Abstract
Even the most effective drug product may be used improperly and thus ultimately prove ineffective if it does not meet the perceptual, motor and cognitive capacities of its target users. Currently, no comprehensive guideline for systematically designing user‐centric drug products that would help prevent such limitations exists. We have compiled a list of approximate but nonetheless useful strategies—heuristics—for implementing a user‐centric design of drug products and drug product portfolios. First, we present a general heuristic for user‐centric design based on the framework of Human Factors and Ergonomics (HF/E). Then we demonstrate how to implement this general heuristic for older drug users (i.e., patients and caregivers aged 65 years and older) and with respect to three specific challenges (use‐cases) of medication management: (A) knowing what drug product to take/administer, (B) knowing how and when to take/administer it, and (C) actually taking/administering it. The presented heuristics can be applied prospectively to include existing knowledge about user‐centric design at every step during drug discovery, pharmaceutical drug development, and pre‐clinical and clinical trials. After a product has been released to the market, the heuristics may guide a retrospective analysis of medication errors and barriers to product usage as a basis for iteratively optimizing both the drug product and its portfolio over their life cycle.
Keywords: consumer health information, drug design and labelling, ergonomics, human engineering, medication errors, patient safety, patient‐centric design, self‐administration, user‐centric design
1. INTRODUCTION
The role of drug users—patients, caregivers and healthcare professionals—in determining the effectiveness of drug products must not be underestimated. If the design and accompanying informational materials of drug products do not match users' needs and capacities, the proper use—hence safety and effectiveness—of even the most efficacious product can be compromised. In other words, drug products that are difficult to take/administer may not be used properly, resulting in unnecessary medication errors, or may not be used at all, jeopardizing therapeutic success.1, 2, 3
Patient‐centric and, more generally, user‐centric drug development have recently gained attention in the pharmaceutical literature,4, 5, 6 the pharmaceutical industry,7 and the regulatory framework8 as a way to address this problem. Although the term “patient‐centric” is frequently used in the literature on drug development, we use “user‐centric” throughout this article to indicate that design efforts should not be limited to patients alone but instead encompass all drug users, including patients, lay caregivers, pharmacists, nurses, physicians, etc. The ultimate goal of user‐centric drug development is to support all drug users in taking and/or administering the right product at the right dose, at the right moment, and in the right way in order to reduce medication errors, especially in vulnerable populations such as older adults9 and children.10 The only complete and coherent guideline that is at present available to help implement user‐centric drug development relates specifically to the development of drug products for children.11 Another guidance document summarizes methods for designing user‐centric medical devices but does not address the specific challenges of user‐centric drug product design.12 Our goal in this paper is to provide approximate but useful strategies—heuristics—for implementing a general user‐centred drug design that can be adapted and applied to different user groups and age groups.
To derive heuristic strategies for user‐centric drug design, we consulted with clinicians about common challenges for drug users and searched for user‐centric solutions in existing regulatory documents and pharmaceutical guidelines and in the research base of Human Factors and Ergonomics (HF/E). HF/E emerged as a field at the intersection between psychology, engineering and economics at the outset of the industrial revolution, when human error started to limit further productivity increases despite ongoing technological improvements.13 Workers had to interact with increasingly sophisticated technology that taxed their sensory, motor and cognitive capabilities—the human factors. The impact of human factors on the effectiveness of human–machine systems was soon discovered in other fields, including the military, aviation and medicine. In medicine, HF/E focuses mainly on the human factors that emerge at the interface between professional users—physicians, nurses, etc.—and medical devices/technologies in order to increase patient safety and health system effectiveness,14, 15 for instance via barcode medication administration technology.16, 17 In comparison, the human factors of non‐professional users of medical devices/technologies (e.g., patients and lay caregivers) have gained little attention in HF/E thus far,18 with the notable exception of users aged 65 and older.19
Based on our analysis, we first elaborate a general heuristic outlining how to structure user‐centric design processes and focus on the most essential steps. On the basis of this outline, we then present more specific heuristics to address three specific tasks for drug users—use‐cases—involved in medication management:
-
Use‐case A
Knowing what drug product to take/administer (i.e., identifying the correct product and being able to discriminate among similar products);
-
Use‐case B
Knowing how to take/administer the drug product (i.e., using a product at the right time, in the right way, and for the right purpose);
-
Use‐case C
Actually taking/administering the drug product (i.e., being able to handle a drug product, for instance by opening drug containers/blisters, applying inhalers/creams or administering multiple drugs)
To demonstrate how to implement the general heuristic with respect to a particular user group, we describe the challenges for patients and caregivers aged 65 and older within each use‐case and present available guidelines and/or evidence to outline heuristic strategies for user‐centric drug design. Older patients and caregivers are the most vulnerable and at the same time the most common group of drug users.9 Thus designing drug products that this group can use despite their specific perceptual, motor and cognitive limitations (see Annex 1 in Ref.9) would more than likely prevent the majority of medication errors, while reducing the severity of those that do occur. The essence of the identified heuristics, which may be applied to all drug users, including health professionals, is summarized in Table 1. Because heuristics are approximate strategies, we point out their limitations and how to address those limitations at the end of the article.
Table 1.
A set of heuristics for user‐centric drug product designa
| 2. A general heuristic for user‐centric design |
|
Step 1: Define what should be done with a product (e.g., Use‐case A, B or C). Step 2: Define what should NOT be done with a product (e.g., prioritize potential medication errors). Step 3: Identify what level of cognitive control is needed for the use‐cases (i.e., perceptual, motor, rule‐based or conceptual control) and minimize related cognitive effort. In order to do so, user‐centric design should support perceptual and/or motor control before requiring rule‐based or conceptual control.20, 21 |
| 3.1 A specific heuristic for Use‐case A: “Knowing what drug product to take/administer” |
|
To support discrimination between products, use unique and consistent combinations of verbal labels and perceptual codes (e.g., colour, shape, size, surface texture). Continuous differences between products (e.g., concentration levels) should be coded using continuous perceptual dimensions (e.g., size, colour saturation), whereas categorical differences between products (e.g., different ingredients) are best specified with categorically distinct perceptual features (e.g., colour hues).22 Given that no guideline exists, perceptual codes must be iteratively usability tested. |
| 3.2 Specific heuristics for Use‐case B: “Knowing how to take/administer a drug product” |
|
3.2.1 Use‐case B1: Being able to read instructions 23, 24, 25, 26 To enhance readability, use a single sans‐serif font at least 12 points in size. Also use line spacing of at least 1.5, with additional spacing between paragraphs to separate distinct units of text. Lines should be no longer than 80 characters and left‐justified without end‐of‐line hyphenation. Text should be printed with high contrast. |
| 3.2.2 Use‐case B2: Being able to comprehend text |
| To design easy‐to‐comprehend text, information on why one should use the drug (purpose) should be provided first, then how much and how often it should be used, and finally, its potential side effects.27 Comprehensibility is further enhanced by short and simple wording, active and positive sentences, argument overlap, a non‐alarmist tone, and a 4th‐ to 5th‐grade reading level. Formatting and layout should underline the logical structure of the text.28 |
| 3.2.3 Use‐case B3: Being able to comprehend numbers |
| To communicate numerical information, provide absolute numbers for risks and benefits with a comparison of two identical reference groups—usually 100 or 1000 people in both the intervention and control group.29, 30 Fact boxes and icon‐arrays are best practice for communicating numerical information.31, 32, 33 |
| 3.2.4 Use‐case B4: Being able to comprehend illustrations/diagrams |
| Illustrations/diagrams may increase comprehensibility. Given that people may differ in reading and graph literacy, illustrations/diagrams must be accompanied by and closely aligned with explanatory text to maximize comprehensibility.28, 34, 35, 36 |
| 3.3 A specific heuristic for Use‐case C: “Actually taking/administering a drug product” |
|
Facilitating motor control is key to preventing medication errors in the majority of users. Almond‐shaped tablets,37 blisters with clearly specified pushmarks, and tablets with clearly specified breakmarks are a good starting point. To avoid confusing users, a perceptual feature should be used consistently and should support only one motor function. Potential trade‐offs between product handling and swallowability (e.g., related to size) should be considered. Given that no guideline exists, design for motor control must be iteratively usability tested. |
Heuristics are defined as approximate but useful problem‐solving strategies.
2. A GENERAL HEURISTIC FOR USER‐CENTRIC DESIGN
According to HF/E, the purpose of user‐centric design is to support smooth and error‐free interactions between user and product so as to meet task‐specific goals (e.g., as implied in the use‐cases above).12 To serve this purpose, drug designers should follow three simple steps: (1) identify relevant use‐cases for a specific user group; (2) identify the most serious challenges of these use‐cases and any resulting complications that must be avoided; and (3) identify design solutions that support users best while minimizing the cognitive effort required to use the product. We will first elaborate this three‐step process and then use it as a blueprint to derive more specific heuristics for the user group of older patients and caregivers.
Step 1 in any user‐centric design process is to define what a user group should be able to do and achieve with a product. For this paper, we identified three main tasks or use‐cases that apply to all drug users (see above). But of course, use‐cases may vary from one user group to another. For instance, medication reconciliation and creating a drug formulary are use‐cases specific to healthcare professionals. Thus Step 1 is intended to define the use‐case (e.g., actually taking a drug product) and the goals and performance criteria for a specific user group (e.g., older patients often take multiple drugs and thus must be able to discriminate among similar products).
Step 2 is to define what should NOT happen when a product is used. That is, in Step 2 drug designers should identify errors that could occur when the requirements of a use‐case cannot be met and rank them according to the severity of the potential consequences. For instance, which error might cause more harm for older patients: not being able to grasp a tablet or not being able to swallow it? Depending on the answer, the drug designer should prioritize solutions for errors that have more severe consequences. The goal of Step 2, then, is both to minimize harm for drug users and, at the same time, help drug designers to prioritize in their planning of the design process.
Step 3 is to find user‐centric solutions to the problems prioritized in Step 2, that is, solutions that help users to prevent errors and minimize the cognitive effort needed for accurate task performance. Such solutions will benefit all users but are essential if cognitive, perceptual and/or motor skills are already limited (e.g., older patients and caregivers) or severely taxed (e.g., health professionals working under stress).
According to HF/E, cognitive effort depends on the level of cognitive control needed to perform a task.38, 39 For instance, to minimize the cognitive effort needed to differentiate between drug products, instructions about how to recognize the visual differences between them (a rule‐based control task) should not be necessary. Instead, product design should provide distinctive perceptual features (e.g., differences in size, colour or shape) so that users can directly “see” differences between the drug products (making it a perceptual control task). Similarly, to extract a product from its packaging (a motor control task), no instructions should be required about where exactly to push on a blister (a rule‐based control task). Instead, physical properties (e.g., shapes, textures, and sizes) should be implemented that allow drug users to “see” where to best open a blister (e.g., using convex rather than concave/flat shapes). In HF/E, perceptual and physical features that specify what users can or cannot do with a product are called affordances. 40 For product designers, they are an effective means of making product functions apparent and thereby reducing cognitive effort.41Of course, more complex tasks may require additional or even all levels of cognitive control. For instance, to accurately take/administer a drug product (Use‐case B), users require simple rules for how and when to take/administer it (rule‐based control) plus perceptual control and motor control to be able to implement these rules. To manage complex diseases such as diabetes, drug users may additionally require a conceptual model of how their disease works and how to adapt medication management in order to control it (conceptual control).39
As a general rule, perceptual control and motor control require the least cognitive effort, followed by rule‐based control and conceptual control.40, 41 Thus in Step 3, designers should first try to find solutions to support product usage (e.g., Use‐cases A, B, C) at the perceptual and motor levels before requiring users to engage in rule‐based or conceptual control.
The three‐step process just outlined is summarized as a general heuristic for user‐centric design in Table 1. Of course, each step of this general heuristic can and should be further specified depending on the specific use‐cases and the particular capacities and limitations of a targeted user group. In what follows, we will show how this can be done with the use‐cases identified above and given the sensory, motor and cognitive capacities of older patients and caregivers (see Annex 1 in Ref.9). Based on this analysis and for each use‐case, we will provide more specific heuristics for user‐centric drug product design. The essence of these heuristics, which may be applied to all drug users, is summarized in Table 1.
3. SPECIFIC HEURISTICS FOR DESIGNING USER‐CENTRIC DRUG PRODUCTS
With the general heuristic in mind, we further specify the use‐cases identified above (Step 1) and outline potential sources of medication errors (Step 2), using the example of older patients and caregivers. Then we present available guidelines and evidence to help design drug products that reduce cognitive effort for older patients and caregivers (Step 3) and identify specific heuristics for user‐centric drug design and each use‐case (Table 1).
3.1. Use‐case A: Knowing what drug product to take/administer
In order for drug users to avoid medication errors, the first challenge is to identify the correct product to use. This is a particularly relevant task for older patients and caregivers who often use multiple drug products (poly‐pharmacy) that, to avoid harm, must not be confused. In clinical practice, discriminability must be supported in at least three types of scenarios. First, products may look alike but contain categorically different ingredients (e.g., round white tablets). Second, they may contain the same ingredient but at different levels of strength or different concentrations. Third, innovator, generic and parallel‐imported products may essentially provide the same ingredient at the same strength or concentration but may look entirely different. Psychologists suggest that our ability to discriminate between objects depends on the availability and reliability of easy‐to‐recognize differentiating features.42, 43 To design drug products that help users to identify the correct product to take/administer in the above‐mentioned clinical practice scenarios, two categories of product‐discriminating features may be used: conceptual distinctions (names or verbal labels) and perceptual features (colour, shape, size, surface texture or combinations thereof).
With respect to product naming/labelling, guidelines focus mainly on text formatting (e.g., font, font size, margins) to increase readability rather than discriminability.23, 24 To help discriminate between similar names for different drug products, the US Food and Drug Administration (FDA)44 and the European Medicines Agency (EMA)8 recommend Tall Man Lettering. That is, letters that help differentiate between similar medication names should be capitalized (e.g., DOBUTamine versus DOPamine). But even if Tall Man Lettering is applied, verbal labels require both a certain level of visual acuity (see Figure 1, example 1) and cognitive resources for the decoding and processing of the verbal information. Both are common challenges for older patients and caregivers9 and sometimes also for health professionals who have to work in busy and stressful environments. To solve these problems, perceptual features may be used to help drug users directly “see” differences between products as well as their immediate and secondary packaging. We review examples of how this can be done using both the literature and clinical practice in turn.
Figure 1.

- Lisinopril 5, 10, 20 mg (strength is coded verbally, but readability may be low due to small font size, especially if visual acuity is low).
- Enalapril 5, 10, 20 mg (strength is coded with shape and colour; switch of codes between 5 mg and 10/20 mg may obscure the fact that the tablets contain the same ingredient).
- Olanzapin velotab 5, 10, 15, 20 mg (strength is coded using proportional changes in size to support comparisons; information on absolute strength cannot be inferred without additional verbal labels).
- Diazepam 2, 5, 10 mg (strength is coded verbally and with colour; colour codes for continuous variables such as strength must be learned and remembered, potentially taxing users' cognitive resources; however, colour may be effective for specifying categorical differences, e.g., between ingredients)
In the literature, very few official standards for how to specify differences between drug products at the perceptual level have been identified. For instance, there is an ISO standard for colour codes to differentiate syringe types (ISO 26825)45 and an American Academy of Ophthalmology (AAO) colour code for caps and labels to help differentiate between topical ocular drugs.46 Nevertheless, although colour codes are often used in marketing to communicate brand and/or product identity, there is no official colour code in use to specify categories of ingredients or levels of strengths or concentrations of drug products. Empirically, however, there is promising yet insufficient evidence on how perceptual features may be used to specify product differences.47, 48 Initial evidence from the laboratory suggests that patients and caregivers tend to use bottle colour, shape and size more frequently and, in the case of colour, more correctly than product names to differentiate between medications.49 But the same study also showed that colours alone are insufficient to support unambiguous patient–doctor communication about drug products.50 Thus the ability to differentiate between medications should be supported with a complementary combination of perceptual and verbal codes.
From a clinical perspective, most ointments are provided in similarly shaped tubes and, apart from a few drug products with consistent colour codes (e.g., red/pink for ibuprofen, light blue for sildenafil), most tablets are white, round and packed in similar‐looking boxes and bottles. In Figure 1, we compare four examples of how perceptual (and verbal) features are currently used to code differences in the strength of the same ingredient, and then we illustrate potential pitfalls. The examples underline an HF/E principle stating that user‐centric design should align perceptual codes with task characteristics.22 Specifically, to differentiate continuous differences between drug products (e.g., strength, dose or concentration of the same ingredient), a continuous perceptual dimension (e.g., size, colour saturation) should be used (e.g., Figure 1, example 3). Categorical differences between drug products (e.g., different ingredients) are best specified using categorically different perceptual features (e.g., distinct colour hues). Figure 2 provides an example where this principle is not followed and further suggests that, for optimal support, differences between drug products must be easy to recognize across all contexts of use, for instance while the product is still packaged and once it has been removed from its packaging.
Figure 2.

The packaging of Olanzapin velotab (Zyprexa) 5, 10, 15 and 20 mg strengths are shown here from left to right. The differences in strength (a continuous variable) are colour coded on the upper part of the packaging using different hues, suggesting categorical differences between the tablets. Once the packaging is removed, all tablets are off‐white and differ only marginally in size between 5 and 10 mg and 15 and 20 mg, hampering recognition of differences in strength
There are also scenarios when using perceptual features to aid in discrimination is problematic. For instance, discrimination based on colour and shape may be compromised if the same features already serve other functions like corporate identity or brand identity. Also, the options for perceptual coding may be limited by drug user characteristics (e.g., red‐green colour blindness) and by cultural conventions. One example of such a convention is that, compared to black‐and‐white stimuli, red and orange colours tend to be associated with warnings or danger in most cultures51, 52 and have a stimulant effect compared to more tranquillizing blue and green colours.53 The essence of this heuristic is summarized in Table 1 (Specific heuristic for Use‐case A: “Knowing what drug product to take/administer”).
3.2. Use‐case B: Knowing how to take/administer a drug product
A second challenge for drug users is to understand which product should be taken when, how and for what purpose. Drug users also need to know what side effects may occur, how to recognize them, and what to do if they occur. Examples from clinical practice suggest that putting this information into action is quite challenging, in particular for older drug users. Older patients and their caregivers often need to fit multiple drugs into a treatment plan (poly‐pharmacy), each with specific but different instructions related to dosing, intake frequencies (e.g., weekly versus daily), etc.2, 3 In addition, if existing drug formulations do not match individual needs, drug users may have to combine several strengths, subdivide, or otherwise modify products prior to use. Without adequate understanding of a product's characteristics, even healthcare professionals may incorrectly crush tablets or open capsules, potentially altering absorption characteristics and putting their patients and themselves at risk.54
If correct drug administration is not well explained and supported, patients may end up taking incorrect doses of drugs at incorrect frequencies, swallowing blisters rather than the tablet itself, taking suppositories orally, or spraying inhalers into the air rather than into their mouths. In combination with potential declines of cognitive functioning, medication management/administration becomes a major challenge for older patients and their caregivers. To help this user group manage medication regimens and avoid errors or, worse, non‐adherence, information about how to correctly use a drug product must be particularly easy to comprehend.2, 3, 9, 55 In our own research we have in fact shown for a cancer self‐screening context that, if health information is tailored to the needs of older patients, errors can be reduced56 and adherence increased.57
Knowing how to accurately take/administer drug products mainly requires rule‐based control and conceptual control, both of which may be best supported with (written) instructions and medication lists summarizing which product to take, when and at what dose.38, 39 As an alternative to written instructions, videos have also been used successfully (e.g., Vision Leaflets, which can be accessed at https://www.kijksluiter.nl/, are available for patients and caregivers in a number of different languages and adjusted to different cultural backgrounds). But given that written text is the most common format for communicating medication instructions, we will focus on designing user‐centric written instructions here. Instructional information for healthcare professionals is provided in the Summary of Product Characteristics (SmPC), which can be retrieved from regulatory databases. For patients, this information is provided in package leaflets or on the package label. Based on the available literature and existing guidelines, we can elaborate specific solutions for several scenarios that can occur within the construct of Use‐case B: users must be able to read instructions, even if visual acuity is reduced (Use‐case B1); and users must comprehend instructional text (Use‐case B2), numbers communicating risks and benefits (Use‐case B3), and illustrations/diagrams (Use‐case B4), even if they are novice healthcare professionals, under stress, anxious or have low health literacy levels. We elaborate on these scenarios in the following and summarize the heuristics for Use‐case B in Table 1.
3.2.1. Use‐case B1: Being able to read instructions
Several guidelines exist for writing instructional text on how to use drug products,23, 24, 25, 26 although they may not always be closely followed.58 Here we present the most important principles. To improve readability of instructions and support (older) drug users with limited visual acuity, contrast should be high (at least 5:1) with black font on white paper. No more than two types of sans‐serif fonts (e.g., Arial or any other font without small extensions called “serifs” at the ends of its characters) should be used (for easy‐to‐read fonts, see DIN 1450:2013‐04), and print should be at least 12 points in size. Headers should be clearly and consistently marked. Lines should be no longer than 80 characters (4.7 inches or 12 cm) and should be left‐justified without end‐of‐line hyphenation. Line spacing should be set at 1.5 with more than 1.5 lines left between paragraphs to help readers separate distinct units.
3.2.2. Use‐case B2: Being able to comprehend instructional text
Instructional text may be made more comprehensible by presenting drug information in a preferred order. Older adults prefer general information about the drug (purpose) to be first, then information about how to take the product (dose and schedule), and finally information about possible side effects.27 The literature suggests that comprehensibility may be enhanced with simple rather than technical wording, a short‐and‐clear rather than complex sentence structure, active rather than passive formulations, positively framed sentences rather than negatively framed ones, and a descriptive text at a 4th‐ to 5th‐grade reading level.59 Sentences must be consistent and should provide sufficient argument overlap to avoid misinterpretations.59 To help readers visually connect related contents in a written text, section headings, list formats,60 flow charts and boxes are effective. Key words can be made bold to facilitate retrieval. To mark a change in content, text structure or topic, new paragraphs and extra spacing are effective.61, 62 Finally, recent guidelines suggest that, to support patient participation, instructional language should be non‐alarmist and non‐patronizing.26
3.2.3. Use‐case B3: Being able to comprehend numbers
Communication of numerical information is hampered mainly by limited numeracy skills63 and ambiguous numerical formats such as relative risks (e.g., a medication decreases the probability of a stroke by 48%) that cannot be interpreted without base rates of the risk.29 To circumvent these problems, risks and benefits of a drug product are best communicated in absolute numbers and as a comparison of two identical reference groups of patients, one which uses and one which does not use the product for a specified period of time (e.g., the probability of a stroke decreases from 28 out of 1,000 men not taking the medication to 15 out of 1,000 men taking the medication over a period of 10 years).29, 30 Ideally, this information is provided in what is known as drug factboxes.31 Factboxes are 2×2 tables summarizing evidence for risks and benefits in intervention and control groups, that is, for those who used and those who did not use the drug product (for a guideline on how to create factboxes, see Ref.64).
Those with low numeracy skills may benefit from visually represented numbers,26 with a symbol (icon) repeated a specific number of times, the repetition typically representing 100 or 1,000 people. An example is provided in Figure 3, where icon arrays visually compare the benefits of an imaginary drug product for 1,000 people taking it as compared to 1,000 people not taking it for a specified period of time. The number of those who are expected to be at risk for a stroke in each population is marked in colour. Such visuals may be provided on Summary of Product Characteristics (SmPC) and package leaflets. They may also be used by healthcare providers in clinical settings in order to transparently communicate the benefits and risks of a drug product to potential users. Numerous studies have demonstrated that icon arrays are an effective tool for communicating health risks,33, 65 enhancing knowledge and reducing common biases (e.g., framing, denominator neglect, anecdotal reasoning), particularly for users with lower numeracy skills.33, 65, 66
Figure 3.
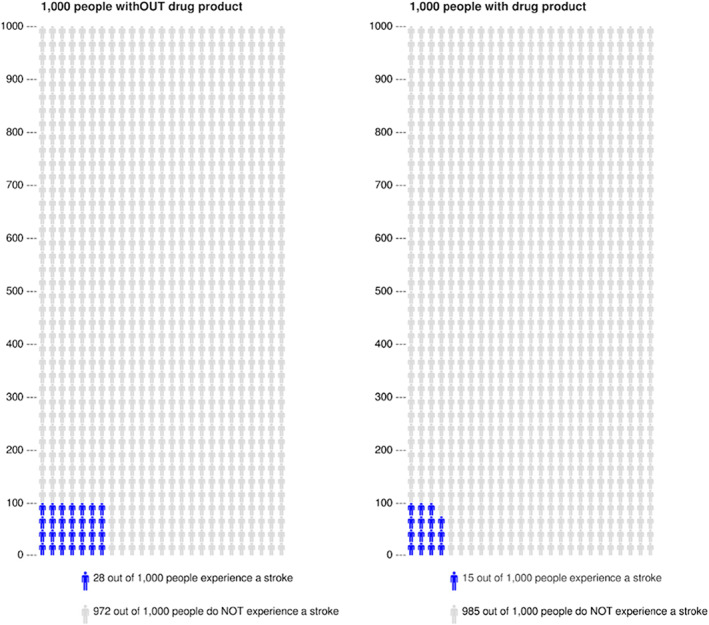
Examples of icon‐arrays communicating the baseline risk of a stroke for a defined population of 1,000 people without drug product (left) compared to 1,000 people taking an imaginary drug product aimed at reducing this risk (right). Potential side effects are omitted in this example but may also be included using another colour. Icon‐arrays were generated using http://www.iconarray.com
3.2.4. Use‐case B4: Being able to comprehend illustrations
Finally, illustrations/diagrams may increase attention to and recall of drug product instructions,34 in particular if clearly linked to written or spoken text.35 Although illustrations/diagrams tend to reduce the need for orthographic decoding, especially for older adults,28, 36 recent research shows that individual differences related to both reading and graph literacy do matter. Populations with low reading literacy seem to benefit from illustrations/diagrams, but only if they are combined with a short oral or written explanation of the visual elements and the messages that they are intended to convey.35 To communicate the numerical risks and benefits of drug products, illustrations/diagrams tend to increase comprehension and recall in people with high graph literacy.67 However, people with low graph literacy tend to be better informed when information is given in numbers. Thus, to maximize benefits, both formats should be provided.
3.3. Use‐case C: Actually taking/administering a drug product
A third important challenge for drug users relates to the handling of a product and its package. Product and package handling are mainly perceptual and motor tasks that should require little rule‐based or conceptual control. However, drug users, especially older patients and caregivers, may have limited perceptual and/or motor functions, such as problems with grasping and unsteady hands (dexterity)68, 69 or problems swallowing (dysphagia).9 If product or package design fails to compensate for limited perceptual and/or motor skills, drug users may invent work‐arounds. In other words, if motor control is hampered, drug users may need to engage in rule‐based and/or conceptual control, taxing their cognitive capacities and increasing the probability of medication errors. For instance, if blisters cannot be readily opened, tablets may end up being crushed, or a user may extract all of the tablets at once and transfer them to a container, increasing the risk of unintended under or overmedication.68 Similarly, if capsules cannot be easily swallowed, a user/administrator may open them, potentially altering drug absorption (e.g., enteric‐coated medications).70 In older patients, both problems may be exacerbated due to poly‐pharmacy.
Based on our knowledge, no guideline exists for designing easy‐to‐open packaging or easy‐to‐swallow drug products for a defined user population, except for the development of drug products for children.11 Until more guidelines become available, the perceptual and motor requirements of the most common and vulnerable population—older patients and their caregivers—should be taken into consideration in order to maximize the potential benefit for this user group (and most probably for the majority of other user groups as well).
To facilitate a user's motor control of drug products, perceptual features that specify where to push on a blister (convex rather than concave/flat shapes) or where and how to break a tablet (breakmarks) should be used. Importantly, each perceptual feature should be used to specify and support one function only. That is, features that look like breakmarks but are meant to support swallowing rather than accurate dosing may mislead drug users, including health professionals, and must be avoided. With respect to package handling, some mechanisms for extracting tablets from a blister may require lower levels of dexterity (e.g., push‐through versus peel‐off blisters) or less opening force (e.g., plastic versus aluminium foil). Even push‐through blisters may be an obstacle for users with limited dexterity, however, so that other solutions, such as easy‐to‐open medication boxes, may be a better choice. On the other hand, such boxes need to be childproof (i.e., not too easy to open) to avoid the risk of accidental poisoning in children. These examples illustrate that, ultimately, what constitutes an easy and safe‐to‐handle drug product is highly dependent on the context of use. Thus to develop user‐centric products, any design must be iteratively usability tested and improved with the involvement of the targeted drug users in their respective contexts of use.68, 69, 71 In this process, designers should also consider that perceptual features may involve trade‐offs (e.g., larger tablets may facilitate grasping but hamper swallowing).
To support swallowability, drug product designers need to consider perceptual and motor features related to tablet shape, size/diameter, surface texture and taste/smell,72 but of course within the boundaries set by pharmacology (e.g., absorption pathways, dosing). First evidence suggests that size/diameter of tablets seems to be less relevant than shape. A recent study shows that, in a population without problems swallowing, almond‐shaped tablets are easiest to swallow compared to elongated and round tablets, even though the almond‐shaped tablet is wider than the latter two.37 Where absorption pathways allow, other routes of administration may be considered. For instance, patients with dysphagia tend to prefer dispersible/effervescent or orally disintegrating tablets over chewable tablets and granules.73 Also, ointments, powder,70 liquids or injections74 may help to facilitate product handling for certain user groups and in certain contexts of use. If the medication must be in tablet form, package leaflets should specify whether the drug may be co‐administered or mixed with food or drink to ease swallowing and, if so, provide simple rules as to how this should be done to avoid medication errors. Again, the route of administration that best supports target users in their various contexts of use must be identified based on the results of iterative usability testing. For a summary, see the specific heuristic for Use‐case C in Table 1.
4. LIMITATIONS OF HEURISTICS AND HOW TO ADDRESS THEM
Heuristics are approximate problem‐solving strategies. One advantage of such strategies is that they are somewhat generic and thus flexible enough to be applied to different user groups and used across various contexts and by various stakeholders. For instance, the presented heuristics can be used by drug designers, but may also be used by health professionals in clinical practice settings to anticipate and overcome obstacles related to correct drug product use (e.g., to explain to patients how to split tablets in order to prevent their having problems swallowing them, or how to use different pill containers for products that contain different substances but look alike, or to clarify that, in the case of generic substitution, a product may contain the same substance even though it is from a different company). The main disadvantage of heuristics, on the other hand, is that they do not provide foolproof solutions for all possible scenarios of drug product design. In the following, we elaborate on some of the limitations of the presented heuristics and on helpful methods for addressing those limitations.
First, what constitutes a user‐centric solution for one use‐case may not be user‐centric for another. For instance, shapes that are easy to discriminate one from another (Use‐case A) may not be easy to swallow (Use‐case C), and easy‐to‐comprehend instructions (Use‐case B) may be ineffective if the product cannot be easily unpacked (Use‐case C). Because trade‐offs between different use‐cases cannot be reliably predicted, iterative prototyping and usability testing before and during the drug development process—in both laboratory and real‐world/clinical contexts of use—are essential.75
Second, we focused mainly on older patients and caregivers as the most common and vulnerable drug users. Other user groups may have different requirements with respect to the presented use‐cases (e.g., children). And other use‐cases may exist, for instance, for healthcare professionals, such as medication reconciliation or compiling a drug formulary. Thus, for user‐centric design, all potential users of a drug product—patients, caregivers, physicians, pharmacists and other healthcare professionals—should be considered, specifically their particular perceptual, motor and cognitive capacities and limitations and the unique tasks and problems they might face in different contexts of use.28
Third, whether a drug product is user‐centric depends not only on the match between a product and user characteristics, but also on how users understand, perceive and take/administer the product in their specific context of use (e.g., at home vs. in the hospital, with vs. without caregivers, with innovator vs. generic products). In other words, sources of medication errors related to misunderstandings and false impressions of drug products can be anticipated only to some degree and will be fully revealed only in practice. To avoid unanticipated problems at the stage of implementation, designers of user‐centric drug products should learn to “walk in the shoes” of the user by applying, for instance, ethnographic in situ observations before and during the early phases of drug development.76 These methods provide valid information about use scenarios (i.e., the situations in which drug products need to be taken) and about how drug users understand, experience and take/administer a drug product in their actual contexts of use. As a result, possible misunderstandings, false impressions, and misuses of drug products may be revealed to inform (re‐)designs and to help avoid similar misconceptions in the future.
Finally, because clinical practice changes over time, user‐centric drug product design is an ongoing challenge. To meet this challenge, pharmaceutical companies and drug developers should focus on a user‐centric development of new drug products (prospective approach) and re‐evaluate patterns of use and medication errors for products already or about to be released to the market (retrospective approach). A combination of methods for prospective and retrospective analyses is best suited to guarantee that drug products and related portfolios meet user requirements for safe and effective drug product use and remain state of the art over their entire life cycle.
For both the prospective and the retrospective approach, HF/E provides a substantial inventory of methods. We give abbreviated descriptions of the most important methods here but recommend a look at the comprehensive list provided in Ref.12 To identify user requirements prospectively, the most widely used methods are observations, interviews and surveys. Whereas observations (also called contextual inquiry) are best used to yield information about use scenarios (see above), interviews—performed individually or in focus groups—are best suited to define user types (often called personas) with their distinctive goals, desires, capacities and limitations. To identify use‐cases (i.e., what users need to do and what they should not do), health professionals should be interviewed. Surveys are the method of choice for quantifying the observed differences between user types and use cases in larger and more representative samples.
For retrospective product evaluations before and after release to the market, methods can be classified as analytical versus empirical and task‐based versus rule‐based. Whereas analytical methods rely mainly on experts of user‐centric design (e.g., HF/E professionals, physicians, pharmacists) for product evaluations, empirical methods analyse interactions between actual users and the product/prototype. For instance, in cognitive walkthroughs, a well‐known analytical, task‐based method, experts are asked to “think aloud” while performing a given task (e.g., the use‐cases) with the product. For heuristic evaluations, 77 a widely used analytical, rule‐based method, experts are asked to evaluate a product based on design heuristics (e.g., the specific heuristics for Use‐case B in Table 1). Empirical methods are generally task‐based and often referred to as usability tests. Here, non‐professional drugs users (e.g., patients, caregivers) and professional drug users (e.g., physicians, nurses, pharmacists) may be asked to “think aloud” while using a product or interviewed and/or requested to fill out standardized usability surveys after product use. Once a product has been released to the market, ongoing usability testing, in situ observations and analyses of case reports (e.g., using pharmacovigilance systems) may provide the best sources for specifying user‐centred redesigns and related improvements of drug products and portfolios.
5. CONCLUSIONS
From an HF/E perspective, even the most effective drug product will not be used properly—or at all—and will therefore ultimately be ineffective if it does not meet the perceptual, motor and cognitive capacities (including health literacy) of the target user population. To date, there is no one recipe, let alone a complete and coherent guideline, for how to implement user‐centric drug design for different user populations and across the product life cycle. But based on lessons learned from HF/E, the heuristics presented in this article will help drug designers to take important first steps towards introducing and/or strengthening a user‐centric perspective in the drug development process, as well as assist health professionals in clinical practice in anticipating, identifying and hopefully overcoming major obstacles to correct drug product use with their patients.
CONTRIBUTORS
M.A.F. contributed the conceptual idea and wrote the first draft. G.R. was responsible for existing guidelines/regulations and visual drug product design. F.K.C. was responsible for providing clinical perspectives and examples. F.C.M. and M.H. co‐authored the drafting of the full manuscript. All authors agree to be accountable for all aspects of the work and ensure the accuracy and integrity of the work. There is no principal investigator for this study.
CONFLICT OF INTEREST
M.A.F., M.H., F.K.C. and F.C.M. received no funding from the public, commercial or non‐profit sector for this research; GR received funding from a public funding agency (Innovationsfonds g‐ba, Grant No. VSF1_2017053).
ACKNOWLEDGEMENTS
We would like to thank Diana van Riet‐Nales and David Messinger for their valuable input on prior versions of this article, and Fran Lorié for editing the manuscript and for providing important suggestions regarding word choices and sentence structure.
Feufel MA, Rauwolf G, Meier FC, Karapinar‐Çarkit F, Heibges M. Heuristics for designing user‐centric drug products: Lessons learned from Human Factors and Ergonomics. Br J Clin Pharmacol. 2020;86:1989–1999. 10.1111/bcp.14134
REFERENCES
- 1. Field TS, Mazor KM, Briesacher B, DeBellis KR, Gurwitz JH. Adverse drug events resulting from patient errors in older adults. J Am Geriatr Soc. 2007;55(2):271‐276. [DOI] [PubMed] [Google Scholar]
- 2. Stauffer Y, Spichiger E, Mischke C. Komplexe Medikamentenregime bei multimorbiden älteren Menschen nach Spitalaufenthalt – eine qualitative Studie. Pflege. 2015;28(1):7‐18. [DOI] [PubMed] [Google Scholar]
- 3. Roux P, Pereira F, Santiago‐Delefosse M, Verloo H. Medication practices and experiences of older adults discharged home from hospital: a feasibility study protocol. Patient Prefer Adherence. 2018;12:1055‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stegemann S. Towards better understanding of patient centric drug product development in an increasingly older patient population. Int J Pharm. 2016;512(2):334‐342. [DOI] [PubMed] [Google Scholar]
- 5. Stegemann S, Onder G, Khan M, van Riet‐Nales D. Defining patient centric pharmaceutical drug product design. AAPS J. 2016;18(5):1047‐1055. [DOI] [PubMed] [Google Scholar]
- 6. Stegemann S. Patient centric drug product design in modern drug delivery as an opportunity to increase safety and effectiveness. Expert Opin Drug Deliv. 2018;15(6):619‐627. [DOI] [PubMed] [Google Scholar]
- 7. Hakes L. The development of medicines for older adults: a personal perspective from industry. Int J Pharm. 2016;512(2):332‐333. [DOI] [PubMed] [Google Scholar]
- 8. European Medicines Agency . Good practice guide on risk minimisation and prevention of medication errors [online]. 2015. Available from: https://www.ema.europa.eu/documents/regulatory-procedural-guideline/good-practice-guide-risk-minimisation-prevention-medication-errors_en.pdf. Accessed 3 December 2019.
- 9. European Medicines Agency . Reflection paper on the pharmaceutical development of medicines for use in the older population [online]. 2017. Available from: https://www.ema.europa.eu/documents/scientific‐guideline/reflection‐paper‐pharmaceutical‐development‐medicines‐use‐older‐population‐first‐version_en.pdf. Accessed 3 December 2019.
- 10. Van Riet‐Nales DA, Kozarewicz P, Aylward B, et al. Paediatric drug development and formulation design – a European perspective. AAPS PharmSciTech. 2017;18(2):241‐249. [DOI] [PubMed] [Google Scholar]
- 11. Committee for Medicinal Products for Human Use (CHMP) . Guideline on pharmaceutical development of medicines for paediatric use [online]. 2013. Report No.: EMA/CHMP/QWP/805880/2012 Rev.2. Available from: https://www.ema.europa.eu/documents/scientific‐guideline/guideline‐pharmaceutical‐development‐medicines‐paediatric‐use_en.pdf. Accessed 3 December 2019.
- 12. Food and Drug Administration . Applying human factors and usability engineering to medical devices: guidance for industry and Food and Drug Administration staff. 2016.
- 13. Meister D. The history of human factors and ergonomics. Boca Raton, FL: CRC Press; 1999. [Google Scholar]
- 14. Carayon P. Handbook of human factors and ergonomics in health care and patient safety. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 15. Making Health Care Safer: A Critical Analysis of Patient Safety Practices [online]. Rockville, MD: Agency for Healthcare Research and Quality; 2001. (Evidence Report/Technology Assessment No. 43 (Prepared by the University of California at San Francisco–Stanford Evidence‐based Practice Center under Contract No. 290‐97‐0013)). Report No.: AHRQ Publication No. 01‐E058. Available from: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.125.2605&rep=rep1&type=pdf. Accessed 3 December 2019.
- 16. Holden RJ, Rivera‐Rodriguez AJ, Faye H, Scanlon MC, Karsh B‐T. Automation and adaptation: nurses' problem‐solving behavior following the implementation of bar‐coded medication administration technology. Cogn Technol Work. 2013;15(3):283‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patterson ES, Cook RI, Render ML. Improving patient safety by identifying side effects from introducing bar coding in medication administration. J Am Med Inform Assoc. 2002;9(5):540‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valdez RS, Holden RJ, Hundt AS, et al. The work and work systems of patients: a new frontier for macroergonomics in health care. Proc Hum Factors Ergon Soc Annu Meet. 2014;58(1):708‐712. [Google Scholar]
- 19. Rogers WA, Fisk AD. Human Factors Interventions for the Health Care of Older Adults. Boca Raton, FL: CRC Press; 2001. [Google Scholar]
- 20. Vicente KJ, Rasmussen J. Ecological interface design: theoretical foundations. IEEE Trans Syst Man Cybern. 1992;22(4):589‐606. [Google Scholar]
- 21. Rasmussen J. Skills, rules, and knowledge; signals, signs, and symbols, and other distinctions in human performance models. IEEE Trans Syst Man Cybern. 1983;SMC‐13(3):257‐266. [Google Scholar]
- 22. Wickens CD, Carswell CM. The proximity compatibility principle: its psychological foundation and relevance to display design. Hum Factors J Hum Factors Ergon Soc. 1995;37(3):473‐494. [Google Scholar]
- 23. European Commission . Guideline on the readability of the labelling and package leaflet of medicinal products for human use [online]. 2009. Available from: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-2/c/2009_01_12_readability_guideline_final_en.pdf. Accessed 3 December 2019.
- 24. European Medicines Agency . QRD convention to be followed for the EMA‐QRD templates [online]. 2011. Available from: https://www.ema.europa.eu/documents/regulatory‐procedural‐guideline/quality‐review‐documents‐qrd‐convention‐be‐followed‐european‐medicines‐agency‐qrd‐templates_en.pdf. Accessed 3 December 2019.
- 25. Lühnen J, Albrecht M, Mühlhauser I, Steckelberg A. Leitlinie evidenzbasierte Gesundheitsinformation [online]. 2017. Available from: https://www.leitlinie-gesundheitsinformation.de/. Accessed 3 December 2019.
- 26. Steckelberg A, Berger B, Köpke S, Heesen C, Mühlhauser I. Kriterien für evidenzbasierte Patienteninformationen. Z Ärztl Fortbild Qual Gesundhwes. 2005;99:343‐351. [PubMed] [Google Scholar]
- 27. Morrow D, Leirer V, Altieri P, Tanke E. Elders' schema for taking medication: implications for instruction design. J Gerontol. 1991;46(6):P378‐P385. [DOI] [PubMed] [Google Scholar]
- 28. Hartley J. Designing instructional text for older readers: a literature review. Br J Educ Technol. 1994;25(3):172‐188. [Google Scholar]
- 29. Gigerenzer G, Gaissmaier W, Kurz‐Milcke E, Schwartz LM, Woloshin S. Helping doctors and patients make sense of health statistics. Psychol Sci Public Interest J Am Psychol Soc. 2007;8(2):53‐96. [DOI] [PubMed] [Google Scholar]
- 30. Feufel MA. Statistische Risiken und Unsicherheit in Patientinneninformationen In: Lesch W, Schütt A, eds. Gesundheitsinformationen kommunizieren, Stakeholder Engagement gestalten. Berlin: Medizinisch Wissenschaftliche Verlagsgesellschaft; 2016:125‐136. [Google Scholar]
- 31. Schwartz LM, Woloshin S. The drug facts box: improving the communication of prescription drug information. Proc Natl Acad Sci. 2013;110(Supplement_3):14069‐14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galesic M, Garcia‐Retamero R, Gigerenzer G. Using icon arrays to communicate medical risks: overcoming low numeracy. Health Psychol. 2009;28(2):210‐216. [DOI] [PubMed] [Google Scholar]
- 33. Garcia‐Retamero R, Galesic M. Who profits from visual aids: overcoming challenges in people's understanding of risks [corrected]. Soc Sci Med. 2010;70(7):1019‐1025. [DOI] [PubMed] [Google Scholar]
- 34. Park J, Zuniga J. Effectiveness of using picture‐based health education for people with low health literacy: an integrative review. Cogent Med. 2016;3(1). 10.1080/2331205X.2016.1264679 [DOI] [Google Scholar]
- 35. Houts PS, Doak CC, Doak LG, Loscalzo MJ. The role of pictures in improving health communication: a review of research on attention, comprehension, recall, and adherence. Patient Educ Couns. 2006;61(2):173‐190. [DOI] [PubMed] [Google Scholar]
- 36. Morrow DG, Hier CM, Menard WE, Leirer VO. Icons improve older and younger adults' comprehension of medication information. J Gerontol B Psychol Sci Soc Sci. 1998;53B(4):P240‐P254. [DOI] [PubMed] [Google Scholar]
- 37. Bar‐Shalom D. The quest for easier‐to‐swallow tablets. Tablets Capsul. 2016;4. [Google Scholar]
- 38. Klein HA, Meininger AR. Self‐management of medication and diabetes: cognitive control. IEEE SMC Part A. 2004;34(6):718‐725. [Google Scholar]
- 39. Lippa KD, Klein HA, Shalin VL. Everyday expertise: cognitive demands in diabetes selfmanagement. Hum Factors. 2008;50(1):112‐120. [DOI] [PubMed] [Google Scholar]
- 40. Gibson JJ. The theory of affordances In: Shaw R, Bransford J, eds. Perceiving, Acting and Knowing: Towards an Ecological Psychology. Hillsdale, NJ: Lawrence Erlbaum; 1979:67‐82. [Google Scholar]
- 41. Norman D. The Design of Everyday Things: Revised and Expanded Edition. New York: Basic Books; 2013. [Google Scholar]
- 42. Tolman EC, Brunswik E. The organism and the causal texture of the environment. Psychol Rev. 1935;42(1):43‐77. [Google Scholar]
- 43. Todd PM, Gigerenzer G. Ecological Rationality: Intelligence in the World. New York: Oxford University Press; 2012. [Google Scholar]
- 44. Institute for Safe Medication Practices . Look‐alike drug names with recommended tall man letters [internet]. 2016. Available from: https://www.ismp.org/sites/default/files/attachments/2017-11/tallmanletters.pdf. Accessed 3 December 2019.
- 45.Produkte für Patientensicherheit: Grundlagen Standard nach ISO 26825 [online]. Available from: https://www.pro-patientensicherheit.de/produkte/spritzenkennzeichnung/grundlagen-standard-nach-iso-26825/. Accessed 3 December 2019.
- 46. The American Academy of Ophthalmology . Color codes for topical ocular medications [online]. 2015. Available from: https://www.aao.org/about/policies/color-codes-topical-ocular-medications. Accessed 3 December 2019.
- 47. Hyland S. Does colour‐coded labelling reduce the risk of medication errors? The ‘con’ side. Can J Hosp Pharm. 2009;62(2):155‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Filiatrault P. Does colour‐coded labelling reduce the risk of medication errors? The ‘pro’ side. Can J Hosp Pharm. 2009;62(2):154‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marando CM, Seibold LK, SooHoo JR, Pantcheva MB, Ramulu PY, Kahook MY. The utility of cap color and bottle characteristics for topical glaucoma therapy. Ophthalmology. 2015;122(12):2577‐2578. [DOI] [PubMed] [Google Scholar]
- 50. Dave P, Villarreal G, Friedman DS, Kahook MY, Ramulu PY. Ability of bottle cap color to facilitate accurate patient–physician communication regarding medication identity in patients with glaucoma. Ophthalmology. 2015;122(12):2373‐2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ng AWY, Chan AHS. Color associations among designers and non‐designers for common warning and operation concepts. Appl Ergon. 2018;70:18‐25. [DOI] [PubMed] [Google Scholar]
- 52. Braun CC, Mine PB, Silver NC. The influence of color on warning label perceptions. Int J Ind Ergon. 1995;15(3):179‐187. [Google Scholar]
- 53. de Craen AJ, Roos PJ, de Vries AL, Kleijnen J. Effect of colour of drugs: systematic review of perceived effect of drugs and of their effectiveness. BMJ. 1996;313(7072):1624‐1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Royal Pharmaceutical Society . Pharmaceutical issues when crushing, opening or splitting oral dosage forms [online]. 2011. Available from: https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Support/toolkit/pharmaceuticalissuesdosageforms-%282%29.pdf. Accessed 3 December 2019.
- 55. Insel K, Morrow D, Brewer B, Figueredo A. Executive function, working memory, and medication adherence among older adults. J Gerontol Ser B. 2006;61(2):P102‐P107. [DOI] [PubMed] [Google Scholar]
- 56. Feufel MA, Schneider TR, Berkel HJ. A field test of the effects of instruction design on colorectal cancer self‐screening accuracy. Health Educ Res. 2010;25(5):709‐723. [DOI] [PubMed] [Google Scholar]
- 57. Schneider TR, Feufel MA, Berkel HJ. Promoting colorectal cancer screening in public health outreach campaigns. Hum Factors. 2011;53(6):637‐646. [DOI] [PubMed] [Google Scholar]
- 58. Beers E, Egberts TCG, Leufkens HGM, Jansen PAF. Information for adequate prescribing to older patients: an evaluation of the product information of 53 recently approved medicines. Drugs Aging. 2013;30(4):255‐262. [DOI] [PubMed] [Google Scholar]
- 59. Morrow D, Leirer V, Sheikh J. Adherence and medication instructions review and recommendations. J Am Geriatr Soc. 1988;36(12):1147‐1160. [DOI] [PubMed] [Google Scholar]
- 60. Morrow D, Leirer V, Altieri P. List formats to improve medication instructions for older adults. Educ Gerontol. 1995;21(2):151‐166. [Google Scholar]
- 61. Hartley J. Eighty ways of improving instructional text. IEEE Trans Prof Commun. 1981;PC‐24(1):17‐27. [Google Scholar]
- 62. Hartley J. Designing instructional and informational text In: Jonassen D, ed. Handbook of research on educational communications and technology. Mahwah, NJ: Lawrence Erlbaum Associates; 2004:917‐947. [Google Scholar]
- 63. Nelson W, Reyna VF, Fagerlin A, Lipkus I, Peters E. Clinical implications of numeracy: theory and practice. Ann Behav Med. 2008;35(3):261‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McDowell M, Rebitschek FG, Gigerenzer G, Wegwarth O. A simple tool for communicating the benefits and harms of health interventions: a guide for creating a fact box. MDM Policy Pract. 2016;1(1). 10.1177/2381468316665365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zikmund‐Fisher BJ, Fagerlin A, Ubel PA. A demonstration of ‘less can be more’ in risk graphics. Med Decis Making. 2010;30(6):661‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Garcia‐Retamero R, Galesic M, Gigerenzer G. Do icon arrays help reduce denominator neglect? Med Decis Making. 2010;30(6):672‐684. [DOI] [PubMed] [Google Scholar]
- 67. Gaissmaier W, Wegwarth O, Skopec D, Müller A‐S, Broschinski S, Politi MC. Numbers can be worth a thousand pictures: individual differences in understanding graphical and numerical representations of health‐related information. Health Psychol. 2012;31(3):286‐296. [DOI] [PubMed] [Google Scholar]
- 68. Philbert D, Notenboom K, Bouvy ML, van Geffen ECG. Problems experienced by older people when opening medicine packaging. Int J Pharm Pract. 2014;22(3):200‐204. [DOI] [PubMed] [Google Scholar]
- 69. van Geffen EC, Meuwese E, Philbert D, Bouvy ML. Problems with medicine packages: experiences reported to a Dutch medicine reporting system. Ann Pharmacother. 2010;44(6):1104‐1109. [DOI] [PubMed] [Google Scholar]
- 70. Bostwick JR, Demehri A. Pills to powder: an updated clinician's reference for crushable psychotropics. Curr Psychiatr Ther. 2017;16(2):46‐49. [Google Scholar]
- 71. Mühlfeld L, Langguth P, Häusler H, Hagels H. Influence of blister package design on usability among older adults. Int J Clin Pharmacol. 2012;34(4):553‐560. [DOI] [PubMed] [Google Scholar]
- 72. Fields J, Go JT, Schulze KS. Pill properties that cause dysphagia and treatment failure. Curr Ther Res. 2015;77:79‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu F, Ghaffur A, Bains J, Hamdy S. Acceptability of oral solid medicines in older adults with and without dysphagia: a nested pilot validation questionnaire based observational study. Int J Pharm. 2016;512(2):374‐381. [DOI] [PubMed] [Google Scholar]
- 74. Gill HS, Prausnitz MR. Does needle size matter? J Diabetes Sci Technol. 2007;1(5):725‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moser C. User experience design In: User Experience Design. In, Berlin: Springer; 2013:1‐22. [Google Scholar]
- 76. Madden R. Being Ethnographic. A Guide to the Theory and Practice of Ethnography. London: Sage; 2010. [Google Scholar]
- 77. Nielsen J. Enhancing the explanatory power of usability heuristics In: Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. Boston, MA: ACM; 1994:152‐158. [Google Scholar]
- 78. Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people's health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25(4):398‐405. [DOI] [PubMed] [Google Scholar]
- 79. Okan Y, Garcia‐Retamero R, Cokely ET, Maldonado A. Improving risk understanding across ability levels: encouraging active processing with dynamic icon arrays. J Exp Psychol Appl. 2015;21(2):178‐194. [DOI] [PubMed] [Google Scholar]
- 80. Shah SGS, Robinson I. Benefits of and barriers to involving users in medical device technology development and evaluation. Int J Technol Assess Health Care. 2007;23(1):131‐137. [DOI] [PubMed] [Google Scholar]


