Abstract
Aims
Racemic ibuprofen is widely used for the treatment of preterm neonates with patent ductus arteriosus. Currently used bodyweight‐based dosing guidelines are based on total ibuprofen, while only the S‐enantiomer of ibuprofen is pharmacologically active. We aimed to optimize ibuprofen dosing for preterm neonates of different ages based on an enantiomer‐specific population pharmacokinetic model.
Methods
We prospectively collected 210 plasma samples of 67 preterm neonates treated with ibuprofen for patent ductus arteriosus (median gestational age [GA] 26 [range 24–30] weeks, median body weight 0.83 [0.45–1.59] kg, median postnatal age [PNA] 3 [1–12] days), and developed a population pharmacokinetic model for S‐ and R‐ibuprofen.
Results
We found that S‐ibuprofen clearance (CLS, 3.98 mL/h [relative standard error {RSE} 8%]) increases with PNA and GA, with exponents of 2.25 (RSE 6%) and 5.81 (RSE 15%), respectively. Additionally, a 3.11‐fold higher CLS was estimated for preterm neonates born small for GA (RSE 34%). Clearance of R‐ibuprofen was found to be high compared to CLS (18 mL/h [RSE 24%]), resulting in a low contribution of R‐ibuprofen to total ibuprofen exposure. Current body weight was identified as covariate on both volume of distribution of S‐ibuprofen and R‐ibuprofen.
Conclusion
S‐ibuprofen clearance shows important maturation, especially with PNA, resulting in an up to 3‐fold increase in CLS during a 3‐day treatment regimen. This rapid increase in clearance needs to be incorporated in dosing guidelines by adjusting the dose for every day after birth to achieve equal ibuprofen exposure.
Keywords: enantiomers, ibuprofen, patent ductus arteriosus, population pharmacokinetics, preterm neonates
1. What is already known about this subject
Ibuprofen is often used for the treatment of patent ductus arteriosus in preterm neonates.
Ibuprofen is administered as a racemic mixture of S‐ and R‐ibuprofen, while only S‐ibuprofen is pharmacologically active.
Dosing strategies vary widely across neonatal intensive care units, but have in common that they are based on racemic pharmacokinetic and pharmacodynamic studies.
What this study adds
Enantiomer specific pharmacokinetic parameters were characterized in 67 preterm neonates and indicate that ibuprofen doses should be based on weight and postnatal age in preterm neonates.
Next to characterization of the maturation of the clearance of both S‐ and R‐ibuprofen over a wide range of postnatal age, an unexpectedly high clearance in small for gestational age neonates was found.
1. INTRODUCTION
Patent ductus arteriosus (PDA) is diagnosed when spontaneous closure of the ductus arteriosus does not occur. Preterm neonates with the lowest gestational age (GA) are at highest risk of PDA. 1 Numerous poor outcomes are associated with PDA, but currently there is no consensus on if, how and when to treat PDA in preterm neonates and PDA treatment policies vary widely. 2
If one decides to treat the PDA, ibuprofen is currently considered as the drug of choice. Ibuprofen showed lower risks for side‐effects and a potential decreased risk for necrotising enterocolitis compared to indomethacin, whereas the role of acetaminophen for PDA still remains to be determined. 3 , 4 , 5 , 6 In addition, the reduction in clearance of the renally excreted antibiotic vancomycin was lower when ibuprofen instead of indomethacin was administered, suggesting less nephrotoxicity. 4
Ibuprofen is available as a racemic mixture of R‐ and S‐ibuprofen, with S‐ibuprofen being the pharmacologically active drug. 7 A 3‐day dosing regimen starting with a loading dose of 10 mg/kg followed by 5 mg/kg/day for 2 days is registered for intravenous ibuprofen. 8 Throughout the years the registered dosing regimen has undergone many off‐label adaptations, such as an increase in dose with PNA on the day of treatment initiation, as suggested by Hirt et al. 9 These authors also defined a minimal target exposure to total ibuprofen of an area under the curve 24 hours after the first dose (AUC24) of 600 mg h/L, at which PDA closure was observed in 91% of their population. 9
Enantiomer specific pharmacokinetic (PK) studies were performed by Gregoire et al., who reported substantial differences between the clearance of S‐ibuprofen (CLS) and R‐ibuprofen (CLR). 10 , 11 A simulation study was recently performed by Flint et al. to translate these findings into a dosing regimen. 12 However, due to the relatively narrow range in PNA (0–8 days) of the population examined by Gregoire et al., further investigation of the maturation of enantiomer specific ibuprofen PK is desirable before implementation of the proposed dosing regimen. 11 , 12
Current ibuprofen dosing advices do not recognize the enantiomer‐specific PK and activity, nor do they take into account the exact influence of covariates such as PNA and GA. In our study, we aim to gain more insight into the enantiomer specific PK of ibuprofen in preterm neonates with PDA. By analysing data from different centres, we account for the varying treatment approaches with respect to PNA at treatment initiation, dosages and treatment duration used in the Netherlands. The overall aim of this study is to develop a population PK model on which to perform a covariate analysis to identify how patient characteristics affect the enantiomer specific PK. Finally, we will perform simulations to explore the exposure in different patient groups, which can guide clinicians to individualize dosing regimens.
2. METHODS
2.1. Study
This study was part of the Drug dosage Improvement in Neonates (DINO) study (NCT 02421068), a prospective study that used opportunistic blood sampling to evaluate and improve current dosage guidelines of drugs in preterm neonates. Next to ibuprofen, 8 other drugs were studied in preterm neonates born before 32 weeks of gestation and admitted to 1 of 4 collaborating neonatal intensive care units (NICUs). Patients were treated with intravenous or gastro‐enteral ibuprofen (Pedea, Recordati Ireland Ltd.) according the standard of care of the participating NICU. Each blood sample was <1% of the total blood volume per day, and the maximal blood sample volume did not exceed 3% of the total blood volume per 4 weeks. The local ethics review board approved the protocol and both oral and written informed consent from parents/legal guardians were obtained prior to study initiation (MEC‐2014‐067). Blood samples were centrifuged and plasma was isolated and frozen (−80°C) until analysis.
2.2. Bioanalytical analysis
S‐ and R‐ibuprofen were quantified based on a validated ultraperformance liquid chromatography UV analysis method, developed by the OECD GLP (Good Laboratory Practice) compliant Department of Pharmacology, Toxicology and Biochemistry of the Faculty of Veterinary Medicine, Ghent University. 13 The lower limit of quantification was set at 1 μg/mL for both enantiomers, the limit of detection was 0.0359 and 0.225 μg/mL for S‐ and R‐ibuprofen, respectively. Intra‐ and interassay accuracies ranged from −4.3 to 5.8% and 2.5 to 4.4%, respectively, for S‐ibuprofen, and from −9.4 to 6.5% and −2.3 to 2.8% for R‐ibuprofen. Intra‐ and interassay imprecision did not exceed 10.1% for S‐ or R‐ibuprofen.
2.3. Population PK analysis
2.3.1. Software
NONMEM version 7.3 (ICON Development Solutions, Ellicott City, MD, USA) was used for model development, supported by Perl‐speaks‐NONMEM version 4.7.0. R version 3.5.1 was used in Rstudio version 1.1.463 to build the dataset and visualize the data and model output. R version 3.1.2 was loaded to perform a normalized prediction distribution error (NPDE) analysis. 14
2.3.2. PK model development
First, we developed a model for S‐ibuprofen using the first‐order conditional estimation with interaction method. As dose, half of the administered dose of the racemic mixture of ibuprofen was assumed to be S‐ibuprofen. 15 Model‐development was based on comparison of objective function value (OFV) for nested models, and numerical and graphical model performance (relative standard error [RSE] < 50% and no trends in conditional weighted residuals [CWRES] vs time after dose and observed concentrations). A stepwise covariate analysis‐based covariate analysis was performed manually, to prevent addition of correlated covariates (e.g. PNA and postmenstrual age [PMA]). Covariates to test were selected by visual examination of plots of interindividual variability (IIV) estimates vs all available covariates. Potential covariate relationships were tested and added based on significant improvement in OFV and improvement of goodness‐of‐fit plots, if not correlated to already included covariates. Significance levels of P ≤ .01 and P ≤ .001 (6.6 and 10.8 points in OFV, respectively) were used for forward inclusion and backward exclusion of the covariates, respectively. Body weight was not measured daily, and therefore interpolated linearly for days on which no measurements were available. Linear interpolation was used in a similar fashion for bilirubin and albumin when data were available. For individuals lacking bilirubin or albumin data (n = 24 and 52, respectively), the median observed in our population was imputed.
For the development of the S‐ and R‐enantiomer model, R‐ibuprofen concentrations were added to the S‐ibuprofen model, thereby allowing description of the separate PK of both enantiomers simultaneously. At this point, the M3 method was applied to include all data below lower limit of quantification (2 and 69% of S‐ and R‐enantiomer, respectively), thereby changing the estimation method to Laplacian with interaction. 16 A potential inversion of R‐ to S‐ibuprofen was estimated as a second rate constant from the R‐ibuprofen compartment to the S‐ibuprofen compartment as described before. 11 The effect of PNA on CLR was tested based on the results of Gregoire et al. 11
2.3.3. Model evaluation
The performance of key models was evaluated by NPDE and bootstrap analyses, based on 1000 simulations of the data. 17 A prediction‐corrected visual predictive check was performed to assess the performance of the M3 method. 18
2.3.4. Evaluation and optimization of the dosing regimen
The final model was used to evaluate ibuprofen exposure for 2 different dosing regimens, intravenous dosing according to the label (10‐5‐5 mg/kg/day) and dosing according to the currently advised dosing regimen suggested by Hirt et al. 9 A set of 18 hypothetic preterm neonates were created, with their PNA and GA set manually to represent the observed variation in these covariates in the study population. Nine were appropriate for GA (AGA) and 9 small for GA (SGA). AGA birth weights were imputed based on the median intrauterine weight, and SGA birth weight was based on the 10th percentile of the intrauterine growth curves. 19 In both the AGA and SGA group, simulations were performed for ibuprofen treatment starting at a PNA of 1, 3 and 7 days for subjects with a GA of 25, 27 and 29 weeks. Body weight was assumed to remain unchanged during the simulation period, and 1000 simulations were run per subject and dosing regimen.
A heat map was made to evaluate the variability in exposure to total ibuprofen of preterm neonates across the range of PNA and GA of the study populations. For this purpose, a population of a total of 33 subjects were created, with a PNA at treatment initiation ranging from 1 to 8 days, and a GA ranging from 24 to 28 weeks. Birth weight of these subjects was imputed based on intrauterine growth curves. 19 The median weights of male and female foetuses at their set GA were averaged to represent 1 birth weight. 19 Body weight was assumed to remain equal to birthweight during the simulation period. Each subject was simulated 1000 times. To prevent extrapolation outside the observed combinations of GA and PNA, combinations missing in our dataset were removed from this plot.
Based on observed trends in the concentration–time profiles and heat maps the currently used dosing regimens were optimized, aiming for similar exposure across the PNA and GA ranges. During the simulations, a more frequent dose adjustment for increasing PNA was also examined.
3. RESULTS
3.1. Patients and samples
A total of 210 plasma samples of 67 neonates were collected in which the concentrations of S‐ and R‐ibuprofen were measured. Four patients, with 1 sample each, who received ibuprofen gastroenterally were excluded a priori since these data were regarded as too sparse to describe the absorption and bioavailability upon oral administration. Patient characteristics are presented in Table 1.
TABLE 1.
Patient characteristics (median (range)) and dosing information of the population, up to the last available sample. For time‐changing characteristics the median of the individual median is presented. Ibuprofen doses are calculated based on absolute dose administered and (interpolated) current weight. Loading and maintenance doses were based on a 3‐day regimen and identified by number of dose
| Appropriate for gestational age | Small for gestational age | Total population | |
|---|---|---|---|
| Patient characteristics | |||
| Preterm infants (n) | 60 | 7 | 67 |
| Sex (male/female) | 32/28 | 3/4 | 35/32 |
| Gestational age (wk) | 26.1 (24.0–30.1) | 26.7 (24.6–28.9) | 26.1 (24.0–30.1) |
| Birth weight (kg) | 0.91 (0.59–1.45) | 0.67 (0.47–0.95) | 0.87 (0.47–1.45) |
| Current weight (kg) | 0.85 (0.53–1.59) | 0.74 (0.45–1.04) | 0.83 (0.45–1.59) |
| Part of twin (n) | 30 | 3 | 33 |
| Postnatal age at treatment initiation (d) | 3 (1–8) | 4 (2–12) | 3 (1–12) |
| Bilirubin (μmol/L) [number of patients] | 97.4 (42.5–177.3) [37] | 75.5 (47.7–104.3) [6] | 94.9 (42.5–177.3) [43] |
| Albumin (g/L) [number of patients] | 28.9 (25.0–36.5) [14] | 26.6 [1] | 28.7 (25.0–36.5) [15] |
| Coadministration of fluconazole (n) | 17 | 2 | 19 |
| Ibuprofen dosing | |||
| Duration of ibuprofen treatment (d) | 3 (0–15) | 2 (1–4) | 3 (0–15) |
| Infusion duration (min) | 15 (3–30) | 15 (0–30) | 15 (0–30) |
| Entire treatment period | |||
| Dose of ibuprofen (mg/kg) | 9.6 (4.6–22.7) | 8.1 (4.5–20.4) | 9.5 (4.5–22.7) |
| First treatment cycle | |||
| Loading dose of ibuprofen (mg/kg) | 11 (5–23) | 10 (7–20) | 11 (5–23) |
| Maintenance dose of ibuprofen (mg/kg) | 6 (5–15) | 5 (4–9) | 6 (4–15) |
| Second treatment cycle | |||
| Loading dose of ibuprofen (mg/kg), n | 18 (5–20) [17] | 19 (7–20) [3] | 18 (5–20) [20] |
| Maintenance dose of ibuprofen (mg/kg), n | 9 (5–19) [11] | 9 (5–19) [11] | |
| Third treatment cycle | |||
| Loading dose of ibuprofen (mg/kg), n | 14 (8–20) [4] | 14 (8–20) [4] | |
| Maintenance dose of ibuprofen (mg/kg), n | 9 (9–10) [3] | 9 (9–10) [3] | |
| Samples | |||
| Time after last dose (h) | 15.9 (0–112.4) | 15.3 (0.5–22.1) | 15.8 (0–112.4) |
| Observed concentrations S‐ibuprofen (mg/L) | 20.61 (0.08–84.17) | 9.22 (0.91–31.78) | 20.02 (0.08–84.17) |
| Observed concentrations R‐ibuprofen (mg/L) |
0 (0–24.73) |
0 (0–9.45) | 0 (0–24.73) |
3.2. Population PK model of S‐ibuprofen
Plasma concentrations of S‐ibuprofen were best described by a 1‐compartment model with IIV on clearance and volume of distribution of S‐ibuprofen (CLS and VS, respectively) and a proportional error to describe the residual variability. Observed concentrations of S‐ibuprofen plotted against time after dose did not suggest any nonlinear PK of S‐ibuprofen (Figure S1) within the concentration range observed in this study. Current weight (WT), birth weight, PNA, PMA, GA, sex, being SGA (birth weight lower than the 10th percentile of their respective gestational growth curves 19 ), bilirubin and albumin plasma concentrations, and being born from a multiple pregnancy were tested as covariates (Figure S2).
Both PNA and GA were found most predictive for CLS (P < .001 [−108 points in OFV] and P < .001 [−18 points in OFV], respectively), and were implemented in the model. Being SGA was identified as an additional significant covariate on CLS, with an estimated 3.11‐fold increase in CLS compared to AGA preterm neonates (P < .001 [−21 points in OFV]). For Vs, both WT and sex were significant covariates, however despite the effect of sex being slightly more significant than WT regarding OFV in the backwards elimination, the effect of WT on Vs was maintained in the model based on goodness‐of‐fit plots. Parameter estimates of the S‐ibuprofen model and the respective bootstrap estimates are provided in Table 2.
TABLE 2.
Parameter estimates of the final models and their corresponding bootstrap estimates
| S‐ibuprofen | R‐ and S‐ibuprofen | |||
|---|---|---|---|---|
| Final model estimates (RSE %) [shrinkage %] | Bootstrap estimate (95% CI) | Final model estimates (RSE %) [shrinkage %] | Bootstrap estimate (95% CI) | |
| S‐ibuprofen parameters | ||||
| CLS,ind = CLS,pop * (PNAind/6 days)ΘPNA,S * ΘSGA * (GAind/26 weeks)ΘGA | ||||
| CLS,pop (mL/h) | 3.96 (9) | 4.20 (3.37–5.28) | 3.98 (8) | 4.27 (3.39–5.23) |
| ΘPNA,S | 2.41 (9) | 2.21 (1.32–2.67) | 2.25 (6) | 1.98 (1.27–2.63) |
| ΘSGA | 3.42 (27) | 3.00 (2.02–3.39) | 3.11 (34) | 3.02 (1.94–4.56) |
| ΘGA | 5.98 (15) | 5.86 (3.82–8.22) | 5.81 (15) | 5.57 (3.43–8.13) |
| VS,ind = VS,pop * (WTind/860 g)ΘWT | ||||
| VS,pop (mL) | 248 (8%) | 239 (192–293) | 231 (5) | 220 (187–259) |
| ΘWT | 0.645 (38%) | 0.633 (0.123–1.22) | 0.60 (0.22–1.21) | |
| Interindividual variability (IIV) | ||||
| IIV on CLS (%) | 42.9 (14%) [16] | 42.9 (29.4–61.4) | 46.7 (21) [16] | 40.9 (28.8–60.2) |
| IIV on VS (%) | 31.2 (32%) [33] | 30.2 (11.3–47.6) | 25.9 (19) [33] | 23.1 (9.8–41.2) |
| Covariance IIV CLS ~ VS (%) | ‐ | ‐ | 48.1 (36) | 20.2 (1.8–32.1) |
| Residual variability | ||||
| Proportional error (%) | 29.8 (21%) | 29.1 (22.1–35.4) | 29.8 (10) | 28.5 (21.5–35.9) |
| R‐ibuprofen parameters | ||||
| CLR, ind = CLR,pop + ΘPNA,R *(PNAind‐6 days) | ||||
| CLR,pop (mL/h) | ‐ | ‐ | 189 (24) | 196 (128–286) |
| ΘPNA,R | ‐ | ‐ | 32.3 (48) | 28.5 (7.6–61.3) |
| VS,ind = VS,p * (WTind/860 g)ΘWT | ||||
| VR,pop (mL) | ‐ | ‐ | 303 (15) | 280 (181–408) |
| Residual variability | ||||
| Proportional error (%) | ‐ | ‐ | 63.0 (23) | 54.5 (29.0–91.0) |
| Additive error (μg/mL) | ‐ | ‐ | 1.09 (14) | 1.08 (0.78–1.44) |
RSE: relative standard error; CI: confidence interval; CLS: clearance of S‐ibuprofen; pop: population mean value of a parameter for an appropriate for gestational age individual with a PNA of 6 days, GA of 26 weeks and current weight of 860 g; PNAind: individual PNA in days; ΘPNA,S: exponent for influence of PNA on CLS; ΘSGA: factor for influence of being small for gestational age on CLS, only applied for SGA preterm neonates; GA: gestational age in weeks; GAind: individual GA; ΘGA: exponent of GA on CLS; VS: distribution volume of S‐ibuprofen; WTind: individual current weight in g; ΘWT: exponent for influence of WT on VS and VR; IIV: interindividual variability; CLR: clearance of R‐ibuprofen; ΘPNA,R: slope of influence of PNA on CLR; VR: volume of distribution of R‐ibuprofen.
3.3. Population PK model of S‐ and R‐ibuprofen
To the final model for S‐ibuprofen, a model for R‐ibuprofen was added in which the R‐ibuprofen data were described (Figure S3). R‐ibuprofen concentrations were best described by a 1‐compartment model with a combined residual error model (Table 2). IIV on CLR or volume of distribution of R‐ibuprofen (VR) could not be identified. The influence of PNA on CLR was described by a linear function, estimating an increase of CLR of 32.3 mL/h per postnatal day. Despite the lack of IIV on VR identified in the R‐ibuprofen model, the effect of WT was included on VR (P < .05). Estimation of 2 different exponents to describe the effects of WT on Vs and VR separately was not found to be statistically better compared to estimating 1 joint exponent (dOFV 0.002, P > .1); therefore, 1 exponent of 0.456 (RSE 36%) was estimated for both volumes. The parameter estimates of the S‐ibuprofen model did not change substantially upon addition of R‐ibuprofen model and data (Table 2). Covariance of IIV on CLS and Vs was included in the enantiomer model.
Figure 1 shows how CLS changes with PNA for AGA and SGA preterm neonates with a GA of 25, 27 and 29 weeks and PNA ranging from 2 to 18 days. In Figure 2, the maturation of both CLS and CLR found in this study is compared to other studies performed in preterm neonates, children and adults.
FIGURE 1.
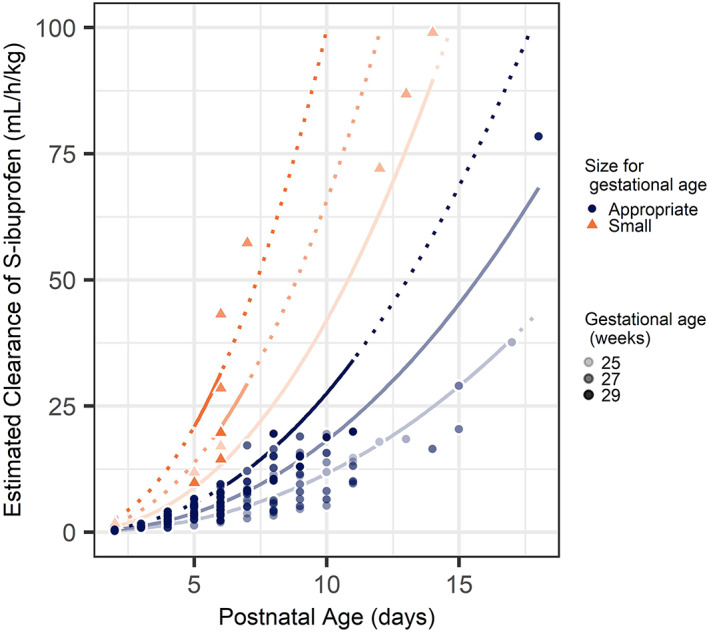
Effect of gestational age and postnatal age on maturation of clearance of S‐ibuprofen (CLS). Dots represent individual estimates of CLS, with increasing colour intensity with GA. Solid lines represent estimated functions for observed CLS, dashed lines represent predicted functions for CLS
FIGURE 2.
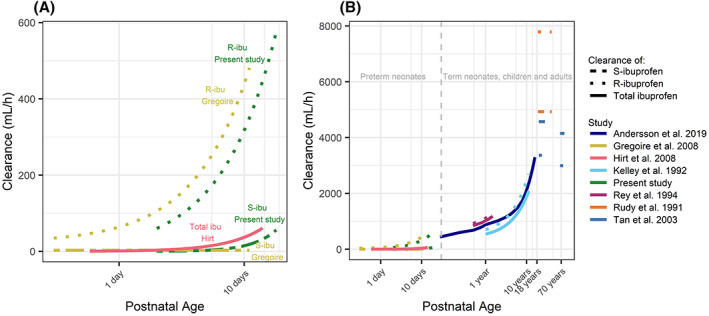
Maturation of clearance of total, S‐ or R‐ibuprofen by the current study and other studies in preterm neonates (A) and preterm neonates, children and adults (B). Studies presented for a postnatal age below 6 months (Hirt et al. 8 and Gregoire et al. 10 ) represent studies performed in preterm neonates, studies in subjects older than 6 months did not distinguish on prematurity. Results of the studies of Rey et al. 20 and Kelley et al. 21 were expressed in mL/min/kg for which the respective clearances were calculated for the subjects with the minimal and maximum age within the study. In the study of Anderson et al. 22 clearance of total ibuprofen was predicted as function of postmenstrual age. The dark blue line is drawn through calculated clearances for postnatal ages 1, 2, 3, 6, 12 months, and 2, 4, 6, 8, 10, 12 and 16 years
3.3.1. Model evaluation
A stratified bootstrap on AGA and SGA preterm neonates was performed because a strong effect of being SGA was observed in both the S‐ibuprofen and the model of S‐ and R‐ibuprofen. A goodness of fit plot is presented in Figure 3. Results of the NPDE analysis stratified on enantiomer are presented in Figure S4. Mean NPDE for S‐ibuprofen was 0.03481 (standard error = 0.062), variance 0.8224 (standard error = 0.08) and global adjusted P‐value .0298. For R‐ibuprofen the mean NPDE was 0.07336 (standard error = 0.081), variance 1.371 (standard error = 0.13) and global adjusted P‐value .000589. PcVPCs of the continuous data vs time after dose, and of S‐ibuprofen vs weight and PMA are presented in Figures S5 and S6, respectively.
FIGURE 3.
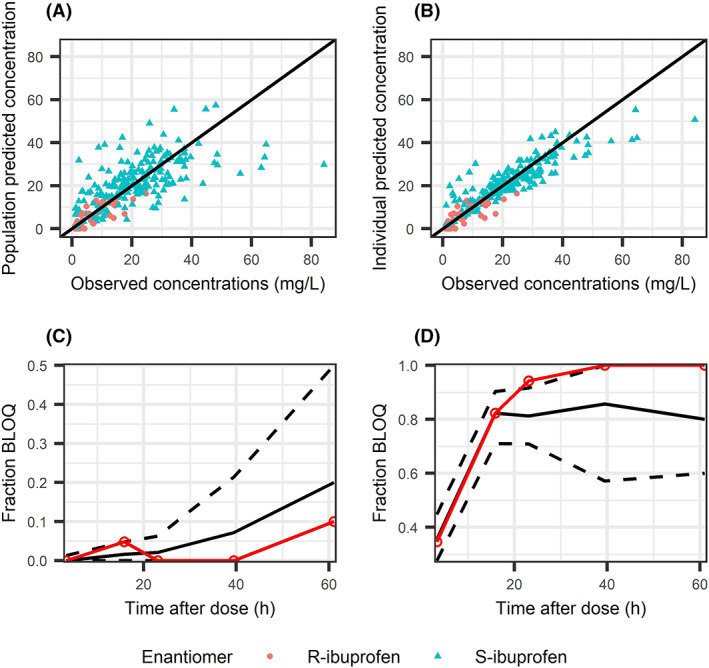
Goodness of fit of the final enantiomer model. (A) Population predicted concentration of S‐ and R‐ibuprofen (orange and blue points respectively). (B) Individual predicted vs predicted concentrations of S‐ and R‐ibuprofen. (C, D) Prediction of the fraction of observations to be below the lower limit of quantification (BLOQ) for S‐ and R‐ibuprofen (C and D, respectively). Solid black line represents the median fraction of samples to be BLOQ based on 1000 simulations of the original dataset, black dashed lines represent the 95% confidence interval of the simulated fraction BLOQ. The observed fraction BLOQ samples are represented with a solid red line
3.4. Evaluation and optimizing of dosing regimen
Concentration–time profiles of S‐ and R‐ibuprofen in hypothetical simulated preterm neonates dosed according to the label (10‐5‐5 mg/kg) and as suggested by Hirt et al. are presented in Figure 4. 9 The highest exposure is observed in preterm neonates with a GA of 27 weeks and a PNA of 1 day, illustrating the low CLS in both SGA and AGA preterm neonates at PNA 1. In preterm neonates with a GA of 27 weeks, a median AUC24 of S‐ibuprofen (AUC24,S) of 467 mg h/L was observed when treatment according to the label was initiated at postnatal day 1, of 426 mg h/L upon treatment initiation at postnatal day 3 and of 318 mg h/L when treatment is initiated at postnatal day 7. In SGA preterm neonates with the same age characteristics these values were 414, 334 and 149 mg h/L, respectively. Increasing the dose with PNA, as suggested by Hirt et al. prevents a drop in AUC24,S after treatment initiation at PNA day 3 compared to initiation at day 1 (620 and 464 mg h/L, respectively, for an AGA preterm neonate with a GA of 27 weeks). AUC24,S decreases, however, when treatment is initiated at PNA day 7 (590 mg h/L; Figure 4).
FIGURE 4.
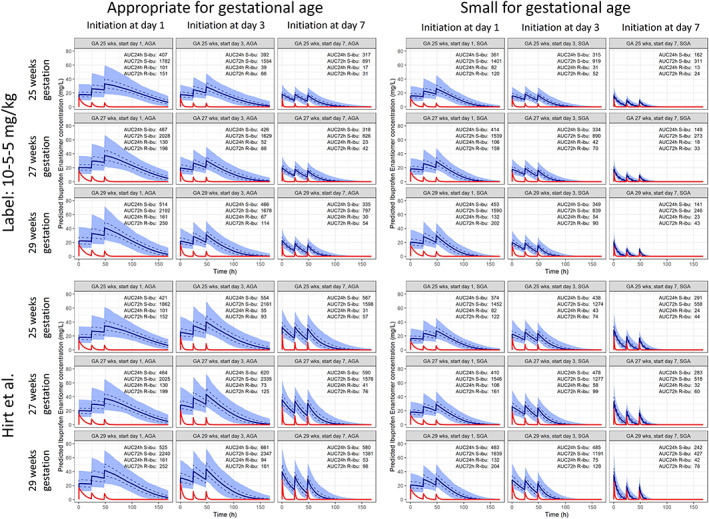
Concentration‐time profiles of S‐ and R‐ibuprofen in subjects dosed according to the label (10–5‐5 mg/kg, top row) and as advised by Hirt et al. (1000 simulations, bottom row).8 Treatment is initiated at postnatal day 1, 3 or 7 in appropriate for gestational age neonates (columns 1–3) and small for gestational age neonates (columns 4–6). Subjects dosed as advised by Hirt et al.8 received a loading dose of 10 mg/kg and 2 maintenance doses of 5 mg/kg every 24 hours when treatment was initiated at PNA 1, a loading dose of 14 mg/kg and 2 maintenance doses every 24 hours of 7 mg/kg when treatment was initiated at PNA 3 days, and a loading dose of 18 mg/kg followed by 2 maintenance doses of 9 mg/kg every 24 upon initiation at PNA 7 days. Both dosing advices are simulated in preterm neonates with gestational ages of 25, 27 and 29 weeks. Solid blue lines represent the median predicted concentration of S‐ibuprofen of 1000 simulations, with a shaded area that represents the 95% prediction interval. The 50% prediction interval is indicated with a dashed line. The red line presents the median predicted concentration of R‐ibuprofen of 1000 simulations
The contribution of R‐ibuprofen to the total exposure to ibuprofen decreases with PNA as result of the higher CLR than CLS and the rapid increase of CLR with PNA. R‐ibuprofen contributes 22% to the total AUC24 when treatment is initiated at PNA 1 day. This contribution drops to 11 and 6% when initiation occurs on postnatal days 3 and 7, respectively, when GA is 27 weeks.
Heat maps in Figure 5 illustrate the variation in exposure to total ibuprofen for a wide range in AGA preterm neonates. Consistent with Figure 4, a lower AUC24 and AUC72 is observed when treatment according to the label is initiated at higher PNAs. After dosing as suggested by Hirt et al. i.e. increasing the dose with PNA, the lowest median AUC24 is observed in preterm neonates with a GA of 24 weeks and treatment initiation at PNA day 2 (410 mg h/L). The highest median AUC24 was observed in a preterm with a GA of 30 weeks and treatment initiation at PNA day 5.
FIGURE 5.
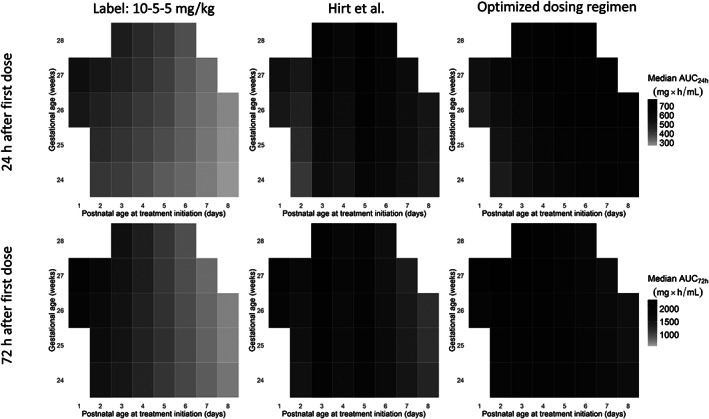
Exposure to total ibuprofen 24 hours (top row) and 72 hours (bottom row) after the first ibuprofen dose of the first treatment cycle, in preterm neonates with different combinations of gestational age and postnatal age at treatment initiation. The colour intensity of a square represents the median AUC of total exposure of 1000 simulations. Left row: dosing following the label; a loading dose of 10 mg/kg followed by 2 maintenance doses of 5 mg/kg every 24 hours. Middle row: dosing as advised by Hirt et al.8: a loading dose of 10 mg/kg and 2 maintenance doses of 5 mg/kg every 24 hours when treatment was initiated at postnatal age (PNA) 1, a loading dose of 14 mg/kg and 2 maintenance doses every 24 hours of 7 mg/kg when treatment was initiated at PNA 3 days, and a loading dose of 18 mg/kg followed by 2 maintenance doses of 9 mg/kg every 24 hours upon initiation at PNA 7 days. In the right column, the optimized dosing regimen was imputed: 10–5–5 mg/kg when initiated at PNA 1, 12–6–6 mg/kg when initiated at PNA 2, 14–7‐7 mg/kg when initiated at PNA 3, 16–8‐8 mg/kg when initiated at PNA 4, 18–9‐9 mg/kg when initiated at PNA 5, 20–10‐10 mg/kg when initiated at PNA 6, 22–11‐11 mg/kg when initiated at PNA 7 and 24–12‐12 mg/kg when initiated at PNA 8. Combinations of initiation postnatal age and gestational age at the edge of both characteristics not present in the original dataset were omitted (grey squares)
Adjusting the dose only on postnatal days 3 and 5 appears to cause a lower exposure in preterm neonate whose treatment initiates at postnatal day 4 and PNA above 5 days. To obtain similar exposure across this population, the dose needs to be increased every postnatal day both at treatment initiation and during treatment. Starting with a loading dose of 10 mg/kg for initiation at PNA 1 day, the loading dose should be increased with 2 mg/kg for every postnatal day of treatment initiation after day 1. The maintenance doses should be adjusted accordingly, starting with a maintenance dose of 5 mg/kg once daily 24 hours after treatment is initiated at PNA day 1 and a dose increase with 1 mg/kg for every postnatal day during the course. An overview of the proposed loading dose and maintenance on each day of treatment initiation is presented in Table 3.
TABLE 3.
Proposed dosing regimen with loading and maintenance doses for each postnatal day of initiation from 1 to 8. Administered doses above 20 mg/kg have not yet been reported and are proposed based on simulation results only. Therefore, the doses above 20 m/kg cannot be directly translated into the clinical practice yet
| Postnatal day of treatment initiation | Loading dose (mg/kg) | First maintenance dose (mg/kg) | Second maintenance dose (mg/kg) |
|---|---|---|---|
| 1 | 10 | 5 | 6 |
| 2 | 12 | 6 | 7 |
| 3 | 14 | 7 | 8 |
| 4 | 16 | 8 | 9 |
| 5 | 18 | 9 | 10 |
| 6 | 20 | 10 | 11 |
| 7 | 22 | 11 | 12 |
| >8 | 24 | 12 | 13 |
Optimization of the dosing regimen results in less variation of AUC24 and AUC72 between patients with different characteristics. The range of AUC24 was 435–766 mg h/L using the optimized dosing regimen, vs 410–761 mg h/L when dosing as advised by Hirt et al. 9 The ranges of AUC72 were 1406–2366 and 1038–2288 mg h/L after dosing according to the optimized dosing regimen and Hirt et al.’s advice, respectively.
4. DISCUSSION
In this study, we were able to describe the PK of S‐ibuprofen and to estimate the exposure to the 2 ibuprofen enantiomers separately. In contrast to previous studies, our cohort of patients has a relatively wide range in PNA, reflecting contemporary practices. We found profound effects of PNA, SGA and GA on CLS, which ask for dose adjustments not only based on body weight, but also on age.
We developed a population PK model for the pharmacologically active enantiomer S‐ibuprofen in which a strong effect of PNA on CLS was identified. A rapid increase of CLS with PNA is previously described by Hirt et al., but might have been obscured by a narrower range in PNA and no distinction between the enantiomers. 9 While CLS cannot directly be compared to clearance of total ibuprofen, Figure 2 shows that the estimated maturation patterns are similar, mutually reassuring estimated maturation within the available PNA range. The maturation of CLS with PNA is expected to reach a plateau at a higher PNA, but the PNA range in our study (0–18 days) was not yet large enough to identify this. Because there is very limited evidence for the treatment of PDA with ibuprofen after the first 2 weeks of life, we do not regard the lack of identifying a plateau a limitation of this study. 23 However, caution is required when extrapolating these results beyond the PNA range with available observations (2–18 days).
The observed higher CLS in SGA vs AGA preterm neonates was unexpected, since usually a decreased drug clearance is observed in this subpopulation. 24 , 25 , 26 , 27 , 28 This finding is substantiated by the lower plasma levels of S‐ibuprofen that can be observed in the raw data (Figure S1A), despite the fact that the 2 groups received a similar dose per kg body weight. Potential effects of the coadministration of fluconazole, known to decrease CLS, as well as differences in albumin and bilirubin plasma levels were examined, but no differences between SGA and AGA groups were identified. 29 Even though the number of SGA preterm neonates just reached the minimum of 10% of the entire population (7 out of 67), only 14 plasma samples were available taken relatively early during treatment (Table 1). We do not consider these data sufficient to propose a dose increase for SGA preterm neonates, but we do see this as a strong signal that SGA preterm neonates are different from their AGA peers in more than just birth weight. A previously reported higher need for surgical ligation of PDA in SGA preterm neonates after pharmacological treatment with indomethacin or ibuprofen might be explained by a higher clearance. 30 More insight into the exposure–response relationship of ibuprofen, and validation of the observed effect of being SGA is needed.
The opportunistic sampling approach used in this study allowed us to study multiple drugs in parallel in a very fragile population, but might not have resulted in the optimal data to study the PK of R‐ibuprofen. 31 , 32 , 33 Despite this limited data we were able to confirm a higher CLR compared to CLS, as also observed in other preterm neonates and older populations (Figure 2). 10 , 11 , 34 , 35 , 36 The limited data available can also explain the lack of identification of chiral inversion of R‐ to S‐ibuprofen, as identified in piglets and well described in adults. 7 , 13 However, the varying fractions of R‐ibuprofen dose to be inverted identified by Gregoire et al. (17 and 61%) already suggested difficulties with identifying this inversion in preterm neonates. Because CLR is estimated to be substantially higher than CLS, potential inverted R‐ibuprofen is expected to have little effect on exposure to S‐ibuprofen. For future studies, it appears to be sufficient to study total ibuprofen, since the clinical impact of R‐ibuprofen seems limited. One other limitation of the present study was the lack of sufficient data on enteral dosing of ibuprofen. Since a higher effectiveness is observed after enteral administration of ibuprofen, identifying PK parameters after gastro‐enteral administration may be of relevance. 5 Evaluation of population predicted‐ and individual predicted concentration plots (Figure S4) on the log‐scale reveals some under‐prediction of the lowest R‐ibuprofen concentrations. The NPDE analysis (Figure S4) confirmed this under‐prediction. However, despite the suboptimal data‐ and predictions of R‐ibuprofen, we believe that this model adds valuable knowledge about enantiomer specific PK of ibuprofen, particularly since minimal knowledge in this population is available at the time of writing.
Heat maps and concentration–time profiles of currently used dosing guidelines suggest a higher exposure in infants with a higher GA, while the model predicts increasing CLS with GA. This appears to be the result of linear scaling of doses with body weight, while Vs is not predicted to increase linearly. Since steady‐state is not yet reached after 72 hours, the relatively lower Vs in preterm neonates with a higher GA results in higher AUC24 and AUC72. Changing plasma albumin levels with GA might be underlying to the nonlinear increase of VS and VR with GA. 37
The generally used 10–5‐5 mg/kg dosing regimen results in large variation in exposure, supporting the findings of Hirt et al.9 that a dose increase is needed when treatment is initiated at a higher PNA. Hirt et al.9 already suggested to increase the dose with 4 mg/kg at PNA 2.9 and 4.5 days, until a PNA of 7.5 days, which results in more similar exposure in preterms starting at a PNA of 1 and 3 days compared to dosing according to the label. However, this dosing regimen stops increasing the dose after a PNA of 7.5 days. Because we had a wider range of data in PNA (observations at 2–18 days), we were able to detect that a further increase in dose would be necessary after 250 hours to maintain an equal exposure. Furthermore, the proposed dosing regimen by Hirt et al.9 consists of a constant maintenance dose, and Figure 4 shows that this will result in varying exposure within the standard 3 days of treatment. A more frequent adjustment of dose resulted in less varying exposure between different treatment days and within 1 treatment regimen.
Based on current knowledge aiming for similar exposure seems to be the first goal. Evidence for target exposure remains, however, limited and is not yet individualized. A described relationship between GA and chance on spontaneous closure of the PDA suggests that similar exposure might not suffice for achieving effective treatment of all preterm neonates and underlines the need for further PK–pharmacodynamic studies. 12
Toxicity is certainly of interest as well, and knowledge on this topic is also limited. The highest dosages of ibuprofen that have been clinically evaluated in preterm neonates include a loading dose of 20 mg/kg and maintenance of 10 mg/kg, but a further increase might be needed. 5 The next step in optimizing the use of ibuprofen to treat PDA is to perform a PK–pharmacodynamic analysis, with which individual targets can be examined, and by including safety measures, a maximum exposure can be determined.
5. CONCLUSIONS
In this study, we described the enantiomer‐specific PK and found that S‐ibuprofen clearance shows important maturation, especially with PNA, resulting in an up to 3‐fold increase in CLS during a 3‐day treatment regimen. This rapid increase in clearance needs to be incorporated in dosing guidelines by adjusting the dose for every day after birth to achieve equal ibuprofen exposure, also during a 3‐day treatment regimen. To obtain similar exposure in AGA preterm neonates, the loading dose should be increased by 2 mg/kg for every postnatal day of treatment initiation, starting with 10 mg/kg. A maintenance of 50% of the loading dose plus 1 mg/kg increase with every postnatal day will result in minimal differences in exposure between different PNAs. Studying enantiomer‐specific PK of ibuprofen did not provide us with new insights, and in future studies measuring total ibuprofen suffices.
ACKNOWLEDGEMENT
The DINO study and all accompanying research were funded by the Netherlands Organisation for Health Research and Development ZonMw (Grant number: 836011022).
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
A.G.J.E., R.B.F, S.V., C.A.J.K. and S.H.P.S wrote the manuscript R.B.F., S.H.P.S., I.K.M.R., J.C.A.d.K., P.A., K.D.L., P.L.J.D., S.C. and J.M. performed the research, A.G.J.E., R.B.F, S.V., C.A.J.K. and S.H.P.S. analysed the data.
Supporting information
FIGURE S1 (A) Observed concentrations of S‐ibuprofen vs time after last dose. Blue points represent concentrations observed in appropriate for gestational age (AGA) preterm neonates, orange points represent concentrations observed in small for gestational age (SGA) preterm neonates. (B) Observed concentrations of S‐ibuprofen stratified by being AGA or SGA and the varying dosing regimens used.
FIGURE S2 (A) Estimated interindividual variability (ETA) on CLS vs available covariates. (B) Estimated interindividual variability (ETA) on VS vs available covariates.
FIGURE S3 Representation of the structure of the enantiomer model.
FIGURE S4 (A, B) Normalized prediction distribution error (npde) evaluation of the final model predictions for S‐ibuprofen (A) and R‐ibuprofen (B). Top left: quantile‐quantile plot, top right: npde quantiles with a normal distribution overlay, bottom left: npde vs time after dose, bottom right: npde vs observed concentrations and predicted concentrations. Red stars represent observations below the lower limit of quantification. (C) Population predicted concentration of S‐ and R‐ibuprofen. (D) Individual predicted vs predicted concentrations of S‐ and R‐ibuprofen. (E) Weighted residuals vs population predicted concentrations of S‐ and R‐ibuprofen. (F) Weighted residuals vs time after dose.
FIGURE S5 Prediction‐corrected visual predictive check (pcVPC) of the final model, stratified by enantiomer. Black dots represent the prediction‐corrected observed concentrations, and the dotted black lines represent the median and 95th and 5th percentile of the data. Red squares represent the 95% confidence interval of the 1000 simulated prediction‐corrected concentrations, and blue squares represent the 95% confidence intervals of the 95th and 5th percentiles of the simulated prediction‐corrected concentrations.
FIGURE S6 Prediction‐corrected visual predictive check (pcVPC) of the performance of the final model on simulating S‐ibuprofen concentrations, vs weight (A) and postmenstrual age (B). Black dots represent the prediction‐corrected observed concentrations, and the dotted black lines represent the median and 95th and 5th percentile of the data. Red squares represent the 95% confidence interval of the 1000 simulated prediction‐corrected concentrations, and blue squares represent the 95% confidence intervals of the 95th and 5th percentiles of the simulated prediction‐corrected concentrations.
Engbers AGJ, Flint RB, Völler S, et al. Enantiomer specific pharmacokinetics of ibuprofen in preterm neonates with patent ductus arteriosus. Br J Clin Pharmacol. 2020;86:2028–2039. 10.1111/bcp.14298
The authors confirm that the PI's for this paper were Sinno Simons, Peter Andriessen, Kian Liem and Pieter Degraeuwe and that they had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Koch J. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117(4):1113‐1121. 10.1542/peds.2005-1528 [DOI] [PubMed] [Google Scholar]
- 2. Sathanandam S, Whiting S, Cunningham J, et al. Practice variation in the management of patent ductus arteriosus in extremely low birth weight infants in the United States: survey results among cardiologists and neonatologists. Congenit Heart Dis. 2019;14(1):6‐14. 10.1111/chd.12729 [DOI] [PubMed] [Google Scholar]
- 3. Ferguson JM. Pharmacotherapy for patent ductus arteriosus closure. Congenit Heart Dis. October 2018;2018:52‐56. 10.1111/chd.12715 [DOI] [PubMed] [Google Scholar]
- 4. Cristea S, Allegaert K, Falcao AC, et al. Larger dose reductions of vancomycin required in neonates with patent ductus arteriosus receiving indomethacin vs. ibuprofen. Antimicrob Agents Chemother. 2019;63(8):1‐8. 10.1128/aac.00853-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitra S, Florez ID, Tamayo ME, et al. Association of placebo, indomethacin, ibuprofen, and acetaminophen with closure of hemodynamically significant patent ductus arteriosus in preterm infants a systematic review and meta‐analysis. JAMA ‐ J am Med Assoc. 2018;319(12):1221‐1238. 10.1001/jama.2018.1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. 2020;(2). 10.1002/14651858.CD003481.pub8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rainsford KD. Ibuprofen: Discovery, Development and Therapeutics. 1st ed. Hoboken, New Jersey: Wiley‐Blackwell; 2015. [Google Scholar]
- 8. NeoProfen (ibuprofen lysine) Injection for intravenous use [FDA label]. 2006.
- 9. Hirt D, Van Overmeire B, Treluyer JM, et al. An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamic study. Br J Clin Pharmacol. 2008;65(5):629‐636. 10.1111/j.1365-2125.2008.03118.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gregoire N, Gualano V, Geneteau A, et al. Population pharmacokinetics of ibuprofen enantiomers in very premature neonates. J Clin Pharmacol. 2004;44(10):1114‐1124. 10.1177/0091270004268320 [DOI] [PubMed] [Google Scholar]
- 11. Gregoire N, Desfrere L, Roze J‐C, Kibleur Y, Koehne P. Population pharmacokinetic analysis of ibuprofen enantiomers in preterm newborn infants. J Clin Pharmacol. 2008;48(12):1460‐1468. 10.1177/0091270008323752 [DOI] [PubMed] [Google Scholar]
- 12. Flint RB, ter Heine R, Spaans E, et al. Simulation‐based suggestions to improve ibuprofen dosing for patent ductus arteriosus in preterm newborns. Eur J Clin Pharmacol. 2018;74(12):1585‐1591. 10.1007/s00228-018-2529-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Millecam J, van Bergen T, Schauvliege S, et al. Developmental pharmacokinetics and safety of ibuprofen and its enantiomers in the conventional pig as potential pediatric animal model. Front Pharmacol. 2019;10 10.3389/fphar.2019.00505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Comets E, Brendel K, Mentré F. Computing normalised prediction distribution errors to evaluate nonlinear mixed‐effect models: the npde add‐on package for R. Comput Methods Programs Biomed. 2008;90(2):154‐166. 10.1016/j.cmpb.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 15. Michailidou A, Trenz H‐J, de Wilde P. Pedea 5 mg/ml solution for injection [EMA label]. 2019. 10.2307/j.ctvdf0dxq.12 [DOI]
- 16. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481‐504. 10.1023/A:1012299115260 [DOI] [PubMed] [Google Scholar]
- 17. Nguyen THT, Mouksassi MS, Holford N, et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol. 2017;6(2):87‐109. 10.1002/psp4.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bergstrand M, Karlsson MO. Handling data below the limit of quantification in mixed effect models. AAPS j. 2009;11(2):371‐380. 10.1208/s12248-009-9112-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoftiezer L, Hof MHP, Dijs‐Elsinga J, Hogeveen M, Hukkelhoven CWPM, van Lingen RA. From population reference to national standard: new and improved birthweight charts. Am J Obstet Gynecol. 2019;220(4):383.e1‐383.e17. 10.1016/j.ajog.2018.12.023 [DOI] [PubMed] [Google Scholar]
- 20. Rey E, Pariente‐Khayat A, Gouyet L, et al. Stereoselective disposition of ibuprofen enantiomers in infants. Br J Clin Pharmacol. 1994;39:373‐375. 10.1111/j.1365-2125.2004.02288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelley MT, Walson PD, Edge JH, Cox S, Mortensen ME. Pharmacokinetics and pharmacodynamics of ibuprofen isomers and acetaminophen in febrile children. Clin Pharmacol Ther. 1992;52(2):181‐189. 10.1038/clpt.1992.128 [DOI] [PubMed] [Google Scholar]
- 22. Anderson BJ, Hannam JA. A target concentration strategy to determine ibuprofen dosing in children. Pediatr Anesth. 2019;29(11):1107‐1113. 10.1111/pan.13731 [DOI] [PubMed] [Google Scholar]
- 23. Frauenfelder O, van Beynum IM, Reiss IKM, Simons SHP. Ibuprofen for ductus arteriosus months after birth. Case Rep Pediatr. 2016;2016:1‐3. 10.1155/2016/2659389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mugabo P, Els I, Smith J, et al. Nevirapine plasma concentrations in premature infants exposed to single‐dose nevirapine for prevention of mother‐tochild transmission of HIV‐1. South African Med J. 2011;101(9):655‐658. [PubMed] [Google Scholar]
- 25. Chaudhuri M, Garg SK, Narang A, Bhakoo ON. Kinetics of theophylline in apnea of prematurity in small for gestational age babies. Indian Pediatr. 1996;33:181‐187. 10.1161/CIRCHEARTFAILURE.112.967299 [DOI] [PubMed] [Google Scholar]
- 26. Lulic‐Botica M, Sheer T, Edwards D, Thomas RL, Natarajan G. Impact of small‐for‐gestational age (sga) status on gentamicin pharmacokinetics in neonates. J Clin Pharmacol. 2014;54(1):39‐45. 10.1002/jcph.190 [DOI] [PubMed] [Google Scholar]
- 27. Allegaert K, Anderson BJ, van den Anker JN, Vanhaesebrouck S, de Zegher F. Renal drug clearance in preterm neonates: relation to prenatal growth. Ther Drug Monit. 2007;29(3):284‐291. 10.1097/FTD.0b013e31806db3f5 [DOI] [PubMed] [Google Scholar]
- 28. Frattarelli DAC, Ergun H, Lulic‐Botica M, Lehr VT, Aranda JV. Vancomycin elimination in human infants with intrauterine growth retardation. Pediatr Infect Dis J. 2005;24(11):979‐983. 10.1097/01.inf.0000186283.95728.34 [DOI] [PubMed] [Google Scholar]
- 29. Hynninen VV, Olkkola KT, Leino K, et al. Effects of the antifungals voriconazole and fluconazole on the pharmacokinetics of S‐(+)‐ and R‐(−)‐ibuprofen. Antimicrob Agents Chemother. 2006;50(6):1967‐1972. 10.1128/AAC.01483-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boghossian NS, Do BT, Bell EF, et al. Efficacy of pharmacologic closure of patent ductus arteriosus in small‐for‐gestational‐age extremely preterm infants. Early Hum Dev. 2017;113:10‐17. 10.1016/j.earlhumdev.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Völler S, Flint RB, Beggah F, et al. Recently registered midazolam doses for preterm neonates Do not Lead to equal exposure: a population pharmacokinetic model. J Clin Pharmacol. 2019;59(10):1300‐1308. 10.1002/jcph.1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Völler S, Flint RB, Stolk LM, et al. Model‐based clinical dose optimization for phenobarbital in neonates: an illustration of the importance of data sharing and external validation. Eur J Pharm Sci. 2017;109:S90‐S97. 10.1016/j.ejps.2017.05.026 [DOI] [PubMed] [Google Scholar]
- 33. Völler S, Flint RB, Andriessen P, et al. Rapidly maturing fentanyl clearance in preterm neonates. Arch Dis Child Fetal Neonatal Ed. 2019;104(6):5‐10. 10.1136/archdischild-2018-315920 [DOI] [PubMed] [Google Scholar]
- 34. Zheng C, Hao H, Wang G, et al. Chiral separation of ibuprofen and chiral pharmacokinetics in healthy Chinese volunteers. Eur J Drug Metab Pharmacokinet. 2008;33(1):45‐51. 10.1007/BF03191018 [DOI] [PubMed] [Google Scholar]
- 35. Ochoa D, Prieto‐Pérez R, Román M, et al. Effect of sex and CYP2C9 and CYP2C8 polymorphisms on the pharmacokinetics of ibuprofen enantiomers. Pharmacogenomics. 2015;16(9):939‐948. 10.2217/PGS.15.40 [DOI] [PubMed] [Google Scholar]
- 36. Anderson BJ, Hannam JA. A target concentration strategy to determine ibuprofen dosing in children. Paediatr Anaesth. 2019;29(11):1107‐1113. 10.1111/pan.13731 [DOI] [PubMed] [Google Scholar]
- 37. Cartlidge PH, Rutter N. Serum albumin concentrations and oedema in the newborn. Arch Dis Child. 1986;61:657‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 (A) Observed concentrations of S‐ibuprofen vs time after last dose. Blue points represent concentrations observed in appropriate for gestational age (AGA) preterm neonates, orange points represent concentrations observed in small for gestational age (SGA) preterm neonates. (B) Observed concentrations of S‐ibuprofen stratified by being AGA or SGA and the varying dosing regimens used.
FIGURE S2 (A) Estimated interindividual variability (ETA) on CLS vs available covariates. (B) Estimated interindividual variability (ETA) on VS vs available covariates.
FIGURE S3 Representation of the structure of the enantiomer model.
FIGURE S4 (A, B) Normalized prediction distribution error (npde) evaluation of the final model predictions for S‐ibuprofen (A) and R‐ibuprofen (B). Top left: quantile‐quantile plot, top right: npde quantiles with a normal distribution overlay, bottom left: npde vs time after dose, bottom right: npde vs observed concentrations and predicted concentrations. Red stars represent observations below the lower limit of quantification. (C) Population predicted concentration of S‐ and R‐ibuprofen. (D) Individual predicted vs predicted concentrations of S‐ and R‐ibuprofen. (E) Weighted residuals vs population predicted concentrations of S‐ and R‐ibuprofen. (F) Weighted residuals vs time after dose.
FIGURE S5 Prediction‐corrected visual predictive check (pcVPC) of the final model, stratified by enantiomer. Black dots represent the prediction‐corrected observed concentrations, and the dotted black lines represent the median and 95th and 5th percentile of the data. Red squares represent the 95% confidence interval of the 1000 simulated prediction‐corrected concentrations, and blue squares represent the 95% confidence intervals of the 95th and 5th percentiles of the simulated prediction‐corrected concentrations.
FIGURE S6 Prediction‐corrected visual predictive check (pcVPC) of the performance of the final model on simulating S‐ibuprofen concentrations, vs weight (A) and postmenstrual age (B). Black dots represent the prediction‐corrected observed concentrations, and the dotted black lines represent the median and 95th and 5th percentile of the data. Red squares represent the 95% confidence interval of the 1000 simulated prediction‐corrected concentrations, and blue squares represent the 95% confidence intervals of the 95th and 5th percentiles of the simulated prediction‐corrected concentrations.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


