smaller fragments and induced surface modifications.
Keywords: Ultrasound, Buckwheat, Alternative protein, Digestibility, Structural property
Abstract
The present work investigated the effects of sonication at different amplitudes and durations on the in vitro digestibility of buckwheat protein isolates (BPIs). The conformation, particle size and microstructures of the BPIs were also studied to explicate the possible mechanisms of the sonication-induced changes. The results showed that sonication conditions of 20 kHz, pulsed on-time 10 s, off-time 5 s, amplitude of 60% and duration of 10 min (SA6T10) improved the digestibility of BPIs from 41.4% (control) to 58.2%. The tertiary structure analysis showed that sonication exposed the hydrophobic core buried inside the protein molecules and broke the intramolecular crosslinks, based on the increase in the surface hydrophobicity and intrinsic fluorescence and the decrease in the disulphide content. The secondary structure analysis showed that SA6T10 decreased the content of β-turn and β-sheet by 40.9% and 22.4%, respectively, and increased the content of anti-parallel β-sheet, random coil, and α-helix by 40.9%, 30.6%, and 25.5%, respectively. The particle size of the control BPIs (427.7 ± 76.7 nm) increased to 2130.8 ± 356.2 nm in the SA6T10 sonicated sample with a corresponding decrease in the polydispersity index from 0.97 ± 0.04 to 0.51 ± 0.13. Moreover, scanning electron microscopy indicated that sonication broke the macroparticles into smaller fragments and changed the surface state of the proteins. Taken together, sonication has proven to be a promising approach for improving the digestibility of buckwheat proteins, which can be explored as a source of plant-based alternative protein for food applications.
1. Introduction
Buckwheat is mostly grown in some European countries and China. It can be categorized into two types, common buckwheat (Fagopyrum esculentum Moench) and Tartary buckwheat (Fagopyrum tataricum L. Gaertn), based on differences in the composition and biological features. The total protein content of buckwheat ranges from 12 to 15% on a wet weight basis [1]. Buckwheat protein is rich in lysine, which is the first limiting amino acid in cereal proteins, such as corn and wheat proteins [2]. The indispensable amino acid composition of buckwheat protein is comparable to the FAO/WHO suggested requirements for children and adults. Presently, studies on buckwheat proteins have focused on preparation of nutraceuticals with different beneficial effects on human health, such as cholesterol-lowering [3], [4], [5], antioxidative [6], [7], antihypertensive [8], [9], antimicrobial and antitumor activities [10], [11], [12]. However, buckwheat proteins are underutilized in terms of human nutrition mostly because of their content of anti-nutritional factors, such as protease inhibitors, tannins, phytic acid and saponins, which decrease protein digestibility in the digestive tract [13]. Furthermore, the molecular structure of proteins also affects their digestibility [14]; for instance, corn protein contains numerous hydrophobic amino acid residues and α-helical domains that make it less digestible [15].
According to an FAO report, over 690 million people globally were affected by hunger in 2019 and 132 million people may be added to the total number of undernourished in the world in 2020 because of COVID-19 pandemic [16]. Apart from lack of food, “hunger” encompasses food insecurity, reduced nutrient bioaccessibility and related health issues. Meanwhile, the requirement for protein supply has increased due to the increasing world population and consumer preference for high-protein foods [17]. Recently, the awareness of the food industry and consumers about plant-based proteins has increased because of their lower carbon footprint compared to animal proteins. However, one of the drawbacks of plant-based proteins is their lower digestibility and bioavailability, which can be improved by suitable processing. Therefore, there is a need to discover alternative proteins and to develop new methods for improving the digestibility and nutritional quality of the proteins.
Power ultrasound, also known as high-intensity ultrasound, with intensity ranging from 10 to 1000 W·cm−2 and frequency of 10–100 kHz, has extensively been used in the food industry. Many studies reported that ultrasonic pretreatment of proteins before enzymolysis hydrolyzed the protein into smaller fragments and improved the reaction rate significantly [18], [19], [20]. This occurred because acoustic cavitation generated drastic physical forces (micro jets, shear forces, shock waves, turbulence, etc.) and highly reactive free radicals [21], [22], which can attack the sidechains and backbone of protein molecules, leading to changes in their secondary and microstructures [23], [24]. These changes result in the exposure of hydrolysis sites buried inside a protein, making them accessible to proteases. Acoustic frequency, power intensity, temperature of the medium, and duration of treatment have been extensively studied as the major parameters of high-intensity ultrasound [25]. However, the effect of the acoustic amplitude, square of which is directly proportional to the amount of energy applied to the sonication system [21], on the protein structure and digestibility has not been investigated. Theoretically, when a higher acoustic amplitude is used at a fixed frequency of an ultrasonic system, the cavitation bubbles can grow and collapse more easily within a few acoustic cycles, and these collapsed bubbles can generate numbers of nuclei bubbles that finally intensify the cavitation effect.
Therefore, this study was aimed at investigating the effects of sonication duration and acoustic amplitude on the in vitro digestibility of buckwheat protein isolates (BPIs). In addition, the effects of sonication on the tertiary structures (surface hydrophobicity, intrinsic fluorescence, sulfhydryl and disulfide bond contents), secondary structure, particle size, zeta-potential and microstructure of BPIs were studied to elucidate the structural mechanism underlying the effect of ultrasound on the digestibility of the proteins.
2. Materials and methods
2.1. Materials and reagents
Buckwheat flour was obtained from Bulk Barn (Ottawa, Ontario, Canada). α-amylase from porcine pancreas (Type VI-B, ≥5 U/mg solid), pepsin (≥250 U/mg solid), pancreatin from porcine pancreas (8 × USP specifications), bile acid, 1-anilino-8-naphthalene-sulfonate (ANS) and Ellman’s reagent (5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) were purchased from Millipore Sigma (St. Louis, MO, USA). Lowry reagent was purchased from Bio-Rad Laboratories (Richmond, Calif., USA). All reagents used in the experiments were of analytical grade.
2.2. Preparation of buckwheat protein isolates
Buckwheat protein isolates (BPIs) were prepared according to a reported method [26] with some modifications. Briefly, 100 g of buckwheat flour was dispersed in 2 L of distilled water and the pH was adjusted to 10.0 using 1 M NaOH. The suspension was stirred for 3 h at 40 °C with a magnetic stirrer (Thermo Scientific). The resulting slurry was centrifuged at 7 500 g and 4 °C for 15 min. The pH of the supernatant was adjusted to the isoelectric point (pI 4.5) by the addition of 1 M HCl to precipitate the protein. After standing undisturbed for 1 h, the precipitated protein was centrifuged at 7 500 g and 4 °C for 15 min. The resulting protein was washed 3 times with distilled water, then dissolved in distilled water and neutralized with 0.1 M NaOH. The solution was lyophilized using a freeze dryer (Labconco, Kansas, MO, USA).
2.3. Sonication of buckwheat protein isolates
BPIs (1 g) was added to 25 mL of distilled water in a 50 mL plastic centrifuge tube placed in ice bath and sonicated using a 20 kHz FB120 sonicator (Fisher Scientific, Hampton, NH, USA). The probe with a diameter of 3 mm was submerged to a depth of 2 cm below the surface of the BPI solutions. First, sonication was conducted at 60% amplitude of the maximum, pulsed on-time 10 s, off-time 5 s for different durations (5, 10, 15, 20, and 30 min). Second, sonication was performed at pulsed on-time 10 s, off-time 5 s for 10 min at different amplitude of the maximum (40%, 60%, 80%, and 100%). The acoustic power (P) was determined by the calorimetric method [27]:
| (1) |
where m is the mass of the medium (g), Cp is the heat capacity of the medium (J·g−1·°C−1), and dT/dt is the slope of the temperature vs. time plot. The dissipated acoustic power was expressed as watts per unit area of the emitting surface (W·cm−2), and the values are presented in Table 1.
Table 1.
Experimental design of sonication.
| Treatments | Protein concentration (g/L) | Temperature (°C) | Pulsed on-time (s) | Pulsed off-time (s) | Duration (min) | Amplitude (%) | Power intensity (W/cm2) |
|---|---|---|---|---|---|---|---|
| SA6T5 | 40 | 20 | 10 | 5 | 5 | 60 | 55.2 ± 0.3 |
| SA6T10 | 40 | 20 | 10 | 5 | 10 | 60 | 55.2 ± 0.3 |
| SA6T15 | 40 | 20 | 10 | 5 | 15 | 60 | 55.2 ± 0.3 |
| SA6T20 | 40 | 20 | 10 | 5 | 20 | 60 | 55.2 ± 0.3 |
| SA6T30 | 40 | 20 | 10 | 5 | 30 | 60 | 55.2 ± 0.3 |
| SA4T10 | 40 | 20 | 10 | 5 | 10 | 40 | 32.8 ± 2.5 |
| SA8T10 | 40 | 20 | 10 | 5 | 10 | 80 | 80.2 ± 2.3 |
| SA10T10 | 40 | 20 | 10 | 5 | 10 | 100 | 104.5 ± 0.9 |
2.4. In vitro digestion of buckwheat protein isolates
The in vitro digestion of the control and sonicated BPIs was conducted according to the COST consensus method [28] with some modifications. Briefly, 200 mg of samples were added to 7 mL of simulated salivary fluid (SSF) electrolyte stock solution. Then, 1 mL of salivary α-amylase (750 U/mL), 25 µL of CaCl2 (0.3 M) and 0.975 µL of distilled water were added to the mixture to reach a total reaction volume of 10 mL, followed by incubation at 37 °C for 2 min without shaking. Thereafter, 7.5 mL of simulated gastric fluid (SGF) electrolyte stock solution, 1.6 mL of pepsin stock solution (25 000 U/mL) dissolved in SGF, and 5 µL of CaCl2 (0.3 M) were added to the reaction mixture. The pH of the mixture was adjusted to 3.0 using 1 M HCl and the mixture was incubated at 37 °C for 2 h with shaking at 120 rpm. Subsequently, 11 mL of simulated intestinal fluid (SIF) electrolyte stock solution, 5 mL of pancreatin solution (800 U/mL) prepared in SIF (based on trypsin activity), 2.5 mL of freshly prepared bile solution (160 mM), and 40 µL of CaCl2 (0.3 M) were added to the gastric digested mixture and the pH was adjusted to 7.0 using 1 M NaOH. The mixture was incubated at 37 °C for another 2 h with shaking at 120 rpm. The in vitro digestion was terminated by boiling the mixtures for 10 min, followed by centrifugation at 7500g for 15 min after cooling to room temperature.
The in vitro digestibility (IVD) of the proteins was calculated using the following equation:
| (2) |
where C is the concentration of peptides released (mg·mL−1) after deduction of a blank (buffer and digestive enzymes), which was determined using the Lowry method [29], V is the volume of digestion (mL), n is the dilution factor, and M is the mass of the protein (mg).
2.5. Surface hydrophobicity (H0) measurements of BPIs
The surface hydrophobicity (H0) of the control and sonicated BPIs was determined using ANS as the fluorescence probe according to a previously reported method [30]. Lyophilized samples dissolved in 0.1 M phosphate buffer solution (pH 7.4) were centrifuged at 12 000g at 4 °C for 15 min. After determining the protein content using the Lowry method, the supernatant was serially diluted with the 0.1 M phosphate buffer solution (pH 7.4) to obtain protein concentrations ranging from 0.5 to 0.1 mg·mL−1. Then, 5 μL of 8.0 mM ANS (prepared in 0.1 M phosphate buffer, pH 7.4) was added to 200 μL of the diluted protein solution, mixed and kept in the dark for 15 min. The relative fluorescence intensity was measured at room temperature (25 ± 1 °C) with a Spark multimode microplate Reader (Tecan Group Ltd., Männedorf, Switzerland) at an excitation wavelength of 390 nm (excitation bandwidth, 20 nm) and an emission wavelength of 470 nm (emission bandwidth, 20 nm). The slope of the fluorescence intensity vs. protein concentration (mg·mL−1) was calculated by linear regression (R2 ≥ 0.99) and used as the surface hydrophobicity (H0).
2.6. Measurement of intrinsic fluorescence of BPIs
The intrinsic fluorescence emission spectra of the control and sonicated BPIs (0.5 mg/mL in 0.1 M phosphate buffer, pH 7.4) were measured at room temperature (25 ± 1 °C) using a Spark multimode microplate Reader (Tecan Group Ltd., Männedorf, Switzerland). The measurements were done at an excitation wavelength of 280 nm and a bandwidth of 20 nm. The emission spectra at the wavelength ranging from 300 to 600 nm, with a bandwidth of 20 nm and a step size of 2 nm, were collected. The spectra were depicted as an average of three scans after deducting the spectrum of the blank (buffer without BPIs).
2.7. Determination of the reactive sulfhydryl, total sulfhydryl, and disulfide bond contents of BPIs
The reactive sulfhydryl (RSH) content of the control and sonicated BPIs was determined using a previously reported method [31], with some modification. Briefly, 20 mg of BPIs was dissolved in 2 mL of Tris buffer solution (86 mM Tris, 90 mM glycine, and 4 mM EDTA, pH8.0), followed by vortex mixing for 60 s and centrifugation at 14 000g at 4 °C for 10 min. The supernatant was collected and diluted 10 folds. Then, 10 μL of Ellman’s reagent (4 mg·mL−1 DTNB in standard buffer) was added to 1 mL of the diluted samples, mixed and incubated at 25 °C for 15 min. Absorbance was read at 412 nm with a Spark multimode microplate Reader (Tecan Group Ltd., Männedorf, Switzerland). A protein blank in which Ellman’s reagent solution was replaced with the buffer was used to determine the turbidity of the solution.
The total sulfhydryl (TSH) content of BPIs was determined using the same protocol as RSH determination, with the exception that the buffer contained 8 M urea and 5 g·L-1 sodium dodecyl sulfate. The RSH and TSH contents were calculated according to the following equation:
| (3) |
where D is the dilution factor (1.01), C is the diluted protein concentration determined by the Lowry method (mg·mL−1), ΔAbs412 is the difference in absorbance measured at 412 nm with and without DTNB:
| (4) |
The SS bond content of the control and sonicated BPIs was determined according to the method developed by Beveridge et al. [32] with some modification. BPIs (20 mg), 2 mL of 10 M urea in the buffer, and 30 μL of β-mercaptoethanol were mixed and incubated at 25 °C for 1 h. Then, 5 mL of 12% (w/v) trichloroacetic acid was added to the mixture and incubated for another 1 h, followed by centrifugation at 7500g at 4 °C for 10 min. The precipitate was washed twice using 4 mL of 12% trichloroacetic acid and centrifuged afterwards. The precipitate was dissolved in 2 mL of 8 M urea and 5 g·L−1 SDS in the standard buffer, then 20 μL of Ellman’s reagent was added to the mixture and incubated at 25 °C for 15 min. The absorbance of the mixture was measured at 412 nm. The SS content was calculated using the following equation:
| (5) |
where is the content of TSH and SH derived from the reduction of SS, which was determined using Eq. (3), and is the total free thiol content which was calculated previously.
2.8. Measurement of ATR/FT-IR spectra of BPIs
The attenuated total reflectance Fourier transform infrared spectra (ATR/FT-IR) of the control and sonicated BPIs were scanned at the wavenumber ranging from 400 to 4 000 cm−1 using a Nicolet 6700 FTIR (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a ‘iTX Smart Orbit Diamond’ ATR accessory. The surface of the crystal was cleaned with 99% IPA before the background was collected. After the background acquisition was completed, the sample was loaded onto the surface of the ATR crystal and the pressing tip was pressed onto the sample to ensure good contact with the crystal surface. The spectra were collected using an OMNIC software (Thermo Fisher Scientific, Waltham, MA, USA) and each spectrum was an average of 128 scans. The amide I band with wavenumbers between 1600 and 1700 cm−1 were processed to find overlapped peaks by deconvolution with the aid of the Peak Fit 4.12 software (Systat Software Inc., San Jose, CA, USA). The secondary structure was assigned (side chain vibrations: 1601–1608 cm−1, anti-parallel β-sheet: 1613–1620 cm−1 and 1677–1683 cm−1, β-sheet: 1623–1639 cm−1 and 1687–1699 cm−1, random coil: 1641–1646 cm−1, α-helix: 1649–1654 cm−1, β-turn: 1659–1673 cm−1) as previously reported [24], [33], [34], [35], [36]. The content of secondary structure was reported as relative peak area to the total area of amide I.
2.9. Particle size and zeta potential measurements of BPIs
The control and sonicated BPIs (2 mg) were dispersed in 2 mL of Milli-Q water. Then, the particle size and zeta-potential of the suspensions were determined with a Nano Zeta-sizer (Malvern Instruments Ltd, UK) after equilibrating for 120 s at 25 °C.
2.10. Scanning electron microscopy (SEM) of BPIs
The morphologies of the control and sonicated BPIs were observed with a JSM-7500F field emission scanning electron microscope (JEOL, Japan) at an acceleration voltage of 2.0 kV, after coating the sample with a layer of gold with a thickness of 6.8 nm using an EM ACE 200 (LEICA, Germany).
2.11. Statistical analysis
All the data were reported as mean ± standard deviation (SD) of triplicate pretreatment experiments. Statistical differences between the groups were analyzed by the one-way analysis of variance. Significant differences between means were determined by Tukey’s test at P < 0.05. All analyses were performed with the statistical software SPSS 18.0 (IBM Corporation, NY, USA) and the graphs were plotted with Origin Pro 8.5 (Origin Lab Corporation, MA, USA).
3. Results and discussion
3.1. Effect of sonication on the in vitro digestibility of buckwheat protein isolates
The results of the in vitro digestibility (IVD) of BPIs are shown in Table 2. In general, all BPIs sonicated at 60% amplitude of the acoustic wave and for 10–30 min had significantly higher IVD than the untreated BPI (P < 0.05). Moreover, the increase in the sonication time from 5 to 10 min resulted in the maximum value of IVD, which was increased by 41% over that of the control. This increase in the IVD might be a result of the ultrasound effect on the molecular conformation and microstructure of BPIs, thus increasing the accessibility of the proteins to digestive enzymes. However, extending the sonication time longer than 10 min led to a decrease in the IVD, especially after sonication for 20 min (SA6T20). This was probably because the protein molecules can undergo both denaturation and renaturation under external factors; denaturation is beneficial for digestion while renaturation makes the protein resistant to digestion. As the protein denaturation-renaturation is at an equilibrium state, proper sonication can prompt the occurrence of denaturation. Moreover, prolonged sonication can induce the formation of protein aggregates. Wang et al. [37] found that prolonged sonication time from 20 to 50 min at an interval of 10 min resulted in a decrease in the degree of hydrolysis of oat protein isolates due to the refolding and burying of the cleavage sites in the protein interior.
Table 2.
Effects of sonication on the In vitro digestibility (IVD), particle size, polydispersity index (PDI), and zeta potential of BPIs.
| Treatments | IVD (%) | Particle size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|---|
| Control | 41.4 ± 1.7ab | 427.7 ± 76.7a | 0.97 ± 0.04c | −51.60 ± 1.48a |
| SA6T5 | 46.0 ± 5.1bc | 2244.1 ± 364.0 cd | 0.30 ± 0.03a | −41.56 ± 2.50 cd |
| SA6T10 | 58.2 ± 4.9e | 2130.8 ± 356.2bc | 0.51 ± 0.13b | −45.62 ± 3.00b |
| SA6T15 | 54.4 ± 6.4de | 1955.5 ± 266.6bc | 0.52 ± 0.03b | −42.77 ± 1.71bc |
| SA6T20 | 48.6 ± 6.2 cd | 1940.9 ± 193.7bc | 0.49 ± 0.12ab | −36.61 ± 3.00e |
| SA6T30 | 54.2 ± 3.6de | 2040.3 ± 211.1bc | 0.80 ± 0.24c | −35.49 ± 2.01e |
| SA4T10 | 51.9 ± 4.5cde | 1772.6 ± 262.4b | 0.43 ± 0.11ab | −53.04 ± 4.16a |
| SA8T10 | 45.6 ± 3.3bc | 1894.3 ± 305.9bc | 0.41 ± 0.07ab | −37.41 ± 1.48e |
| SA10T10 | 38.6 ± 3.7a | 2663.3 ± 363.5d | 0.89 ± 0.17c | −38.30 ± 1.61de |
※ Mean ± SD (n = 9). Within the column, means having different superscript letters are significantly different (P < 0.05). SA6T5, sonication at 60% amplitude for 5 min; SA6T10, sonication at 60% amplitude for 10 min; SA6T15, sonication at 60% amplitude for 15 min; SA6T20, sonication at 60% amplitude for 20 min; SA6T30, sonication at 60% amplitude for 30 min; SA4T10, sonication at 40% amplitude for 10 min; SA8T10, sonication at 80% amplitude for 10 min; SA10T10, sonication at 100% amplitude for 10 min.
Acoustic amplitude is another parameter that influences the cavitation. Generally, the intensity is proportional to the square of the amplitude of pressure waves [21]. As shown in Table 2, the IVD of BPIs first increased to a certain value owing to the increase in the amplitude of the ultrasound, and later decreased significantly (P < 0.05). The IVD for SA4T10 (40% amplitude), SA6T10 (60% amplitude), SA8T10 (80% amplitude) and SA10T10 (100% amplitude) were 51.9%, 58.2%, 45.6% and 38.6%, respectively. Similarly, the increase in ultrasound power was reported to decrease the degree of hydrolysis of oat protein isolates [37]. This observation may be because the smaller amplitude generated with lower intensity was dispersed in the protein solution and thus was not strong enough to disrupt the protein structure. However, the high intensity may have induced the formation of aggregates resulting in more compact protein particles that were less enzyme-digestible.
3.2. Effects of sonication on the surface hydrophobicity and intrinsic fluorescence of BPIs
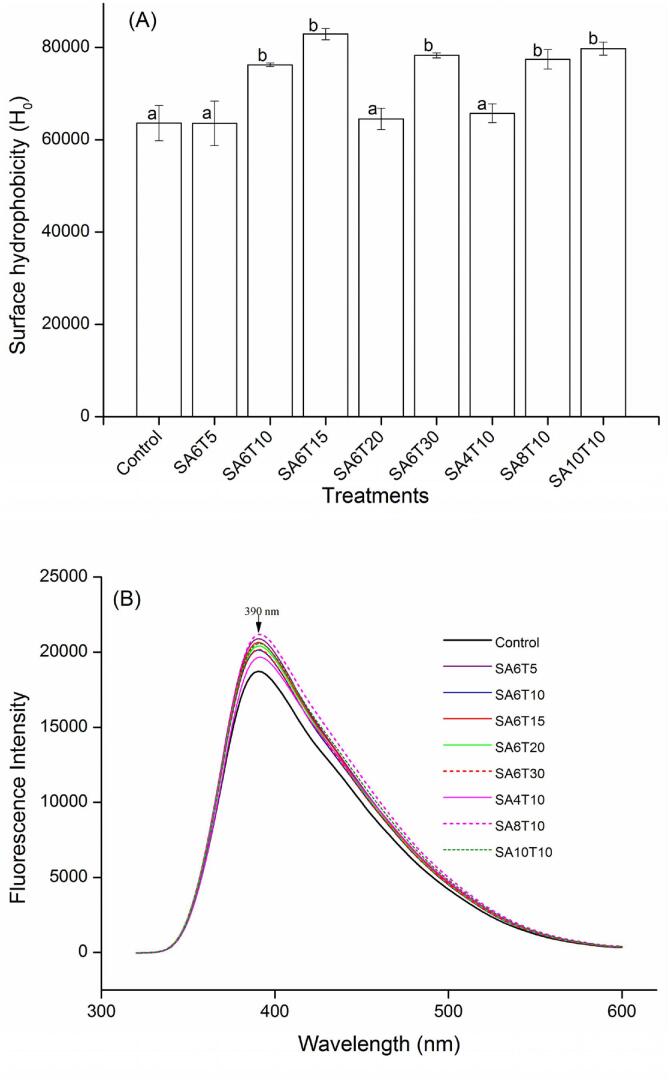
Surface hydrophobicity, which correlates with the number of polar and non-polar groups on the surface of proteins, can reflect the change in molecular conformation of proteins. As shown in Fig. 1(A), higher amplitude and longer duration of sonication increased the surface hydrophobicity of BPIs significantly (P < 0.05). This is possibly because acoustic amplitude is directly proportional to the amount of energy applied to the system. This increase in H0 resulted from unfolding of the BPIs and exposure of hydrophobic amino acid residues buried in the interior of the protein molecules, due to the disruptive effects of the micro jets, shear forces, shock waves, and turbulence generated by ultrasound waves. The unfolding of a protein may expose its cleavage sites, thus facilitating interactions with the enzymes and increasing digestibility. Similar findings have been reported on studying sonicated plant proteins [23], [38], [39], [40] and animal proteins [27], [41], resulting in improved enzymatic hydrolysis of proteins.
Fig. 1.
Effects of sonication on the surface hydrophobicity (A) and fluorescence intensity (B) of buckwheat protein isolates.
Some hydrophobic amino acid residues such as tryptophan, tyrosine and phenylalanine can emit fluorescence when they are exposed outside or located at the surface of proteins. This indicator can be used to characterize changes in tertiary structures of proteins because these residues form the hydrophobic core of proteins where they interact by hydrophobic bonding. As shown in Fig. 1(B), sonication increased the fluorescence intensity of the BPIs. At 60% amplitude, the fluorescence intensity decreased due to the increase in the ultrasound treatment duration, except for SA6T10. This result is because the exposed hydrophobic groups aggregated under the turbulence effect of sound waves. On the other hand, the fluorescence intensity of sonicated BPIs increased with increase in ultrasound amplitude, which might be ascribed to protein unfolding resulting from the higher intensity. However, extremely high intensity may have led to refolding or aggregation of the proteins, as observed in the decreased fluorescence intensity of SA10T10. Taken together, the increase in fluorescence intensity of BPIs may be because sonication destroyed the hydrophobic interactions, hydrogen bonds and Van der Waals forces between the protein molecules [23].
3.3. Effects of sonication on the reactive sulfhydryl, total sulfhydryl, and disulfide bond content of BPIs
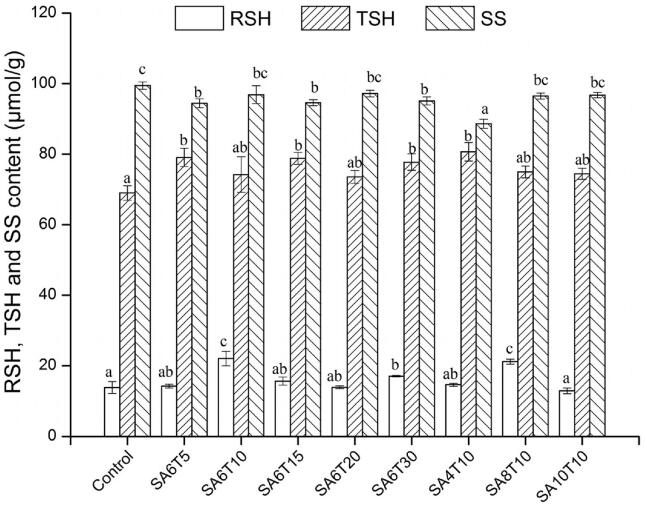
Reactive sulfhydryl (RSH) groups, which are mostly located on the surface of protein molecules, can be involved in the formation of noncovalent bonds, such as hydrogen bonds. Total sulfhydryl (TSH) is the SH group that exists in the free form rather than as SS bond, including RSH and SH groups buried inside the protein molecules. Depending on the acoustic amplitude applied (Fig. 2), some sonication intensities increased both RSH and TSH contents significantly (P < 0.05). Compared to the control, sonication at SA6T10 increased the content of RSH from 13.86 to 22.05 μmol/g (by 59%), while sonication at SA4T10 increased the content of TSH from 69 to 80.67 μmol/g (by 16.9%). However, sonication at SA10T10 decreased the RSH content (P < 0.05) with no significant differences in TSH (P > 0.05). Under the denaturation conditions, the content of TSH increased as a result of unfolding of the protein molecules. The increases in the content of RSH and TSH observed with the treatment may be ascribed to that sonication exposed the SH buried inside protein molecules and the ultrasound broke the disulfide bonds because of the physical and chemical effects of cavitation.
Fig. 2.
Effects of sonication on the reactive sulfhydryl (RSH), total sulfhydryl (TSH), and disulfide bond (SS) contents of buckwheat protein isolates.
The disulfide bond (SS) is one of the major forces that stabilize the structures of proteins. As shown in Fig. 2, sonication reduced the SS content of BPIs, especially for SA6T5, SA6T15, SA6T30 and SA4T10 (P < 0.05). Among sonication, longer duration (T5-T30) and higher acoustic amplitude (A6-A10) of treatment, both providing higher energy, did not change the SS content significantly (P > 0.05). It is likely the SS was under a dynamic equilibrium state, i.e., degradation and formation of SS bonds might have happened simultaneously. This phenomenon was demonstrated by the in situ monitoring of SH and SS contents of wheat gluten under ultrasound irradiation [42]. Generally, the increase in SH content and the decrease in SS content is one of the mechanisms by which sonication improves the digestibility or enzymolysis of proteins [23], [40].
3.4. Effects of sonication on the secondary structure of BPIs
Peaks located in the amide I region of ATR/FT-IR are widely used to determine the secondary structure of proteins. After deconvolution, extraction of the overlapping components (see supplementary information, Fig. S1) and assignments of attribution, the secondary structure contents of BPIs were determined. The β-sheet, random coil, α-helix, and β-turn contents of the native buckwheat protein isolate (Table 3) were close to the values reported by Choi and Ma [33] for globulin from common buckwheat, i.e., 34.5% β-sheet, 14.4% random coil, 16.0% α-helix and 20.0% β-turn. Notably, the increase in the in vitro digestibility of BPIs by SA6T10, SA6T15, SA6T20 and SA6T30 (Table 2) might be because these treatments decreased the content of β-turn, which has a compact structure, compared to the control. Moreover, these treatments, except SA6T15, increased the content of the anti-parallel β-sheet and, except SA6T10, increased the content of the β-sheet. However, only SA6T10 decreased the content of the β-sheet and increased the content of the random coil substantially; this treatment also had the most pronounced effect on IVD. Jin et al. [23] attributed the improvement in enzymolysis of corn gluten meal to the increase in the β-sheet, but the study did not structurally differentiate the anti-parallel β-sheet from the β-sheet structure. In the present study, the total content of anti-parallel β-sheet and β-sheet of BPIs treated by SA6T10 (45.6%) was close to that of the control (46.6%). Interestingly, all the secondary structure contents of the SA6T15-treated sample decreased and this might be attributed to the increase in the relative peak area of side-chain vibration (1608 cm−1) (Table S1). The random-coil, found in the control, was not detected in the SA8T10-treated sample, but the contents of other secondary structures increased compared to the control. This is likely because sonication made the protein molecules arrange more regularly or because of the shift of bands. The increase in content of the ordered structure might have occurred during the renaturation of the proteins. In general, sonication can increase the digestibility of proteins because of changes in the content of secondary structures resulting from cavitation-induced physical forces and free radicals [21].
Table 3.
Effects of sonication on the secondary structure content of BPIs.
| Treatments | Anti-parallel β-sheet (%) | β-Sheet (%) | Random coil (%) | α-Helix (%) | β-Turn (%) |
|---|---|---|---|---|---|
| Control | 14.9 | 31.7 | 14.4 | 14.5 | 20.8 |
| SA6T5 | 11.0 (−26.2%) | 38.2 (+20.5%) | 14.4 | 13.9 (−4.1%) | 18.9 (−9.1%) |
| SA6T10 | 21.0 (+40.9%) | 24.6 (−22.4%) | 18.8 (+30.6%) | 18.2 (+25.5%) | 12.3 (−40.9%) |
| SA6T15 | 14.9 | 29.4 (−7.2%) | 13.6 (−5.6%) | 13.5 (−6.9%) | 18.1 (−13.0%) |
| SA6T20 | 17.0 (+14.1%) | 35.8 (+12.9%) | 13.7 (−4.9%) | 20.7 (+42.8%) | 12.8 (−38.5%) |
| SA6T30 | 18.1 (+21.5%) | 33.6 (+6.0%) | 13.4 (−6.9%) | 13.0 (−10.3%) | 18.2 (−12.5%) |
| SA4T10 | 17.0 (+14.1%) | 40.4 (+27.4%) | 8.1 (−43.8%) | 10.1 (−30.3%) | 20.6 |
| SA8T10 | 17.8 (+15.5%) | 37.4 (+18.0%) | n.d. | 16.7 (+15.2%) | 22.7 (+9.1%) |
| SA10T10 | 14.2 (−4.7%) | 31.6 | 15.0 (+4.2%) | 14.8 (+2.1%) | 20.7 |
※ SA6T5, sonication at 60% amplitude for 5 min; SA6T10, sonication at 60% amplitude for 10 min; SA6T15, sonication at 60% amplitude for 15 min; SA6T20, sonication at 60% amplitude for 20 min; SA6T30, sonication at 60% amplitude for 30 min; SA4T10, sonication at 40% amplitude for 10 min; SA8T10, sonication at 80% amplitude for 10 min; SA10T10, sonication at 100% amplitude for 10 min; n.d., not detected.
3.5. Effects of sonication on particle size, polydispersity index and zeta potential of BPIs
The particle sizes of the control and sonicated BPIs, which were presented as z-average values, and polydispersity index (PDI) are shown in Table 2. The particle size of BPIs was increased significantly by sonication, with the maximum 5-fold increase observed when the protein was sonicated at 100% amplitude for 10 min (SA10T10). Some studies reported that ultrasound reduced the particle size of some animal and plant proteins [27], [43]. O'Sullivan et al. [43] found that sonication of rice protein isolates by using a frequency of 20 kHz and power intensity of ~34 W·cm−2 for 2 min slightly increased the particle size by 2.3%. Jiang et al. [38] also reported that sonication increased the particle size of black bean protein isolates owing to protein aggregation. The large increase in particle size of BPIs might be due to the disruption of the protein microstructure by the ultrasound, leading to protein particle swelling after dispersion in water or induction of protein self-assembly due to hydrophobic interaction between the unfolded regions.
PDI represents the disperse homogeneity of particles, with higher values indicating more heterogeneous particles in the system. As shown in Table 2, the PDI of the control BPIs indicated that the dispersed protein particles had a wide range of molecular weight distribution. Sonication mostly decreased the PDI, suggesting that the dispersion of proteins occurred in a narrower molecular weight distribution, possibly ascribed to the formation of aggregates or to swelling of protein particles. Similarly, Stefanović et al. [44] reported that an increase in the sonication duration from 5 min to 20 min, decreased the PDI and increased the particle size of egg white proteins; the reduced PDI was attributed to the random aggregation induced by excessive protein denaturation. Jiang et al. [38] indicated that the particle size of sonicated black bean protein isolates became larger because of the formation of soluble and unstable aggregates, and that the particle size distribution broadened due to the breakdown of the unstable aggregates.
The surface of proteins shows a net charge resulting from the amino acid groups exposed to the exterior. Typically, higher zeta potential means stronger electrostatic repulsion between protein molecules, and vice versa; thus, it plays a role in stabilizing the protein dispersion. In Table 2, the control sample had the highest zeta potential magnitude, which was decreased significantly (P < 0.05) by sonication, except for SA4T10. Moreover, longer sonication duration and higher amplitude resulted in the most pronounced decrease in zeta potential magnitude because of higher energy input into the system. The zeta potential values indicated that the sonication-induced structural changes (Table 3) and protein aggregation, based on the increased particle size (Table 2), may have reduced the number of negatively charged residues on the protein surface. In contrast, Jiang et al. [38] reported that sonication increased the zeta potential of black bean protein isolates, resulting in the strengthening of the inter-particle electrostatic repulsion, thus disrupting protein aggregation and improving the dispersion stability of the proteins.
3.6. Effects of sonication on the microstructure and surface morphology of BPIs
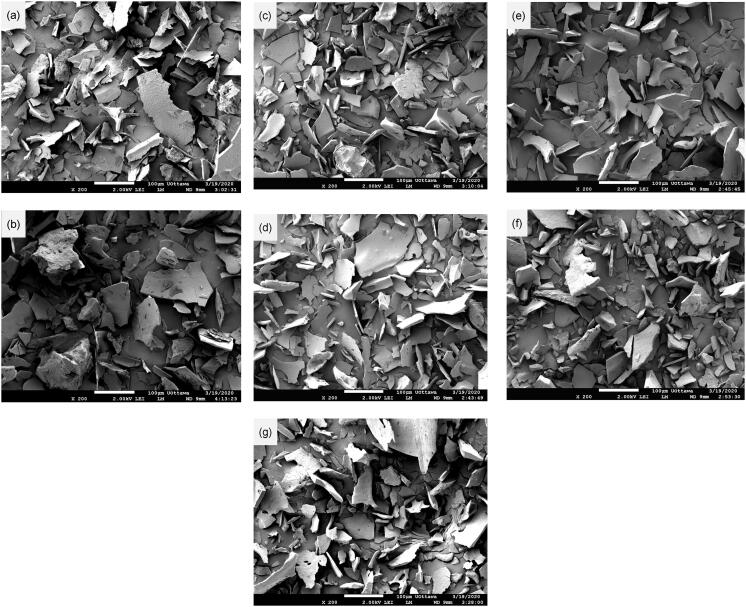
The microstructures of the control and sonicated BPIs are shown in Fig. 3. The thin lamellar structure is the typical feature of BPIs; however, some blocks were present in Fig. 3(a)-(c). Fig. 3(a) showed that the structure of the control BPI was compact. The SA4T10-treated sample revealed a loose structure having a larger size (Fig. 3(b)), which might be attributed to the destruction of the bonds between the protein subunits by the ultrasound waves, resulting in the extension of the distance between particles. In Fig. 3(c), the SA6T10 sample showed both blocks (marked with a circle) and multi-layer structure (marked with an arrow), which possibly resulted from the aggregation of the proteins. High intensity sonication can break the protein particles into smaller fragments, as observed in Fig. 3(c)-(g). The quantity and size of the fragments correlated positively with the input energy of ultrasound waves to the protein solutions. The formation of fragments might be a result of the destruction of the crosslinks between the polypeptide chains of proteins, including the SS bonds, hydrogen bond and Van der Waals interactions, due to effects of shear forces, micro streaming, shock waves and free radicals generated by the ultrasound radiation [21], [23]. Nonetheless, the fragmentation did not result in the reduction of particle size of the proteins (Table 2). Therefore, we postulated that the proteins might absorb and hold water in their inner structures when dispersed in the aqueous solution, thus increasing the particle size.
Fig. 3.
Microstructures of native and sonicated BPIs. (a) Control, (b) SA4T10, (c) SA6T10, (d) SA6T20, (e) SA6T30, (f) SA8T10, (g) SA10T10.
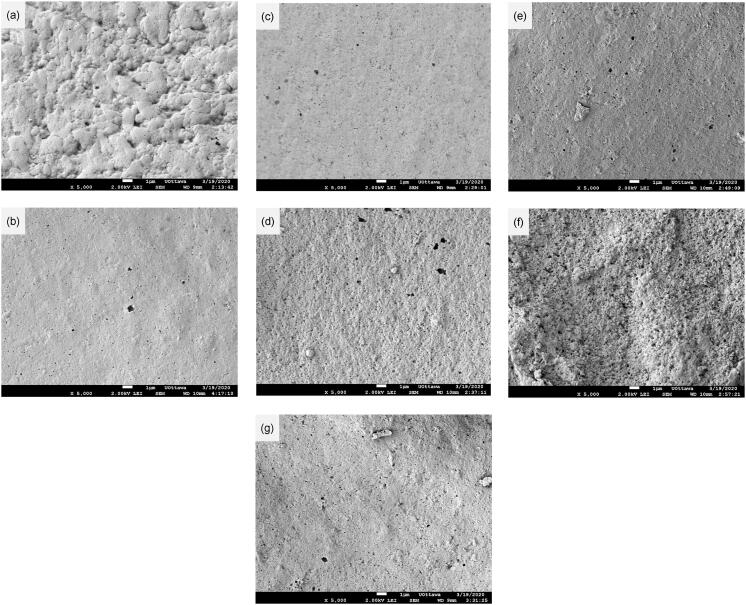
Surface morphology, including roughness, has substantial influence on the bio-adsorption [45] and mass transfer. As previously mentioned, the surface of the control BPI was tight (Fig. 4(a)), which may have protected the proteins from adsorption of water, thus the protein particles had smaller sizes when dispersed in water (Table 2). Sonicated samples (Fig. 4(b)-(g)), especially SA8T10 (Fig. 4(f)), presented a rough surface of proteins with many micropores, which can allow water to enter into the proteins, thus increasing the degree of swelling and leading to larger particle sizes. Furthermore, the adsorption between the proteins and enzymes can be enhanced because of the larger contact areas of the sonicated BPIs. Longer sonication duration led to rougher protein surfaces because of the extension of cavitation. On the other hand, the surface of SA10T10 sample (Fig. 4(g)) appeared smoother than that of SA8T10 (Fig. 4(f)), which might be because the surface protruding edges were excised by the high intensity ultrasound. A previous report had demonstrated the flattened surface of glutelin extracted from the sonicated corn gluten meal [24]. In general, the surface of the SA8T10-treated sample was substantially different from those of the other treated samples, which might be related to the lack of the random coil in the SA8T10 sample (Table 3). Our previous research on sonication of corn gluten meal indicated that sonication decreased the height and surface roughness of the proteins [24], suggesting differences that may originate from the nature of the materials.
Fig. 4.
Surface morphologies of native and sonicated BPIs. (a) Control, (b) SA4T10, (c) SA6T10, (d) SA6T20, (e) SA6T30, (f) SA8T10, (g) SA10T10.
4. Conclusions
Treatment of buckwheat protein by sonication improved the in vitro digestibility of the proteins significantly. Specifically, sonication under 20 kHz, pulsed on-time 10 s, off-time 5 s, amplitude of 60% and duration of 10 min (SA6T10) improved the digestibility significantly by 41% compared to the control. The structural analysis showed that sonication changed the tertiary structure by increasing the surface hydrophobicity, intrinsic fluorescence intensity, and total sulfhydryl contents. The secondary structure analysis showed that SA6T10 decreased the content of the β-turn and increased the content of the anti-parallel β-sheet, and the random coil. Furthermore, sonication changed the particle size, dispersion characteristics, zeta-potential, and microstructures of the proteins. In conclusion, sonication can be further explored as an effective method to improving the digestibility of buckwheat proteins towards their utilization in food product development.
CRediT authorship contribution statement
Jian Jin: Conceptualization, Investigation, Formal analysis, Validation, Visualization, Writing - original draft. Ogadimma D. Okagu: Investigation, Validation, Writing - review & editing. Abu ElGasim Ahmed Yagoub: Writing - review & editing. Chibuike C. Udenigwe: Conceptualization, Validation, Funding acquisition, Resources, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
J. Jin was supported by Key Laboratory of Coarse Cereals Processing, Ministry of Agriculture, Chengdu University (2018CC14), and China Scholarship Council (No. 201908510042), and Longshan Talents program of Southwest University of Science and Technology (17LZX549). This project was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) through the Discovery Grant Program (RGPIN-2018-06839), and by the University of Ottawa through the University Research Chair Program (C.C. Udenigwe). The authors wish to acknowledge Yun Liu (Department of Chemistry, University of Ottawa) for providing technical assistance with the SEM and ATR.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2020.105348.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bejosano F.P., Corke H. Properties of protein concentrates and hydrolysates from Amaranthus and Buckwheat. Ind. Crops Prod. 1999;10:175–183. [Google Scholar]
- 2.Belitz H.D., Grosch W., Schieberle P. Springer; 2009. Food Chemistry. [Google Scholar]
- 3.Tomotake H., Shimaoka I., Kayashita J., Yokoyama F., Nakajoh M., Kato N. A buckwheat protein product suppresses gallstone formation and plasma cholesterol more strongly than soy protein isolate in hamsters. J. Nutr. 2000;130:1670–1674. doi: 10.1093/jn/130.7.1670. [DOI] [PubMed] [Google Scholar]
- 4.Tomotake H., Yamamoto N., Kitabayashi H., Kawakami A., Kayashita J., Ohinata H., Karasawa H., Kato N. Preparation of tartary buckwheat protein product and its improving effect on cholesterol metabolism in rats and mice fed cholesterol-enriched diet. J. Food Sci. 2007;72:S528–S533. doi: 10.1111/j.1750-3841.2007.00474.x. [DOI] [PubMed] [Google Scholar]
- 5.Metzger B.T., Barnes D.M., Reed J.D. Insoluble fraction of buckwheat (Fagopyrum esculentum Moench) protein possessing cholesterol-binding properties that reduce micelle cholesterol solubility and uptake by Caco-2 cells. J. Agric. Food. Chem. 2007;55:6032–6038. doi: 10.1021/jf0709496. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y., Xiong Y.L., Zhai J., Zhu H., Dziubla T. Fractionation and evaluation of radical scavenging peptides from in vitro digests of buckwheat protein. Food Chem. 2010;118:582–588. doi: 10.1016/j.foodchem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang C.H., Peng J., Zhen D.W., Chen Z. Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates. Food Chem. 2009;115:672–678. [Google Scholar]
- 8.Li C.H., Matsui T., Matsumoto K., Yamasaki R., Kawasaki T. Latent production of angiotensin I converting enzyme inhibitors from buckwheat protein. J. Pept. Sci. 2002;8:267–274. doi: 10.1002/psc.387. [DOI] [PubMed] [Google Scholar]
- 9.Ma M.S., Bae I.Y., Lee H.G., Yang C.-B. Purification and identification of angiotensin I-converting enzyme inhibitory peptide from buckwheat (Fagopyrum esculentum Moench) Food Chem. 2006;96:36–42. [Google Scholar]
- 10.Guo X., Zhu K., Zhang H., Yao H. Purification and characterization of the antitumor protein from Chinese tartary buckwheat (Fagopyrum tataricum Gaertn.) water-soluble extracts. J. Agric. Food. Chem. 2007;55:6958–6961. doi: 10.1021/jf071032+. [DOI] [PubMed] [Google Scholar]
- 11.Leung E.H., Ng T. A relatively stable antifungal peptide from buckwheat seeds with antiproliferative activity toward cancer cells. J. Pept. Sci. 2007;13:762–767. doi: 10.1002/psc.891. [DOI] [PubMed] [Google Scholar]
- 12.Wang C., Yuan S., Zhang W., Ng T., Ye X. Buckwheat antifungal protein with biocontrol potential to inhibit fungal (Botrytis cinerea) infection of cherry tomato. J. Agric. Food. Chem. 2019;67:6748–6756. doi: 10.1021/acs.jafc.9b01144. [DOI] [PubMed] [Google Scholar]
- 13.Deng Y., Padilla-Zakour O., Zhao Y., Tao S. Influences of high hydrostatic pressure, microwave heating, and boiling on chemical compositions, antinutritional factors, fatty acids, in vitro protein digestibility, and microstructure of buckwheat. Food Bioprocess Technol. 2015;8:2235–2245. [Google Scholar]
- 14.Markus G. Protein substrate conformation and proteolysis. Proc. Natl. Acad. Sci. U.S.A. 1965;54:253. doi: 10.1073/pnas.54.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Q., Ren X., Ma H., Li S., Xu K., Oladejo A.O. Effect of low-frequency ultrasonic-assisted enzymolysis on the physicochemical and antioxidant properties of corn protein hydrolysates. J. Food Qual. 2017;2017 [Google Scholar]
- 16.FAO, IFAD, UNICEF, WFP, WHO . FAO; Rome: 2020. The State of Food Security and Nutrition in the World 2020. Transforming Food Systems for Affordable Healthy Diets. https://doi.org/10.4060/ca9692en. [Google Scholar]
- 17.Berryman C.E., Lieberman H.R., Fulgoni V.L., III, Pasiakos S.M. Protein intake trends and conformity with the Dietary Reference Intakes in the United States: analysis of the National Health and Nutrition Examination Survey, 2001–2014. Am. J. Clin. Nutr. 2018;108:405–413. doi: 10.1093/ajcn/nqy088. [DOI] [PubMed] [Google Scholar]
- 18.Qu W., Ma H., Liu B., He R., Pan Z., Abano E.E. Enzymolysis reaction kinetics and thermodynamics of defatted wheat germ protein with ultrasonic pretreatment. Ultrason. Sonochem. 2013;20:1408–1413. doi: 10.1016/j.ultsonch.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C., Ma H., Yu X., Liu B., Yagoub A.E.-G.A., Pan Z. Pretreatment of defatted wheat germ proteins (by-products of flour mill industry) using ultrasonic horn and bath reactors: Effect on structure and preparation of ACE-inhibitory peptides. Ultrason. Sonochem. 2013;20:1390–1400. doi: 10.1016/j.ultsonch.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Jin J., Ma H., Qu W., Wang K., Zhou C., He R., Luo L., Owusu J. Effects of multi-frequency power ultrasound on the enzymolysis of corn gluten meal: Kinetics and thermodynamics study. Ultrason. Sonochem. 2015;27:46–53. doi: 10.1016/j.ultsonch.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Mason T.J., Lorimer J.P. Wiley-VCH Verlag GmbH; Weinheim, Germany: 2002. Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing. [Google Scholar]
- 22.Ashokkumar M. Springer Science & Business Media; 2010. Theoretical and Experimental Sonochemistry Involving Inorganic Systems. [Google Scholar]
- 23.Jin J., Ma H., Wang K., Yagoub A.E.-G.A., Owusu J., Qu W., He R., Zhou C., Ye X. Effects of multi-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason. Sonochem. 2015;24:55–64. doi: 10.1016/j.ultsonch.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Jin J., Ma H., Wang B., Yagoub A.E.-G.A., Wang K., He R., Zhou C. Effects and mechanism of dual-frequency power ultrasound on the molecular weight distribution of corn gluten meal hydrolysates. Ultrason. Sonochem. 2016;30:44–51. doi: 10.1016/j.ultsonch.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Feng H., Barbosa-Cánovas G.V., Weiss J. Springer; 2011. Ultrasound Technologies for Food and Bioprocessing. [Google Scholar]
- 26.Tomotake H., Shimaoka I., Kayashita J., Nakajoh M., Kato N. Physicochemical and functional properties of buckwheat protein product. J. Agric. Food. Chem. 2002;50:2125–2129. doi: 10.1021/jf011248q. [DOI] [PubMed] [Google Scholar]
- 27.Jambrak A.R., Mason T.J., Lelas V., Paniwnyk L., Herceg Z. Effect of ultrasound treatment on particle size and molecular weight of whey proteins. J. Food Eng. 2014;121:15–23. [Google Scholar]
- 28.Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C., Carriere F., Boutrou R., Corredig M., Dupont D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014;5:1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- 29.Waterborg J.H. The Protein Protocols Handbook. Springer; 2009. The Lowry method for protein quantitation; pp. 7–10. [Google Scholar]
- 30.Alizadeh-Pasdar N., Li-Chan E.C. Comparison of protein surface hydrophobicity measured at various pH values using three different fluorescent probes. J. Agric. Food. Chem. 2000;48:328–334. doi: 10.1021/jf990393p. [DOI] [PubMed] [Google Scholar]
- 31.Ou S., Kwok K., Wang Y., Bao H. An improved method to determine SH and–S–S–group content in soymilk protein. Food Chem. 2004;88:317–320. [Google Scholar]
- 32.Beveridge T., Toma S., Nakai S. Determination of SH- and SS- groups in some food proteins using Ellman's reagent. J. Food Sci. 1974;39:49–51. [Google Scholar]
- 33.Choi S.-M., Ma C.Y. Conformational study of globulin from common buckwheat (Fagopyrum esculentum Moench) by Fourier transform infrared spectroscopy and differential scanning calorimetry. J. Agric. Food. Chem. 2005;53:8046–8053. doi: 10.1021/jf051040v. [DOI] [PubMed] [Google Scholar]
- 34.Ellepola S.W., Choi S.M., Ma C.Y. Conformational study of globulin from rice (Oryza sativa) seeds by Fourier-transform infrared spectroscopy. Int. J. Biol. Macromol. 2005;37:12–20. doi: 10.1016/j.ijbiomac.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Secundo F., Guerrieri N. ATR-FT/IR study on the interactions between gliadins and dextrin and their effects on protein secondary structure. J. Agric. Food. Chem. 2005;53:1757–1764. doi: 10.1021/jf049061x. [DOI] [PubMed] [Google Scholar]
- 36.Byler D.M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986;25:469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- 37.Wang B., Atungulu G.G., Khir R., Geng J., Ma H., Li Y., Wu B. Ultrasonic treatment effect on enzymolysis kinetics and activities of ACE-inhibitory peptides from oat-isolated protein. Food Biophys. 2015;10:244–252. [Google Scholar]
- 38.Jiang L., Wang J., Li Y., Wang Z., Liang J., Wang R., Chen Y., Ma W., Qi B., Zhang M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014;62:595–601. [Google Scholar]
- 39.Jin J., Ma H., Wang W., Luo M., Wang B., Qu W., He R., Owusu J., Li Y. Effects and mechanism of ultrasound pretreatment on rapeseed protein enzymolysis. J. Sci. Food Agric. 2016;96:1159–1166. doi: 10.1002/jsfa.7198. [DOI] [PubMed] [Google Scholar]
- 40.Li S., Yang X., Zhang Y., Ma H., Liang Q., Qu W., He R., Zhou C., Mahunu G.K. Effects of ultrasound and ultrasound assisted alkaline pretreatments on the enzymolysis and structural characteristics of rice protein. Ultrason. Sonochem. 2016;31:20–28. doi: 10.1016/j.ultsonch.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Gülseren İ., Güzey D., Bruce B.D., Weiss J. Structural and functional changes in ultrasonicated bovine serum albumin solutions. Ultrason. Sonochem. 2007;14:173–183. doi: 10.1016/j.ultsonch.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y., Li Y., Li S., Zhang H., Ma H. In situ monitoring of the effect of ultrasound on the sulfhydryl groups and disulfide bonds of wheat gluten. Molecules. 2018;23:1376. doi: 10.3390/molecules23061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osullivan J., Murray B., Flynn C., Norton I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocolloids. 2016;53:141–154. [Google Scholar]
- 44.Stefanović A.B., Jovanović J.R., Dojčinović M.B., Lević S.M., Nedović V.A., Bugarski B.M., Knežević-Jugović Z.D. Effect of the controlled high-intensity ultrasound on improving functionality and structural changes of egg white proteins. Food Bioprocess Technol. 2017;10:1224–1239. [Google Scholar]
- 45.Mendez-Vilas A., Bruque J., González-Martín M. Sensitivity of surface roughness parameters to changes in the density of scanning points in multi-scale AFM studies. Application to a biomaterial surface. Ultramicroscopy. 2007;107:617–625. doi: 10.1016/j.ultramic.2006.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.