Abstract
Aims
Adequate medication management is a key condition to ensuring effective pharmacotherapy. However, it is well acknowledged that older people may encounter difficulties self‐administering medicines in a correct manner.
Methods
A mixed method pilot study was performed to investigate medication self‐management in older and multimorbid patients with polypharmacy. The pilot study involved medication management tasks followed by semi‐structured interviews in 20 patients. The tasks and interviews were based on the patients' individual medication plans, which had been prepared earlier by the pharmacy for each patient on basis of all their prescriptions.
Results
The patients' self‐reported medication management skills differed from their actual observed medication management performance. In addition, the routines and coping strategies used by the patients to deal with the complexity of their overall medication regimen were not in accordance with the medication plan and the instructions for use on the product labels. Issues were observed on all stages of the medication process that can be considered relevant to patient adherence, especially medication plan recall, product identification, product selection, product handling and product recognition in a multicompartment compliance aid.
Conclusions
The pilot study suggested that medication management issues by older and multimorbid patients remain widely undetermined and unrecognized in primary care. Further investigation and interdisciplinary collaboration will be required to resolve the user problems and ensure adequate patient adherence.
Keywords: age 65 and over, medication errors, medication issues, medication management, older adults, therapeutic complexity
What is already known about this subject
Polypharmacy is associated with an increase in medication issues e.g. nonadherence.
Problems of handling pharmaceutical packaging by older and multimorbid patients have been reported.
Increasing therapeutic complexity might transcend patients' capacity to manage medication.
What this study adds
Evidence on the complexity of medication self‐management.
Patient problems occurring at various steps of medication management.
Insights into problem root causes and patient behaviour to deal with these problems.
1. INTRODUCTION
The increasing global wealth and effective healthcare provision continues to contribute to progressing longevity and hence a growth of the number of old and very old people in our society. While increasing chronological age correlates with the occurrence of chronic diseases and multimorbidity, the aging trajectories differ between individuals due to their genetic, societal and life‐style factors.1, 2, 3 Moreover, there is a direct relationship between multimorbidity, functional impairments, the prevalence of disabilities and polypharmacy.4, 5
Declining functional abilities and increasing disabilities affect the activities of daily living of patients, whereby specific multimorbidity patterns may lead to a specific set of impairments6 and acute hospitalization may accelerate the functional decline.7 However, home dwelling polypharmacy patients have to self‐prepare and administer medicinal products according to a defined schedule whilst respecting defined instructions for use. This requires a certain degree of cognitive, sensory and functional capabilities.8 There is evidence that the increasing workload and demand encompasses the capacity of the patients to deal with the complexity of the requirements.9, 10, 11 Problems that patients encounter when using medications have recently being reviewed revealing physical, sensory and/or cognitive limitations in handling medicines or using the devices, in reading and understanding the labels,12, 13 and in administering each medicinal product correctly14 at the intended time as important factors.15 All these problems contribute to medication errors, which are the third leading cause of death in the USA.16, 17, 18 The multicompartment compliance aid (MCA) is considered as a medication management tool to ease medication use19 as they are reusable plastic devices consisting of 3–5 different chambers in which the daily medication doses can be filled according to the dosing time (e.g. morning, midday, evening, night).
The filling of an MCA according to the user (dosing and where appropriate storage) instructions of each medicinal product is a process involving the recognition of the products, the retrieval of the information on the instructions for use, the removal of the product from its packaging, the identification of the product and the allocation into the right MCA compartment. The objective of this pilot study was to compare the subjective views of older and multimorbid patients on their individual medication management problems and performance with the way they actually handled their medication upon expert observation.
2. METHODS
2.1. Study design
This pilot study used a mixed method design using a practical preparation task and a semi structured interview methodology to investigate the medication self‐management in older patients with polypharmacy. The study was approved by the ethical committee of the Charité Medical University of Berlin, Germany.
2.2. Intervention
Older patients were asked to prepare their personal daily medication into their own or other familiar MCA. The medicinal products were provided in their original secondary packaging (carton boxes) and their primary packaging, which were either blisters, vials or glass bottles fulfilling the regulatory requirements for child resistance. All products were placed in front of the patients on a table randomly including a copy of their individual medication plan. Each plan was made by a pharmacy based on all the prescriptions for a patient and each plan had been discussed with the relevant patient before the study. After the filling of the MCA compartments according to the medication plan, the patients were interviewed using a semi‐structured interview capturing six domains of medication use: (i) medication history and biography; (ii) attitude and behaviour towards the drug therapy; (iii) experience with own medication and medication management; (iv) handling and usage; (v) perception of compliance and adherence; and (vi) perceived issues or preferences, wishes or any other feedback on their therapy. The study design is summarized in Figure 1.
Figure 1.

Study design and data analysis. MMSE, mini mental state examination
2.3. Pilot study population
Twenty patient volunteers were randomly recruited from the day care unit and hospital of the Evangelisches Geriatriezentrum Berlin, the geriatric centre of the Charité Medical University of Berlin. The patients included were aged ≥65 years, lived independently, did not receive professional support in medication management and administration, were under polypharmacy defined as regular use of ≥5 drug products and had a mini mental state examination (MMSE) score >23. The patients were recruited after an incidence of hospitalization when they were in transitional care back into their independent living environment. None of the patient was foreseen to be transferred to institutional care, supervised drug therapy by a caregiver or legal guardianship or showed cognitive limitation or depressive disorders, acute disease state with psychological and physical stress or acute injuries or disabilities of upper limb functions.
2.4. Data collection
The individual patient data were collected from the medical records of the University clinics included age, sex, insurance status, care support, diagnosed diseases, prescribed medications and MMSE. Additional geriatric assessments performed included Barthels Index,20 the maximum isometric grip strength in both hands (Smedley Dynamometr, Scandidact, Denmark), timed test of money counting according to Nikolaus,21 MMSE and a patient perceived adherence interview.22
2.5. Data analysis
The patient data and characteristics were collected in the patient survey form. The preparation of the daily medications and semi‐structured interviews were videotaped. A coding system for thematic task identification was developed to analyse the videos using MAXQDA software. The video sequences of the individual task performance were comparatively evaluated to qualify themes of common issues during the medication preparation process. For the comparison, the chronological sequence of tasks for the medication preparation process were decomposed into 7 major preparation steps as shown in Figure 2.
Figure 2.
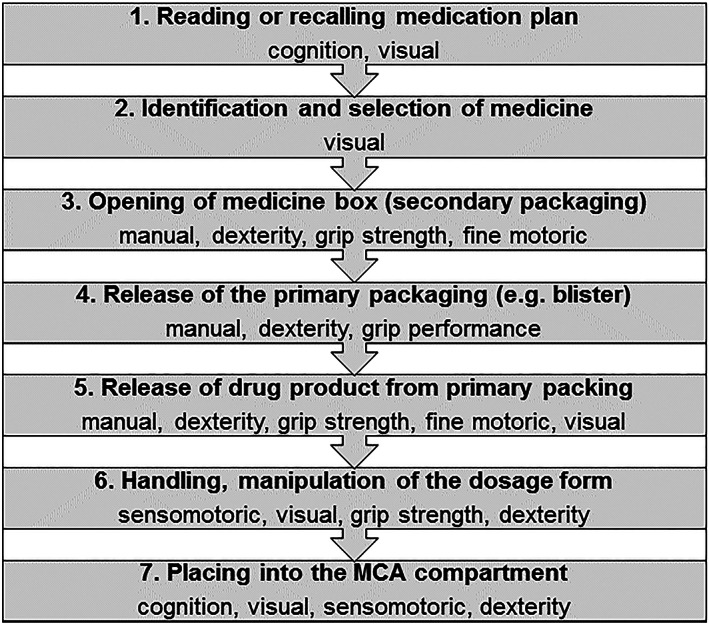
Task decomposition and chronological sequence of the medication preparation process and the major human capabilities required. MCA, multicompartment compliance aid
The subjective perception of patient performance and issues with the preparation of the medication were captured in the semi‐structured interviews and compared to the objective performance of the relevant item. The videos (e.g. task sequence and fulfilment) and interviews (e.g. narrative expression and body language) were analysed by A.S.C. (PhD student) and in case of doubt in consultation with S.S.T. (pharmacist) and R.E.F. (medical doctor) until no further themes of medication preparation issues could be identified. The identified issues were further analysed by A.S.C. and in case of doubt in consultation with S.S.T. and R.E.C., regarding patient and product related contributing factors as a source for the issues observed (Figure 2).
3. RESULTS
3.1. Study population
For the pilot study, 20 patients were recruited, of whom 8 (40%) were male and 12 (60%) female. The average age was 78 years with a minimum of 71 and a maximum of 88 years. The patients were prescribed to 5 to 20 different medications with an average of 9 medications per day. The patients had a least 2 different dosing moments per day whereby the average number was 3.5 dosing moments. The patients received chronic treatments on average for about 19 years ranging from 4 years to a maximum of 65 years. The average Barthel Index was 69 with a range of 50–100. The timed test of money counting required 23–124 seconds, with an average performance of 47 seconds. The handgrip strength was higher for males then for females as well as for the right hand compared to the left hand. The patients rated their medication adherence as very high. Only 5 (25%) confirmed that they sometimes forget to take their medicines and only 3 (15%) to have problems in remembering to take them. The characteristics of the recruited patients for this pilot study are summarized in Table 1.
Table 1.
Characteristics and geriatric assessment of the study population included in the pilot study (MMSE, mini mental state examination)
| Study population (n = 20) | ||
|---|---|---|
| Sex (male) | 8 | |
| Age, average (range) (y) | 78 (71–88) | |
| Drug therapy, average number (range) | ||
| Drug products average number | 9 (5–20) | |
| Dosing moments per day | 3.5 (2–4) | |
| Drug therapy experience | 18.7 (4–65) | |
| Barthel index, average number (range) | 69 (50–100) | |
| Timed test of money counting, average (range) (s) | 47.42 (23–124) | |
| Hand‐grip strength (kg; n = 19) | Right | Left |
| Male (n = 8) | 36 (24–51) | 28 (21–43) |
| Female (n = 11) | 20 (12–28) | 16 (10–26) |
| All | 27 (n‐n) | 21 (n‐n) |
| MMSE | ≥ 23 | |
| Perceived adherence performance | Yes (n = 20) | |
| 1. Sometimes forget to take my medicines? | 5 | |
| 2. Sometimes I don't remember to take my medicine | 3 | |
| 3. Tend to stop my medication when I when I fell good | 0 | |
| 4. Tend to stop my medication when I do not feel good | 0 | |
A total of 71 different drugs were prescribed to the patients, of which 10 accounted for about 50% of the prescriptions (Table 2). The prescribed drugs were all orally administered and included solid (e.g. tablets, capsules) and liquid (e.g. solutions, drops) dosage forms.
Table 2.
The 10 most prescribed medicines and patients receiving these medicines (n = 20)
| Medicine | Patients (%) |
|---|---|
| Metamizole | 13 (65) |
| Pantoprazole | 13 (65) |
| Acetylsalicylic acid | 10 (50) |
| Simvastatin | 10 (50) |
| Ramipril | 9 (45) |
| Vitamin D3 | 9 (45) |
| Metoprolol | 8 (40) |
| Torasemide | 8 (40) |
| Atorvastatin | 5 (25) |
| L‐tyroxine | 4 (20) |
3.2. Preparation of a daily medication schedule
3.2.1. General observations
All patients self‐express a high degree of respect for the medicinal products (e.g. express the risk for harm of medicines, referred to the physician to decide) and they were convinced to use them according to the physicians' instructions. However, the prescribed medicines were not considered of equal importance and as such the patients did not deal with them in the same manner. For example, patients expressed that the anticoagulant had the highest priority and should always be taken, while they would not bother too much if they forget to take the antihypertensive. The self‐rated medication management and administration performance was judged as proficient and free from any perceived specific problems. Patients reported that over time they have developed their own strategies and routines to manage the therapy and deal with issues and the increasing therapeutic complexity. These strategies were derived from learning effects (e.g. tablet splitting), intuition (e.g. tablet crushing) and health beliefs (e.g. medicines are better tolerated after food). The own capabilities and coping strategies to reduce the therapeutic complexity or overcome issues with medicine handling and use, were either perceived as being fully compliant with the instructions for use or done without reflection about their coping strategy yet being firmly convinced it can be done.
3.2.2. Reading or recalling medication plan
The patients involved in this study expressed their belief that they know and recall their medication schedules. However, all patients had to refer to the medication plan when starting the preparation task. While all patients expressed high respect for medicines as well as an intention to avoid medications as much as possible, the level of successful recall of their own medication varied substantially between individuals. Medicines that were considered to be very important (e.g. noticeable effects) or already prescribed over many years were recalled more often compared to medicines perceived less important or newly prescribed. The patients confirmed to be familiar with medication plans and being in the possession of their medication plan. All patients were familiar with MCA and all except one confirmed using them at home routinely when preparing the medication for later use.
3.2.3. Identification and selection of medicine
All medicines were displayed on the table in front of the patients. Three different behavioural approaches were identified. One group of the patients used a random selection approach causing some duplications as well as omission of medicines. Another group followed the medication plan strictly, which required substantial time and efforts leading to fatigue and declining attention. The third group of the patients sorted the medicines according to their recognition with the tendency to sort out and omit the newly prescribed or generically substituted medicines. The identification of the medicines were primarily related to familiarity with a package design. Brand or drug names were only used in a few cases and one patient used a numbering system.
There was a lack of knowledge on the prescribed dose and the existence of different dose strengths for the medicinal products. When asked about the dose they are prescribed to, patients reported to take one tablet. These issues were especially prominent in products where the dose strength appeared only very modest or with poor contrast beside the clearly printed product name as well as when a product was subject of generic substitution (e.g. replacement of one ramipril 2.5 mg tablet by ½ ramipril 5.0 mg tablet).
The observation led us to believe that the identification of the medicine by the patient occurred only after release from the primary package whereby minor distinctions of tablets were being reported by the patient for identification and differentiation, e.g. special embossing, imprint or breaking mark.
3.2.4. Opening of carton medicine box and release of dosage form from packaging
The handling of the carton medicine box occurred in a similar fashion by all patients, where the box is taken in one hand (mainly left hand) while trying to push down the side flap with the thumb of the right hand. When the side flap opens in the opposite direction (bottom up instead of top‐down), patients turned the box several times until the side flap was in the right position. Despite arranging the packaging to be opened with the thumb top‐down (highest degree of opening forces), several attempts were required for the first opening. In case an original seal is applied, the patients sensory and grip strength skills were insufficient to effectively open the packaging and several attempts were required including the use of tools (e.g. scissors, knifes). The side flap opening issues were pronounced in carton boxes with a small height. After opening the side flap successfully, the blisters or vial was often enclosed in the embedded leaflet, which leads to a high level of frustration including re‐closing and opening of the opposite side flap to directly access the blister. The release of the blister or vial from the box required sufficient dexterity and grip strength. To compensate for limitations, some patients poured out the entire content on the table. Due to blister deformation after use and leaflet fold‐up issues, patients tended to withdraw the leaflet and box after the first use.
The main type of primary packaging of the products used by the patient in this study were blisters, followed by vials or glass bottles. The release of the dosage forms (e.g. tablets) from the blister were an effortful process requiring sufficient grip strength, dexterity and manual precision. Differences in the opening times observed suggest depending on the blister types (e.g. PVD/PVdC‐alu and alu‐alu blister) as well as dimensions of the cavity and proximity of cavities on a blister strip. Thirteen patients tried to release the tablet by holding the flat side of the blister downwards with four fingers and pushing with both thumbs top down, waiting for the tablet to fall out into the other four fingers or directly into the MCA. The other technique, used by 6 patients. was to turn the flat site of the blister upwards, cut the alu‐foil with the thumb and push with the other four fingers from underneath the tablet through the foil. Only one patient used both techniques during the preparation. The high‐pressure force required to push out the tablets lead to issues such as tablet breakage, unintended dropping or falling out of the tablet on the table or in a wrong compartment of the MCA. One patient did not realize that the release of the tablet from the blister had occurred and was not able to locate the tablet afterwards, and 2 patients were unsure if the tablet was released or in which compartment it was. Tablets that fall on the table, especially small and round tablets with smooth surfaces, were difficult to pick up and transfer to the MCA compartment. Differences in the emptying patterns were also observed, whereby some patients emptied a single blister systematically, while others used any blister of a box.
The opening of child resistant vials and bottles were confirmed to be a major barrier to retrieve the medication. The opening procedures for the closure systems were understood, but the grip, push and twist strength were insufficient to overcome the original seal and/or the locking forces. A vial filled with tablets used a soft cotton layer to prevent tablet damage during transport. Despite relatively good manual functioning, the embedded cotton could not be removed by the patient. For the liquid forms, additional issues were encountered with the measuring device (e.g. measuring cup pulled on the screw cap was not recognized, poorly visible or insufficient volume grading) and patient understanding and capability of dose measuring (e.g. visual accuracy). Similar issues were observed with drops that were related to the product (e.g. malfunctioning drop system) or the patient capabilities (e.g. visual acuity, drop counting).
3.2.5. Handling and/or manipulation of the dosage form
Tablet splitting was a common process that the patients were asked to perform by the healthcare professional in order to get their individual dose. Tablet splitting was perceived as a cumbersome process requiring sufficient visual acuity, dexterity and grip strength. About half of the patients were unable to split the tablet and those who were capable usually needed several attempts until tablets could be halved. To deal with the problem, patients reported to use tools such as knifes or claws and only a few used commercially available tablet splitters, which were not considered by them as very helpful. Patients reported sharp tablet edges, tablet crumbling and inaccurate division as major issues. Patients perceived tablet splitting as an option for all tablets as long as they can physically achieve it. The absence of a visible break mark or leaflet instruction not to split the tablet was not considered or did not prevent patients from splitting. Patients reported of their habit to split larger tablets in order to deal with swallowing issues when they experience them. The storage of the second half of the split tablet was a general problem and often dealt with by pushing the half back into the deformed blister cavity or the vial, or the patients used a separate cup for storing the tablet halves of one or different products.
3.2.6. Placing into the MCA compartment and drug administration
The placement of the products into the right compartments of the MCA required sufficient cognition, visual acuity and fine motor control. Misplacement of a tablet occurred occasionally and was often not recognized by the patient due to the similarity of the tablet shape and white colour. This poor product differentiation also affected patient ability to identify the tablets once they were placed into the MCA. When a misplacement was recognized, the removal of the misplaced tablet from the compartment was very difficult due to grasping issues and was often resolved by inverting the MCA and pouring the entire contents on the table, which led to mix up of the different dosing compartments.
Independent from the instructions, 1/3 of patients reported taking all medicinal products at once (e.g. after breakfast) in order to reduce the complexity of the medication regimen and save time. Swallowing issues were commonly mentioned for larger tablets as well as for porous and uncoated tablets. Issues with liquid forms were related to an unpleasant taste and/or odour.
4. DISCUSSION
Twenty multimorbid older patients from a day care unit were enrolled in this pilot study, representing a cohort of independent living patients requested to self‐manage their medications at home. According to our best knowledge, this is the first pilot study focused on understanding patient views and investigating issues with own medication use across each step in the medication preparation process, combined with patient objective (medication preparation task) and subjective (semi‐structured interview) performance endpoints. The semi‐structured interview revealed a high degree of self‐confidence in the patients' capability to manage their own medications and not being aware of any problems with the use of the products. The patients who reported subjective medication management and use skills were in contrast to the observed objective performance during the tasks. All patients experienced issues with preparing the medication schedules of which manual issues (e.g. open packaging, splitting tablets), recognition issues (e.g. not knowing medication, confusion about products, identification) and complexity issues (e.g. omitting medicines, wrong dose) were the most prominent. These important discrepancies could be explained by the different factors listed in Table 3. Consequently, usability issues with the drug products are not perceived or considered by patients as being related to the product design and they do not feel that it is appropriate for them to report usability.
Table 3.
Factors and reasoning for the discrepancy between subjective and objective patient performance
| Factors | Reasoning |
|---|---|
| Development of own coping strategies to deal with problems (resilience) | Circumvent problems or simplifying process |
| Overconfidence in self‐management | Believe to use the medicines as intended |
| Answer in a social desirability way | Avoid conflict |
| Do not want to acknowledge own limitations | Keep self‐esteem |
| Self‐confirmation of independence | Fear of autonomy loss |
| Lack of knowledge on medicines | Ignoring or not understanding package leaflet or other patient information |
Older patients with multimorbidity have complex therapeutic schedules.22 The medication preparation process requires an increasingly high level of organizational, temporal, operational, cognitive and emotional input from the patients.23, 24, 25 Successful performance requires that both cognitive demand and level of focused attention increases with the number of medicines, which might overload the patient's capabilities.9 To reduce the level of demand and complexity, patients develop routines and their own procedures, e.g. by taking all medications after breakfast, which can be the root cause for unintended medication errors.26 The routines are to a great extent derived from prior learning with medication use and the application to all other medications (e.g. tablet splitting or crushing, administration after breakfast). That neither drug brand nor chemical names are used by the patients can be explained by the lack of pronounceability (e.g. artificial names containing X, Y, Z, W), lack of meaning (chemical name, INN) or incompatibility with their mother tongue. The developed routine and familiarization with an established medicine use is very difficult to change, preventing implementation of a new dose strength, generic substitution or alterations in product design.27
The knowledge about the medication is very different between patients and depends on the personality and the perceived importance of a medicine and/or disease. Even though the study population was very limited, different distinct personality behaviours and attitudes could be observed. The critical personality (“Each pill is one too much”), the organized personality (“I take my tablets regularly, independent from the circumstances”), the helpless personality (“This should be decided by the doctor, I don't have a clue on it”) and the routine personality (“I am used to it. The idea to not take anything—I would be concerned”). Similar differences in attitudes towards medications have been reported for involving older patients in medication decision‐making.28 The perceived importance of the medicines was higher for drugs with diagnosable effect (e.g. phenprocoumon), with known risk (e.g. digitoxin) or expected effect (e.g. tamsulosin, pregabalin) compared to medicines with no perceived efficacy or symptom relief. In the preparation of the medication schedule, patients tend to select the perceived important and known medicines first and the unknowns were often disregarded. The limited knowledge on the existence of different dose strengths can lead to patient confusion and the administration of a wrong dose. Dosing issues observed with volume measurement and drop counting are mainly related to difficulties with the usability of the dosing aids (e.g. measuring cup, dropper functioning).
The preparation of the drug therapy requires several higher‐level functions, specifically visual, sensory‐motoric and cognitive functions. The observed problems in this study were related to limitations in visual and sensory‐motoric functions by normal aging process29, 30 or specific morbidity patterns.31 For example, tablet splitting requires a high degree and hand sensory functioning, fine motor coordination, grip strength and visual acuity. These functions are known to decline with age32, 33, 34, 35, 36 and impact medication use and management.37, 38, 39 The successful tablet splitting task also depends on the tablet size, shape and hardness as well as potential use of tablet splitting device.40 Our study revealed that small and round tablets were more difficult to split then larger and oblong tablets. Patients reported to use knifes, claws and tablet splitting devices but found the task very time consuming and effortful. Those patients who reported splitting tablets that were not designed for splitting did so as a way of helping swallowing of the tablet. The issues observed with poor product recognition after release from the packaging in the context of polypharmacy was related to insufficient differentiation of the tablets (similar looking). A tablet that fell in a wrong compartment could often not be identified and transferred safely into the right compartment. To deal with this issue for the perceived important medicines, some patients have developed unique skills to identify the medication based on very minor difference in shape, size or tablet marks (e.g. embossing/imprinting, score line). However, only one patient was able to identify all the medicines in the filled MCA.
The medication management issues observed in this study were driven by two overarching patient trajectories: (i) increasing therapeutic complexity and (ii) declining functional and cognitive capacity. In Table 4, the major product factors and related issues as well patient problem self‐solving strategies are summarized. Despite being different in extent, the progression of the trajectories follows similar pathways. As multimorbidity and polypharmacy is a silent and long‐term process, patients develop their own routines and strategies to deal with the medicines and therapeutic schedules. In addition to this, the lack of pharmaceutical and medical knowledge as well as the desire for independence might explain the important discrepancy between subjective and objective performance as well as preventable medication errors. In this regard, it is important to reconsider that patients were only included who were neither disabled nor cognitively impaired and lived independently, suggesting that the problems observed might be more accentuated in real world populations.
Table 4.
Issues derived from product factors in patients with increasing therapeutic complexity and patients self‐solving approaches
| Product characteristics | Potential consequences | Patient self‐solving approach |
|---|---|---|
| Product identification and secondary packaging | ||
| Carton box labelling information not relevant or too complex for patient | • poor product recognition | • rely on recognizable features |
| • dose errors | ||
| • omission | ||
| • medication errors | ||
| Brand name and inn poor linguistic compatibility | • not considered for product search and identification | • search for other recognizable words (e.g. gastro‐resistant) or number medicines |
| • confusion | ||
| Changing packaging design or generic substitution | • product not recognized | • consult doctor |
| • create confusion | • omission | |
| • nonacceptance | ||
| Strength of original seal and/or high first opening force | • nonopening | • use knife/scissors |
| • frustration | • omission | |
| • nonadherence | ||
| Tight closing and/or locking of side‐flap in carton box | • frustration | • cut off side‐flap |
| • several attempts | • discard carton box | |
| • nonadherence | ||
| Side flap opening not adapted to the right‐hand user patterns | • frustration | • turning in hand |
| • several attempts | • several attempts | |
| • discard carton box | ||
| Primary packaging design | ||
| Blister design | ||
| • cavities too close to each other | • nonopening | • de‐blistering all dose units and storage in e.g. open cup |
| • cavity depth too small (flat blister) | • nonadherence | • use knife or scissor |
| Blister material | ||
| • back‐foil tensile strength too high | • nonopening | • de‐blistering all dose units and storage in e.g. open cup |
| • tablet breakage | ||
| • crumbling of back‐foil | • tablet retaining in back‐foil | • use knife or scissor |
| • loss of tablet (e.g. Falls on ground) | ||
| • nonadherence | ||
| Irreversible blister deformation | • unsystematic emptying of the blisters (discard of product), discard secondary packaging (including leaflet) | • storage of all blisters in e.g. bowl without secondary packaging and leaflet |
| Leaflet design | ||
| • structure of information too complex | • leaflet not considered as a source of information | • rely on own use assumptions |
| • language not suitable for patient | • shake out entire content | • screening leaflet for relevant information (when, why, how?) |
| • discard leaflet | ||
| • important information for patient difficult to find | ||
| • covers the blisters after opening side flap | ||
| • difficult to fold up after use | ||
| Vial and bottle closing system | ||
| • different opening mechanism | • fail to open and access medicine | • no reclosing |
| • high dexterity and grip strength required | • discard closure system | • omission |
| • product stability issues | ||
| • tablets in vials covered by cotton | • loss of child resistance | |
| • nonadherence | ||
|
Liquid dosing cup design | ||
|
• transparent grading (poor visibility) • grading not according to dosing requirement • dosing cup attached to closure system • sensitivity of drop counter to use procedure (poor drop formation) |
• dosing inaccuracy • product loss • drug contamination • microbiological contamination • medication errors |
• rely on own use assumptions |
| High tensile strength of sachet (high tear resistance) |
• failure to open • product leakage and loss • contamination • nonadherence |
• use of knife/scissors |
| Drug product design and usage | ||
| Small, round tablet shape and smooth surface | • handling issues (pick up from table, filling in the right compartment), tablet loss (fall to ground), medication errors | • wet fingers to pick up tablets, try to release directly in MCA compartment |
|
Tablet splitting • tablet too small • high breaking force • insufficient tablet hardness/integrity • formation of sharp edges • bad taste at break zone |
• failure to split • inaccuracy in dose • drug contamination • tablet crumbling • swallowability issues (sharp edges) • storage issue of second tablet half • instability • taste issues • nonadherence |
• use of tablet splitter or knife • change from 2 × ½ to 1 × 1 tablet regimen |
| Rough and/or dry tablet surface structure |
• swallowability issues • nonadherence |
• omission • crushing and disperse in water |
| Dosage form size too big | • medication errors (e.g. Crushing, splitting), nonadherence |
• omission • crush and disperse in water |
|
Product preparation tasks • dropping speed too low or too high (drop counter) • poor powder suspendability • unclear sequence of tasks |
• preparation or counting errors • inaccuracy in dose, medication errors |
• rely on own use capabilities |
| Polypharmacy related issues | ||
| Lack of (visual) use guidance | Inappropriate medicines use based on prior learning and routine |
• nonawareness • accept error |
| Poor product differentiation |
• confusion • medication errors |
• rely on correct MCA fill, accept errors |
| Look‐alike design (e.g. white, round tablet) |
• confusion • loss of identification in MCA • medication errors |
• search differences in colour/shape, create comparison card with similar tablets |
| Special use requirements/restrictions | • inappropriate use, medication errors, nonadherence | • rely on learned and intuitive use capabilities |
| Standard design (e.g. round white tablet) | • medication errors, dosing errors, nonadherence | • skip control, rely on correct MCA fill |
| Product similarity | • use of nonprescribed medicines, medication errors, dosing errors, nonadherence | • trust routine use behaviour and own capabilities |
| Product complexity add on to therapeutic complexity | • increasing cognitive demand, medication errors, nonadherence |
• attention replaced by routine • skip control process |
MCA, multicompartment compliance aid.
The patient characteristics and trajectories are not reversible and can only be modified to a very limited degree by patient education and cognitive training41, 42, 43, 44 because of lack of awareness and comprehension. Traditional pharmaceutical product design factors for secondary and primary packaging, formulation and dosage forms, product design and differentiation as well as restrictive use requirements might be appropriate for younger, single disease patient populations, which are the typical subjects, included in the pivotal clinical trials. For the older and multimorbid patients in our pilot study, the pharmaceutical design factors have been found to be suboptimal causing several issues with medication self‐management. More emphasis has to be given to modifying and adapting the pharmaceutical product design process and product design to address the needs of the multimorbid patient population as well as integrate patients social and behavioural factors into the healthcare provision to increase drug safety and effectiveness.45, 46
4.1. Limitations of the study
This pilot study included a limited number of patients (20) living independently in a major city (Berlin) and as such represents only a small portion of older and multimorbid patients. In addition, the patients were recruited from a day care unit following a recent incidence of hospitalization. This might have led to a higher degree of medication problems observed. The pilot study was performed using semistructured interviews and medication preparation tasks based on the individual medication plans, which differed in terms of drug products and complexity from each other. This pilot study design was found to be very useful in the determination of potential problems with the use of specific products and overall medication management, but is limited in terms of quantification of specific issues or correlation of issues to certain disease or patient pattern. The study served the purpose to investigate the self‐management capabilities of older and multimorbid patients with regard to the product design characteristics in order to identify the critical areas for further research.
5. CONCLUSION
Using a typical older and multimorbid patient population, this pilot study confirmed findings from previous studies describing issues of patients with handling medicinal products and self‐managing complex medication schedules. However, the extent to which patients experience issues with handling, preparing and managing their medication was surprising. The results suggest that patients' self‐reported problems and perceived medication management skills are in contrast to the observed medication preparation and managing performance. The findings revealed a high degree of unrecognized, undetermined and unreported medication issues in older and multimorbid patients when managing their medications at home. The issues derive from patient, product, prescribing and monitoring factors that have to be addressed through interdisciplinary collaboration. Mitigating the risk through product design, reduced therapeutic complexity and problem awareness, such as diverging advices in approved product information and healthcare professionals' instructions to patients, are achievable targets of such collaborations. For example, since patient self‐reports are in contrast to the observed performance, an objective assessment instrument to evaluate the patients' therapeutic management capabilities would be required.
COMPETING INTERESTS
There are no competing interests to declare.
Schenk A, Eckardt‐Felmberg R, Steinhagen‐Thiessen E, Stegemann S. Patient behaviour in medication management: Findings from a patient usability study that may impact clinical outcomes. Br J Clin Pharmacol. 2020;86:1958–1968. 10.1111/bcp.13946
The authors confirm that the PI for this paper is Ariane Schenk and that she had direct clinical responsibility for patients.
REFERENCES
- 1. Langie SAS, Lara J, Mathers JC. Early determinants of the aging trajectory. Best Pract Res Clin. 2012;26(5):613‐626. [DOI] [PubMed] [Google Scholar]
- 2. Gerstorf D, Smith J, Baltes PB. A systematic‐wholistic approach to differential aging: longitudinal findings from the Berlin aging study. Psychol Aging. 2006;21(4):645‐663. [DOI] [PubMed] [Google Scholar]
- 3. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education; a cross sectional study. Lancet. 2012;380(9836):37‐43. [DOI] [PubMed] [Google Scholar]
- 4. Stenholm S, Westerlund H, Head J, et al. Comorbidity and functional trajectories from midlife to old age: the health and retirement study. J Gerontol a Biol Sci Med Sci. 2015;70(3):330‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charlesworth CJ, Smit E, Lee DSH, Alramadhan F, Odden MC. Polypharmacy among adults aged 65 years and older in the United States: 1988–2010. J Gerontol a Biol Sci Med Sci. 2015;70(8):989‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson CA, Jones M, Mishra GD, Byles J, Dobson A. Multimorbidity patterns are differently associated with functional ability and decline in a longitudinal cohort of older women. Age Ageing. 2015;44(5):810‐816. [DOI] [PubMed] [Google Scholar]
- 7. Ehlenbach WJ, Larson EB, Curtis JR, Hough CL. Physical function and disability after acute care and critical illness hospitalization in a prospective cohort of older adults. J Am Geriatr Soc. 2015;63(10):2061‐2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kairuz T, Bye L, Birdsall R, et al. Identifying compliance issues with prescription medicines among older people. Drugs Aging. 2008;25(2):153‐162. [DOI] [PubMed] [Google Scholar]
- 9. Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cummulative complexity: a functional, patient centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012;65(10):1041‐1051. [DOI] [PubMed] [Google Scholar]
- 10. Beckman AGK, Parker MG, Thorslund M. Can elderly people take their medication? Patient Educ Couns. 2005;59(2):186‐191. [DOI] [PubMed] [Google Scholar]
- 11. Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly looking beyond cost and regimen complexity. Am J Geriatr Pharmacother. 2011;9(1):11‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barnett N. Medication problems of older people. Annotated bibliography of publications. Burnham National Electronic Library of Medicine UK 2012.. (https://www.medicinesresources.nhs.uk/upload/documents/Evidence/Medicines%20Management/mm‐elderly‐problems‐final.pdf)
- 13. Noteboom K, Beers E, Van Riet‐Nales DA, et al. Practical problems with medication use that older people experience: a qualitative study. J Am Geriatr Soc. 2014;62(12):2339‐2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stegemann Gosch M, Breitkreutz J. Swallowing dysfunction and dysphagia is an unrecognized challenge for oral drug therapy. Int J Pharm. 2012;430(1‐2):197‐206. [DOI] [PubMed] [Google Scholar]
- 15. Elliott J. Problems with medication use in the elderly: an Australian perspective. J Pharm Pract Res. 2006;36(1):58‐66. [Google Scholar]
- 16. Makary MA, Daniel M. Medical error: the third leading cause of death in the USA. BMJ. 2016;353:i2139. [DOI] [PubMed] [Google Scholar]
- 17. FDA . Safety consideration for product design to minimize medication errors. April 2016..
- 18. EMA . Good practice guide on minimization and prevention on medication errors. EMA/606103/2014 November 18, 2015..
- 19. Lecouturier J, Cunningham B, Campbell D, Copeland R. Medication compliance aids: a qualitative study of users' views. Br J Gen Pract. 2011;61(583):93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61‐65. [PubMed] [Google Scholar]
- 21. Nikolaus T, Bach M, Specht‐Leible N, Oster P, Schierf G. The timed test of money counting: a short physical performance test for manual dexterity and cognitive capacity. Age Ageing. 1995;24(3):257‐258. [DOI] [PubMed] [Google Scholar]
- 22. Choudhry NK, Fischer MA, Avron J, et al. The implication of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med. 2011;171(9):814‐822. [DOI] [PubMed] [Google Scholar]
- 23. Stoehr GP, Lu S‐Y, Lavery L, et al. Factors associated with adherence to medication regimens in older primary care patients: the steel valley senior survey. Am J Geriatr Pharmacother. 2008;6(5):255‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roth MT, Ivey JL. Self‐reported medication use in community‐residing older adults: a pilot study. Am J Geriatr Pharmacother. 2005;3(3):196‐204. [DOI] [PubMed] [Google Scholar]
- 25. Kripalani S, Henderson LE, Chiu EY, Robertson R, Kolm P, Jacobson TA. Predictors of medication self‐management skills in a low‐literacy population. J Gen Intern Med. 2006;21(8):852‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pound P, Britten N, Morgan M, et al. Resisting medicines: a synthesis of quality studies of medicine taking. Soc Sci Med. 2005;61(1):133‐155. [DOI] [PubMed] [Google Scholar]
- 27. Haslbeck JW, Schaeffer D. Routine in medication management: the perspective of people with chronic conditions. Chronic Illn. 2009;5(3):184‐196. [DOI] [PubMed] [Google Scholar]
- 28. Belcher VN, Fried TR, Agostini JV, Tinetti ME. Views of older adults on patient participation in medication related decision making. J Gen Intern Med. 2006;21(4):298‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nieves JW, Li T, Zion M, et al. The clinically meaningful change in physical performance scores in an elderly cohort. Aging Clin Exp Res. 2007;19(6):484‐491. [DOI] [PubMed] [Google Scholar]
- 30. Bendayan R, Cooper R, Wlock EG, Hofer SM, Piccinin AM, Muniz‐Terrera G. Hierarchy and speed of loss in physical functioning: a comparison across older U.S. and English men and women. J Gerontol a Biol Sci Med Sci. 2017;72(8):1117‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Scherder E, Dekker W, Eggermont L. Higher‐level hand function in aging and (preclinical) dementia: its relationship with (instrumental) activities of daily life – a mini review. Gerontology. 2008;54(6):333‐341. [DOI] [PubMed] [Google Scholar]
- 32. Carmeli E, Patish N, Coleman R. The aging hand. J Gerontol Med Sci. 2003;58A:146‐152. [DOI] [PubMed] [Google Scholar]
- 33. Hardin M. Assessment of hand function and fine motoric coordination in the geriatric population. Top Geriatr Rehabil J. 2002;18(2):18‐27. [Google Scholar]
- 34. Ferrer‐Blasco T, Gonzalez‐Meijome JM, Monter‐Mico R. Age‐related changes in the human visual systems and prevalence of refractive conditions in patients attending the eye‐clinic. J Cataract Refract Surg. 2008;34(3):424‐432. [DOI] [PubMed] [Google Scholar]
- 35. The Eye Disease Prevalence Research Group . Prevalence of age‐related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564‐572. [DOI] [PubMed] [Google Scholar]
- 36. Reidy A, Minassian DC, Vafidis G, et al. Prevalence of serious eye disease and visual impairment in a North London population: population based, cross sectional study. BMJ. 1998;316(7145):1643‐1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Skomrock LK, Richardson VE. Simulating age‐related changes in color vision to assess the ability of older adults to take medication. Consult Pharm. 2010;25(3):163‐170. [DOI] [PubMed] [Google Scholar]
- 38. Windham BG, Griswold ME, Fried LP, Rubin GS, Xue QL, Carlson MC. Impaired vision and the ability to take medication. J am Geriatr Soc. 2005;53(7):1179‐1190. [DOI] [PubMed] [Google Scholar]
- 39. Wilbur K, Wong RYM. Assessing geriatric patients' ability to functionally manage medication packaging. Can J Hosp Pharm. 2007;60(4):238‐244. [Google Scholar]
- 40. Van Riet‐Nales DA, Doeve ME, Nicia AE, et al. The accuracy, precision and sustainability of different techniques for tablet subdivision: breaking by hand and the use of tablet splitter or a kitchen knife. Int J Pharm. 2014;466(1‐2):44‐51. [DOI] [PubMed] [Google Scholar]
- 41. Ball K, Bersch DB, Helmer KF, et al. Effects of cognitive training interventions with older adults. JAMA. 2002;288(18):2271‐2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lowe CJ, Raynor DK, Purvis J, Farrin A, Hudson J. Effects of medicine review and education programme for older people in general practice. Br J Clin Pharmacol. 2000;50(2):172‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith GE, Housen P, Yaffe K, et al. A cognitive training program based on principles of brain plasticity: results from the improvement in memory with plasticity‐based adaptive cognitive training (IMPACT) study. J Am Geriatr Soc. 2009;57(4):594‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wilson EAH, Wolf MS. Working memory and the design of health materials: a cognitive factor perspective. Patient Educ Couns. 2009;74(3):318‐322. [DOI] [PubMed] [Google Scholar]
- 45. Stegemann S, Ternik RL, Onder G, Khan MA, van Riet‐Nales DA. Defining patient centric pharmaceutical drug product design. AAPS J. 2016;18(5):1047‐1055. [DOI] [PubMed] [Google Scholar]
- 46. Hignett S, Lang A. Human factors for Health & Social Care. Chartered Institute of Ergonomics & Human Factors White Paper 2018. (https://www.ergonomics.org.uk/Public/News_Events/News_Items/Healthcare‐White‐Paperpublished.aspx)


