Abstract
Life expectancy is rising in most parts of the world as is the prevalence of chronic diseases. Suboptimal adherence to long‐term medications is still rather the norm than the exception, although it is well known that suboptimal adherence compromises the therapeutic effectiveness. Information and communications technology provides new concepts for improving adherence to medications. These so‐called telehealth concepts or services help to implement closed‐loop healthcare paradigms and to establish collaborative care networks involving all stakeholders relevant to optimising the overall medication therapy. Together with data from Electronic Health Records and Electronic Medical Records, these networks pave the way to data‐driven decision support systems. Recent advances in machine learning, predictive analytics, and artificial intelligence allow further steps towards fully autonomous telehealth systems. This might bring advances in the future: disburden healthcare professionals from repetitive tasks, enable them to timely react to critical situations, and offer a comprehensive overview of the patients' medication status. Advanced analytics can help to assess whether patients have taken their medications as prescribed, to improve adherence via automatic reminders. Ultimately, all relevant data sources need to be collated into a basis for data‐driven methods, with the goal to assist healthcare professionals in guiding patients to obtain the best possible health status, with a reasonable resource utilisation and a risk‐adjusted safety and privacy approach. This paper summarises the state‐of‐the‐art of telehealth and artificial intelligence applications in medication management. It focuses on 3 major aspects: latest technologies, current applications, and patient related issues.
Keywords: adherence, artificial intelligence, machine learning, medication, telehealth
1. INTRODUCTION
Life expectancy in many parts of the world is on the rise, resulting in an increased number of patients suffering from 1 or more chronic disease, which may reduce the patient and caregiver quality of life, and which may increase healthcare costs. The most prevalent chronic diseases in older adults (age ≥65 years) are cardiovascular diseases, which are also the most frequent cause of death.1 Heart failure (HF), for example, has a prevalence of >10% among people aged >70 years. In HF patients, the 12‐month all‐cause mortality rates are up to 17% and there are also high 12‐month readmission rates of up to 44%.2 For HF patients, postdischarge medication reconciliation is critical to achieve adequate adherence to medications and avoid hospital readmission. Patients must acknowledge the need to take their medications as prescribed and to change their lifestyle where appropriate. Both tasks can be supported by the use of upcoming technologies.3
Although it is well known that nonadherence to medications bears high risks,4 adherence to long‐term medications is frequently suboptimal.5 Information and communications technology (ICT) provides new tools for improving adherence to medications with the ultimate goal to maintain or improve patient health, and to avoid hospitalisation or a transition from outpatient to institutionalised care. These ICT concepts and systems will be designated telehealth in the following, and are also known as connected health, remote monitoring, personal health or mHealth.
This paper focuses on 3 major aspects of telehealth and artificial intelligence (AI) in medication management: latest technologies, current applications and patient‐related issues using HF as representative chronic disease example (see Table 1). The used terminology is consistent with the ABC taxonomy.6
Table 1.
Outline of this paper
| Technologies | Data sources in telehealth settings, near‐field communication in telehealth, near‐field communication‐based technologies for monitoring adherence to medications, other technologies for monitoring adherence to medications, data‐driven technologies for monitoring adherence to medications, safety and security for data‐driven management of adherence. |
| Applications | Closed‐loop healthcare, medication management in telehealth applications, methods for quantification of adherence to medications, application of artificial intelligence in telehealth settings, second opinion and expert feedback in telehealth applications. |
| Patient issues | Challenges for typical telehealth patients, user interfaces and usability, advances of telehealth in monitoring adherence to medications (early recognition, completeness, validation, reminders). |
2. MONITORING CLINICAL PARAMETERS AND ADHERENCE TO MEDICATIONS IN TELEHEALTH: STATE‐OF‐THE‐ART AND UPCOMING TECHNOLOGIES
2.1. Data sources in telehealth settings
Common data sources in telehealth settings are medical sensor devices like blood pressure meters and body weight scales. Additionally, patients can enter data regarding their subjective wellbeing and their medication intake.7 The actual selection of the devices to enable telehealth are determined by the underlying disease that would need to be monitored, e.g. blood glucose meters for diabetes, electrocardiogram recorders or pedometers for HF.
To monitor adherence to medications, a commonly used approach is smartphone apps, where app (abbreviation for application) denotes software specifically designed for mobile devices like tablets or smartphones.8 Such apps can e.g. scan the EAN‐13 barcodes of medication packages using the smartphone's camera to ensure that the right medication is taken at the right time.8, 9 However, in hospitals and other inpatient settings, longer lists of monitored drugs may exist. Especially in these scenarios answering prompts on a touchscreen can provide a more comfortable alternative to physically interacting with every single medication packaging.10
In both situations, the prescribed medications can be displayed on the screens together with default values for the administration of a certain medication, e.g. 1–3 times daily, 100–200 mg, single dose.
By displaying questions to the patient, apps could also include a digital version of self‐reported questionnaires.
2.2. Near‐field communication in telehealth
Newer smartphones and medical devices frequently have built‐in near‐field communication (NFC) capabilities, which can simplify the workflow of patient self‐monitoring. NFC is a short‐range communication standard that allows data transmission within a distance of some centimetres between 2 NFC devices close together (touching). In case of NFC, an appropriate app and a smartphone build the key element of an intuitive telehealth system that can be used to monitor the patient's clinical parameters and adherence to medications.7, 8, 9, 11 Passive NFC tags can store and provide data enabling the identification of certain items, like for example medication packages.8 NFC can provide multiple interactions and it is a core technology of the so‐called Internet of things.9, 12 Figure 1 shows a depiction of NFC‐based data collection. A comprehensive description on how NFC can be used to design easy‐to‐learn and easy‐to‐use telehealth solutions is described by Abibi et al.13
Figure 1.
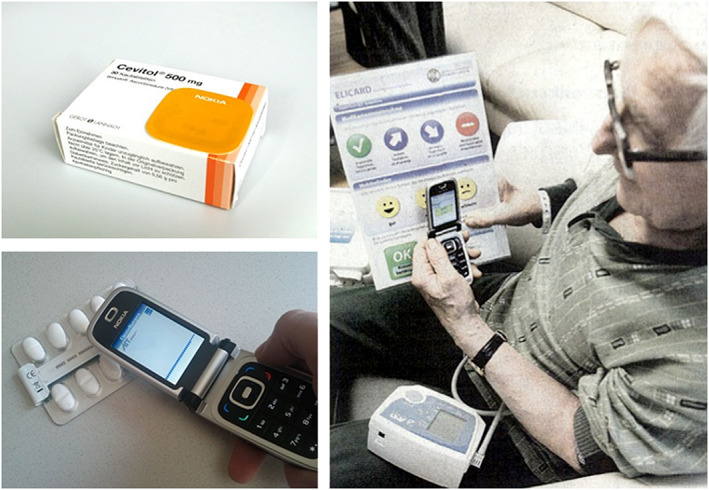
Data collection via near‐field communication (NFC). Left top: medication packaging with attached NFC tag; left bottom: collecting data from a smart blister; right: collecting data via NFC by touching a blood pressure meter and the fields of a chart, which are equipped with NFC tags. (© AIT Austrian Institute of Technology GmbH)
2.3. NFC‐based technologies for monitoring adherence to medications
Instead of manual data entry or scanning bar codes, NFC tags can be used for monitoring adherence to medications.7, 11 By bringing such NFC tags in touch with a smartphone, patients can record the intake of their medications.
Alternatively, special blisters can be used to monitor the intake of medications via NFC. Such smart blisters are covered with a foil that includes an electronic circuit.11, 14 A microcontroller recognises interruptions of the conductive paths when the tablets or capsules are taken out of the blister and will then record the time and date. Via an accompanying app and telehealth link, the data can be shared in real time with healthcare professionals and, where appropriate, other caregivers. As smart blisters do not record the actual intake of the tablet or capsule, the accuracy of the measurement is limited.11, 14
2.4. Other technologies for monitoring adherence to medications
Further concepts of monitoring adherence to medications involve medicine bottles or boxes, which recognise and store opening events8, 15 as well as eDispensers, which are able to remind patients that they need to take their medication, and that can directly supply them with it.16 Other concepts relate to recording and analysing the patient's hand movements with triaxial accelerometers of wireless wearable devices.17 Systems that monitor medication ingestion are swallowing the medication in front of a camera, which tracks the patient by motion detection,18 the addition of a fluorophore to the medication, which can be detected in the bloodstream with a monitoring device on the patient's wrist17 or with a placebo medication that is coadministered with the drug and that is equipped with a microchip that sends out a signal when it is digested.19 The development of tablets or capsules with an integrated sensor that would record ingestion or digestion would be another idea.
2.5. Data‐driven technologies for monitoring adherence to medications
In addition to these more or less established technologies, technical advances have resulted in an increasing popularity of highly data‐driven technologies.
A first step towards autonomous telehealth are decision support systems, which are used to evaluate predefined decision rules on measured vital signs and subsequently suggest suitable medication changes to healthcare professional, caregiver or patient.20, 21 When it comes to detecting patterns in data, machine learning approaches show high potential (see Table 2 for a description of data science related terms). In the field of HF, up to now, most machine learning approaches were based on electronic health record (EHR) data and claims for reimbursement. The machine learning was aimed at assisting healthcare professionals in diagnosis, risk assessment and prediction of adverse events.22
Table 2.
Explanation of selected terms related to data science
| Artificial intelligence | The technical replication of human intelligence. |
| Data‐driven | Relying on big amounts of data. |
| Decision support | The automated suggestion of a beneficial option. |
| Deep learning | An approach to technically replicate the cognitive abilities of the human brain by consecutively passing information through several layers of an artificial neural network. |
| Machine learning | The application of algorithms that can learn from data. In supervised machine learning, the algorithms are trying to correctly estimate predefined classes (e.g. naïve Bayes, support vector machine, regression trees). In unsupervised machine learning, the algorithms try to find patterns in the data coming up with classes themselves (e.g. clustering). Deep learning can either be supervised or unsupervised. |
| Predictive analytics | The prediction of future outcomes by analysing existing data. |
For direct monitoring of patients' medication intake, AI has been utilised by a mobile app that supervises the patient through the camera of her/his mobile device. This way, the patients and their medications were identified and the intake was documented with high reliability.23, 24 Up to 2018, only 1 single study related to the use of daily measured vital signs that were recorded in a HF telehealth setting. Using multiresolution analysis and a naïve Bayes classifier, the highest predictive value was gained for weight measurements and diastolic blood pressure curves.25 For chronic obstructive pulmonary disease and asthma telehealth, a variety of machine learning approaches (naïve Bayes classifiers, support vector machines, regression trees and clustering) have been applied to symptoms, peripheral oxygen saturation (SpO2), lung function, blood pressure, heart rate, weight, temperature, respiratory rate, lung sounds and physical activity measurements. However, the resulting prediction accuracy was found to be not clinically useful by now.26 Further research is needed because there is poor performance of conventional algorithms and too little integration of such systems into routine healthcare.
2.6. Safety and security for data‐driven management of adherence
For application in medicine, computer algorithms need to be safe and useful. Thus, if a software is considered as a medical device, there are regulatory issues that need to be met. In the EU, currently, medical devices need to be fit for their intended purpose, which is assessed through compliance with the Essential Requirements of Directive 93/42/EEC. In the US, medical devices need to get clearance from the U.S. Food and Drug Administration (FDA). However, validation of AI‐based systems is difficult, as on the one hand, decisions can usually not be understood easily and on the other hand the skills and experience of an expert can hardly be compared to a software. Due to the question of accountability, many manufacturers provide their software as supportive tools for experts, who take the final decision and are thus responsible.27
The collection of sensitive patient information from various sources (telehealth, EHRs etc.) is related to privacy risks and needs ethical considerations. Access to personal health data has to be carefully controlled and the patients' privacy strongly needs to be protected. Security risks incurred by inappropriate behaviour of the user (patient, caregiver, healthcare professional) as well as external attacks need to be prevented by carefully designed security system architectures (privacy by design). Data integrity and appropriate information supply for use by healthcare professionals are of equal importance to the wellbeing of patients.28 Access to telehealth systems needs to be protected by state‐of‐the‐art security concepts. For example, Austria's EHR system (ELGA) supports access via the Handy‐Signature, which requires manual entry of (i) a telephone number, (ii) a password and (iii) a confirmation code received via SMS.10 Although this procedure is considered highly secure in terms of access control, it should be reminded that the login procedure for telehealth systems should be tailored to the target group, which are commonly older adults, and for whom the indicated approach may not work, i.e. there would be a need for a user friendly interface.
3. STATUS AND CONCEPT OF TELEHEALTH APPLICATIONS AND THEIR FUTURE POTENTIAL
3.1. Closed‐loop healthcare
The technologies described above offer new possibilities to healthcare professionals, caregivers and patients and will be beneficial for all if they are embedded into a corresponding telehealth‐enabled infrastructure and healthcare processes. A typical example on how to utilise telehealth services, is to apply the so‐called closed‐loop healthcare paradigm (see Figure 2), which offers a collaborative care network involving all stakeholders to achieve the best results in the therapy of chronic diseases.3, 9, 14, 19 In closed‐loop healthcare, general practitioners (GPs), hospital physicians, disease‐specific specialists, nurses, other caregivers, patients and their relatives share a common communication platform. Thus, no information gets lost and stakeholders can easily interact with each other.3
Figure 2.
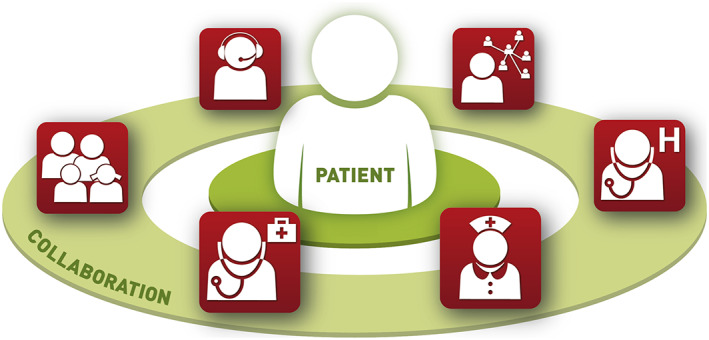
Closed‐loop healthcare paradigm in the heart failure scenario. Clockwise: Coordinator, clinician, nurse, general practitioner, relatives, helpdesk. (© AIT Austrian Institute of Technology GmbH)
3.2. Medication management in telehealth applications
In HF patients in particular, medication is used to control blood pressure and fluid retention, which has been proven to enhance clinical outcomes when applied as intended. The HF guidelines of the European Society of Cardiology give clear recommendations for therapy adjustments to avoid deteriorations.2 For instance, diuretic dose adjustment should be considered, if the body weight of the patient increases more than 2 kg in 3 days, as a matter of increased fluid retention.2 Rulesets for medication intake can be applied in telehealth settings through the implementation of reminders or notification messages, which are triggered by events derived from automated analysis of biosignals.20, 21
3.3. Methods for quantification of adherence to medications
To quantify adherence to medications, various methods can be applied.9 There are straight forward methods, such as counting the number of bottle openings or tablets or capsules taken per defined time interval.29, 30 Also, calculating the percentage of days or weeks, when the patient took their medicine in accordance with the treatment plan is possible.31 Another option is to count the number of so‐called drug holidays, which are defined as 1 or more days without following the expected scheme of medication intake.31 Furthermore, there are more complex methods such as estimating the time the patient's drug concentration was on an appropriate level9 or asking the patients to give feedback via predefined questionnaires.32, 33 Figure 3 shows common methods for quantification of adherence to medications. A comprehensive description on how adherence to medications can be assessed and quantified can be found in Stegemann.9
Figure 3.
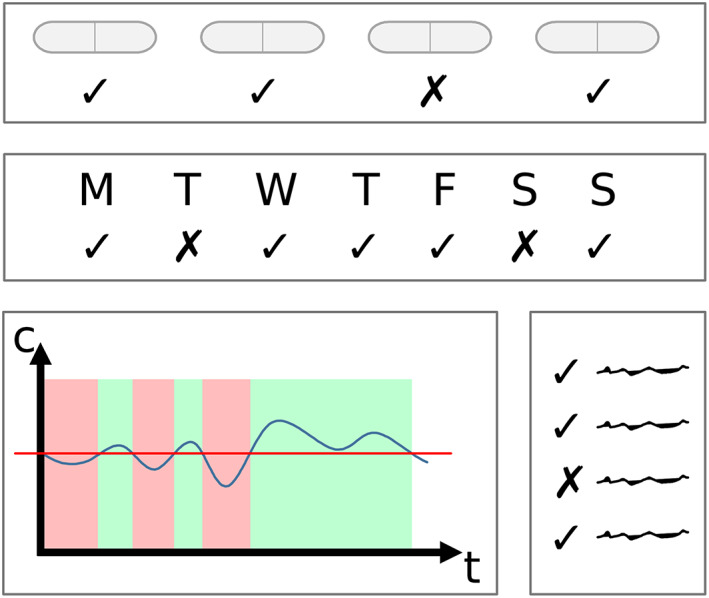
Common methods for quantification of adherence to medications. Top: calculating the percentage of pills taken; middle: calculating the fraction of adherent time intervals (e.g. days); bottom left: calculating the fraction of time, when the concentration of the drug was on an appropriate level; bottom right: evaluating a questionnaire, which was answered by a patient
3.4. Application of AI in telehealth settings
Advances in ICT and data analytics lead to the new concept of AI. The concept has been applied to more and more fields recently, starting with voice‐controlled personal assistants (Alexa, Siri), fraud detection, image recognition, web‐search or autonomous driving.34 Even if it is a buzzword today, AI could potentially enable fully autonomous telehealth in the future.8 Previously, computer programs relied on expert knowledge, which was used to derive rules and patterns that could be applied in specific scenarios. With methods such as deep learning, AI is capable of learning from source data without human supervision. Based on example cases with known outcomes, the algorithms come up with complex functions by themselves to predict the clinical outcomes for individual patients and alert healthcare professionals when appropriate. One obstacle for applying such methods is the concern about the black‐box process, i.e. it is often difficult to give information on WHY a specific result or decision has been obtained.34 Without the chance to do a plausibility check, such systems may face scepticism, even if clinical trials provide evidence that these systems potentially perform better than a skilled healthcare professional.35
Combining all available data sources into a comprehensive dataset (see Figure 4), which is used to gain a deeper understanding of an individual patient's health state and to optimise treatment and adherence, might be beneficial. However, there are many open regulatory issues regarding privacy, security and standardisation of data that still need to be addressed and solved before such methods can be used at a larger scale.9 Early consideration of novel approaches by the regulatory domain can be fostered through scientific advice procedures or studies into regulatory knowledge gaps.36
Figure 4.
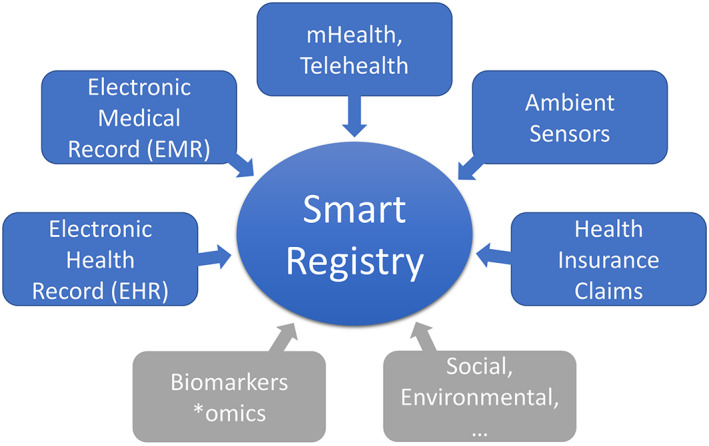
Combining all available data sources into a comprehensive dataset
3.5. Second opinion and expert feedback in telehealth applications
For the long‐term treatment of chronic diseases, expertise that is specific to multimorbid patients can be used to guide physicians in prescribing medicines according to up‐to‐date knowledge. The same applies to knowledge provided by AI‐based systems.9
4. PATIENT RELATED ISSUES AND POTENTIAL ADVANCES THROUGH TELEHEALTH
4.1. Challenges for typical telehealth patients
Patients who benefit most from telehealth applications are usually (i) older, (ii) affected by multimorbidity and (iii) on polypharmacy (i.e. using 5 drugs or more concurrently), as all this implies a challenging situation for the patient, caregiver or healthcare professional.9, 11 Since clinical trials usually include younger adults suffering from 1 disease only and on single therapy i.e. as clinical trials usually exclude geriatric or even frail older people who are suffering from multimorbidity and who are on polypharmacy,19 the effects of a medication in a real world setting can hardly be predicted from the clinical trial data. Telehealth has a huge potential to generate real‐world evidence when the special needs of the affected patients are considered while setting up a telehealth system. Cognitive and physical impairments should be considered, as older and multimorbid adults may e.g. have usability problems with common smartphone apps.8
4.2. User interfaces and usability
Long‐term monitoring of vital signs and medication intake remains a challenge as it requires active collaboration by the patients and/or their caregivers. Patients need to be highly motivated and willing to actively follow the treatment plan over a long period. Methods that require the patient's judgement (e.g. retrospective self‐estimation of adherence) were shown to be unreliable during long‐term monitoring.5, 17 The best long‐term adherence was observed for simple methods, which could be integrated into daily routines.5 The related devices need to be stable and ready for use at any time.9, 19 The underlying technology should support a fast and smooth workflow without lags or other interruptions, as any inconvenience could potentially lead to frustration and rejection. To keep the expenses low, the used technologies should also be cost‐effective and broadly available.19
However, exaggerated simplification of data entry (e.g. by presenting predefined default values), can reduce the data quality. Also, patients or caregivers might get caught in daily routine and confirm prompts without checking these in detail.10
4.3. Advances of telehealth in monitoring adherence to medications
Telehealth brings many potential advances regarding the medication therapy:
4.3.1. Early recognition
In standard outpatient settings, healthcare professionals cannot react to adverse or undesired effects without a significant delay (i.e. at the time of the next visit). Adherence monitoring systems including the transmission of vital signs can enable healthcare professionals to timely recognise deteriorations in the patient's health status and to adapt the therapeutic setting accordingly.20
4.3.2. Completeness
Comprehensive documentation of all patient information anytime and anywhere is a key criterion for optimal healthcare provision. Data completeness on drug therapy include all drugs that have been prescribed, dispensed and taken. Linking the monitoring system to a comprehensive EHR system, containing medication information — e.g. the e‐Medikation application in Austria's national EHR system37 — can give healthcare professionals a comprehensive overview of the patient's current medication status.10 A combination of these data with patient reported medication intake from telehealth systems can prevent adverse effects resulting from e.g. drug–drug interactions, intolerance or double prescriptions.
4.3.3. Validation
A major challenge for systems measuring adherence to medications is the validation of medication intake confirmations.19 While direct validation of the intake via smart blisters with built‐in electronics remains difficult, even the resulting effects as illustrated via vital sign monitoring can provide adherence information. For example, healthcare professionals can observe the reactions of blood pressure, heart rate, etc., and potentially determine whether a patient has taken the medication as prescribed or not.14
4.3.4. Reminders
A major reason for nonadherence is forgetfulness.5 When patients leave their daily routine, they are especially prone to either miss the correct time point or even completely forget to take their medication. Even when adherence to medications stays on a high level from Monday to Thursday, it often significantly drops during weekends and holidays.38 Automatic reminders can improve adherence, when they are sent in cases of detected nonadherence. In terms of the content of such reminders, personalised message texts did not show improved adherence.5 The time of day seems to be relevant, as better adherence was observed for morning doses compared to other doses.38 Reminder systems should notify the patients in situations, when they have the time and possibility to immediately take their medications.19 Ideally, systems for management of adherence should be able to learn from all the available data sources such as the time points of previously confirmed intakes, the patients' feedback or additional data from e.g. calendar apps or GPS tracking.
5. CONCLUSION
Healthcare professionals have to prescribe many medicines to most effectively treat a growing number of patients who are affected by multiple complex diseases. However, beneficial effect to the patients' health will be limited, as long as they do not or cannot use their medication in accordance with the prescribed regimen. Especially in case of medications for long‐term use, adherence remains a challenge.
Recent developments of ICT have brought numerous possibilities to support healthcare professionals in supervising and interacting with their patients and/or their caregivers in a comfortable and effective way. Telehealth can provide healthcare professionals with a comprehensive overview of the patients' health status and adherence to the prescribed medications. Important vital signs, current prescriptions and medication intake are collected and this information can be shared among patients, caregivers and other healthcare professionals so that all will have access to the actual information.
There are still many additional ICT applications in telehealth that could be of further use. Decision support systems could be utilised to deal with routine monitoring tasks, such as identifying patients at risk early on. Such systems will increasingly incorporate machine learning and AI. In normal conditions, automated processes will be more able to manage the whole telehealth process, guiding patients with advice and therapy adaptations, while only deviations from routine will need the intervention of a healthcare professional.
To reach this goal, on the one hand, predictive modelling methods need to be applied retrospectively to telehealth data to reveal patterns predicting the patients' clinical outcomes. On the other hand, prospective clinical studies are urgently needed to further assess the many opportunities and challenges of telehealth in the context of AI‐based medication adjustments.
Finally, all relevant data sources need to be made available and combined to a comprehensive dataset to create the basis for data‐driven methods, with the ultimate goal to help healthcare professionals in guiding their patients to the best possible health status, with a reasonable amount of resources and a risk adjusted safety and privacy approach.
COMPETING INTERESTS
There are no competing interests to declare.
Eggerth A, Hayn D, Schreier G. Medication management needs information and communications technology‐based approaches, including telehealth and artificial intelligence. Br J Clin Pharmacol. 2020;86:2000–2007. 10.1111/bcp.14045
REFERENCES
- 1. Brennan P, Perola M, van Ommen GJ, Riboli E, Consortium EC. Chronic disease research in Europe and the need for integrated population cohorts. Eur J Epidemiol. 2017;32(9):741‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37:2129‐2200. [DOI] [PubMed] [Google Scholar]
- 3. Modre‐Osprian R, Pölzl G, Von Der Heidt A, Kastner P. Closed‐loop healthcare monitoring in a collaborative heart failure network. Stud Health Technol Inform. 2014;198:17‐24. [PubMed] [Google Scholar]
- 4. De Geest S, Sabaté E. Adherence to long‐term therapies: evidence for action. Eur J Cardiovasc Nurs. 2003;2(4):323. [DOI] [PubMed] [Google Scholar]
- 5. Vervloet M, Linn AJ, van Weert JC, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J am Med Inform Assoc. 2012;19(5):696‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kastner P, Morak J, Modre R, et al. Innovative telemonitoring system for cardiology: from science to routine operation. Appl Clin Inform. 2010;1(2):165‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schreier G, Schwarz M, Modre‐Osprian R, Kastner P, Scherr D, Fruhwald F. Design and evaluation of a multimodal mHealth based medication management system for patient self administration. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:7270‐7273. [DOI] [PubMed] [Google Scholar]
- 9. Stegemann S. Developing Drug Products in an Aging Society: From Concept to Prescribing. Cham: Springer; 2016. [Google Scholar]
- 10. Ebner H, Modre‐Osprian R, Kastner P, Schreier G. Integrated medication management in mHealth applications. Stud Health Technol Inform. 2014;198:238‐244. [PubMed] [Google Scholar]
- 11. Morak J, Schwarz M, Hayn D, Schreier G. Feasibility of mHealth and near field communication technology based medication adherence monitoring. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:272‐275. [DOI] [PubMed] [Google Scholar]
- 12. Schreier G. The internet of things for personalized health. Stud Health Technol Inform. 2014;200:22‐31. [PubMed] [Google Scholar]
- 13. Adibi S. Mobile Health: a Technology Road Map. Cham: Springer; 2015. [Google Scholar]
- 14. Brath H, Morak J, Kästenbauer T, et al. Mobile health (mHealth) based medication adherence measurement ‐ a pilot trial using electronic blisters in diabetes patients. Br J Clin Pharmacol. 2013;76(Suppl 1):47‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosen MI, Rigsby MO, Salahi JT, Ryan CE, Cramer JA. Electronic monitoring and counseling to improve medication adherence. Behav Res Ther. 2004;42(4):409‐422. [DOI] [PubMed] [Google Scholar]
- 16. Wiegratz I, Elliesen J, Paoletti AM, Walzer A, Kirsch B. Adherence with ethinylestradiol 20 μg/drospirenone 3 mg in a flexible extended regimen supported by the use of a digital tablet dispenser with or without acoustic alarm: an open‐label, randomized, multicenter study. Int J Womens Health. 2015;7:19‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang R, Sitova Z, Jia X, et al. Automatic identification of solid‐phase medication intake using wireless wearable accelerometers. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:4168‐4171. [DOI] [PubMed] [Google Scholar]
- 18. Bilodeau GA, Ammouri S. Monitoring of medication intake using a camera system. J Med Syst. 2011;35(3):377‐389. [DOI] [PubMed] [Google Scholar]
- 19. Stegemann S, Baeyens JP, Cerreta F, et al. Adherence measurement systems and technology for medications in older patient populations. Eur Geriatr Med. 2012;3(4):254‐260. [Google Scholar]
- 20. Kropf M, Modre‐Osprian R, Hayn D, Fruhwald F, Schreier G. Telemonitoring in heart failure patients with clinical decision support to optimize medication doses based on guidelines. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:3168‐3171. [DOI] [PubMed] [Google Scholar]
- 21. Kropf M, Modre‐Osprian R, Gruber K, Fruhwald F, Schreier G. Evaluation of a clinical decision support rule‐set for medication adjustments in mHealth‐based heart failure management. Stud Health Technol Inform. 2015;212:81‐87. [PubMed] [Google Scholar]
- 22. Tripoliti EE, Papadopoulos TG, Karanasiou GS, Naka KK, Fotiadis DI. Heart failure: diagnosis, severity estimation and prediction of adverse events through machine learning techniques. Comput Struct Biotechnol J. 2017;15:26‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bain EE, Shafner L, Walling DP, et al. Use of a novel artificial intelligence platform on Mobile devices to assess dosing compliance in a phase 2 clinical trial in subjects with schizophrenia. JMIR Mhealth Uhealth. 2017;5(2):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Labovitz DL, Shafner L, Reyes Gil M, Virmani D, Hanina A. Using artificial intelligence to reduce the risk of nonadherence in patients on anticoagulation therapy. Stroke. 2017;48(5):1416‐1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koulaouzidis G, Iakovidis DK, Clark AL. Telemonitoring predicts in advance heart failure admissions. Int J Cardiol. 2016;216:78‐84. [DOI] [PubMed] [Google Scholar]
- 26. Sanchez‐Morillo D, Fernandez‐Granero MA, Leon‐Jimenez A. Use of predictive algorithms in‐home monitoring of chronic obstructive pulmonary disease and asthma: a systematic review. Chron Respir Dis. 2016;13(3):264‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pesapane F, Volonté C, Codari M, Sardanelli F. Artificial intelligence as a medical device in radiology: ethical and regulatory issues in Europe and the United States. Insights Imaging. 2018;9(5):745‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ozair FF, Jamshed N, Sharma A, Aggarwal P. Ethical issues in electronic health records: a general overview. Perspect Clin Res. 2015;6(2):73‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diaz E, Levine HB, Sullivan MC, et al. Use of the medication event monitoring system to estimate medication compliance in patients with schizophrenia. J Psychiatry Neurosci. 2001;26(4):325‐329. [PMC free article] [PubMed] [Google Scholar]
- 31. Vrijens B, Goetghebeur E. Comparing compliance patterns between randomized treatments. Control Clin Trials. 1997;18(3):187‐203. [DOI] [PubMed] [Google Scholar]
- 32. Gabr WM, Shams ME. Adherence to medication among outpatient adolescents with epilepsy. Saudi Pharm J. 2015;23(1):33‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self‐report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16(2):269‐277. [DOI] [PubMed] [Google Scholar]
- 34. Fogel AL, Kvedar JC. Artificial intelligence powers digital medicine. Npj Digital Medicine. 2018;1(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haenssle HA, Fink C, Schneiderbauer R, et al. Groups Rsl‐Ial‐I. man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists. Ann Oncol. 2018;29(8):1836‐1842. [DOI] [PubMed] [Google Scholar]
- 36. European Medicines Agency . Scientific advice and protocol assistance. https://www.ema.europa.eu/en/human‐regulatory/research‐development/scientific‐advice‐protocol‐assistance. Accessed Jan 10, 2019.
- 37. Gall W, Aly AF, Sojer R, Spahni S, Ammenwerth E. The national e‐medication approaches in Germany, Switzerland and Austria: a structured comparison. Int J Med Inform. 2016;93:14‐25. [DOI] [PubMed] [Google Scholar]
- 38. Vervloet M, Spreeuwenberg P, Bouvy ML, Heerdink ER, de Bakker DH, van Dijk L. Lazy sunday afternoons: the negative impact of interruptions in patients' daily routine on adherence to oral antidiabetic medication. A multilevel analysis of electronic monitoring data. Eur J Clin Pharmacol. 2013;69(8):1599‐1606. [DOI] [PubMed] [Google Scholar]


