Abstract
Aoniraptor libertatem is a mid‐sized megaraptoran that comes from the Late Cretaceous (Turonian) Huincul Formation at Río Negro province, Patagonia, Argentina. In this study, we conducted a detailed analysis of pneumaticity of the sacrum and tail of Aoniraptor. This shows a complex structure within these vertebrae, being composed by small diverticulae surrounding large pneumatic canals and a central chamber that opens outside through pleurocoels or pneumatic canals. Further, we carried out a histologic analysis which confirms the pneumatic nature of these anatomical features. Both analyses found that chevrons in Aoniraptor were invaded by pneumaticity, a feature that appears to be unique to this taxon. In addition, a comparative analysis between Aoniraptor and other theropods (e.g. Gualicho and other megaraptorans) was carried out. This resulted in the modification of previous schemes about the evolution of pneumaticity through Theropoda, the finding of some evolutionary pneumatic traits through Megaraptora, and the usefulness of pneumatic traits as a taxonomic tool.
Keywords: anatomy, cretaceous, megaraptora, tail, theropoda
Segmentation of the last sacral centrum in right lateral (a, d and g), ventral (b, e and h) and posterior (c, f and i) views and close‐up of selected parts of the bone in anterodorsal (j) and ventrolateral views (k). (a–c) Complete modeling: the blue parts are the pneumaticity reaching the external surface in the pleurocoels and some broken parts. (d–f) Model with the internal pneumatic traits in blue. (g–i) Main pneumatic internal traits (central chamber in red; right longitudinal canal in yellow; left longitudinal canal in light blue; long ventral canal in blue and pleurocoels in green). (j–k) Close‐up of pneumatic features of the neural arch. CDF?, centrodiapophyseal fossa?; PCDL, posterior centrodiapophyseal lamina; STP, sacral transverse process. Not to scale.

1. INTRODUCTION
One of the most intriguing features of Aves, together with the presence of flight feathers, elongated wings and extensive modifications of the skeleton (Makovicky and Zanno, 2011), is the presence of a complex respiratory system that invades, through pneumatic diverticula, the postcranial skeleton (e.g. limbs, vertebrae and ribs; Hunter, 1774; Duncker, 1971; Wedel, 2006). These diverticula are not involved in the gas exchange but replace metabolically expensive bone and reduce bone mass and consumption of metabolic energy, enabling the capability of flight (Welty, 1982; Currey and Alexander, 1985).
However, postcranial pneumaticity is not unique to birds, being observed in non‐avian dinosaurs (Britt, 1993; Wedel, 2003; O´Connor, 2006, 2007; O’Connor and Claessens, 2006; Sereno et al., 2008; Makovicky and Zanno, 2011; Benson et al., 2012; Xu et al., 2014; Watanabe et al., 2015; Lambertz et al., 2018) as well as pterosaurs (Britt, 1993; Butler et al., 2009; Claessens et al., 2009; Martin and Palmer, 2014). The presence of this condition in non‐volant taxa indicates that the interaction between postcranial pneumaticity and flight capability was secondarily acquired and suggests that the main function of postcranial pneumatization is not flight. In this regard, many hypotheses have been proposed, such as thermoregulation (Farmer, 2006), locomotory balance (Wedel, 2003; Ruben et al., 2003; O´Connor, 2006) and reduction of high metabolic needs in large‐sized (and flying) taxa (Scheid, 1982; Wedel, 2003; Ruben et al., 2003; O´Connor, 2006, 2007; O’Connor and Claessens, 2006; Makovicky and Zanno, 2011; Benson et al., 2012).
Several authors have analyzed the evolution of postcranial pneumaticity towards the avian line (Wedel, 2003; O´Connor, 2006; O’Connor and Claessens, 2006; Benson et al., 2010; Watanabe et al., 2015). Most of those studies rest on the presence of external pneumatic openings and, with the exception of Watanabe et al. (2015), do not provide an extensive description of the internal structure of the postcranial pneumaticity. Benson et al. (2012) noted that the pneumatization of the column in Theropoda is widespread in the cervical, dorsal and sacral regions, whereas the caudal pneumaticity is present in few theropods. However, the analysis by Benson and colleagues is based on external and macroscopic pneumatic features along the backbone. Thus it is uncertain whether pneumatic internal features change along the vertebral column.
Aoniraptor libertatem (Motta et al., 2016) is a mid‐sized megaraptoran from the Huincul Formation (Middle Cenomanian‐Early Turonian) of Río Negro, Argentina. This specimen is represented by the last sacral vertebrae, more than 10 caudal elements and five chevrons.
Here, we conduct an extensive description of the internal structure of the vertebrae of the megaraptoran Aoniraptor libertatem (Motta et al., 2016). This affords new anatomical information that has important implications for the evolution of postcranial pneumaticity of theropods as well as new evidence about the anatomy of Megaraptora.
Recently, a few works (Lambertz et al., 2018; Aureliano et al., 2019) reveal that the presence of an air‐sac system could be inferred from the presence of pneumosteal tissue. This gives another tool for recognizing and improving knowledge of the distribution and evolution of the pneumaticity within Dinosauria. A correlation between air‐sac system invasion and its histological signal in bones has been proposed (Lambertz et al., 2018). Within non‐avian dinosaurs, pneumosteal tissue has been found in the sauropods Europasaurus holgeri, Diplodocus sp. (Lambertz et al., 2018) and Uberabatitan ribeiroi (Aureliano et al., 2019). We expand here the occurrence of pneumosteum to non‐avian theropod dinosaurs by identification of this tissue in the caudal vertebrae and chevron in Aoniraptor libertatem.
2. METHODS
The holotype of Aoniraptor libertatem (MPCA‐Pv 804/1 to 804/25; Motta et al., 2016) consists of the last sacral and first caudal vertebrae in articulation, 11 caudal verterbrae and some isolated transverse processes (Figure 1). The nomenclature used here is that proposed by Wilson et al. (2011) for vertebral fossae and Wilson (2012) for laminae, and by Wedel et al. (2000) and Wedel (2003, 2006) for pneumatic structures. We follow the criteria of O’Connor and Claessens (2006) and O´Connor (2006) about the nomenclature of the pneumatic structures in dinosaurs. We employ the anatomical terms anterior and posterior instead of cranial and caudal.
FIGURE 1.

Interpretative drawing of the preserved materials of Aoniraptor libertatem (Motta et al., 2016). Scale: 50 cm
The specimen was CT‐scanned at the TCba Salguero Diagnostic Center (Buenos Aires, Argentina) using a CT 64 Ingenuity Core medical tomograph. The material was scanned in two rounds: a low energy round (parameters: 283 mA and 140 kV) and a high energy round (parameters: 30 mA and 120 kV). The slice thickness was 0.5 mm in both cases. Virtual three‐dimensional images were obtained and visualized using the software amira 5.3.3. Final illustrations were made with Adobe photoshop (CS6).
Some mechanical preparation was made after the CT scan with the aim to corroborate the scanning information. In this regard, we re‐prepared some areas of interest by removing the matrix and plaster overlaying the bone, using needles and airscribes.
A comparative survey within Megaraptora was made to identify evolutionary phylogenetic trends and internal variations within the skeleton. This survey includes direct observations on the holotype of Aoniraptor libertatem, the second specimen of Megaraptor namunhuaiquii (MUCPv 341) located in Museo de la Universidad Nacional del Comahue, Proyecto Dino Collection, (Neuquén, Argentina); the holotype of Aerosteon riocoloradensis (MCNA 3137) housed in the Museo de Ciencias Naturales y Antropológicas ‘J. C. Moyano’; and the holotype of Giganotosaurus carolinii (MUCPv‐CH‐1) housed in the Museo Ernesto Bachmann.
2.1. Paleohistological analysis
Bone tissue samples were extracted as longitudinal sections of caudal vertebra and chevron bone belonging to the holotype specimen MPCA‐Pv 804/1‐25. Thin sections were prepared following the method outlined by Chinsamy and Raath (1992). The bones were embedded in a clear epoxy resin (Araldite© GY 279, catalyzed with Aradur® hY 951) and left for 24 hr to set. They were cut into smaller blocks perpendicular to the long axis of the bone using a cut diamond‐tipped saw within a Ken 9025 grinding machine. One surface of each resin block was then affixed to a frosted petrographic glass slide using the same resin that was used for embedding and left to set for a further 24 hr. The sections were wet‐ground to approximately 60 µm thickness and polished using a Prazis APL‐S polishing machine with abrasive papers of increasing grit size (P80, P120, P320, P400, P600, P1200, P1500, P2000, P3000). Histological samples were studied using a Nikon E400 petrographic polarizing microscope under normal, polarized and lambda types of light.
Nomenclature and definitions of structures used in this study follow Francillon‐Vieillot et al. (1990). In the case of the term ‘intrinsic fibers’, we follow the definition of de Ricqlès (1991), that is, collagenous fibers deposited by osteoblasts during the ossification process, forming the fibrous component of the bone matrix. Regarding growth marks, we follow the terminology of Francillon‐Vieillot et al. (1990), in which lines of arrested growth (LAGs) are a type of cementing line that represents temporary but complete cessation of appositional growth. The annuli are defined as narrow layers of parallel‐fibered bone or lamellar bone that reflect periods of relatively slow growth rates. The term ‘growth mark’ is used here for both LAGs and annuli (Francillon‐Vieillot et al., 1990).
3. RESULTS
3.1. Sacral vertebra
The neural arch of this element is poorly preserved both externally and internally (revealed by the CT imaging) (Figure 2). It shows numerous fossae and foramina connected by internal chambers (Motta et al., 2016), indicating that the last sacral neural arch of Aoniraptor was strongly pneumatized. Sacral pneumatization is widespread among theropods (Benson et al., 2010), including megaraptorans (Porfiri et al., 2014; Motta et al., 2016; Aranciaga‐Rolando et al., 2019). Aoniraptor shows a camellate vertebral centrum, but with some big chambers surrounding the neural canal that perforate the transverse process. The distortion of the neural arch precludes more detailed observations. An elliptical centrodiapophyseal fossa is observed ventral to the right transverse process (Figure 2k). The CT scan reveals a small foramen entering the bone at the bottom of that fossa. This small foramen is distorted by a wide internal fracture of the bone that obscured its morphology. Watanabe et al. (2015) note the presence of a small foramen on the centrodiapophyseal fossa of ornithomimosaurs and interpreted it as a neurovascular foramen because ‘it does not lead into any extensive internal chamber’. The short anterior centrodiapophyseal lamina shows, on its anterior surface, a subtriangular foramen divided by a bony septum (Figure 2j). The CT scan shows that this foramen pierces the centrum but internal distortion does not allow further observations to be made. Dorsal to this foramen, at the anterodorsal surface of the centrodiapophyseal lamina, a subtriangular‐shaped and very deep accessory fossa is observed (Motta et al., 2016) (Figure 2j). This accessory fossa leads to an extensive internal chamber on the right side of the neural arch. The CT scan shows that this chamber runs along the lateral side of the neural arch and enters the transverse process of the vertebra. This chamber could be observed via external observation of a broken part of the transverse process (Motta et al., 2016). A similar internal chamber is observed in ornithomimosaurs but it is not associated with any pneumatic foramen (Watanabe et al., 2015). Medial to the lateral internal chamber, a wide central chamber is observed. This chamber is elliptical in shape, notably higher dorsoventrally than anteroposteriorly long, and runs along the neural canal. Anterolaterally, this chamber seems to be connected with pleurocoels located within the prezygapophyseal‐centrodiapophyseal fossae. Also, both fossae connect medially to each other and with the central chamber. However, the borders of both pleurocoels are distorted and the CT scan did not reveal determinant data so, for these reasons, such connection is considered to be ambiguous.
FIGURE 2.
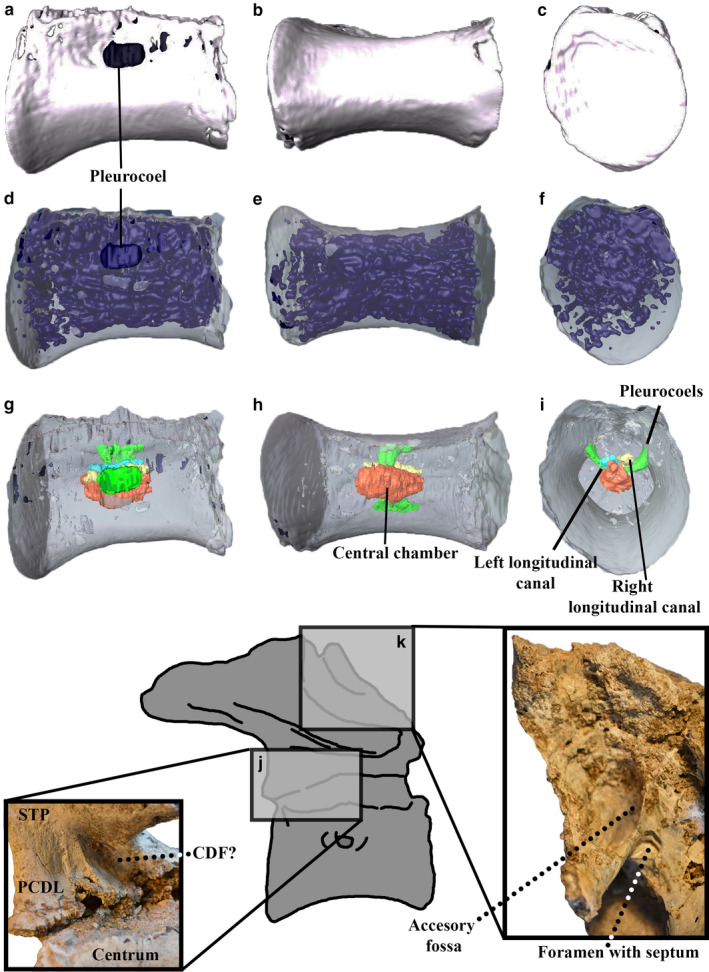
Segmentation of the last sacral centrum in right lateral (a, d and g), ventral (b, e and h) and posterior (c, f and i) views and close‐up of selected parts of bone in anterodorsal (j) and ventrolateral views (k). (a–c) Complete modeling: blue parts are the pneumaticity reaching the external surface in the pleurocoels and some broken parts. (d–f) Model with the internal pneumatic traits in blue. (g–i) Main pneumatic internal traits (central chamber in red; right longitudinal canal in yellow; left longitudinal canal in light blue; long ventral canal in blue and pleurocoels in green). (j–k) Close‐u\p of pneumatic features of the neural arch. CDF?, centrodiapophyseal fossa?; PCDL, posterior centrodiapophyseal lamina; STP, sacral transverse process. Not to scale [Colour figure can be viewed at wileyonlinelibrary.com]
In contrast with the neural arch, the centrum is well‐preserved. Both sides of the centrum show pleurocoels located at the dorsal third of its height. A large and single pleurocoel is observed on the right side (Figure 2a,d,g) and two small pleurocoels are present on the left side (Motta et al., 2016). Pleurocoels are placed within oval‐shaped pleurofossae. The right pleurocoel is rounded in contour and connects with a large and deep canal that ends on an extensive pneumatic chamber located at the center of the vertebral centrum. Left pleurocoels are rounded in shape and also reach the central chamber through two small foramina. The central chamber has two different parts: the dorsal and lateral canals and the main space of the chamber (Figure 2g–i). The dorsolateral canals are anteroposteriorly long and resemble in morphology the longitudinal canals described for more posterior caudal. Probably, these projections are homologous to those canals but they are described here as part of the central chamber because of their extensive connection. The central pneumatic chamber anastomoses externally, filling, through smaller camellae, the entire volume of the centrum (Figure 2d–f). Such camellae become smaller close to the margins of the centrum and to the anterior and posterior articular surfaces. Dorsally, a wide internal canal connecting the centrum with the neural arch is absent, in contrast to the caudal vertebrae.
3.2. First caudal vertebra
The first caudal vertebra is articulated with the last sacral (Motta et al., 2016). Its neural arch is missing except for the right anterior and posterior centrodiapophyseal and prezygadiapophyseal laminae (Motta et al., 2016). Prezygapophyseal‐centrodiapophyseal and the centrodiapophyseal fossae are present; both seem to perforate, but this condition is uncertain due to incomplete images. Watanabe et al. (2015) note a foramen in the centrodiapophyseal fossa of some ornithomimosaurs and identify it as a true pneumatic foramen.
The centrum of the first caudal shows a deep and a rounded pleurocoel (Figure 3) within a fossa on its left lateral side (Motta et al., 2016). Contrarily, on the right side of centrum there is no foramen, but a shallow fossa. The pleurocoel connects with the internal camellae of the centrum. These camellae are similar to those of the sacral centrum because the bigger ones are in the center of the bone and the smaller closer to the anterior and posterior articular surfaces (Figure 3d–f). All the internal spaces in the centrum are interconnected, indicating that they may be pneumatic in origin. The pleurocoel conducts primarily to a long, longitudinal and relatively large canal. Later it connects with the central chamber and finally communicates with the rest of the diverticulae (Figure 3g–i). Interestingly, this canal has its counterpart on the right side of the centrum. Both are very similar in morphology but differ in that the canal of the right side of the centrum lacks its pleurocoel. Ventral to these two longitudinal canals, the central chamber is much wider and rhomboidal in contour. This central chamber is observed in the CT scans of other theropods but has never been identified as pneumatic (Watanabe et al., 2015).
FIGURE 3.

Segmentation of the first caudal centrum in left lateral (a, d and g), dorsal (b, e and h), anterior (c and f) views and cross‐section (i). (a–c) Complete modeling; the green parts are the pneumatic traits reaching the external surface. (d–f) Modeling (transparent) with the internal pneumatic parts in green. (g–i) Main pneumatic traits (central chamber in red; left longitudinal canal in yellow; right longitudinal canal in light blue; and sub‐vertical canals and pleurocoel in green). CFL, caudofemoralis longus scar (see Supporting Information). Not to scale [Colour figure can be viewed at wileyonlinelibrary.com]
Ventrally in the neurocentral suture, a rounded foramen is observed in the left side. This foramen, observed in the CT imaging, does not communicate with any internal cavity so it is not pneumatic in nature (Figure 4j).
FIGURE 4.

Segmentation of other proximal caudal centra in right lateral (a, d and g), dorsal (b, e and h), anterior (c and f) and left lateral views (j). (a–c) Photographs of the original bones. (d–f) Complete modeling: the blue areas are pneumatic traits visible through some broken parts and the green parts are the external entrance of the sub‐vertical pneumatic canals. (g–i) Model (transparent) with the internal pneumatic traits in blue. (j) Close‐up of the right non‐pneumatic foramina. CFL, caudofemoralis longus scar (see Supporting Information). Not to scale [Colour figure can be viewed at wileyonlinelibrary.com]
The CT scan reveals a second type of pneumatic invasion of the vertebrae that differs from that at the lateral side of the centrum. It consists in two sub‐vertically oriented canals communicating the neural arch with the centrum (Figure 3d–f). Both canals end in the paired longitudinal canals mentioned above. The left sub‐vertical canal is slightly bigger in the right one. This second type of pneumatization differs from the ‘pleurocoel‐derived’ pneumatization observed in the lateral side of the centrum of these caudal and sacral vertebrae. Furthermore, the ‘arch‐derived’ pneumatization is observed at middle and mid‐posterior caudals of Aoniraptor but the ‘pleurocoel‐derived’ is absent. In sum, there is a change in the way that pneumaticity invades the centrum along the tail of Aoniraptor. In this sense, the first caudal has a transitional character between both types of pneumatization.
3.3. Proximal caudals
Only three isolated and poorly preserved transverse processes have been recovered (Motta et al., 2016). Postzygapophyseal‐centrodiapophyseal (Pocdf sensu Wilson et al., 2011) and prezygapophyseal‐centrodiapophyseal fossae (Prcdf sensu Wilson et al., 2011) were present. The transverse process shows a trabeculated internal structure, suggesting that proximal caudal vertebrae probably show pneumaticity in the neural arch resembling the condition exhibited by the first caudal vertebrae. However, due to the absence of more complete materials, whether these transverse processes have a pneumatic character or such pneumaticity is connected to those of the centra will remain ambiguous.
Proximal caudal centra (Motta et al., 2016) lack pleurocoels on the lateral surfaces (Figure 4a,d,g). The CT scan reveals one small foramen (diameter 1 mm) placed at the same level at which a pleurocoel should be located (Figure 4j). Upon closer inspection and some mechanical preparation on the external surface, we observed these foramina in almost all the remaining distal caudal vertebrae and on the right side of the first caudal vertebrae. Although these foramina connect with the internal spaces of the vertebrae, we refer to them as neurovascular in origin because of their very small diameter (only extensive foramina that connect with extensive internal chambers are unambiguously considered pneumatic, following the criterion of O´Connor, 2006). Conversely, at the left side of the first caudal and the sacral vertebra, this foramen is replaced by a pleurocoel. In this sense, this reinforces previous evidence that that the pneumatic system uses preexisting vascular foramina (Bremer, 1940; O´Connor, 2006). In Aoniraptor, its morphology suggests that in the last sacral and first caudal vertebra the air‐sac diverticulae invaded the vertebra through the vascular foramina located within the pleurocoel, whereas in more distal caudals the pneumaticity invaded the vertebrae through the neural arch, and the foramina located at the ventral surface of the centrum have a strictly vascular function.
CT scanning shows an internal structure very similar to that described for the first caudal. The centrum is trabeculated, with smaller camellae close to the external margins of the bone (Figure 4g–i). At mid‐length of the centrum, two large, sub‐vertically oriented canals end with two main longitudinally oriented canals. These canals are connected with a large cavity located at the ventral third of the centrum.
3.4. Proximal‐mid‐caudal vertebrae
Preserved neural arches show deep and wide pocdf and prcdf below the transverse processes (Figure 5a–c). The prcdf are oval or subtriangular in contour and are relatively larger than the pocdf. The latter is rounded in contour. Both fossae become progressively smaller in more posterior vertebrae. Both fossae communicate with each other through short but wide canals within the internal structure of the vertebrae (Figure 5d–f).
FIGURE 5.

Segmentation of a proximo‐medial vertebrae in right lateral (a, d and g), posterior (b, e and h), dorsal (c and f) views and close‐up of the postspinal fossa (i). (a–c) Complete modeling: the blue parts are the pneumatic traits visible through some pneumatic conduits and some broken parts. (d–f) Model (transparent) with the internal pneumatic traits in blue. (g–i) Main pneumatic conduits (central chamber in red; right longitudinal canal in yellow; left longitudinal canal in light blue; and sub‐vertical canals in green and long ventral canal in blue). PRCDF, prezygapophyseal‐centrodiapophyseal fossa; POCDF, postzygapophyseal‐centrodiapophyseal fossa. Not to scale [Colour figure can be viewed at wileyonlinelibrary.com]
A pleurocoel is observed in the prespinal fossa. It enters the neural arch through a wide and sub‐vertically oriented canal that is placed in the right half of the fossa. The postspinal fossa shows one small pleurocoel in the left half of the fossa (Figure 5i). The lateral walls of the neural spines are smooth and lack pleurocoels on their base, like those of carcharodontosaurids (Britt, 1993; Benson et al., 2012). In the same way, Aoniraptor vertebrae do not show pneumatic foramina closer to the articular surface of the postzygapophyses.
Internally, the resolution of the CT scan of proximal‐mid‐caudals is not good and thus some features of the internal anatomy remain obscure. The structure seems to be constituted of trabeculae, being proportionally bigger than those of more anterior caudals (Figure 5d–f). In the centra, such trabeculae are less dense when compared with trabeculae of more anterior caudals. Trabeculae occupy all the internal structure of the neural arch and do not form canals or chambers. Trabeculae extend along the neural spines, transverse processes and post‐ and prezygapophyses. The pneumaticity in these structures does not appear to be extensive. The neural spine shows two separate pneumatic canals: one is located near the anterior end of the neural spine and is sub‐vertically oriented; the other is posteriorly placed and is obliquely oriented. Both canals exit by means single pneumatic foramina at the anterior and posterior surfaces of the dorsal end of the neural spine.
Close to the mid‐height of the vertebral centra, the internal pneumaticity is mainly represented by two short sub‐vertically oriented canals that connect the pneumaticity of the neural arch with that of the centrum (Figure 5g–h). These canals could be observed with the naked eye in one isolated vertebral centrum. These sub‐vertically oriented canals are connected with two longitudinal canals. These are shorter than in more anterior vertebrae. These converge with a large central chamber surrounded by subsidiary trabeculae (Figure 5g–h). Such a chamber is ovoidal in contour and anteroposteriorly longer that dorsoventrally tall. Ventral to the central chamber, there exists a long and longitudinal canal.
3.5. Middle caudals
Aoniraptor has five mid‐caudal vertebrae (Motta et al., 2016). Compared with more anterior caudals, the external pneumatic traits here are reduced (Figure 6a–c). The prcdf is observed in the two more anterior vertebrae (and only at the left side of the neural arch). Such fossa is oval in contour and enter the neural arch via a short, wide and oval‐shaped pneumatic foramen. The pocdf is only observed in the left side of the most anterior vertebrae. This fossa is subcircular in contour and is connected with the internal pneumaticity of the vertebrae. The prespinal fossa shows two ovoid pneumatic openings that enter the bone (Figure 6i). The CT imaging shows that such openings communicate with the internal structure of the vertebrae (Figure 6d–f). The postspinal fossa shows a narrow pneumatic foramen on its left side. This foramen and those of the prespinal fossa are observed in the posteriormost preserved caudal. The anterior surface of the neural spine shows a small foramen at its dorsal end. The CT scan reveals that such a foramen is connected with a small and anteriorly located sub‐vertical canal.
FIGURE 6.

Segmentation of a medial vertebra in right lateral (a, d and g), dorsal (b, e and h), posterior (c and f) views and close‐up of its prespinal fossa (i). Segmentation of two articulated mid‐caudal vertebrae (g–h). (a–c) Complete modelling: the blue parts are the pneumatic traits visible through some pneumatic conduits and some broken parts. (d–f) Model (transparent) with the internal pneumatic traits in blue. (g) Main pneumatic conduits (central chamber in red; connection between central chamber and pneumaticity of the neural arch in green). (h) Close‐up of the central chambers of two articulated elements. Black arrows point anteriorly. PRCDF: prezygapophyseal‐centrodiapophyseal fossa; POCDF: postzygapophyseal‐centrodiapophyseal fossa. Not to scale [Colour figure can be viewed at wileyonlinelibrary.com]
The CT scan shows a profuse pneumatization in the neural arch; its internal structure is composed of trabeculae that are relatively larger and smaller in number than in more anterior caudals (Figure 6d–f). Such trabeculae enter within the pre‐ and postzygapophyses, the base of the neural spine and transverse processes. A pair of lateral and sub‐vertically oriented canals connects the trabeculae of the neural arch with those of the centrum (Figure 6g–h). The centrum shows, as in more anterior caudals, two longitudinally oriented canals that ventrally connect with a large and ovoid central chamber. This chamber is wider than in more anterior vertebrae and is not placed at mid‐length but is slightly anteriorly displaced. The connection between the central chamber and the longitudinal canals is wide. Ventrally, the central chamber shows a long and longitudinal canal. This canal is anteroposteriorly as long as the chamber. The neural spine shows rounded but isolated trabeculae on its posterodorsal corner. Because of the absence of connection with other external or internal pneumatic structures, this space does not represent a pneumatic feature. However, because of its position, it could represent a cavity that may be the remnant of the oblique posterior canal of more anterior caudals.
3.6. Chevrons
Five chevrons of Aoniraptor are preserved: two proximal, two mid‐proximal and one distal. The proximal chevrons show a pleurocoel at the posterior surface of the haemal canal (Figure 7a–f). This pleurocoel enters into the bone as a tall and sub‐triangular sub‐vertically oriented cavity. Such a cavity extends along most of the proximal one‐third of the bone. The same is observed in the mid‐proximal chevrons but, in this case, such a cavity is dorsoventrally shorter (Figure 7g–l). A pleurocoel is present at the posterior surface of the haemal canal and a smaller one on its anterior surface. The only preserved distal chevrons lack pneumaticity.
FIGURE 7.

Segmentation of proximal (a–f) and proximo‐medial (g–l) chevrons in anterior (a, d, g and j), posterior (b, e, h and k) and right lateral (c, f, i and l) views. (a–c, g–i) Complete modeling: the blue parts are the pneumatic traits visible through some pneumatic conduits. (d–f, j–l) Model (transparent) with the internal pneumatic traits in blue. Not to scale [Colour figure can be viewed at wileyonlinelibrary.com]
3.7. Results from the histological analysis
The primary bone of the caudal vertebra in Aoniraptor is restricted to the walls of the vertebra. This primary tissue is formed mainly by fibrolamellar bone and primary osteons. The primary tissue does not show any other pneumosteum. Aoniraptor exhibits trabeculae in most of its vertebral sections; these trabeculae are comprised of secondary lamellar bone. Dorsally, there are signs of pneumosteal tissue in the vertical canals that enter the bone and the central chamber (Figure 8; images 1–3). The secondary osteons are not rare within the trabeculae; however, they are much more abundant close to the vertebral walls and within the trabeculae in the ventral side of the vertebra. Ventrally, these secondary osteons are formed by a Haversian system and the pneumosteal tissue is present only in the avascular and lamellar tissue (Figure 8; image 4). Pneumosteal tissue is recognized and distinguished from the secondary lamellar trabecular or endosteal tissues by an undulose extinction and a densely packed fabric of tiny ‘hair‐like’ fibers (Aureliano et al., 2019). The appearance of these resembles the asbestiform parallel fibrous aggregates of serpentines (Da Mommio, 2018) but at a smaller scale (Aureliano et al., 2019). These fibers are markedly different (much finer and thinner) from Sharpey’s fibres associated with tendinous insertions of muscles (Petermann and Sander, 2013). Presence of pneumosteal tissue could indicate the existence of diverticula of an air‐sac in this area.
FIGURE 8.

General view of the transverse section of the caudal vertebra of Aoniraptor libertaten (a) showing the areas with presence of pneumosteum (white arrows). (1–3) Pneumosteal tissue on the sub‐vertical canals that enters the bone. (4) Pneumosteal tissue in the ventral side of the caudal vertebra. Scale bars: (a) 1 cm; 1, 3 and 4 = 100 µm; 2 = 1 mm [Colour figure can be viewed at wileyonlinelibrary.com]
The longitudinal section of chevron of Aoniraptor shows primary bone formed by fibrolamellar bone along its dorsolateral margin, closer to its contact with the caudal vertebrae and towards its distal end. This fibrolamellar bone shows a high density of vascular canals. The primary bone of the chevron is characterized by the absence of pneumosteal tissue. However, the avascular and lamellar endosteal bone of the chevron presents pneumosteum (Figure 9; images 1–2). In this case, this tissue is composed of thin parallel fibers inclined at about 30–45° towards the endosteal surface.
FIGURE 9.

General view of the longitudinal section of the chevron of Aoniraptor libertaten (a) showing the areas where pneumosteum is present (white arrows). (1–2) Avascular and lamellar endosteal bone of the chevron with pneumosteum. Scale bars: (a) 1 cm; 1–2 = 100 µm [Colour figure can be viewed at wileyonlinelibrary.com]
The histological features of the vertebrae of Aoniraptor resemble the sauropods Europasaurus holgeri and Diplodocus sp., because of the presence of a surface not composed of cortical bone but rather of secondary trabecular bone. In all cases, the secondary trabecular bone shows a pneumosteal tissue (Lambertz et al., 2018). Similar observations in the sauropod Urebatitan corroborate the presence of pneumosteum in this taxon (Aureliano et al., 2019). Previous results have shown that pneumosteum can be diagenetically distorted before the complete obliteration of trabeculae (Aureliano et al., 2019). This could explain the partial absence of pneumosteum in some areas of caudal vertebrae and chevron of Aoniraptor. Therefore, caution is needed when selecting specimens for sampling and searching of pneumosteum, as its absence may be due to a sampling bias.
It is important to note that the unambiguous presence of pneumosteum corroborates the pneumatic character of some anatomical traits noted through anatomical analysis. In this sense, the pneumosteum in proximal caudals was found in the vertical canals and in the central chamber, whereas in the chevron, this tissue was found in the cavity of the bone but not in the rest of the body, reinforcing its pneumatic nature.
3.8. Anatomical changes in pneumaticity along the tail of Aoniraptor libertatem
The detailed analysis has revealed a series of progressive changes in pneumatic features along the tail. In the base of the tail, the pneumaticity of the centrum is initially ‘pleurocoel‐derived’ (as in the sacral and first caudal element), but it changes to ‘arch‐derived’ posteriorly. Once inside the vertebrae, the pneumaticity passes into a system of canals and chambers (e.g. sub‐vertically oriented and longitudinal canals and central chamber), although this morphology varies slightly along the tail. Anteriorly, in the sacral vertebra this canal‐chamber system is not completely formed, as the longitudinal canals and the central chamber are fused to each other (Figure 2g–i). However, more posteriorly such a complex is easier to observe in the proximal and proximo‐medial centra because the longitudinal canals and central chamber are more separated from each other (Figure 3g–i). Finally, in the mid‐caudal centra the canals and the chambers fuse again (Figure 6g–h) to create a single, large chamber.
In addition, the central chamber becomes proportionally larger more posteriorly in the tail, becoming predominant in the mid‐caudals. Furthermore, the size and number of the trabeculae progressively changes towards the tip of the tail, becoming larger and less numerous more posteriorly as the central chamber progressively gains importance (Figure 6g–h).
3.9. Comparisons
3.9.1. Aoniraptor and its implications in theropod tail pneumaticity
Caudal pneumaticity in theropods is an unusual condition restricted to Torvosaurus, carcharodontosaurids, and within Coelurosauria to ornithomimosaurs, megaraptorans, therizinosaurids and oviraptorosaurs (Benson et al., 2012; Novas et al., 2013; Watanabe et al., 2015). Furthermore, pneumaticity up to the middle caudals has only been observed, aside from in megaraptorans, in some oviraptorosaurs, selected therizinosauroids and Deinocheirus (Benson et al., 2010; Lee et al., 2014).
Clearly, caudal pneumaticity is more common among the coelurosaurian clades, being very scarce or absent in more basal taxa. Benson et al. (2012) indicate that megalosaurids are the first theropods that exhibit caudal pneumatization. However, Benson et al. (2012; Suppl. Inf.) recognize that among megalosaurids only Torvosaurus has caudal pneumatizaticity (Britt, 1991). Britt (1991) mentions the presence of ‘subneurocentral fossae’ in the first 10 caudals of Torvosaurus. First, following Benson et al. (2012; Suppl. Inf.) Torvosaurus is a unique megalosaur that exhibits tail pneumatization and thus its condition cannot necessarily be extrapolated to that of other Megalosaurids. Second, the presence of blind fossae in vertebrae alone, presents ambiguous evidence of pneumatization (O’Connor and Claessens, 2006). Third, Britt on his PhD thesis (Britt, 1993) presented an extensive description of the pneumaticity of Torvosaurus and indicated that caudal vertebrae are devoid of pneumaticity. In sum, without further evidence, the presence of tail pneumaticity in Torvosaurus (and other megalosaurs) is considered here as ambiguous at least.
Allosauroid carcharodontosaurids show clear evidence of tail pneumatization (Britt, 1993; Benson et al., 2012). Caudal and sacral vertebrae of these theropods exhibit a deep, vertical and round pleurocoel at the lateral surface of the base of the neural spine, as is observed in Acrocanthosaurus (Britt, 1993) and up to the fifth caudal in Giganotosaurus (Aranciaga Rolando, pers. obs.). The presence of this deep and vertical pleurocoel seems unique to carcharodontosaurids. In megaraptorans, this portion of the neural arch is flat in all preserved caudal vertebrae of Aoniraptor, Megaraptor, Orkoraptor and Murusraptor (Calvo et al., 2004; Novas et al., 2008; Coria and Currie, 2016; Motta et al., 2016). The available evidence suggests that carcharodontosaurids follow the ‘neural arch‐pattern’ invasion sensu Benson et al. (2012), because the pneumatization first invades the neural arch and later the centrum.
Within Coelurosauria, pneumatization of the tail is shared by intermediate clades within Coelurosauria, whereas paravians are devoid of tail pneumaticity (Benson et al., 2012). This contrasts with the increasing of pneumatization of the skeleton closer to Aves, related to flight capabilities (Watanabe et al., 2015).
Ornithomimosaurs exhibit the lesser degree of tail pneumatization being present in two (or maybe three) species (Watanabe et al., 2015). Pneumaticity is restricted to proximal (in Archaeornithomimus) and middle (in Deinocheirus) caudals. Compared with Aoniraptor, ornithomimosaur pneumaticity does not invade the centrum (lateral pleurocoels are absent). However, ornithomimosaurs share with megaraptorans the presence of a pneumatic centrodiapophyseal fossa that connects with a longitudinal internal pneumatic chamber associated with transverse processes (Watanabe et al., 2015).
In therizinosauroids, pneumaticity invades the vertebral centrum through pleurocoels in the proximal and mid‐centra (e.g. in Nothronychus, Erliansaurus, Neimongosaurus; Zhang et al., 2001; Zanno et al., 2009). The neural arch exhibits well‐developed and pneumatic prcdf and pocdf fossae. This condition extends up to the fourth caudal (Zanno, 2010a).
Oviraptorosaurs are, together with megaraptorans, the coelurosaur groups that show the highest degree of pneumatization of the tail (Clark et al., 2001; Xu et al., 2007; Balanoff and Norell, 2012). In oviraptorosaurs the diverticula invade the distal part of the tail (Clark et al., 2001) but the neural arches remain apneumatic (Xu et al., 2007; Balanoff and Norell, 2012). Furthermore, the transverse processes exhibit pneumatic foramina that could not be homologized with other known pneumatic fossae (e.g. cdf, prcdf or pocdf; Xu et al., 2007). The centrum exhibits lateral pleurocoels up to distal caudal elements.
In recent years, some incomplete sacral elements have been described for megaraporans (e.g. SMNS 58023, CPPLIP 1324, Phuwiangvenator, Murusraptor, Megaraptor and Tratayenia; Martinelli et al., 2013; Coria and Currie, 2016; Porfiri et al., 2014; Porfiri et al., 2018, Aranciaga‐Rolando et al., 2019; Samathi et al., 2019). The internal pneumatic structure of all these sacral elements is camellate. Almost all these megaraptorans show strong pneumatic features at the lateral surface of sacral vertebrae, with the exception of Phuwiangvenator (Samathi et al., 2019), which lacks any sign of pleurocoels or fossae and SMNS 58023 (Aranciaga‐Rolando et al., 2019), the latter which shows well‐developed pneumatization on its neural arch but small pleurocoels on its centra. Interestingly, these two specimens represent one of the oldest and probably basal members of the group (Aranciaga‐Rolando et al., 2019; Samathi et al., 2019). In contrast to those specimens, remaining megaraptorans show large pleurocoels at the lateral walls of the sacral centrum (e.g. Aoniraptor, CPPLIP 1324, Megaraptor, Murusraptor and Tratayenia; Martinelli et al., 2013; Porfiri et al., 2014; 2018; Coria and Currie, 2016; Motta et al., 2016; Aranciaga‐Rolando et al., 2019) and a strong pneumatization of the neural arch (e.g. Aoniraptor, Murusraptor, Megaraptor and Tratayenia). This is comprised of deep fossae located at the ventral side of the transverse process (Porfiri et al., 2018). Further, Aoniraptor and Tratayenia exhibit pneumatic cdf and prcdf (Porfiri et al., 2018) and an apomorphic accessory fossa is observed in Aoniraptor.
Caudal vertebrae are known in Megaraptor (Calvo et al., 2004), Orkoraptor (Novas et al., 2008), Australovenator (Poropat et al., 2019), Murusraptor (Coria and Currie, 2016), Aerosteon (Sereno et al., 2008), Fukuiraptor (Azuma and Currie, 2000), MPMA 08‐003‐94 (Méndez et al., 2012), an isolated caudal of Sengüer River (Huene, 1929; Méndez et al., 2012) and a probable first caudal of SMNS 58023 (Aranciaga‐Rolando et al., 2019). Tails of Megaraptora are still poorly known and often composed of one or two isolated caudal elements, which makes the comparison with other members of the group very difficult. In this case, Aoniraptor represent the most complete tail of Megaraptora known to date.
The presence of pleurocoels in the caudal centra is widely distributed in Megaraptora. In Aoniraptor, pleurocoels are restricted to the first element and are absent from other proximal and middle caudals. Interestingly, other basal members of Megaraptora, such as Fukuiraptor and Australovenator (Azuma and Currie, 2000; Poropat et al., 2019), also lack pleurocoels on middle caudals. In more derived megaraptorans, pleurocoels reach at least the proximal caudals, as in Orkoraptor (Novas et al., 2008), or spread up to the middle caudals, as in Aerosteon (Sereno et al., 2008), Megaraptor (Calvo et al., 2004), the isolated caudal of the Sengüer River and MPMA 08‐003‐94 (Méndez et al., 2012). This suggests that pleurocoels become a more important path of pneumatic invasion in the middle of the tail in more derived megaraptorans. However, until more CT scans of other megaraptorans come to light we can not corroborate this statement. Furthermore, the internal pneumaticity has already been observed in the middle caudals of megaraptorans but does not invade the vertebrae through pleurocoels.
There are three main ways of caudal pneumatization via the neural arches of Aoniraptor : along its proximal, proximo‐middle and middle caudal vertebrae.
First, pneumatic fossae below the transverse process, which is comprised of pocdf, cdf and prcdf housing pneumatic foramina, are observed in the proximal caudals of Aerosteon, Murusraptor, Megaraptor and Orkoraptor. However, in Orkoraptor the prcdf seems to be apneumatic (Novas et al., 2008). Second, there are pneumatic foramina in prespinal and postspinal fossae, which are observed in the proximal caudals of Murusraptor and Orkoraptor (only in the postspinal fossa in the latter). Third, there are pneumatic foramina in the dorsal tip of the neural spine, which has been observed in Murusraptor; in Megaraptor and Orkoraptor, however, this condition is uncertain because the neural spine is badly preserved. Australovenator shows at least one mid‐caudal with an apneumatic prespinal fossa (Poropat et al., 2019).
Following Benson et al. (2012) analysis, the pneumatization of the tail of Megaraptora fits the ‘neural arch‐first pattern’, as the pneumatization of the neural arch is much more widely distributed on the most basal members of the clade. In concordance, the pleurocoels of the centra are restricted to the proximal part of the tail in basal forms of the group (e.g. in Aoniraptor), extending toward the middle caudal elements in derived megaraptorids (e.g. Megaraptor; Aerosteon).
4. DISCUSSION
Benson et al. (2012) elaborated the most comprehensive analysis about evolution of pneumaticity within Theropoda; however, in the light of new discoveries and observations, we propose some modifications of this scheme (Figure 10). For example, there is no unambiguous evidence about the pneumatic condition of the tail of Torvosaurus (and consequently megalosaurids). For this reason, we consider the tail of megalosaurs as at least as dubious. In consequence, the pneumaticity of the tail outside Coelurosauria has been restricted, until now, to Carcharodontosauria. In the original scheme of Benson et al. (2012), megaraptorans are included as carcharodontosaurids following the hypothesis of Benson et al. (2010). However, we follow the hypothesis of Novas et al. (2013) that proposes Megaraptora as basal coelurosaurs. For this reason, caudal pneumatization of Carcharodontosauria is restricted to Giganotosaurus, Carcharodontosaurus and Acrocanthosaurus (Stromer, 1931; Britt, 1993).
FIGURE 10.
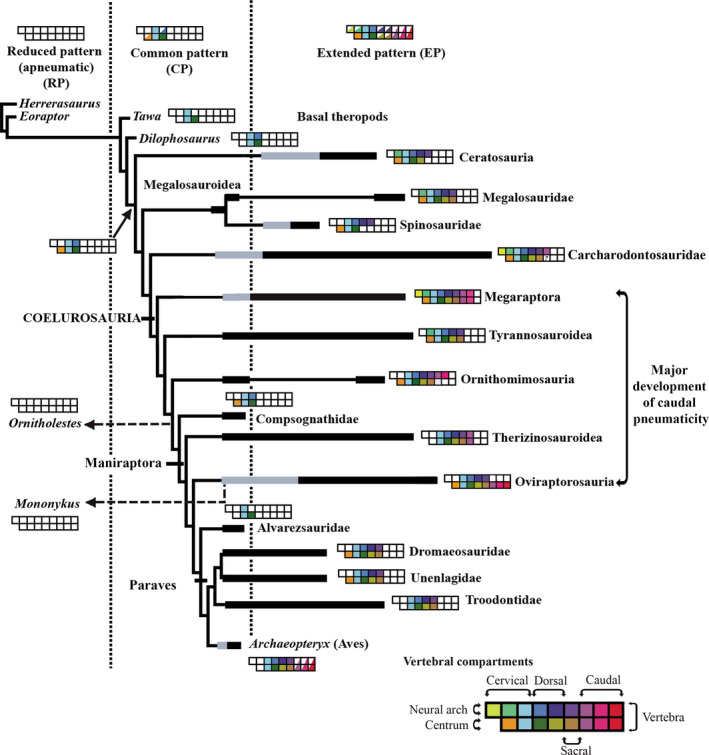
Modifications on Benson´s et al. (2012) scheme on evolution of theropod pneumaticity. Colored boxes represent the anatomical compartments occupied by pneumaticity. Half‐filled boxes indicate variable pneumatization in the clade within the common (CP) or extended (EP) pattern. Reduced pattern is considered apneumatic. Black bars indicate within‐clade range in number of pneumatic compartments, gray portions indicate ambiguously reconstructed states. See original work for more information [Colour figure can be viewed at wileyonlinelibrary.com]
Carcharodontosaurids show an apomorphic condition on their proximal caudals that presents round and big pleurocoels at the base of the neural spine. This condition is not observed in caudal vertebrae of Megaraptora (Calvo et al., 2004; Novas et al., 2008; Coria and Currie, 2016; Motta et al., 2016; Poropat et al., 2019). Also, Megaraptorans differ from Carcharodontosaurids in having pneumatic pre‐ and postspinal fossae, and three pneumatic fossae below the transverse processes (Novas et al., 2013). Moreover, pneumaticity in Megaraptora is more widespread along the tail. In carcharodontosaurids (e.g. Giganotosaurus) the pneumaticity does not pass beyond the fifth caudal. Further, is indicated above, in Megaraptora, both centra and neural arches are highly pneumatized, whereas in carcharodontosaurids, only the neural arch appears to shows pneumatization (Britt, 1993).
Within Coelurosauria (Figure 10) the pneumaticity of the tail is more widespread on basal forms but is reduced on basal paravians (Benson et al., 2012). Among basal coelurosaurs, Megaraptora and Oviraptorosauria show the most extensive caudal pneumaticity. Notwithstanding, Megaraptora differs from Ornithomimosauria because the latter has apneumatic centra (Watanabe et al., 2015) and from Oviraptorosauria because this group shows apneumatic neural arches (Clark et al., 2001; Xu et al., 2007; Balanoff and Norell, 2012). Therizinosauroids, share with megaraptorans the presence of pleurocoels in the lateral wall of centra and the presence of pneumatic fossae related to transverse processes (prcdf and pocdf) (Zhang et al., 2001; Zanno et al., 2009). However, in therizinosauroids the pneumaticity does not extend beyond the fourth caudal element (Zanno et al., 2009).
Interestingly, in non‐megaraptoran coelurosaur (Figure 10) pneumaticity is absent on basal forms of each clade. In this sense, in ornithomimosaurs, tail pneumaticity is observed in Archaeornithomimus and Deinocheirus (Watanabe et al., 2015), whereas basal members of the group (e.g. Shenzhousaurus) show apneumatic caudal vertebrae. Therizinosauroids Nothronychus, Erliansaurus and Neimongosaurus (Zhang et al., 2001; Zanno et al., 2009) show pneumatic tails, whereas the basal taxon Falcarius (Zanno, 2010b) is completely apneumatic. Further, within Oviraptorosauria, derived forms (e.g. Citipati, Khaan, Heyuannia, Gigantoraptor; Clark et al., 2001; Xu et al., 2009; Balanoff and Norell, 2012; Funston et al., 2018) show signs of pneumatization in the tail, which are absent in Similicaudipteryx and Caudipteryx (Zhou et al, 2000; He et al., 2008). Megaraptora shows a strong pneumatization of the tail in its basal forms (e.g. Aoniraptor); however, this statement could not be proved because there are no preserved caudal elements of its more basalmost forms (e.g. Phuwiangvenator, Fukuiraptor, SMNS 58023).
In Aoniraptor, pneumatic diverticula enter the vertebrae ‘through‐centrum’ in the sacrum and first caudal, and ‘through‐arch’ in the first and posteriormost caudals. In contrast, in more derived megaraptorans (e.g. Megaraptor or Aerosteon) the ‘through‐centrum’ assumes a major role because of the presence of middle caudals with well‐developed lateral pleurocoels. This pattern fits with the ‘neural‐arch first’ pattern of Benson et al. (2012).
Schwarz et al. (2019) described pneumatic features for the tail (and other parts of the skeleton) of Archaeopteryx. These authors mentioned the presence of pneumatic foramina and internal cavities within almost the entire tail. However, without a CT scan analysis it is not possible to corroborate whether such foramina are connected with the internal spaces; in this way we consider presence of tail pneumaticity ambiguous for Archaeopteryx (Figure 10).
4.1. Chevron pneumaticity in theropods
The present work reveals for the first time in theropods the invasion of the pneumatic system in chevrons (Figures 7 and 9). This feature has not been previously noted for other theropod dinosaurs (Benson et al., 2012), including theropod groups that exhibit extensive caudal pneumaticity, such as oviraptorosaurs and ornithomimosaurs (Benson et al., 2012; Persons et al., 2013, 2015; Watanabe et al., 2015).
Regarding megaraptorans, the pneumatic character of the chevrons was mentioned in Motta et al. (2016) but is unambiguously confirmed here. However, personal observations of one of the authors (A.M.A.R.) in some chevrons of Megaraptor namunhuaiquii (Calvo et al., 2004) reveal that this taxon shows similar openings. Notwithstanding, Brusatte et al. (2012) describes and portrays chevrons of Alioramus altai with similar openings to those of Aoniraptor. Nevertheless, without more exhaustive analyses the presence of pneumatic traits in chevrons of Megaraptor and Alioramus should be considered ambiguous.
However, in Aoniraptor this trait was confirmed by both CT scan and histologic analyses, which suggest that the abdominal air‐sac has a more complex structure that the previously thought. In this sense, in Aoniraptor the pneumatic diverticulae invade the caudal vertebrae through at least three single (postspinal and prespinal fossa and those of the chevrons) and two paired openings (PRCDF and POCDF). This suggests that the air‐sacs surround the vertebrae from all sides and pass through large muscles (e.g. CFL). This differs with those noted by O´Connor (2006) for some extant birds, where the abdominal air‐sacs do not reach the dorsal part of the tail. Further, in Aoniraptor the abdominal air‐sacs extend approximately 2 m forwards, whereas in derived megaraptorans (e.g. Aerosteon) such air‐sacs could reach 3–4 m in length. This clearly contrasts with the massive tails (completely composed of bone and muscles) of previous analyses (Persons and Currie, 2011a, 2011b) and reveals a more complex scenario in the internal structure for the tails of theropods.
4.2. Pneumaticity as a taxonomic tool: distinctiveness of Gualicho and Aoniraptor
The Violante Farm Locality houses a large variety of carnivorous theropods such as the mid‐sized abelisaurid Tralkasaurus (Cerroni et al., 2020), large‐sized carcharodontosaurid Taurovenator (Motta et al., 2016), mid‐sized megaraptoran Aoniraptor (Motta et al., 2016) and a small‐sized paravian (Motta et al., 2020), as well as the enigmatic Gualicho (Apesteguía et al., 2016). In the original description, Apesteguía and collaborators placed Gualicho at the base of Coelurosauria, very close to Megaraptora. Because Gualicho and Aoniraptor were considered to have similar phylogenetic positions (Motta et al., 2016) and because both come from the same fossiliferous locality, comparison between both taxa would appear mandatory.
The only overlapping material between Gualicho and Aoniraptor consists of caudal vertebrae (Figure 11). Despite both taxa showing superficially similar morphology, a detailed look indicates important differences between both taxa.
FIGURE 11.

Anatomical comparison between the proximo‐medial and mid‐caudals of Aoniraptor (a and c) and Gualicho (b and d). (a) Posterior (left) and lateral (right) views of a proximo‐medial caudal of Aoniraptor. (b) Posterior (left) and lateral (right) views of a proximo‐medial caudal of Gualicho. (b′) Close‐up showing the reduced transverse process. (b″) Close‐up showing the horizontal shelf between postzygapophyses. (c) Anterior (left) and lateral (right) views of a mid‐caudal of Aoniraptor. (d) Anterior (left) and lateral (right) views of two mid‐caudals of Gualicho. POCDF, postzygapophyseal‐centrodiapophyseal fossa. Poz, postzygapophyses. Prz, prezygapophyses. Not to scale [Colour figure can be viewed at wileyonlinelibrary.com]
All the preserved caudals of Gualicho show broken parts, such as external surfaces, pre‐ and postzygapophyses and one of the elements is even cut in half, which reveals that the internal structure of the vertebrae of Gualicho is apneumatic. Upon closer observation, and extensive search and mechanical preparation of some specific parts of the holotype of Gualicho, we observed that this theropod does not exhibit the pneumatic traits found in Aoniraptor or any other theropod, such as lateral pleurocoels in the centrum, pneumatic fossae related to the transverse process or the base of the neural spine, or pneumatic foramina within the pre‐ and postspinal fossae (Figure 11b,c).
Aoniraptor shows caudal centra with an articular surface in contour, being dorsoventrally taller that transversely wide (vs. the subcircular‐shaped articular surface in Gualicho; Figure 11b); not flared articular surfaces, resulting in flat lateral surfaces of centrum (vs. flared articular surfaces with notably longitudinally concave surfaces of centrum in Gualicho; Figure 11b); strongly transversely convex ventral surface of centrum (vs. flat in Gualicho), and absence of transversely sub‐horizontal shelf connecting both postzygapophyses (vs. present and prominent in Gualicho; Figure 11b″).
Further, the transverse processes of Gualicho are represented by a faint and short ridge located at the dorsal third of the vertebral centrum (Figure 11b′). Aoniraptor preserves 12 neural arches, and even at the more posterior ones the processes are placed within the neural arch (Figure 11a,c). The prezygapophyses of the mid‐caudals in Gualicho (Figure 11b,d) are notably longer and more horizontally projected than those mid‐caudals of Aoniraptor (Figure 11a,c), which are short and strongly dorsally oriented. The articular surfaces of the prezygapophyses are also much longer in Gualicho than in Aoniraptor. The postzygapophyses of mid‐caudals in Gualicho (Figure 11b,c) are well‐developed, projecting posteroventrally from the body of the neural arch. In contrast, in Aoniraptor (Figure 11a–c) the postzygapophyses of mid‐caudals are notably reduced to a very small articular surface located in the posterior corner of the neural arch. In Gualicho the prespinal fossae are shallow, anteroposteriorly short but transversally wide, which results in a sub‐triangular‐shaped fossa. In contrast, the prespinal fossae of Aoniraptor are well‐developed, deep, notably longer than wide and sub‐quadrangular in contour. Postspinal fossae of Gualicho (Figure 11b,c) are almost absent, in contrast to the well‐developed and deep ones of Aoniraptor (Figure 11a–c).
Personal inspection on the holotype specimen of Gualicho (MPCN PV 0001.) reveals that the pleurocoels of dorsal vertebrae represent half of the total length of the centrum and are not surrounded by a pleurofossa; they enter the vertebrae by a wide canal. This combination of characters is absent in Megaraptora (Novas et al., 2013; present study). Furthermore, the dorsal vertebrae of Gualicho exhibit a camerate internal structure (Aranciaga Rolando, pers. obs.), which contrasts with the camellated condition present in megaraptorans and several tetanuran theropods (Benson et al., 2012).
This evidence indicates that Aoniraptor and Gualicho are not the same taxa, and even suggests that both taxa may not be phylogenetically related.
5. CONCLUSIONS
We provide the most complete description of pneumatic traits of a vertebral region within Theropoda and the first comprehensive analysis for Megaraptora. Further, comparisons with other theropods result in a modification of Benson et al. (2012) scheme where the caudal pneumaticity is almost uniquely restricted to Coelurosauria, with the exception of some species of Carcharodontosauridae and one dubious case of megalosaurid. This shows that basal megaraptorans show strong pneumatic traits and pneumatic characters become more evident and extend posteriorly in the tail of derived megaraptorids.
In agreement with previous work (Lambertz et al., 2018; Aureliano et al., 2019), histological evidence shows that the intimate relationship between air‐sac diverticula and skeleton is visible throughout pneumosteum tissue. This is associated with secondary trabecular and endosteal bone. Interestingly, the pneumosteum is observed in the sub‐vertical canal and central chamber of the vertebrae and the main cavity of the chevron, corroborating the pneumatic character of these structures.
This study represents the first evidence of pneumosteal tissue within non‐avian theropods and corroborates with histology and CT scanning the presence of pneumatic chevrons within Megaraptora, a unique feature of the clade.
AUTHOR CONTRIBUTIONS
A.M.A.R. was involved in the scanning, image processing, writing of the manuscript and configuration of the images. J.G.M. made the histologic samples, analyzed the histologic sections and wrote the histologic part of the manuscript. F.E.N revised the manuscript and gave advice throughout the investigation.
DATA AVAILABILITY STATEMENT
Data available in article supplementary material.
Supporting information
Video S1
Video S2
Video S3
Video S4
Acknowledgements
We thank Dr Ignacio Poyo and all the technicians and medics from the TCba Salguero Diagnostic Center (Buenos Aires, Argentina), who kindly allowed us to do the scanning. We thank Victoria Sanchez and Mirtha González for their assistance with the petrographic microscope. Also, we thank M. Motta, M. Cerroni, A. Gentil, S. Rozadilla, F. Brissón Egli, and N. Chimento (Museo Argentino de Ciencias Naturales) for comments and discussion on the anatomy of Aoniraptor as well as occasional help with the scans and manuscript. We especially thank Dr. F. Agnolin for comments and suggestions about the manuscript. We are grateful to the editor of Journal of Anatomy, Juan I. Canale and an anonymous reviewer for useful comments about the manuscript.
Aranciaga Rolando M, Garcia Marsà J, Novas F. Histology and pneumaticity of Aoniraptor libertatem (Dinosauria, Theropoda), an enigmatic mid‐sized megaraptoran from Patagonia. J. Anat. 2020;237:741–756. 10.1111/joa.13225
References
- Apesteguía, S. , Smith, N.D. , Juárez‐Valieri, R. and Makovicky, P.J. (2016) An unusual new theropod with a didactyl manus from the upper cretaceous of Patagonia, Argentina. PLoS ONE, 11(7), e0157793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranciaga‐Rolando, A.M. , Novas, F.E. and Agnolín, F.L. (2019) A reanalysis of Murusraptor barrosaensis Coria & Currie (2016) affords new evidence about the phylogenetical relationships of Megaraptora. Cretaceous Research, 99, 104–127. [Google Scholar]
- Aureliano, T. , Ghilardi, A.M. , Silva‐Junior, J.C.G. , Martinelli, A.G. , Ribeiro, L.C.B. , Marinho, T. et al. (2019) Influence of taphonomy on histological evidence for vertebral pneumaticity in an upper cretaceous titanosaur from South America. Cretaceous Research, 108, 104337. [Google Scholar]
- Azuma, Y. and Currie, P.J. (2000) A new carnosaur (Dinosauria: Theropoda) from the lower cretaceous of Japan. Canadian Journal of Earth Sciences, 37, 1735–1753. [Google Scholar]
- Balanoff, A.M. and Norell, M.A. (2012) Osteology of Khaan mckennai (Oviraptorosauria: Theropoda). Bulletin of the American Museum of Natural History, 372, 1–77. [Google Scholar]
- Benson, R.B.J. , Carrano, M.T. and Brusatte, S.L. (2010) A new clade of archaic large bodied predatory dinosaurs (Theropoda: Allosauroidea) that survived to the latest Mesozoic. Naturwissenschaften, 97, 71–78. [DOI] [PubMed] [Google Scholar]
- Benson, R.B.J. , Butler, R.J. , Carrano, M.T. and O’Connor, P.M. (2012) Air‐filled postcranial bones in theropod dinosaurs: physiological implications and the ‘reptile’‐bird transition. Biological Reviews, 87, 168–193. [DOI] [PubMed] [Google Scholar]
- Bremer, J.L. (1940) The pneumatization of the humerus in common fowl and the associated activity of theelin. Anatomical Record, 77, 197–211. [Google Scholar]
- Britt, B.B. (1991) Theropods of Dry Mesa Quarry (Morrison Formation, Late Jurassic), Colorado, with emphasis on the osteology of Torvosaurus tanneri. Brigham Young University Geology Studies, 37, 1–72. [Google Scholar]
- Britt, B.B. (1993) Pneumatic postcranial bones in dinosaurs and other archosaurs. Ph.D. Thesis, University of Calgary. [Google Scholar]
- Brusatte, S.L. , Carr, T.D. and Norell, M.A. (2012) The osteology of Alioramus, a gracile and long‐snouted tyrannosaurid (Dinosauria: Theropoda) from the Late Cretaceous of Mongolia. Bulletin of the American Museum of Natural History, 366, 1–197. [Google Scholar]
- Butler, R.J. , Barrett, P.M. and Gower, D.J. (2009) Postcranial skeletal pneumaticity and air‐sacs in the earliest pterosaurs. Biology Letters, 5, 557–5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo, J.O. , Porfiri, J.D. , Veralli, C. , Novas, F. and Poblete, F. (2004) Phylogenetic status of Megaraptor namunhuaiquii Novas based on a new specimen from Neuquén, Patagonia, Argentina. Ameghiniana, 41, 565–575. [Google Scholar]
- Cerroni, M.A. , Motta, M.J. , Agnolín, F.L. , Rolando, A.A. , Egli, F.B. and Novas, F.E. (2020) A new abelisaurid from the Huincul Formation (Cenomanian‐Turonian; Upper Cretaceous) of Río Negro province, Argentina. Journal of South American Earth Sciences, 98, 102–445. [Google Scholar]
- Chinsamy, A. and Raath, M.A. (1992) Preparation of fossil bone for histological examination. Palaeontology Africana, 29, 39–44. [Google Scholar]
- Claessens, L.P.A.M. , O’Connor, P.M. and Unwin, D.M. (2009) Respiratory evolution facilitated the origin of pterosaur flight and aerial gigantism. PLoS ONE, 4, e4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, J.M. , Norell, M.A. and Barsbold, R. (2001) Two new oviraptorids (Theropoda: Oviraptorosauria), Upper Cretaceous Djadokhta Formation, Ukhaa Tolgod, Mongolia. Journal of Vertebrate Paleontology, 21(2), 209–213. [Google Scholar]
- Coria, R.A. and Currie, P.J. (2016) A new megaraptoran dinosaur (Dinosauria, Theropoda, Megaraptoridae) from the late Cretaceous of Patagonia. PLoS ONE, 11(7), e0157973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currey, J.D. and Alexander, R.M. (1985) The thickness of the walls of tubular bones. Journal of Zoology, 206, 453–468. [Google Scholar]
- Da Mommio, A. (2018) ALEX STREKEISEN: Serpentine. Alex Strekeisen. Available at: http://www.alexstrekeisen.it/english/meta/serpentine.php [Accessed 28 June 2019]. [Google Scholar]
- Duncker, H.‐R. (1971) The lung air sac system of birds. Advances in Anatomy, Embryology and Cell Biology, 45(6), 1–171. [Google Scholar]
- Farmer, C.G. (2006) On the origin of avian air sacs. Respiratory Physiology and Neurobiology, 154, 89–106. [DOI] [PubMed] [Google Scholar]
- Francillon‐Vieillot, H. , De Buffrénil, V. , Castanet, J.D. , Géraudie, J. , Meunier, F.J., Sire, J.Y. et al (1990) Microstructure and mineralization of vertebrate skeletal tissues. Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends, 1, 471–530. [Google Scholar]
- Funston, G.F. , Mendonca, S.E. , Currie, P.J. and Barsbold, R. (2018) Oviraptorosaur anatomy, diversity and ecology in the Nemegt Basin. Palaeogeography, Palaeoclimatology, Palaeoecology, 494, 101–120. [Google Scholar]
- He, T. , Wang, X.L. and Zhou, Z.H. (2008) A new genus and species of caudipterid dinosaur from the Lower Cretaceous Jiufotang Formation of western Liaoning, China. Vertebrata PaleAsiatica, 46(3), 178–189. [Google Scholar]
- von Huene, F.R.F. (1929) Los saurisquios y ornitisquios del Cretácico argentino. Anales Museo de La Plata, 3(2), 194. [Google Scholar]
- Hunter, J. (1774) An account of certain receptacles of air, in birds, which communicate with the lungs, and are lodged both among the fleshy parts and in the hollow bones of those animals. Philosophical Transactions of the Royal Society of London, 64, 205–213. [Google Scholar]
- Lambertz, M. , Bertozzo, F. and Sander, P.M. (2018) Bone histological correlates for air sacs and their implications for understanding the origin of the dinosaurian respiratory system. Biology Letters, 14(1), 20170514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y.‐N. , Barsbold, R. and Currie, P.J. (2014) Resolving the long‐standing enigmas of a giant ornithomimosaur Deinocheirus mirificus . Nature, 515, 257–260. [DOI] [PubMed] [Google Scholar]
- Makovicky, P.J. and Zanno, L.E. (2011) Theropod diversity and the refinement of avian characteristics. Living Dinosaurs: the Evolutionary History of Modern Birds, 9–29. [Google Scholar]
- Martin, E.G. and Palmer, C. (2014) Air space proportion in pterosaur limb bones using computed tomography and its implications for previous estimates of pneumaticity. PLoS ONE, 9(95), e97159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli, A.G. , Ribeiro, L.C.B. and Neto, F.M. (2013) Insight on the theropod fauna from the Uberaba Formation (Bauru Group), Minas Gerais State: new megaraptoran specimen from the Late Cretaceous of Brazil. Rivista Italiana di Paleontologia e Stratigrafia, 119(2), 205–214. [Google Scholar]
- Méndez, A.H. , Novas, F.E. and Iori, F.V. (2012) First record of Megaraptora (Theropoda, Neovenatoridae) from Brazil. Comptes Rendus Palevol, 11, 251–256. [Google Scholar]
- Motta, M.J. , Aranciaga‐Rolando, A.M. , Rozadilla, S. , Agnolín, F.E. , Chimento, N.R. , Egli, F.B. et al (2016) New theropod fauna from the Upper Cretaceous (Huincul Formation) of northwestern Patagonia, Argentina. New Mexico Museum of Natural History and Science Bulletin, 7, 231–253. [Google Scholar]
- Motta, M.J. , Agnolín, F.L. and Brissón‐Eglí, F. (2020) New theropod dinosaur from the Upper Cretaceous of Patagonia sheds light on the paravian radiation in Gondwana. The Science of Nature. In press. [DOI] [PubMed] [Google Scholar]
- Novas, F.E. , Ezcurra, M.D. and Lecuona, A. (2008) Orkoraptor burkei nov.gen. et sp., a large theropod from the Maastrichtian Pari Aike Formation, Southern Patagonia, Argentina. Cretaceous Research, 29, 468–480. [Google Scholar]
- Novas, F.E. , Agnolin, F.L. and Ezcurra, M.D. (2013) Evolution of the carnivorous dinosaurs during the Cretaceous: The evidence from Patagonia. Cretaceous Research, 45, 174–215. [Google Scholar]
- O´Connor, P.M. (2006) Postcranial pneumaticity: An evaluation of Soft‐Tissue influences on the postcranial skeleton and the reconstruction of pulmonary anatomy in archosaurs. Journal of morphology, 267, 1199–1226. [DOI] [PubMed] [Google Scholar]
- O’Connor, P.M. (2007) The postcranial axial skeleton of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the late Cretaceous of Madagascar. Journal of Vertebrate Paleontology, 27(suppl 2), 127–162. [Google Scholar]
- O’Connor, P.M. and Claessens, L.P.A.M. (2006) Basic avian pulmonary design and flow‐through ventilation in non‐avian theropod dinosaurs. Nature, 436, 253–256. [DOI] [PubMed] [Google Scholar]
- Persons, W.S. and Currie, P.J. (2011a) Dinosaur speed demon: The caudal musculature of Carnotaurus sastrei and implications for the evolution of South American abelisaurids. PloS one, 6(10), e25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persons, W.S. and Currie, P.J. (2011b). The tail of Tyrannosaurus: Reassessing the size and locomotive importance of the M. caudofemoralis in non‐avian theropods. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology, 294(1), 119–131. [DOI] [PubMed] [Google Scholar]
- Persons, W.S. , Currie, P.J. and Norell, M.A. (2013) Oviraptorosaur tail forms and functions. Acta Palaeontologica Polonica, 59(3), 553–567. [Google Scholar]
- Persons, W.S. , Funston, G.F. , Currie, P.J. and Norell, M.A. (2015) A possible instance of sexual dimorphism in the tails of two oviraptorosaur dinosaurs. Scientific Reports, 5, 9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann, H. and Sander, M. (2013) Histological evidence for muscle insertion in extant amniote femora: implications for muscle reconstruction in fossils. Journal of Anatomy, 222(4), 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porfiri, J.D. , Novas, F.E. , Calvo, J.O. , Agnolín, F.L. , Ezcurra, M.D. and Cerda, I.A. (2014) Juvenile specimen of Megaraptor (Dinosauria, Theropoda) sheds light about tyrannosauroid radiation. Cretaceous Research, 51, 35–55. [Google Scholar]
- Porfiri, J.D. , Juárez‐Valieri, R.D. , Santos, D.D.D. and Lamanna, M.C. (2018) A new megaraptoran theropod dinosaur from the Upper Cretaceous Bajo de la Carpa Formation of northwestern Patagonia. Cretaceous Research, 89, 302–319. [Google Scholar]
- Poropat, S.F. , White, M.A. , Vickers‐Rich, P. and Rich, T.H. (2019) New megaraptorid (Dinosauria: Theropoda) remains from the Lower Cretaceous Eumeralla Formation of Cape Otway, Victoria, Australia. Journal of Vertebrate Paleontology, 39(4), e1666273. [Google Scholar]
- de Ricqlès, A. (1991) Comparative microstructure of bone. In: Bone Matrix and Bone Specific Products, 1–78. [Google Scholar]
- Ruben, J.A. , Jones, T.D. and Geist, N.R. (2003) Respiratory and reproductive paleophysiology of dinosaurs and early birds. Physiological and Biochemical Zoology, 76, 141–164. [DOI] [PubMed] [Google Scholar]
- Samathi, A. , Chanthasit, P. and Sander, P.M. (2019) Two new basal coelurosaurian theropod dinosaurs from the Lower Cretaceous Sao Khua Formation of Thailand. Acta Palaeontologica Polonica, 64(2), 239–260. [Google Scholar]
- Sereno, P.C. , Martínez, R.N. , Wilson, J.A. , Varricchio, D.J. , Alcober, O.A. and Larsson, H.C.E. (2008) Evidence for avian intrathoracic air sacs in a new predatory dinosaur from Argentina. PLoS ONE, 3, e3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid, P. (1982) A model for comparing for comparing gas‐exchange systems in vertebrates In: Taylor C.R., Johansen K. and Bolis L. (Eds.) A Model for Comparing Gas‐Exchange Systems in Vertebrates: A Companion to Animal Physiology. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Schwarz, D. , Kundrát, M. , Tischlinger, H. , Dyke, G. and Carney, R.M. (2019) Ultraviolet light illuminates the avian nature of the Berlin Archaeopteryx skeleton. Scientific Reports, 9(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromer, E. (1931) Ergebnisse der Forschungsrisen Prof. E. Stromers in den Wüsten Ägyptens. II. Wirbeltierreste der Baharje‐Stufe (unterstes Cenoman). 10. Ein Skelett‐Rest von Carcharodontosaurus no. gen. Abhandlungen der Bayerischen Akademie der Wissenschaften, Mathematisch‐Naturwissenschaftliche Abteilung, Neue Folge, 9, 1–23. [Google Scholar]
- Watanabe, A. , Eugenia Leone Gold, M. , Brusatte, S.L. , Benson, R.B.J. , Choiniere, J. , Davidson, A. et al (2015) Vertebral pneumaticity in the ornithomimosaur Archaeornithomimus (Dinosauria: Theropoda) Revealed by computed tomography imaging and reappraisal of axial pneumaticity in Ornithomimosauria. PLoS ONE, 10(12), e0145168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedel, M.J. (2003) Vertebral pneumaticity, air sacs, and the physiology of sauropod dinosaurs. Paleobiology, 29, 243–255. [Google Scholar]
- Wedel, M. (2006) Origin of postcranial skeletal pneumaticity in dinosaurs. Integrative Zoology, 2, 80–85. [DOI] [PubMed] [Google Scholar]
- Wedel, M.J. , Cifelli, R.L. and Sanders, R.K. (2000) Osteology, paleobiology, and relationships of the sauropod dinosaur Sauroposeidon . Acta Palaeontologica Polonica, 45, 343–388. [Google Scholar]
- Welty, J.C. (1982) The Life of Birds. Philadelphia, PA: Saunders College Publishing. [Google Scholar]
- Wilson, J.A. (2012) New vertebral laminae and patterns of serial variation in vertebral laminae of sauropod dinosaurs. Contributions from the Museum of Paleontology, University of Michigan, 32, 91–110. [Google Scholar]
- Wilson, J.A. , D’Emic, M.D. , Ikejiri, T. , Moacdieh, E.M. and Whitlock, J.A. (2011) A nomenclature for vertebral fossae in sauropods and other saurischian dinosaurs. PLoS ONE, 6(2), e17114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, X. , Clark, J.M. , Mo, J. , Choiniere, J. , Forster, C.A. , Erickson, G.M. et al (2009) A Jurassic ceratosaur from China helps clarify avian digital homologies. Nature, 459, 940–944. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Tan, Q. , Wang, J. , Zhao, X. and Tan, L. (2007) A gigantic bird‐like dinosaur from the Late Cretaceous of China. Nature, 447(7146), 844–847. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Zhou, Z. , Dudley, R. , Mackem, S. , Chuong, C.M. , Erickson, G.M. et al (2014) An integrative approach to understanding bird origins. Science, 346, 1253293. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Xu, X. , Zhao, X. , Sereno, P. , Kuang, X. and Tan, L. (2001) A long‐necked therizinosauroid dinosaur from the Upper Cretaceous iron Dabasu Formation of Nei Mongol, People’s Republic of China. Vertebrata PalAsiatica, 39(4), 282–290. [Google Scholar]
- Zanno, L.E. (2010a) A taxonomic and phylogenetic re‐evaluation of Therizinosauria (Dinosauria: Maniraptora). Journal of Systematic Palaeontology, 8(4), 503–543. [Google Scholar]
- Zanno, L.E. (2010b) Osteology of Falcarius utahensis (Dinosauria: Theropoda): characterizing the anatomy of basal therizinosaurs. Zoological Journal of the Linnean Society, 158(1), 196–230. [Google Scholar]
- Zanno, L.E. , Gillette, D.D. , Albright, L.B. and Titus, A.L. (2009) A new North American therizinosaurid and the role of herbivory in ‘predatory’ dinosaur evolution. Proceedings of the Royal Society B: Biological Sciences, 276(1672), 3505–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Z.H. , Wang, X.L. , Zhang, F.C. and Xu, X. (2000) Important features of Caudipteryx evidence from two nearly complete new specimens. Vertebrata PalAsiatica, 38(4), 243–265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1
Video S2
Video S3
Video S4
Data Availability Statement
Data available in article supplementary material.


