Abstract
Primates live in very diverse environments and, as a consequence, show an equally diverse locomotor behaviour. During locomotion, the primate hand interacts with the superstrate and/or substrate and will therefore probably show adaptive signals linked with this locomotor behaviour. Whereas the morphology of the forearm and hand bones have been studied extensively, the functional adaptations in the hand musculature have been documented only scarcely. To evaluate whether there are potential adaptations in forelimb musculature to locomotor behaviour, we investigated the forearm and hand musculature of the highly arboreal gibbons (including Hylobates lar, Hylobates pileatus, Nomascus leucogenys, Nomascus concolor, Symphalangus syndactylus) and compared this with the musculature of the semi‐terrestrial rhesus macaques (Macaca mulatta) by performing complete and detailed dissections on a sample of 15 unembalmed specimens. We found that the overall configuration of the upper arm and hand musculature is highly comparable between arboreal gibbons and semi‐terrestrial macaques, and follows the general primate condition. Most of the identified differences in muscle configuration are located in the forearm. In macaques, a prominent m. epitrochleoanconeus is present, which potentially helps to extend the forearm and/or stabilize the elbow joint during quadrupedal walking. The m. flexor carpi radialis shows a more radial insertion in gibbons, which might be advantageous during brachiation, as it can aid radial deviation. The fingers of macaques are controlled in pairs by the m. extensor digiti secondi et tertii proprius and the m. extensor digiti quarti et quinti proprius—a similar organization can also be found in their flexors—which might aid in efficient positioning of the hand and fingers on uneven substrates during quadrupedal walking. In contrast, extension of the little finger in gibbons is controlled by a separate m. extensor digiti minimi, whereas digits 2 to 4 are extended by the m. extensor digitorum brevis, suggesting that simultaneous extension of digits 2–4 in gibbons might be important when reaching or grasping an overhead support during brachiation. In conclusion, the overall configuration of the forelimb and hand musculature is very similar in gibbons and macaques, with some peculiarities which can be linked to differences in forelimb function and which might be related to the specific locomotor behaviour of each group.
Keywords: adaptation, anatomy, hylobatids, locomotion, macaques, primates
The overall configuration of the forelimb and hand musculature is very similar in gibbons and macaques, with some peculiarities which can be linked to differences in forelimb function and which might be related to the specific locomotor behaviour of each group.

1. INTRODUCTION
The primate hand displays a large variety of phenotypes which reflects an equally diverse functional repertoire (Horn, 1972; Vereecke, D’Août and Aerts, 2006; Marzke, 2009; Williams, 2010; Almécija, Smaers and Jungers, 2015; Liu, Xiong and Hu, 2016; Thompson et al., 2018). Understanding how these phenotypes correlate to different locomotor behaviours of distinct primate taxa may facilitate the interpretation of hand function from primate fossil remains. Nonhuman primates use their hands for manipulation as well as for locomotion, and adaptive signals to these specific functions in the morphology of the forearm and hand bones have been studied extensively. For example, gibbons have a large wrist mobility in all directions as a potential adaptation to suspensory locomotion (Richmond, 2001), which is associated with a ball‐and‐socket configuration of the midcarpal joint (Lemelin and Schmitt, 1998; Orr et al., 2010; McMahon, Zijl and Gilad, 2015; Prime and Ford, 2016; Orr, 2017; 2018). In macaques, the articular surface of the basal manual phalanges is proximodorsally excavated to enable hyperextension, which is a specialization for digitigrade locomotion (Hayama, Chatani and Nakatsukasa, 1994; Lemelin and Schmitt, 1998). In addition, macaques have a broad midcarpal joint morphology which is interpreted as being advantageous for loading during quadrupedal walking (Lewis, 1985; Lemelin and Schmitt, 1998; Richmond, 2001; Daver, Berillon and Grimaud‐Hervé, 2012). These previous studies focused on adaptations in skeletal morphology, while functional adaptations in the hand musculature have been documented only scarcely. Detailed descriptions of forelimb musculature in different primate taxa, as well as comparative analyses between those taxa, are limited in the current literature (Tuttle, 1967; 1969; Lemelin and Diogo, 2016). In 2009, Michilsens et al. conducted a study on the functional anatomy of the gibbon forelimb, with a detailed account on the upper and lower arm musculature in four different gibbon species (Hylobates lar, Hylobates pileatus, Hylobates moloch, Symphalangus syndactylus; n = 11; Michilsens et al., 2009). Here, we extend this dataset with eight additional gibbon specimens of five different species (H. lar, H. pileatus, Nomascus concolor, N. leucogenys, S. syndactylus) and also include detailed information on intrinsic hand musculature. In addition, we compare the forelimb muscle configuration of the highly arboreal gibbons with that of the semi‐terrestrial macaques (Fam. Cercopithecidae) to evaluate whether there are specific adaptations in forelimb musculature that could be related to locomotor behaviour. A full quantification of the forelimb and hand musculature in both primate taxa will be presented as Part 2 of this study.
2. METHODS
2.1. Specimen collection
As multiple species of the hylobatid family are included in this study, they are further referred to as ‘gibbons’. The forearm and hand of eight (sub)adult gibbons were obtained via collaborations with different European Zoos and institutes: the National Museum of Scotland (Edinburgh, UK), Ghent University (campus Merelbeke, Belgium), the Zoological and Botanical Park of Mulhouse (France), Pakawi Park (Belgium). The forearm and hand of seven adult rhesus macaques were obtained via collaboration with the Ghent University (campus Merelbeke, Belgium) and KU Leuven (campus Gasthuisberg, Belgium). All specimens were collected opportunistically, no animals were sacrificed for this study. The forelimbs were disarticulated at level of the shoulder in all specimens. Full specimen details are provided in Table 1.
TABLE 1.
Specimen details
| Code | Subject identifier | Sex | Age | Injury | Sample | Collection |
|---|---|---|---|---|---|---|
| Hl1 | SR192.17 | F | $ | X, + | L | NMS, Edinburgh, UK |
| Hl2 | GH161.17 | U | $ | X, + | R | NMS, Edinburgh, UK |
| Hl3 | Gent1 | U | $ | R | Ghent University, campus Merelbeke, Belgium | |
| Hp1 | SR100.18 | M | Adult | X, + | R | NMS, Edinburgh, UK |
| Nc1 | 2007BE404 | M | Adult | L | Pakawi Park, Belgium | |
| Nl1 a | M09103 | M | Adult | R | Zoological and Botanical Park of Mulhouse, France | |
| Ss1 | M7139 | M | Adult | X | R | NMS, Edinburgh, UK |
| Ss2 | SR93.17 | M | Adult | X | L | NMS, Edinburgh, UK |
| Mm1 | Gent1 | U | Adult | R | Ghent University, campus Merelbeke, Belgium | |
| Mm2 | Gent2 | F | Adult | DP 4 | L | Ghent University, campus Merelbeke, Belgium |
| Mm3 | Gent3 | U | Adult | L | Ghent University, campus Merelbeke, Belgium | |
| Mm4 | Gent4 | U | Adult | L | Ghent University, campus Merelbeke, Belgium | |
| Mm5 | Gent5 | U | Adult | PIP 3 joint | L | Ghent University, campus Merelbeke, Belgium |
| Mm6 | Gent6 | M | Adult | L | Ghent University, campus Merelbeke, Belgium | |
| Mm7 | Leuven1 | U | Adult | IP 2 and 3 joints | R | KU Leuven, campus Gasthuisberg, Belgium |
Hl: Hylobates lar, Hp: Hylobates pileatus, Nc: Nomascus concolor, Nl: Nomascus leucogenys, Ss: Symphalangus syndactylus, Mm: Macaca mulatta.
DP, distal phalanx; F, female; IP, interphalangeal joint; M, male; NMS, National Museum of Scotland; PIP, proximal interphalangeal joint; RZSA, Royal Zoological Society of Antwerp; +, extrinsic muscles of tendons damaged due to skinning postmortem; U, unknown; X, thenar muscles damaged due to skinning postmortem.
Wild born, $ (Young) subadult based on presence of unfused growth plates.
2.2. Dissection procedure
The specimens were stored at −18°C and were thawed at room temperature 24 hr prior to the dissections. A complete dissection of the left or right forearm and hand was performed for all specimens (unilateral sampling). All muscles were isolated one by one and their origins and insertions were documented and compared with previous studies (Tuttle, 1969; Gibbs, Collard and Wood, 2002; Michilsens et al., 2009; Diogo and Wood, 2012a; Aversi‐Ferreira et al., 2016; van Leeuwen et al., 2018). Presence or absence of muscles or abnormalities was also noted. The dissections were documented extensively using a dedicated photography setup. Some specimens were skinned prior to transport to the university, which caused damage to the thenar and hypothenar muscles, and/or the tendons of the extrinsic muscles in some specimens (see Table 1).
3. RESULTS
The description of the extrinsic and intrinsic hand muscles discussed below are based on detailed dissections of a macaque (n = 7) and gibbon (n = 8) sample. As some specimens were damaged, due to either skinning or the dislocation at the level of the shoulder, the number of included specimens varies for each muscle. The exact number of specimens is shown each time between parentheses. Details on the origin and insertion of all extrinsic and intrinsic muscles are listed in (Table S1). Anatomical data on bonobos and humans from previous dissections are also added for comparison (van Leeuwen et al., 2018).
3.1. Upper arm musculature
The long head of the m. biceps brachii (Bb) originates from the supraglenoid tubercle of the scapula (11/11) and inserts onto the radial tuberosity (14/14) in all macaque and gibbon specimens. In macaques, the short head originates from the coracoid process of the scapula, similar to modern humans and most other primates, and fuses with the muscle belly of the long head (6/6). In gibbons, however, the short head originates from the crest of the lesser tubercle of the humerus (7/7) and inserts on the bicipital aponeurosis into the deep fascia on the medial forearm (connection with FDS) (8/8). This supports previous findings that in most primates both the long head and short head of the Bb cross the shoulder joint, whereas in gibbons only the long head crosses the shoulder (Jungers and Stern, 1980; Michilsens et al., 2009). The short head works as an elbow flexor and forearm supinator, without action at the shoulder joint. This could imply that the humeral flexion capacity of the Bb is reduced in gibbons compared with macaques and other primates with a bi‐articular configuration of the short head of the Bb. In gibbons, the short head of the Bb forms a ventral muscle chain between the m. pectoralis major (PM) and m. flexor digitorum superficialis (FDS) (see Jungers and Stern, 1980). The fusion between these multiple‐joint muscles is thought to conduct the flexor force of the PM distally across the shoulder, elbow and wrist joints so that active or passive tension in this muscle results in automatic flexion of the forearm and fingers without requiring activity in the distal muscles of the chain. While such ventral muscle chain would indeed be advantageous for brachiating gibbons, the function of this chain remains debated (Jungers and Stern, 1980; Michilsens et al., 2009).
The m. triceps brachii (Tb) consists of three heads in all specimens (15/15). The Tb originates from the infraglenoid tubercle of the scapula (long head) (12/12) and the humeral shaft (lateral and medial head) (14/14), and inserts onto the olecranon (14/14). In most macaques the long and medial head are completely separate (5/6), whereas in all gibbons, the three heads are fused at the insertion (8/8), which is also seen in modern humans. In macaques, the Tb is an important muscle during quadrupedal walking, as it produces the torque at the elbow joint during the first three‐quarters of the step (Manter, 1938). During brachiation in gibbons, the Tb will probably primarily act at the shoulder (Michilsens et al., 2009).
The m. dorso‐epitrochlearis (DET) originates in both macaques and gibbons from the muscle belly of the m. latissimus dorsi (12/12), yet the insertion is variable. The DET inserts onto the olecranon and the fascia of Bb and Tb in all macaques (6/6) and some gibbons (3/8), but in most gibbons it inserts via a tendon sheet onto the medial epicondyle of the humerus (5/8). The DET, clearly present in both macaques and gibbons, is rarely seen in humans (Cheng and Scott, 2000) as fewer than 30 cases have been reported over the past 200 years (Natsis et al., 2012). The function of the DET is still debated. It has long been speculated to facilitate force transmission from the shoulder to the fingertips by acting as a dorsal muscle chain (Sonntag, 1922; Andrews and Groves, 1976); however, EMG studies have shown that the DET might only be a morphological consequence of the rearrangement of the origin of the short head of the Bb (Jungers and Stern, 1980). The DET has also been labelled as a ‘climbing muscle’ because of its connection with the m. latissimus dorsi, as fusion of these muscles contributes to increased concerted contraction (Sonntag, 1922).
The m. brachialis (B) originates from the distal half (13/14) or complete (1/14) shaft of the humerus and inserts onto the tuberosity of the ulna (14/14). Occasional fusion with the m. supinator (SUP) in macaques (1/6) or with the m. pronator teres (PT) in gibbons (1/8) can occur. It is considered a pure elbow flexor in primates, including humans.
The m. coracobrachialis (CB) consists of a long (middle) and medial (deep) head in macaques (6/6) (cf. Aversi‐Ferreira et al., 2016). Both heads originate from the coracoid process of the scapula and the common coracoid tendon (6/6), and the long head is always fused with the short head of the Bb (6/6). The long head inserts midway on the humeral shaft (6/6) and the medial head inserts more proximally, onto the surgical neck of the humerus (6/6). In gibbons, the CB shows a one‐headed configuration similar to modern humans, originating from the coracoid process of the scapula (7/7) and a direct, muscle fibre insertion onto the periosteum of the proximal (1/8) or middle (7/8) humeral shaft. In macaques, the two‐headed configuration might increase internal rotation and adduction of the arm during quadrupedal walking (Aversi‐Ferreira et al., 2016).
The m. epitrochleoanconeus (ETA) is a separate, well‐defined muscle in macaques that originates from the medial epicondyle of the humerus and inserts onto the medial border of the olecranon (7/7) (Figure 1). A well‐developed ETA is absent in gibbons (8/8), but a strong ligament—similar to the ulnar collateral ligament in humans—can be found on the position of the macaque ETA (8/8). There is some confusion about the presence or absence of the ETA in primates. In bonobos and other great apes, the presence is debated, as it can easily be missed or considered part of the FCU during dissections of the forearm (Diogo and Wood, 2012a; Diogo, Molnar and Wood, 2017). In humans as well, there is still no agreement about whether the ETA is present (Hirasawa, Sawamura and Sakakida, 1979; Gessini et al., 1981; Uscetin et al., 2014; de Ruiter and van Duinen, 2017) or absent (Diogo, Richmond and Wood, 2012a). In present or past dissections that we conducted we never found a distinct ETA in either bonobos or human cadavers (Marie JM Vanhoof, pers. obs.). In macaques, the ETA covers the cubital tunnel, where it protects the ulnar nerve as it passes through the elbow. Furthermore, activation of the ETA potentially facilitates forearm extension and/or stabilization of the elbow joint during quadrupedal walking (Diogo and Wood, 2012a).
FIGURE 1.
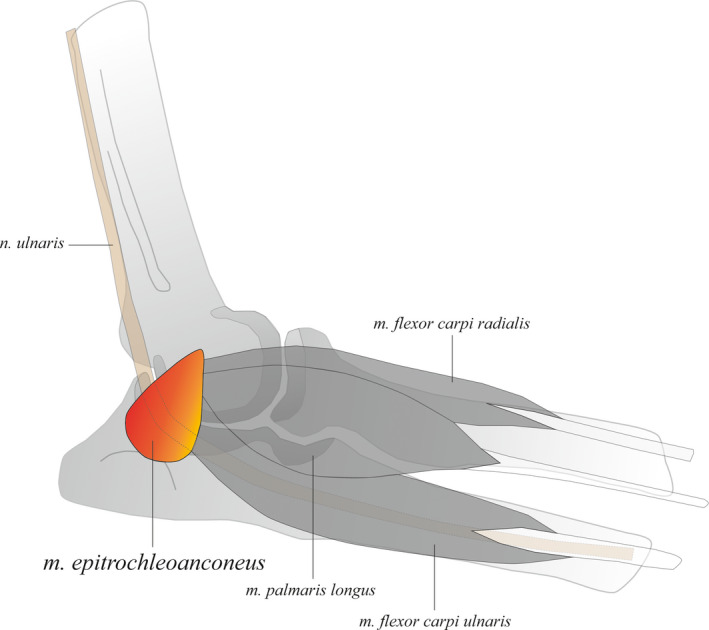
The well‐developed m. epitrochleoanconeus (ETA) of macaques. It originates from the medial epicondyle of the humerus and inserts onto the medial border of the olecranon. As it crosses the cubital tunnel, the ETA protects the ulnar nerve. Activation of the ETA potentially facilitates forearm extension and/or stabilization of the elbow joint during quadrupedal walking [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. Forearm rotators
The m. brachioradialis (BR) invariably originates from the supracondylar ridge of the humerus (15/15) and shows incidental proximal fusion with the m. brachialis (B) in both macaques (2/7) and gibbons (2/8). In macaques, the BR muscle belly runs underneath the m. extensor carpi radialis longus (ECRL) and m. extensor carpi radialis brevis (ECRB) at the origin, and inserts with a long tendon onto the styloid process of the radius (7/7). In gibbons, the BR runs superficial to the ECRL and ECRB and its long tendon inserts either directly onto the styloid process (3/8) or onto the shaft of the radius proximal to the styloid (5/8), with the tendon running further along the radius to end on the styloid process (1/8) or just proximal to it (4/8), a situation similar to bonobos. In humans, the BR is a strong elbow flexor when the forearm is in a mid‐position between pronation and supination at the radioulnar joint, and works synergistic with the B and Bb, a function which is also important during brachiation in gibbons. During pronation, the BR is more active during elbow flexion because the Bb is at a mechanical disadvantage, as is probably also the case in macaques during quadrupedal walking (Boland, Spigelman and Uhl, 2008).
The m. supinator (SUP) originates from the lateral epicondyle of the humerus and inserts onto the proximal half of the radius in all specimens (15/15), similar to humans. In all macaques (7/7) and most gibbon specimens, the SUP has an additional origin from the proximal third of the ulna, whereas in bonobos this is the main origin of the SUP. In macaques, the SUP probably acts as a supinator of the forearm as in modern humans, although the exact activity pattern of the SUP during quadrupedal locomotion has not yet been investigated. In gibbons, EMG studies have indicated that the SUP mainly acts in the support phase of brachiation, during which time the forearm passes increasingly into supination (Stern and Larson, 2001).
The m. pronator teres (PT) consists of a single humeral head in macaques with its origin on the medial epicondyle of the humerus (7/7). In gibbons, either a humeral head (7/8), as seen in macaques, or an ulnar head, which originates from the proximal ulna (1/8), is present. Although Miller (1932) stated that the PT of all hominoids has two heads (Miller, 1932), a configuration we also found in bonobos, a two‐headed configuration with a humeral and ulnar head as seen in humans and great apes was never observed in the gibbon specimens. In all primate specimens, the PT inserts halfway the radius (15/15), and fusion with the FCR (macaque: 3/7, gibbon: 1/8), the FDS (macaque: 3/7) or B (gibbon: 1/8) may occur. Poor development or absence of the ulnar head seems to be a common variation in humans (Jamieson and Anson, 1952; Caetano et al., 2017), which is supported by phylogenetic development, as in most mammals (except anthropoid apes) the ulnar head is completely missing (Macalister, 1868; McMurrich, 1903). In humans, the median nerve passes between both heads of the PT, which enhances the risk of entrapment of the median nerve, also called the ‘pronator teres syndrome’ (Nigst and Dick, 1979; Hartz et al., 1981; Fuss and Wurzl, 1990). Absence of the ulnar head in all macaques and most of the gibbons might be important to avoid such entrapment during locomotion. Another possible explanation is that the presence of an ulnar head in humans allows pronation of the forearm independent of the position of the elbow. In macaques, the angle of the elbow joint is relatively constant during quadrupedal locomotion (Demes et al., 1998), and an ulnar head might not be needed. In gibbons, the PT is primarily active, as the elbow is flexed in the middle of the swing phase of brachiation (Stern and Larson, 2001), which might indicate that forearm pronation independent of elbow position is also not important in gibbons. Indeed, an ulnar head in gibbons was only observed in one individual.
The m. pronator quadratus (PQ) has a rather consistent configuration in macaques and gibbons. It originates from the distal ulna (13/15) and inserts onto the distal interosseous membrane and the distal radius (13/15), similar to bonobos. In two gibbon specimens (2/8), the PQ appears as two fused muscle bellies. The proximal belly (deep head) inserts with a tendinous portion onto the distal radius. The distal belly (superficial head) is larger and originates from the distal radius and inserts with tendinous fibres onto the distal ulna. As reported in literature, this configuration with two bellies is also commonly seen in humans (Johnson and Shrewsbury, 1976; Stuart, 1996). In macaques and gibbons, the fibres of the PQ consistently show an oblique orientation, which is also seen in bonobos, whereas in humans, only the fibres of the deep head show an oblique orientation, as the fibres of the superficial head are transversely oriented from origin to insertion. It has been suggested that the human superficial head, due to its transverse fibre orientation, is the initiator and rotator for pronation, whereas the deep head is mostly involved in stabilizing the distal radioulnar joint (Johnson and Shrewsbury, 1976). This indicates that the primary function of the PQ in gibbons and macaques, due to the oblique fibre orientation, is stabilization of the distal radioulnar joint, which implies that the PT is the most important forearm pronator in these primates. The idea that the PQ in gibbons and macaques plays a role as a dynamic ligament corresponds to its positioning close to the distal radioulnar joint (small moment arm) and its relatively small size (distal one‐quarter to one‐fifth of the forearm) compared with modern humans (distal one‐third of the forearm). In gibbons, the PQ is also used to position the hand prior to grasping a new support (Stern and Larson, 2001).
3.3. Extrinsic hand musculature
The m. extensor carpi radialis longus (ECRL) and m. extensor carpi radialis brevis (ECRB) show a similar configuration in both macaques and gibbons. The ECRL originates from the lateral supracondylar ridge of the humerus, distal to the BR (15/15), and inserts onto the base of metacarpal 2 (MC2) (15/15), similar to the configuration in bonobos. In two gibbon specimens, the ECRL sends a tendon slip to the base of MC1 at the insertion (2/8). This means that in these specimens, the ECRL could also assist in thumb extension and abduction and/or stabilization of the trapeziometacarpal joint. The ECRB originates from the lateral supracondylar ridge of the humerus, distal from the ECRL and inserts onto the dorsoradial base of MC3 in all macaques (7/7). Fusion with the m. extensor digitorum communis (EDC) (4/7) can occur. In gibbons, the ECRB originates either solely from the lateral epicondyle of the humerus (1/8) or from the lateral supracondylar ridge of the humerus (7/8) in combination with the lateral epicondyle (1/8). In bonobos, the ECRB originates solely from the lateral epicondyle of the humerus. As in macaques, fusion with the EDC is possible but is only observed in one gibbon specimen (1/8). The ECRB inserts onto the dorsoradial base of MC3 (8/8). Both the ECRL and ECRB, synergist muscles with a similar function, can be proximally fused in macaques (6/7) and gibbons (2/8), which leads to a concerted action between both muscles.
The m. extensor carpi ulnaris (ECU) originates from the common extensor tendon at the lateral epicondyle of the humerus and inserts with a long tendon onto the ulnar base of MC5 in all macaques (7/7). In gibbons, the ECU also originates from the lateral epicondyle of the humerus (8/8), sometimes in combination with the proximal ulna (1/8), which is the main origin in bonobos, or the olecranon (1/8). The insertion on MC5 is similar to that of macaques in all gibbon specimens (8/8). Given its position in the forearm, the ECU functions as wrist extensor and ulnar deviator in macaques and gibbons, common to what is observed in other primates, including humans.
The m. extensor digitorum communis (EDC) originates with a common tendon from the lateral epicondyle of the humerus in all specimens (15/15), similar to bonobos and humans, and is proximally fused with the m. extensor digiti minimi (EDM) in half of the gibbons (4/8). The EDC splits into four individual tendons at the dorsum of the hand in macaques (7/7), whereas in gibbons, the tendon to digit 2 splits off proximally to the wrist, the tendon to digit 5 splits off at the level of the wrist, and the tendons to digits 3 and 4 split off at the dorsum of the hand and commonly interconnect with the tendons of digits 2 and 5 (juncturae tendineum) (Figure S1). These juncturae tendineum are also found in bonobos, between the tendons to digits 4 and 5. Each tendon inserts on the distal phalanx, after forming the extensor mechanism with the m. lumbricalis and mm. interossei (see intrinsic musculature) (15/15). The EDC acts as a wrist and digital extensor.
In the following paragraphs, the m. extensor digiti quarti et quinti proprius (EDQQ), m. extensor digiti secundi et tertii proprius (EDST), m. extensor digitorum brevis (EDB), m. extensor digiti minimi (EDM), and m. extensor indicis (EI) are discussed together because they are developmentally related (Diogo et al., 2009).
Macaques show the most primitive condition, with a m. extensor digiti secundi et tertii proprius (EDST) and m. extensor digiti quarti et quinti proprius (EDQQ), inserting onto digits 2–3 and digits 4–5, respectively, which corresponds to the m. extensores digitorum breves (EDB) of digits 2–3 and digits 4–5 of other tetrapods (Diogo et al., 2009). The EDST originates from the proximal half of the ulna (7/7) and the tendons insert onto the ulnar side of the extensor mechanism of digits 2 and 3 (Figure 2a). The EDQQ originates from the lateral epicondyle of the humerus, from the same extensor tendon as the EDC, and the tendons insert on the ulnar side of the extensor mechanism of digits 4 and 5, near the proximal phalanx (Figure 2a). Extension of the fingers is thus controlled in pairs in macaques.
FIGURE 2.
(a) The m. extensor digiti secundi et tertii proprius (EDST) and m. extensor digiti quarti et quinti proprius (EDQQ) of macaques; (b) m. extensor digitorum brevis (EDB) and m. extensor digiti minimi (EDM) of gibbons. Note that in macaques the fingers are controlled in pairs, which might aid in efficient positioning of the hand and fingers on uneven terrain during quadrupedal walking. In contrast, extension of the little finger in gibbons is separate from the extension of digits 2–4, suggesting that simultaneous extension of digits 2–4 in gibbons might be important when reaching or grasping an overhead support during brachiation
In contrast to macaques, gibbons show a more derived configuration, as they all possess a m. extensor digitorum brevis (EDB) (Figure 2b), which inserts onto digits 2–4, and a true m. extensor digiti minimi (EDM), which inserts onto digit 5 (Figure 2b). The EDB originates from the proximal half of the interosseous membrane (8/8), and either the proximal (4/8) or distal half (4/8) of the ulna. The individual tendons insert either onto the proximal phalanx of digits 2, 3 and 4 (6/8) or only onto digits 3 and 4 (1/8). Occasional insertions onto the base of MC2, MC3 and MC4 may occur (1/8). The most important function of the EDB is the coordinated extension of digits 2, 3 and 4. The EDM originates from the lateral epicondyle of the humerus (7/8) or the distal half of the ulna (1/8), and inserts onto the distal phalanx of digit 5 together with the tendon of the EDC (8/8). Proximal fusion of the EDM with the ED can occur (4/8).
Modern humans show the most derived condition. They possess an EDM—similar to gibbons—in combination with a separate m. extensor indicis (EI), which inserts onto the distal phalanx of digit 2, a configuration also seen in great apes (Aversi‐Ferreira et al., 2010; Zihlman, Farland and Mi, 2011). The EI and EDM of modern humans and great apes are phylogenetically derived from the EDB of other tetrapods (Diogo et al., 2009) and replace the EDB. Gibbons present an intermediate configuration in that they preserve an EDB and have an EDM. In one gibbon specimen, we even identified an EI, originating from the distal third of the ulna and the interosseous membrane and inserting onto the distal phalanx of the index finger together with the tendon of the EDC (1/8). Variation in the extensor musculature is, however, also present in humans. The EDB has been reported as a rare anatomical variation in humans (2.3% of the human population; Suwannakhan, Tawonsawatruk and Meemon, 2016; Georgiev et al., 2018) and an ‘EI’ with a tendon running to both the index and middle finger (i.e. m. extensor indicis et medii communis), as seen in macaques, also occurs in humans (0%–6%) (Suwannakhan et al., 2016; Georgiev et al., 2018).
The specific configuration of these extensors in macaques, gibbons and humans has important functional implications. In macaques, the fingers are controlled in pairs by the EDST and EDQQ. A similar organization is found in the finger flexors of macaques, where the fingers are also controlled in pairs. This specific organization might aid in efficient positioning of the hand and fingers during palmi‐ or digitigrade quadrupedal walking, such as pairwise extension (and little abduction) of the fingers to accommodate to uneven substrates, which might prevail over individual finger control. Although one could argue that the substrates that macaques need to move along are no more uneven than those of gibbons, the hand positioning in palmi/digitigrade quadrupedalism is very different from that used in brachiation. Gibbons typically use a hook grip during brachiation, and the hands are positioned on the superstrate in an overhead position, without visual input. In such a hook grip position, individual positioning of the fingers seems less important and simultaneous flexion of the four fingers prevails (Tuttle, 1969; Susman, Jungers and Stern, 1982). In gibbons, extension of the little finger is controlled by a separate EDM, and digits 2–4 are extended by the EDB. This suggests that simultaneous extension of digits 2–4 in gibbons might be important when reaching or grasping a support during brachiation (cf. hook grip position described above). Humans and bonobos have a separate EDM and EI, resulting in a functional dissociation between the extension of the index finger and little finger, which is also distinct to that of digits 3 and 4, which is primarily mediated by the EDC. This individualization of finger extension, in combination with a separate m. flexor pollicis longus to the thumb, is likely linked to the high manual dexterity of humans.
The m. abductor pollicis longus (APL) originates from the interosseous membrane and the proximal shaft of the ulna in all macaque and gibbons specimens (15/15). In macaques, the APL consists of one muscle belly with a tendon that splits at the level of the trapezium, inserting with one slip onto the base of MC1 and with the other onto the prepollex (7/7) (Figure 3a). In gibbons, however, the APL consists of two muscle bellies, APL I and II, each with its own tendon, and the bellies are either proximally fused (6/8) or easily separable (2/8). The tendon of APL I always inserts on the base of the MC1 (8/8), whereas the tendon of APL II inserts most often on the trapezium (7/8), with an additional insertion on the prepollex (2/8), or it may insert solely on the capitate (1/8) (Figure 3b). The configuration seen in gibbons, with a distinct APL I and II, is also observed in bonobos and humans (van Leeuwen et al., 2018), even though this is largely overlooked in other literature. This specific configuration means that only the APL I can be considered a true abductor of the thumb, whereas the APL II functions as radial deviator of the wrist and has no function on the thumb. The insertion onto the prepollex, as seen in macaques and some gibbons (and bonobos), might not entail a functional difference to an insertion on the trapezium, given the close association between the prepollex and trapezium in most nonhuman primates (Le Minor, 1994).
FIGURE 3.
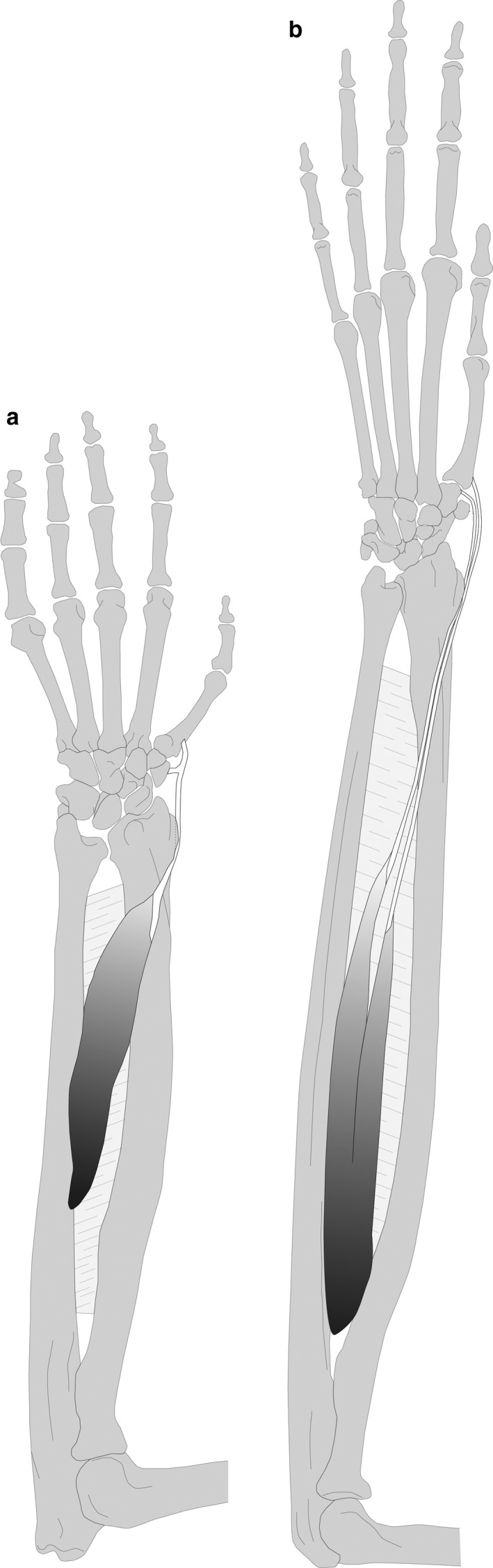
Illustration of the m. abductor pollicis longus (APL). (a) In macaques, the APL consists of one muscle belly with a tendon that splits at the level of the trapezium, inserting with one slip onto the base of MC1 and with the other onto the prepollex. (b) In gibbons, the APL consists of two muscle bellies each with its own tendon, APL I (insertion on the base of MC1) and APL II (insertion on the trapezium)
The m. extensor pollicis longus (EPL) has its origin on the proximal ulna (15/15), and often also from the interosseous membrane both in macaques (4/7) and gibbons (3/8). It inserts with a long tendon onto the distal phalanx of digit 1 (14/15). This configuration is also seen in bonobos. In one gibbon specimen, the insertion could not be reported due to tissue damage. Both macaques and gibbons (and bonobos) lack a m. extensor pollicis brevis (EPB), in contrast to humans, although absence of the EPB in humans has also been reported (Nayak et al., 2008). The EPB in humans displays some anatomical variations, such as the m. extensor pollicis et indicis accessorius with a tendon to digits 1 and 2 (Yoshida, 1995). It has been proposed in the literature that the EPB of modern humans is a derivative of APL I (Straus, 1941; Diogo et al., 2009), as the primitive mammalian condition shows an APL with a single tendon (Aversi‐Ferreira et al., 2010), whereas the APL in macaques splits distally and gibbons clearly show two separate tendons (APL I and II). However, this interpretation is still debated, as both the APL I and EPB have a different insertion, i.e. the base of MC1 versus the proximal phalanx of the thumb. In addition, humans can have an EPB present next to an APL with multiple tendons (cf. APL I) inserting around the first carpometacarpal joint (Lacey, Goldstein and Tobin, 1951; Celik, Sendemir and Simsek, 1994; Sehirli, Cavdar and Yüksel, 2001).
The m. flexor carpi radialis (FCR) shows a similar configuration in both macaques and gibbons (and bonobos). It originates from the common flexor tendon at the medial epicondyle of the humerus and inserts with a long tendon onto the palmar base of MC2, running deep to the thenar muscles (15/15). Proximal fusion with the muscle belly of the FDS (1/7) and/or PT (3/7) might occur in macaques. In one gibbon specimen, the FCR also originates from the proximal ulna (1/8). Given its position in the macaque and gibbon forearm, it functions as a wrist flexor and radial deviator, and probably a weak pronator.
The m. flexor carpi ulnaris (FCU) originates from the common flexor tendon at the medial epicondyle of the humerus (caput humerale) in all macaque and gibbon specimens (15/15). An additional origin from the olecranon (caput ulnare) occurs in both macaques (4/7) and gibbons (1/8). This configuration is also seen in bonobos. The FCU inserts with a long tendon onto the pisiform bone in all specimens (15/15). The FCU functions as wrist flexor and ulnar deviator in both macaques and gibbons. In macaques, the long pisiform, which is directed perpendicular to the palmar surface of the hand, gives the FCU the optimal leverage for flexing an extended wrist (Lewis, 1985; Sarmiento, 1988), which is important during quadrupedal walking. In gibbons, the pisiform has a proximodistal orientation which increases the lever arm of the FCU for wrist flexion and ulnar deviation (Sarmiento, 1988). These wrist movements are important during brachiation (Michilsens et al., 2010).
The m. palmaris longus (PL) originates from the common flexor tendon at the medial epicondyle of the humerus and its long and slender tendon extends into the palmar aponeurosis at the level of the wrist in all macaques (7/7). This configuration is similar to that of modern humans, though the PL tendon of macaques runs more ulnarly into the palmar aponeurosis. In gibbons, the origin of the PL is the same as in macaques, with an additional origin from the fascia of the aponeurosis bicipitis of the Bb in two specimens (2/8). At the insertion, the configuration in gibbons is distinct from that observed in macaques, with a radially positioned PL tendon at the wrist (5/7), as is also seen in bonobos, or even with an insertion onto the tendon of the FCR (2/7). The more radial insertion found in gibbons could be important during brachiation, as it can aid in radial deviation. However, more important is that the PL is always present in macaques and gibbons (and bonobos), but in modern humans the PL is unilaterally absent in 16% of the population (Thompson, Mockford and Cran, 2001).
The m. flexor digitorum superficialis (FDS) originates from the common flexor tendon at the medial epicondyle of the humerus in all macaques (7/7) and inserts most commonly with four separate tendons onto the middle phalanx of digits 2 to 5 (6/7). In one specimen, the tendons to digits 2 and 3 are vestigial and insert on the tendon sheaths of the FDP at the level of the lumbricals, whereas the tendons to digits 4 and 5 insert onto the proximal phalanx (1/7). The FDS has a rather complex architecture in macaques, which is also commonly seen in bonobos, consisting of three partially fused muscle bellies that are folded together (Figure S2):
a muscle running to digit 2 (FDS II), which shows a distinct belly‐tendon‐belly‐tendon configuration (cf. bonobos van Leeuwen et al., 2018),
a muscle belly with two tendons inserting onto digits 3 and 4 (FDS III‐IV),
a muscle with one tendon inserting onto digit 5 (FDS V).
Moreover, in all macaque specimens, the FDS is connected with the m. flexor digitorum profundus (FDP) with an additional muscle belly, at the level of the FDS for digits 2 to 3 (7/7). In gibbons, the configuration of the FDS is even more variable than in macaques. In half of the specimens, the FDS consists of one muscle belly (4/8), but two (2/8), three (1/8) or four muscle bellies (1/8) have also been observed. The distribution of tendons to digits 2 to 5 differs from specimen to specimen. Moreover, in gibbons the FDS originates not only from the medial epicondyle but also from the proximal ulna (4/8) or from the proximal ulna and radius (1/8). In gibbons, the deep flexors of the toes, like the FDS, also show considerable variation in the specific distribution of the tendons towards the digits (Langdon, 1990; Vereecke et al., 2005), indicating that the tendon organization has no major influence on the functionality of the FDS.
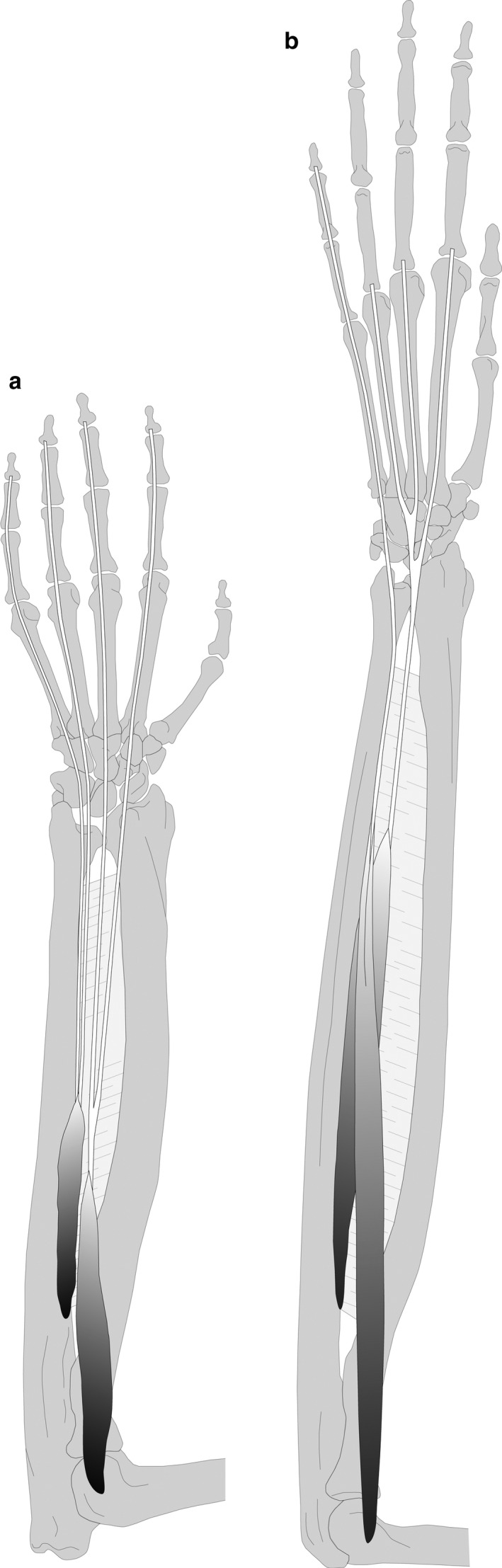
The m. flexor digitorum profundus (FDP) originates from the medial epicondyle of the humerus (situated deep to the FDS), the shaft of the radius (in between the SUP and PQ), the interosseous membrane and the shaft of the ulna (from the olecranon to the PQ) in all specimens (15/15). In bonobos, the FDP does not originate from the medial epicondyle of the humerus, but from the interosseous membrane and the shaft of the radius and ulna. In macaques and gibbons, the FDP has five tendons inserting respectively onto the distal phalanges of each digit, with some exceptions (see Table S1). In gibbons, the FDP usually consists of two muscle bellies that are partially fused, one for digit 1 (FDP I) and one for digits 2 to 5 (FDP II‐V) (5/8) (Figure 4a). The other specimens do not have a separate FDP I (3/8). In two specimens, the FDP II‐V sends a tendon to digit 1, which splits off from the tendon running to digit 2 (2/8). In another gibbon specimen, two individual muscle bellies occur, one for digits 1 and 2 (FDP I‐II) and one for digits 3 to 5 (FDP II‐V). Here, the tendons to digits 2 and 3 partly originate from the FDS (1/8) (Figure 4b). In macaques, the configuration of the FDP is more variable, with the majority of the specimens showing a configuration with three fused muscle bellies, one for digits 1 to 3 (FDP I‐III), one for digit 4 (FDP IV) and one for digit 5 (FDP V) (5/7) (Figure 4c). One specimen shows a slightly different configuration, with a division in FDP I‐III‐IV, FDP II and FDP V (1/7), and a second macaque specimen displays an unusual FDP configuration with two muscle bellies—FDP I‐II‐III and FDP IV‐V. In addition, the tendons to digits 2 and 3 show a tendon‐lumbrical‐tendon configuration in which the first two lumbricals form a single unit with the FDP tendons instead of originating from these tendons (Figure 4d). In macaques, the tendons of the FDP are clustered together at wrist level, and the tendons to the digits split off more distally than in gibbons. In addition, the tendon to the thumb originates from the middle of the tendon cluster, not from FDP II as seen in gibbons. Also notable is the connection between FDP and FDS in macaques, as described above. However, crucial is the decoupling between the thumb (and index finger) and the lateral digits in gibbons, a configuration common to humans, compared with the division between the medial and lateral digits in macaques (also seen at the extensors, cf. EDST and EDQQ).
FIGURE 4.
The m. flexor digitorum profundus (FDP) in macaques (a,b) and gibbons (c,d). (a) Common FDP configuration with three fused muscle bellies, one for digits 1 to 3 (FDP I‐III), one for digit 4 (FDP IV) and one for digit 5 (FDP V); the FDP is connected to the FDS with an additional muscle belly (*). (b) Unusual FDP configuration with two muscle bellies (FDP I‐III and FDP IV‐V); the tendons to digits 2 and 3 show a tendon‐lumbrical‐tendon configuration in which the first two lumbricals form a single unit with the FDP tendons instead of originating from these tendons. (c) Common FDP configuration with two partially fused muscle bellies, one for digit 1 (FDP I) and one for digits 2 to 5 (FDP II‐V). (d) Unusual FDP configuration with two muscle bellies (FDP I‐II and FDP III‐V); the tendons to digits 2 and 3 partly originate from the FDS (*) [Colour figure can be viewed at wileyonlinelibrary.com]
3.4. Intrinsic hand musculature
The intrinsic hand musculature consists of the thenar muscles (APB, FPB, ADP, OPP), the hypothenar muscles (ADM, FDM, ODM), the lumbricals (LUMB) and intermediate hand muscles (IM, FBP, IOP, IOD, mm. contrahentes) (Figure 5, Table S1). These intrinsic hand muscles of macaques and gibbons are described in detail below.
FIGURE 5.
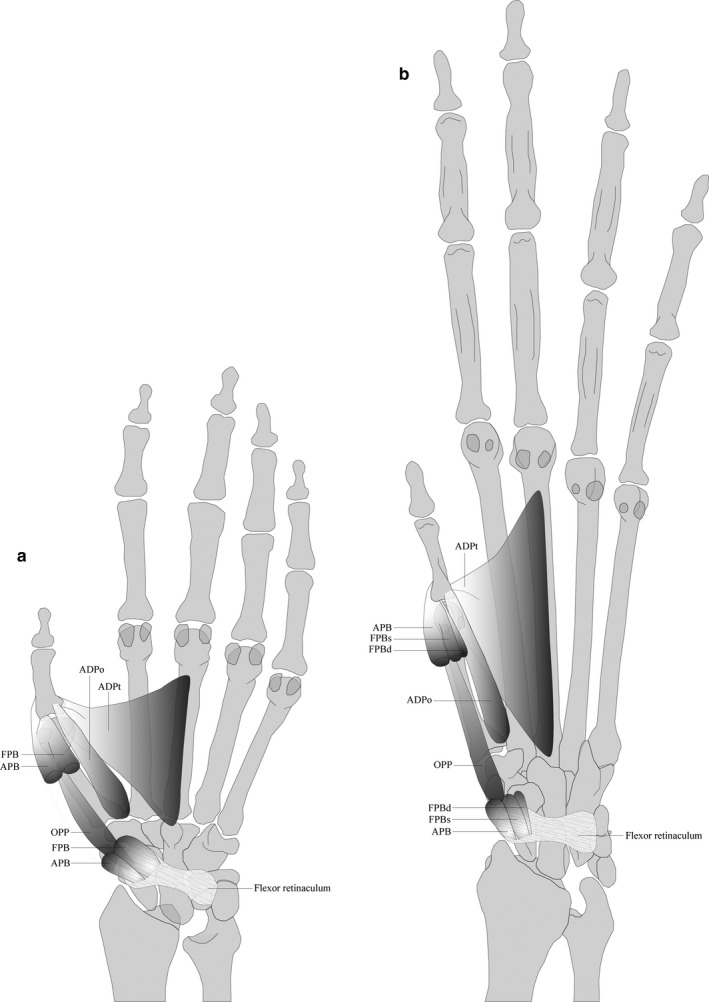
Overview of the general organization of the thenar musculature in (a) macaques and (b) gibbons. In gibbons, there is a clear distinction between the superficial and deep head of the FPB. APB (m. abductor pollicis brevis), FPB (m. flexor pollicis brevis), FPBs (m. flexor pollicis brevis, superficial head), FBPd (m. flexor pollicis brevis, deep head), OPP (m. opponens pollicis), ADPo (m. adductor pollicis, oblique head), ADPt (m. adductor pollicis, transverse head)
The m. abductor pollicis brevis (APB) has a similar configuration in both macaques and gibbons. It originates from the flexor retinaculum and inserts with a short tendon onto the radial sesamoid bone of the first metacarpophalangeal joint (MCP1) in all specimens (11/11), similar to the configuration in bonobos. In some macaques, the APB also originates from the palmar aponeurosis (4/7), while the insertion can extend to the radial side of the proximal phalanx (3/7), which is common in humans (Gupta and Michelsen‐Jost, 2012). In one gibbon specimen, the APB is fused with the OPP (1/4), whereas some macaque specimens show fusion between the APB and FPB (3/7). The APB functions as abductor of the thumb both in gibbons and macaques, as well as a stabilizer for the TMC joint.
The m. flexor pollicis brevis (FPB), situated underneath the APB, originates from the flexor retinaculum and inserts with a short tendon onto the radial sesamoid bone of the MCP1 joint in all specimens (13/13). A clear distinction between a superficial and deep head, as commonly observed in humans, may occur in both gibbon (4/6) and macaques (2/7). In macaques, the FPB shows some fusion with the APB at the origin (3/7), and with the oblique head of the ADP at insertion (3/7). In gibbons, the FPB can also originate from both the flexor retinaculum and the volar side of MC1 (1/6), while its insertion can be located at the ulnar side of the APB insertion (5/6) or at the base of the proximal phalanx of digit 1 (1/6). In two gibbon specimens, the FPB shows some fusion with the OPP (2/6).
The m. adductor pollicis (ADP) always consists of a clearly separable transverse and oblique head in macaques (7/7). The transverse head originates from the palmar base and shaft of MC3 (7/7) and inserts onto the ulnar sesamoid bone of the MCP1 joint (7/7), in combination with the MCP joint (3/7) and/or the proximal phalanx of digit 1 (4/7). In one specimen, muscle tissue extends towards the radial side of MC2. The oblique head originates from the palmar base of MC1 (7/7), sometimes together with the palmar base of MC2 (1/7) or MC3 (1/7). It also has its insertion onto the ulnar sesamoid bone of the MCP1 joint (7/7), along with the ulnar (1/7) or radial (1/7) side of the proximal phalanx of digit 1 or the MCP1 joint (1/7). In gibbons, both heads are usually clearly separable (4/6); however, occasionally they are indistinguishable (2/6), in which case the ADP originates from the palmar base of MC1 and MC3 and inserts onto the ulnar sesamoid bone of the MCP 1 joint. The transverse head is similar to that of macaques, with its origin on the palmar base and shaft of MC3 (4/4). It inserts onto the ulnar sesamoid bone of the MCP1 joint (4/4) and may extend to the base of the proximal phalanx of digit 1 (1/4). The oblique head originates from the palmar base of MC1 (2/4), the base of MC2 (1/4) or the flexor retinaculum (1/4). Like the transverse head, it inserts onto the ulnar sesamoid bone of the MCP1 joint (4/4) with the occasional extension to the base of the proximal phalanx of digit 1 (2/4). The main function of the ADP is adduction of the thumb.
The m. opponens pollicis (OPP) is a clearly separate muscle in macaques. It originates from the flexor retinaculum, with some fibres originating from the APB and FPB (1/7) or the prepollex (2/7), and inserts onto the radial side of the MC1 shaft. It has no contact with the sesamoid bones of the MCP1 joint. In gibbons (and bonobos), the OPP is either completely fused with the FPB (1/5) or the APB (1/5), partially fused with the FPB (1/5) or is present as a separate muscle (2/5). When separate, it originates from the flexor retinaculum (2/2) in combination with the palmar base of MC1 (1/2), and it inserts onto the radial side of the MC1 shaft (2/2). The OPP assists in opposition and adduction of the thumb.
The m. palmaris brevis (PB) is a well‐developed muscle in macaques (7/7), whereas in gibbons (and bonobos) no distinct PB can be identified (mostly fat tissue) in half of the specimens (2/4). The PB originates from the flexor retinaculum and inserts onto the palmar aponeurosis in all primate specimens (11/11). The PB of gibbons is similar in appearance to that of bonobos and humans, whereas the bulkier PB in macaques likely acts as a cushion to protect the ulnar artery and nerve during quadrupedal walking.
The m. abductor digiti minimi (ADM) originates from the pisiform bone (7/7) in combination with the flexor retinaculum and pisohamate ligament (4/7) in macaques (and bonobos). It inserts onto the ulnar side of the MCP5 joint (7/7), in combination with the proximal phalanx (2/7), as seen in bonobos, or joining the FDM tendon (5/7). In two macaque specimens, the ADM is proximally fused with the FDM. In gibbons, the ADM originates from either the pisiform bone (2/5) or the base of MC5 (3/5) and inserts onto the ulnar side of the MCP5 joint (5/5). In one gibbon specimen, the ADM is partially fused with the FDM. The ADM acts as abductor of digit 5.
The m. flexor digiti minimi (FDM) originates from the flexor retinaculum (7/7) and pisiform bone (2/7) in macaques. It inserts onto the MCP5 joint (7/7), along with the ADM tendon (2/7) or the proximal phalanx of digit 5 (4/7). In two macaque specimens, the FDM shows proximal fusion with the ADM. In gibbons, the origin is more variable. The FDM can originate from the flexor retinaculum (3/5), the base of MC5 (1/5) or the palmar aponeurosis (1/5). The FDM inserts onto the ulnar base of the proximal phalanx of digit 5 in all gibbon specimens (5/5) and also bonobos. Proximal fusion with the ODM (1/5) or distal fusion with the ADM (2/5) in gibbons is possible. The FDM acts as flexor of digit 5.
The m. opponens digiti minimi (ODM) originates from the flexor retinaculum and inserts onto the ulnar side of the MC5 shaft in all macaque and gibbon specimens (11/11), similar to the bonobo configuration. In one gibbon specimen, the ODM is completely fused with the FDM. In macaques, an additional origin from the base of MC5 can be present (2/7). The function of the ODM is opposition of digit 5.
The mm. lumbricales (LUMB) of digit II‐V each originate from the corresponding FDP tendon and insert with a well‐developed tendon onto the radial side of the extensor sheath at the proximal phalanx of the corresponding digit in more than half of the specimens (9/15). However, some variation is possible regarding the origin. In most macaque specimens, and also bonobos, LUMB III‐V originate from two FDP tendons (LUMB III from FDP II and III [5/6], LUMB IV from FDP III and IV [7/7], and LUMB V from FDP IV and V [6/7]), which might aid force transmission. One macaque specimen shows a particular configuration in which the lumbrical muscle was positioned in series with the FDP tendon (see FDP description). In gibbons, LUMB II (1/8) and LUMB III (3/8) can originate from the FDP II and III tendons, LUMB IV from FDP III and IV (2/8), and LUMB V from FDP IV and V (2/8) or solely from FDP IV (1/8). In one gibbon specimen, proximal fusion of LUMB II‐IV occurs near the origin on the FDP tendons. The LUMB act as flexors of the MCP joints and extensors of the IP joints.
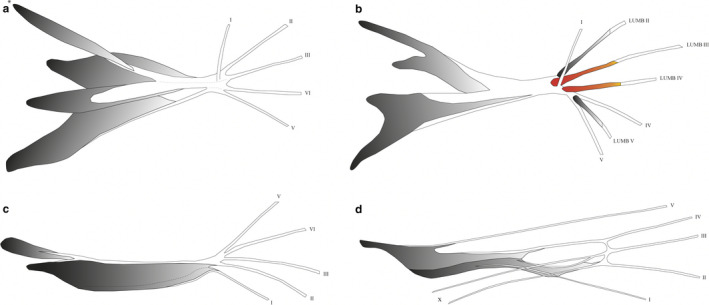
The primitive mammalian condition for the intermediate hand muscles is the presence of four mm. intermetacarpales (IM) and 10 mm. flexores breves profundi (FBP) (Diogo et al., 2009; Diogo and Tanaka, 2012b; Diogo and Molnar, 2014; Lemelin and Diogo, 2016). In primates, two FBP have differentiated: FBP I forms the FPB and OPP, and FBP X forms the FDM and ODM. In humans, the IM (I‐IV) are fused with FBP (III, V, VI, VIII) to form the mm. interossei dorsales (IOD I‐IV) (Diogo et al., 2009). In all macaque specimens, both the IM and FBP are fused to form the IOD (7/7). In gibbons, however, some individuals display an intermediate configuration where only one, two or three IOD are present and the other IM and FBP remain present as separate muscles (5/8). This configuration is also seen in bonobos (van Leeuwen et al., 2018). In macaques, the presence of IOD might be important for specific hand movements during quadrupedal walking, such as abduction of fingers to accommodate to uneven terrain. A detailed visualization of individual specimens’ intermediate hand muscle configurations is reported in Figure S3. In addition to the IOD and IOP, a m. contrahens (C5) is present in all but one macaque specimen (7/8). The C5 originates from the palmar base of MC3 (partially fused with IOD II and III) and inserts on the radial side of the MCP5 joint, joining the extensor mechanism (Figure 6a). No other contrahens muscles are observed in the macaque sample. In contrast, the gibbon sample shows that contrahens muscles associated with digits 2, 3 and 4 are present in some specimens. Four specimens have a C2 inserting onto the ulnar side of the MCP2 joint (4/8) (Figure 6b) and three specimens have a C4 and C5 inserting onto the ulnar side of the MCP3 and MCP4 joints, respectively (3/8). In the literature, however, the insertion of C4 and C5 has been described onto the radial side of the MCP3 and MCP4 joint in gibbons (one H. lar: Yamamoto, Murakami and Ohtsuka, 1988), macaques (two macaques: Yamamoto, Murakami and Ohtsuka, 1988) and three Japanese monkeys (Homma and Sakai, 1994). In one gibbon specimen, an additional muscle distinct from the contrahens muscles described above is present. It originates from the IOD I and inserts onto the radial side of the proximal phalanx of digit 1. This muscle is similar to the m. contrahens digitorum (CD) of modern humans, as described by Tubbs, Salter and Oakes (2005).
FIGURE 6.
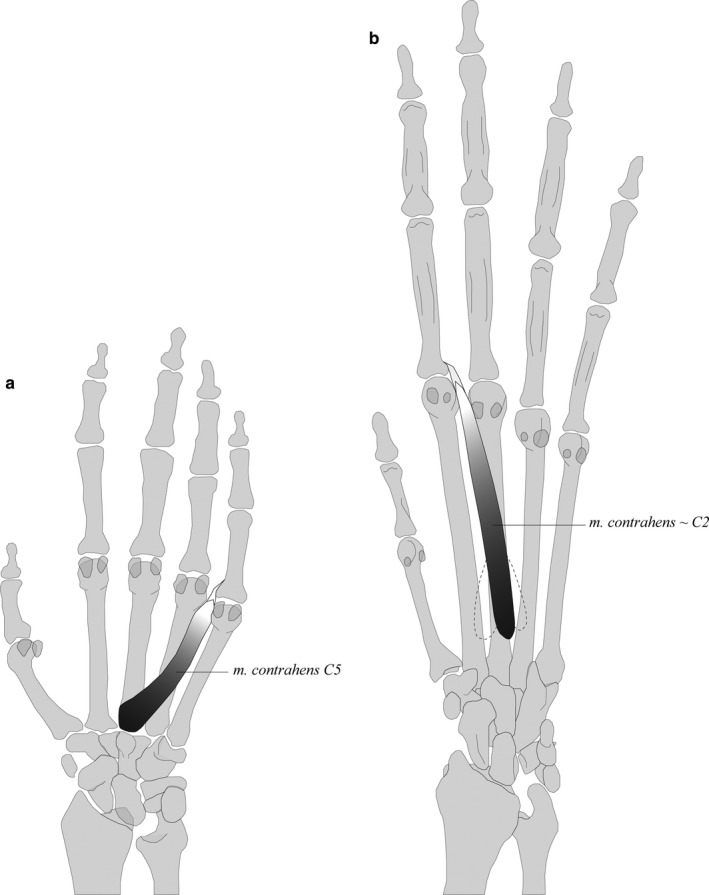
Two examples of a contrahens muscle: (a) C5 of macaques, which originates from the palmar base of MC3 (partially fused with IOD II and III) and inserts on the radial side of the MCP5 joint (joining the extensor mechanism); (b) C2 of gibbons, which has a varying origin and inserts onto the ulnar side of the MCP2 joint
4. DISCUSSION
In this study, the configuration of the forearm and hand muscles of arboreal gibbons is compared with that of terrestrial macaques. In addition, anatomical data from previous dissections on knuckle‐walking bonobos and bipedal humans are included to allow us to evaluate whether the forearm and hand musculature shows functional adaptations to locomotor behaviour (van Leeuwen et al., 2018).
4.1. Upper arm musculature
The most notable trait in the gibbon upper limb is the configuration of the short head of the m. biceps brachii (Bb), which originates from the lesser tubercle of the humerus as such, losing its function at the glenohumeral joint, which is most likely a derived condition. In gibbons, the short head therefore primarily acts as elbow flexor and forearm supinator (Michilsens et al., 2009), whereas in macaques, bonobos and humans the short head of the Bb functions as a shoulder flexor as it crosses the shoulder joint and originates from the coracoid process of the scapula. According to Jungers and Stern (1980), in gibbons the short head of the Bb forms a ventral muscle chain between the m. pectoralis major (PM) and m. flexor digitorum superficialis (FDS) (Jungers and Stern, 1980), although this specific action remains debated.
Other remarkable muscles are the m. dorso‐epitrochlearis (DET) and m. epitrochleoanconeus (ETA). The DET is present in both macaques and gibbons but has a slightly different configuration. The DET inserts onto the medial epicondyle in most gibbons, as opposed to the lateral side of the elbow in macaques (Sonntag, 1922; Jungers and Stern, 1980; Michilsens et al., 2009). In great apes, the DET also inserts onto the medial epicondyle of the humerus (Diogo et al., 2010, 2013a; Diogo, Potau and Pastor, 2013b), whereas in other primate taxa (Alouatta, Saimiri, Callithrix), the DET inserts onto the olecranon as seen in macaques (Diogo and Wood, 2012a). Although Aversi‐Ferreira et al. (2016) suggest that the DET favours arboreal locomotion when it inserts onto the olecranon and quadrupedal locomotion when it inserts onto the epicondyle of the humerus, this contradicts our results. The insertion onto the olecranon in macaques might be important to help stabilize the elbow during terrestrial quadrupedalism, whereas through its insertion onto the medial epicondyle of the humerus in gibbons and bonobos, the DET could produce elbow and digital flexion (i.e. dorsal muscle chain), which could be an advantage during brachiation in gibbons and climbing/clambering in bonobos (Jungers and Stern, 1980), although this function is still debated, as is the case for the ventral muscle chain (see above).
The ETA is a prominent muscle in macaques but is not observed in gibbons (or humans). It may serve to protect the ulnar nerve, running superficially through the cubital tunnel, and it potentially helps to extend the forearm and/or stabilize the elbow joint during quadrupedal walking. However, there is still some discussion about the presence or absence of this muscle across different primate taxa, which requires anatomical data from a larger nonhuman primate sample (Uscetin et al., 2014; de Ruiter and van Duinen, 2017; Diogo, Molnar and Wood, 2017).
4.2. Forearm rotators
The forearm rotators (BR, SUP, PT, PQ) have a very similar configuration in macaques and gibbons (and bonobos), with a low variability in muscle architecture. This conserved morphology might indicate that these muscles are under strong selective pressure and that their specific configuration is tightly linked to forearm functionality. A two‐headed configuration of the PT, as in modern human, has never been observed in either the macaque or the gibbon sample. In the PQ, on the other hand, a two‐headed configuration similar to humans was observed in two gibbon specimens. Due to the oblique orientation of the muscle fibres, the primary function of the PQ is likely stabilization of the distal radioulnar joint in macaques, gibbons and bonobos (Johnson and Shrewsbury, 1976). This implies that the PT is the most important forearm pronator in these primates.
4.3. Extrinsic hand musculature
The dorsal compartment of the forearm shows a different configuration in macaques and gibbons. In macaques, the fingers are controlled in pairs by the EDST and EDQQ. A similar organization is found in the finger flexors of macaques, where the fingers are also controlled in pairs. We suggest that this specific organization might aid in efficient positioning of the hand and fingers during palmi‐ or digitigrade quadrupedal locomotion on uneven substrates. In gibbons, the little finger is controlled by a separate EDM, and extension of digits 2–4 is coupled. This might indicate that simultaneous extension of digits 2–4 in gibbons is important when reaching for and grasping an overhead support during brachiation. Humans and bonobos have a separate EDM and EI, resulting in a functional dissociation between the extension of the index finger and little finger. In humans, this is likely linked to the high manual dexterity.
The ventral compartment shows an extraordinary variability within the FDS and FDS, both in gibbons and macaques. This variability is also seen in bonobos. This high inter‐individual variation might indicate that these muscles are under mild selective pressure and that the differences in configuration of these muscles have no major influence on the functionality of the hand/fingers.
4.4. Intrinsic hand musculature
The thenar (APB, FPB, OPP, ADP) and hypothenar (ADM, FDM, ODM) muscles have a very similar configuration in gibbons and macaques (and bonobos), with a varying degree of fusion between the different muscles. The intermediate hand musculature is much more variable and a different configuration is seen in macaques compared with gibbons. The intermediate hand muscles are organized in palmar and dorsal interossei in macaques, similar to the human configuration, whereas gibbons display a highly variable configuration with at least some unfused FBP and IM, a configuration also seen in bonobos. In gibbons, various contrahens muscles may be present, whereas in macaques, only a C5 is observed. The intermediate hand muscles of gibbons show a higher degree of variation than those of macaques, which might suggest that they have no major implications for the functionality of the hand.
4.5. Critical considerations
Our findings are based on a detailed dissection of eight gibbon and seven macaque specimens. Although this is a limited sample size compared with human studies, it forms a unique and valuable sample of nonhuman primates that was studied using a consistent protocol. Inherent to working with primate cadavers is the lack of an equal distribution across species, sexes or ages, and most importantly, sampling from captivity. However, given a healthy gene pool, we do not expect an impact of captivity on muscle configuration. Given the genetic distance between macaques and gibbons, we cannot be certain that the differences in muscle configuration are due to variation in locomotor behaviour and not genetics. This is challenging to test, although it should not go unremarked, as only two taxa are being compared in detail, and there is no relative context of variation across other arboreal or terrestrial primate taxa. We have tried to mitigate this issue by adding information on the forelimb and hand musculature of two additional taxa, the bonobo and human, with different locomotor behaviours. However, the gibbon group contains different genera and species in contrast to the homologous sampling of rhesus macaques. This could explain the difference in variation of the FDS, FDP and intermediate hand muscles between gibbons and macaques, although we also observe a high variation in bonobos. The contrasting results on the DET also stress the importance of broad phylogenetic sampling.
Despite these limitations, this research is not only important to obtain a detailed insight in the anatomy of the gibbon and macaque forelimb and hand, but in combination with in vivo research and behavioural studies it can be translated to complete form‐function relationships of the primate hand which will aid functional interpretation of fossil remains of nonhuman primates and hominins.
5. CONCLUSION
The overall configuration of the forelimb and hand musculature is highly comparable between the different primate groups and follows the general primate condition. Most of the identified differences in muscle configuration between arboreal gibbons, terrestrial macaques, knuckle‐walking bonobos and bipedal humans seem to be related to the specific locomotor behaviour of each group, though sampling in a wider range of primate taxa is needed to substantiate these functional adaptations further.
AUTHOR CONTRIBUTIONS
EEV conceived the study. EEV, MJMV and TvL designed the study. MJMV and TvL performed the dissections. MJMV and EEV analysed the data and wrote the manuscript. All authors reviewed and approved the manuscript.
Supporting information
Fig S1
Fig S2
Fig S3
Table S1
Acknowledgements
The authors thank the different zoos and institutes which provided additional primate specimens: Georg Hantke (National Museum of Scotland, Edinburgh), Pieter Cornillie (Ghent University, campus Merelbeke), Koen Nelissen (KU Leuven, campus Gasthuisberg), François Druelle (Zoological and Botanical Park of Mulhouse, France), Robby Van der Velden (Pakawi Park, Belgium). Furthermore, we thank Olivier Vanovermeire and Henk Lacaeyse from the Medical Imaging Department, AZ Groeninge (Kortrijk, Belgium) for CT‐scanning of the specimens. Finally, we would like to thank one of the students who assisted during the dissections and Tracy Kivell for constructive comments on the manuscript. Funding for this project was obtained from KU Leuven (project C14/16/082).
Vanhoof MJM, van Leeuwen T, Vereecke EE. The forearm and hand musculature of semi‐terrestrial rhesus macaques (Macaca mulatta) and arboreal gibbons (Fam. Hylobatidae). Part I. Description and comparison of the muscle configuration. J. Anat. 2020;237:774–790. 10.1111/joa.13222
REFERENCES
- Almécija, S. , Smaers, J.B. and Jungers, W.L. (2015) The evolution of human and ape hand proportions. Nature Communications, 6(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, P. and Groves, C. (1976) Gibbons and brachiation In: Rumbaugh D.M. (ed.) Gibbon and Siamang: Suspensory Behavior, Locomotion and other Behaviors of Captive Gibbons: Cognition. Gibbons and Siamang: A Series of Volumes on the Lesser Apes, Vol. 4 Basel, Switzerland: Karger, pp. 167–218. [Google Scholar]
- Aversi‐Ferreira, T.A. , Diogo, R. , Potau, J.M. , Bello, G. , Pastor, J.F. and Aziz, M.A. (2010) Comparative anatomical study of the forearm extensor muscles of Cebus libidinosus (Rylands et al, (Rylands . Anatomical Record, 293(12), 2056–2070. [DOI] [PubMed] [Google Scholar]
- Aversi‐Ferreira, T.A. , Aversi‐Ferreira, R.A. , Bretas, R.V. , Nishimaru, H. and Nishijo, H. (2016) Comparative anatomy of the arm muscles of the Japanese monkey (Macaca fuscata) with some comments on locomotor mechanics and behavior. Journal of Medical Primatology, 45(4), 165–179. [DOI] [PubMed] [Google Scholar]
- Boland, M.R. , Spigelman, T. and Uhl, T.L. (2008) The Function of Brachioradialis. Journal of Hand Surgery. American Society for Surgery of the Hand, 33(10), 1853–1859. [DOI] [PubMed] [Google Scholar]
- Caetano, E.B. , Vieira, L.Â. , Sprovieri, F.A. , Petta, G.C. , Nakasone, M.T. and Serafim, B.L. (2017) Anatomical variations of pronator teres muscle: Predispositional role for nerve entrapment. Revista Brasileira de Ortopedia, 52(2), 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik, H. , Sendemir, E. and Simsek, C. (1994) Anomalous insertion of abductor pollicus longus. Journal of Anatomy, 184(3), 643. [PMC free article] [PubMed] [Google Scholar]
- Cheng, E.J. and Scott, S.H. (2000). Morphometry of Macaca mulatta forelimb. I. Shoulder and elbow muscles and segment inertial parameters. Journal of Morphology, 245(3), 206–224. [DOI] [PubMed] [Google Scholar]
- Daver, G. , Berillon, G. and Grimaud‐Hervé, D. (2012) Carpal kinematics in quadrupedal monkeys: Towards a better understanding of wrist morphology and function. Journal of Anatomy, 220(1), 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demes, B. , Stern, J.T. , Hausman, M.R. , Larson, S.G. , McLeod, K.J. and Rubin, C.T. (1998) Patterns of strain in the macaque ulna during functional activity. American Journal of Physical Anthropology, 106(1), 87–100. [DOI] [PubMed] [Google Scholar]
- Diogo, R. , Abdala, V. , Aziz, M.A. , Lonergan, N. and Wood, B.A. (2009) From fish to modern humans ‐ Comparative anatomy, homologies and evolution of the pectoral and forelimb musculature. Journal of Anatomy, 214(5), 694–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo, R. , Potau, J.M. , Pastor, F. , De Paz, F.J. , Ferrero, E.M. , Bello, G. , Barbosa, M. , Aziz, A. , Martorell, J.A. and Wood, B.A. (2010) Photographic and Descriptive Musculoskeletal Atlas of Gorilla: With Notes on the Attachments, Variations, Innervation, Synonymy and Weight of the Muscles. Boca Raton, FL: CRC Press. [Google Scholar]
- Diogo, R. , Shearer, B. , Potau, J.M. , Pastor, J.F. , de Paz, F.J. , Arias‐Martorell, J. , Turcotte, C. , Hammond, A. , Vereecke, E. , Vanhoof, M. , Nauwelaerts, S. and Wood, B. (2013a) Photographic and Descriptive Musculoskeletal Atlas of Orangutans: with notes on the attachments, variations, innervations, function and synonymy and weight of the muscles. Boca Raton, FL: CRC Press. [Google Scholar]
- Diogo, R. and Molnar, J. (2014) Comparative anatomy, evolution, and homologies of tetrapod hindlimb muscles, comparison with forelimb muscles, and deconstruction of the forelimb‐hindlimb serial homology hypothesis. Anatomical Record, 297(6), 1047–1075. [DOI] [PubMed] [Google Scholar]
- Diogo, R. , Molnar, J.L. and Wood, B. (2017) Bonobo anatomy reveals stasis and mosaicism in chimpanzee evolution, and supports bonobos as the most appropriate extant model for the common ancestor of chimpanzees and humans, Scientific Reports, 7(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo, R. , Potau, J.M. and Pastor, J.F. (2013b) Photographic and Descriptive Musculoskeletal Atlas of Chimpanzees: With Notes on the Attachments, Variations, Innervation, Function and Synonymy and Weight of the Muscles. Boca Raton, FL: CRC Press. [Google Scholar]
- Diogo, R. , Richmond, B.G. and Wood, B. (2012a) Evolution and homologies of primate and modern human hand and forearm muscles, with notes on thumb movements and tool use. Journal of Human Evolution. Elsevier Ltd, 63(1), 64–78. [DOI] [PubMed] [Google Scholar]
- Diogo, R. and Tanaka, E.M. (2012b) Anatomy of the pectoral and forelimb muscles of wildtype and green fluorescent protein‐transgenic axolotls and comparison with other tetrapods including humans: a basis for regenerative, evolutionary and developmental studies. Journal of Anatomy, 221(6), 622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo, R. and Wood, A. (2012) Comparative Anatomy and Phylogeny of Primate Muscles and Human Evolution. Boca Raton, FL: CRC Press. [Google Scholar]
- Fuss, F. and Wurzl, G. (1990) Median nerve entrapment. Pronator teres syndrome. Surgical and Radiologic Anatomy, 12(4), 267–271. [DOI] [PubMed] [Google Scholar]
- Georgiev, G.P. , Tubbs, R.S. , Iliev, A. , Kotov, G. and Landzhov, B. (2018) Extensor indicis proprius muscle and its variants together with the extensor digitorum brevis manus muscle: a common classification. Clinical significance in hand and reconstructive surgery. Surgical and Radiologic Anatomy. Springer Paris, 40(3), 271–280. [DOI] [PubMed] [Google Scholar]
- Gessini, L. , Jandolo, B. , Pietrangeli, A. and Occhipinti, E. (1981) Ulnar nerve entrapment at the elbow by persistent epitrochleoanconeus muscle. Case report. Journal of Neurosurgery, 55(5), 830–831. [DOI] [PubMed] [Google Scholar]
- Gibbs, S. , Collard, M. and Wood, B.A. (2002) Soft‐tissue anatomy of the extant hominoids: A review and phylogenetic analysis. Journal of Anatomy, 200, 3–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, S. and Michelsen‐Jost, H. (2012) Anatomy and function of the thenar muscles. Hand Clinics, 28(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Hartz, C.R. , Linscheid, R.l. , Gramse, R.R. and Daube, J.R. (1981) The pronator teres syndrome: Compressive neuropathy of the median nerve. Journal of Bone and Joint Surgery ‐ Series A, 63(6), 885–890. [PubMed] [Google Scholar]
- Hayama, S. , Chatani, K. and Nakatsukasa, M. (1994) The digitigrade hand and terrestrial adaptation in Japanese Macaques. Anthropological Science, 102, 115–125. [Google Scholar]
- Hirasawa, Y. , Sawamura, H. and Sakakida, K. (1979) ‘Entrapment neuropathy due to bilateral epitrochleoanconeus muscles: A case report’. Journal of Hand Surgery, 4(2), 181–184. [DOI] [PubMed] [Google Scholar]
- Homma, T. and Sakai, T. (1994) Intrinsic hand muscles of the Japanese Monkey Macaca fuscata . Anthropological Science, 102, 85–95. [Google Scholar]
- van Horn, R.N. (1972) Structural adaptations to climbing in the Gibbon hand. American Anthropologist, 74(3), 326–334. [Google Scholar]
- Jamieson, R.W. and Anson, B.J. (1952) The relation of the median nerve to the heads of origin of the pronator teres muscle, a study of 300 specimens. Quarterly Bulletin Northwestern University (Evanston, Ill.) Medical School, 26(1), 34–35. [PMC free article] [PubMed] [Google Scholar]
- Johnson, R.K. and Shrewsbury, M.M. (1976) The pronator quadratus in motions and in stabilization of the radius and ulna at the distal radioulnar joint. Journal of Hand Surgery, 1(3), 205–209. [DOI] [PubMed] [Google Scholar]
- Jungers, W.L. and Stern, J.T. (1980) Telemetered electromyography of forelimb muscle chains in gibbons (Hylobates lar). Science, 208(4444), 617–619. [DOI] [PubMed] [Google Scholar]
- Lacey, T.I. , Goldstein, L.A. and Tobin, C.E. (1951) Anatomical and clinical study of the variations in the insertions of the abductor pollicis longus tendon, associated with Stenosing tendovaginitis. Journal of Bone and Joint Surgery, 33(2), 347–350. [PubMed] [Google Scholar]
- Langdon, J.H. (1990) Variations in cruropedal musculature. International Journal of Primatology, 11(6), 575–606. [Google Scholar]
- van Leeuwen, T. , Vanhoof, M.J.M. , Kerkhof, F.D. , Stevens, J.M.G. and Vereecke, E.E. (2018) Insights into the musculature of the bonobo hand. Journal of Anatomy, 233(3), 328–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemelin, P. and Diogo, R. (2016) Anatomy, Function, and Evolution of the Primate Hand Musculature The Evolution of the Primate Hand. Developments in Primatology: Progress and Prospects New York, NY: Springer, pp. 155–193. [Google Scholar]
- Lemelin, P. and Schmitt, D. (1998) The relation between hand morphology and quadrupedalism in primates. American Journal of Physical Anthropology, 105(2), 185–197. [DOI] [PubMed] [Google Scholar]
- Lewis, O.J. (1985) Derived morphology of the wrist articulations and theories of hominoid evolution. Part II. The midcarpal joints of higher primates. Journal of Anatomy, 142(3), 151–172. [PMC free article] [PubMed] [Google Scholar]
- Liu, M.‐J. , Xiong, C.‐H. and Hu, D. (2016) Assessing the manipulative potentials of monkeys, apes and humans from hand proportions: Implications for hand evolution. Proceedings of the Royal Society B: Biological Sciences, 283, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macalister, A. (1868) On the nature of the coronoid portion of the Pronator Radii Teres. Journal of Anatomy and Physiology, 2(1), 8–12. [PMC free article] [PubMed] [Google Scholar]
- Manter, J.T. (1938) The dynamics of quadrupedal locomotion. Journal of Experimental Biology, 15, 522–540. [Google Scholar]
- Marzke, M.W. (2009) Upper‐limb evolution and development. Journal of Bone and Joint Surgery ‐ Series A, 91‐A, 26–30. [DOI] [PubMed] [Google Scholar]
- McMahon, T. , Van Zijl, P.C.M. and Gilad, A.A. (2015) The advantage of throwing the first stone: How understanding the evolutionary demands of Homo sapiens is helping us understand carpal motion. Journal of American Academy of Orthopaedic Surgeons, 27(3), 320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurrich, J.P. (1903) The phylogeny of the forearm flexors. American Journal of Anatomy, 2(2), 177–209. [Google Scholar]
- Michilsens, F. , Vereecke, E.E. , D'Août, K. and Aerts, P. (2009) Functional anatomy of the gibbon forelimb: Adaptations to a brachiating lifestyle. Journal of Anatomy, 215(3), 335–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michilsens, F. , Vereecke, E.E. , D’Août, K. and Aerts, P. (2010) Muscle moment arms and function of the siamang forelimb during brachiation. Journal of Anatomy, 217(5), 521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, R.A. (1932) Evolution of the pectoral girdle and fore limb in the Primates. American Journal of Physical Anthropology, 17(1), 1–56. [Google Scholar]
- Le Minor, J.M. (1994) The sesamoid bone of Musculus abductor pollicis longus (Os radiale externum or Prepollex) in Primates. Cells Tissues Organs, 150(3), 227–231. [DOI] [PubMed] [Google Scholar]
- Natsis, K. , Totlis, T. , Vlasis, K. , Sofidis, G. , Lazaridis, N. and Tsitouridis, I. (2012) Dorsoepitrochlearis muscle: An unknown cause of shoulder motion limitation and axilla deformity. Journal of Orthopaedic Science, 17(2), 186–188. [DOI] [PubMed] [Google Scholar]
- Nayak, S.R. , Krishnamurthy, A. , Pai, M.M. , Prabhu, L.V. , Ramanathan, L.A. , Ganesh Kumar, C. and Thomas, M.M. (2008) Multiple variations of the extensor tendons of the forearm. Romanian Journal of Morphology and Embryology, 49(1), 97–100. [PubMed] [Google Scholar]
- Nigst, H. and Dick, W. (1979) Syndromes of compression of the median nerve in the proximal forearm (pronator teres syndrome; anterior interosseous nerve syndrome). Archives of Orthopaedic and Traumatic Surgery, 93(4), 307–312. [DOI] [PubMed] [Google Scholar]
- Orr, C.M. , Leventhal, E.L. , Chivers, S.F. , Marzke, M.W. , Wolfe, S.W. and Crisco, J.J. (2010) Studying primate carpal kinematics in three dimensions using a computed‐tomography‐based markerless registration method. Anatomical Record, 293(4), 692–701. [DOI] [PubMed] [Google Scholar]
- Orr, C.M. (2017) Locomotor hand postures, carpal kinematics during wrist extension, and associated morphology in anthropoid primates. Anatomical Record, 300(2), 382–401. [DOI] [PubMed] [Google Scholar]
- Orr, C.M. (2018) Kinematics of the anthropoid os centrale and the functional consequences of scaphoid‐centrale fusion in African apes and hominins. Journal of Human Evolution, 114, 102–117. [DOI] [PubMed] [Google Scholar]
- Prime, J.M. and Ford, S.M. (2016) Hand Manipulation Skills in Hylobatids In: Evolution of Gibbons and Siamang. New York, NY: Springer, pp. 269–289. [Google Scholar]
- Richmond, B.G. (2001) Functional morphology of the midcarpal joint in knuckle‐walkers and terrestrial quadrupeds In: Hidemi I., Tuttle R., Pickford M., Ogihara N. & Nakatsukasa M. (Eds.) Human Origins and Environmental Backgrounds. New York, NY: Springer, pp. 105–122. [Google Scholar]
- de Ruiter, G.C.W. and van Duinen, S.G. (2017) Complete removal of the epitrochleoanconeus muscles in patients with cubital tunnel syndrome: results from a small prospective case series. World Neurosurgery, 104, 142–147. [DOI] [PubMed] [Google Scholar]
- Sarmiento, E.E. (1988) Anatomy of the hominoid wrist joint: Its evolutionary and functional implications. International Journal of Primatology, 14(A), 1–345. [Google Scholar]
- Sehirli, Ü.S. , Cavdar, S. and Yüksel, M. (2001) Bilateral variations of the abductor pollicis longus. Annals of Plastic Surgery, 47(5), 582–583. [DOI] [PubMed] [Google Scholar]
- Sonntag, C.F. (1922) On the anatomy of the drill (Mandrillus leucophaeus). Proceedings of the Zoological Society of London, 92(2), 429–453. [Google Scholar]
- Stern, J.T. and Larson, S.G. (2001) Telemetered electromyography of the supinators and pronators of the forearm in gibbons and chimpanzees: Implications for the fundamental positional adaptation of hominoids. American Journal of Physical Anthropology, 115(3), 253–268. [DOI] [PubMed] [Google Scholar]
- Straus, W.L. (1941) The phylogeny of the human forearm extensors (Concluded). Human Biology, 13(2), 23–50. [Google Scholar]
- Stuart, P.R. (1996) Pronator quadratus revisited. Journal of Hand Surgery (European volume), 21(6), 714–722. [DOI] [PubMed] [Google Scholar]
- Susman, R.L. , Jungers, W.L. and Stern, J.T. (1982) The functional morphology of the accessory interosseous muscle in the gibbon Hand: Determination of locomotor and manipulatory compromises. Journal of Anatomy, 134(1), 111–120. [PMC free article] [PubMed] [Google Scholar]
- Suwannakhan, A. , Tawonsawatruk, T. and Meemon, K. (2016) Extensor tendons and variations of the medial four digits of hand: a cadaveric study. Surgical and Radiologic Anatomy. Springer Paris, 38(9), 1083–1093. [DOI] [PubMed] [Google Scholar]
- Thompson, N.E. , Ostrofsky, K.R. , McFarlin, S.C. , Robbins, M.M. , Stoinski, T.S. and Almécija, S. (2018) Unexpected terrestrial hand posture diversity in wild mountain gorillas. American Journal of Physical Anthropology, 166(1), 84–94. [DOI] [PubMed] [Google Scholar]
- Thompson, N.W. , Mockford, B.J. and Cran, G.W. (2001) Absence of the palmaris longus muscle: A population study. Ulster Medical Journal, 70(1), 22–24. [PMC free article] [PubMed] [Google Scholar]
- Tubbs, R.S. , Salter, E.G. and Oakes, W.J. (2005) Contrahentes digitorum muscle. Clinical Anatomy, 18(8), 606–608. [DOI] [PubMed] [Google Scholar]
- Tuttle, R.H. (1967) Knuckle‐walking and the evolution of hominoid hands. American Journal of Physical Anthropology, 26(2), 171–206. [Google Scholar]
- Tuttle, R.H. (1969) Quantitative and functional studies on the hands of the Anthropoidea. I. The Hominoidea. Journal of Morphology, 128(3), 309–363. [DOI] [PubMed] [Google Scholar]
- Uscetin, I. , Bingol, D. , Ozkaya, O. , Orman, C. and Akan, M. (2014) Ulnar nerve compression at the elbow caused by the epitrochleoanconeus muscle: A case report and surgical approach. Turkish Neurosurgery, 24(2), 266–271. [DOI] [PubMed] [Google Scholar]
- Vereecke, E.E. , D'Aout, K. , Payne, R. and Aerts, P. (2005) Functional analysis of the foot and ankle myology of gibbons and bonobos. Journal of Anatomy, 206(5), 453–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke, E.E. , D’Août, K. and Aerts, P. (2006) Locomotor versatility in the white‐handed gibbon (Hylobates lar): A spatiotemporal analysis of the bipedal, tripedal, and quadrupedal gaits. Journal of Human Evolution, 50(5), 552–567. [DOI] [PubMed] [Google Scholar]
- Williams, S.A. (2010) Morphological integration and the evolution of knuckle‐walking. Journal of Human Evolution, 58(5), 432–440. [DOI] [PubMed] [Google Scholar]
- Yamamoto, C. , Murakami, T. and Ohtsuka, A. (1988) Homology of the adductor pollicis and contrahentes muscles: a study of monkey hands. Acta Medica Okayama, 42(4), 215–226. [DOI] [PubMed] [Google Scholar]
- Yoshida, Y. (1995) Anatomical studies on the Extensor Pollicis et Indicis Accessorius Muscle and the Extensor Indicis Radialis Muscle in Japanese. Okajimas Folia Anatomica Japonica, 71(6), 355–363. [DOI] [PubMed] [Google Scholar]
- Zihlman, A.L. , Farland, R.K.M.C. and Mi, G. (2011) Functional anatomy and adaptation of male Gorillas (Gorilla gorilla gorilla) with comparison to male Orangutans (Pongo pygmaeus). Anatomical Record, 294, 1842–1855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Table S1


